Abstract
Objective
Osteoarthritis is the most prominent form of arthritis, affecting approximately 15% of the population in the United States. Knee osteoarthritis (KOA) has become one of the leading causes of disability in older adults. Besides knee replacement, there are no curative treatments for KOA, so persistent pain is commonly treated with opioids, acetaminophen, and nonsteroidal anti-inflammatory drugs. However, these drugs have many unpleasant side effects, so there is a need for alternative forms of pain management. We sought to test the efficacy of a dietary intervention to reduce KOA.
Design
A randomized controlled pilot study to test the efficacy of two dietary interventions.
Subjects
Adults 65–75 years of age with KOA.
Methods
Participants were asked to follow one of two dietary interventions (low-carbohydrate [LCD], low-fat [LFD]) or continue to eat as usual (control [CTRL]) over 12 weeks. Functional pain, self-reported pain, quality of life, and depression were assessed every three weeks. Serum from before and after the diet intervention was analyzed for oxidative stress.
Results
Over a period of 12 weeks, the LCD reduced pain intensity and unpleasantness in some functional pain tasks, as well as self-reported pain, compared with the LFD and CTRL. The LCD also significantly reduced oxidative stress and the adipokine leptin compared with the LFD and CTRL. Reduction in oxidative stress was related to reduced functional pain.
Conclusions
We present evidence suggesting that oxidative stress may be related to functional pain, and lowering it through our LCD intervention could provide relief from pain and be an opioid alternative.
Keywords: Diet, Knee Pain, Osteoarthritis, Oxidative Stress, Intervention, Leptin
Introduction
Osteoarthritis (OA) is the most predominant form of arthritis, affecting about 15% of the population [1]. Because of its affinity for the lower, weight-bearing joints, knee osteoarthritis (KOA) has become one of the leading causes of disability in the lower extremities in an ever-aging population, with a lifetime risk estimated at 40% in men and 47% in women [2]. The risks are even higher in those who are classified as obese, and the prevalence is expected to double from 2014 to 2020 in the United States due to the increased presence of an aging, obese population [3]. Thus, treatments must be developed that can address both the obesity and resulting pain due to OA.
Risk factors for developing KOA, aside from age and obesity, include major mechanical injury, repetitive use and abnormal loading of the joints, sex, race/ethnicity, diet, and other genetic factors [4]. Currently there are no curative treatments for KOA besides total replacement of the joint, and the symptomatic pain that persists in this population is commonly treated with opioid medications, acetaminophen, and nonsteroidal anti-inflammatory drugs. These treatments often reduce pain but come with the cost of substantial adverse side effects [5]. As there is a lack of balance between pain relief and side effects, there needs to be a solution to the experienced pain of KOA without the downfalls of medication.
The damage to the cartilage and bone in KOA prompts an immune response that leads to inflammation and subsequent pain within the joint [6]. Specifically, levels of C-reactive protein (CRP) and inflammatory cytokines such as interleukin-6 (IL-6), IL-1β, IL-18, and tumor necrosis factor alpha (TNF-α) were elevated in individuals with KOA [7,8]. In addition, anti-inflammatory mediators such as IL-10 were shown to be decreased in those with KOA [8]. Given that inflammation plays a key role in OA patients, it is also important to consider how diet may play a role in inflammation and subsequent pain. Typical American diets are high in refined carbohydrates and saturated fats [9]. Previous studies have shown that high levels of carbohydrates consumed in the diet can lead to the formation of advanced glycation end products (AGEs), a pro-inflammatory process that appears to increase the production of pro-inflammatory cytokines [10]. IL-6 and TNF-α can induce the expression of pro-inflammatory mediators and degradative enzymes that inhibit cartilage matrix synthesis and slow down the already subdued cartilage repair process [8]. In addition, increased CRP levels are a risk factor for chronic pain and are also associated with higher pain sensitivity [11]. Our previous work in rodents has shown that a diet high in carbohydrates and fats increases microglial activation in the spinal cord, increases peripheral inflammation, and prolongs hypersensitivity after inflammatory injury in rats [12] and mice [13,14]. In addition, we have shown that a low-glycemic diet leads to more rapid recovery and lower pain hypersensitivity after the same inflammatory injury [13]. It is possible that the high levels of carbohydrates and unhealthy fats in the American diet could be contributing to the inflammation in OA and therefore worsening the pain that patients are experiencing. By lowering the intake of refined carbohydrates, we speculate that 1) the amount of thiobarbituric acid reactive substances (TBARS; a measure of oxidative stress as a product of AGEs) will decrease and 2) a reduction in dietary carbohydrates will improve functional pain. Diets such as the Mediterranean diet (a partial low-carbohydrate diet) have been shown to reduce inflammation in arthritis patients [15] and self-reported pain in OA [16] and rheumatoid arthritis [17,18], supporting our hypothesis. However, diet intervention studies to date have focused exclusively on self-reported pain [16–20], and not assessment of functional pain, which may be a better indicator of efficacy. This randomized controlled pilot study aims to provide support for the use of a low-carbohydrate diet to reduce pain related to KOA, irrespective of weight loss, as a potential alternative to pharmacological therapies.
Methods
Participants
Twenty-one adults (nine males, 12 females) between the ages of 65 and 75 years with medically diagnosed knee osteoarthritis (based on the American College of Rheumatology criteria [21]) were recruited via telephone from research databases or through community advertisements. Inclusion criteria also included the ability to understand verbal or written English and willingness to follow a prescribed diet. Exclusion criteria included preexisting metabolic conditions (e.g., diabetes), inability to read/speak English, unwillingness to follow a prescribed diet, currently on a prescribed diet, history of joint replacement, history of eating disorders, difficulty chewing or swallowing, current or recent smoker (last six months), dependence on others for food preparation, cardiovascular disease or renal failure (last six months), recent significant weight loss (>4 kg in last two months), uncontrolled blood pressure (SBP >159 or DBP >95 mmHg), daily opioids for pain, and other medications that may interfere with study outcomes (e.g., proton pump inhibitors). All participants provided written informed consent, as approved by the University of Alabama at Birmingham Institutional Review Board.
Dietary Interventions
Following determination of eligibility, participants were randomly assigned to one of three diet groups (Table 1): low-carbohydrate (LCD; N = 8), low-fat (LFD; N = 6), and control (CTRL; N = 7). The LCD group was instructed to restrict their daily total (not net) carbohydrate intake to ≤20 g/d for the first three weeks and was further permitted to increase daily intake to 40 g, if required. Fats were not restricted, nor were protein (meats, eggs). Fruits were restricted, and vegetables were permitted in limited quantities (two cups per day of leafy greens, one cup per day of nonstarchy vegetables, etc.). Participants were instructed as to the types and quantities of beverages that were permitted to accompany the LCD. Artificial carbohydrate-free sweeteners (stevia or sucralose) were permitted, but powdered sweeteners (aspartame, saccharin, stevia, sucralose) were allowed in limited quantities as they contain maltodextrin (1 g of rapidly digesting carbohydrate). The LFD group was used as a weight loss control, in which males (500 kcal/d) and females (250–300 kcal/d) had their daily calories reduced primarily through reduction of fats, according to the United States Department of Agriculture guidelines (www.choosemyplate.gov). This diet results in approximately 60% of daily kcal from carbohydrates, 20% from protein, and 20% from fats. The LFD consists of fruits and vegetables, low-fat foods, whole grains, low-fat dairy, and limited cholesterol and saturated fats. The CTRL group was permitted to eat as usual, though they were given educational documents related to portion control.
Table 1.
Macronutrient overview of the three different diet groups
| Diet | Calories/Day | Fat, g/d | Carbohydrate, g/d | Protein, g/d |
|---|---|---|---|---|
| Low-fat diet | 800–1,200 | 50–67 | Unlimited | 100 |
| Low-carbohydrate diet | Unlimited | Unlimited | 20 | 100 |
| Control diet | Unlimited | Unlimited | Unlimited | Unlimited |
Outcome Measures
A summary of all measures and their timing is shown in Table 2.
Table 2.
Testing parameters, frequency, and references for each outcome measure
| Measure | Type of Test | Frequency | Reference |
|---|---|---|---|
| Body weight, kg | Anatomical | Every 3 wk | N/A |
| Body mass index | Anatomical | Every 3 wk | N/A |
| Fat mass | Anatomical | Every 3 wk | N/A |
| Food journal | Adherence | Daily | N/A |
| Temporal summation | Functional | Every 3 wk | [21] |
| Timed walk | Functional | Every 3 wk | [21] |
| Repeated chair stands | Functional | Every 3 wk | [21] |
| Leptin | Blood/serum | Every 6 wk | N/A |
| IFN-γ | Blood/serum | Every 6 wk | N/A |
| IL-6 | Blood/serum | Every 6 wk | N/A |
| TNF-α | Blood/serum | Every 6 wk | N/A |
| CRP | Blood/serum | Every 6 wk | N/A |
| TBARS | Blood/serum | Every 6 wk | N/A |
| Knee Injury and Osteoarthritis Outcome Score | Self-report/behavioral | Every 3 wk | [22] |
| Brief Pain Inventory | Self-report/behavioral | Every 3 wk | [23] |
| Quick Inventory of Depressive Symptomology | Behavioral | Every 3 wk | [24] |
CRP = C-reactive protein; IFN = interferon; IL = interleukin; TBARS = thiobarbituric acid reactive substances; TNF = tumor necrosis factor.
Body weight (kg), body mass index (BMI), and fat mass (FM) were obtained using a body composition analyzer, TBF-310 (Tanita, Tokyo, Japan). Waist circumference (cm) and height (cm) were obtained using a measuring tape at each visit. Participants were given a food journal to log their meals each week. The type of food, beverages, sauces, and added sugars/sweeteners were recorded, as well as the amounts consumed. Adherence was verbally confirmed, and food journals were assessed by a Registered Dietician and the study administrator at each visit to ensure compliance with the assigned diet. In the event of nonadherence, participants were counseled with respect to the assigned diet. Persistent nonadherence (more than seven days with excess carbohydrates or fats) resulted in removal from the study. At each visit, a physician assessed their overall physical health (blood pressure, heart rate, temperature, weight loss/gain, adverse symptoms) to ensure the diet was not compromising health. Participants returned every three weeks for testing, for a total of 12 weeks (T0 = baseline, T1 = 3 weeks, T2 = 6 weeks, T3 = 9 weeks, T4 = 12 weeks). All data were collected at the Susan Mott Webb Building for Nutrition Sciences at the University of Alabama at Birmingham.
Functional Pain Testing
The participants rated the intensity/unpleasantness of any pain in the affected knee on a scale from 0 to 100 (0 = no pain, 100 = the worst pain imaginable) before and after participating in the functional pain testing tasks. Functional pain scores were defined as the difference in pain scores (intensity and unpleasantness) given before and after completing the tasks. Instructions for rating scales were played on each visit on an Android tablet to ensure consistency of instruction. For each task, instructions and the description of the task were played on the tablet before each test. Participants were given a five-minute break between the tasks to allow for recovery. The repeated chair stands and timed walk are components of the Short Physical Performance Battery, and all procedures were followed as described (see below) [22].
Temporal Summation
Participants were asked to sit in a chair with both feet flat on the floor and both knees bent at approximately 90º. Participants were asked to rate the intensity and unpleasantness of the pain in the affected knee separately on a scale from 0 to 100 before beginning the test. A 300-g von Frey filament was presented once on the central anterior patella of the affected knee for one second. After one presentation, participants again rated intensity and unpleasantness. Ten presentations of the filament were then applied to the same area of the knee (1/sec). After 10 presentations, participants again rated the intensity and unpleasantness. The increase following the single presentation and the repeated presentation was taken as a measure of temporal summation.
Timed Walk
A distance of eight feet was marked out in a straight line on the floor of the testing room with two pieces of tape. Before the walk, participants were asked to rate the intensity and unpleasantness of the pain in the affected knee while standing. Participants were asked to stand at one end and walk to the other end (tape to tape) and stop. The walk was timed (ms) using a handheld stopwatch from the moment the participant initiated movement to the moment they crossed the second piece of tape (eight feet). The participant completed the timed walk twice. The mean of the two the times was assigned to the participant as an ordinal score (0 = could not do, 1 = >5.7 sec [<0.43 m/sec], 2 = 4.1–6.5 sec [0.44–0.60 m/sec], 3 = 3.2–4.0 sec [0.61–0.77 m/sec], 4 = <3.1 sec [>0.78 m/sec]). If the task was started but not completed, an ordinal score of 0 was assigned.
Repeated Chair Stands
Participants were asked to sit in a chair with their knees bent and with feet flat on the floor. Before starting the test, participants rated the intensity and unpleasantness of the pain in their affected knee. Participants were asked to place their hands on the opposite shoulders (right hand/left shoulder, left hand/right shoulder) and then progress from sitting to standing and back a total of five times without using their arms. The test was timed from the moment the participant initiated movement until they sat down for a fifth time. Ordinal scores were assigned (0 = could not do, 1 = >16.7 sec, 2 = 16.6–13.7 sec, 3 = 136.6–11.2 sec, 4 = <11.1 sec) based on how long it took the individual took to complete all five chair stands. If the task was too difficult and/or not completed, the participants were allowed to stop and given an ordinal score of 0, and the number of chair stands completed was recorded.
Blood Draw and Analysis Methods
Blood was collected at the first visit and at six and 12 weeks by a Registered Nurse. Tiger Top tubes (Fisher Scientific, 8.5 mL) with clot-activating gel were used to collect the blood and separate serum from the cells. Samples were left at room temperature for 15 minutes after blood draw. The tubes were then centrifuged at 15,000 rpm at 4ºC for 15 minutes. The supernatant was then pipetted into two 1-mL screw-top lid tubes, labeled and stored at –80ºC until analysis. Analyses were performed at the Diabetes Research Center Human Physiology Core Laboratory (P30DK056336) at The University of Alabama at Birmingham. CRP (mg/L) was analyzed on the Stanbio Sirrus Clinical Chemistry Analyzer (Thermo Fisher Scientific, Pittsburgh, PA, USA) using turbidometric reagent (Pointe Scientific, Canton, MI, USA). Leptin (ng/mL) was analyzed using the Millipore RIA Assay kit (Millipore Sigma/Merck KGaA, Darmstadt, Germany), with a minimum sensitivity (90% bound) of 1.269 ng/mL and an intra-assay CV of 7.044%. IFN-γ, IL-6, and TNF-α (pg/mL) were analyzed using the MSD Human Pro-Inflammatory Panel on an MSD Imager (Meso Scale Discovery, Rockville, MD, USA) with minimum sensitivities of 0.396 mg/mL, 0.130 pg/mL, and 0.120 pg/mL, respectively. Finally, a TBARS assay was completed to measure oxidative stress using an R&D Parameter (R & D Systems, Minneapolis, MN, USA) chemical assay, with a minimum sensitivity of 1.10 ng/mL and an intra-assay CV of 4.34%.
Questionnaires
Between each functional test (during the five-minute rest period), questionnaires were given to participants that assessed knee pain and mood. The Knee Injury and Osteoarthritis Outcome Score (KOOS) [23] questionnaire was used to assess the participants’ opinions on their pain and associated problems with regards to their KOA. The Brief Pain Inventory (BPI) allowed patients to report the severity of their pain and the degree to which their pain interfered with physical function, as well as providing a location for reporting of pain medications. BPI pain was computed as a composite score that incorporated all reported time points (hours, days, weeks, months) into consideration, as per scoring directions [24]. Finally, the Quick Inventory of Depressive Symptomology was used for the assessment of the patients’ depressive symptoms [25]. All questionnaires were scored according to the questionnaire protocols.
Statistical Analysis
Statistical analyses were carried out using IBM SPSS Statistics, version 24 (IBM Corp, Chicago IL, USA). All data were first tested for normality. Chi-square tests were used to examine group differences in sex, and the Kruskal-Wallis test was used to compare age between groups. All other variables of interest were found to be normally distributed, so a univariate analysis of variance (ANOVA) was carried out comparing all groups at baseline to confirm that the groups did not differ at the outset. Repeated-measures ANOVAs were used to detect change over time (BL-12 weeks), and two-sided Dunnett tests were used to compare the diet groups with the CTRL. Changes within each group from baseline to 12 weeks were examined by paired t tests. To determine the impact of change in TBARS on other variables of interest, we conducted a multivariate ANOVA with TBARS as a covariate. Leptin is an adipokine released by adipose tissue. Thus, we assessed the change in leptin levels between the diet groups as a function of weight. A significance level of P < 0.05 was used in all cases.
Results
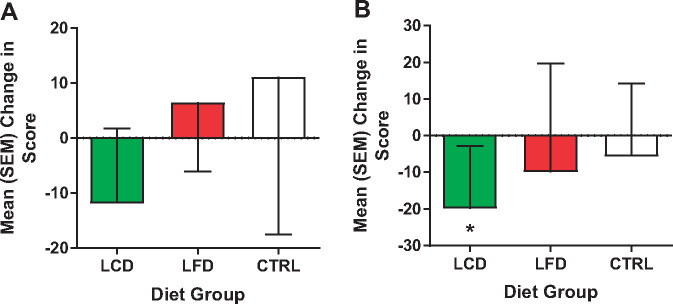
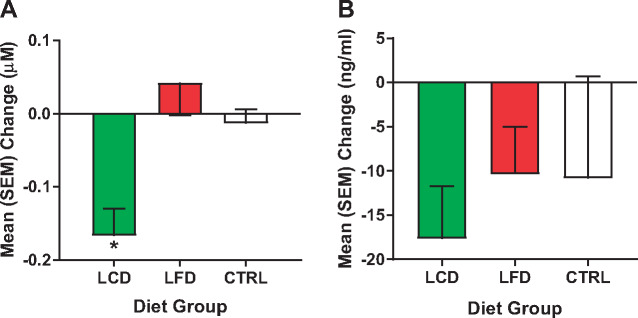
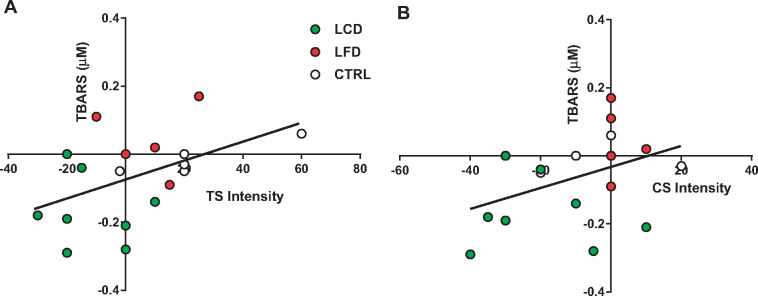
Data are reported for the 21 men and women who completed the study (Table 3). Two participants in the CTRL group failed to complete the study, and one in the LFD was lost to nonadherence. As displayed, sex was relatively evenly distributed between the three groups, but race was weighted more toward non-Hispanic whites (NHWs). No group differences were found at the baseline time point for all variables. Paired t tests (Table 4) revealed a significant decrease in weight (Figure 1) and BMI in the LCD and LFD groups, but not the CTRL group. However, the tests also revealed that only in the LCD group were there significant improvements in pain interference scores (Figure 2), quality of life (Figure 3), pain intensity (temporal summation and chair stands) (Figure 4A, B;Supplementary Data), TBARS (Figure 5A;Supplementary Data), and leptin (Figure 5B). These differences were not evident in the LFD group or the CTRL group. In addition to the results of the paired t tests, Table 4 also shows the results of a repeated-measures ANOVA, confirming a significant time × diet interaction in the LCD group for TBARS (P = 0.018), but no other interactions. The change in TBARS level correlated with changes in pain intensity following temporal summation (r2 = 0.2264, P < 0.05) (Figure 6A), and there was a trend suggesting a relation between change in TBARS and pain intensity associated with the chair stand test (r2 = 0.1633, P = 0.090 (Figure 6B). As expected, the change in weight was related to the change in leptin levels between the groups (F(1, 1 4) = 5.556, P < 0.05). Ordinal scores and performance measures for TW and CS tests are shown in the Supplementary Data for all groups at baseline and at the end of the 12 weeks.
Table 3.
Demographic data for participant sample
| Variable | CTRL | LFD | LCD | P Value |
|---|---|---|---|---|
| Sex (M/F) | 3/4 | 3/3 | 3/5 | 0.896 |
| Race (NHW/AA/HSP) | 2/4/1 | 5/1/0 | 7/1/0 | 0.120 |
| Age, y | 68.71 ± 7.11 | 72.33 ± 1.97 | 71.00 ± 3.12 | 0.230 |
| No. randomized (M/F) | 4/5 | 3/4 | 3/5 | N/A |
AA = African American; CTRL = control; F = female; LCD = low-carbohydrate diet; LFD = low-fat diet; M = male; NHW = non-Hispanic white; HSP = Hispanic.
Table 4.
Statistical results of all analyses
| Measure | Baseline | 12 wk | Change Within Group* | Time × Diet (Unadjusted)† | Post Hoc Analysis‡ |
|---|---|---|---|---|---|
| Weight, kg | 0.001 | ||||
| CTRL | 77.64 ± 7.07 | 75.87 ± 7.55 | 0.144 | ||
| LFD | 88.02 ± 18.07 | 81.30 ± 16.70 | 0.001 | 0.553 | |
| LCD | 98.53 ± 18.56 | 89.59 ± 17.86 | 0.001 | 0.072 | |
| BMI, kg/m2 | 0.003 | ||||
| CTRL | 26.9 ± 3.02 | 26.6 ± 3.46 | 0.332 | ||
| LFD | 29.65 ± 4.48 | 27.33 ± 3.68 | 0.002 | 0.788 | |
| LCD | 35.64 ± 7.35 | 32.53 ± 7.11 | 0.001 | 0.029 | |
| BPI pain | 0.283 | ||||
| CTRL | 3.64 ± 2.35 | 3.86 ± 2.19 | 0.682 | ||
| LFD | 2.87 ± 1.93 | 2.50 ± 1.84 | 0.267 | 0.802 | |
| LCD | 3.06 ± 1.73 | 2.43 ± 1.77 | 0.137 | 0.474 | |
| BPI interference | 0.713 | ||||
| CTRL | 3.41 ± 0.708 | 2.65 ± 1.19 | 0.113 | ||
| LFD | 2.87 ± 1.92 | 1.65 ± 1.84 | 0.267 | 0.407 | |
| LCD | 2.46 ± 1.64 | 0.986 ± 1.11 | 0.022 | 0.081 | |
| KOOS pain | 0.893 | ||||
| CTRL | 3.23 ± 0.542 | 2.78 ± 0.759 | 0.071 | ||
| LFD | 2.56 ± 0.734 | 2.24 ± 0.729 | 0.337 | 0.140 | |
| LCD | 2.68 ± 0.360 | 2.20 ± 0.730 | 0.058 | 0.150 | |
| KOOS QOL | 0.605 | ||||
| CTRL | 3.67 ± 0.718 | 3.26 ± 0.759 | 0.047 | ||
| LFD | 2.91 ±0.785 | 2.08 ± 0.954 | 0.534 | 0.167 | |
| LCD | 3.28 ± 0.604 | 2.75 ±0.627 | 0.031 | 0.344 | |
| QIDS severity | 0.910 | ||||
| CTRL | 1.86 ±1.07 | 1.43 ±0.787 | 0.200 | ||
| LFD | 1.67 ± 0.817 | 1.33 ± 0.516 | 0.175 | 0.895 | |
| LCD | 1.63 ± 0.744 | 1.13 ± 0.354 | 0.104 | 0.652 | |
| TS intensity | 0.084 | ||||
| CTRL | 14.57 ± 20.80 | 25.71 ± 21.49 | 0.344 | ||
| LFD | 26.83 ± 36.69 | 33.33 ± 32.66 | 0.263 | 0.606 | |
| LCD | 11.25 ± 11.25 | –0.6250 ± 10.16 | 0.043 | 0.307 | |
| TS unpleas | 0.819 | ||||
| CTRL | 22.42 ± 29.57 | 21.42 ± 18.64 | 0.933 | ||
| LFD | 35.5 ± 41.9 | 26.67 ± 32.65 | 0.358 | 0.706 | |
| LCD | 14.00 ± 13.02 | 7.500 ± 15.11 | 0.295 | 0.560 | |
| CS intensity | 0.454 | ||||
| CTRL | 31.42 ± 17.72 | 25.71 ± 15.11 | 0.476 | ||
| LFD | 26.7 ± 26.6 | 16.7 ± 18.6 | 0.447 | 0.562 | |
| LCD | 27.50 ± 14.14 | 7.500 ± 11.65 | 0.013 | 0.218 | |
| CS unpleas | 0.899 | ||||
| CTRL | 34.3 ± 23.7 | 25.71 ± 15.11 | 0.407 | ||
| LFD | 26.7 ± 26.6 | 16.7 ± 18.6 | 0.447 | 0.491 | |
| LCD | 25.63 ± 15.68 | 11.25 ± 14.57 | 0.100 | 0.235 | |
| TW intensity | 0.066 | ||||
| CTRL | 5.71 ± 9.75 | 18.57 ± 21.15 | 0.163 | ||
| LFD | 3.67 ± 8.04 | –6.67 ± 16.32 | 0.186 | 0.047 | |
| LCD | 11.25 ± 14.57 | 3.750 ± 7.440 | 0.265 | 0.586 | |
| TW unpleas | 0.283 | ||||
| CTRL | 7.86 ± 11.49 | 10.0 ± 11.54 | 0.589 | ||
| LFD | 3.67 ± 8.04 | –6.67 ± 16.32 | 0.186 | 0.689 | |
| LCD | 7.500 ± 10.35 | 3.750 ± 7.440 | 0.476 | 0.080 | |
| IL-6 | 0.141 | ||||
| CTRL | 0.793 ± 0.415 | 1.74 ± 2.03 | 0.329 | ||
| LFD | 0.975 ± 0.460 | 0.965 ± 0.377 | 0.894 | 0.800 | |
| LCD | 1.131 ± 1.129 | 1.090 ± 0.7045 | 0.825 | 0.932 | |
| CRP | 0.383 | ||||
| CTRL | 5.92 ± 6.28 | 3.93 ± 3.76 | 0.578 | ||
| LFD | 2.08 ± 2.12 | 3.91 ± 5.38 | 0.242 | 0.632 | |
| LCD | 3.10 ± 5.54 | 2.33 ± 2.18 | 0.605 | 0.525 | |
| TNF-α | 0.117 | ||||
| CTRL | 2.19 ± 0.418 | 2.58 ± 0.572 | 0.386 | ||
| LFD | 2.42 ± 0.497 | 2.24 ± 0.383 | 0.052 | 0.989 | |
| LCD | 3.41 ± 0.949 | 3.35 ± 0.839 | 0.578 | 0.048 | |
| IFN-γ | 0.277 | ||||
| CTRL | 11.3 ± 14.7 | 14.9 ± 20.2 | 0.289 | ||
| LFD | 4.90 ± 2.28 | 4.71 ± 1.35 | 0.854 | 0.211 | |
| LCD | 7.44 ± 2.90 | 8.98 ± 3.40 | 0.184 | 0.500 | |
| Leptin | 0.688 | ||||
| CTRL | 34.67 ± 21.53 | 23.85 ± 10.27 | 0.417 | ||
| LFD | 34.55 ± 32.81 | 24.2 ± 20.4 | 0.110 | 1.000 | |
| LCD | 54.9 ± 29.6 | 37.3 ± 32.1 | 0.021 | 0.462 | |
| TBARS | 0.002 | ||||
| CTRL | 0.656 ± 0.149 | 0.643 ± 0.116 | 0.537 | ||
| LFD | 0.552 ± 0.105 | 0.595 ± 0.115 | 0.397 | 0.591 | |
| LCD | 0.691 ± 0.205 | 0.525 ± 0.122 | 0.003 | 0.816 |
BMI = body mass index; BPI = Brief Pain Inventory; CRP = C-reactive protein; CS = chair stands; CTRL = control; IFN = interferon; IL = interleukin; KOOS QOL = Knee Injury and Osteoarthritis Outcome Score quality of life; LCD = low-carbohydrate diet; LFD = low-fat diet; QIDS = Quick Inventory of Depressive Symptomology; TBARS = thiobarbituric acid reactive substances; TNF = tumor necrosis factor; TS = temporal summation; TW = timed walk.
T test.
Repeated measures analysis of variance.
Dunnett (two-sided).
Figure 1.
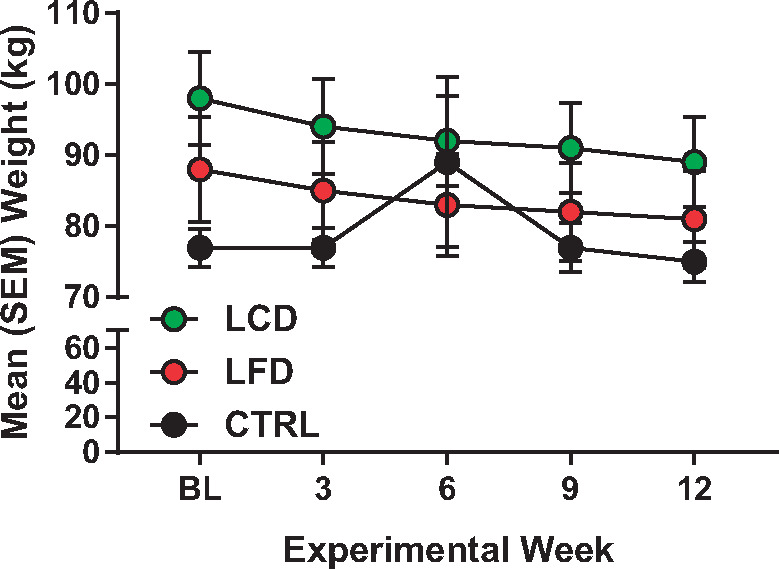
Mean (standard error of the mean) weight (kg) of all groups (low-carbohydrate diet, top; low-fat diet, middle; control, bottom) over the 12-week intervention period.
Figure 2.
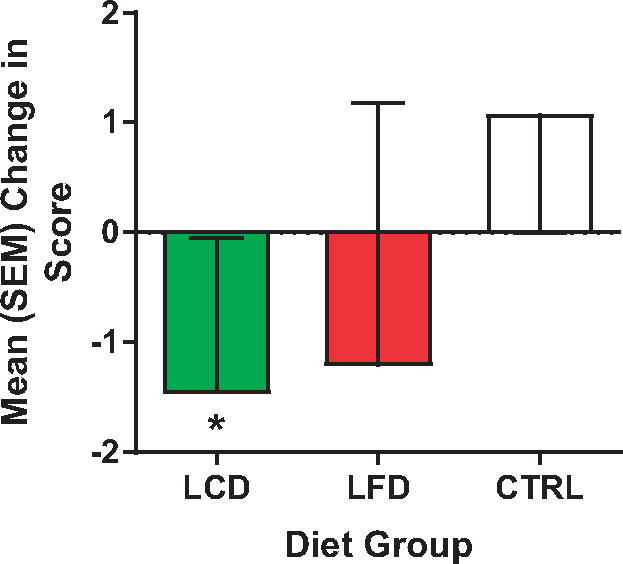
Mean (standard error of the mean) change in pain interference scores on the Brief Pain Inventory between week 12 and baseline for all groups. *P < 0.05.
Figure 3.

Mean (standard error of the mean) change in quality of life measure in the Knee Injury and Osteoarthritis Outcome Score for all groups over the 12-week intervention. *P < 0.05.
Figure 4.
Mean (standard error of the mean) change in intensity ratings for all groups between week 12 and baseline for functional tests. A) Temporal summation. B) Chair stands. *P < 0.05.
Figure 5.
Mean (standard error of the mean) change in serum concentrations of (A) thiobarbituric acid reactive substances (µM) and (B) leptin (ng/mL) for all groups between week 12 and baseline. *P < 0.05.
Figure 6.
Change in thiobarbituric acid reactive substances (week 12 – baseline, y-axis) as a function of the change in pain intensity rating (x-axis) following the temporal summation task (A) or the repeated chair stands task (B). Participants are illustrated by group (CTRL, LFD, LCD). Regression line was calculated using data from all participants. Values below 0 represent a reduction in values at the end of the intervention when compared to baseline.
Discussion
KOA is a widespread public health issue that has many effects on patients’ physical, cognitive, and emotional functioning. The prevalence rate of KOA is higher in the older population, wherein pharmacological intervention is often inadequate and carries a greater risk of side effects. In addition, opioid abuse rates have continued to increase [26], demonstrating the critical need for alternative forms of pain management. Thus, a holistic, multimodal approach may be a viable alternative for this population of chronic pain patients. Based on the assumption that oxidative stress may be one of the causes of the pain experience in patients [27,28], we investigated the potential of a dietary intervention to reduce pain in older adults with KOA. Our pilot data showed encouraging results, whereby our LCD reduced functional pain, leptin, oxidative stress, and self-reported pain measures while increasing quality of life.
Based on the role that carbohydrates play in oxidative stress, we hypothesized that excess carbohydrates in the diet would result in oxidative stress, pain, and inflammation. Consequently, these effects would decrease if consumption of carbohydrates was also reduced in patients with KOA. Because there were no significant differences in weight loss between the diet groups, we believe that the differences in pain scores between the groups are unrelated to weight loss, but are an effect of the quality of the diet. This belief has been reinforced in the literature in studies of arthritis [29]. Our data show that the LCD group had dramatically reduced self-reported pain scores (intensity and unpleasantness) in the chair stands, temporal summation, and timed walk. Quality of life scores (KOOS) significantly increased. TBARS decreased in the blood in the LCD group when compared with the LFD and CTRL groups. There were no significant differences in pro-inflammatory cytokine levels over the 12 weeks. This suggests that KOA pain may not be directly related to peripheral inflammation. However, our data indicate that oxidative stress may be linked to functional KOA pain as the change in TBARS was related to the change in pain intensity ratings following temporal summation and, possibly, repeated chair stands. The reduction of TBARS in the LCD group was primarily driven by women, so further investigation into the sex-specific effects of diet on TBARS may be warranted.
Nutritional stress caused by poor diet promotes a state of oxidative stress by increasing systemic free radical damage and reducing antioxidant status [30]. Recently, the interaction of AGEs and oxidative stress and the effects it has on the body have become an extensive field of interest. Glycation is a nonenzymatic reaction between excess carbohydrates and other macronutrients such as proteins, lipids, and nucleic acids [31,32] that produce AGEs. AGEs can be ingested and created endogenously in the presence of excess carbohydrates, as well as through other cellular processes [33]. Under normal conditions, cells have innate mechanisms that help with detoxification and prevent accumulation of AGEs [34]. However, when the system becomes overloaded, these compounds create a state that ultimately leads to cellular breakdown [35,36] and cell death. AGEs not only exert their deleterious actions this way, but also through their interactions with specific receptors.
Receptor for AGEs (RAGE) is a member of the immunoglobulin cell surface receptor family [31,37]. The binding of AGEs to RAGE stimulates various intracellular signaling pathways, such as mitogen-activated protein kinases, extracellular signal–regulated kinases 1 and 2, cyclin-dependent kinase inhibitor 1, and several other kinases [38], which leads to apoptosis, inflammation, and cell differentiation. In addition, stimulation of RAGE also activates nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) and the subsequent transcription of many pro-inflammatory genes [39,40]. Even more interestingly, RAGE-induced activation of NFkB is self-perpetuated and maintained through positive feedback, creating a cycle of self-renewing inflammatory signals and cytokines [39]. RAGE can also directly induce oxidative stress in several other ways, including activation of nicotinamide adenine dinucleotide phosphate-oxidase, decreasing the activity of superoxide dismutase and catalase pathways [41]. RAGE activation also reduces cellular antioxidant defenses, such as glutathione and ascorbic acid, which in turn supports the production of AGEs [42]. AGEs also directly stimulate reactive oxygen species (ROS) production [43]. ROS are pro-inflammatory mediators for immune cells and can cause cell damage and apoptosis [33]. Thus, it is likely that poor-quality diet can promote the production of ROS, activation of immune cells, and, subsequently, inflammation that contributes to the development of KOA and pain.
Although our present study showed promise for the treatment of KOA with an LCD, there were some limitations. First, given that this was a pilot study, the sample size was small and not racially diverse, and the distribution of racial groups was unequal between the diet groups. Therefore, a larger, more diverse sample would allow investigations of race and sex, both known to be differentially affected by pain [44–48] and diet [49–52]. Second, subsequent long-term follow-up assessments will be able to determine whether participants in the LCD group maintained their lifestyle change, or a version of it, when positive results were experienced. Honesty on self-reporting measures for food intake, long-term adherence, and acceptance will be an important factor in reducing the re-emergence of KOA pain as subjects age. Studying aspects that increase these factors will be critical to widespread adoption of these treatments.
Conclusions
Our exploratory pilot study demonstrates an improvement in self-reported and functional pain in older adults with KOA following an LCD intervention. In only 12 weeks, the quality of life and functional pain of this population were significantly improved, which may have been the result of a reduction in oxidative stress. These preliminary data are reassuring and support the use of the LCD as an effective therapeutic approach for older patients with KOA. Further clinical trials will be performed to ensure a greater sample size and to examine other factors affecting the outcome such as race/ethnicity and sex differences. Finally, as oxidative stress is a factor in many inflammatory conditions, it may be possible to utilize our LCD intervention for other pain conditions and disorders.
Supplementary Material
Acknowledgments
The authors are grateful to Maryellen Williams and Heather Hunter for serum analyses, Dr. D’Ann Somerall for assistance with blood draws, and the nursing staff in the Susan Mott Webb Building at the University of Alabama at Birmingham.
Funding sources: This work was supported in part by a Translational Nutrition and Aging Research Academic Career leadership Award (K07AG043588-03) to JLL. RES contributed startup funds.
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1. Zhang Y, Jordan JM.. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26(3):355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21(9):1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sowers MR, Karvonen-Gutierrez CA.. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol 2010;22(5):533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 2000;43(5):995–1000. [DOI] [PubMed] [Google Scholar]
- 5. O'Neil CK, Hanlon JT, Marcum ZA.. Adverse effects of analgesics commonly used by older adults with osteoarthritis: Focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother 2012;10(6):331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mobasheri A, Henrotin Y.. Biomarkers of (osteo)arthritis. Biomarkers 2015;20(8):513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sokolove J, Lepus CM.. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther Adv Musculoskelet Dis 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herrero-Beaumont G, Roman-Blas JA, Bruyere O, et al. Clinical settings in knee osteoarthritis: Pathophysiology guides treatment. Maturitas 2017;96:54–7. [DOI] [PubMed] [Google Scholar]
- 9. Freedman MR, King J, Kennedy E.. Popular diets: A scientific review. Obes Res 2001;9(Suppl 1):1S–40S. [DOI] [PubMed] [Google Scholar]
- 10. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB.. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2017;20(7):1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Afari N, Mostoufi S, Noonan C, et al. C-reactive protein and pain sensitivity: Findings from female twins. Ann Behav Med 2011;42(2):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Totsch SK, Quinn TL, Strath LJ, et al. The impact of the Standard American Diet in rats: Effects on behavior, physiology and recovery from inflammatory injury. Scand J Pain 2017;17(1):316–24. [DOI] [PubMed] [Google Scholar]
- 13. Totsch SK, Meir RY, Quinn TL, et al. Effects of a Standard American Diet and an anti-inflammatory diet in male and female mice. Eur J Pain 2018; 22(7):1203–13. [DOI] [PubMed] [Google Scholar]
- 14. Totsch SK, Waite ME, Tomkovich A, et al. Total Western diet alters mechanical and thermal sensitivity and prolongs hypersensitivity following complete freund’s adjuvant in mice. J Pain 2016;17(1):119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliviero F, Spinella P, Fiocco U, et al. How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis. Swiss Med Wkly 2015;145:w14190.. [DOI] [PubMed] [Google Scholar]
- 16. Veronese N, Stubbs B, Noale M, et al. Adherence to the Mediterranean diet is associated with better quality of life: Data from the Osteoarthritis Initiative. Am J Clin Nutr 2016;104(5):1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKellar G, Morrison E, McEntegart A, et al. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann Rheum Dis 2007;66(9):1239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sköldstam LB, Hagfors L, Johansson G.. Weight reduction is not a major reason for improvement in rheumatoid arthritis from lacto-vegetarian, vegan or Mediterranean diets. Nutr J 2005;4(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guldbrand H, Lindstrom T, Dizdar B, et al. Randomization to a low-carbohydrate diet advice improves health related quality of life compared with a low-fat diet at similar weight-loss in type 2 diabetes mellitus. Diabetes Res Clin Pract 2014;106(2):221–7. [DOI] [PubMed] [Google Scholar]
- 20. Hagen KB, Byfuglien MG, Falzon L, Olsen SU, Smedslund G.. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst Rev 2009;(1):CD006400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29(8):1039–49. [DOI] [PubMed] [Google Scholar]
- 22. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 23.Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS). 2003.
- 24. Cleeland CS, ed. Brief Pain Inventory. Houston, TX: MD Anderson Cancer Ceter; 1991. [Google Scholar]
- 25. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573–83. [DOI] [PubMed]
- 26. Palmer RE, Carrell DS, Cronkite D, et al. The prevalence of problem opioid use in patients receiving chronic opioid therapy: Computer-assisted review of electronic health record clinical notes. Pain 2015;156(7):1208–14. [DOI] [PubMed] [Google Scholar]
- 27. Battisti E, Albanese A, Guerra L, Argnani L, Giordano N.. Alpha lipoic acid and superoxide dismutase in the treatment of chronic low back pain. Eur J Phys Rehabil Med 2013;49(5):659–64. [PubMed] [Google Scholar]
- 28. Kolberg C, Horst A, Moraes MS, et al. Peripheral oxidative stress blood markers in patients with chronic back or neck pain treated with high-velocity, low-amplitude manipulation. J Manipulative Physiol Ther 2015;38(2):119–29. [DOI] [PubMed] [Google Scholar]
- 29. Skoldstam LBL, Hagfors L, Johansson G.. Weight reduction is not a major reason for improvement in rheumatoid arthritis from lacto-vegetarian, vegan or Mediterranean diets. Nutr J 2005;4:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI Jr.. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 2016;473(24):4527–50. [DOI] [PubMed] [Google Scholar]
- 31. Gkogkolou P, Bohm M.. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol 2012;4(3):259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldin A, Beckman JA, Schmidt AM, Creager MA.. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006;114(6):597–605. [DOI] [PubMed] [Google Scholar]
- 33. Bayir H, Kagan VE.. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis–there is nothing more practical than a good theory. Crit Care 2008;12(1):206.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thornalley PJ. The enzymatic defence against glycation in health, disease and therapeutics: A symposium to examine the concept. Biochem Soc Trans 2003;31(6):1341–2. [DOI] [PubMed] [Google Scholar]
- 35. Conner JR, Beisswenger PJ, Szwergold BS.. Some clues as to the regulation, expression, function, and distribution of fructosamine-3-kinase and fructosamine-3-kinase-related protein. Ann N Y Acad Sci 2005;1043:824–36. [DOI] [PubMed] [Google Scholar]
- 36. Conner JR, Beisswenger PJ, Szwergold BS.. The expression of the genes for fructosamine-3-kinase and fructosamine-3-kinase-related protein appears to be constitutive and unaffected by environmental signals. Biochem Biophys Res Commun 2004;323(3):932–6. [DOI] [PubMed] [Google Scholar]
- 37. Chavakis T, Bierhaus A, Nawroth PP.. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect 2004;6(13):1219–25. [DOI] [PubMed] [Google Scholar]
- 38. Fleming TH, Humpert PM, Nawroth PP, Bierhaus A.. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: A mini-review. Gerontology 2011;57(5):435–43. [DOI] [PubMed] [Google Scholar]
- 39. Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 2005;83(11):876–86. [DOI] [PubMed] [Google Scholar]
- 40. Miyata T, Taneda S, Kawai R, et al. Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci U S A 1996;93(6):2353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114(12):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramasamy R, Vannucci SJ, Yan SS, et al. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005;15(7):16R–28R. [DOI] [PubMed] [Google Scholar]
- 43. Rains JL, Jain SK.. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011;50(5):567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berkley KJ. Sex differences in pain. Behav Brain Sci 1997;20(3):371–80; discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 45. Carey TS, Freburger JK, Holmes GM, et al. Race, care seeking, and utilization for chronic back and neck pain: Population perspectives. J Pain 2010;11(4):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carey TS, Garrett JM.. The relation of race to outcomes and the use of health care services for acute low back pain. Spine 2003;28(4):390–4. [DOI] [PubMed] [Google Scholar]
- 47. Anderson KO, Green CR, Payne R.. Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain 2009;10(12):1187–204. [DOI] [PubMed] [Google Scholar]
- 48. Totsch SK, Quinn TL, Strath LJ, et al. The impact of the Standard American Diet in rats: Effects on behavior, physiology and recovery from inflammatory injury. Scand J Pain 2017;17:316–24. [DOI] [PubMed] [Google Scholar]
- 49. Kodama K, Tojjar D, Yamada S, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 2013;36(6):1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Q, Wang Y, Huang ES.. Changes in racial/ethnic disparities in the prevalence of type 2 diabetes by obesity level among US adults. Ethn Health 2009;14(5):439–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuk JL, Ross R.. Influence of sex on total and regional fat loss in overweight and obese men and women. Int J Obes (Lond) 2009;33(6):629–34. [DOI] [PubMed] [Google Scholar]
- 52. Tirosh A, de Souza RJ, Sacks F, et al. Sex differences in the effects of weight loss diets on bone mineral density and body composition: POUNDS LOST trial. J Clin Endocrinol Metab 2015;100(6):2463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.