Abstract
Arcobacter spp. are emerging waterborne and foodborne zoonotic pathogens responsible for gastroenteritis in humans. In this work, we evaluated the occurrence and the antimicrobial resistance profile of Arcobacter isolates recovered from different aquatic sources. Besides, we searched for Arcobacter spp. in seaweeds and the corresponding seawater samples. Bacteriological and molecular methods applied to 100 samples led to the isolation of 28 Arcobacter isolates from 27 samples. The highest prevalence was detected in rivers followed by artificial ponds, streams, well waters, and spring waters. Seaweeds contained a higher percentage of Arcobacter than the corresponding seawater samples. The isolates were identified as Arcobacter butzleri (96.4%) and Arcobacter cryaerophilus (3.6%). All the isolates showed a multi-drug resistance profile, being resistant to at least three different classes of antibiotics. Molecular analysis of genetic determinants responsible for tetracycline resistance in nine randomly chosen isolates revealed the presence of tetO and/or tetW. This work confirms the occurrence and the continuous emergence of antibiotic-resistant Arcobacter strains in environmental samples; also, the presence of quinolone-resistant Arcobacter spp. in aquatic sources used for water supply and irrigation represents a potential risk for human health.
Keywords: Arcobacter butzleri, water samples, multiplex PCR, antibiotic susceptibility, tetO, tetW
1. Introduction
The members of the Arcobacter genus are Gram-negative, slender, spiral-shaped rods and belong to the family Campylobacteraceae [1]: they are distinguished from Campylobacter genus by their ability to grow in aerobic conditions and at lower temperatures from 15 to 30 °C [2].
Arcobacter spp. are emerging entero-pathogens that can be isolated worldwide from different aquatic matrices, such as lakes and rivers [3,4,5,6], groundwater [7,8], wastewater [9,10,11], drinking water [12], seawater [13,14], and food of both animal and non-animal origin [15,16,17,18]. Thus, food and water are considered the main vehicle of the pathogen [19,20]. A. butzleri, A. cryaerophilus, and A. skirrowii have been associated with animal and human infections and A. butzleri has been classified as a serious hazard to human health by the International Commission on Microbiological Specifications for Foods in 2002 [21] and is often correlated with bacteremia, gastroenteritis, and watery diarrhea in humans [22,23,24]. Alarming outbreak episodes of A. butzleri have been reported in a nursery and primary school in Italy [25], associated with groundwater, which served as the drinking water source in Idaho, USA [7], and related with the consumption of water contaminated with wastewater from sewage treatment plants [8]. The presence and the persistence of A. butzleri in the environment could be dependent upon its ability to form biofilms that allow its survival in various conditions, favoring bacterial diffusion and transmission within the different food chains [26].
A. butzleri is known to contain numerous virulence genes and to cause intestinal and extra-intestinal infections, that are often self-limited [27,28]. A. butzleri infection can be treated with antibiotics, i.e., β-lactams, fluoroquinolones, macrolides [28]. However, Arcobacter species frequently display a multidrug-resistant profile, hampering the antibiotic treatment of A. butzleri infections. Recent studies have indicated an increase of resistance against fluoroquinolones as well as tetracycline of A. butzleri and A. cryaerophilus isolates from food and aquatic sources [27,29,30].
The foods that usually are consumed raw, such as vegetables, have been found to carry A. butzleri and strains isolated from these matrices have been demonstrated to possess many virulence and antibiotic resistance genes [26]. The high prevalence of antimicrobial resistance among bacteria may be dependent upon the use of antibiotics in animal production and human medicine [31,32]. Aquatic environments and sea animals are considered as reservoirs of antibiotic resistance genes [33,34,35] and Arcobacter spp. can be isolated worldwide from different aquatic matrices, such as lakes and rivers [3,4,5,6]. In recent years, an increasing number of scientific papers concerning Arcobacter focused on the growing importance of this emerging entero-pathogen [36].
Recently, the antibiotic-resistance profile and the genomic diversity of this pathogen were exploited by comparing 49 A. butzleri strains isolated from various environments and samples [37]. All isolates were resistant to nalidixic acid, followed by cefotaxime, ampicillin, levofloxacin, ciprofloxacin, and erythromycin. Comparison of the antibiotic-resistance profile and the genome sequences revealed that A. butzleri contains many genes coding for efflux pumps and other antibiotic resistant determinants, for example, quinolone resistance is due to the mutation Thr-85-Ile of the gyrA gene [29].
This study aimed to detect and identify Arcobacter spp. from different environmental water sources using bacteriological and molecular methods, to determine the antibiotic resistance profile of the isolates and to investigate the antibiotic genetic determinants providing tetracycline resistance.
2. Results
2.1. Isolation and Identification of Arcobacter Species
Twenty-eight Arcobacter strains were isolated out of 100 samples (28%). Specifically, 9 Arcobacter strains were found in 11 rivers (81.8%), 6 in 8 artificial ponds (75%), 2 in 5 streams (40%), 2 in 20 well waters (10%), and 1 in 17 spring waters (5.8%) used for water supply (Table 1). All the analyzed drinking water samples were negative. However, Arcobacter spp. was identified in a sample of non-chlorinated source water (spring water) used for drinking.
Table 1.
Prevalence and molecular identification of Arcobacter spp. in the examined samples.
| Sample (Source) | N. of Samples | Arcobacter spp. (%) | A. butzleri | A. cryaerophilus |
|---|---|---|---|---|
| Rivers | 11 | 9 (81.8) | 9 | |
| Ponds | 8 | 6 (75) | 5 | 1 |
| Streams | 5 | 2 (40) | 2 | |
| Well water | 20 | 2 (10) | 2 | |
| Spring water | 17 | 1 (5.8) | 1 | |
| Drinking water | 12 | 0 | - | |
| Seawater | 21 | 3 (14.2) | 3 | |
| Seaweeds | 6 | 5 (83.3) | 5 | |
| Total | 100 | 28 (28%) | 27 (96.4%) | 1 (3.6%) |
Furthermore, Arcobacter spp. was searched in seaweeds belonging to the genus Enteromorpha and in the corresponding seawater samples, collected from six independent locations of the northern coast of Sicily. Interestingly, while 3 Arcobacter spp. were isolated from seawater samples (50%), 5 out of 6 algal samples were positives to A. butzleri (83.3%) (Table 2).
Table 2.
Arcobacter spp. in the examined seawater and seaweeds.
| Sample Number | Area of Origin | Type of Sample | Arcobacter spp. |
|---|---|---|---|
| 1 | Messina | Seawater | ND |
| Seaweed | A. butzleri | ||
| 2 | Palermo | Seawater | ND |
| Seaweed | ND | ||
| 3 | Palermo | Seawater | A. butzleri |
| Seaweed | A. butzleri | ||
| 4 | Messina | Seawater | ND |
| Seaweed | A. butzleri | ||
| 5 | Palermo | Seawater | A. butzleri |
| Seaweed | A. butzleri | ||
| 6 | Messina | Seawater | A. butzleri |
| Seaweed | A. butzleri |
ND: Not Detected.
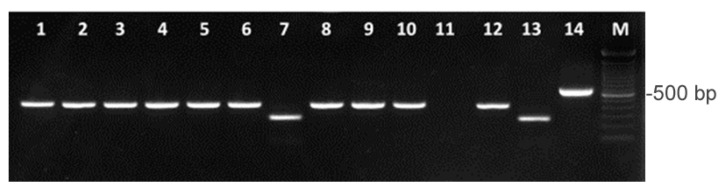
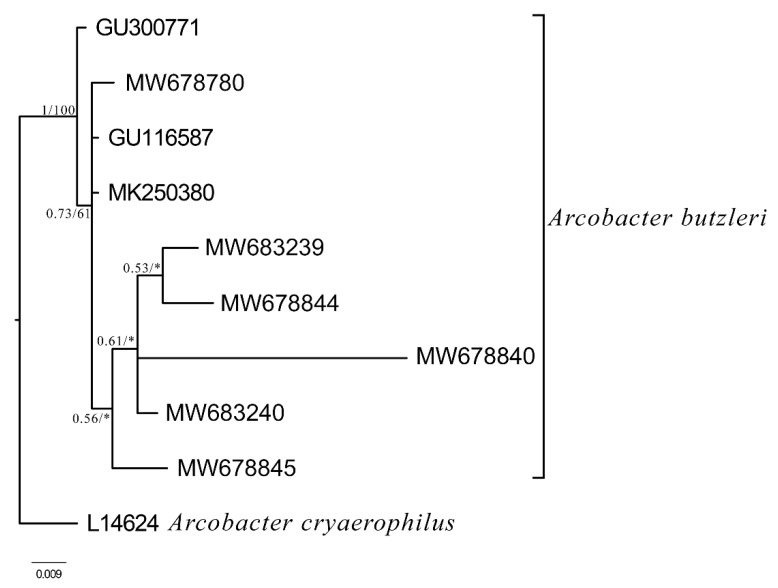
Multiplex PCR (mPCR) demonstrated that 27 out of the 28 isolates corresponded to A. butzleri (96.4%) and only one to the A. cryaerophilus (3.8%) (Figure 1). A. butzleri and A. cryaerophilus were co-isolated from a water sample of an artificial pond populated by aquatic birds. A. skirrowii was not detected in any of the samples. PCR amplicons were sequenced and BLAST alignment revealed a 98–99% identity with A. butzleri 16S rDNA gene and 99% identity with A. cryaerophilus 23S rDNA gene. Phylogenetic tree of the sequences of the amplification product obtained by A. butzleri (Figure 2) indicated a well-structured clade.
Figure 1.
Multiplex PCR results of ten Arcobacter isolates. Lanes 1–6: A. butzleri; lane 7: A. cryaerophilus; lanes 8–10: A. butzleri; lane 11: negative control; lane 12: positive control (A. butzleri, NCTC 12481); lane 13: positive control (A. cryaerophilus, NCTC 11885); lane 14: positive control (A. skirrowii, NCTC 12713); lane M: 100 bp DNA Ladder (Invitrogen).
Figure 2.
Bayesian phylogram of A. butzleri. based on the 358 bp fragment of the 16S rDNA. Sample of A. cryaerophilus (A.N. L14624) was used as outgroup to root the tree. Node statistical support is reported as nodal posterior probabilities (Bayesian Inference of phylogeny, BI)/bootstrap values (Maximum Likelihood, ML). Asterisks indicate a bootstrap support value lower than 50. GenBank Accession Numbers reported in bold refer to novel sequences obtained in this study.
2.2. Antimicrobial Susceptibility Testing
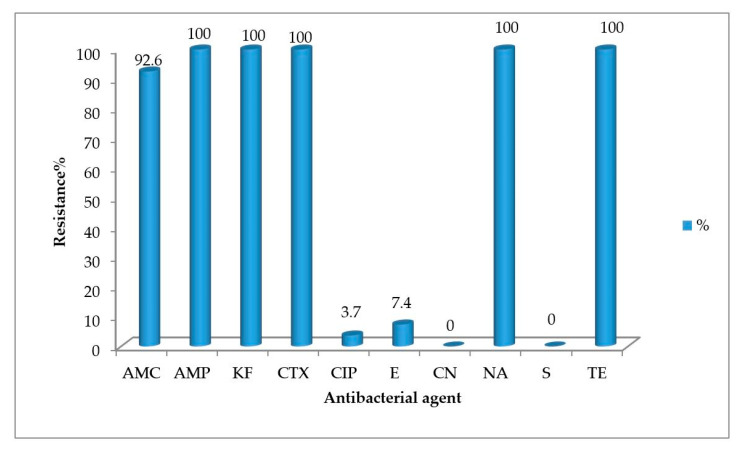
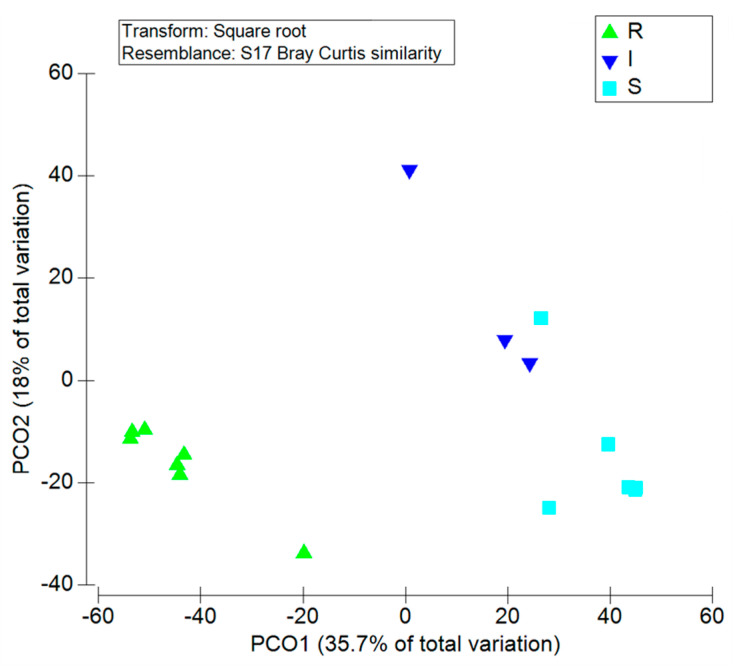
All A. butzleri isolates were susceptible to gentamicin (CN) and streptomycin (S), and resistant to ampicillin (AMP), cefalotin (KF), cefotaxime (CTX), nalidixic acid (NA), and tetracycline (TE). A. butzleri strains were resistant to amoxicillin-clavulanic acid (AMC), erythromycin (E), and ciprofloxacin (CIP) at the rate of 92.6%, 7.4%, and 3.7%, respectively (Figure 3 and Table 3). A. cryaerophilus strain showed resistance to 9 out of the 10 tested antibiotics and sensitivity to gentamicin (CN). Multidrug-resistance, defined as resistance to three or more tested antibiotics, was observed in all the isolates. PCoA (Figure 4) indicated 3 distinct clusters, containing the resistant, the intermediate, and the sensitive isolates.
Figure 3.
The percentage of antibiotic resistance of A. butzleri isolates (AMC: amoxicillin-clavulanic acid, AMP: ampicillin, KF: cefalotin, CTX: cefotaxime, CIP: ciprofloxacin, E: erythromycin, CN: gentamycin, NA: nalidixic acid, S: streptomycin, TE: tetracycline). The resistance percentage was calculated as the ratio of number of antibiotic resistant isolates divided by the total number of A. butzleri isolates (n = 27).
Table 3.
Antibiotic resistance of A. butzleri and A. cryaerophilus strains isolated from water samples and seaweeds (n = 28).
| Antibiotics | Isolates from | Total (n = 28) |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rivers (n = 9) |
Streams (n = 2) |
Ponds (n = 6) |
Well Waters (n = 2) |
Spring Waer (n = 1) |
Seawater (n = 3) |
Seaweed (n = 5) |
|||||||||||||||||||||
|
AB (n = 5) |
AC (n = 1) |
||||||||||||||||||||||||||
| R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | |
| Amoxicillin-clavulanic acid (AMC) | 7 | 0 | 2 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 26 | 0 | 2 |
| Ampicillin (AMP) | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 28 | 0 | 0 |
| Cefalotin (KF) | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 28 | 0 | 0 |
| Cefotaxime (CTX) | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 28 | 0 | 0 |
| Ciprofloxacin (CIP) | 1 | 0 | 8 | 0 | 0 | 2 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 2 | 0 | 26 |
| Erythromycin (E) | 0 | 2 | 7 | 0 | 0 | 2 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 4 | 3 | 3 | 22 |
| Gentamycin (CN) | 0 | 0 | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 28 |
| Nalidixic acid (NA) | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 28 | 0 | 0 |
| Streptomycin (S) | 0 | 0 | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 1 | 0 | 27 |
| Tetracycline (TE) | 9 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 28 | 0 | 0 |
R: resistant; I: intermediate; S: susceptible; AB: A. butzleri; AC: A. cryaerophilus; n indicates the number of isolates from each aquatic environment.
Figure 4.
Principal coordinates analysis of the resistant, intermediate, and sensitive isolates of A. butzleri. R: resistant; I: intermediate; S: susceptible.
2.3. Analysis of the Quinolone and Tetracycline Resistance Genes
The search for tetracycline resistance genes by PCR in the genome of nine randomly chosen tetracycline-resistant isolates revealed that all carried tetW or tetO, even simultaneously (Table 4). No isolates contained the tetA gene. The two ciprofloxacin-resistant isolates were tested for the presence of the resistance gene qnrS by PCR and sequencing of the gyrA amplification product. The sequence of the gyrA gene did not show the mutation associated with a quinolone resistance phenotype, nor the PCR of qnrS gave the amplicon of the expected size (data not shown).
Table 4.
Tetracycline resistance genes in nine strains isolated from water samples.
| Sample | Specie | tet Resistance Genes |
|---|---|---|
| Seawater | A. butzleri | tetW−, tetO+, tetA− |
| River | A. butzleri | tetW+, tetO+, tetA− |
| Pond with aquatic animals | A. butzleri | tetW+, tetO−, tetA− |
| River | A. butzleri | tetW+, tetO+, tetA− |
| River | A. butzleri | tetW+, tetO+, tetA− |
| Seawater | A. butzleri | tetW+, tetO+, tetA− |
| River | A. butzleri | tetW+, tetO−, tetA− |
| Pond with turtles | A. butzleri | tetW+, tetO−, tetA− |
| Pond with aquatic animals | A. cryaerophilus | tetW+, tetO−, tetA− |
3. Discussion
This study enlarges the knowledge on the spread and the antibiotic resistance profile of the emerging enteropathogen Arcobacter in water samples. Even if the Arcobacter genus is widespread in various environments, water may play an important role in its transmission to animals and humans. Our results showed a higher prevalence of Arcobacter in the surface waters (streams, rivers, ponds) and a very low occurrence in waters dedicated to human consumption (well water, spring water, and drinking water) in accordance with other reports which correlated the presence of Arcobacter species with fecal contamination [9,38]. Specifically, in this study, A. butzleri was the predominant species and was isolated in 81.8% of the river water samples and 75% of the artificial ponds where aquatic animals (birds, turtles) lived. A. cryaerophilus was co-isolated together with A. butzleri from an artificial pond, while A. skirrowii was never detected in any of the samples. Indeed, A. butzleri is the most frequent species isolated from different water samples such as 23% in river water [5] and 55.1% in freshwater, seawater, and sewage samples [9]. A. butzleri was detected in the creek (26.31%) and stream water samples (18.36%) and not isolated from ponds and drinking water samples in the Kars region [39]. A. butzleri was also isolated from surface water samples (25.6%) and treated wastewater samples (77.9%) in southwestern Alberta, Canada [4]. Talay et al. (2016) reported a prevalence of 35.7% from various aquatic sources including sewages, rivers, and spring waters, of which 34% were positive for A. butzleri [11]. In Sicily, Arcobacter spp. was detected from surface waters and in estuarine waters of rivers [13,40]. Water birds (ducks, geese, etc.) can be reservoirs of Arcobacter [41,42] and this would probably explain the higher incidence of isolation in the artificial ponds inhabited by these animals. The waters collected, in this study, from the ponds contained Arcobacter, while the spring water samples, did not. Besides, the samples of drinking waters here analyzed were negative; however, A. butzleri was isolated from a sample of non-chlorinated source water (spring water) used for human consumption, considered as drinkable water for the absence of the fecal contamination markers, i.e., Escherichia coli and enterococci. The presence of Arcobacter was reported in drinking water in Turkey [43] and in treated water samples in Malaysia [44] with percentages of 3% and 11.1%, respectively. Cases of Arcobacter outbreaks associated with contaminated water have been documented worldwide [7,12,26]. Because Arcobacter is sensitive to chlorination [7,45], its isolation in drinking water might indicate either ineffective chlorination or recontamination after chlorination [43]. Due to its ability to form biofilms [2,46], chlorination could not suppress Arcobacter colonization, in fact, biofilms of this strain were found in drinking water distribution pipes [47].
Seawater is also mentioned as a potential source of Arcobacter spp. In our study, A. butzleri was identified in three out of 21 samples of seawater examined (14.2%). Interestingly, we found that algae of the genus Enteromorpha, collected together with seawater samples, were more frequently colonized by A. butzleri with a value of 83.3% than the corresponding seawater samples (50%) (Table 2). Algae could represent a suitable microhabitat for Arcobacter and other bacteria. Previous studies reported that A. butzleri was more abundant in seawater and plankton samples collected from the Straits of Messina, Italy, when associated with plankton than free-living [13,36].
Although the illness caused by Arcobacter can be self-limited, antibiotics, such as aminoglycosides, tetracyclines, and fluoroquinolones are recommended as the drugs of choice for the treatment for Arcobacter infection in human and animals [19,48]. However, strains resistant to these antibiotics have been detected in food and water sources [18,45,46,47]. The resistance of A. butzleri isolates to β-lactams is widespread in water sources as well as in other environments [49]. The resistance of Arcobacter spp. to cephalosporins is known; in fact, these antibiotics are commonly used for the isolation of Campylobacteraceae in selective media [50]. Recently, the genetic determinants associated with the resistance mechanisms have been exploited by comparing the genome sequences of 49 strains [37].
In our results, multidrug resistance was observed in all Arcobacter isolates tested. Precisely, all Arcobacter isolates were resistant to tetracycline and nalidixic acid, and the β-lactam antibiotics, ampicillin, cefalotin, cefotaxime, and AMC (except two isolates). Only three A. butzleri isolates, collected from seawater, seaweed, and a river were erythromycin- and ciprofloxacin-resistant and one A. butzleri isolate was ciprofloxacin-resistant; the A. cryaerophilus isolate displayed resistance to all the tested antibiotics, except gentamycin. Šilha et al. [49] reported that A. cryaerophilus strains collected from water sources were sensitive only to tetracycline and gentamicin.
It is interesting to note that the majority of studies report that A. butzleri isolates are highly susceptible to tetracycline, so that it can be used for human Arcobacter infections [50,51], while out study demonstrated an increasing resistance of the isolates.
Tetracycline resistance is dependent upon more than 40 genes (tet genes). Our molecular analysis demonstrated that among three different genetic determinants known to be involved in tetracycline resistance (tetA, tetO, and tetW) results, all the tetracycline-resistant isolates carried tetO and tetW, even together, while no isolates contained tetA.
The tetO and tetW genes are found more frequently than other tet-genes (e.g., tetA) in commensal bacteria isolated from fecal and water samples [52]. The fact that four out of nine isolates contained two genetic determinants is worrying. tetO and tetW genes confer ribosomal protection from the inhibiting effect of tetracycline [53,54,55], and they appear to be promiscuous in environmental organisms through different transfer mechanisms [56,57]. All isolates were resistant to nalidixic acid and two to ciprofloxacin. The search for qnrS and the sequencing of the gyrA gene did not explain the resistance to ciprofloxacin. To the best of our knowledge, the quinolone-resistance of A. butzleri is due to the mutation Thr-85-Ile of the gyrA gene [37]. Thus, further investigation is necessary to understand the molecular basis of quinolone resistance in these isolates.
The results obtained in this work show that aquatic sources can be a vehicle of potential pathogenic Arcobacter spp. Water is a likely key component to Arcobacter transmission, particularly, in intensive farming operations where water is consumed by the animals or in processing plant [58] or used for vegetable products [59] and water contamination could be due to feces of livestock animals [60] and farm effluents [9,61]. These bacteria can adapt and survive promptly in environmental waters, such as rivers, canals, and irrigation water; indeed, Arcobacter may survive in environmental waters, replicate at refrigeration temperatures [2], and develop the viable nonculturable state, thus representing a potential risk for human health [62,63].
To better assess the risks for human health, it is important to deepen these investigations and to search Arcobacter strains in non-chlorinated water that can be used for water supply and for irrigation of raw consumed vegetables.
4. Materials and Methods
4.1. Sampling
One hundred samples (Table 1) including rivers (n = 11), streams (n = 5), artificial ponds (n = 8), well waters (n = 20), spring waters (n = 17), drinking waters (n = 12), and seawater (n = 21), were collected between February and December 2017 in all around Sicily; in addition, seaweed samples (n = 6) were taken in consideration.
All the water samples, collected in 1 L sterile flasks, were transported under cold storage temperature (4 °C) to the laboratory and analyzed within 24 h.
4.2. Isolation of Arcobacter
For the isolation of Arcobacter spp. from water samples, the protocol reported in Collado et al. 2008 [9] was followed. Specifically, 200 mL of water were filtered using a 0.45 µm nitrocellulose membrane filter (Sartorius). For Arcobacter isolation from seaweeds, 10 g were weighed. Filters or seaweeds were placed into sterile bags containing 30 or 90 mL, respectively, of Arcobacter enrichment broth (Oxoid, UK) added with cefoperazone, amphotericin B, and teicoplanin (CAT) selective supplement (SR0174, Oxoid, UK) and incubated a 30 °C for 48 h under aerobic condition. After incubation, 200 µL of the broth were then dropped onto the surface of 0.45 µm nitrocellulose membrane filter (Sartorius), placed onto two selective agar plates: trypticase soy agar (TSA) plus 5% Laked Horse blood (Oxoid) with CAT and modified charcoal cefoperazone deoxycholate agar (mCCDA) supplemented with CAT. Plates were incubated at room temperature for 30 min and after removal of the filters, incubated at 30 °C for 48 h to 72 h under aerobic conditions [16]. Subsequently, suspected colonies grown within the filter area with a diameter between 0.5 and 2 mm, were picked, subcultured onto blood agar, and incubated at 30° C for 48 h [6]. Presumptive identification tests (Gram staining, catalase, oxidase, urease tests, and motility) were performed on at least five suspected colonies. The isolates referable as Arcobacter genus (Gram negative, spiral- shaped, motile, oxidase and catalase positive, urease negative), were stored in 20% (v/v) nutrient broth–glycerol at −80 °C, for subsequent molecular identification.
4.3. Identification of Arcobacter Species by Multiplex PCR
DNA was extracted by using the protocol previously reported [15] and utilized as template in a multiplex PCR assay to amplify the 16S and 23S rRNA genes in order to obtain a specific and simultaneous identification of A. butzleri, A. cryaerophilus, and A. skirrowii [64]. The selected primers amplified a 401-bp fragment from A. butzleri, a 257-bp fragment from A. cryaerophilus, and a 641-bp fragment from A. skirrowii. The amplification products were then separated by electrophoresis on 1.5% agarose gels, stained by SYBR Safe DNA gel stain, at 100 V for 40 min, and the bands were visualized under UV transilluminator (GelDoc-It, UVP Cambridge, UK). DNA from reference strains A. butzleri (NCTC 12481), A. cryaerophilus (NCTC 11885) and A. skirrowii (NCTC 12713) were used as positive controls and sterile distilled water was used as negative control.
4.4. Identification of Arcobacter Species by Sequence Analysis
The PCR products of Arcobacter spp. were purified using Illustra GFX PCR DNA and Gel Band Purification kit (GE Healthcare) following the manufacturer’s instructions for sequencing analysis. The purified products were sent to Macrogen Company (Amsterdam, Holland) for Sanger sequencing. The identification was performed by the alignment of the sequences against a reference database (GenBank). Novel sequences are available online in the GenBank™ database under the accession numbers MW678780, MW683239, MW678840, MW678844, MW678845, MW683240. The software packages MrBayes v. 3.2.7) [65] and MEGAX [66] were used for inferring phylogenetic relationships through Bayesian inference of phylogeny (BI) and maximum likelihood analysis (ML). As support measures for the nodes, bootstrap values were calculated with 1000 replicates in the ML trees, whereas in the BI tree, the posterior probability values were reported. PartitionFinder v. 1.0.1 [67] was used to choose the best evolutionary model following the Akaike information criterion (AIC). For the 16S rDNA fragment, the general time-reversible model of evolution with gamma-distributed rate variation among sites (GTR+Γ; nst = 6) was used for both the BI and ML analyses. Six sequences were used as representatives.
4.5. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing of the Arcobacter isolates against 10 antibiotics was performed on Mueller-Hinton agar (Oxoid) by disk diffusion method according to the guidelines of the Clinical and Laboratory Standard Institute (CLSI) for Campylobacter jejuni/coli. Amoxicillin-clavulanic acid (AMC, 30 µg), ampicillin (AMP, 10 µg), cefalotin (KF 30 µg), cefotaxime (CTX, 30 µg), ciprofloxacin (CIP, 5 µg), erythromycin (E, 10 µg), gentamicin (CN, 10 μg), nalidixic acid (NA 30 µg), streptomycin (S, 10 µg), tetracycline (TE, 30 μg) disks (OXOID, UK) were used. Fluoroquinolone ciprofloxacin, tetracyclines, and aminoglycosides, such as gentamicin, kanamycin, and streptomycin, represent the most common antimicrobial agents for treatment of Arcobacter infections, because of high frequency of susceptibility towards them. The isolates were sub-cultured on blood agar base (Oxoid) and after incubation at 37 °C in aerobic condition for 24 h, a 0.5 MacFarland bacterial suspension was prepared in saline solution and spread on Mueller-Hinton agar. The plates were incubated at 37 °C for 24 h under aerobic condition. Then, the diameters of inhibition zones were measured and the isolates were classified as resistant (R), susceptible (S), or intermediate (I). The experiments were performed in duplicate. The A. butzleri LMG 10828T (RM4018) strain was used for comparison purposes. Principal coordinate analysis (PCoA) was performed using the software package PRIMER 6 [68]. The analyses were based on Bray–Curtis distance matrix.
4.6. Analysis of the Quinolone and Tetracycline Resistance Genes
DNA was extracted from 9 samples and utilized as template to amplify the genes coding for products responsible for the resistance to tetracycline (tetA, tetO, and tetW) and to quinolones (qnrS and gyrA) using the primer pairs reported in Table 5. Amplicons were detected using a 6% polyacrylamide non denaturing gel in TBE 0.5X, except gyrA amplicon that was detected using a 1.5% agarose gel. The presence of the expected amplification product was considered as a positive sample. All the PCR products were sequenced.
Table 5.
List of the primers used in this study.
| Target Name | Primer Sequence (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|
| 16s rDNA | cggtgaatacgttcycgg | 142 | [63] |
| gghtaccttgttacgactt | |||
| tetA | gctacatcctgcttgccttc | 210 | [64] |
| catagatcgccgtgaagagg | |||
| tetW | acatcattgatactccaggtcacg | 120 | [51] |
| tttcactttgtggttgaacccctc | |||
| tetO | ggaggggttcaaccacaaag | 88 | [51] |
| ctatgtaaataaaatggatag | |||
| gyrA | tggattaaagccagttcatagaag | 344 | [29] |
| tcatmgwatcatcataatttggwac | |||
| qnrS | gacgtgctaacttgcgtgat | 118 | [59] |
| tggcattgttggaaacttg | |||
| butz | cctggacttgacatagtaagaatga | 401 | [64] |
| arco | cgtattcaccgtagcatagc | ||
| skir | ggcgatttactggaacaca | 641 | [64] |
| arco | cgtattcaccgtagcatagc | ||
| cry1 | tgctggagcggatagaagta | 257 | [64] |
| cry2 | aacaacctacgtccttcgac |
5. Conclusions
In the present study, we evaluated the presence of Arcobacter species in different environmental water sources. Our results showed the spread of this important zoonotic agent in the environment, which can be considered a potential risk for food safety. The determination of Arcobacter in water sources might be important to better understand the epidemiology and the ecology of these bacteria.
All the Arcobacter isolates displayed an alarming multi-drug resistance. Molecular analysis of genetic determinants responsible for tetracycline resistance in nine randomly chosen isolates revealed the presence of tetO and/or tetW. This work confirms the occurrence and the continuous emergence of antibiotic-resistant Arcobacter strains in environmental samples; besides, the presence of antibiotic-resistant Arcobacter spp. in aquatic sources used for water supply and irrigation represents a potential risk for human health.
Acknowledgments
The authors acknowledge all colleagues and volunteers for their assistance in collecting samples. We wish to thank Ylenia Di Leto for technical support and Luca Vecchioni for bioinformatics support.
Author Contributions
Conceptualization, M.A., S.S., A.C. and R.A.; methodology, P.A., V.A., C.C., L.N.; investigation, P.A., V.A., C.C., L.N. and R.A.; writing—original draft preparation, S.S. and A.C.; writing—review and editing, R.A.; supervision, S.S.; funding acquisition, S.S. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Research Project IZS SI 06/18 RC.
Data Availability Statement
Nucleotide sequence data reported in this study are available in the GenBank™ database under the accession numbers MW678780, MW683239, MW678840, MW678844, MW678845, MW683240 (Arcobacter butzleri 16S rRNA gene, partial sequence).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vandamme P., De Ley J. Proposal for a New Family, Campylobacteraceae. Int. J. Syst. Bacteriol. 1991;41:451–455. doi: 10.1099/00207713-41-3-451. [DOI] [Google Scholar]
- 2.Kjeldgaard J., Jørgensen K., Ingmer H. Growth and Survival at Chiller Temperatures of Arcobacter butzleri. Int. J. Food Microbiol. 2009;131:256–259. doi: 10.1016/j.ijfoodmicro.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Collado L., Kasimir G., Perez U., Bosch A., Pinto R., Saucedo G., Huguet J.M., Figueras M.J. Occurrence and Diversity of Arcobacter Spp. along the Llobregat River Catchment, at Sewage Effluents and in a Drinking Water Treatment Plant. Water Res. 2010;44:3696–3702. doi: 10.1016/j.watres.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Webb A.L., Taboada E.N., Selinger L.B., Boras V.F., Inglis G.D. Prevalence and Diversity of Waterborne Arcobacter butzleri in Southwestern Alberta, Canada. Can. J. Microbiol. 2017;63:330–340. doi: 10.1139/cjm-2016-0745. [DOI] [PubMed] [Google Scholar]
- 5.Morita Y., Maruyama S., Kabeya H., Boonmar S., Nimsuphan B., Nagai A., Kozawa K., Nakajima T., Mikami T., Kimura H. Isolation and Phylogenetic Analysis of Arcobacter Spp. in Ground Chicken Meat and Environmental Water in Japan and Thailand. Microbiol. Immunol. 2004;48:527–533. doi: 10.1111/j.1348-0421.2004.tb03548.x. [DOI] [PubMed] [Google Scholar]
- 6.Musmanno R.A., Russi M., Lior H., Figura N. In Vitro Virulence Factors of Arcobacter butzleri Strains Isolated from Superficial Water Samples. New Microbiol. 1997;20:63–68. [PubMed] [Google Scholar]
- 7.Rice E.W., Rodgers M.R., Wesley I.V., Johnson C.H., Tanner S.A. Isolation of Arcobacter butzleri from Ground Water. Lett. Appl. Microbiol. 1999;28:31–35. doi: 10.1046/j.1365-2672.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 8.Fong T.-T., Mansfield L.S., Wilson D.L., Schwab D.J., Molloy S.L., Rose J.B. Massive Microbiological Groundwater Contamination Associated with a Waterborne Outbreak in Lake Erie, South Bass Island, Ohio. Environ. Health Perspect. 2007;115:856–864. doi: 10.1289/ehp.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collado L., Inza I., Guarro J., Figueras M.J. Presence of Arcobacter Spp. in Environmental Waters Correlates with High Levels of Fecal Pollution. Environ. Microbiol. 2008;10:1635–1640. doi: 10.1111/j.1462-2920.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- 10.Merga J.Y., Royden A., Pandey A.K., Williams N.J. Arcobacter Spp. Isolated from Untreated Domestic Effluent. Lett. Appl. Microbiol. 2014;59:122–126. doi: 10.1111/lam.12256. [DOI] [PubMed] [Google Scholar]
- 11.Talay F., Molva C., Atabay H.I. Isolation and Identification of Arcobacter Species from Environmental and Drinking Water Samples. Folia Microbiol. 2016;61:479–484. doi: 10.1007/s12223-016-0460-0. [DOI] [PubMed] [Google Scholar]
- 12.Jacob J., Lior H., Feuerpfeil I. Isolation of Arcobacter butzleri from a Drinking Water Reservoir in Eastern Germany. Zent. Hyg. Umw. 1993;193:557–562. [PubMed] [Google Scholar]
- 13.Fera M.T., Maugeri T.L., Gugliandolo C., Beninati C., Giannone M., La Camera E., Carbone M. Detection of Arcobacter Spp. in the Coastal Environment of the Mediterranean Sea. Appl. Environ. Microbiol. 2004;70:1271–1276. doi: 10.1128/AEM.70.3.1271-1276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghane F.G.M.M. Isolation of Arcobacter butzleri from Caspian Sea’s Water. J. Appl. Environ. Microbiol. 2014;2:61–64. doi: 10.12691/jaem-2-2-5. [DOI] [Google Scholar]
- 15.Noto A.M.D., Sciortino S., Cardamone C., Ciravolo C., Napoli C., Alio V., Arculeo P., Oliveri G., Costa A. Detection of Arcobacter spp. in Food Products Collected from Sicilia Region: A Preliminary Study. Ital. J. Food Saf. 2018;7 doi: 10.4081/ijfs.2018.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collado L., Guarro J., Figueras M.J. Prevalence of Arcobacter in Meat and Shellfish. J. Food Prot. 2009;72:1102–1106. doi: 10.4315/0362-028X-72.5.1102. [DOI] [PubMed] [Google Scholar]
- 17.Mottola A., Bonerba E., Bozzo G., Marchetti P., Celano G.V., Colao V., Terio V., Tantillo G., Figueras M.J., Di Pinto A. Occurrence of Emerging Food-Borne Pathogenic Arcobacter spp. Isolated from Pre-Cut (Ready-to-Eat) Vegetables. Int. J. Food Microbiol. 2016;236:33–37. doi: 10.1016/j.ijfoodmicro.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Parisi A., Capozzi L., Bianco A., Caruso M., Latorre L., Costa A., Giannico A., Ridolfi D., Bulzacchelli C., Santagada G. Identification of Virulence and Antibiotic Resistance Factors in Arcobacter butzleri Isolated from Bovine Milk by Whole Genome Sequencing. Ital. J. Food Saf. 2019;8:7840. doi: 10.4081/ijfs.2019.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collado L., Figueras M.J. Taxonomy, Epidemiology, and Clinical Relevance of the Genus Arcobacter. Clin. Microbiol. Rev. 2011;24:174–192. doi: 10.1128/CMR.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueras M.J., Levican A., Pujol I., Ballester F., Rabada Quilez M.J., Gomez-Bertomeu F. A Severe Case of Persistent Diarrhoea Associated with Arcobacter cryaerophilus but Attributed to Campylobacter Sp. and a Review of the Clinical Incidence of Arcobacter Spp. New Microbes New Infect. 2014;2:31–37. doi: 10.1002/2052-2975.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skovgaard N. Microorganisms in Foods 7: Microbiological Testing in Food Safety Management—By International Commission for the Microbiological Specifications of Foods (ICMSF). Kluwer Academic/Plenum Publishers, New York, Xiii+367 Pages, Hardbound, ISBN 0-306-47262-7, Euro 144.50/ US$ 125.00/GP@$ 88.50. Http://Www.Wkap.Nl . Int. J. Food Microbiol. 2003;2–3:291–292. doi: 10.1016/S0168-1605(03)00163-6. [DOI] [Google Scholar]
- 22.Lerner J., Brumberger V., Preac-Mursic V. Severe Diarrhea Associated With Arcobacter butzleri. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13:660–662. doi: 10.1007/BF01973994. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberg O., Dediste A., Houf K., Ibekwem S., Souayah H., Cadranel S., Douat N., Zissis G., Butzler J.-P., Vandamme P. Arcobacter Species in Humans. Emerg. Infect. Dis. 2004;10:1863–1867. doi: 10.3201/eid1010.040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arguello E., Otto C.C., Mead P., Babady N.E. Bacteremia Caused by Arcobacter butzleri in an Immunocompromised Host. J. Clin. Microbiol. 2015;53:1448–1451. doi: 10.1128/JCM.03450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandamme P., Pugina P., Benzi G., Etterijck R.V., Vlaes L., Kersters K., Butzler J.P., Lior H., Lauwers S. Outbreak of Recurrent Abdominal Cramps Associated with Arcobacter butzleri in an Italian School. J. Clin. Microbiol. 1992;30:2335–2337. doi: 10.1128/JCM.30.9.2335-2337.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chieffi D., Fanelli F., Fusco V. Arcobacter butzleri: Up-to-Date Taxonomy, Ecology, and Pathogenicity of an Emerging Pathogen. Compr. Rev. Food Sci. Food Saf. 2020;19:2071–2109. doi: 10.1111/1541-4337.12577. [DOI] [PubMed] [Google Scholar]
- 27.Vidal-Veuthey B., Jara R., Santander K., Mella A., Ruiz S., Collado L. Antimicrobial Resistance and Virulence Genes Profiles of Arcobacter butzleri Strains Isolated from Back Yard Chickens and Retail Poultry Meat in Chile. Lett. Appl. Microbiol. 2020;72:126–132. doi: 10.1111/lam.13404. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira S., Queiroz J.A., Oleastro M., Domingues F.C. Insights in the Pathogenesis and Resistance of Arcobacter: A Review. Crit. Rev. Microbiol. 2016;42:364–383. doi: 10.3109/1040841X.2014.954523. [DOI] [PubMed] [Google Scholar]
- 29.Abdelbaqi K., Ménard A., Prouzet-Mauleon V., Bringaud F., Lehours P., Mégraud F. Nucleotide Sequence of the GyrA Gene of Arcobacter Species and Characterization of Human Ciprofloxacin-Resistant Clinical Isolates. FEMS Immunol. Med. Microbiol. 2007;49:337–345. doi: 10.1111/j.1574-695X.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 30.Van den Abeele A.-M., Vogelaers D., Vanlaere E., Houf K. Antimicrobial Susceptibility Testing of Arcobacter butzleri and Arcobacter cryaerophilus Strains Isolated from Belgian Patients. J. Antimicrob. Chemother. 2016;71:1241–1244. doi: 10.1093/jac/dkv483. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira S., Luís Â., Oleastro M., Pereira L., Domingues F.C. A Meta-Analytic Perspective on Arcobacter Spp. Antibiotic Resistance. J. Glob. Antimicrob. Resist. 2019;16:130–139. doi: 10.1016/j.jgar.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Vitale M., Galluzzo P., Buffa P.G., Carlino E., Spezia O., Alduina R. Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples. Antibiotics (Basel) 2019;8:97. doi: 10.3390/antibiotics8030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sucato A., Vecchioni L., Savoca D., Presentato A., Arculeo M., Alduina R. A Comparative Analysis of Aquatic and Polyethylene-Associated Antibiotic-Resistant Microbiota in the Mediterranean Sea. Biology. 2021;10:200. doi: 10.3390/biology10030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blasi M.F., Migliore L., Mattei D., Rotini A., Thaller M.C., Alduina R. Antibiotic Resistance of Gram-Negative Bacteria from Wild Captured Loggerhead Sea Turtles. Antibiotics (Basel) 2020;9:162. doi: 10.3390/antibiotics9040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alduina R., Gambino D., Presentato A., Gentile A., Sucato A., Savoca D., Filippello S., Visconti G., Caracappa G., Vicari D., et al. Is Caretta Caretta a Carrier of Antibiotic Resistance in the Mediterranean Sea? Antibiotics (Basel) 2020;9:116. doi: 10.3390/antibiotics9030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu T.-T.D., Lee J. Global Distribution and Prevalence of Arcobacter in Food and Water. Zoonoses Public Health. 2015;62:579–589. doi: 10.1111/zph.12215. [DOI] [PubMed] [Google Scholar]
- 37.Isidro J., Ferreira S., Pinto M., Domingues F., Oleastro M., Gomes J.P., Borges V. Virulence and Antibiotic Resistance Plasticity of Arcobacter butzleri: Insights on the Genomic Diversity of an Emerging Human Pathogen. Infect. Genet. Evol. 2020;80:104213. doi: 10.1016/j.meegid.2020.104213. [DOI] [PubMed] [Google Scholar]
- 38.Lee C., Agidi S., Marion J.W., Lee J. Arcobacter in Lake Erie Beach Waters: An Emerging Gastrointestinal Pathogen Linked with Human-Associated Fecal Contamination. Appl. Environ. Microbiol. 2012;78:5511–5519. doi: 10.1128/AEM.08009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Çelik E., Ünver A. Isolation and Identification of Arcobacter spp. by Multiplex PCR from Water Sources in Kars Region. Curr. Microbiol. 2015;71:546–550. doi: 10.1007/s00284-015-0883-x. [DOI] [PubMed] [Google Scholar]
- 40.Maugeri T., Irrera G.P., Lentini V., Carbone M.H., Fera M.T., Gugliandolo C. Detection and Enumeration of Arcobacter spp. in the Coastal Environment of the Straits of Messina (Italy) New Microbiol. 2005;28:177–182. [PubMed] [Google Scholar]
- 41.Atabay H.I., Wainø M., Madsen M. Detection and Diversity of Various Arcobacter Species in Danish Poultry. Int. J. Food Microbiol. 2006;109:139–145. doi: 10.1016/j.ijfoodmicro.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Atabay H.I., Unver A., Sahin M., Otlu S., Elmali M., Yaman H. Isolation of Various Arcobacter Species from Domestic Geese (Anser Anser) Vet. Microbiol. 2008;128:400–405. doi: 10.1016/j.vetmic.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Ertas N., Dogruer Y., Gonulalan Z., Guner A., Ulger I. Prevalence of Arcobacter Species in Drinking Water, Spring Water, and Raw Milk as Determined by Multiplex PCR. J. Food Prot. 2010;73:2099–2102. doi: 10.4315/0362-028X-73.11.2099. [DOI] [PubMed] [Google Scholar]
- 44.Shah A.H., Saleha A.A., Zunita Z., Cheah Y.K., Murugaiyah M., Korejo N.A. Genetic Characterization of Arcobacter Isolates from Various Sources. Vet. Microbiol. 2012;160:355–361. doi: 10.1016/j.vetmic.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Moreno Y., Alonso J.L., Botella S., Ferrús M.A., Hernández J. Survival and Injury of Arcobacter after Artificial Inoculation into Drinking Water. Res. Microbiol. 2004;155:726–730. doi: 10.1016/j.resmic.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira S., Fraqueza M.J., Queiroz J.A., Domingues F.C., Oleastro M. Genetic Diversity, Antibiotic Resistance and Biofilm-Forming Ability of Arcobacter butzleri Isolated from Poultry and Environment from a Portuguese Slaughterhouse. Int. J. Food Microbiol. 2013;162:82–88. doi: 10.1016/j.ijfoodmicro.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Assanta M.A., Roy D., Lemay M.J., Montpetit D. Attachment of Arcobacter butzleri, a New Waterborne Pathogen, to Water Distribution Pipe Surfaces. J. Food Prot. 2002;65:1240–1247. doi: 10.4315/0362-028X-65.8.1240. [DOI] [PubMed] [Google Scholar]
- 48.Fera M.T., Maugeri T.L., Giannone M., Gugliandolo C., La Camera E., Blandino G., Carbone M. In Vitro Susceptibility of Arcobacter butzleri and Arcobacter cryaerophilus to Different Antimicrobial Agents. Int. J. Antimicrob. Agents. 2003;21:488–491. doi: 10.1016/S0924-8579(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 49.Šilha D., Pejchalová M., Šilhová L. Susceptibility to 18 Drugs and Multidrug Resistance of Arcobacter Isolates from Different Sources within the Czech Republic. J. Glob. Antimicrob. Resist. 2017;9:74–77. doi: 10.1016/j.jgar.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Ramees T.P., Dhama K., Karthik K., Rathore R.S., Kumar A., Saminathan M., Tiwari R., Malik Y.S., Singh R.K. Arcobacter: An Emerging Food-Borne Zoonotic Pathogen, Its Public Health Concerns and Advances in Diagnosis and Control—A Comprehensive Review. Vet. Q. 2017;37:136–161. doi: 10.1080/01652176.2017.1323355. [DOI] [PubMed] [Google Scholar]
- 51.Zambri M., Cloutier M., Adam Z., Lapen D.R., Wilkes G., Sunohara M., Topp E., Talbot G., Khan I.U.H. Novel Virulence, Antibiotic Resistance and Toxin Gene-Specific PCR-Based Assays for Rapid Pathogenicity Assessment of Arcobacter faecis and Arcobacter lanthieri. BMC Microbiol. 2019;19:11. doi: 10.1186/s12866-018-1357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang H.Y., Byelashov O.A.B.A., Geornaras I.G., Goodridge L.D.G.D., Nightingale K.K.N.K., Belk K.E.B.E., Smith G.C.S.C., Sofos J.N.S.N. Presence of Antibiotic-Resistant Commensal Bacteria in Samples from Agricultural, City, and National Park Environments Evaluated by Standard Culture and Real-Time PCR Methods. Can. J. Microbiol. 2010;56:761–770. doi: 10.1139/W10-060. [DOI] [PubMed] [Google Scholar]
- 53.Schnappinger D., Hillen W. Tetracyclines: Antibiotic Action, Uptake, and Resistance Mechanisms. Arch. Microbiol. 1996;165:359–369. doi: 10.1007/s002030050339. [DOI] [PubMed] [Google Scholar]
- 54.Roberts M.C. Update on Acquired Tetracycline Resistance Genes. FEMS Microbiol. Lett. 2005;245:195–203. doi: 10.1016/j.femsle.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 55.Levy S.B., McMurry L.M., Barbosa T.M., Burdett V., Courvalin P., Hillen W., Roberts M.C., Rood J.I., Taylor D.E. Nomenclature for New Tetracycline Resistance Determinants. Antimicrob. Agents Chemother. 1999;43:1523–1524. doi: 10.1128/AAC.43.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Billington S.J., Songer J.G., Jost B.H. Widespread Distribution of a Tet W Determinant among Tetracycline-Resistant Isolates of the Animal Pathogen Arcanobacterium pyogenes. Antimicrob. Agents Chemother. 2002;46:1281–1287. doi: 10.1128/AAC.46.5.1281-1287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chopra I., Roberts M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houf K., De Zutter L., Van Hoof J., Vandamme P. Occurrence and Distribution of Arcobacter Species in Poultry Processing. J. Food Prot. 2002;65:1233–1239. doi: 10.4315/0362-028X-65.8.1233. [DOI] [PubMed] [Google Scholar]
- 59.Hausdorf L., Neumann M., Bergmann I., Sobiella K., Mundt K., Fröhling A., Schlüter O., Klocke M. Occurrence and Genetic Diversity of Arcobacter Spp. in a Spinach-Processing Plant and Evaluation of Two Arcobacter-Specific Quantitative PCR Assays. Syst. Appl. Microbiol. 2013;36:235–243. doi: 10.1016/j.syapm.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 60.van Driessche E., Houf K., van Hoof J., De Zutter L., Vandamme P. Isolation of Arcobacter Species from Animal Feces. FEMS Microbiol. Lett. 2003;229:243–248. doi: 10.1016/S0378-1097(03)00840-1. [DOI] [PubMed] [Google Scholar]
- 61.Chinivasagam H.N., Corney B.G., Wright L.L., Diallo I.S., Blackall P.J. Detection of Arcobacter Spp. in Piggery Effluent and Effluent-Irrigated Soils in Southeast Queensland. J. Appl. Microbiol. 2007;103:418–426. doi: 10.1111/j.1365-2672.2007.03275.x. [DOI] [PubMed] [Google Scholar]
- 62.Wesley I.V. Helicobacter and Arcobacter Species: Risks for Foods and Beverages. J. Food Prot. 1996;59:1127–1132. doi: 10.4315/0362-028X-59.10.1127. [DOI] [PubMed] [Google Scholar]
- 63.Di Cesare A., Eckert E.M., D’Urso S., Bertoni R., Gillan D.C., Wattiez R., Corno G. Co-Occurrence of Integrase 1, Antibiotic and Heavy Metal Resistance Genes in Municipal Wastewater Treatment Plants. Water Res. 2016;94:208–214. doi: 10.1016/j.watres.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 64.Houf K., Tutenel A., De Zutter L., Van Hoof J., Vandamme P. Development of a Multiplex PCR Assay for the Simultaneous Detection and Identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol. Lett. 2000;193:89–94. doi: 10.1111/j.1574-6968.2000.tb09407.x. [DOI] [PubMed] [Google Scholar]
- 65.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanfear R., Calcott B., Ho S.Y.W., Guindon S. Partitionfinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 68.Clarke K., Gorley R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research) Primer-e Ltd; Plymouth, MA, USA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nucleotide sequence data reported in this study are available in the GenBank™ database under the accession numbers MW678780, MW683239, MW678840, MW678844, MW678845, MW683240 (Arcobacter butzleri 16S rRNA gene, partial sequence).