Abstract
Aims
This study aimed to investigate whether human umbilical cord mesenchymal stem cells (UC-MSCs) can prevent articular cartilage degradation and explore the underlying mechanisms in a rat osteoarthritis (OA) model induced by monosodium iodoacetate (MIA).
Methods
Human UC-MSCs were characterized by their phenotype and multilineage differentiation potential. Two weeks after MIA induction in rats, human UC-MSCs were intra-articularly injected once a week for three weeks. The therapeutic effect of human UC-MSCs was evaluated by haematoxylin and eosin, toluidine blue, Safranin-O/Fast green staining, and Mankin scores. Markers of joint cartilage injury and pro- and anti-inflammatory markers were detected by immunohistochemistry.
Results
Histopathological analysis showed that intra-articular injection of human UC-MSCs significantly inhibited the progression of OA, as demonstrated by reduced cartilage degradation, increased Safranin-O staining, and lower Mankin scores. Immunohistochemistry showed that human UC-MSC treatment down-regulated the expression of matrix metalloproteinase-13 (MMP13) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5), and enhanced the expression of type II collagen and ki67 in the articular cartilage. Furthermore, human UC-MSCs significantly decreased the expression of interleukin (IL)-1β and tumour necrosis factor-α (TNF-α), while increasing TNF-α-induced protein 6 and IL-1 receptor antagonist.
Conclusion
Our results demonstrated that human UC-MSCs ameliorate MIA-induced OA by preventing cartilage degradation, restoring the proliferation of chondrocytes, and inhibiting the inflammatory response, which implies that human UC-MSCs may be a promising strategy for the treatment of OA.
Cite this article: Bone Joint Res 2021;10(3):226–236.
Keywords: Human umbilical cord mesenchymal stem cells, Osteoarthritis, Cartilage degradation, Inflammatory response
Article focus
This study investigated the role of human umbilical cord mesenchymal stem cells (UC-MSCs) in the treatment for osteoarthritis (OA) and explored the underlying mechanisms.
Key messages
Intra-articular injection of human UC-MSCs ameliorate OA and prevent the degradation of articular cartilage in the MIA-induced OA rat injury models.
Human UC-MSCs down-regulated the expression of MIA-induced cartilage-degrading enzymes and restored the proliferation of the injured chondrocytes.
Human UC-MSCs attenuated the MIA-induced inflammatory response and enhanced the expression of anti-inflammatory factors, tumour necrosis factor-α-induced protein 6 (TSG-6) and interleukin-1 receptor antagonist (IL-1RA).
Strengths and limitations
Our findings highlight a promising therapeutic strategy for OA via intra-articular injection of human UC-MSCs.
The exact molecular mechanisms by which human UC-MSCs inhibit inflammation and alleviate OA need to be further studied.
Introduction
Osteoarthritis (OA) is defined as a chronic degenerative and inflammatory joint disorder, involving mainly the weight-bearing joints, such as the knees and hips. Currently, there are no curative therapeutic approaches for OA, especially for severe OA treatment. Several cell-based therapies, such as autologous chondrocyte implantation, osteochondral transplantation, and autologous perichondrial and periosteal grafts have been applied in an attempt to restore the injured joint surface. Although some improvements in function and pain relief have been observed, these therapies can only temporarily alleviate symptoms,1-3 therefore stem cell-based therapies have attracted increased attention.
Mesenchymal stem cells (MSCs) possess multipotency including osteogenic and chondrogenic differentiation.4 Recent studies have demonstrated the beneficial therapeutic effects of MSCs for bone and joint diseases, which include stimulating native cells to proliferate and regenerate tissue and immunomodulation,5 as well as paracrine6 and anti-inflammatory activities.7 In animal experimental models8,9 and early clinical studies,10,11 stem cell therapy with MSCs derived from bone marrow (BM-MSCs) and adipose tissue (AD-MSCs) improved function and pain of the injured joint, and reduced cartilage defects without adverse events.
Human umbilical cord mesenchymal stem cells (UC-MSCs) have become popular for MSC-based therapy because they are easy to obtain and have high expansion capacity and low immunogenicity.12,13 In comparison to BM-MSCs, UC-MSCs were shown to possess the gene expression profile more similar to embryonic stem (ES) cells,14 and have higher potential and efficiency of multilineage differentiation and a different secretome with a variety of trophic factors, growth factors, and cytokines.12,13 Multiple animal models have been used to test the therapeutic effects of UC-MSCs on different diseases.13 It was also reported that UC-MSCs have a better capacity to stimulate chondrocyte regeneration than BM-MSCs.15 However, the potential mechanisms of UC-MSCs for the treatment of OA are not completely understood.
Although the surgically induced OA models and osteochondral injury models can be used as models for OA, the selection of appropriate models in preclinical studies should be specified and relevant to the clinical scenario.16 Intra-articular injection of monosodium iodoacetate (MIA) is widely employed to induce OA-like lesions in the knee.17,18 The histological changes in the articular cartilage induced by MIA resemble those induced by human OA. It has not yet been clarified if UC-MSC transplantation assists in recovery from cartilage damage in MIA-induced OA.
In the present study, we have applied a rat knee OA model by intra-articular injection of MIA and evaluated the therapeutic effects of human UC-MSCs in ameliorating MIA-induced OA in rats, and explored the underlying mechanisms.
Methods
Isolation and expansion of human UC-MSCs
Written informed consent was obtained from the participants before the umbilical cord collection. Detailed methods for human UC-MSCs isolation and expansion have been reported previously with modifications.19 The primary human UC-MSCs were harvested when the cell confluence reached 80% and then expanded by regular subculture. The human UC-MSCs at passage 5 (P5) were harvested as working cells and used in this study. An ARRIVE checklist is included in the Supplementary Material to show that the ARRIVE guidelines were adhered to in this study.
Characterization of human UC-MSCs for phenotype and multilineage potential
At P5, the UC-MSCs were characterized by flow cytometry using the following fluorescently labelled antibodies (BioLegend, USA): anti-human CD73 (cat. No. 344016), CD90 (cat. no. 328110), CD105 (cat. no. 323204), CD34 (cat. no. 343604), CD44 (cat. no. 103007), CD45 (cat. no. 304008), CD19 (cat. no. 302208), and HLA-DR (cat. no. 307606) antibodies as described previously.20 Cell phenotype was determined using a flow cytometer (Beckman Coulter, USA). For multipotent differentiation potential, human UC-MSCs were maintained in adipogenic (cat. no. A10070), chondrogenic (cat. no. A10071), and osteogenic (cat. no. A10072) differentiation media, respectively, following the manufacturer’s instruction (Gibco, USA). Successful inductions of adipogenesis, osteogenesis, and chondrogenesis were confirmed by staining with Oil Red O, Alizarin Red, and Alcian Blue (Gibco), and the expressions of specific differentiation genes were also detected by quantitative real-time polymerase chain reaction (qRT-PCR).
Monosodium iodoacetate-induced osteoarthritis model
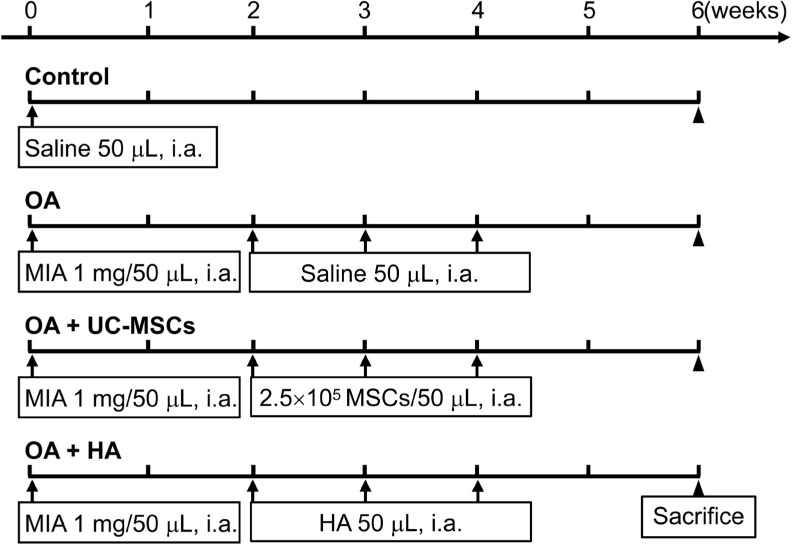
Male Wistar rats (180 g to 200 g; Charles Service, China) were housed in a pathogen-free facility with standard conditions of temperature 24°C, 12 hours light/dark cycle, and food and water ad libitum. The animals were anaesthetized with intraperitoneal injection of 1% sodium pentobarbital. After that, an OA rat model was induced by intra-articular injection of 1.0 mg/50 μl MIA (Sigma-Aldrich, USA) into the right knee cavity under aseptic conditions. Rats were randomly divided into four groups (n = 7 per group): 1) normal group; 2) OA group (saline, 50 μl); 3) OA+ UC-MSCs (2.5 × 105 cells/50 μl) group; and 4) OA+HA (hyaluronic acid, 0.5 mg/50 μl) group. All treatments were started at two weeks after MIA injection by intra-articular injection, once a week for three times under anaesthesia. Two weeks after the last treatment, all rats were killed with 1% sodium pentobarbital, some knees were collected for further histological analysis, and chondrocytes of the other knees were isolated according to a previously described method.21 Cartilage slices were harvested from the knee joints of Wistar rats (n = 3 to 4), cut into small pieces, and immediately frozen in liquid nitrogen for qRT-PCR and enzyme-linked immunosorbent assay (ELISA) analysis.
Histological analysis and immunohistochemistry
The joints collected for histology were fixed in 4% paraformaldehyde, decalcified in ethylenediaminetetraacetic acid (EDTA) solution (0.25 mol/l, pH 7.4), and embedded in paraffin. Haematoxylin and eosin (H&E), Toluidine blue, and Safranin-O/Fast green staining were conducted with 6 μm-thick tissue sections. The severity of OA in different groups was assessed using the Mankin Histological-Histochemical Grading System21,22 by three independent investigators (including WR and YQZ) in a blinded fashion. The evaluation parameters were as follows: 1) cartilage structure (0 to 5); 2) chondrocytes (0 to 4); 3) Safranin-O/Fast green staining (0 to 5); and 4) tidemark integrity (0 to 1), with higher total scores out of 15 indicating a higher grade of OA. Immunohistochemistry was performed following a standard protocol. Briefly, tissue sections were regularly deparaffinized and rehydrated. The antigen was retrieved with citric acid buffer (pH 6.0) microwave antigen retrieval for 15 minutes. After blocking with hydrogen peroxide and serum, the tissue sections were incubated with the following antibodies (Bioss, USA): ADAMTS-5 (1:200), MMP13 (1:200), type II collagen (1:200), IL-1β (1:100), tumour necrosis factor-α-induced protein 6 (TSG-6) (1:100); and ki67 (1:500, Abcam, USA), TNF-α (1:200, Proteintech, USA), anti-human nuclei antibody MAB1281 (1:100, Millipore, USA), and interleukin-1 receptor antagonist (IL-1RA) (1:100, ABclonal, China) overnight at 4°C. Horseradish peroxidase-labelled secondary antibody was incubated at 37°C for 60 minutes. The colour was developed by applying 3,3′-diaminobenzidine tetrahydrochloride (DAB) (DakoCytomation, USA) and the sections were counterstained with haematoxylin. The positively stained cells were counted from three high magnification fields per slide, and the mean ratio of positive cells over total cells counted was calculated from three rats per group for statistical analysis.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the cells or cartilage of rat knees using TRIzol (Life Technologies, USA). First strand complementary DNA (cDNA) was synthesized using PrimeScript RT Kit (TaKaRa, China), and used as a template to determine the expression of specific genes (adipocytes, osteoblasts, and chondrocyte-related genes) with the indicated primers (Supplementary Table i) using SYBR Green reagent (Thermo Fisher Scientific, USA) in qRT-PCR. All the primers were optimized for one thermocycler protocol. The cycling parameters: PCR reaction was carried out on 50 ng of cDNA samples using 0.5 μmol/l of each primer and 5 μl 2× SYBR Green PCR Master Mix. The following conditions were used: 95°C for ten minutes; 40 cycles at 95°C for ten seconds; 60°C for ten seconds, and 72°C for 30 seconds in a BIO-RAD CFX Connect (Bio-Rad, USA), and analyzed with the Bio-Rad CFX Manager software (ver. 3.2.2; Bio-Rad). Relative expression of the target gene was normalized to endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCT comparative method.21,23
Enzyme-linked immunosorbent assay
The articular cartilage tissues were homogenized for protein extraction. The interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α) expressions were determined using ELISA kits (Mlbio, China) according to the manufacturer’s instruction.
Statistical analysis
All quantitative data were presented as mean and standard deviation by GraphPad Prism 5 (GraphPad Software, USA). Statistical analysis was performed using SPSS v19.0 (IBM, USA). Data for morphological and histological scores were analyzed by independent-samples t-test. The difference was considered as statistically significant when p < 0.05.
Results
Characterization of human UC-MSCs
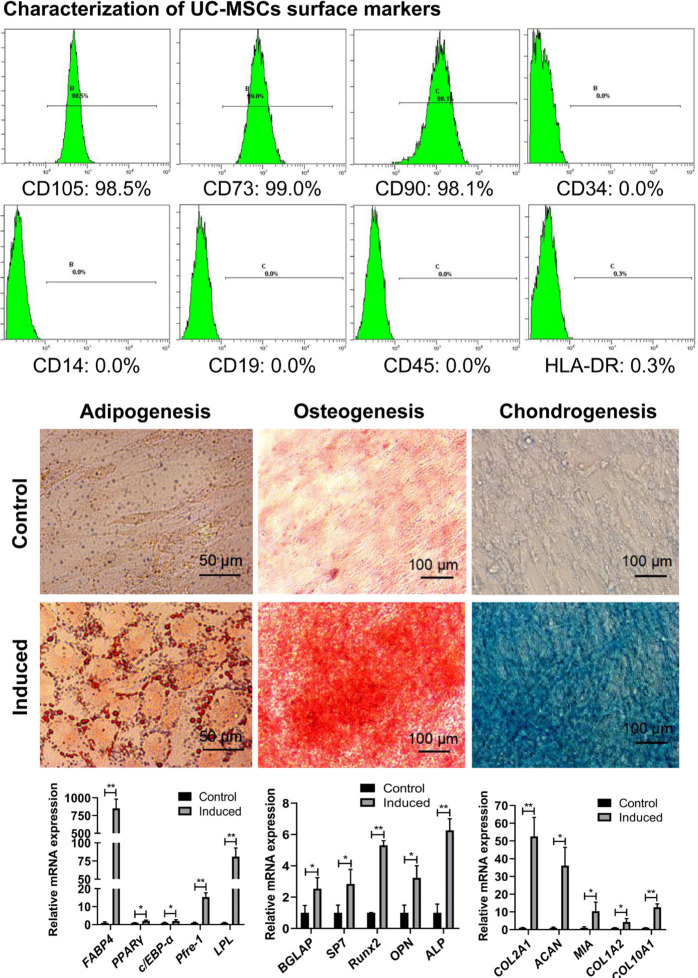
The cell surface markers of human UC-MSCs were characterized by flow cytometry, which showed a positive expression (> 98%) of CD105, CD90, CD73, and negative expression (< 0.3%) of CD19, CD14, CD45, CD34, and human leucocyte antigen-DR (HLA-DR). The multilineage differentiation of human UC-MSCs was induced by adopting different induction media, and the adipogenic, osteogenic, and chondrogenic differentiations were demonstrated by positive staining with Oil Red O for the accumulation of lipid droplets, Alizarin Red for calcium deposition, and Alcian Blue for glycosaminoglycan (GAG) synthesis, respectively. More evidence was obtained by qRT-PCR for the expressions of adipogenic, osteogenic, and chondrogenic genes, which were significantly enhanced after designated induction (Figure 1).
Fig. 1.
Characterization of human umbilical cord mesenchymal stem cells (UC-MSCs). Isolated primary UC-MSCs were cultured to passage 5, and then characterized for their surface markers and multipotent differentiation capacity. A typical expression pattern of surface markers for mesenchymal stem cells (MSCs) was determined by flow cytometry with high positive expression of CD105, CD90, CD73 (> 98%), and negative expression of CD19, CD34, CD14, CD45, and human leucocyte antigen-DR (HLA-DR) (< 0.3% positive). Multiple differentiation capacity of UC-MSCs was determined with different induction media as described in Methods. The phenotypes of induced differentiation were visualized by staining with Oil Red O for adipogenesis (scale bars = 50 μm), Alizarin Red for osteogenesis, and Alcian blue for chondrogenesis (scale bars = 100 μm) as indicated, respectively. After induction, a significant enhanced expression of adipogenic, osteogenic, and chondrogenic genes was detected by quantitative real-time polymerase chain reaction (*p < 0.05, **p < 0.01 vs control). Adipoblast related genes were as follows: FABP4, fatty-acid-binding protein 4; PPARγ, peroxisome proliferation-activated receptor γ; C/EBP-α, CCAAT/enhancer-binding proteins α; Pref-1, preadipocyte factor 1; LPL, lipoprteinlipase. Osteoblast related genes were as follows: BGLAP, bone gamma carboxyglutamate protein; SP7, Sp7 transcription factor; Runx2, runt-related transcription factor-2; OPN, secreted phosphoprotein 1; ALP, alkaline phosphatase. Chondrocyte related genes were as follows: ACAN, aggrecan; MIA, melanoma inhibitory activity; COL1A2, collagen type I α 2 chain; COL2A1, collagen type II α 1 chain; COL10A1, collagen type X α 1 chain. mRNA, messenger RNA.
Human UC-MSC treatment ameliorated MIA-induced OA and inhibited the degradation of articular cartilage
To assess the therapeutic effects, human UC-MSCs were directly transplanted into the articular cavity of rats two weeks after the establishment of MIA-induced OA. The same volume of saline or hyaluronic acid (HA) was injected as control (Figure 2).
Fig. 2.
Schematic diagram of the experimental procedure. HA, hyaluronic acid; i.a., intra-articular injection; MIA, monoiodoacetate; MSCs, mesenchymal stem cells; OA, osteoarthritis; UC-MSCs, human umbilical cord mesenchymal stem cells.
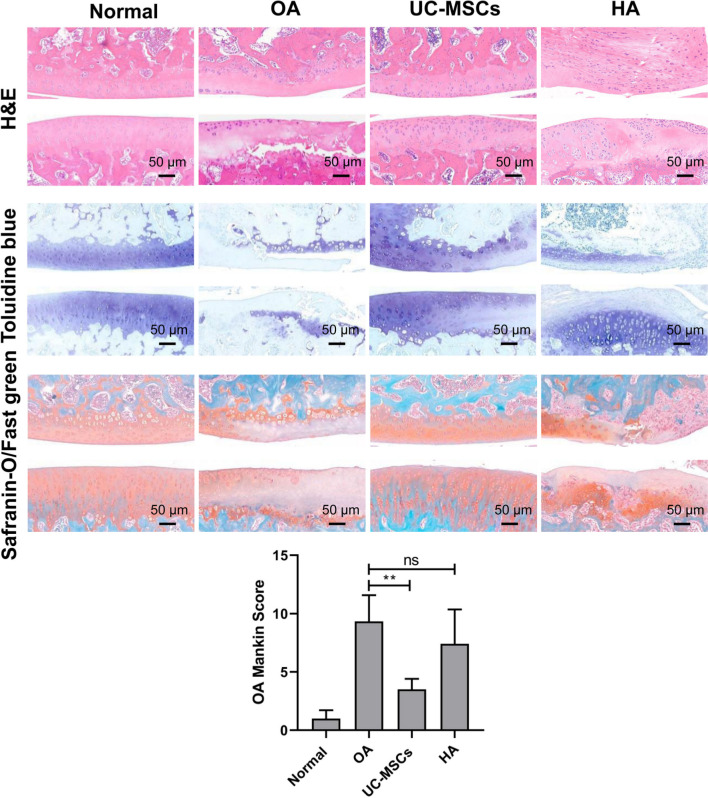
Intra-articular injection of MIA-induced typical OA damages in the rat knee joint. Safranin-O staining of the cartilage matrix after two weeks of MIA injection showed that the surfaces of the articular cartilage were rough with higher Mankin scores (Supplementary Figure a). Compared with the normal rats, the injured articular cartilages of OA rats showed rough surface and/or formation of fissures/cracks, reduction of chondrocytes, and cartilage volume. Toluidine blue and Safranin-O staining demonstrated a significant reduction of GAGs and proteoglycan (PG), respectively (Figure 3).
Fig. 3.
Human umbilical cord mesenchymal stem cell (UC-MSC) but not hyaluronic acid (HA) treatment attenuated monosodium iodoacetate (MIA)-induced osteoarthritis (OA). Haematoxylin and eosin (H&E) staining shows a loss of chondrocytes, uneven surface and/or fissures/cracks formation, and volume reduction of articular cartilage in the MIA-induced OA rats. Toluidine blue and Safranin-O/Fast green staining demonstrate a significant reduction of glycosaminoglycans (GAGs) and proteoglucan (PG), respectively, in the OA articular cartilage. The pictures shown are representative of each group (scale bars = 50 μm). The severity of OA was assessed using the Mankin score system by three independent blinded investigators (including WR and YQZ). Data shown are mean and standard deviation from three rats/group. **p < 0.01. ns, no significance.
After human UC-MSC treatment, we observed a significant improvement in joint damage caused by MIA: fissures/cracks had disappeared, the cartilage surface was almost normal, and the chondrocytes and cartilage volume also were increased; compared to the saline-treated OA group, the expression of matrix components, GAG, and PG was enhanced. Mankin score assessment for OA severity was conducted. Mankin score confirmed that human UC-MSC treatment significantly attenuated the severity of MIA-induced OA. In HA treated group, however, no improvement of MIA-induced OA was observed (Figure 3).
Human UC-MSCs down-regulated the expression of MIA-induced cartilage-degrading enzymes and restored the proliferation of the injured cartilage
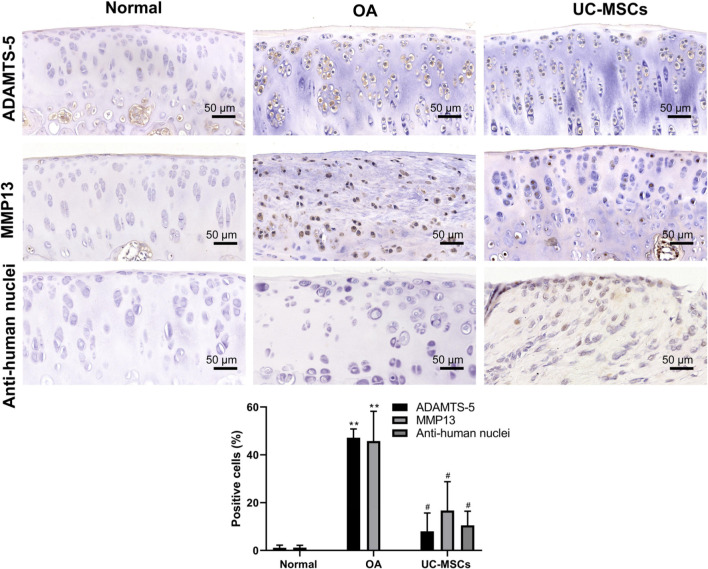
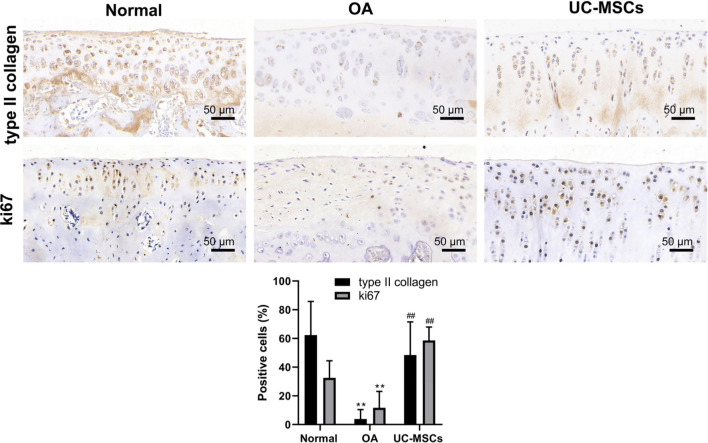
Immunohistochemical staining results showed significantly higher expressions of ADAMTS-5 and MMP13 in MIA-induced OA articular cartilage that is significantly down-regulated after treatment with human UC-MSCs (Figure 4, Supplementary Figures b and c; p < 0.05). The human nucleus signal was positive in the field of articular cartilage in UC-MSC treatment group, whereas it was negative in normal and OA groups (Figure 4, Supplementary Figure d; p < 0.05). These results indicate that UC-MSCs directly engrafted into the cartilage and the injected cells could be tracked after two weeks by immunohistochemical staining. We further detected the expression of type II collagen and ki67 to determine whether human UC-MSCs can induce the proliferation of the injured chondrocytes. MIA injection led to a clear low expression of type II collagen, a hyaline cartilage-specific matrix protein, and ki67, a cell proliferation marker, indicating a reduced matrix synthesis and low proliferation rate of chondrocytes in the injured cartilage. In contrast, human UC-MSC treatment recovers the expression of type II collagen and ki67 significantly (Figure 5, Supplementary Figures e and f; p < 0.01).
Fig. 4.
Human umbilical cord mesenchymal stem cells (UC-MSCs) down-regulated monosodium iodoacetate (MIA)-induced matrix metalloproteinase-13 (MMP13) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) expression. Immunohistochemical staining was performed for the expression of MMP13, ADAMTS-5, and anti-human nuclei in the articular cartilage; the pictures shown are representative staining in each group. The percentage of positive stained cells (in brown colour) for both MMP13 and ADAMTS-5 was calculated, respectively. Data shown are mean and standard deviation from three rats/group. **p < 0.01 versus normal group, #p < 0.05, ##p < 0.01 versus UCMSC-treated groups. OA, osteoarthritis.
Fig. 5.
Human umbilical cord mesenchymal stem cells (UC-MSCs) promoted matrix synthesis and chondrocyte proliferation in monosodium iodoacetate (MIA)-induced osteoarthritis (OA) cartilage. The articular cartilage was immunostained for the expression of collagen II and ki67. The percentage of positive cells (in brown colour) for both collagen II and ki67 was calculated, respectively. Data shown are mean and standard deviation from three rats/group. **p < 0.01 versus normal group, ##p < 0.01 versus UC-MSC-treated groups.
Human UC-MSCs attenuated MIA-induced inflammatory response and enhanced the expression of anti-inflammatory factors, TSG-6 and IL-1RA
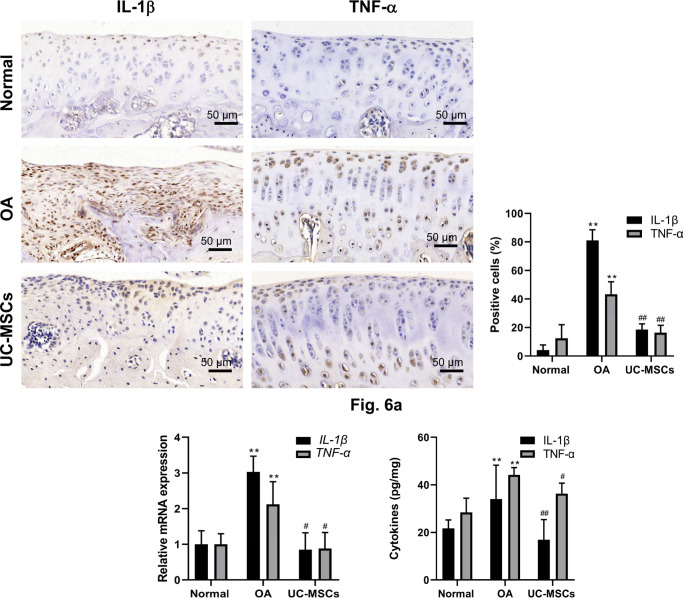
Inflammatory response plays a critical role in the pathogenesis of the disease. The levels of local pro-inflammatory and anti-inflammatory cytokines were determined by immunostaining, qRT-PCR, and ELISA. In MIA-induced OA group, both IL-1β and TNF-α immunostaining in chondrocytes in the cartilage were significantly enhanced, which were reserved almost back to normal control in human UC-MSCs treated group (Figure 6a, Supplementary Figures g and h; p < 0.01); the enhancement of IL-1β and TNF-α was further confirmed by qRT-PCR and ELISA (Figure 6b). In addition, both immunostaining (Figure 7a, Supplementary Figures i and j; p < 0.01) and qRT-PCR (Figure 7b; p < 0.01) showed that human UC-MSC therapy led to significantly increased expression of anti-inflammatory factors TSG-6 and IL-1RA in the articular chondrocytes isolated from knees of rats (Supplementary Figure k).
Fig. 6.
Human umbilical cord mesenchymal stem cells (UC-MSCs) inhibited inflammatory response in monosodium iodoacetate-induced osteoarthritis (OA) cartilage. The expressions of interleukin (IL)-1β and tumour necrosis factor-α (TNF-α) in the articular cartilage were analyzed by immunostaining and quantified as a) the percentage of positive cells, which was further confirmed by b) quantitative real-time polymerase chain reaction and enzyme-linked immunosorbent assay. Data represent mean and standard deviation. **p < 0.01 versus normal group, #p < 0.05, ##p < 0.01 versus OA group. mRNA, messenger RNA.
Fig. 7.
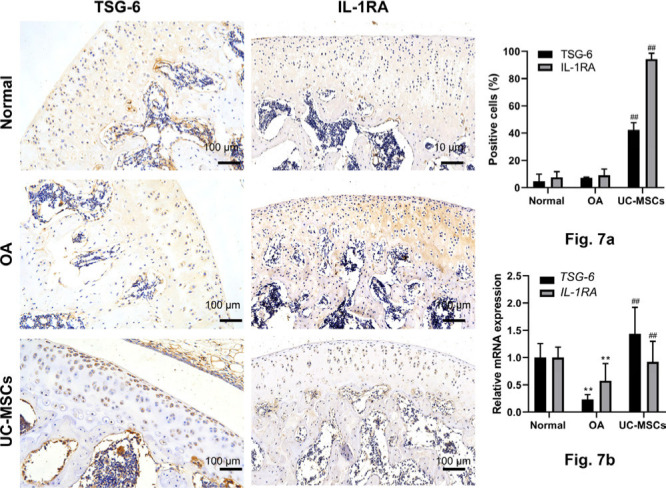
Human umbilical cord mesenchymal stem cells (UC-MSCs) enhanced the expression of tumour necrosis factor-α-induced protein 6 (TSG-6) and interleukin-1 receptor antagonist (IL-1RA) in monosodium iodoacetate (MIA)-induced osteoarthritis (OA) cartilage. a) The expression of TSG-6 and IL-1RA in the articular cartilage was determined by immunostaining and analyzed with the percentage of positive cells. b) The expression of TSG-6 and IL-1RA was further confirmed by quantitative real-time polymerase chain reaction. Data represent mean and standard deviation. **p < 0.01 versus normal group, ##p < 0.01 versus OA group. mRNA, messenger RNA.
Discussion
In this study, we demonstrated that: 1) intra-articular injection of human UC-MSCs significantly ameliorates OA damages in a MIA-induced rat OA model; 2) human UC-MSCs protected the articular cartilage from OA damage by down-regulating the expression of cartilage-degrading enzymes and preventing the cartilage degeneration; and 3) human UC-MSCs alleviated inflammatory response by inhibiting the expression of pro-inflammatory cytokines and promoting the expression of anti-inflammatory factors, which was critical for the therapeutic effect of human UC-MSCs on OA. These findings implied a clinical potential of human UC-MSCs as a promising strategy for OA treatment.
Although UC-MSCs may have a superior chondrogenesis capacity compared to MSCs from other tissues, relatively fewer in vivo studies have been done to assess the therapeutic effect of UC-MSCs on OA in animal models. In this study, a clear therapeutic effect of human UC-MSCs was observed in a MIA-mediated rat knee OA model, while little effect was achieved by HA treatment. In an attempt to achieve a better therapeutic effect, multiple intra-articular injections of human UC-MSCs were applied in this study, which was well tolerated by all animals tested. This result may imply a proper dose and regimen for the clinical treatment of OA with human UC-MSCs. It should be noted that the lack of an additional cell type (e.g. fibroblasts or BM-MSCs) to compare with the UC-MSCs is a limitation of this study.
The original design of MSC-based therapy was to replace and regenerate the degraded and damaged tissues, including the injured articular cartilage in OA. However, the result from the present study showed that UC-MSC engraftment and subsequent differentiation into cartilage was rare, even when transplanted by intra-articular injection, most likely due to a low survival rate of MSCs after in vivo transplantation,24,25 and implied that tissue regeneration by UC-MSCs does contribute to repairment of the cartilage defects, but may not constitute the major mechanisms for their therapeutic effect. Similar results were observed in our study. Hallmark damage of OA is matrix degradation of articular cartilage due to increased expression and activation of cartilage-degrading enzymes, such as ADAMTS-526 and MMP13.27,28 It has been shown that knockout of either ADAMTS-5 or MMP13 protects the animal from OA progression and cartilage erosion.29,30 In the present study, the expression of ADAMTS-5 and MMP13 was higher in the cartilage of MIA-induced OA rats, and completely down-regulated by human UC-MSC treatment, indicating a protective mechanism of human UC-MSCs through manipulating the expression of cartilage-degrading enzymes. Human UC-MSC treatment prevents the cartilage degeneration significantly, demonstrated by an increased number of chondrocytes with higher expression of type II collagen and ki67 in the articular cartilage.
The intensive and sustained inflammatory response is one of the most important underlying mechanisms for OA pathogenesis, which is the main target for developing novel OA therapy.31 Local enhancement of pro-inflammatory cytokines in the articular tissues and fluid has been observed in OA model animals32 and patients.33,34 MIA induces local inflammation from the synovial fluid to the membrane of the knee joint, which results in the production of inflammatory mediators such as IL-1β and TNF-α. These mediators further promote the pathogenesis of OA by increasing inflammatory cytokines and matrix metalloproteinases in cartilage, which is followed by chondrocyte apoptosis and cartilage degradation.35-37 However, one of the fundamental properties for the application of MSCs in immune and inflammatory diseases is their immunomodulatory and anti-inflammatory capacity.7 In an inflammatory environment, MSCs secrete factors that cause multiple anti-inflammatory effects and influence matrix turnover in synovium and cartilage.23 We have demonstrated in this study that human UC-MSC treatment significantly inhibits the inflammatory response in the cartilage of OA rats by depressing the expression of pro-inflammatory cytokines, TNF-α and IL-1β, and elevating the production of anti-inflammatory factors TSG-6 and IL-1RA. It should be noted that the expression of human TSG-6 and IL-1RA was undetectable in MIA + UC-MSCs groups. Although there is controversy about GAPDH as a reference gene under some conditions, the relative levels of gene expression were normalized against GAPDH in MIA-induced rat OA model and a rabbit OA model.23,38 Moreover, there are few human UC-MSCs located in the field of articular cartilage. These results suggested that most of anti-inflammatory factors (TSG-6 and IL-1RA) were derived from the native rat chondrocytes and that UC-MSCs could influence the micro-environment via paracrine actions to regulate the inflammatory response. These results may identify one of the most critical mechanisms for the therapeutic effect of human UC-MSCs on OA treatment.
Multiple intra-articular administrations of human UC-MSCs exert a significant therapeutic effect on severe OA injury by preventing disease progression and promoting an almost complete recovery in structure, cellularity, and matrix quality of the injured articular cartilage. In addition to in situ engrafting and differentiation, human UC-MSCs exert their therapeutic effect by down-regulating the expression of cartilage-degrading enzymes, stimulating chondrocyte proliferation, and alleviating inflammatory response. While the exact molecular mechanisms need further investigation, the results from this study suggest beneficial effects of human UC-MSCs for OA and offer a potential dosage and regimen for clinical application.
Author contributions
Q. Zhang: Performed the experimental studies, Analyzed and synthesized the data, Wrote the manuscript.
E Xiang: Synthesized the data, Reviewed and proofed the manuscript.
W. Rao: Conducted the experiments, Reviewed the manuscript.
Y. Q. Zhang: Performed the experimental studies.
C. H. Xiao: Conducted the experiments.
C. Y. Li: Analyzed the data, Reviewed the manuscript.
B. Han: Designed the study, Participated in the research design, Drafted the manuscript.
D. Wu: Designed the study, Drafted and reviewed the manuscript.
Q. Zhang and E. Xiang are joint first authors.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. This work was supported by a grant from Wuhan Municipal Science and Technology Bureau (2019030703011513) and the 3551 Talents Plan of Wuhan Optical Valley (WXG [2017] No.129).
Acknowledgements
We thank Lian Chen for her help in Mankin Histological-Histochemical score, and also thank Cheng Chang, Xi Le, Liang Gong, Mengting Liu, Zhexiong Yan, and Zhao Lei for their excellent technical assistance.
Ethical review statement
Isolation and expansion of human umbilical cord mesenchymal stem cells (UC-MSCs): the protocol for isolation and use of human UC-MSCs was approved by the Institutional Ethics Review Board of the Union Hospital, Tongji Medical College (permit No. [2018] SC02). Monosodium iodoacetate (MIA)-induced osteoarthritis model: the animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Hubei Provincial Center for Safety Evaluation of Food and Drug (permit No.: 201802004).
Supplementary material
Supplementary Table i shows the primers used for quantitative real-time polymerase chain reaction. Supplementary Figures a to j show histological evaluation of the articular cartilage and immunohistochemistry, and Supplementary Figure k shows the isolation of chondrocytes from the articular cartilage of Wistar rats.
© 2021 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med Overseas Ed. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 2. Astur DC, Arliani GG, Binz M, et al. . Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96-A(10):816–823. [DOI] [PubMed] [Google Scholar]
- 3. Mundi R, Bedi A, Chow L, et al. . Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44(7):1888–1895. [DOI] [PubMed] [Google Scholar]
- 4. Pittenger MF, Mackay AM, Beck SC, et al. . Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 5. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman CM, Curtin C, Barry FP, O'Flatharta C, Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010;21(10):1239–1250. [DOI] [PubMed] [Google Scholar]
- 7. Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, et al. . Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22(21):2825–2835. [DOI] [PubMed] [Google Scholar]
- 8. Singh A, Goel SC, Gupta KK, et al. . The role of stem cells in osteoarthritis: an experimental study in rabbits. Bone Joint Res. 2014;3(2):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo Monaco M, Merckx G, Ratajczak J, et al. . Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018;2018:9079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PLoS One. 2017;12(4):e0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kong L, Zheng L-Z, Qin L, Ho KKW. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding D-C, Chang Y-H, Shyu W-C, Lin S-Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. [DOI] [PubMed] [Google Scholar]
- 14. Fong C-Y, Chak L-L, Biswas A, et al. . Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011;7(1):1–16. [DOI] [PubMed] [Google Scholar]
- 15. Miranda JP, Camões SP, Gaspar MM, et al. . The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front Immunol. 2019;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tawonsawatruk T, Sriwatananukulkit O, Himakhun W, Hemstapat W. Comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection and osteochondral injury in rat models. Bone Joint Res. 2018;7(3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang G, Evans CH, Benson JM, et al. . Safety and biodistribution assessment of sc-rAAV2.5IL-1Ra administered via intra-articular injection in a mono-iodoacetate-induced osteoarthritis rat model. Mol Ther Methods Clin Dev. 2016;3:15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu Y, Wu X, Liang Y, et al. . Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 2016;16(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S, Wang Y, Guan L, Ji M. Characteristics of human umbilical cord mesenchymal stem cells during ex vivo expansion. Mol Med Rep. 2015;12(3):4320–4325. [DOI] [PubMed] [Google Scholar]
- 20. Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J Biomed Mater Res A. 2016;104(3):600–610. [DOI] [PubMed] [Google Scholar]
- 21. Mei L, Shen B, Ling P, et al. . Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS One. 2017;12(4):e0176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JE, Lee SM, Kim SH, et al. . Effect of self-assembled peptide-mesenchymal stem cell complex on the progression of osteoarthritis in a rat model. Int J Nanomedicine. 2014;9 Suppl 1:141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park JW, Yun Y-P, Park K, et al. . Ibuprofen-loaded porous microspheres suppressed the progression of monosodium iodoacetate-induced osteoarthritis in a rat model. Colloids and Surfaces B: Biointerfaces. 2016;147:265–273. [DOI] [PubMed] [Google Scholar]
- 24. Chiang E-R, Ma H-L, Wang J-P, Liu C-L, Chen T-H, Hung S-C. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS One. 2016;11(2):e0149835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agung M, Ochi M, Yanada S, et al. . Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1307–1314. [DOI] [PubMed] [Google Scholar]
- 26. Li W, Wu M, Jiang S, Ding W, Luo Q, Shi J. Expression of ADAMTS-5 and TIMP-3 in the condylar cartilage of rats induced by experimentally created osteoarthritis. Arch Oral Biol. 2014;59(5):524–529. [DOI] [PubMed] [Google Scholar]
- 27. Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neuhold LA, Killar L, Zhao W, et al. . Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Little CB, Barai A, Burkhardt D, et al. . Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glasson SS, Askew R, Sheppard B, et al. . Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. [DOI] [PubMed] [Google Scholar]
- 31. Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. [DOI] [PubMed] [Google Scholar]
- 32. Wang B-W, Jiang Y, Yao Z-L, Chen P-S, Yu B, Wang S-N. Aucubin protects chondrocytes against IL-1β-induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Devel Ther. 2019;13:3529–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Attur M, Belitskaya-Lévy I, Oh C, et al. . Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63(7):1908–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoff P, Buttgereit F, Burmester G-R, et al. . Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37(1):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Héraud F, Héraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59(12):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. López-Armada MJ, Caramés B, Lires-Deán M, et al. . Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14(7):660–669. [DOI] [PubMed] [Google Scholar]
- 37. Ye Z, Chen Y, Zhang R, et al. . c-Jun N-terminal kinase - c-Jun pathway transactivates Bim to promote osteoarthritis. Can J Physiol Pharmacol. 2014;92(2):132–139. [DOI] [PubMed] [Google Scholar]
- 38. Kim H, Yang G, Park J, Choi J, Kang E, Lee B-K. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci Rep. 2019;9(1):13854. [DOI] [PMC free article] [PubMed] [Google Scholar]