Abstract
Aflatoxins (AFs) are some of the most agriculturally important and harmful mycotoxins. At least 20 AFs have been identified to this date. Aflatoxin B1 (AFB1), the most potent fungal toxin, can cause toxicity in many species, including humans. AFs are produced by 22 species of Aspergillus section Flavi, 4 species of A. section Nidulantes, and 2 species of A. section Ochraceorosei. The most important and well-known AF-producing species of section Flavi are Aspergillus flavus, A. parasiticus, and A. nomius. AFs contaminate a wide range of crops (mainly groundnuts, pistachio nuts, dried figs, hazelnuts, spices, almonds, rice, melon seeds, Brazil nuts, and maize). Foods of animal origin (milk and animal tissues) are less likely contributors to human AF exposure. Despite the efforts to mitigate the AF concentrations in foods, and thus enhance food safety, AFs continue to be present, even at high levels. AFs thus remain a current and continuously pressing problem in the world.
Keywords: aflatoxigenic microfungi, aflatoxins, food
1. Introduction
Aflatoxins (AFs) are some of the most important and harmful mycotoxins. As of 2020, 60 years have already passed since their discovery. AFs are one of the five agriculturally most important mycotoxins [1,2,3,4]. Chemically, the AFs are difuranocoumarin derivatives with a bifuran group attached to the coumarin nucleus and a pentanone ring (in the case of aflatoxin AFBs) or a lactone ring (in case of aflatoxin AFGs) [5]. There are more than 20 known AFs, but the most common are aflatoxin B1 (AFB1) (PubChem CID: 186907), aflatoxin B2 (AFB2) (PubChem CID: 2724360), aflatoxin G1 (AFG1) (PubChem CID: 14421), and aflatoxin G2 (AFG2) (PubChem CID: 2724362) (PubChem, 2020), from which AFB1 is the major representative in food crops [6]. Aflatoxin M1 (AFM1) (PubChem CID: 15558498) and M2 (AFM2) (PubChem CID: 10903619) are the hydroxylated metabolites of AFB1 and AFB2 [7,8,9].
AFs are acutely toxic, hepatotoxic, immunosuppressive, mutagenic, teratogenic, and carcinogenic compounds [10,11,12,13,14]. The International Agency for Research on Cancer (IARC) evaluated the carcinogenicity of naturally occurring AFs (AFB1, AFB2, AFG1, and AFG2) for humans as Group 1 ”carcinogenic to humans” in 1987 [10,15], and re-evaluated in 2012 [16,17]. AFM1 is often misclassified in the literature as Group 1; however, it was classified as Group 2B “possibly carcinogenic to humans” in 1993 [1] and has not been re-evaluated since. For these reasons, AFs need to be monitored and their concentrations in food should be kept at the lowest possible levels.
While acute exposure to a high dose can result in vomiting, abdominal pain, and even death, chronic exposure to low doses may lead to liver cancer [18,19], which is generally considered to be the most significant impact of AFs on human health [10,20]. According to the latest data from the Global Cancer Observatory, liver cancer is the sixth most common cancer for both sexes of all ages, with a total of 905,677 new cases estimated in 2020 [21]. It has been estimated that AFs contribute to 4.6% to 28.2% of all global hepatocellular carcinomas [22].
Nowadays, AFs are of great interest as they are one of the most serious contaminants that can significantly affect the food chain. Humans, at the top of the food chain, often consume contaminated foodstuffs of both plant and animal origins. Besides human health, food insecurity caused by AFs contamination can also affect humanity at the social, political, and economic levels [23].
Therefore, in this article, attention is paid to AFs in terms of AF producers and the occurrence of AFs in foods around the world.
2. Producers of Aflatoxins
To date, AFs are produced by 28 species of the genus Aspergillus. Aspergillus subgenus Circumdati section Flavi contains some of the most important species in the genus, which usually produce AFs [24,25,26].
The accurate identification of Aspergillus section Flavi requires a polyphasic approach that includes the morphological characters (the microscopic structures, such as the uni- or biseriate conidial heads, the production of dark-colored sclerotia by certain species, and yellow-green to brown shades of conidia), and the chemical (extrolite data) and molecular (partial sequences of calmodulin, β-tubulin, and internal transcribed spacer region) approaches, as these species are closely related and could not be easily distinguished by morphological characteristics alone [24,25,26].
Aspergillus section Flavi currently contains a total of 34 species in 8 clades: the Aspergillus alliaceus-, A. avenaceus-, A. bertholletius-, A. coremiiformis-, A. flavus-, A. leporis-, A. nomius-, and A. tamarii-clade [24,25,26,27]. The three new clades A. texensis-, A. agricola-, and A. toxicus-clade with three species were presented in the year 2020 [28,29].
Table 1 gives an overview of the current identity of Aspergillus species from Aspergillus section Flavi as AF producers focus on foodstuffs [24,25,26,27,28,29,30].
Table 1.
Aflatoxigenic Aspergillus species from Aspergillus section Flavi.
| Species | AF Producer | Year of Identification | Occurrence |
|---|---|---|---|
| A. flavus | B1, B2 | 1962 | Peanuts, maize, spices |
| A. parasiticus | B1, B2 G1, G2 | 1963 | Maize, peanuts |
| A. nomius | B1, B2 G1, G2 | 1987 | Wheat, turmeric |
| A. pseudonomius | B1, B2 G1, G2 | 1997 | Brazil nut |
| A. pseudotamarii | B1, B2 | 2001 | Brazil nut |
| A. parvisclerotigenes | B1, B2 G1, G2 | 2005 | Peanuts |
| A. arachidicola | B1, B2 G1, G2 | 2008 | Carob flour |
| A. luteovirescens a | B1, B2 G1, G2 | 2008 | Brazil nut |
| A. minisclerotigenes | B1, B2 G1, G2 | 2008 | Peanuts, curry, red chili |
| A. pseudocaelatus | B1, B2 G1, G2 | 2011 | Peanuts, Brazil nut |
| A. togoensis | B1, B2 | 2011 | Fruit of Landolphia spp. |
| A. mottae | B1, B2 G1, G2 | 2012 | Maize |
| A. novoparasiticus | B1, B2 G1, G2 | 2012 | No occurrence in food b |
| A. sergii | B1, B2 G1, G2 | 2012 | Almond |
| A. transmontanensis | B1, B2 G1, G2 | 2012 | Almond |
| A. texensis | B1, B2 G1, G2 | 2018 | Maize |
| A. aflatoxiformans | B1, B2 G1, G2 | 2019 | Peanuts, sesame |
| A. austwickii | B1, B2 G1, G2 | 2019 | Rice, sesame |
| A. cerealis | B1, B2 G1, G2 | 2019 | Rice, maize, peanut |
| A. pipericola | B1, B2 G1, G2 | 2019 | Black pepper |
| A. agricola sp. nov. | B1, B2 | 2020 | Maize |
| A. toxicus sp. nov. | B1, B2 | 2020 | Maize |
a Formerly named Aspergillus bombycis; b Sputum of leukemic patient.
The most important and most well-known AF-producing species of section Flavi in foodstuffs are Aspergillus flavus [31,32], A. parasiticus [33,34,35], and A. nomius [36,37]. While Aspergillus flavus produces AFB1 and AFB2, A. parasiticus and A. nomius can produce AFB1, AFB2, AFG1, and AFG2.
Aspergillus minisclerotigenes and A. parvisclerotigenes also belong to section Flavi. Both have morphological and physiological similarities to A. flavus; however, they produce more but smaller sclerotia. In contrast to A. flavus, this is usually coupled with a high and consistent production of both the B and G type of AFs [24].
In addition to Aspergillus flavus, four other A. species (A. agricola, A. pseudotamarii, A. togoensis, and A. toxicus) only produce AFB1 and AFB2. Seventeen other Aspergillus species can produce AFB1, AFB2, AFG1, and AFG2. It is generally accepted that A. flavus is unable to produce AFs type G, but it is also reported that some Korean strains are capable of producing both AFG1 and AFG2 [25]. However, some Aspergillus species from Aspergillus section Nidulantes [38] or Aspergillus section Ochraceorosei [32,39] can also produce AFs.
The identification of Aspergillus section Nidulantes requires a polyphasic approach which includes the morphological characters (the microscopic structures such as the color, shape, size, and ornamentation of ascospores, the shape and size of conidia and vesicles, and growth temperatures), and the chemical (extrolite data) and molecular (internal transcribed spacer region, partial β-tubulin, calmodulin, and RNA polymerase II the second largest subunit (RPB2) gene sequences) approaches [38]. Based on this polyphasic approach, Aspergillus section Nidulantes was subdivided into 7 clades and 65 species [38]. The majority of section Nidulantes species can produce a sexual state, and those species were, in the dual name nomenclature system, assigned to the genus Emericella. Because of the adoption of the “one fungus: one name” nomenclatural system, all Emericella species were transferred to Aspergillus [40]. AFB1 was produced by four species: Aspergillus astellatus [41], A. miraensis [42,43], A. olivicola [44], and A. venezuelensis [45]. Aspergillus ochraceoroseus and A. rambellii belong to section Ochraceorosei [32]. A. ochraceoroseus produce AFB1 [11,39,46,47], and A. rambellii also produce AFB1 [32,39].
With the development of modern molecular biological and chromatographic methods, other new AF producers will certainly be identified soon and bring new research to this area.
3. Aflatoxin Occurrence in Foods
The contamination of foods with AFs, like with other mycotoxins, has become a global problem [48]. For several years, a statement claiming that a total amount of 25% of the world’s crops are affected by molds and mycotoxins, supposedly estimated by the Food and Agriculture Organization (FAO), has been circulating worldwide [12,49]. However, this estimation has been challenged in the most recent studies dealing with the background of this matter, as this statement was not possible to trace back, since even FAO experts were not able to do so [50]. On the basis of an extensive study by the BIOMIN Company in 2004–2011, 72% of samples of feed (mainly maize, wheat, barley, and silage) and feed raw materials (especially for swine, poultry, and cows) from all over the world, but mainly from Asia (40%) and Europe (38%), contained a detectable amount of at least one mycotoxin including AFs. Moreover, a co-occurrence of two or more mycotoxins was confirmed in 38% of samples [51], and of course, AFs can interact in synergy with other mycotoxins. This fact is alarming since the major intake of mycotoxins into human organisms is usually due to dietary exposure [52], and even a low concentration of AFs is hazardous for humans [53].
In general, inappropriate storage is considered a major cause of foods contamination with mycotoxins—especially in developing countries [54,55], in which approximately 20% of the global volume of potentially highly contaminated commodities originate [56]. In some cases, contamination of crops with mycotoxins may already occur in the field due to stress factors such as insects or drought that facilitate the contamination [57]. Climate conditions, such as high temperatures, heavy rainfalls, and high relative humidity, are likely to contribute to crop contamination as well, as they make plants more susceptible to fungal, and thus mycotoxin, contamination [58,59]. Contamination during transport and processing is also possible [23]. Good agricultural, manufacturing, and hygienic practices, good plant disease management, and adequate storage conditions can limit mycotoxin levels in the food chain, yet these practices do not eliminate mycotoxins completely [60,61].
Fortunately, some contamination-reducing chemical (ammonization, hydrogen peroxide, sodium bisulfate, organic acids, ozone, and plant extracts), physical (separation, solvent extraction, mineral adsorbents, heating, extrusion, microwaving, irradiation, and UV radiation) and biological (enzymes, bacterial cells, yeast cells, and non-toxigenic strains) technologies have been developed to enhance food safety [20,23]. However, the European Union legislation, in Section 2 of the Annex “Mycotoxins”, does not allow any foods contaminated with mycotoxins to be detoxified by the chemical approach [62]. Moreover, foods treated by sorting or other physical means must not be mixed with foods intended for direct human consumption nor with foods intended to be used as food ingredients [62]. Biological control, depending on the competition between non-toxigenic and toxigenic strains, is the most commonly used method, especially in countries where AFs pose a significant threat [63]. For example, a product AflasafeTM has begun to be applied to reduce AFs with an average efficiency of 99% (76%–100%) in maize and groundnuts [64,65,66]. The principle of its use lies in the contamination of crops with non-toxigenic strains before they are contaminated by toxigenic strains of Aspergillus flavus. AflasafeTM is a relatively cheap and easy-to-apply product that ensures a long-lasting reduction of AFs (up to consumption level) [64].
AFs contaminate a wide range of foods of both plant and animal origin. AFB1, AFB2, AFG1, and AFG2 are major contaminants in commodities of plant origin, mainly groundnuts, tree nuts, spices, seeds, dry fruits, and cereals [67,68,69]. The daily intake of AFs at the level of nanograms to micrograms per person per day is mainly achieved through the consumption of contaminated maize and groundnuts [70]. Animal products are less likely substrates for AF producers; however, the metabolites AFM1 and AFM2 are typical in milk, including human breast milk [71,72], and dairy products of lactating ruminants that have been fed with contaminated feed (carry-over to dairy milk) [73,74,75,76]. AFM1 has also been detected in cheese worldwide [77,78,79] and AFs (in low concentrations) have been reported to occur in certain products of animal origin, such as meat and meat products, or eggs, etc. (carry-over of AFs in products of animal origin) [74].
Drought periods combined with high temperatures significantly increase AF production in the fields [80]. It has been estimated that at least 4.5 billion people worldwide are chronically exposed to AFs from foods, especially in “hot zones” in the regions situated between 40° N and 40° S latitude [81]. Climate change and the trend of global warming may lead to an increased occurrence of mycotoxins, for the production of which higher temperatures are needed, and the same goes for AFs [82]. This might be the case in Northern [82] or Western [83] Europe, for example, where AFB1 contamination of maize was recently observed [84]. It should be emphasized that even in the current modern age, cases of acute aflatoxicosis leading to human death may occur due to climate change [85]. Climate change is dealt with in more detail in the Special Issue of Toxins entitled “Mycotoxins in Relation to Climate Change”.
On the other hand, it is known that the AFs belong to the dominant mycotoxins in the African and Asian continents, as well as North and South America and the Australian continent [86]. Additionally, despite all efforts to mitigate AFs in foods, there are still cases of high AF concentrations in foods. Therefore, to enhance food safety, there is a global need for regulatory limits and food contaminant monitoring tools.
3.1. The Occurrence of Aflatoxins in Food in the African Continent
In African countries, maize and groundnuts represent the largest exposure to AFs [87,88], where maize is a staple crop for the majority of the African population [88,89]. The case of highly contaminated (1–46, 400 µg/kg) maize in Kenya in 2004, associated with 125 human deaths, is historically relevant [85].
There are still cases of concentrations exceeding the limits set in many countries. Recently, high concentrations of AFs in maize grains of up to 9091.8 µg/kg (AFB1) were found in Kenya [89], up to 3760 µg/kg for total AFs (where AFT is the sum of AFB1, AFB2, AFG1, and AFG2) in Uganda [90], up to 2806.5 µg/kg (AFT) in the Democratic Republic of Congo [91], up to 1460 µg/kg (AFT) in Nigeria [88], up to 945 µg/kg (AFT) in Ghana [92], and up to 107.6 µg/kg (AFT) in Zambia [93].
3.2. The Occurrence of Aflatoxins in Food in the Asian Continent
Practically all tropical countries face the problem of AFs [94]. The climate of Asian countries is very favorable for AF-producing microfungi [95], especially when it comes to commodities such as cereals—mainly maize and rice, cereal products, beans, groundnuts, and other oily products—which is alarming, as cereals and groundnuts are considered major items in the Asian diet [94].
AFs were found in maize in concentrations of up to 1572 µg/kg (AFB1) in Vietnam [96]. However, in Asia, rice is the most important crop in terms of its consumption [55,97], and especially production, as approximately 90% of the world’s rice is produced in Asia, of which nearly two-thirds are produced by China, India, and Indonesia [98]. High concentrations of AFs in rice have been reported in many scientific studies. In the case of AFB1, reported concentrations reached up to 361.0 µg/kg in India [99], up to 185.0 µg/kg in Sri Lanka [100], and up to 26.6 µg/kg in Thailand [101]. In the case of AFT concentrations, they were found to reach up to 96.3 µg/kg in Malaysia [102], up to 77.8 µg/kg in Vietnam [55], up to 21.4 µg/kg in Turkey [103], and up to 21.0 µg/kg in China [104].
3.3. The Occurrence of Aflatoxins in Food in the American Continent
America is the largest producer of maize (565 million tons in 2019; 49.2% of world production). The United States, Brazil, Argentina, and Mexico belong to the top 10 producers worldwide [98]. Alongside sub-Saharan Africa and Southeast Asia, maize is a staple food in Latin America [105], especially in Guatemala [106] and Mexico [107].
However, concentrations of AFB1 of up to 2656 µg/kg were observed in maize in Guatemala and are potentially high throughout the rest of Central America and Mexico [106]. Lower concentrations of up to 282.5 µg/kg (AFB1) and 303.9 µg/kg (AFT) were detected in maize kernels in South Haiti [108], and concentrations of up to 49.9 µg/kg (AFT) were found in Brazil [109]. Processed maize products are also contaminated with AFs. For example, tortillas and popcorn have been reported to be contaminated with up to 287.23 µg/kg (AFB1) [110] and up to 120 µg/kg (AFT) [111], respectively, in Mexico.
Of course, the problem is not only maize as a staple food, as high levels of AFs are also found in other local commodities, including up to 33.3 µg/kg (AFT) in nuts, up to 176.4 µg/kg (AFT) in Capsicum spices in Chile [112], and up to 70.9 µg/kg (AFT) in the case of Brazilian rice [113].
3.4. The Occurrence of Aflatoxins in Food in the Australian Continent
In Australia, hot and dry conditions typical for the arid and semi-arid areas covering much of the continent are the main stress factors that allow for the contamination of crops with AFs. This represents a major problem in Australia in terms of peanut degradation [114,115]. The occurrence of AFs is not quite as common in Australian maize [116], and when it occurs, it is in low or moderate concentrations [117] for unknown reasons [115]. Nevertheless, maize is only a small part of the human and animal diet in Australia [115].
The occurrence of AFs in Australian maize is usually in the range of 1–5 µg/kg, but can also occasionally reach higher concentrations of up to 200 µg/kg [118]. However, higher concentrations of AFT in maize (up to 311.1 µg/kg), and also in peanuts (up to 384.8 µg/kg), sorghum (up to 138.3 µg/kg), and wheat (up to 26.8 µg/kg), have been found in Australia [115,119,120,121].
3.5. Aflatoxin Regulations in the European Union and around the World
The discovery of AFs and their serious negative effects on human and animal health in the early 1960s led many countries in the world to establish certain regulations of mycotoxins in foods to protect consumers from the harmful effects caused by mycotoxins [122,123]. The first limit regulating mycotoxins, namely AFs, was set in the late 1960s, and by 2003 approximately 100 countries in the world had already regulated mycotoxins in foods [123]. Although the number of countries regulating mycotoxins in foods is increasing [123], most African countries and other developing countries lack regulations [92], as the compliance with the limits in developing countries would result in a shortage of food, and thus an increase in its price.
From the perspective of all mycotoxins, the regulations of AFB1, AFT, and AFM1 are the greatest concern of worldwide legislation [124]. The Codex Alimentarius specifies an AF maximum limit of 15 µg/kg (for almonds, hazelnuts, Brazil nuts, peanuts, and pistachio nuts for further processing) and 10 µg/kg (for almonds, Brazil nuts, hazelnuts, and pistachio nuts for direct consumption and dried figs), and AFM1 maximum limit of 0.5 µg/kg for milk [125]. However, the maximum levels of AFs in foods vary throughout different countries depending on the type of product and also on the import/export regime [69].
The European Union (EU) has one of the most comprehensive and strictest regulations on AF levels, set by the commission regulation 1881/2006 [62], and later on by its amending supplement 165/2010 [126], that are binding upon the 27 member states of the EU. These levels are in ranges 0.1–12 µg/kg, 4–15 µg/kg, and 0.025–0.05 µg/kg for AFB1, AFT, and AFM1, respectively, in the case of various foods [62,126].
For comparison with other countries, the maximum limit/regulatory limit/action level (or the range) for AFB1 has been set at 30 µg/kg in India, at 20 µg/kg in the Philippines, at 15–20 µg/kg in Indonesia [127], at 0.5–20 µg/kg in China [128], at 5–10 µg/kg in Japan, and at 0.1–10 µg/kg in Korea [127].
The maximum/action limit (or the range) for AFT has been set at 20–35 µg/kg in Indonesia [127]; at 5–15 µg/kg in Malaysia [129]; at 30 µg/kg in Sri Lanka [127]; at 20 µg/kg in the United States [130,131,132,133], Thailand, the Philippines [127], and Nigeria [134]; at 15–20 µg/kg in Hong Kong [127]; at 1–20 in Brazil µg/kg [135]; at 15 µg/kg in Canada [136], Korea [69,127], Australia [137], and Zimbabwe [134]; at 0–15 µg/kg in Taiwan, at 10 µg/kg in Japan, Vietnam [69,127], Kenya, Mozambique, South Africa, and Uganda [134]; and at 5 µg/kg in Singapore [127].
If a country has any regulation on AFM1 in milk or dairy products, it is usually set at 0.5 µg/kg [128,135,138,139], which is in line with the Codex Alimentarius. However, in the EU legislation, the AFM1 maximum limits (0.025–0.05 µg/kg) are 10–20 times lower compared to the Codex Alimentarius (0.5 µg/kg) [62,125].
3.6. The Occurrence of Aflatoxins Based on Data by INFOSAN (2016–2020)
The International Food Safety Authorities Network (INFOSAN) is a global information network jointly managed by the World Health Organization (WHO) and the FAO [140]. The INFOSAN has facilitated urgent international communication during food safety emergencies between more than 600 members from 188 of the 194 FAO and WHO member states since 2004. The INFOSAN aims to reduce the incidence of foodborne diseases that have a significant impact on public health and international trade [140,141]. Regarding AFs, only two cases, both of which concerned maize in Tanzania, were reported in 2016 and 2017 [142]. There have been no reports on AFs in foods since.
3.7. The Occurrence of Aflatoxins in Food Based on Data by RASFF (2015–2020)
The Rapid Alert System for Food and Feed (RASFF) is an important warning system for food and feed safety from the perspective of the EU countries [143]. Regarding the number of notifications reported by RASFF in 2015-2020, most mycotoxin notifications were related to AFs (approximately 88%), of which most were of the food category (approximately 94%) and less were of the feed category (approximately 6%), as shown in Table 2 [144].
Table 2.
The share of aflatoxin notifications in 2015-2020.
| Substance/Year | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Mycotoxins | 495 | 549 | 579 | 655 | 584 | 423 |
| AFs a | 441 (89.1%) | 478 (87.1%) | 539 (93.0%) | 567 (86.6%) | 497 (85.1%) | 370 (87.5%) |
| AFs in food | 423 (95.9%) | 461 (96.4%) | 515 (95.5%) | 510 (89.9%) | 467 (94.0%) | 348 (94.1%) |
a AFs = aflatoxins; processed according to the Rapid Alert System for Food and Feed (RASFF) database [143].
Based on data from the last years (2015–2020), the vast majority of notified products contaminated with AFs belong to the “nuts, nut products, and seeds” category, followed behind by “fruits and vegetables”, “herbs and spices”, “cereals and bakery products”, and others. Namely, the most often notified foods are, in descending order, groundnuts, pistachio nuts, dried figs, hazelnuts, spices, almonds, rice, melon seeds, Brazil nuts, and maize [144].
Throughout the years 2015–2020, cases of very high concentrations of AFs in foods were notified. Based on these “high-level” notifications, groundnuts, pistachio nuts, almonds, dried figs, hazelnuts, chilies, melon seeds, and apricot kernels appear to be highly contaminated (with the maximum concentration of AFB1 or AFT exceeding 1000 µg/kg). Spices (other than chilies), tiger nuts, Brazil nuts, rice, pecan nuts, walnuts, and maize represent the less contaminated foods [144]. There is a concern for the development of aflatoxicosis associated with the consumption of foods with an AF concentration of at least 1000 µg/kg [145]. This implies that the group of above-mentioned highly contaminated commodities may tend to cause aflatoxicosis in humans or animals. Some of the highest values of aflatoxin contamination in 2015–2020 are shown in Table 3.
Table 3.
The highest concentrations of aflatoxin B1 and total aflatoxins in foods notified by RASFF in 2015–2020.
| No. | Product | AFB1 (µg/kg) |
AFT a (µg/kg) |
Origin | Year |
|---|---|---|---|---|---|
| 1 | Peanut paste | 707,000 | 907,000 | Senegal | 2016 |
| 2 | Peanuts | 180,200 | 220,900 | China | 2015 |
| 3 | Groundnuts in shell | 42,100 | 46,800 | Egypt | 2019 |
| 4 | Groundnuts | 17,000 | 38,000 | Turkey | 2016 |
| 5 | Pistachios | − | 26,300 | Germany | 2020 |
| 6 | Peanut in shell | 24,000 | 26,000 | China | 2015 |
| 7 | Almonds | − | 24,000 | US | 2018 |
| 8 | Dried figs | 15,300 | − | Turkey | 2020 |
| 9 | Roasted chopped hazelnuts | 4000 | 15,200 | Turkey | 2015 |
| 10 | Shelled nuts | 12,890 | 14,420 | Turkey | 2019 |
| 11 | Organic groundnut kernels | 11,000 | 14,000 | Egypt | 2020 |
| 12 | Dried red chilies | 13,700 | 14,000 | India | 2020 |
| 13 | Roasted and salted watermelon seeds | 13,700 | − | Turkey | 2020 |
| 14 | Shelled almonds | 10,440 | 11,420 | US | 2019 |
| 15 | Hazelnut kernels | 7200 | − | Georgia | 2019 |
a AFT = sum of aflatoxins B1, B2, G1, and G2; processed according to the RASFF database [144].
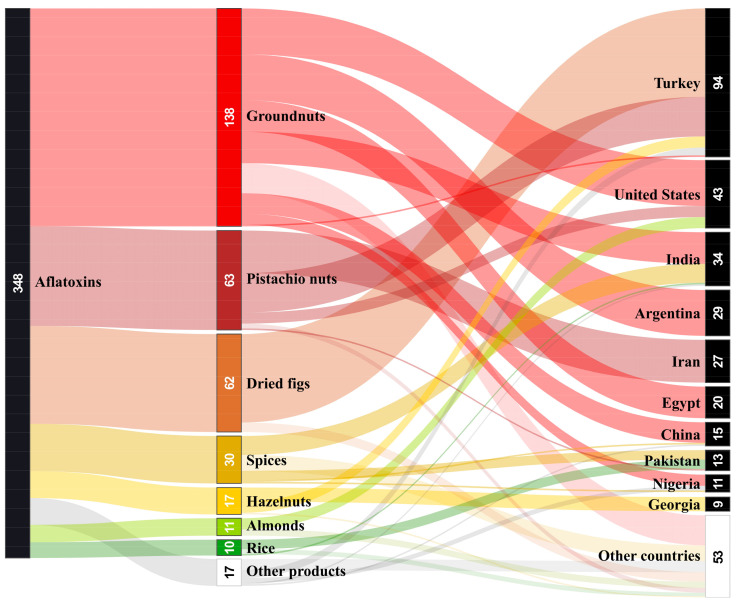
In the year 2020, groundnuts, pistachio nuts, dried figs, spices, hazelnuts, almonds, and rice were the most notified products in relation to AF contamination. The other notified products were mostly various seeds (melon, ogbono, sunflower, lotus, and sesame seeds) and flours (wheat flour, chestnut flour, and banku mix). Single notifications concerned Brazil nuts, apricot kernels, soya, milk, and date syrup. Most notifications originated in Turkey (mainly dried figs and pistachio nuts), followed far behind by the United States (mainly groundnuts) and India (mainly groundnuts and spices). A significant number of notifications originated in Argentina (groundnuts only), Iran (pistachio nuts only), Egypt (groundnuts only), China (mainly groundnuts), Pakistan (mainly spices and rice), Nigeria (mainly groundnuts), and Georgia (hazelnuts only) (see Figure 1) [144]. Fewer notifications (the number is given in brackets) originated in other countries: Spain (7); Sri Lanka (6); Brazil (5); Italy and Ghana (3); Ethiopia, United Kingdom, Germany, Ukraine, and Cameroon (2); and Angola, Vietnam, Hong Kong, South Africa, Jordan, Togo, Hungary, Nepal, Bolivia, Cambodia, Paraguay, Indonesia, Belgium, Malaysia, Tunisia, Senegal, and Azerbaijan (1). Two notifications were of unknown origin [144].
Figure 1.
Aflatoxin notifications in food by the RASFF in 2020. Note: All products in the “Other products” category were notified less than four times in 2020. The category “Other countries” includes notifications from 24 countries, in which less than 9 notifications originated and 2 notifications were of unknown origin. Processed according to RASFF database [144].
The amount of the world production of these commodities should be taken into consideration as demonstrated in Table 4. Although groundnuts are the most often notified product, pistachio nuts can be labelled as the relatively most frequently notified product, with approximately one notification per 16,787 tons produced. In contrast, there is one notification per 344,886 tons of groundnuts produced [144].
Table 4.
The number of RASFF aflatoxin notifications concerning certain food products in relation to their average world production.
| Product | Average Annual Production (2015–2019) a (Tons) |
Number of Notifications by RASFF (2020) | Tons Produced per RASFF Notification |
|---|---|---|---|
| Groundnuts | 47,591,548 | 138 | 344,866 |
| Pistachio nuts | 1,057,587 | 63 | 16,787 |
| Dried figs | 1,185,768 | 62 | 19,125 |
| Spices | 14,541,902 | 30 | 484,730 |
| Hazelnuts | 939,927 | 17 | 55,290 |
| Almonds | 3,039,020 | 11 | 276,275 |
| Rice | 748,304,354 | 10 | 74,830,435 |
a Average annual spice production includes these categories: “Anise, badian, fennel, coriander”, “Chilies and peppers, dry”, “Cinnamon”, “Cloves”, “Ginger”, “Nutmeg, mace, cardamoms”, “Mustard seed”, “Pepper, Piper pp.”, “Peppermint”, “Vanilla”, and “Spice not elsewhere specified”. Processed according to FAOSTAT and RASFF databases [98,144].
4. Conclusions
The year 2020 has already passed 60 years of AF discovery. Since then, despite the scientific progress in the knowledge on AFs and the efforts made to reduce the risk they pose to public health, developing countries still have to tolerate a high level of AF contamination of foods to not compromise the food supply. Selected research topics concerning AFs continue to draw attention worldwide, such as research on the diversity and genetic variability of AF production in Aspergillus flavus and other AF producers, or on the problem of using biocontrol strategies for the non-aflatoxigenic strains of A. flavus with the goal of the better protection of public health and the prevention of economic losses. The recent occurrence data, the recent food consumption data, and the recent toxicological data of AFs in foodstuffs are required for the assessment of the severity of AF toxicity, the estimation of human dietary exposure, and health risk assessments.
Acknowledgments
Dedicated to the memory of all researchers around the world who substantially contributed to AFs research and helped to build general knowledge on AFs.
Author Contributions
Conceptualization, D.P., V.O., and F.M.; methodology, D.P., V.O., and F.M.; investigation, D.P., V.O., and F.M.; data curation, D.P., V.O., and F.M.; writing—original draft preparation, D.P., V.O., and F.M.; writing—review and editing, D.P., V.O. and, F.M.; visualization, D.P.; supervision, V.O. and F.M.; project administration, D.P.; funding acquisition, D.P. and V.O. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Ministry of Health, Czech Republic for theconceptual development of a research organization (“National Institute of Public Health—NIPH, IN 75010330”) and a specific research project (no. 2115/2020) of the Faculty of Science, University of Hradec Kralove, Czech Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
As of 2020, 60 years have passed since the discovery of aflatoxins. A total of 22, 4, and 2 Aspergillus producers of section Flavi, Nidulantes, and Ochracerosei produce aflatoxins, respectively. Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius are the most important aflatoxin producers of section Flavi. Groundnuts, pistachio nuts, dried figs, hazelnuts, spices, almonds, rice, melon seeds, Brazil nuts, and maize are the most common commodities contaminated with aflatoxins.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occuring Substances: Food Items and Costituents, Heterocyclic Aromatic Amines and Mycotoxins. Vol. 56. IARC Press; Lyon, France: 1993. [Google Scholar]
- 2.Miller J.D. Fungi and Mycotoxins in Grain: Implications for Stored Product Research. J. Stored Prod. Res. 1995;31:1–16. doi: 10.1016/0022-474X(94)00039-V. [DOI] [Google Scholar]
- 3.Santos L., Marín S., Sanchis V., Ramos A.J. Screening of Mycotoxin Multicontamination in Medicinal and Aromatic Herbs Sampled in Spain. J. Sci. Food Agric. 2009;89:1802–1807. doi: 10.1002/jsfa.3647. [DOI] [Google Scholar]
- 4.Winter G., Pereg L. A Review on the Relation between Soil and Mycotoxins: Effect of Aflatoxin on Field, Food and Finance. Eur. J. Soil Sci. 2019;70:882–897. doi: 10.1111/ejss.12813. [DOI] [Google Scholar]
- 5.Schuda P.F. Syntheses of Natural Products. Springer; Berlin/Heidelberg, Germany: 1980. Aflatoxin Chemistry and Syntheses; pp. 75–111. [Google Scholar]
- 6.European Food Safety Authority Effect on Public Health of a Possible Increase of the Maximum Level for ‘Aflatoxin Total’ from 4 to 10 µg/kg in Peanuts and Processed Products Thereof, Intended for Direct Human Consumption or Use as an Ingredient in Foodstuffs. EFSA J. 2018;16:1–32. doi: 10.2903/j.efsa.2018.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giray B., Girgin G., Engin A.B., Aydın S., Sahin G. Aflatoxin Levels in Wheat Samples Consumed in Some Regions of Turkey. Food Control. 2007;18:23–29. doi: 10.1016/j.foodcont.2005.08.002. [DOI] [Google Scholar]
- 8.Hussain I., Anwar J. A Study on Contamination of Aflatoxin M1 in Raw Milk in the Punjab Province of Pakistan. Food Control. 2008;19:393–395. doi: 10.1016/j.foodcont.2007.04.019. [DOI] [Google Scholar]
- 9.PubChem. [(accessed on 17 December 2020)]; Available online: https://pubchem.ncbi.nlm.nih.gov/
- 10.Peraica M., Radić B., Lucić A., Pavlović M. Toxic Effects of Mycotoxins in Human. Bull. World Health Organ. 1999;77:754–766. [PMC free article] [PubMed] [Google Scholar]
- 11.Klich M.A., Cary J.W., Beltz S.B., Bennett C.A. Phylogenetic and Morphological Analysis of Aspergillus Ochraceoroseus. Mycologia. 2003;95:1252–1260. doi: 10.1080/15572536.2004.11833033. [DOI] [PubMed] [Google Scholar]
- 12.Bhat R., Rai R.V., Karim A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010;9:57–81. doi: 10.1111/j.1541-4337.2009.00094.x. [DOI] [PubMed] [Google Scholar]
- 13.Kensler T.W., Roebuck B.D., Wogan G.N., Groopman J.D. Aflatoxin: A 50-Year Odyssey of Mechanistic and Translational Toxicology. Toxicol. Sci. 2011;120:S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P., Mahato D.K., Kamle M., Mohanta T.K., Kang S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017;7:1–10. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risks to Humans: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. Vol. 1. IARC Press; Lyon, France: 1987. [Google Scholar]
- 16.Ostry V., Malir F., Toman J., Grosse Y. Mycotoxins as Human Carcinogens-the IARC Monographs Classification. Mycotoxin Res. 2017;33:65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risks to Humans: Chemical Agents and Related Occupations. A Review of Human Carcinogens. Vol. 100. IARC Press; Lyon, France: 2012. [PMC free article] [PubMed] [Google Scholar]
- 18.Etzel R. Mycotoxins. J. Am. Med. Assoc. 2002;287:425–427. doi: 10.1001/jama.287.4.425. [DOI] [PubMed] [Google Scholar]
- 19.Sherif S., Salama E., Abdel-Wahhab P.M. Mycotoxins and Child Health: The Need for Health Risk Assessment. Int. J. Hyg. Environ. Health. 2009;212:347–368. doi: 10.1016/j.ijheh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Ismail A., Gonçalves B.L., de Neeff D.V., Ponzilacqua B., Coppa C.F.S.C., Hintzsche H., Sajid M., Cruz A.G., Corassin C.H., Oliveira C.A.F. Aflatoxin in Foodstuffs: Occurrence and Recent Advances in Decontamination. Food Res. Int. 2018;113:74–85. doi: 10.1016/j.foodres.2018.06.067. [DOI] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer/World Health Organization Cancer Today, Data Visualization Tools for Exploring the Global Cancer Burden in 2020. [(accessed on 10 January 2021)]; Available online: https://gco.iarc.fr/today/home.
- 22.Liu Y. Wu Felicia Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ. Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udomkun P., Wiredu A.N., Nagle M., Müller J., Vanlauwe B., Bandyopadhyay R. Innovative Technologies to Manage Aflatoxins in Foods and Feeds and the Profitability of Application–A Review. Food Control. 2017;76:127–138. doi: 10.1016/j.foodcont.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varga J., Frisvad J.C., Samson R.A. Two New Aflatoxin Producing Species, and an Overview of Aspergillus Section Flavi. Stud. Mycol. 2011;69:57–80. doi: 10.3114/sim.2011.69.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisvad J.C., Hubka V., Ezekiel C.N., Hong S.-B., Novakova A., Chen A.J., Arzanlou M., Larsen T.O., Sklenar F., Mahakarnchanakul W., et al. Taxonomy of Aspergillus Section Flavi and Their Production of Aflatoxins, Ochratoxins and Other Mycotoxins. Stud. Mycol. 2019;93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norlia M., Jinap S., Nor-Khaizura M.A.R., Radu S., Samsudin N.I.P., Azri F.A. Aspergillus Section Flavi and Aflatoxins: Occurrence, Detection, and Identification in Raw Peanuts and Peanut-Based Products along the Supply Chain. Front. Microbiol. 2019;10:1–17. doi: 10.3389/fmicb.2019.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godet M., Munaut F. Molecular Strategy for Identification in Aspergillus Section Flavi. FEMS Microbiol. Lett. 2010;304:157–168. doi: 10.1111/j.1574-6968.2009.01890.x. [DOI] [PubMed] [Google Scholar]
- 28.Singh P., Orbach M.J., Cotty P.J. Aspergillus Texensis: A Novel Aflatoxin Producer with S Morphology from the United States. Toxins. 2018;10:513. doi: 10.3390/toxins10120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh P., Callicott K.A., Orbach M.J., Cotty P.J. Molecular Analysis of S-Morphology Aflatoxin Producers from the United States Reveals Previously Unknown Diversity and Two New Taxa. Front. Microbiol. 2020;11:1–16. doi: 10.3389/fmicb.2020.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mom M.P., Romero S.M., Larumbe A.G., Iannone L., Comerio R., Smersu C.S.S., Simón M., Vaamonde G. Microbiological Quality, Fungal Diversity and Aflatoxins Contamination in Carob Flour (Prosopis Flexuosa) Int. J. Food Microbiol. 2020;326:1–7. doi: 10.1016/j.ijfoodmicro.2020.108655. [DOI] [PubMed] [Google Scholar]
- 31.Nesbitt B.F., O’Kelly J., Sargeant K., Sheridan A.N.N. Aspergillus Flavus and Turkey X Disease. Toxic Metabolites of Aspergillus Flavus. Nature. 1962;195:1062–1063. doi: 10.1038/1951062a0. [DOI] [PubMed] [Google Scholar]
- 32.Varga J., Frisvad J., Samson R. A Reappraisal of Fungi Producing Aflatoxins. World Mycotoxin J. 2009;2:263–277. doi: 10.3920/WMJ2008.1094. [DOI] [Google Scholar]
- 33.Codner R.C., Sargeant K., Yeo R. Production of Aflatoxin by the Culture of Strains of Aspergillus Flavus-Oryzae on Sterilized Peanuts. Biotechnol. Bioeng. 1963;5:185–192. doi: 10.1002/bit.260050303. [DOI] [Google Scholar]
- 34.Buchanan R.L., Ayres J.C. Effect of Sodium Acetate on Growth and Aflatoxin Production by Aspergillus Parasiticus NRRL 2999. J. Food. Sci. 1976;41:128–132. doi: 10.1111/j.1365-2621.1976.tb01117.x. [DOI] [Google Scholar]
- 35.Schmidt-Heydt M., Rüfer C.E., Abdel-Hadi A., Magan N., Geisen R. The Production of Aflatoxin B1 or G1 by Aspergillus Parasiticus at Various Combinations of Temperature and Water Activity Is Related to the Ratio of AflS to AflR Expression. Mycotoxin Res. 2010;26:241–246. doi: 10.1007/s12550-010-0062-7. [DOI] [PubMed] [Google Scholar]
- 36.Kurtzman C.P., Horn B.W., Hesseltine C.W. Aspergillus Nomius, a New Aflatoxin-Producing Species Related to Aspergillus Flavus and Aspergillus Tamarii. Antonie Leeuwenhoek. 1987;53:147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- 37.Yunes N.B.S., Oliveira R.C., Reis T.A., Baquião A.C., Rocha L.O., Correa B. Effect of Temperature on Growth, Gene Expression, and Aflatoxin Production by Aspergillus Nomius Isolated from Brazil Nuts. Mycotoxin Res. 2020;36:173–180. doi: 10.1007/s12550-019-00380-w. [DOI] [PubMed] [Google Scholar]
- 38.Chen A.J., Frisvad J.C., Sun B.D., Varga J., Kocsubé S., Dijksterhuis J., Kim D.H., Hong S.-B., Houbraken J., Samson R.A. Aspergillus Section Nidulantes (Formerly Emericella): Polyphasic Taxonomy, Chemistry and Biology. Stud. Mycol. 2016;84:1–118. doi: 10.1016/j.simyco.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frisvad J.C., Skouboe P., Samson R.A. Taxonomic Comparison of Three Different Groups of Aflatoxin Producers and a New Efficient Producer of Aflatoxin B1, Sterigmatocystin and 3-O-Methylsterigmatocystin, Aspergillus Rambellii Sp. Nov. Syst. Appl. Microbiol. 2005;28:442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Samson R.A., Visagie C.M., Houbraken J., Hong S.-B., Hubka V., Klaassen C.H.W., Perrone G., Seifert K.A., Susca A., Tanney J.B., et al. Phylogeny, Identification and Nomenclature of the Genus Aspergillus. Stud. Mycol. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frisvad J.C., Samson R.A., Smedsgaard J. Emericella Astellata, a New Producer of Aflatoxin B1, B2 and Sterigmatocystin. Lett. Appl. Microbiol. 2004;38:440–445. doi: 10.1111/j.1472-765X.2004.01520.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L.-C., Chen J., Lin W.-H., Guo S.-X. A New Species of Emericella from Tibet, China. Mycotaxon. 2013;125:131–138. doi: 10.5248/125.131. [DOI] [Google Scholar]
- 43.Hubka V., Novakova A., Peterson S.W., Frisvad J.C., Sklenář F., Matsuzawa T., Kubatova A., Kolarik M. A Reappraisal of Aspergillus Section Nidulantes with Descriptions of Two New Sterigmatocystin-Producing Species. Plant Syst. Evol. 2016;302:1267–1299. doi: 10.1007/s00606-016-1331-5. [DOI] [Google Scholar]
- 44.Zalar P., Frisvad J.C., Gunde-Cimerman N., Varga J., Samson R.A. Four New Species of Emericella from the Mediterranean Region of Europe. Mycologia. 2008;100:779–795. doi: 10.3852/08-078. [DOI] [PubMed] [Google Scholar]
- 45.Frisvad J.C., Samson R.A. Emericella Venezuelensis, a New Species with Atellate Ascospores Producing Sterigmatocystin and Aflatoxin B1. Syst. Appl. Microbiol. 2004;27:672–680. doi: 10.1078/0723202042369910. [DOI] [PubMed] [Google Scholar]
- 46.Klich M.A., Mullaney E.J., Daly C.B., Cary J.W. Molecular and Physiological Aspects of Aflatoxin and Sterigmatocystin Biosynthesis by Aspergillus Tamarii and A. Ochraceoroseus. Appl. Microbiol. Biotechnol. 2000;53:605–609. doi: 10.1007/s002530051664. [DOI] [PubMed] [Google Scholar]
- 47.Cary J.W., Ehrlich K.C., Beltz S.B., Harris-Coward P., Klich M.A. Characterization of the Aspergillus Ochraceoroseus Aflatoxin/Sterigmatocystin Biosynthetic Gene Cluster. Mycologia. 2009;101:352–362. doi: 10.3852/08-173. [DOI] [PubMed] [Google Scholar]
- 48.Hussein H.S., Brasel J.M. Toxicity, Metabolism, and Impact of Mycotoxins on Humans and Animals. Toxicology. 2001;167:101–134. doi: 10.1016/S0300-483X(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 49.Park D.L., Njapau H., Boutrif E. Minimizing Risks Posed by Mycotoxins Utilizing the HACCP Concept. Food Nutr. Agric. 1999;3:49–54. [Google Scholar]
- 50.Eskola M., Kos G., Elliott C.T., Hajšlová J., Mayar S., Krska R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25% Crit. Rev. Food Sci. Nutr. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- 51.Streit E., Naehrer K., Rodrigues I., Schatzmayr G. Mycotoxin Occurrence in Feed and Feed raw Materials Worldwide: Long-Term Analysis With Special Focus on Europe and Asia. J. Sci. Food Agric. 2013;93:2892–2899. doi: 10.1002/jsfa.6225. [DOI] [PubMed] [Google Scholar]
- 52.Zinedine A., Mañes J. Occurrence and Legislation of Mycotoxins in Food and Feed from Morocco. Food Control. 2009;20:334–344. doi: 10.1016/j.foodcont.2008.07.002. [DOI] [Google Scholar]
- 53.Mahato D.K., Lee K.E., Kamle M., Devi S., Dewangan K., Kumar P., Kang S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019;10:1–10. doi: 10.3389/fmicb.2019.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heussner A.H., Bingle L.E.H. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins. 2015;7:4253–4282. doi: 10.3390/toxins7104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huong B.T.M., Do T.T., Madsen H., Brimer L., Dalsgaard A. Aflatoxins and Fumonisins in Rice and Maize Staple Cereals in Northern Vietnam and Dietary Exposure in Different Ethnic Groups. Food Control. 2016;70:191–200. doi: 10.1016/j.foodcont.2016.05.052. [DOI] [Google Scholar]
- 56.Food and Agriculture Organization Basic Facts on the World Cereal Situation. [(accessed on 13 January 2021)];1996 Available online: http://www.fao.org/3/w1690e/w1690e16.htm#I1.
- 57.Klaassen C. Casarett & Doull’s Toxicology: The Basic Science of Poisons. 7th ed. McGraw-Hill; New York, NY, USA: 2007. [Google Scholar]
- 58.Marroquín-Cardona A.G., Johnson N.M., Phillips T.D., Hayes A.W. Mycotoxins in a Changing Global Environment–a Review. Food Chem. Toxicol. 2014;69:220–230. doi: 10.1016/j.fct.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 59.Botana L.M., Sainz M.J., editors. Climate Change and Mycotoxins. Walter de Gruyter GmbH; Berlin, Germany: 2015. [Google Scholar]
- 60.Karlovsky P., Suman M., Berthiller F., De Meester J., Eisenbrand G., Perrin I., Oswald I.P., Speijers G., Chiodini A., Recker T. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016;32:179–205. doi: 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iha M.H., Trucksess M.W. Management of Mycotoxins in Spices. J. AOAC Int. 2019;102:1732–1739. doi: 10.1093/jaoac/102.6.1732. [DOI] [PubMed] [Google Scholar]
- 62.European Commission Commission Regulation (EC) No. 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union. 2006;364:5–24. [Google Scholar]
- 63.Savić Z., Dudaš T., Loc M., Grahovac M., Budakov D., Jajić I., Krstović S., Barošević T., Krska R., Sulyok M., et al. Biological Control of Aflatoxin in Maize Grown in Serbia. Toxins. 2020;12:162. doi: 10.3390/toxins12030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitt J.I. The Pros and Cons of Using Biocontrol by Competitive Exclusion as a Means for Reducing Aflatoxin in Maize in Africa. World Mycotoxin J. 2019;12:103–112. doi: 10.3920/WMJ2018.2410. [DOI] [Google Scholar]
- 65.Senghor L.A., Ortega-Beltran A., Atehnkeng J., Callicott K.A., Cotty P.J., Bandyopadhyay R. The Atoxigenic Biocontrol Product Aflasafe SN01 Is a Valuable Tool to Mitigate Aflatoxin Contamination of Both Maize and Groundnut Cultivated in Senegal. Plant Dis. 2019;104:510–520. doi: 10.1094/PDIS-03-19-0575-RE. [DOI] [PubMed] [Google Scholar]
- 66.Agbetiameh D., Ortega-Beltran A., Awuah R.T., Atehnkeng J., Elzein A., Cotty P.J., Bandyopadhyay R. Field Efficacy of Two Atoxigenic Biocontrol Products for Mitigation of Aflatoxin Contamination in Maize and Groundnut in Ghana. Biol. Control. 2020;150:1–13. doi: 10.1016/j.biocontrol.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Miranda M.M., Rosero-Moreano M., Taborda-Ocampo G. Occurrence, Dietary Exposure and Risk Assessment of Aflatoxins in Arepa, Bread and Rice. Food Control. 2019;98:359–366. doi: 10.1016/j.foodcont.2018.11.046. [DOI] [Google Scholar]
- 68.Pickova D., Ostry V., Malir J., Toman J., Malir F. A Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins. 2020;12:789. doi: 10.3390/toxins12120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kabak B. Aflatoxins in Foodstuffs: Occurrence and Risk Assessment in Turkey. J. Food Compost. Anal. 2021;96:103734. doi: 10.1016/j.jfca.2020.103734. [DOI] [Google Scholar]
- 70.International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicine, Some Mycotoxins, Naphthalene and Strene. Vol. 82. IARC Press; Lyon, France: 2002. [Google Scholar]
- 71.Warth B., Braun D., Ezekiel C.N., Turner P.C., Degen G.H., Marko D. Biomonitoring of Mycotoxins in Human Breast Milk: Current State and Future Perspectives. Chem. Res. Toxicol. 2016;29:1087–1097. doi: 10.1021/acs.chemrestox.6b00125. [DOI] [PubMed] [Google Scholar]
- 72.Fakhri Y., Rahmani J., Oliveira C.A.F., Franco L.T., Corassin C.H., Saba S., Rafique J., Khaneghah A.M. Aflatoxin M1 in Human Breast Milk: A Global Systematic Review, Meta-Analysis, and Risk Assessment Study (Monte Carlo Simulation) Trends Food Sci. Technol. 2019;88:333–342. doi: 10.1016/j.tifs.2019.03.013. [DOI] [Google Scholar]
- 73.Fink-Gremmels J. Mycotoxins in Cattle Feeds and Carry-over to Dairy Milk: A Review. Food Addit. Contam. 2008;25:172–180. doi: 10.1080/02652030701823142. [DOI] [PubMed] [Google Scholar]
- 74.Herzallah S.M. Determination of Aflatoxins in Eggs, Milk, Meat and Meat Products Using HPLC Fluorescent and UV Detectors. Food Chem. 2009;114:1141–1146. doi: 10.1016/j.foodchem.2008.10.077. [DOI] [Google Scholar]
- 75.Hassan H.F., Kassaify Z. The Risks Associated with Aflatoxins M1 Occurrence in Lebanese Dairy Products. Food Control. 2014;37:68–72. doi: 10.1016/j.foodcont.2013.08.022. [DOI] [Google Scholar]
- 76.Benkerroum N. Mycotoxins in Dairy Products: A Review. Int. Dairy J. 2016;62:63–75. doi: 10.1016/j.idairyj.2016.07.002. [DOI] [Google Scholar]
- 77.Mulunda M., Ngoma L., Nyirenda M., Motsei L., Bakunzi F. A Decade of Aflatoxin M1 Surveillance in Milk and Dairy Products in Developing Countries (2001-2011): A Review. In: Hussaini A.M., editor. Mycotoxin and Food Safety in Developing Countries. IntechOpen; Rijeka, Croatia: 2013. pp. 39–60. [Google Scholar]
- 78.Rojas-Marín V., Carvajal-Moreno M., González-Villaseñor M.C., García-Hernández E.A., González–Mendoza A. Presence of Aflatoxin Carcinogens in Fresh and Mature Cheeses. Pharm. Anal. Acta. 2018;9:1–6. doi: 10.4172/2153-2435.1000581. [DOI] [Google Scholar]
- 79.Sengun I., Yaman D., Gonul S. Mycotoxins and Mould Contamination in Cheese: A Review. World Mycotoxin J. 2008;1:291–298. doi: 10.3920/WMJ2008.x041. [DOI] [Google Scholar]
- 80.Sanders T.H., Cole R.J., Blankenship P.D., Dorner J.W. Aflatoxin Contamination of Peanuts from Plants Drought Stressed in Pod or Root Zones. Peanut Sci. 1993;20:5–8. doi: 10.3146/i0095-3679-20-1-2. [DOI] [Google Scholar]
- 81.Williams J.H., Phillips T.D., Jolly P.E., Stiles J.K., Jolly C.M., Aggarwal D. Human Aflatoxicosis in Developing Countries: A Review of Toxicology, Exposure, Potential Health Consequences, and Interventions. Am. J. Clin. Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 82.Paterson R. Further Mycotoxin Effects from Climate Change. Food Res. Int. 2011;44:2555–2566. doi: 10.1016/j.foodres.2011.05.038. [DOI] [Google Scholar]
- 83.Miraglia M., Marvin H.J.P., Kleter G.A., Battilani P., Brera C., Coni E., Cubadda F., Croci L., De Santis B., Dekkers S., et al. Climate Change and Food Safety: An Emerging Issue with Special Focus on Europe. Food Chem. Toxicol. 2009;47:1009–1021. doi: 10.1016/j.fct.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Battilani P., Toscano P., Van der Fels-Klerx H.J., Moretti A., Leggieri M.C., Brera C., Rortais A., Goumperis T., Robinson T. Aflatoxin B1 Contamination in Maize in Europe Increases Due to Climate Change. Sci. Rep. 2016;6:1–7. doi: 10.1038/srep24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis L., Onsongo M., Njapau H., Schurz-Rogers H., Luber G., Kieszak S., Nyamongo J., Backer L., Dahiye A.M., Misore A. Aflatoxin Contamination of Commercial Maize Products during an Outbreak of Acute Aflatoxicosis in Eastern and Central Kenya. Environ. Health Perspect. 2005;113:1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devegowda G., Raju M.V.L.N., Afzali N., Swamy H.V.L.N. Mycotoxin Picture Worldwide: Novel Solutions for Their Counteraction. In: Lyons T.P., Jacques K.A., editors. Biotechnology in Feed Industry Proceedings of the Alltech’s 14th Annual Symposium. Nottingham University Press; Nottingham, UK: 1998. [Google Scholar]
- 87.Wild C.P., Miller J.D., Groopman J.D. Mycotoxin Control in Low-and Middle-Income Countries. Vol. 9. International Agency for Research on Cancer; Lyon, France: 2015. [PubMed] [Google Scholar]
- 88.Liverpool-Tasie L.S.O., Turna N.S., Ademola O., Obadina A., Wu F. The Occurrence and Co-occurrence of Aflatoxin and Fumonisin along the Maize Value Chain in Southwest Nigeria. Food Chem. Toxicol. 2019;129:458–465. doi: 10.1016/j.fct.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Mahuku G., Nzioki H.S., Mutegi C., Kanampiu F., Narrod C., Makumbi D. Pre-Harvest Management Is a Critical Practice for Minimizing Aflatoxin Contamination of Maize. Food Control. 2019;96:219–226. doi: 10.1016/j.foodcont.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sserumaga J.P., Ortega-Beltran A., Wagacha J.M., Mutegi C.K., Bandyopadhyay R. Aflatoxin-Producing Fungi Associated with Pre-Harvest Maize Contamination in Uganda. Int. J. Food Microbiol. 2020;313:1–8. doi: 10.1016/j.ijfoodmicro.2019.108376. [DOI] [PubMed] [Google Scholar]
- 91.Kamika I., Ngboula K.-T.-N., Tekere M. Occurrence of Aflatoxin Contamination in Maize throughout the Supply Chain in the Democratic Republic of Congo. Food Control. 2016;69:292–296. doi: 10.1016/j.foodcont.2016.05.014. [DOI] [Google Scholar]
- 92.Dadzie M.A., Oppong A., Ofori K., Eleblu J.S., Ifie E.B., Blay E., Obeng–Bio E., Appiah-Kubi Z., Warburton M.L. Distribution of Aspergillus Flavus and Aflatoxin Accumulation in Stored Maize Grains across Three Agro-Ecologies in Ghana. Food Control. 2019;104:91–98. doi: 10.1016/j.foodcont.2019.04.035. [DOI] [Google Scholar]
- 93.Kachapulula P.W., Akello J., Bandyopadhyay R., Cotty P.J. Aflatoxin Contamination of Groundnut and Maize in Zambia: Observed and Potential Concentrations. J. Appl. Microbiol. 2017;122:1471–1482. doi: 10.1111/jam.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardona T., Ilangantileke S., Noomhorm A. Aflatoxin research on grain in Asia: Its problems and possible solutions. In: Semple R.L., Frio A.S., Hicks P.A., Lozare J.V., editors. Mycotoxin Prevention and Control in Foodgrains. FAO; Bankok, Thailand: 1991. pp. 309–322. [Google Scholar]
- 95.Bilgrami K.S., Sinha K.K. Aflatoxin in India I. In: Zuber M.S., Lillehoj E.B., Renfro B.L., editors. Aflatoxin in Maize. CIMMYT, UNDP and USAID; El Batan, Mexico: 1986. pp. 349–358. [Google Scholar]
- 96.Do T.H., Tran S.C., Le C.D., Nguyen H.-B.T., Le P.-T.T., Le H.-H.T., Le T.D., Thai-Nguyen H.-T. Dietary Exposure and Health Risk Characterization of Aflatoxin B1, Ochratoxin A, Fumonisin B1, and Zearalenone in Food from Different Provinces in Northern Vietnam. Food Control. 2020;112:107108. doi: 10.1016/j.foodcont.2020.107108. [DOI] [Google Scholar]
- 97.Ali N. Aflatoxins in Rice: Worldwide Occurrence and Public Health Perspectives. Toxicol. Rep. 2019;6:1188–1197. doi: 10.1016/j.toxrep.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.FAOSTAT Food and Agriculture Organization of the United Nations. [(accessed on 25 February 2020)]; Available online: http://www.fao.org/faostat/en/#data/QC/visualize.
- 99.Toteja G.S., Mukherjee A., Diwakar S., Singh P., Saxena B.N., Sinha K.K., Sinha A.K., Kumar N., Nagaraja K.V., Bai G. Aflatoxin B1 Contamination of Parboiled Rice Samples Collected from Different States of India: A Multi-Centre Study. Food Addit. Contam. 2006;23:411–414. doi: 10.1080/02652030500442490. [DOI] [PubMed] [Google Scholar]
- 100.Bandara J., Vithanege A.K., Bean G.A. Occurrence of Aflatoxins in Parboiled Rice in Sri Lanka. Mycopathologia. 1991;116:65–70. doi: 10.1007/BF00436366. [DOI] [PubMed] [Google Scholar]
- 101.Panrapee I., Phakpoom K., Thanapoom M., Nampeung A., Warapa M. Exposure to Aflatoxin B1 in Thailand by Consumption of Brown and Color Rice. Mycotoxin Res. 2016;32:19–25. doi: 10.1007/s12550-015-0236-4. [DOI] [PubMed] [Google Scholar]
- 102.Abdullah N., Nawawi A., Othman I. Survey of Fungal Counts and Natural Occurrence of Aflatoxins in Malaysian Starch-Based Foods. Mycopathologia. 1998;143:53–58. doi: 10.1023/A:1006945514876. [DOI] [PubMed] [Google Scholar]
- 103.Aydin A., Aksu H., Gunsen U. Mycotoxin Levels and Incidence of Mould in Turkish Rice. Environ. Monit. Assess. 2011;178:271–280. doi: 10.1007/s10661-010-1688-9. [DOI] [PubMed] [Google Scholar]
- 104.Lai X., Liu R., Ruan C., Zhang H., Liu C. Occurrence of Aflatoxins and Ochratoxin A in Rice Samples from Six Provinces in China. Food Control. 2015;50:401–404. doi: 10.1016/j.foodcont.2014.09.029. [DOI] [Google Scholar]
- 105.Nuss E.T., Tanumihardjo S.A. Maize: A Paramount Staple Crop in the Context of Global Nutrition. Compr. Rev. Food Sci. Food Saf. 2010;9:417–436. doi: 10.1111/j.1541-4337.2010.00117.x. [DOI] [PubMed] [Google Scholar]
- 106.Torres O., Matute J., Gelineau-van Waes J., Maddox J.R., Gregory S.G., Ashley-Koch A.E., Showker J.L., Voss K.A., Riley R.T. Human Health Implications from Co-exposure to Aflatoxins and Fumonisins in Maize-Based Foods in Latin America: Guatemala as a Case Study. World Mycotoxin J. 2015;8:143–159. doi: 10.3920/WMJ2014.1736. [DOI] [Google Scholar]
- 107.Sandoval I.G., Wesseling S., Rietjens I.M. Aflatoxin B1 in Nixtamalized Maize in Mexico; Occurrence and Accompanying Risk Assessment. Toxicol. Rep. 2019;6:1135–1142. doi: 10.1016/j.toxrep.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aristil J., Venturini G., Maddalena G., Toffolatti S.L., Spada A. Fungal Contamination and Aflatoxin Content of Maize, Moringa and Peanut Foods from Rural Subsistence Farms in South Haiti. J. Stored Prod. Res. 2020;85:1–8. doi: 10.1016/j.jspr.2019.101550. [DOI] [Google Scholar]
- 109.Oliveira M.S., Rocha A., Sulyok M., Krska R., Mallmann C.A. Natural Mycotoxin Contamination of Maize (Zea Mays L.) in the South Region of Brazil. Food Control. 2017;73:127–132. doi: 10.1016/j.foodcont.2016.07.033. [DOI] [Google Scholar]
- 110.Zuki-Orozco B.A., Batres-Esquivel L.E., Ortiz-Pérez M.D., Juárez-Flores B.I., Díaz-Barriga F. Aflatoxins Contamination in Maize Products from Rural Communities in San Luis Potosi, Mexico. Ann. Glob. Health. 2018;84:300–305. doi: 10.29024/aogh.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morales-Moo T., Hernández-Camarillo E., Carvajal-Moreno M., Vargas-Ortiz M., Robles-Olvera V., Salgado-Cervantes M.A. Human Health Risk Associated with the Consumption of Aflatoxins in Popcorn. Risk Manag. Healthc. Policy. 2020;13:2583–2591. doi: 10.2147/RMHP.S274767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Foerster C., Muñoz K., Delgado-Rivera L., Rivera A., Cortés S., Müller A., Arriagada G., Ferreccio C., Rios G. Occurrence of Relevant Mycotoxins in Food Commodities Consumed in Chile. Mycotoxin Res. 2020;36:63–72. doi: 10.1007/s12550-019-00369-5. [DOI] [PubMed] [Google Scholar]
- 113.Katsurayama A.M., Martins L.M., Iamanaka B.T., Fungaro M.H.P., Silva J.J., Frisvad J.C., Pitt J.I., Taniwaki M.H. Occurrence of Aspergillus Section Flavi and Aflatoxins in Brazilian Rice: From Field to Market. Int. J. Food Microbiol. 2018;266:213–221. doi: 10.1016/j.ijfoodmicro.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 114.Blaney B.J. Mycotoxin Surveillance in Australia. J. Appl. Toxicol. 1982;2:83–87. doi: 10.1002/jat.2550020206. [DOI] [Google Scholar]
- 115.Pitt J.I., Hocking A.D. Mycotoxins in Australia: Biocontrol of Aflatoxin in Peanuts. Mycopathologia. 2006;162:233–243. doi: 10.1007/s11046-006-0059-0. [DOI] [PubMed] [Google Scholar]
- 116.Blaney B.J., Ramsey M.D., Tyler A.L. Mycotoxins and Toxigenic Fungi in Insect-Damaged Maize Harvested during 1983 in Far North Queensland. Aust. J. Agric. Res. 1986;37:235–244. doi: 10.1071/AR9860235. [DOI] [Google Scholar]
- 117.Blaney B.J., K’Keeffe K., Bricknell L.K. Managing Mycotoxins in Maize: Case Studies. Aust. J. Exp. Agric. 2008;48:351–357. doi: 10.1071/EA06095. [DOI] [Google Scholar]
- 118.Blaney B.J. Aflatoxin Survey of Maize from the 1978 Crop in the South Burnett Region of Queensland [Aspergillus Fungal Infection; Stock Feed] Qld. J. Agric. Animal. Sci. 1981;38:7–12. [Google Scholar]
- 119.Blaney B.J. Mycotoxins in crops grown in different climatic regions of Queensland. In: Lacey J., editor. Trichothecenes and Other Mycotoxins. John Wiley and Sons Ltd.; Chichester, UK: 1985. pp. 97–108. [Google Scholar]
- 120.Chauhan Y., Wright G., Rachaputi N.C. Modelling Climatic Risks of Aflatoxin Contamination in Maize. Aust. J. Exp. Agric. 2008;48:358–366. doi: 10.1071/EA06101. [DOI] [Google Scholar]
- 121.Kennedy I.R. ((Univesrity of Sydney, Sydney, Australia)). Personal communication. 2017.
- 122.Mazumder P.M., Sasmal D. Mycotoxins–Limits and Regulations. Anc. Sci. Life. 2001;20:1–19. [PMC free article] [PubMed] [Google Scholar]
- 123.Food and Agriculture Organization . Worldwide Regulations for Mycotoxins in Food and Feed in 2003. Food and Agriculture Organization of the United Nations; Roma, Italy: 2004. [Google Scholar]
- 124.Blanc M. Sampling: The Weak Link in the Sanitary Quality Control System of Agricultural Products. Mol. Nutr. Food Res. 2006;50:473–479. doi: 10.1002/mnfr.200600009. [DOI] [PubMed] [Google Scholar]
- 125.Codex Alimentarius Commission . Codex Alimentarius: General Standard for Contaminants and Toxins in Food and Feed (CXS 193-1995) Food and Agriculture Organization/World Health Organization; Geneva, Switzerland: 2019. Mycotoxins; pp. 13–44. [Google Scholar]
- 126.European Commission Commission Regulation (EU) No. 165/2010 of 26 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. Off. J. Eur. Union. 2010;50:8–12. [Google Scholar]
- 127.Anukul N., Vangnai K., Mahakarnchanakul W. Significance of Regulation Limits in Mycotoxin Contamination in Asia and Risk Management Programs at the National Level. J. Food Drug. Anal. 2013;21:227–241. doi: 10.1016/j.jfda.2013.07.009. [DOI] [Google Scholar]
- 128.United States Department of Agriculture China Releases Standard for Maximum Levels of Mycotoxins in Foods. [(accessed on 10 February 2020)]; Available online: https://gain.fas.usda.gov/Recent%20GAIN%20Publications/China%20Releases%20Standard%20for%20Maximum%20Levels%20of%20Mycotoxins%20in%20Foods%20_Beijing_China%20-%20Peoples%20Republic%20of_5-9-2018.pdf.
- 129.Mohd-Redzwan S., Jamaluddin R., Abd-Mutalib M.S., Ahmad Z. A Mini Review on Aflatoxin Exposure in Malaysia: Past, Present and Future. Front. Microbiol. 2013;4:1–8. doi: 10.3389/fmicb.2013.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.US Food and Drug Administration CPG Sec. 570.200 Brazil Nuts-Adulteration with Aflatoxin. [(accessed on 9 February 2020)]; Available online: https://www.fda.gov/media/72053/download.
- 131.US Food and Drug Administration CPG Sec. 555.400 Foods-Adulteration with Aflatoxin. [(accessed on 9 February 2020)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-555400-foods-adulteration-aflatoxin.
- 132.US Food and Drug Administration CPG Sec. 570.375 Aflatoxin in Peanuts and Peanut Products. [(accessed on 9 February 2020)]; Available online: https://www.fda.gov/media/72073/download.
- 133.US Food and Drug Administration CPG Sec. 570.500 Pistachio Nuts-Aflatoxin Adulteration. [(accessed on 9 February 2020)]; Available online: https://www.fda.gov/media/72084/download.
- 134.Ncube J., Maphosa M. Current State of Knowledge on Groundnut Aflatoxins and Their Management from a Plant Breeding Perspective: Lessons for Africa. Sci. Afr. 2020;7:1–7. doi: 10.1016/j.sciaf.2020.e00264. [DOI] [Google Scholar]
- 135.Ministério da Saúde/Agência Nacional de Vigilância Sanitária Resolução RDC nº 7, de 18 de fevereiro de 2011. [(accessed on 25 February 2020)]; Available online: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0007_18_02_2011_rep.html.
- 136.Government of Canada List of Contaminants and Other Adulterating Substances in Foods. [(accessed on 25 February 2020)]; Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/contaminants-adulterating-substances-foods.html.
- 137.Australia Government New Zealand Food Standards Code–Schedule 19–Maximum Levels of Contaminants and Natural Toxicants. [(accessed on 25 February 2020)]; Available online: https://www.legislation.gov.au/Details/F2017C00333.
- 138.US Food and Drug Administration CPG Sec. 527.400 Whole Milk, Lowfat Milk, Skim Milk-Aflatoxin M1. [(accessed on 9 February 2020)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-527400-whole-milk-lowfat-milk-skim-milk-aflatoxin-m1.
- 139.Ministry of Food and Drug Safety Food Code. [(accessed on 10 February 2020)]; Available online: https://www.mfds.go.kr/eng/brd/m_15/view.do?seq=69982.
- 140.Food and Agriculture Organization Preventing Food Safety Emergencies: INFOSAN, FAO/WHO International Food Safety Authorities Network. [(accessed on 29 May 2020)]; Available online: http://www.fao.org/3/a-i8024e.pdf.
- 141.Savelli C.J., Bradshaw A., Ben Embarek P., Mateus C. The FAO/WHO International Food Safety Authorities Network in Review, 2004-2018: Learning from the Past and Looking to the Future. Foodborne Pathog. Dis. 2019;16:480–488. doi: 10.1089/fpd.2018.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.International Food Safety Authorities Network . INFOSAN Activity Report 2016/2017. World Health Organization and Food and Agriculture Organization of the United Nations; Geneva, Switzerland: 2018. [Google Scholar]
- 143.Pigłowski M. Food Hazards on the European Union Market: The Data Analysis of the Rapid Alert System for Food and Feed. Food Sci. Nutr. 2020;8:1603–1627. doi: 10.1002/fsn3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rapid Alert System for Food and Feed Portal Database. [(accessed on 9 February 2020)]; Available online: https://webgate.ec.europa.eu/rasff-window/portal/
- 145.World Health Organization Aflatoxins. [(accessed on 9 February 2020)]; Available online: https://www.who.int/foodsafety/FSDigest_Aflatoxins_EN.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.