RP describes a symptom complex comprising pain, numbness, colour changes and impaired physical function of the fingers and other extremities in response to cold and/or emotional stress [1]. RP is a major cause of pain and disease-related morbidity in SSc [2, 3]. Despite the availability of a wide range of vasodilator drug therapies to treat SSc-RP, treatment can be poorly tolerated and seldom fully effective [2]. At present, the decision to initiate and assess treatment response in RP, including the need for dose escalation, is largely based upon clinician–patient discussions around symptom severity, the perceived effectiveness of existing/planned interventions, and drug tolerability. The management of SSc-RP forms part of treatment recommendations published by the British Society of Rheumatology [4], the European League against Rheumatism [5], the Scleroderma Clinical Trials Consortium/Canadian Scleroderma Research group [6] and the UK Scleroderma Study group [7]. Each recommendation has focused on different vasodilator drug classes, rather than specific dose escalation strategies designed to optimize drug tolerability, treatment adherence and efficacy. The positioning of specific classes of vasodilator medications has been determined as much by drug cost as by efficacy considerations [4, 5]. There is no existing clinical trial– or practice-based evidence available from which to establish optimal drug dosing (for tolerability and efficacy) or to explore and fully exploit the potential value of combination approaches in the management of SSc-RP.
The ‘treat to target’ (T2T) approach has delivered improved outcomes and revolutionized management approaches in RA and PsA [8, 9]. These approaches have devised criteria for ‘remission’ and ‘low/minimal disease activity’ [9]. T2T in arthritis has resulted in significantly improved long-term structural and functional outcomes for patients [9]. However, at present a T2T approach cannot be applied to RP. There are a number of required requisites, which include reliable and feasible outcome measures of efficacy, effective treatments, and superiority of T2T over initial maximum combination therapy (including drug tolerability and survival). If drug escalation of vasodilator therapies for RP were to adopt a T2T approach, then what might be the target and/or goal of treatment? Would such an approach in SSc solely focus on RP symptom severity or incorporate other clinical aspects of digital vasculopathy, such as digital ulcer healing/occurrence. It is possible that the target may extend to subclinical features, such as evolution of capillary morphological changes at the nailfold that may antedate digital vascular complications of SSc [10] or include visceral vascular manifestations such as pulmonary arterial hypertension. Such a T2T approach for RP could also be applied to primary RP, which can also have a negative impact on patient quality of life and function [2]. The ‘target’ in primary RP, however, may be different to that developed for SSc-RP.
If RP symptom severity is the primary goal, then should the ‘target’ solely focus on RP attack frequency/duration (the principle outcome in existing diary-based approaches to assessing RP severity, such as the Raynaud’s Condition Score diary) or include outcomes that reflect broader aspects relating to how patients ‘feel’ and ‘function’ such as pain, numbness, and hand function? Would a composite measure incorporating established outcome measures be adequate, or would new outcome measures need to be devised and tested?
Would the target require complete resolution, an arbitrary proportional reduction, or a specific target threshold based on patient acceptable symptom states? Balancing treatment efficacy with drug tolerability and adherence would also require consideration. On this, and other aspects, target patient population involvement would be essential to the development of a ‘target’ that was accepted as clinically meaningful and acceptable for patients. Expert consensus would also need to be sought on a number of important considerations. For example, if RP is to be considered part of a broader continuum of digital vasculopathy in SSc that extends to the occurrence of digital ischaemic lesions, then expert consensus would need to first establish whether the SSc community is in agreement that disease modification (and the putative approach for assessing this) is a feasible and achievable goal of any T2T strategy. On this, and a number of other issues, the community may first need to establish an evidence-base that provides robust scientific justification for any future attempt at T2T strategies for SSc-RP or SSc-related digital vasculopathy.
There is limited data available from clinical trials and experts for guiding the initiation and escalation of oral therapies for RP, including after treatment failure. Our existing rather haphazard approach to managing SSc-RP has historical parallels with the previous management of RA and PsA. T2T strategies have revolutionized the management of inflammatory arthritis, with a reduced burden of erosive disease, joint destruction, and major disability. The success of T2T strategies in inflammatory arthritis can largely be attributed to more effective use of existing conventional DMARDs, although the emergence of new treatment targets has undeniably contributed to recent success. With the availability of a broad repertoire of existing and new vasodilator drugs, can we hope to achieve similar advances in the management of digital vasculopathy in SSc? For example, it is now standard practice to use initial combination drug therapies in patients with pulmonary artery hypertension, reflecting the intrinsic vascular pathology in this complication. A T2T treatment strategy trial for digital vasculopathy in SSc could explore the relative merits of different approaches (e.g. initial combination vs goal-directed sequential therapy) to modifying the progressive (structural and functional) microangiopathy of SSc and clinical sequelae within the digits (e.g. RP severity, digital ulcer occurrence) and other organs (e.g. pulmonary artery hypertension occurrence).
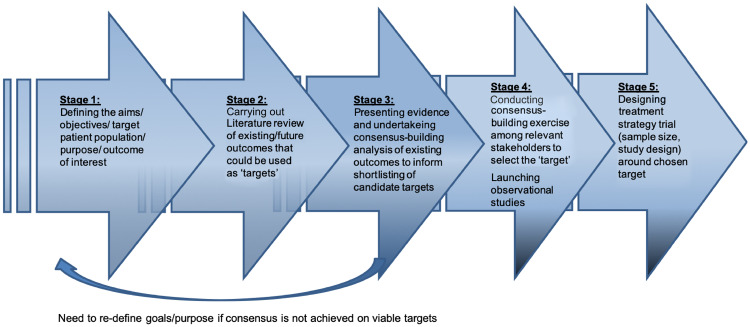
There are many barriers to the successful development of a T2T strategy for SSc-RP and digital vasculopathy, and in Fig. 1 we have proposed a roadmap with five stages that may support the development of a T2T strategy for SSc-RP. Key initial steps are to define the study population and the goals of developing a T2T strategy (stage 1) and to review and to shortlist candidate target items (stages 2 and 3, respectively). If consensus regarding viable targets is not agreed at this point, then the goals and purpose will need to be refined. Subsequently, a consensus-building exercise among relevant stakeholders (e.g. rheumatologists with an interest in SSc-RP and patient representatives) would allow the ‘target’ to be defined. Ultimately, well-designed studies (stage 5) will be required to investigate the feasibility and treatment benefit of a T2T strategy in patients with SSc-RP. Much can be learned from the initial studies of T2T for RA, including randomized trials that compared T2T with routine care, and those comparing different treatment approaches (e.g. monotherapy vs combination therapy) to reach a defined target [9]. A key feature of these studies was that patients were frequently reviewed, and there was clear guidance on how to intensify treatment in patients who had not reached the target [9]. In conclusion, it is hoped that such efforts could optimize treatment approaches for RP and herald the emergence of disease-modifying vasodilator therapies for SSc-related digital vasculopathy.
Fig. 1.
A proposal of a roadmap for developing a treat-to-target approach for SSc-RP
Acknowledgements
D.K. is supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) K24 AR063120.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: M.H. has received speaker honoraria from Actelion pharmaceuticals. J.D.P. has undertaken consultancy work and received speaker honoraria from Actelion pharmaceuticals, and undertaken consultancy work for Boehringer Ingelheim. D.K. is a consultant to Actelion, Acceleron, Arena, Bayer, Boehringer Ingelheim, Bristol-Myer Squibb, CSL Behring, Chemomab, Corbus, GSK, Genentech/Roche, Galapagos, Merck, and Mitsubishi Tanabi; has received grants as part of investigator-initiated trials (to the University of Michigan) from Bayer, Bristol-Myer Squibb, and Pfizer; and has stock options in Eicos Sciences, Inc.
References
- 1. Pauling JD, Hughes M, Pope JE.. Raynaud’s phenomenon—an update on diagnosis, classification and management. Clin Rheumatol 2019, doi: 10.1007/s10067-019-04745-5. [DOI] [PubMed] [Google Scholar]
- 2. Hughes M, Snapir A, Wilkinson J. et al. Prediction and impact of attacks of Raynaud’s phenomenon, as judged by patient perception. Rheumatology 2015;54:1443–7. [DOI] [PubMed] [Google Scholar]
- 3. Pauling JD, Domsic RT, Saketkoo LA. et al. A multi-national qualitative research study exploring the patient experience of Raynaud’s phenomenon in systemic sclerosis. Arthritis Care Res 2018;70:1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denton C, Hughes M, Gak N. et al. BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatology 2016;55:1906–10. [DOI] [PubMed] [Google Scholar]
- 5. Kowal-Bielecka O, Fransen J, Avouac J. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327–39. [DOI] [PubMed] [Google Scholar]
- 6. Fernández-Codina A, Walker KM, Pope JE; Scleroderma Algorithm Group . Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol 2018;70:1820–8. [DOI] [PubMed] [Google Scholar]
- 7. Hughes M, Ong VH, Anderson ME. et al. Consensus best practice pathway of the UK Scleroderma Study Group: digital vasculopathy in systemic sclerosis. Rheumatology 2015;54:2015–24. [DOI] [PubMed] [Google Scholar]
- 8. Coates LC, Moverley AR, McParland L. et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon DH, Bitton A, Katz JN. et al. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol 2014;66:775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paxton D, Pauling JD.. Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum 2018;48:482–94. [DOI] [PubMed] [Google Scholar]