Abstract
Clematis plants play an important role in botanical gardens. Heat stress can destroy the activity, state and conformation of plant proteins, and its regulatory pathway has been well characterized in Arabidopsis and some crop plants. However, the heat resistance response mechanism in horticultural plants including Clematis has rarely been reported. Here, we identified a heat-tolerant clematis species, Clematis vitalba. The relative water loss and electrolytic leakage were significantly lower under heat treatment in Clematis vitalba compared to Stolwijk Gold. Differential expression heat-tolerant genes (HTGs) were identified based on nonparametric transcriptome analysis. For validation, one heat shock transcription factor, CvHSF30-2, extremely induced by heat stimuli in Clematis vitalba, was identified to confer tolerance to heat stress in Escherichia coli and Saccharomyces cerevisiae. Furthermore, silencing of HSF30-2 by virus-induced gene silencing (VIGS) led to heat sensitivity in tobacco and Clematis, suggesting that the candidate heat-resistant genes identified in this RNA-seq analysis are credible and offer significant utility. We also found that CvHSF30-2 improved heat tolerance of Clematis vitalba by elevating heat shock protein (HSP) expression, which was negatively regulated by CvHSFB2a. Taken together, this study provides insights into the mechanism of Clematis heat tolerance and the findings can be potentially applied in horticultural plants to improve economic efficiency through genetic approaches.
Keywords: Clematis, heat stress, transcriptome analysis, CvHSF30-2, CvHSFB2a, VIGS
1. Introduction
Clematis, also known as the clematis peony, is a genus of about 355 species of buttercup, mainly distributed in the temperate regions north of the Earth’s equator, which are typical woody vines. Clematis plants play an important role in botanical gardens due to their unique flowers, rich colors and wire-like stems and are praised as the “Queen of Vines” [1]. Clematis can be seen in most botanical gardens, parks and family gardens. Additionally, Clematis is a plant source of many medicinal active ingredients and its specialized metabolites can be used as medicine to disperse wind damp, unclog channels and ease pain [2].
Thermal stress is a major threat to the cell, causing protein denaturation and compromising membrane integrity, leading to the death of plant cells [3,4]. Understanding the balance between temperature perception and plant defense can provide a foundation for enhancing plant tolerance (defined as the ability to compensate in part for fitness decrements caused by stress [5]) to thermal stress and improving economic efficiency. Eukaryotic cells respond to heat shock by elevating the transcription of heat shock proteins (HSPs), many of which function in preventing or repairing the damage caused by heat stress and thus confer enhanced thermotolerance [3,6]. Recent work has revealed that plant HSPs are classified into five major groups: HSP100s, HSP90s, HSP70s, HSP60s and small HSPs (sHSPs) [7,8]. Arabidopsis cytosolic HSP90 regulates the heat shock response and is responsible for heat acclimation [9]. The chloroplast small heat shock protein HSP21 is essential for the development of chloroplasts during heat stress [10]. The sHsp26 family protects the photosynthetic machinery during episodes of high-temperature stress in wheat [11].
HSPs are transcriptionally induced by heat shock factor (HSF)-class transcription factors (TFs) upon activation by heat stress [6,12]. The HsfA1 group is considered as the master regulator of heat stress response (HSR) [13]. Genetic evidence shows that HsfA1a and HsfA1b are involved in the induction of a number of Hsp genes in the early phase of HSR in Arabidopsis [14]. In tomato (Lycopersicon esculentum), HsfA1a plays an important role in regulating HSR [15]. HsfA2 is highly and exclusively expressed under heat stress and becomes the dominant HSF under long-term heat stress (HS) [13]. Excessive expression of Arabidopsis HsfA2 was found to improve heat tolerance in an hsfA1a,b,d,e mutant [16]. Increased thermotolerance was also obtained by ectopic expression of rice HsfA2a and wheat HsfA2d in Arabidopsis [17,18]. Some HSF proteins have been reported to be involved in HsfA1-independent pathways under heat shock [19]. A rice (Oryza sativa) mutant, spl7, which is sensitive to elevated temperature, has been shown to have a missense mutation in an Hsf gene belonging to the HsfA4 group [20]. It has also been reported that HSFB TFs, e.g., Arabidopsis HsfB1 and HsfB2b, function as repressors in the expression of HsfA and several heat shock proteins [21]. Unlike that in Arabidopsis and some crop plants, the heat resistance response mechanism in horticultural plants including Clematis has been scarcely reported on.
Virus-induced gene silencing (VIGS) is a burgeoning and useful technology that is based on the plant autoimmune system. When plants face viral infection, the endogenous gene homologous to the insert fragment in the VIGS vector can be degraded by post-transcriptional gene silencing (PTGS) [22,23]. Some plant viruses have been used to develop VIGS vectors; e.g., the tobacco rattle virus (TRV) [24], tobacco mosaic virus (TMV) [25], tomato golden mosaic virus (TGMV) [26] and potato virus X (PVX) [27]. VIGS is an effective reverse-genetics tool for the gene function study of plants [28]. Knockdown of endogenous F3′H in Phalaenopsis via VIGS to assess whether delphinidin could accumulate has been conducted previously [29], and in another study, RcAP1 was silenced by using VIGS in Rose “Old Blush” to verify its function in flower bud formation [30].
In this study, one heat-tolerant Clematis species, Clematis vitalba, was identified based on physiological indexes. Transcriptome analysis showed that HSF TFs are essential for thermotolerance in Clematis vitalba. For validation, one heat-shock transcription factor, CvHSF30-2, extremely induced by heat stimuli in Clematis vitalba, was identified as conferring resistance to a species of stresses to Escherichia coli and Saccharomyces cerevisiae. The effects of CvHSF30-2 on heat stress in tobacco were verified by the VIGS technique. In addition, we established the method of Clematis VIGS to provide a theoretical basis for the study of its gene function. We also found that CvHSF30-2 improved the heat tolerance of Clematis vitalba by elevating HSP expression, which was negatively regulated by CvHSFB2a. Our findings uncover the thermotolerance mechanism in Clematis varieties and have potential applications in agriculture in improving the economic efficiency of horticultural plants through genetic approaches.
2. Results
2.1. Heat Shock Phenotypes of Different Clematis Species
Clematis vitalba (Cv) is an original species of Clematis with branched, grooved stems, deciduous leaves and scented green-white flowers with fluffy underlying sepals. Due to its disseminatory reproductive system, vitality and climbing behavior, Clematis vitalba is an invasive plant in many places, such as New Zealand (http://www.iucngisd.org/gisd/species.php?sc=157 (accessed on 3 May 2020)). Clematis alpina “Stolwijk Gold” (SG) belongs to the ornamental cultivars of Clematis (Figure 1A), as recognized by the Royal Horticultural Society of England (https://www.rhs.org.uk/Plants/236939/Clematis-alpina-Stolwijk-Gold-(A)/Details (accessed on 3 May 2020)).
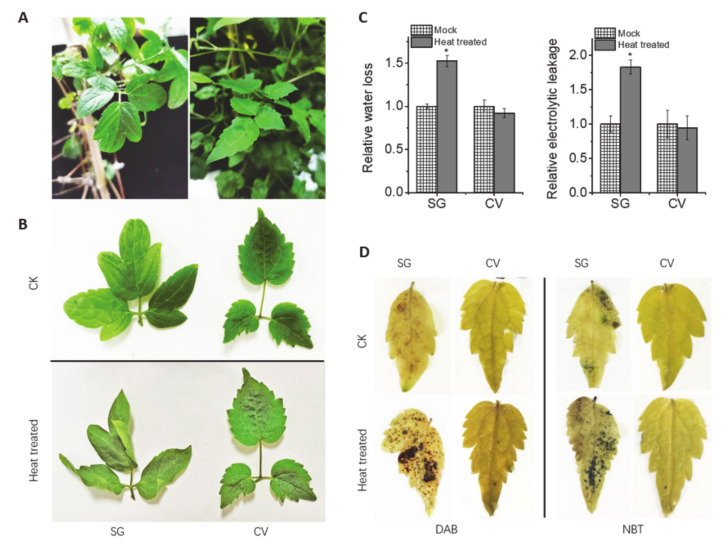
Figure 1.
Two Clematis varieties and physiological changes after high-temperature treatment. (A) Two Clematis plants: Clematis vitalba (Cv, left) and Clematis “Stolwijk Gold” (SG, right). (B) Phenotype of the two Clematis plants after heat treatment. (C) Change of relative water loss and relative electrolytic leakage after high-temperature treatment (42 °C for 2 h) in Clematis varieties. (D) Diaminobenzidine (DAB) and nitro blue tetrazolium (NBT) staining of the two Clematis varieties’ leaves after high-temperature treatment (42 °C for 2 h).
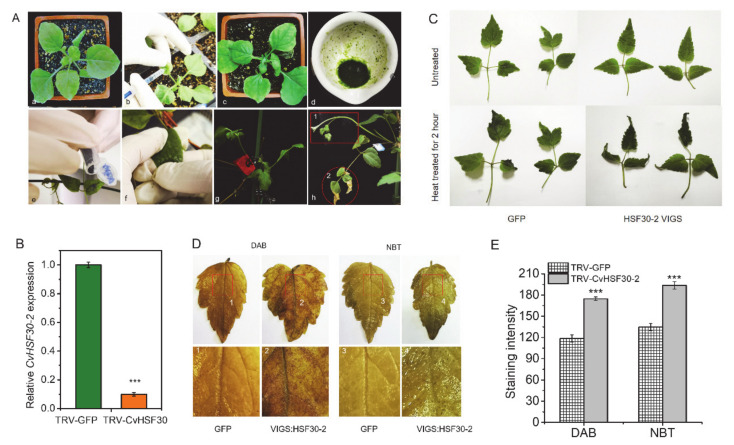
In order to identify heat tolerance differences, Cv and SG were treated with 42 °C for 2 h. It can be seen that the leaves of SG were largely wilting, while those of Cv were totally normal (Figure 1B), suggesting that Cv is one species with high thermotolerance. In addition, relative water loss and electrolytic leakage significantly increased in SG, whereas Cv did not show much difference from the control (Figure 1C), meaning it suffered less membrane damage under heat stress. Moreover, nitro blue tetrazolium (NBT) and diaminobenzidine (DAB) staining revealed that SG had high reactive oxygen species (ROS) accumulation (Figure 1D) after heat treatment, but ROS accumulation was almost unchanged in Cv, indicating that Cv had a very strong antioxidant system under high temperature. All these results illustrated that Cv is tolerant to high temperature while SG is sensitive to heat shock.
2.2. Identification of Heat-Tolerant Genes and Their Differential Expression in Clematis vitalba
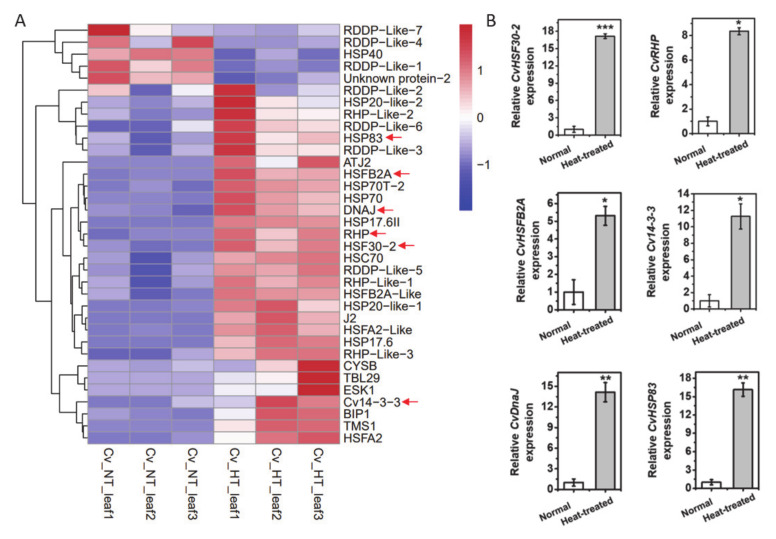
To reveal the molecular mechanism of heat tolerance in Cv, an RNA-seq analysis was performed for leaves from Cv and SG kept at 22 °C (NT) or treated at 42 °C (HT) for 2 h, for which each treatment included three biological replicates. Principal components analysis (PCA) was performed to show the similarity of replicates (Figure S1A,B). Compared with the control, totals of 6506 and 6470 differently expressing genes (DEGs) were detected in heat-shocked Cv and SG, respectively (p < 0.05) (Supplementary Figure S1C). In Cv, 656 transcription factors were identified in the RNA-seq and 58 of them were differently expressed in response to heat shock, including NAC domain-containing protein 100, MADS-box protein AGL80, R2R3-myb transcription factor, transcription repressor MYB6-like, ethylene-responsive transcription factor ERF014 and heat shock factor (Supplementary Figure S1D; Supplementary Table S1). Furthermore, 35 heat-related genes (HRGs) identified by Gene Ontology (GO) annotation were detected in the Cv heat response, including heat shock transcription factors (HSFs), heat shock proteins, 14-3-3 proteins, ribonuclease H protein (RHP) and dnaJ protein (Figure 2A; Supplementary Table S2 shows the description of HRGs), which function in heat signal transduction, transcription regulation and protein homeostasis. In addition, we examined the expression of some genes participating in heat response with a qPCR assay (Figure 2B). The mRNA levels of CvHSF30-2, CvHSFB2a, CvHSP83, ribonuclease H protein (RHP), 14-3-3 proteins and dnaJ protein were significantly upregulated in Cv (Figure 2B), which was consistent with the RNA-seq results. Notably, hardly any heat-related genes overlapped between SG and CV after heat shock (Supplementary Figure S1C), indicating that the heat response mechanism of Cv differs from that of SG and that Cv might have its own special heat tolerant mechanism. It has been reported that transcriptional factors play vital roles in plant development and environmental response, among which HSFs have been reported to participate in plant thermotolerance [6]. Thus, we gave priority to the function Hsf in Clematis thermotolerance, although other transcription factors may also play a part in this process. Pertinent to our interest, CvHSF30-2, which intersected in differently expressing transcriptional factors and HRGs, showed great induction of heat shock. Thus, we speculate that CvHSF30-2 may play an important part in the thermotolerance of Cv.
Figure 2.
RNA-seq analysis of Clematis vitalba after high-temperature treatment. (A) Heat map of differentially expressing heat-related genes (HRGs). The genes chosen for the qPCR assay are marked by arrows. The descriptions of the names of HRGs are shown in Supplementary Table S2. (B) qPCR analysis of some HRGs. * p < 0.05; ** p < 0.01. *** p < 0.001.
2.3. CvHSF30-2 Serves as a Typical Type-A Heat Shock Transcription Factor
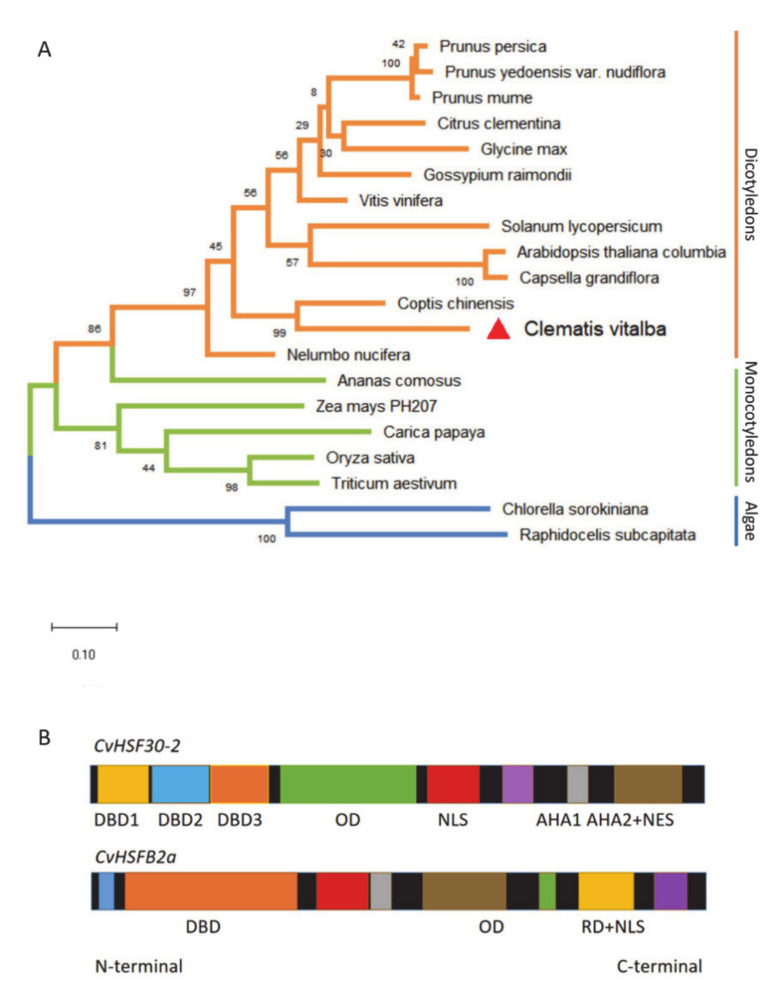
With the combined the RNA-seq and qPCR analysis, CvHSF30-2 was inferred as a hub gene that may play a critical role in the heat tolerance of Clematis vitalba. For further functional characterization of CvHSF30-2, we conducted phylogenetic analysis of HSFs from different organisms and found that HSFs can be divided into three groups: those from dicotyledons, monocotyledons and algae, respectively (Figure 2A). CvHSF30-2 was widely dissimilar to other HSF genes available in GenBank and had the highest homology with Vitis vinifera (Figure 3A). Motif prediction revealed that CvHSF30-2 had a DNA binding domain (DBD), oligomerization domain (OD), nuclear localization signal (NLS), two activator peptide motifs (AHA motifs) and a nuclear export signal (NES; also functions as a type-specific signature region in the C-terminus of class A HSFs) (Figure 3B), indicating that CvHSF30-2 is a typical type-A heat shock transcription factor.
Figure 3.
Phylogenetic and motifs analysis of heat shock transcription factors (HSFs). (A) Phylogenetic analysis of HSF30-2 in different species. The accession numbers of HSFs are shown top to bottom as follows: XP_008220492.1, Prupe.1G410400.7, PQM38437.1, NP_001290015.1, Gorai.013G220400.5, Ciclev10008618m, Glyma.14G096800.3, Solyc08g062960.2.1, AT2G26150.1, Cagra.7662s0001.1, XP_010262066.1, KAF9613066.1, Cv, Aco005862.1, Zm00008a004344_T01, evm.model.supercontig_44.122, LOC_Os07g08140.1, Traes_2DS_B6872CB84.1, PRW33971.1 and GBF94047.1. (B) Motifs of CvHSF30-2 and CvHSFB2a. Motif prediction and visualization were performed in HEATSTER (https://applbio.biologie.uni-frankfurt.de/hsf/heatster/home.php (accessed on 25 July 2020)).
2.4. CvHSF30-2 Enhances the Viability of Tobacco to Survive in Thermal Stress
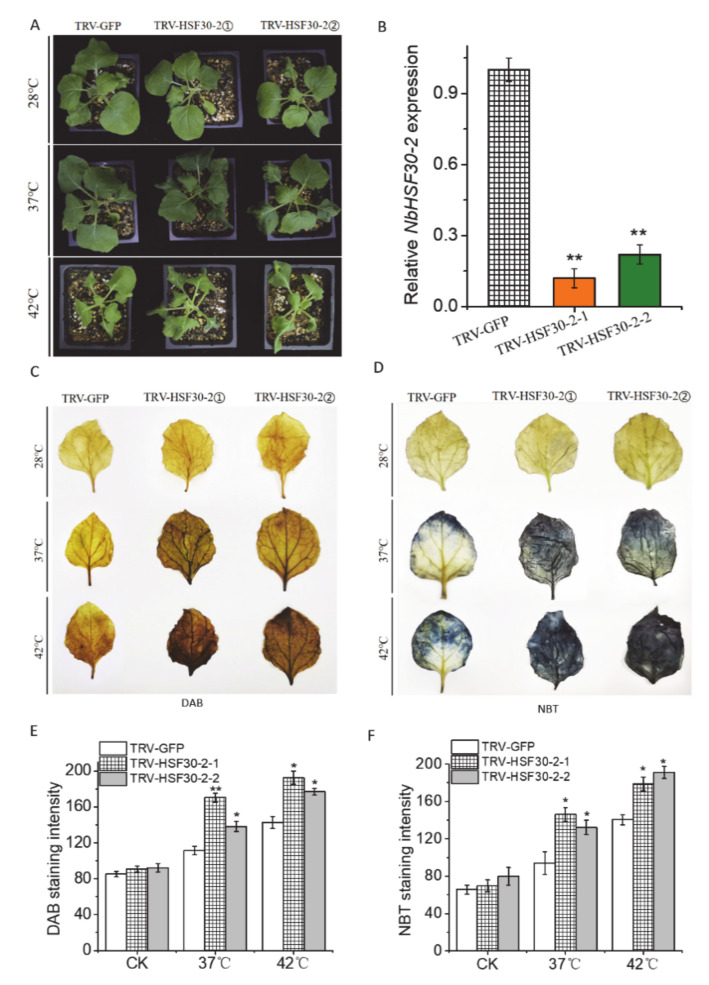
CvHSF30-2 was chosen to verify whether it can endow plant hosts with high-temperature resistance. We used TRV-based VIGS to silence the homologous genes of CvHSF30-2 in N. benthamiana (Niben101Scf01063g03005). HSF30-2 expression in newly emerged leaves of N. benthamiana significantly decreased after inoculation (Figure 4B). After 37 °C treatment, the TRV-NbHSF30-2 transgenic line showed a perfect heat sensitive phenotype compared with TRV-GFP (Figure 4A). Heat stress of 42 °C showed a more severe restraint on tobacco. However, TRV-NbHSF30-2 plants still exhibited a greater wilting phenotype (Figure 4A). Furthermore, more intense DAB and NBT staining was observed in the leaves of TRV-NbHSF30-2 plants than TRV-GFP after 37 °C and 42 °C treatment (Figure 4C,D); that is, the contents of superoxide dismutase and peroxidase in TRV-NbHSF30-2 were higher than those in TRV-GFP. The staining intensity was accurately quantified by the ImageJ2x program with inverse phase of the imagers; the higher the grey value, the deeper the DAB/NBT staining. After heat treatment, the grey value was significantly higher in TRV-NbHSF30-2 plants compared with the control (Figure 4E,F). The result indicated that the silencing of NbHSF30-2 improved heat sensitivity in N. benthamiana.
Figure 4.
Phenotype of HSF30-2 silencing in tobacco under heat stress. (A) Phenotype of HSF30-2-silencing in tobacco by virus-induced gene silencing (VIGS) before and after heat shock. TRV-HSF30-2 means the silencing of the CvHSF30-2 homologous gene in tobacco. TRV-HSF30-2① and TRV-HSF30-2② represent two different HSF30-2-silencing lines. (B) Silencing effect of TRV-HSF30-2 plants based on qPCR. (C,D) DAB and NBT staining of HSF30-2-silenced tobacco after heat treatment. (E,F) DAB and NBT staining quantified using the ImageJ2x program. The unit on the x-axis is the average grey value. The staining intensity was quantified using the ImageJ2x program. The image (inverse phase) was changed into an 8-bit grey image, and the average grey values from the upper, middle and lower areas of root tips calculated by ImageJ2x reflect the staining intensity. Error bars represent the standard error of three biological replicates. * p < 0.05; ** p < 0.01. TRV: tobacco rattle virus; CK: control check (normal temperature).
2.5. CvHSF30-2 Confers Tolerance to Heat Stress in Clematis vitalba Plants
In order to further study the function of CvHSF30-2 in heat response, VIGS technology was used to silence CvHSF30-2 in Cv. Since the leaf structure of Cv is not suitable for injection inoculation, virus-containing tobacco juice was used to infect Clematis. After friction inoculation, Cv plants were cultured for about 20 days (Figure 5A). The newly emerged leaves were treated with 42 °C heat shock for 2 h. The silencing effect of CvHSF30-2 in Cv leaves was detected by qPCR. CvHSF30-2 expression decreased in newly emerged leaves of Cv (by about two-thirds) after inoculation, which verified the success of virus infection and silencing application in Clematis (Figure 5B). After heat shock, it could be seen that the virus-silenced leaves of Cv showed obvious wilting and curling phenomena, but there was no significant difference in the control group (Figure 5C), indicating that the function of CvHSF30-2 positively regulated heat tolerance in Cv. Then, DAB and NBT staining showed that the leaves of TRV-HSF30-2 had obvious staining spots compared with the control group (Figure 5D,E), suggesting its higher ROS accumulation than that of TRV-GFP.
Figure 5.
Silencing of CvHSF30-2 on Clematis. (A) Operation flow chart of VIGS in Cv. (a–c) Four-week-old tobacco, N. benthamiana, was injected with GV3101 harboring TRV2-CvHSF30-2, and the leaves were taken three days later. (d–f) The leaves injected with TRV2-NBHSF30-2 virus were thoroughly ground. Then, juice was taken and placed on the clematis leaves with quartz sand added. The clematis leaves were inoculated by rubbing by hand. (g,h) The clematis inoculated by friction was marked and placed in a constant-temperature incubator for cultivation until new branches and leaves had grown. (B) Silencing effect of CvHSF30-2 in control (GFP) and virus-silenced (VIGS: HSF30-2) clematis leaves based on qPCR. (C) Heat-shock phenotype of CvHSF30-2-silenced Cv by VIGS. (D) Staining of Cv leaves after heat shock by DAB and NBT. DAB- and NBT-stained pictures of each group of clematis leaves; the parts of each picture marked with red squares and labelled 1, 2, 3 and 4 correspond to the enlarged pictures below. (E) DAB and NBT staining quantified using the ImageJ2x program. Error bars represent the standard error of three biological replicates. *** p < 0.001.
2.6. CvHSF30-2 Improves Heat Tolerance of Clematis vitalba by Elevating HSP Expression
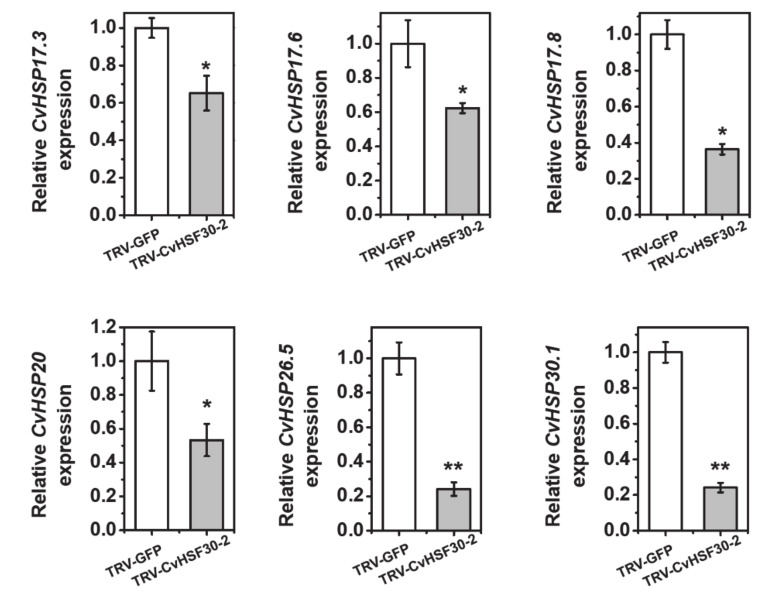
Under transcriptional control of heat shock transcription factors, HSPs respond rapidly to heat stress and stabilize, renature or help to degrade unfolded proteins [31]. Since heat shock transcription factor CvHSF30-2 was greatly induced after heat shock in Clematis vitalba, we speculated that the expressions of HSPs were activated by CvHSF30-2 and found that HSPs were highly expressing in Clematis vitalba when suffering heat shock (Table 1). Furthermore, it has been reported that HSFs function upstream of HSPs [32,33]. Thus we tested whether the expressions of HSPs were affected in TRV-CvHSF30-2 plants. The result showed that the silencing of CvHSF30-2 by VIGS led to a significant decrease in CvHSP17.3, CvHSP17.6, CvHSP17.8, CvHSP20, CvHSP26.5 and CvHSP30.1 expression (Figure 6), which suggested that these HSPs may be positively regulated by CvHSF30-2 in Clematis vitalba.
Table 1.
Differently expressing heat shock proteins (HSPs) before and after heat treatment (p < 0.05).
| Gene_id | Gene Name | Fold Change | P-Adjust | NR Description |
|---|---|---|---|---|
| TRINITY_DN2523_c0_g1 | CvHSP17.3 | 18.24959617 | 5.84 × 10−72 | Predicted: 17.3 kDa class I heat shock protein-like (Ipomoea nil) |
| TRINITY_DN55579_c0_g1 | CvHSP17.6 | 15.30769231 | 0.149868384 | Predicted: 17.6 kDa class I heat shock protein 1-like (Solanum tuberosum) |
| TRINITY_DN28316_c0_g1 | CvHSP30-1 | 8.297101449 | 0.010914936 | 30 kDa heat shock protein-like (Quercus suber) |
| TRINITY_DN19999_c0_g1 | CvHSP30-2 | 7.306338028 | 0.022396861 | 30 kDa heat shock protein-like (Quercus suber) |
| TRINITY_DN1775_c1_g2 | CvHSP17.8 | 5.753496246 | 7.18 × 10−36 | Predicted: 17.8 kDa class I heat shock protein-like (Daucus carota subsp. sativus) |
| TRINITY_DN316_c0_g2 | CvHSP70-1 | 4.37847865 | 2.61 × 10−16 | Heat shock protein 70 (Chimonanthus praecox) |
| TRINITY_DN60388_c0_g1 | CvHSP83 | 4.170731707 | 0.014590875 | Predicted: heat shock protein 83-like (Nelumbo nucifera) |
| TRINITY_DN3156_c0_g1 | CvHSP70-2 | 4.145122415 | 1.61 × 10−13 | HSP70 domain-containing protein (Cephalotus follicularis) |
| TRINITY_DN1775_c0_g2 | CvHSP20 | 3.12634968 | 0.002560726 | Alpha crystallin/Hsp20 domain (Macleaya cordata) |
| TRINITY_DN11240_c0_g1 | CvHSP26.5 | 3.125766871 | 0.051866641 | 26.5 kDa heat shock protein, mitochondrial (Sesamum indicum) |
Figure 6.
Expressions of CvHSP17.3, CvHSP17.6, CvHSP17.8, CvHSP20, CvHSP26.5 and CvHSP30.1 in CvHSF30-2-silenced plants by VIGS. Error bars represent the standard error of three biological replicates. Asterisks indicate a significant difference between TRV-GFP and TRV-CvHSF30-2 by t-test. * p < 0.05; ** p < 0.01.
2.7. CvHSFB2a May Be the Negative Regulator for CvHSF30-2
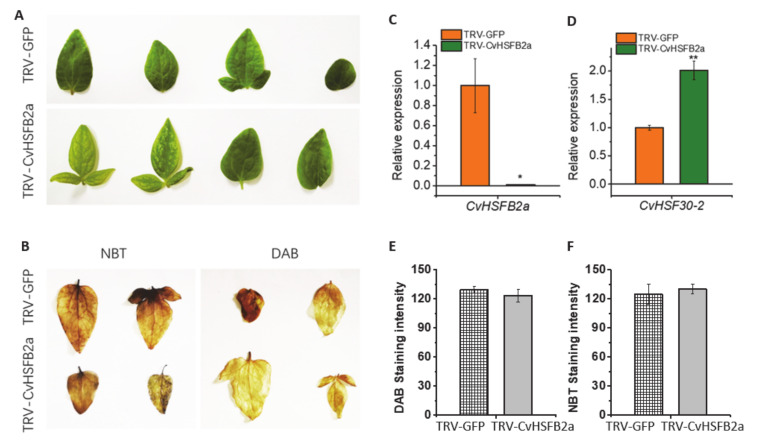
It has been reported that HsfB TFs function as negative regulators of some members of the HsfA family [21]. To compare differences between class A and B HSFs and research the function of class B HSFs, CvHSFB2a was also selected as a representative of class B for further study. Motif prediction revealed that CvHSF30-2 and CvHSFB2a were similar in N-termini while different in C-termini, which indicated a functional difference of the two HSFs. As classical class A and B HSFs, CvHSF30-2 had two activator peptide motifs (AHA motifs) and a nuclear export signal all located in the C-terminus, while CvHSFB2a had a repressor domain (RD) (Figure 3B). In order to investigate the role of CvHSFB2a in regulating CvHSF30-2, we also established a VIGS method for clematis CvHSFB2a. Following friction infection of clematis leaves, heat treatment was conducted after the new leaves grew. Both phenotype and staining proved that the heat resistance of the plants did not change significantly (Figure 7A,B,E,F). In the CvHSFB2a-silenced Clematis plant, the expression of the CvHSFB2a gene was obviously reduced (Figure 7C), while the expression of CvHSF30-2 was significantly increased (Figure 7D), which further demonstrated that CvHSFB2a inhibited the expression of CvHSF30-2.
Figure 7.
Silencing of CvHSFB2a in Clematis. (A) Phenotype of CvHSFB2a-silenced Clematis under heat treatment. (B) DAB and NBT staining of TRV-GFP and TRV-CvHSFB2a Clematis before and after heat treatment. (C) Silencing effect of CvHSFB2a. (D) The expression of CvHSF30-2 in TRV-CvHSFB2a plants. (E,F) DAB and NBT staining as quantified using the ImageJ2x program. Error bars represent the standard error of three biological replicates. * p < 0.05; ** p < 0.01.
3. Discussion
How to resist high temperatures is an important problem for the plants. Compared to traditional model plants such as Arabidopsis and rice, research seldom focuses on the heat-tolerant mechanisms of horticultural plants, especially of those without reference genomes. In our study, one heat-tolerant Clematis species, Clematis vitalba, was identified based on physiological indexes (Figure 1). Transcriptome analysis revealed differentially expressed HTGs, involving heat signal transduction, transcription regulation and protein homeostasis, which have been reported as participating in heat tolerance [31,34]. Among these HTGs, CvHSF30-2 was highly induced after heat treatment in Clematis vitalba, compared with Stolwijk Gold (Figure 2). Further functional verification showed that the overexpression of CvHSF30-2 conferred resistance to heat stress in E. coli and yeast, while the silencing of HSF30-2 by VIGS led to an enhanced thermosensitivity in tobacco and Clematis (Figure 4 and Figure 5). These results confirmed that highly expressing HSFs may be the main reason for thermotolerance in Clematis vitalba.
Following heat shock, the response is strictly modulated by heat shock factors and heat shock proteins to inhibit stress injury [35]. Heat shock transcription factors are the vital regulators of the plant heat response. Several HSFs in Arabidopsis, such as HSFA2; HSF A1a, A1b, A1d and A1e; HSFA3; HSFA6b or HSFB1; and B2b, can obviously affect plant heat tolerance [21,36]. Heat shock proteins also play an important role in response to thermal stress. Heat treatment greatly increased HSP90 expression, which directly interacted with transport inhibitor response 1 (TIR1) to prevent its degradation and modulate auxin-mediated plant growth [37,38]. It has been reported that HSFs function upstream of HSPs in heat response [32,33]. Heat signals can activate the transcription activity of the HSFA1s, which further regulates the expression of heat-responsive genes such as HSP70 to enhance plant heat resistance [39]. HSFA2 is required to maintain high levels of specific histone modifications at the 50 regions of APX2, HSP22 and HSP18.2 [40]. In this study, HSPs were also highly expressing in Clematis vitalba when suffering heat shock (Table 1). Additionally, the silencing of CvHSF30-2 by VIGS led to a significant decrease of CvHSP17.3, CvHSP17.6, CvHSP17.8, CvHSP20, CvHSP26.5 and CvHSP30.1 expression (Figure 6), which suggested that these HSPs may be positively regulated by CvHSF30-2 in Clematis vitalba. Yeast one-hybrid and electrophoretic mobility shift assay (EMSA) analysis should be performed to gain a further understanding of the regulatory relationship between CvHSF30-2 and candidate CvHSPs.
The model plant Arabidopsis contains 21 HSF homologs, which are sorted into three classes (classes A, B and C; [41]). The heat-inducible B-type HSFs, lacking the transactivation domain, may play a different role from HsfA [41]. AtHsfB1 and AtHsfB2b can directly bind to the promoter of HsfA2, as indicated by yeast one-hybrid assay. When transiently coexpressed with AtHsfA4a, AtHsfB1 could restrain the transcription of AtHsfA2 and heat-induced marker genes [21]. Thus, it seemed that HsfB functions as a negative transcription coactivator of some HsfA members. In our study, when CvHSFB2a was silenced by VIGS, the expression of CvHSF30-2 gene was significantly increased (Figure 7C,D), indicating the inhibitor effect of CvHSFB2a in regulating CvHSF30-2. Interestingly, no significant heat-sensitive phenotype was observed for the CvHSFB2a knockdown line, which was consistent with a previous study of AtHsfB1 [21]. The T-DNA knockout the HsfB1 line did not show a heat-resistant defective phenotype, and the expressions of some HSPs were not substantially influenced during heat stress, which is probably due to the functional redundancy of AtHsfB2a [42]. Class B HSFs also function as positive regulators of heat response. The tomato heat stress transcription factor HsfB1 cooperated with HsfA1a by recruiting histone acetyl transferase HAC1 and activated HS-inducible genes [13,43]. Additionally, tomato HsfB1 could maintain or restore the expression of housekeeping genes in heat-stressed cells by cooperation with other transcriptional activators [43,44]. In Arabidopsis, HsfB1 and HsfB2b significantly promote acquired thermotolerance, probably by repressing HSPs that interfere with the nuclear migration of HSFA1s [21]. Thus, it seems that class B HSFs play a complex and important role in modulating the plant heat response. Future work on the interaction of between CvHSFB2a and class A HSFs might help us better understand the function of HSFB transcription factors in response to heat stimuli.
VIGS is a powerful tool for the study of gene function in plants and has been successfully used in many plants, e.g., N. benthamiana [45], wheat [46], tomato [47], Phalaenopsis [29] and rose [30]. In this study, we established a method for Clematis VIGS by tobacco rattle virus-based vectors. Briefly, TRV was first injected into leaves of N. benthamiana for proliferation, and then ground leaf juice was used to infect clematis leaves by friction with quartz sand. The newly growing leaves were used for silencing effect identification and heat tolerance access (Figure 4 and Figure 5). This procedure was similar to the reported tomato VIGS method [47,48]. VIGS integrating intermediate host and friction infection may be a strategy for gene function verification in plants that are difficult to infect by injection. More applications on other plants should be further conducted to confirm its practicability.
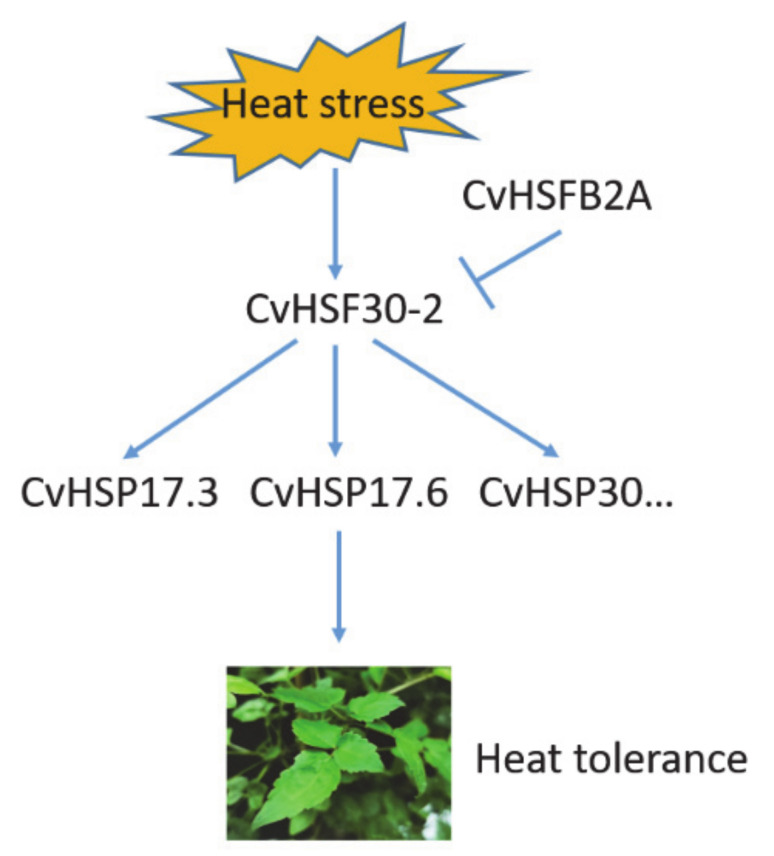
We developed a hypothetical working model for Clematis vitalba thermotolerance based on the results of the current study (Figure 8). When Clematis plants are exposed to heat stress, heat shock transcription factor CvHSF30-2 expression is greatly induced. CvHSF30-2 subsequently elevates the expressions of its candidate downstream HSP targets, e.g., CvHSP17.3, CvHSP17.6 and CvHSP30, and then leads to the tolerance of Clematis plants in response to heat stress. CvHSFB2a may be the negative regulator of CvHSF30-2 in this process.
Figure 8.
Working model for Clematis vitalba thermotolerance.
4. Materials and Methods
4.1. Plant Material Growth Conditions and High-Temperature Treatment Conditions
Triennial potted plants of two Clematis varieties (Clematis vitalba (S-0938 (FR-07)) and Stolwijk Gold) were used in this study. The seeds were derived from Jardin Botanique in France, sown into autoclaved nutrient soil and grown for three years in 12-inch pots in a greenhouse of Shanghai Botanical Garden. For each species, six plants in great and similar growth conditions were taken and placed in two constant-temperature incubators (one at 42 °C, the other at 22 °C) for 2 h, respectively (three plants per constant-temperature incubator). Treated potted plants were used for the subsequent analysis and RNA-seq of the physiological indexes.
4.2. Measurement of Physiological Indexes before and after High-Temperature Treatment
Relative conductivity: 0.2 g of leaves was placed in a 20 mL tube, vacuumized and left to stand for 30 min at normal temperature (the tube was shaken gently every 5 min during the period). The conductivity was measured with a DDS-11A-type conductivity meter. After a boiling water bath for 10 min and cooling, the conductivity value was again measured and the relative conductivity was calculated. The relative conductivity per gram of fresh weight was used to represent relative conductivity. Measuring relative water content: 0.2 g of chopped leaves was weighed and placed in a weighing bottle, then put in an oven for 30 min at 105 °C for dehumidification. The oven was then set to 80 °C for drying to constant weight, the sample was taken out and cooled to normal temperature, and the dry weight was weighed and the relative water content calculated.
4.3. Nitro Blue Tetrazole and Diaminobenzidine Staining before and after High-Temperature Treatment
In DAB staining, leaves of two Clematis varieties subjected to normal or high-temperature treatments were soaked in DAB staining solution at 25 °C for 24 h in the dark, and then soaked in 95% ethanol to remove chlorophyll. In NBT staining, the leaves of two Clematis varieties subjected to normal or high-temperature treatments were immersed in the NBT staining solution at 25 °C for 12 h in the dark, and then immersed in 95% ethanol to remove chlorophyll.
4.4. RNA Extraction and Quantitative PCR Assay
The young leaves (three- to four-weeks-old, heat-treated or untreated controls) of Clematis plants were harvested for the detection of heat-related gene expressions. The newly growing leaves of control and VIGS tobacco (two-weeks-old) or Clematis plants (three- to four-weeks-old) were harvested for silencing effect identification. Total RNA was extracted using a SteadyPure Plant RNA Extraction Kit (Code: AG21019; Accurate Biotechnology Co., Ltd., Hunan, China). cDNA synthesis was performed with the RT reagent kit (Takara) according to the manufacturer’s protocol. Real-time PCRs were done on a Chromo 4™ continuous fluorescence detector with the SYBR RT-PCR Kit (Takara) in a 20 μL reaction volume, which contained 10 μL of SYBR Green I PCR mix, 0.5 μM each of forward and reverse primer, 1 μL of cDNA template and appropriate amounts of sterile ddH2O. Amplification conditions were: 2 min at 95 °C; 40 cycles of 15 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C. Fold changes of RNA transcripts were calculated by the 2−ΔΔCt method [49]. We used CvUBC2D (TRINITY_DN89495_c0_g1) in Clematis plants and NbEF1α in tobacco as the internal controls. The entire experiments were repeated three times. Primers used in the qPCR are listed in Supplementary Table S7.
4.5. RNA-Seq Data Processing, Reassembly and Annotation
RNA purification, reverse transcription, library construction and sequencing were performed at Shanghai Majorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China) according to the manufacturer’s instructions (Illumina, San Diego, CA, USA). The RNA-seq transcriptome libraries were prepared using an Illumina TruSeqTM RNA Sample Preparation Kit (San Diego, CA, USA). RNAseq libraries were sequenced in single lane on an Illumina Hiseq xten/NovaSeq 6000 sequencer (Illumina, San Diego, CA, USA) for 2 × 150 bp paired-end reads. The RNA-seq raw read data was processed using the software fastx_toolkit_0.0.14 (http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 5 May 2020)), SeqPrep (https://github.com/jstjohn/SeqPrep (accessed on 5 May 2020)) and Sickle (https://github.com/najoshi/sickle (accessed on 5 May 2020)) to evaluate and discard sequences of low quality and those affected by adaptor contamination, with quality control shown in Supplementary Tables S3 and S4. After obtaining high-quality RNA-seq sequencing data, Trinity (Version v2.8.5, https://github.com/trinityrnaseq/trinityrnaseq (accessed on 5 May 2020)) was used to assemble all clean data from scratch due to the lack of a reference genome for Clematis. Then, the assembly results were optimized with TransRate (http://hibberdlab.com/transrate/ (accessed on 5 May 2020)) and CD-HIT (http://weizhongli-lab.org/cd-hit/ (accessed on 5 May 2020)). BUSCO (Benchmarking Universal Single-Copy Orthologs, http://busco.ezlab.org (accessed on 5 May 2020)) was used for a re-evaluation of the optimized sequences. The assembly quality is shown in Supplementary Table S5. The assembled high quality transcriptome sequences were annotated by six major databases (NR, Swiss-Prot, Pfam, COG, GO and KEGG databases). The versions and sources of the software and databases we used are shown in Supplementary Table S6.
4.6. Functional Verification of VIGS with Clematis or Tobacco as Host
For the tobacco VIGS assay [24], part of the homologous nucleotide sequence of CvHSF30-2 in N. benthamiana was amplified using the primers in Supplementary Table S7 and determined by Sangon Biotech Co., Ltd. (Shanghai, Chana). Then, the fragments were separately linked to the pTRV2 virus vector. The constructed pTRV2 vectors mentioned above were introduced into the A. tumefaciens strain GV3101. Agrobacterium harboring TRV1- or TRV2-derived vectors was mixed in a 1:1 ratio and infiltrated into the leaves of two-week-old N. benthamiana plants. The newly growing leaves were used for heat tolerance access and silencing effect identification two weeks after injection.
For the Clematis VIGS assay, 246 bp in the 5′-region of CvHSF30-2 mRNA were amplified using the primers in Supplementary Table S7 and determined by Sangon Biotech Co., Ltd. (Shanghai, China). Then, the fragments were separately linked to the pTRV2 virus vector. A. tumefaciens strain GV3101 harboring TRV1- or TRV2-derived vectors was mixed in a 1:1 ratio and infiltrated into the leaves of four-week-old N. benthamiana plants as the intermediate hosts. Three days after inoculation, the inoculated tobacco leaves were ground into juice and inoculated into Clematis by friction with quartz sand. After inoculation, the plants were cultivated at 20–25 °C for three to four weeks. The silencing effect of the target gene was detected on every branch using newly growing apical leaves. The appropriate newly growing leaves on the same branch were used for heat tolerance assessment.
Acknowledgments
We thank Jardin Botanique in France for providing the Clematis seeds.
Supplementary Materials
The Supplementary Materials for this article can be found at https://www.mdpi.com/1422-0067/22/6/2900/s1. Figure S1: PCA and Venn analysis of Cv and SG, Table S1: A total of 58 differentially expressing transcription factors were found in Cv before and after heat treatment, Table S2: Description of differently expressing heat-related genes shown in the heat map, Table S3: Quality control of Cv transcriptome analysis, Table S4: Quality control of SG transcriptome analysis, Table S5: The parameters of the transcriptome assembly with Trinity, Table S6: The versions and sources of the software/databases used in transcriptome analysis, Table S7: Primers used in this study.
Author Contributions
F.M. and S.F. conceived the project and designed the study; R.W. and C.M. performed the experiments; C.J. provided the RNA-seq data; L.Z., S.P. and Y.Z. provided technical assistance to R.W. and C.M.; R.W. and C.M. wrote the article; F.M. and C.M. revised the article. All of the authors discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Commission of Shanghai Municipality (Grant No. 18DZ2283500), the Shanghai Engineering Research Center of Plant Germplasm Resources (17DZ2252700), the Science and Technology Commission of Shanghai Municipality (18DZ2260500) and the Shanghai Key Laboratory of Plant Molecular Sciences National Key R&D Program of China (2018YFD1000400).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
RNA-seq reads are available at https://submit.ncbi.nlm.nih.gov/subs/sra/ (accessed on 17 January 2021) with accession number PRJNA692678.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beutler L. The Rogerson Clematis Collection: Where Every Garden Tells a Story. Pacific Horticulture Society; Berkeley, CA, USA: 2014. [Google Scholar]
- 2.Chohra D., Ferchichi L., Selim Y., Gokhan C., Sarah Z., Alsheikh M. Phenolic profiles, antioxidant activities and enzyme inhibitory effects of an Algerian medicinal plant (Clematis cirrhosa L.) S. Afr. J. Bot. 2020;132:164–170. doi: 10.1016/j.sajb.2020.04.026. [DOI] [Google Scholar]
- 3.Hassan M.U., Chattha M.U., Khan I., Chattha M.B., Aslam M.T. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies - A review. Plant Biosyst. 2020;7:1–56. doi: 10.1080/11263504.2020.1727987. [DOI] [Google Scholar]
- 4.Janni M., Gullì M., Maestri E., Marmiroli M., Valliyodan B., Nguyen H.T., Marmiroli N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020;71:3780–3802. doi: 10.1093/jxb/eraa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simms E.L., Triplett S.J. Cost and benefits of plant response to disease: Resistance and tolerance. Evolution. 1994;48:1973–1985. doi: 10.1111/j.1558-5646.1994.tb02227.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohama N., Sato H., Shinozaki K., Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22:53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Waters E.R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 2013;64:391–403. doi: 10.1093/jxb/ers355. [DOI] [PubMed] [Google Scholar]
- 8.Sharma D., Mamrutha H.M., Gupta V.K., Tiwari R., Singh R. Association of SSCP variants of HSP genes with physiological and yield traits under heat stress in wheat. Res. Crops. 2015;16:139–146. doi: 10.5958/2348-7542.2015.00020.0. [DOI] [Google Scholar]
- 9.Yamada K., Fukao Y., Hayashi M., Fukazawa M., Nishimura M. Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J. Biol. Chem. 2007;282:37794–37804. doi: 10.1074/jbc.M707168200. [DOI] [PubMed] [Google Scholar]
- 10.Zhong L., Zhou W., Wang H., Ding S., Lu Q., Wen X., Peng L., Zhang L., Lu C. Chloroplast Small Heat Shock Protein HSP21 interacts with plastid nucleoid protein pTAC5 and is essential for chloroplast development in Arabidopsis under heat stress. Plant Cell. 2013;25:2925–2943. doi: 10.1105/tpc.113.111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comastri A., Janni M., Simmonds J., Uauy C., Pignone D., Nguyen H.T., Marmiroli N. Heat in wheat: Exploit reverse genetic techniques to discover new alleles within the Triticum durum sHsp26 family. Front. Plant Sci. 2018;19:1337. doi: 10.3389/fpls.2018.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob P., Hirt H., Bendahmane A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017;15:405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fragkostefanakis S., Röth S., Schleiff E., Klaus-Dieter S. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2015;38:1881–1895. doi: 10.1111/pce.12396. [DOI] [PubMed] [Google Scholar]
- 14.Lohmann C., Eggers-Schumacher G., Wunderlich M., Schoffl F. Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol. Genet. Genom. 2004;271:11–21. doi: 10.1007/s00438-003-0954-8. [DOI] [PubMed] [Google Scholar]
- 15.Mishra S.K., Tripp J., Winkelhaus S., Tschiersch B., Theres K., Nover L., Scharf K.D. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002;16:1555–1567. doi: 10.1101/gad.228802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H.C., Charng Y.Y. Common and distinct functions of Arabidopsis Class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 2013;163:276–290. doi: 10.1104/pp.113.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokotani N., Ichikawa T., Kondou Y., Matsui M., Hirochika H., Iwabuchi M., Oda K. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta. 2008;227:957–967. doi: 10.1007/s00425-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 18.Harsh C., Neetika K., Preeti A., Khurana J.P., Paramjit K., Peter C. A seed preferential heat shock transcription factor from wheat provides abiotic stress tolerance and yield enhancement in transgenic Arabidopsis under heat stress environment. PLoS ONE. 2013;8:e79577. doi: 10.1371/journal.pone.0079577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Koskull-Döring P., Scharf K.D., Nover L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007;12:452–457. doi: 10.1016/j.tplants.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Yamanouchi U., Yano M., Lin H., Ashikari M., Yamada K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA. 2002;99:7530–7535. doi: 10.1073/pnas.112209199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda M., Mitsuda N., Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011;157:1243–1254. doi: 10.1104/pp.111.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baulcombe D.C. Fast forward genetics based on virus-induced gene silencing. Curr. Opin. Plant Biol. 1999;2:109–113. doi: 10.1016/S1369-5266(99)80022-3. [DOI] [PubMed] [Google Scholar]
- 23.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Schiff M., Marathe R., Dinesh-Kumar S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002;30:415–429. doi: 10.1046/j.1365-313X.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai M.H., Donson J., Della-Cioppa G., Harvey D., Grill K.H.K. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc. Natl. Acad. USA. 1995;92:1679–1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peele C., Jordan C.V., Muangsan N., Turnage M., Robertson D. Silencing of a meristematic gene using geminivirus-derived vectors. Plant J. 2010;27:357–366. doi: 10.1046/j.1365-313x.2001.01080.x. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz M.T., Voinnet O., Baulcombe D.C. Initiation and maintenance of virus induced gene silencing. Plant Cell. 1998;10:937–946. doi: 10.1105/tpc.10.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson D. VIGS vectors for gene silencing: Many targets, many tools. Annu. Rev. Plant. Biol. 2003;55:495–519. doi: 10.1146/annurev.arplant.55.031903.141803. [DOI] [PubMed] [Google Scholar]
- 29.Liang C.Y., Rengasamy K.P., Huang L.M., Hsu C.C., Chen H.H. Assessment of violet-blue color formation in Phalaenopsis orchids. BMC Plant Biol. 2020;20:212. doi: 10.1186/s12870-020-02402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Y., Tang A., Yu J., Cheng T., Zhang Q. RcAP1, a Homolog of APETALA1, is associated with flower bud differentiation and floral organ morphogenesis in Rosa chinensis. Int. J. Mol. Sci. 2019;20:3557. doi: 10.3390/ijms20143557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Li X., Lin H., Chong K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019;70:753–780. doi: 10.1146/annurev-arplant-050718-100016. [DOI] [PubMed] [Google Scholar]
- 32.Liu H.C., Liao H.T., Charng Y.Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34:738–751. doi: 10.1111/j.1365-3040.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T., Ohama N., Nakajima J., Kidokoro S., Mizoi J., Nakashima K., Maruyama K., Kim J.M., Seki M., Todaka D., et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet. Genom. 2011;286:321–332. doi: 10.1007/s00438-011-0647-7. [DOI] [PubMed] [Google Scholar]
- 34.Li X.R., Deb J., Kumar S.V., Østergaard L. Temperature modulates tissue-specification program to control fruit dehiscence in Brassicaceae. Mol. Plant. 2018;11:598–606. doi: 10.1016/j.molp.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu S.F., Jinn L.T.L. Cytosol-localized heat shock factor-binding protein, AtHSBP, functions as a negative regulator of heat shock response by translocation to the nucleus and is required for seed development in Arabidopsis. Plant Physiol. 2010;153:773–784. doi: 10.1104/pp.109.151225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schramm F., Larkindale J., Kiehlmann E., Ganguli A., Englich E., Vierling E., Von Koskull-Döring P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008;53:264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang R., Zhang Y., Kieffer M., Yu H., Kepinski S., Estelle M. Corrigendum: HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat. Commun. 2016;7:11677. doi: 10.1038/ncomms11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B., Wu S., Zhang Y.E., Xu T., Xue Y. A high temperature-dependent mitochondrial lipase EXTRA GLUME1 promotes floral phenotypic robustness against temperature fluctuation in rice (Oryza sativa L.) PLoS Genet. 2016;12:e1006152. doi: 10.1371/journal.pgen.1006152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickinson P., Kumar M., Martinho C., Yoo S., Lan H., Artavanis G., Charoensawan V., Schöttler M.A., Bock R., Jaeger K.E., et al. Chloroplast signaling gates thermotolerance in Arabidopsis. Cell Rep. 2018;22:1657–1665. doi: 10.1016/j.celrep.2018.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lämke J., Brzezinka K., Altmann S., Bäurle I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2016;35:162–175. doi: 10.15252/embj.201592593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharf K.D., Berberich T., Ebersberger I., Nover L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta. 2012;1819:104–119. doi: 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Charng Y.Y., Liu H.C., Liu N.Y., Chi W.T., Wang C.N., Wang C.T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007;143:251–262. doi: 10.1104/pp.106.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bharti K., Koskull-Döring P.V., Bharti S., Khumar P., Tintschl-Körbitzer A., Treuter E., Nover L. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell. 2004;16:1521–1535. doi: 10.1105/tpc.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn A., Bublak D., Schleiff E., Scharf K.D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 2011;23:741–755. doi: 10.1105/tpc.110.076018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velásquez A.C., Chakravarthy S., Martin G.B. Virus-induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. J. Vis. Exp. 2009;28:1292. doi: 10.3791/1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perochon A., Jia J., Kahla A., Arunachalam C., Scofield S., Bowden S., Wallington E., Doohan F.M. TaFROG encodes a pooideae orphan protein that interacts with SnRK1 and enhances resistance to the mycotoxigenic fungus Fusarium graminearum. Plant Physiol. 2015;169:2895–2906. doi: 10.1104/pp.15.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fantini E., Giuliano G. Virus-induced gene silencing as a tool to study tomato fruit biochemistry. Methods Mol. Biol. 2016;1363:65–78. doi: 10.1007/978-1-4939-3115-6_7. [DOI] [PubMed] [Google Scholar]
- 48.Fu D.Q., Zhu B.Z., Zhu H.L., Jiang W.B., Luo Y.B. Virus-induced gene silencing in tomato fruit. Plant J. 2005;43:299–308. doi: 10.1111/j.1365-313X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- 49.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq reads are available at https://submit.ncbi.nlm.nih.gov/subs/sra/ (accessed on 17 January 2021) with accession number PRJNA692678.