Abstract
Simple Summary
Members of the genus Sarcocystis are worldwide distributed protozoan parasites. Sarcocystis infections cause great losses in economically important animals. There is a lack of studies on Sarcocystis in naturally infected wild predators, especially of the family Mustelidae which represent a presumably important group of definitive hosts of these parasites. The objective of the present study was to examine the small intestine samples of various mustelid species from Lithuania serving as a possible source of Sarcocystis spp. using cattle as intermediate hosts. Overall, 84 samples collected from five mustelid species were analyzed. Oocysts/sporocysts of Sarcocystis spp. were detected in 75 animals (89.3%). Using molecular methods four Sarcocystis spp., S. bovifelis, S. cruzi, S. hirsuta and S. hominis were identified, with the first two being the most prevalent. These results indicate that mustelids are involved in the transmission of Sarcocystis spp. using cattle as intermediate hosts. The determined high prevalence of Sarcocystis spp. rates cause concerns about food safety issues. To control the spread of infection, further studies on the way carcasses of cattle or beef waste become accessible to mustelids are needed.
Abstract
There is a lack of research on the role of mustelids in the transmission of various Sarcocystis spp. In the present study we tested the hypothesis that widespread mustelids in Lithuania could be involved in the transmission of Sarcocystis spp. using cattle as intermediate hosts. In 2016–2020, intestinal samples of 84 mustelids were examined. Sarcocystis spp. were identified by species-specific PCR targeting the cox1 gene and subsequent sequencing. Under a light microscope, oocysts/sporocysts of Sarcocystis spp. were observed in 40 samples (47.6%), while using molecular methods, they were detected in 75 animals (89.3%). Four Sarcocystis spp. were identified in the intestinal samples of American mink (Neovison vison), Beech marten (Martes foina), European pine marten (Martes martes), European badger (Meles meles) and European polecat (Mustela putorius). The prevalence of predominant Sarcocystis spp., S. bovifelis (89.3%) and S. cruzi (73.8%) was significantly higher than that of S. hirsuta (3.6%) and S. hominis (1.2%). In an individual sample, most frequently two Sarcocystis spp. were identified (69.0%), then a single species (15.5%) and three species (4.8%). The present study provides strong evidence that mustelids serve as definitive hosts for Sarcocystis spp. using cattle as intermediate hosts.
Keywords: Sarcocystis, cattle, mustelidae, life cycle, cox1, molecular identification
1. Introduction
Representatives of the genus Sarcocystis (Apicomplexa: Sarcocystidae) are cyst forming coccidians with an obligatory prey-predator two-host life cycle. Asexual multiplication with the formation of sarcocysts takes place in the extra-intestinal tissues of the intermediate host (IH), while sexual stages (oocysts-sporocysts) develop in the small intestine of the definitive host (DH) [1]. Predators and scavengers serve as DH for Sarcocystis spp., whereas prey animals become IH [2].
Members of the family Mustelidae may act as IH or DH for several Sarcocystis spp. The agent of equine protozoal myeloencephalitis, S. neurona was also detected in the muscles of a fisher (Martes pennanti), ferret (Mustela putorius furo) and American mink (Neovison vison) [3]. Additionally, eight species of Sarcocystis have been observed in the muscles of various mustelids [4]. Recently described S. lutrae [5] was identified in the muscles of several Carnivora families, Canidae, Mustelidae and Procyonidae [5,6,7]. The role of mustelids as DH of Sarcocystis spp. has not been investigated [8].
Mustelidae is the largest and most diverse family in the order of Carnivora in Lithuania, with nine species present [9]. Representatives of mustelids occur in all habitats, including the urban ones [10,11]. The broad habitat niches of the American mink, the Beech marten (Martes foina), European badger (Meles meles), European pine marten (Martes martes) and European polecat (Mustela putorius) are reflected in their diverse diets [10,11]. In general, members of the family Mustelidae are opportunistic predators and their diet consists of birds, various mammals, fish, amphibians, invertebrates, fruits, ungulate carcasses, plants and mushrooms [12,13,14,15,16]. In Lithuania, the food chains of mustelids, including cattle carrion, were not investigated in detail, with exception of the European pine marten [17]. Diet of this species in the cold period included 5.3% of carcasses of domestic animals according to the biomass consumed. Thus, far no studies on the role of mustelids in the transmission of Sarcocystis in Lithuania have been undertaken.
Recently, a high prevalence of Sarcocystis spp. in cattle from Lithuania has been recorded [18]. By performing trypsinization of the diaphragm muscles and species-specific PCR targeting the cox1 (mitochondrial gene encoding subunit 1 of cytochrome c oxidase), S. cruzi was identified in 96.1% of the samples, S. bovifelis was detected in 71.6% of the samples, S. hirsuta was confirmed in 30.4% of the samples and S. hominis was observed in 13.7% of the samples [19]. Canids are DH for S. cruzi, humans are DH for S. hominis, whereas S. hirsuta and S. bovifelis are transmitted via felids [19]. The Eurasian lynx (Lynx lynx) is the only wild member of the felids in Lithuania [9]. However, this species is not abundant and there were approximately 160 lynx individuals in Lithuania in 2018 [20]. Thus, the high prevalence of S. bovifelis implies that it is not solely felids that contribute to the spread of this species. Therefore, we put forward the hypothesis that mustelids can act as DH of S. bovifelis. In order to test the assumption, the aim of the present study was to examine the small intestines of various mustelids from Lithuania for the presence of Sarcocystis spp. employing cattle as IH.
2. Materials and Methods
2.1. Sample Collection
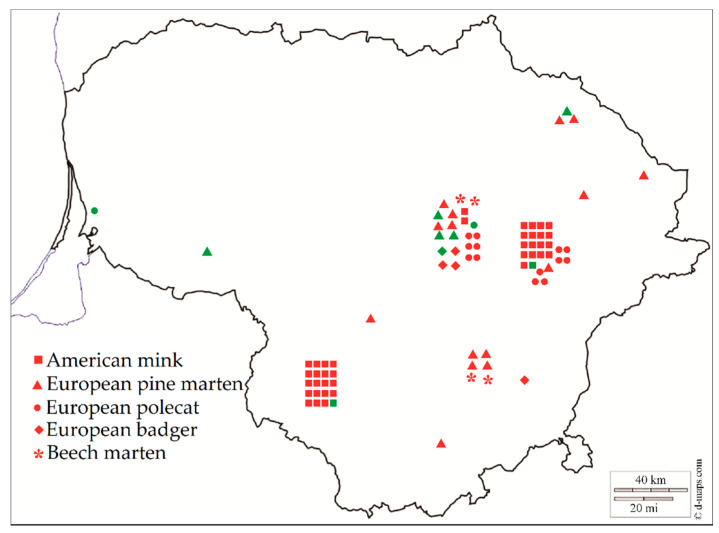
Between 2016 and 2020, intestine samples of 84 mustelids (40 American mink, 4 Beech marten, 5 European badger, 20 European pine marten and 15 European polecat) were studied for the presence of Sarcocystis spp. The animals were collected from hunters, taxidermists, or biologists who found dead animals on the roadways in different regions of Lithuania (Figure 1).
Figure 1.
Sarcocystis spp. in the species of Mustelidae in Lithuania. Red color means positive individuals, green color represents negative individuals.
2.2. Examination of Intestines
Oocysts/sporocysts of Sarcocystis spp. were excreted from the entire intestine of each mustelids using a slightly modified Verma et al. [21] technique. At first, faeces of each intestine were squeezed and the entire intestine was cut lengthwise. The intestinal epithelium was lightly scraped with the help of a scalpel blade and suspended in 50 mL of water. The homogenate was centrifuged for 10 min at 1000 rpm, 25 °C in 50 mL centrifuge tubes. The supernatant was discarded and sediments were re-suspended in 50 mL water. Subsequently, the homogenate was centrifuged for 10 min at 1000 rpm, 25 °C and the supernatant was discarded. The examination of the sediments for oocysts/sporocysts under a light microscope was repeated. The 200 μL of re-suspended sediments were taken from each sample and used for DNA extraction. DNA was isolated from all mustelid samples.
2.3. Molecular Analysis
DNA extraction from mucosal suspension was performed using the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania). Sarcocystis spp. were identified by nested PCR of partial cox1 sequences. Primers used in the present study are listed in Table 1. PCRs were conducted in the final volume of 25 μL made of 12.5 μL of DreamTaq PCR Master Mix (Thermo Fisher Scientific, Vilnius, Lithuania), 0.5 μM of each primer, 0.04 μg template DNA and nuclease-free water. The first run of nested PCR began with one cycle at 95 °C for 5 min followed by 35 cycles of 94 °C for 45 s, 58–60 °C, depending on primer pair for 60 s and 72 °C for 80 s and ending with one cycle at 72 °C for 7 min. For the second PCR assay, 1 μL from the first PCR assay was used. Visualization, purification and sequencing of PCR products were carried out using a previously described protocol [22]. The obtained cox1 sequences were compared with the Nucleotide BLAST program (megablast option) [23]. The cox1 sequences generated in the present study are available in GenBank with Acc. No. MW595468–MW595608.
Table 1.
The primers used for the nested PCR.
| Species | Primer Name | Primer Sequence | Orientation | The Run of Nested PCR |
|---|---|---|---|---|
| S. bovifelis | SF1 1 | ATGGCGTACAACAATCATAAAGAA | Forward | First |
| SkatR | CAGGCTGAACAGHABTACGA | Reverse | First | |
| V2bo3 | ATATTTACCGGTGCCGTACTTATGTT | Forward | Second | |
| V2bo4 | GCCACATCATTGGTGCTTAGTCT | Reverse | Second | |
| S. cruzi | SF1 1 | ATGGCGTACAACAATCATAAAGAA | Forward | First |
| SsunR2 | GTGCCTCCCAGGCTGAAYAG | Reverse | First | |
| GsScruF | TGTATCTACTTACGGCAGGTATCTTT | Forward | Second | |
| GsScruR | CGTAGTTAGATCCATATCACTCGGTA | Reverse | Second | |
| S. hirsuta | SF1 1 | ATGGCGTACAACAATCATAAAGAA | Forward | First |
| SkatR | CAGGCTGAACAGHABTACGA | Reverse | First | |
| GaHiEF 2 | GTTGTGCGGTATGAATTATCAACCT | Forward | Second | |
| GaHiER 2 | GGTAAGAACTGGAATGGTTAATATCAG | Reverse | Second | |
| S. hominis | VohoF | GTGCGGTATGAACTGTCTACTGCT | Forward | First |
| VohoR | AATACCTGCCCGGCCTTAAC | Reverse | First | |
| GaHoEF 2 | TCTCTGGTTTTGGTAACTACTTCGT | Forward | Second | |
| GaHoER 2 | CAGACACTGGGATATAATACCGAAC | Reverse | Second |
2.4. Statistical Tests
The prevalence and 95% CI for prevalence were calculated using OpenEpi epidemiological software [25], following the Wilson method for calculating score interval [26]. Differences in the prevalence of the identified Sarcocystis spp. were evaluated using the Chi-squared test, calculated in WinPepi, ver. 11.39 and using Upton’s approximation for small and medium sample sizes [27]. Comparing the prevalence of Sarcocystis spp., the effect size was expressed according to adjusted Cohen’s w [28].
3. Results
3.1. Differences in Prevalence of Sarcocystis spp. Using Microscopic and Molecular Methods
Based on microscopic examination, the prevalence of Sarcocystis spp. in mucosal scrapings was 47.6% (Table 2). Under a light microscope usually free sporocysts measuring 11.8 × 8.3 µm (7.1–14.5 × 6.5–10.9 μm; n = 219) were seen. Sporulated oocysts of Sarcocystis 17.7 × 13.1 µm (12.5–23.7 × 10.5–18.3 μm; n = 100) were also noticed. With the help of nested PCR and subsequent sequencing Sarcocystis spp. were confirmed in 75 animals (89.3%). In general, as compared with morphological examination, the detection rate of Sarcocystis spp. was significantly higher (χ2 = 33.56, p < 0.0001; adjusted Cohen’s w = 0.709, large effect size) when a molecular method was employed. The molecular method yielded significantly more detections in the American mink, European polecat and European badger (Cohen’s w = 1.083, 0.606 and 1.061, respectively, large effect size). Differences between the two methods in the Beech marten and European pine marten were not significant (Table 2). In one American mink and three Beech marten samples, oocysts/sporocysts were detected microscopically, however, these samples were negative for the examined Sarcocystis spp. using a molecular analysis.
Table 2.
Identification of Sarcocystis spp. oocysts/sporocysts in mustelids using microscopic and molecular examination.
| Host Species | N | Sarcocystis spp. Positive Animals | |||||
|---|---|---|---|---|---|---|---|
| Microscopic Analysis | Molecular Analysis | ||||||
| n | % | 95% CI | N | % | 95% CI | ||
| American mink | 40 | 15 | 37.5 | 24.2–53.0 | 38 | 95.0 *** | 83.5–98.6 |
| Beech marten | 4 | 3 | 75.0 | 30.1–95.4 | 4 | 100 NS | 51.0–100.0 |
| European pine marten | 20 | 12 | 60.0 | 38.7–78.1 | 15 | 75.0 NS | 53.1–88.8 |
| European badger | 5 | 1 | 20.0 | 36.2–62.5 | 4 | 80.0 * | 37.6–96.4 |
| European polecat | 15 | 9 | 60.0 | 35.8–80.2 | 14 | 93.3 ** | 70.2–98.8 |
| Total | 84 | 40 | 47.6 | 37.3–58.2 | 75 | 89.3 *** | 80.9–94.34 |
Significance of differences between methods is shown in superscript: * p < 0.05, ** p < 0.01, *** p < 0.0001, NS not significant.
Based on molecular analysis, the highest prevalence of Sarcocystis spp. was observed in the Beech marten, followed by the American mink and European polecat; however, even the lowest prevalence of Sarcocystis spp. detected in the European badger and European pine marten were 75% and higher (Table 2). The prevalence of Sarcocystis spp. observed in the Beech marten, American mink and European polecat did not differ statistically (species cluster with the highest prevalence). The prevalence of Sarcocystis spp. observed in the American mink was significantly higher (χ2 = 5.09, p < 0.025; Cohen’s w = 0.435, medium effect size) than that detected in the European pine marten. Other differences were not significant and the effect size was either small or absent.
3.2. Molecular Identification of Sarcocystis spp.
The comparison of sequences generated in the present study showed the presence of four Sarcocystis spp. (S. bovifelis, S. cruzi, S. hirsuta and S. hominis) in the analyzed samples of Mustelidae (Table 3).
Table 3.
Intra- and inter-specific genetic variability of identified Sarcocystis spp.
| Sarcocystis spp. | GenBank Accession No. (Length in bp) |
Sequence Similarity (%) | ||
|---|---|---|---|---|
| Comparing Obtained sequences | Comparing Isolates of the Same Species | Comparing Isolates with Other Closely Related Species |
||
| S. bovifelis | MW595468–MW595542 (361) | 98.4–100 | 97.2–100% S. bovifelis (KT900961–KT900998, KC209690–KC209696, MK962347–MK962348, MT796903–MT796925) |
92.5–94.5% S. bovini (KT900999–KT901022, LC171858) |
| S. cruzi | MW595543–MW595604 (556) | 98.2–100 | 96.0–100% S. cruzi (KC209597–KC209600, KT901078–KT901095, LC171859–LC171862, MG787071–MG787076, MT796926–MT796945) | 90.8–93.4% S. pilosa (KU753903–KU753910, LC349942, LC349966–LC349967, LC466196–LC466201, LC481077–LC481081, LC496070, MT070670– MT070677) |
| S. hirsuta | MW595605–MW595607 (461) | 98.9–99.8 | 98.9–99.8% S. hirsuta (KC209634, KT901023–KT901077, LC171863, MT796946–MT796951, MT796958–MT7969) | 95.6–96.3% S. buffalonis (KU247868–KU247873, MG792800–MG792802) |
| S. hominis | MW595608 (501) | - | 97.6–99.0% S. hominis (MH021119, MK497840–MK497843, MT796961–MT796964) | 87.1–87.8% S. bovifelis |
3.3. Distribution of Sarcocystis spp. in the Intestine Samples of Mustelids
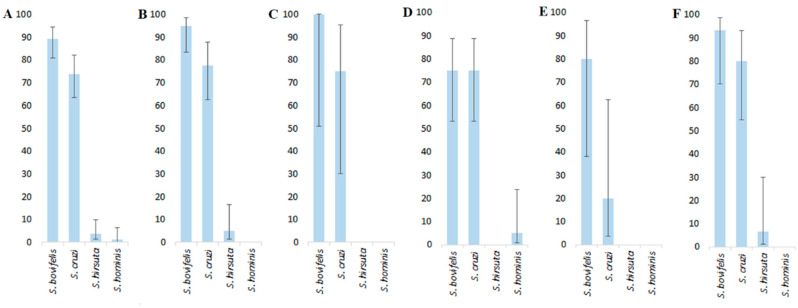
Irrespective of the host species, S. bovifelis in the examined samples was identified most often (Figure 2A). The prevalence of S. bovifelis (89.3%) was significantly higher than that of S. cruzi (73.8%, a small effect size), S. hirsuta (3.6%, a large effect size) and S. hominis (1.2%, a large effect size). The prevalence of S. cruzi was significantly higher than that of S. hirsuta (3.6%) and S. hominis (a large effect size both).
Figure 2.
Prevalence of Sarcocystis spp. in the examined samples of mustelids. (A)—in the pooled sample of all host species, (B)—in American mink, (C)—in Beech marten, (D)—in European pine marten, (E)—in European badger, (F)—in European polecat. Differences of prevalence in A: S. bovifelis > S. cruzi (χ2 = 6.65, p < 0.01; Cohen’s w = 0.288), >S. hirsuta (χ2 = 123.32, p < 0.001; w = 2.376) and >S. hominis (χ2 = 130.79, p < 0.001; w = 2.688); S. cruzi > S. hirsuta (3.6%, χ2 = 86.83, p < 0.001; w = 1.472) and >S. hominis (χ2 = 93.94, p < 0.001; w = 1.604); in B: S. bovifelis >S. cruzi (χ2 = 5.10, p < 0.025; w = 0.372); in E: S. bovifelis > S. cruzi (χ2 = 3.24, p < 0.075; w = 1.064).
The prevalence of S. bovifelis was the highest, exceeding that of S. cruzi in the examined samples of the American mink (a medium effect size, Figure 2B) and European badger (a large effect size, Figure 2E). The prevalence of S. bovifelis and S. cruzi did not differ significantly in European polecat (Figure 2F) and Beech marten (Figure 2C); in European pine marten they were equal (Figure 2D). The prevalence of predominant Sarcocystis spp., S. bovifelis and S. cruzi, was significantly higher than that of S. hirsuta and S. hominis, in all host species (Figure 2B–F). Both predominant species were observed in all five examined host species. Sarcocystis hirsuta was identified in two American mink individuals and one European polecat individual; whereas S. hominis was confirmed in one European pine marten individual.
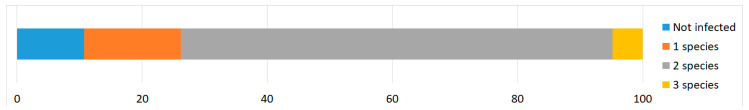
Up to three Sarcocystis spp. were identified in one host individual (Figure 3). No examined Sarcocystis spp. were found in approximately one tenth of the investigated animals (10.7%). The prevalence of single species infections was 15.5%; in all cases when a single species was detected in individual samples, it was S. bovifelis. Two Sarcocystis spp. (69.0%) were most frequently identified in one host individual and in all such cases it was S. cruzi/S. bovifelis co-infection. Three Sarcocystis spp. were confirmed in four animals (4.8%), one European polecat individual, one European pine marten individual and two American minks. In three of these cases, it was S. bovifelis/S. cruzi/S. hirsuta co-infection, in one case—S. bovifelis/S. cruzi/S. hominis co-infection.
Figure 3.
Distribution of the number of Sarcocystis spp. identified in the examined samples of mustelids.
4. Discussion
In the present study, high rates (89.3%) of Sarcocystis spp. employing cattle as IH were observed in mustelids from Lithuania. Under a light microscope oocysts/sporocysts were detected in 40 out of 84 samples (47.6%). In comparison, the presence of Sarcocystis spp. in 75 (89.3%) mucosal scrapings of mustelids were confirmed by molecular methods. Usually, molecular analysis is performed when oocysts/sporocysts of Sarcocystis spp. are microscopically detected in intestine mucosal or faecal samples [2,29,30,31]. However, the results of the present study reveal that molecular methods should be applied in testing all examined samples rather than only microscopically positive ones. No Sarcocystis spp. were identified in the mucosal scrapings of a single American mink and three European pine martens using species-specific PCR; however, oocysts/sporocysts were detected in these samples under a light microscope. Thus, these animals were most likely infected with oocysts/sporocysts of Sarcocystis spp., which employ other than cattle IH. There are a few reports on mustelids as DH for Sarcocystis spp. Transmission experiments have shown that mustelids are DH of several Sarcocystis spp., S. campestris, S. muris, S. putorii, S. undulati and S. citellivulpes (invalid species by Dubey [1]) using members of the order Rodentia as IH [8]. Further studies are needed to reveal the role of mustelids in the transmission of Sarcocystis spp. using various mammals and birds as IH.
Sarcocystis spp. identified in the present study, namely, S. bovifelis, S. cruzi, S. hirsuta and S. hominis, are specific to their IH [32]. Molecular data suggest that S. cruzi might occasionally infect water buffaloes (Bubalus bubalis) [33]. However, sheep, goats, pigs, horses and other domestic animals raised in Lithuania cannot serve as IH of the above-mentioned Sarcocystis spp. [1]. Of the Lithuanian wild fauna, only the European bison (Bison bonasus) can possibly act as an IH of some Sarcocystis spp. detected in this study [34,35,36]. However, the B. bonasus population in Lithuania is not large, it stands at less than 300 individuals and their distribution range does not intersect with the sites of our material on mustelids [9,10,11]. Therefore, it is impossible for B. bonasus to be responsible for the high rates of S. bovifelis and S. cruzi in the intestinal samples of mustelids.
The forest is considered a primary habitat of two mustelid species, European pine marten and European badger, though they are frequent visitors to the surrounding woodlots, meadows and riversides [9]. The habitat of the American mink is related to water—they inhabit banks of rivers, lakes and ponds. These mustelid species are not closely related to human settlements. Two other investigated mustelids, American mink and European polecat, are more often related to settlements than to other habitats, such as forests and shrubby areas [9]. Habitats preferred by mustelids in Lithuania are similar to those in other countries [37]. Diet peculiarities of the investigated mustelids are not directly related to the involvement of these species in the transmission of Sarcocystis spp. using cattle as IH. All the investigated mustelid species are opportunistic feeders. Among such diet sources as fruits, berries and other plant materials, invertebrates, fish, amphibians, birds and various mammals [12,13,14,15,16,17], only one source, namely, cattle carrion, or other sources of cattle meat may be related to Sarcocystis spp. we have identified. Mustelid species that we have investigated [12,13,14,15,16,17], with the exception of the American mink [38], use carrion of wild ungulates.
Cattle are too large prey for mustelids to hunt; therefore, mustelids become infected with S. bovifelis, S. cruzi, S. hirsuta and S. hominis species by scavenging carcasses of cattle. However, habitat distribution of the five investigated mustelid species in Lithuania (see above) should exclude contact with carrion of at least two species, American mink and European pine marten. Therefore, the first assumption about high rates of Sarcocystis spp. employing cattle as IH is related to food safety issues. In further studies we are going to examine in what way cattle carcasses or beef waste become accessible to mustelids in Lithuania. It is important to understand whether there are gaps in the management of anthropogenic carrion [39] and if this has already become a source of predictable resources accessible to mustelids. Improper carrion management may be related to (i) dumping sites, (ii) treatment of the waste from meat processing factories, especially small ones and located in the countryside and (iii) raw meat waste from homesteads and farms. The two last sources may be neighboring forests and water bodies, therefore becoming sources of possible infection and available even to the American mink and European pine marten, otherwise having no contact with cattle carrion.
Historically, the disclosure of DH of Sarcocystis spp. was performed by transmission experiments [40]. Among carnivorous mammals, transmission experiments of Sarcocystis spp. have mainly been carried out with dogs, foxes and cats [41,42]. Recently, molecular methods have been applied for the identification of Sarcocystis spp. from fecal or mucosal scraping samples of various wild predators or scavengers infected under natural conditions [2,29,30,31]. The present work is the first study of the molecular identification of Sarcocystis spp. in mustelids. Further molecular examination of oocysts/sporocysts detected in the intestine or fecal samples of mustelids can help to clarify the role of these carnivorous mammals in the transmission of Sarcocystis parasites.
It is well known that Sarcocystis spp. transmitted via canids cannot be spread via felids and vice versa [1]. However, there is a lack of data on whether Sarcocystis spp. transmitted via canids and/or felids can be spread via mustelids. It was demonstrated that mustelids and canids could serve as DH of S. undulati and S. citellivulpes [8,43], whereas mustelids and felids could act as DH for S. muris [8]. Two species, S. bovifelis (89.3%) and S. cruzi (73.8%), were most common in the analyzed intestinal samples of mustelids (Figure 2), whereas S. hirusta and S. hominis were confirmed in three and single samples, respectively. Canids serve as DH for S. cruzi, felids act as DH for S. hirsuta and S. bovifelis and humans are DH for S. hominis [19]. Thus, our results indicate that mustelids might be involved in the transmission of Sarcocystis spp. which were confirmed to be transmitted via canids and felids. Nevertheless, further detailed studies on this subject are required. Considering a low abundance of wild felids in Lithuania, we speculate that S. hirsuta is mainly transmitted via felids and S. bovifelis is mainly transmitted via mustelids. To test the hypothesis, the prevalence of S. hirsuta and S. bovifelis in muscles of cattle can be examined in European countries where wild felids are more prevalent. Estonia and Finland are the nearest countries with similar environments and with similar abundances of mustelids but with the high abundances of Eurasian lynx, while Germany or Belgium may be the reference countries with the European wildcat (Felis silvestris) populations [44].
5. Conclusions
Using a molecular analysis four Sarcocystis spp. employing cattle as IH (S. bovifelis, S. cruzi, S. hirsuta and S. hominis) were identified in the intestine mucosal scrapings of five Mustelidae species for the first time. Thus, the results of the present study indicate that a wide range of mustelids serve as DH of these Sarcocystis spp. Therefore, it is necessary to identify gaps in the management of cattle carrion and beef waste.
Acknowledgments
This study was supported by the Open Access research infrastructure of the Nature Research Centre under the Lithuanian open access network initiative. The authors are grateful to Valentinas Pabrinkis (Nature Research Centre, Vilnius, Lithuania) who provided samples for the study.
Author Contributions
Conceptualization, P.P. and D.B.; methodology, P.P.; software, L.B.; validation, D.B. and P.P.; formal analysis, L.B. and P.P.; investigation, E.J.-N.; resources D.B.; data curation, P.P.; writing—original draft preparation, P.P., L.B., E.J.-N. and D.B.; writing—review and editing, P.P., L.B., E.J.-N. and D.B.; visualization L.B. and E.J.-N.; supervision, P.P.; project administration, P.P. and D.B.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Research Council of Lithuania (grant number S-MIP-20-24).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data supporting the conclusions of this article are included in the article. The sequences generated in the present study were submitted to the GenBank database under accession numbers MW595468–MW595608.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dubey J.P., Calero-Bernal R., Rosenthal B.M., Speer C.A., Fayer R. Sarcocystosis of Animals and Humans. 2nd ed. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 2.Moré G., Maksimov A., Conraths F.J., Schares G. Molecular Identification of Sarcocystis spp. in Foxes (Vulpes vulpes) and Raccoon Dogs (Nyctereutes procyonoides) from Germany. Vet. Parasitol. 2016;220:9–14. doi: 10.1016/j.vetpar.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Britton A.P., Dubey J.P., Rosenthal B.M. Rhinitis and Disseminated Disease in a Ferret (Mustela putorius furo) Naturally Infected with Sarcocystis neurona. Vet. Parasitol. 2010;169:226–231. doi: 10.1016/j.vetpar.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Prakas P., Strazdaitė-Žielienė Ž., Rudaitytė-Lukošienė E., Servienė E., Butkauskas D. Molecular Identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) in Muscles of Five Species of the Family Mustelidae. Parasitol. Res. 2018;117:1989–1993. doi: 10.1007/s00436-018-5880-0. [DOI] [PubMed] [Google Scholar]
- 5.Gjerde B., Josefsen T.D. Molecular Characterisation of Sarcocystis lutrae n. sp. and Toxoplasma gondii from the Musculature of Two Eurasian Otters (Lutra lutra) in Norway. Parasitol. Res. 2015;114:873–886. doi: 10.1007/s00436-014-4251-8. [DOI] [PubMed] [Google Scholar]
- 6.Kirillova V., Prakas P., Calero-Bernal R., Gavarāne I., Fernández-García J.L., Martínez-González M., Rudaitytė-Lukošienė E., Martinez-Estellez M.A.H., Butkauskas D., Kirjušina M. Identification and Genetic Characterization of Sarcocystis arctica and Sarcocystis lutrae in Red Foxes (Vulpes vulpes) from Baltic States and Spain. Parasites Vectors. 2018;11:173. doi: 10.1186/s13071-018-2694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Máca O. Molecular Identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) from the Raccoon Dog, Nyctereutes procyonoides, and the Common Raccoon, Procyon lotor, in the Czech Republic. Parasites Vectors. 2020;13:231. doi: 10.1186/s13071-020-04108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odening K. The Present State of Species-Systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia) Syst. Parasitol. 1998;41:209–233. doi: 10.1023/A:1006090232343. [DOI] [Google Scholar]
- 9.Atlas of Lithuanian Mammals. [(accessed on 29 December 2020)]; Available online: https://gamtostyrimai.lt/lt/users/viewGroup/id.24/pageId.26.
- 10.Kontrimavičius V., Januškis V., Virbickas J., Augustauskas J., Eitminavičiūtė I., Kazlauskas R., Logminas V., Pileckis S., Prūsaitė J., Valenta V., et al. Lietuvos Fauna. Žinduoliai. Mokslas; Vilnius, Lithuania: 1988. [Google Scholar]
- 11.Balčiauskas L., Trakimas G., Juškaitis R., Ulevičius A., Balčiauskienė L. Atlas of Lithuanian Mammals, Amphibians and Reptiles. 2nd ed. Akstis; Vilnius, Lithuania: 1999. [Google Scholar]
- 12.Baghli A., Engel E., Verhagen R. Feeding Habits and Trophic Niche Overlap of Two Sympatric Mustelidae, the Polecat Mustela putorius and the Beech Marten Martes foina. Z. Jagdwiss. 2002;48:217–225. doi: 10.1007/BF02189692. [DOI] [Google Scholar]
- 13.Lanszki J., Heltai M. Feeding Habits of Sympatric Mustelids in an Agricultural Area of Hungary. Acta Zool. Acad. Sci. Hung. 2011;57:291–304. [Google Scholar]
- 14.Newman C., Zhou Y.B., Buesching C.D., Kaneko Y., Macdonald D.W. Contrasting Sociality in Two Widespread, Generalist, Mustelid Genera, Meles and Martes. Mammal Study. 2011;36:169–188. doi: 10.3106/041.036.0401. [DOI] [Google Scholar]
- 15.Malecha A.W., Antczak M. Diet of the European Polecat Mustela putorius in an Agricultural Area in Poland. J. Vertebr. Biol. 2013;62:48–53. doi: 10.25225/fozo.v62.i1.a7.2013. [DOI] [Google Scholar]
- 16.Nováková L., Vohralík V. Diet of Martes foina in Bohemia, Czech Republic (Carnivora: Mustelidae) Lynx New Ser. 2017;48:155–164. doi: 10.2478/lynx-2017-0009. [DOI] [Google Scholar]
- 17.Baltrūnaitė L. Diet Composition of the Red Fox (Vulpes vulpes L.), Pine Marten (Martes martes L.) and Raccoon Dog (Nyctereutes procyonoides Gray) in Clay Plain Landscape, Lithuania. Acta Zool. Litu. 2002;12:362–368. doi: 10.1080/13921657.2002.10512525. [DOI] [Google Scholar]
- 18.Januškevičius V., Januškevičienė G., Prakas P., Butkauskas D., Petkevičius S. Prevalence and Intensity of Sarcocystis spp. Infection in Animals Slaughtered for Food in Lithuania. Vet. Med. Czech. 2019;64:149–157. doi: 10.17221/151/2017-VETMED. [DOI] [Google Scholar]
- 19.Prakas P., Strazdaitė-Žielienė Ž., Januškevičius V., Chiesa F., Baranauskaitė A., Rudaitytė-Lukošienė E., Servienė E., Petkevičius S., Butkauskas D. Molecular Identification of Four Sarcocystis Species in Cattle from Lithuania, Including S. hominis, and Development of a rapid Molecular Detection Method. Parasites Vectors. 2020;13:610. doi: 10.1186/s13071-020-04473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balčiauskas L., Balčiauskienė L., Litvaitis J.A., Tijušas E. Citizen Scientists Showed a Four-fold Increase of Lynx Numbers in Lithuania. Sustainability. 2020;12:9777. doi: 10.3390/su12229777. [DOI] [Google Scholar]
- 21.Verma S.K., Lindsay D.S., Grigg M.E., Dubey J.P. Isolation, Culture and Cryopreservation of Sarcocystis species. Curr. Protoc. Microbiol. 2017;45:11–127. doi: 10.1002/cpmc.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakas P., Butkauskas D., Rudaitytė E., Kutkienė L., Sruoga A., Pūraitė I. Morphological and Molecular Characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the Sika Deer (Cervus nippon) in Lithuania. Parasitol. Res. 2016;115:3021–3032. doi: 10.1007/s00436-016-5057-7. [DOI] [PubMed] [Google Scholar]
- 23.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 24.Gjerde B. Phylogenetic Relationships among Sarcocystis Species in Cervids, Cattle and Sheep Inferred from the Mitochondrial Cytochrome c Oxidase Subunit I Gene. Int. J. Parasitol. 2013;43:579–591. doi: 10.1016/j.ijpara.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Dean A.G., Sullivan K.M., Soe M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. [(accessed on 19 January 2021)]; Available online: www.OpenEpi.com.
- 26.Brown L.D., Cat T.T., DasGupta A. Interval Estimation for a Proportion. Stat. Sci. 2001;16:101–133. [Google Scholar]
- 27.Abramson J.H. WINPEPI Updated: Computer Programs for Epidemiologists, and their Teaching Potential. Epidemiol. Perspect. Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas J.R., Salazar W., Landers D.M. What is Missing in p < 05? Effect Size. Res. Q. Exerc. Sport. 1991;62:344–348. doi: 10.1080/02701367.1991.10608733. [DOI] [PubMed] [Google Scholar]
- 29.Prakas P., Liaugaudaitė S., Kutkienė L., Sruoga A., Švažas S. Molecular Identification of Sarcocystis rileyi Sporocysts in red Foxes (Vulpes vulpes) and Raccoon Dogs (Nyctereutes procyonoides) in Lithuania. Parasitol. Res. 2015;114:1671–1676. doi: 10.1007/s00436-015-4348-8. [DOI] [PubMed] [Google Scholar]
- 30.Basso W., Alvarez Rojas C.A., Buob D., Ruetten M., Deplazes P. Sarcocystis Infection in Red Deer (Cervus elaphus) With Eosinophilic Myositis/Fasciitis in Switzerland and Involvement of Red Foxes (Vulpes vulpes) and Hunting Dogs in the Transmission. Int. J. Parasitol. Parasites Wildl. 2020;13:130–141. doi: 10.1016/j.ijppaw.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irie T., Uraguchi K., Ito T., Yamazaki A., Takai S., Yagi K. First Report of Sarcocystis pilosa Sporocysts in Feces from red fox, Vulpes vulpes schrencki, in Hokkaido, Japan. Int. J. Parasitol. Parasites Wildl. 2020;11:29–31. doi: 10.1016/j.ijppaw.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gjerde B. Molecular Characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from Cattle (Bos taurus) and Sarcocystis sinensis from Water Buffaloes (Bubalus bubalis) Parasitol. Res. 2016;115:1473–1492. doi: 10.1007/s00436-015-4881-5. [DOI] [PubMed] [Google Scholar]
- 33.Gjerde B., Hilali M., Abbas I.E. Molecular Differentiation of Sarcocystis buffalonis and Sarcocystis levinei in Water Buffaloes (Bubalus bubalis) from Sarcocystis hirsuta and Sarcocystis cruzi in Cattle (Bos taurus) Parasitol. Res. 2016;115:2459–2471. doi: 10.1007/s00436-016-4998-1. [DOI] [PubMed] [Google Scholar]
- 34.Odening K., Wesemeier H.H., Walter G., Bockhardt I. The Wisent (Bison bonasus, Bovidae) as an Intermediate Host of Three Sarcocystis species (Apicomplexa: Sarcocystidae) of Cattle. Folia Parasitol. 1994;41:115–121. [PubMed] [Google Scholar]
- 35.Pyziel A.M., Demiaszkiewicz A.W. Sarcocystis cruzi (Protozoa: Apicomplexa: Sarcocystidae) Infection in European Bison (Bison bonasus) from Białowieza Forest, Poland. Wiad. Parazytol. 2009;55:31–34. [PubMed] [Google Scholar]
- 36.Calero-Bernal R., Verma S.K., Seaton C.T., Sinnett D., Ball E., Dunams D., Rosenthal B.M., Dubey J.P. Sarcocystis cruzi Infection in Wood Bison (Bison bison athabascae) Vet. Parasitol. 2015;210:102–105. doi: 10.1016/j.vetpar.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Bright P.W. Lessons from Lean Beasts: Conservation Biology of the Mustelids. Mamm. Rev. 2000;30:217–226. doi: 10.1046/j.1365-2907.2000.00068.x. [DOI] [Google Scholar]
- 38.Zschille J., Stier N., Roth M., Mayer R. Feeding Habits of Invasive American mink (Neovison vison) in Northern Germany—Potential Implications for Fishery and Waterfowl. Acta Theriol. 2013;59:25–34. doi: 10.1007/s13364-012-0126-5. [DOI] [Google Scholar]
- 39.Moreno-Opo R., Margalida A. Human-Mediated Carrion: Effects on Ecological Processes. In: Olea P., Mateo-Tomás P., Sánchez-Zapata J., editors. Carrion Ecology and Management. Wildlife Research Monographs. Volume 2. Springer; Cham, Switzerland: 2019. pp. 183–211. [DOI] [Google Scholar]
- 40.Dahlgren S.S., Gjerde B. The red fox (Vulpes vulpes) and the Arctic fox (Vulpes lagopus) are Definitive Hosts of Sarcocystis alces and Sarcocystis hjorti from Moose (Alces alces) Parasitology. 2010;137:1547–1557. doi: 10.1017/S0031182010000399. [DOI] [PubMed] [Google Scholar]
- 41.Khan R.A., Evans L. Prevalence of Sarcocystis spp. in Two Subspecies of Caribou (Rangifer tarandus) in Newfoundland and Labrador, and Foxes (Vulpes vulpes), Wolves (Canis lupus), and Husky Dogs (Canis familiaris) as Potential Definitive hosts. J. Parasitol. 2006;92:662–663. doi: 10.1645/GE-753R1.1. [DOI] [PubMed] [Google Scholar]
- 42.Gjerde B., Hilali M. Domestic cats (Felis catus) are Definitive Hosts for Sarcocystis sinensis from Water Buffaloes (Bubalus bubalis) J. Vet. Med. Sci. 2016;78:1217–1221. doi: 10.1292/jvms.16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pak S.M., Perminova V.V., Yeshtokina N.V. Sarcocystis citellivulpes sp. n. from the Yellow Suslik Citellus fulvus Lichtenstain, 1923. In: Beyer T.V., Bezukladnikova N.A., Galuzo I.G., Konovalova S.I., Pak S.M., editors. Toksoplazmidy, Protozoologiya. Akademii Nauk Sovetskoi Sotsialisticheskoi Respubliki; Moscow, Russia: 1979. pp. 111–114. [Google Scholar]
- 44.Mitchell-Jones A.J., Amori G., Bogdanowicz W., Krystufek B., Reijnders P.J.H., Spitzenberger F., Stubbe M., Thissen J.B.M., Vohralik V., Zima J. The Atlas of European Mammals. 1st ed. Academic Press; London, UK: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this article are included in the article. The sequences generated in the present study were submitted to the GenBank database under accession numbers MW595468–MW595608.