Abstract
Dendritic cells (DCs), including conventional DCs (cDCs) and plasmacytoid DCs (pDCs), serve as the sentinel cells of the immune system and are responsible for presenting antigen information. Moreover, the role of DCs derived from monocytes (moDCs) in the development of inflammation has been emphasized. Several studies have shown that the function of DCs can be influenced by gut microbes including gut bacteria and viruses. Abnormal changes/reactions in intestinal DCs are potentially associated with diseases such as inflammatory bowel disease (IBD) and intestinal tumors, allowing DCs to be a new target for the treatment of these diseases. In this review, we summarized the physiological functions of DCs in the intestinal micro-environment, their regulatory relationship with intestinal microorganisms and their regulatory mechanism in intestinal diseases.
Keywords: dendritic cell, immune tolerance, IBD, intestinal tumor, intestinal flora
1. Introduction
In 1973, Steinman and Cohn described a population of cells in the spleen of mice that exhibited a different cellular appearance and behavior from that of monocytes and Mφs, which were hence named dendritic cells (DCs) [1]. DCs were observed to be highly capable of initiating and regulating immune responses with a high level expression of major histocompatibility complex II (MCH-II) and integrin X (complement component 3 receptor 4 subunit) [2,3]. As professional antigen-presenting cells (APC), DCs interact with other innate and adaptive immune cells to ensure the specificity of the adaptive immune response. DCs can largely recognize pathogen-associated molecular patterns (PAMPs) through many receptors, like toll-like receptors (TLRs), which bind to a large number of molecules produced by bacteria, viruses, and fungi. At the same time, circulating DCs have been proven to exhibit intestinal homing characteristics and were found to be regulated by different gliadin-derived peptides in celiac patients [4,5]. During the process of embryonic development and postnatally, DC progenitors migrate into non-lymphoid organs so as to differentiate into immature DCs, which gradually develop a dense network of sentinel cells from the body to the viscera [6].
Many studies have demonstrated that several common diseases are associated with changes in DC distribution or functions [7,8,9,10,11,12]. DCs can be regulated to restore T-cell tolerance and modulate autoantibody production, and functional heterogeneity exists within each DC subset [7]. For example, it was shown that the function of DCs lacking TIR-domain-containing adapter-inducing interferon-β (TRIF) is altered and T cell activation is reduced, which facilitates the progression of diabetes induced by diabetogenic T cells [8]. DCs were also proven to have an influence on the process of diseases like transmissible spongiform encephalopathy [9], autoimmune uveitis [10], acute graft-versus-host disease [11], inflammatory bowel disease (IBD) [12], and intestinal tumors. Therefore, it is of great importance to exhaustively survey the interaction between the physiological characteristics of DCs and diseases.
2. Types of DC
The DC family is very heterogeneous and consists of different DC subsets, each with specific functional characteristics. In general, two different DC subtypes can be distinguished, for example, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). These distinct subsets express various surface receptors and pattern recognition receptors (PRRs), which determine their specialized functions. The cDC subset can be identified and subdivided into three different subtypes by the expression of CD11c, in combination with their unique surface molecules CD1c (BDCA1), CD141 (BDCA3), and CD16. In addition, there are several special classifications, such as dendritic cells responding to specific microorganisms, CD14+ DC, microglia, moDCs, etc. Recently, another subset of DC, termed merocytic DCs (mcDCs), was defined and was found to likely resemble the cDC subset in mice [12]. It was shown that CD34+ hematopoietic stem cells produce granulocytes, monocytes, and progenitor cells of human DCs [13]. Rapamycin (mTOR) networks, which combine pattern recognition and growth factor receptor activation with nutritional information in cells and surrounding tissues, are critical in the proper development of mouse DCs [14]. In this section, we selectively focus on three subtypes of DCs, which may play a vital role in intestinal diseases.
2.1. cDCs
Conventional DCs, also called myeloid DCs (mDCs), express typical myeloid antigens CD11c, CD13, CD33, and CD11b in mice and CD11c in humans [15]. Mouse cDCs derive from common DC precursors in the bone marrow and comprise two main subsets, the CD8α+ and/or CD103+ cDC1 subset and the more heterogeneous CD11b+ cDC2 subset [16,17]. Human cDC1 expressed CD141/BDCA-1, CLEC9A, CADM1, and XCR1, and cDC2 expressed CD1c/BDCA-1, CD11c, CD11b, CD2, and FCER1 [18].
In mice, cDC1s expressed TLR2–TLR4, TLR11–TLR13, and C-type lectin-like receptor (CLEC)12A, and cDC2s expressed TLR1, TLR2, TLR4–TLR9, TLR13, retinoic acid-inducible gene I-like receptor (RLR), NOD-like receptor (NLR), STING, CLEC4A, CLEC6A, and CLEC7A [17]. In humans, cDC1s expressed TLR1, TLR3, TLR6, TLR8, TLR10, STING, and CLEC12A, and cDC2s expressed TLR1–TLR9, RLR, NLR, STING, CLEC4A, CLEC6A, CLEC7A, CLEC10A, and CLEC12A [17]. Tomer Granot [19] combined the technology of flow cytometry and fluorescence imaging, and indicated that human tissue CD13hi CD141hi Clec9A+ cells are cDC1s which maintain a stable quantity, whereas CD1c+Sirp-α+ cells are cDC2s with the specific ability to act as sentinel cells of mucosal tissues.
In the development of cDCs, basic leucine zipper ATF-like transcription factor 3 [20] and RELB proto-oncogene, which is also known as NF-κB subunit, are of great importance [21]. Mice lacking IFN-regulatory factor 8 (IRF8) [22], DNA-binding protein inhibitor ID2 [23], or nuclear factor IL-3-regulated protein (NFIL3) [24] exhibit a severe defect in the development of cDC1s. Some researchers found that although all these factors are required for CD103+ DC development, only IRF8 is essential for CD8α+ DCs [25]. Furthermore, cDC2 development is controlled by RELB63 and PU.1 [26].
cDC precursors travel through the blood to the lymphatic organs and peripheral tissues and then develop into immature DCs and migratory DCs, respectively [27]. These immature cDCs are dedicated to antigen sampling and are characterized by low levels of the expression of T cell co-stimulated molecules and MHCII classes, and then they may remain in resident tissues until an activation signal is encountered [28]. Recent research revealed that tissue-specific factors, including ATF-like transcription factor 3 and RELB proto-oncogene, program the expression levels of different proteases during DC differentiation, thus conferring tissue-specific functions to the different DC subsets in the spleen or thymus [29].
2.2. pDCs
In mice, pDC expressed Siglec-H, bone marrow stromal antigen-2 (BST2), lymphocyte activation marker 3 (LAG-3), B220, Ly6C, CD11c, CD8α, and Ly49Q [29]. Human pDCs are usually short of myeloid antigen, expressing CD45RA, CD4, and variable CD2 and CD7, and are distinguished by the expression of CD123, CD303, CD304, and ILT7 [15,30,31].
Research revealed that human pDCs are composed of two subsets, which can be distinguished by the expression of CD2. CD2hi pDCs are involved in initiating T cell immune responses and can be detected in some tumor biopsies, whereas CD2low pDCs display a limited capacity to induce allogeneic T cell proliferation [32]. pDCs are mainly developed from IL-7R+ lymphoid progenitor cells with the assistance of transcription factors E2-2 and IRF8 [19,33]. pDCs selectively express TLR2, TLR7, TLR9, TLR11, amd TLR12, and it is endosomal sensors TLR7 and TLR9 that mainly mediate the recognition of microbial pathogens [34].
Compared to cDCs, pDCs have a limited effect on antigen presentation and are always regarded as immunomodulating cells [35]. pDCs sense and respond to viral infection by rapidly generating numerous type I and III interferons and secreting cytokines [18,36]. Instead of leaving from bone marrow and differentiating in peripheral organs like cDC progenitor cells, pDCs differentiate completely in the bone marrow. The transcription factor Runx2, specifically expressed in an E2-2-dependent manner in the pDC, is necessary for the migration of the pDC from the bone marrow to the peripheral organs [37]. As considerable efforts have been focused on cDCs, some scientists have proposed that pDCs may be used in therapy to fight against cancer, because pDCs express a wide variety of PRRs, which can be harnessed to facilitate the targeted delivery of antigens to pDCs, leading to antigen presentation and activation of both CD4+ and CD8+ T cells [38].
2.3. moDCs
Human moDCs express CD13, CD33, CD11b, CD11c, CD172a, and MHCII36. Furthermore, short chain fat acids (SCFAs) have immunomodulatory effects on moDCs, which downregulate CXCL9, CXCL10, and CXCL11 production [39], and SCFA receptors such as GPR41, GPR43, and GPR109A are expressed on the surface of human monocyte-derived DCs.
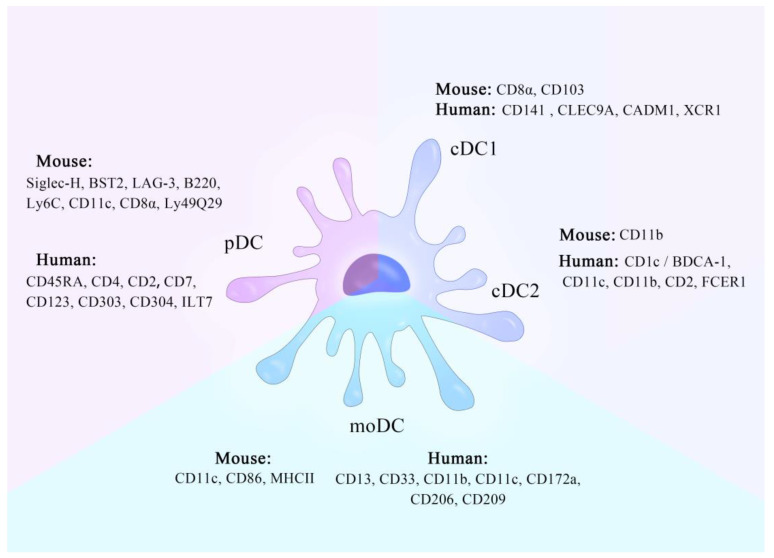
During colitis in mice, a large amount of Ly6Chi mononuclear cells (MOs) can invade the colon and then differentiate into proinflammatory CD103− CX3CR1int CD11b+ DCs, producing IL-12, IL-23, iNOS, and TNF [18,40]. Some investigations demonstrated that moDCs (CD11c+ MHCIIhi cells that were also CD86hi and F4/80lo) were specifically produced by monocyte-dendritic cell progenitors and not granulocyte-monocyte progenitor-derived Ly6Chi MOs [41]. The process of producing moDCs requires granulocyte/Mφ colony stimulating factor (GM-CSF) [27]. Other studies have shown that constitutively migrating LY6C+ MOs can maintain their own properties instead of differentiating into DCs [42]. In this respect, a further study revealed that Ly6C+ cells include three sub-communities, among which CD11c- Flt3+ MOs may be the precursor of CD209a+ PDL2+ moDCs, promoted by the increase in transcription factor PU.1 [43]. The summary of DC types refers to Figure 1 and Box 1.
Figure 1.
A schematic diagram of characters of different dendritic cell (DC) subsets. There are three DC subtypes, which are conventional DCs (cDCs), plasmacytoid DCs (pDCs), and DCs derived from monocytes (moDCs). cDCs can be divided into two subtypes, cDC1 and cDC2. They can be distinguished depending on the specific molecules on the human or mouse DC surface.
Box 1. Types of DC.
DCs have two main types (mDCs and pDCs) and several special classifications, like DC responding to specific microorganisms, CD14+ DC, microglia, moDC.
cDC1 and cDC2 are the subsets of cDCs, with different molecules and functions.
Both cDCs and pDCs contain two subtypes that require a variety of immune factors and immune cells to assist their growth or function.
3. Physiological Function of DCs in the Gut
Approximately 1000 trillion microbes, composed of an estimated 4000 strains, are living in the human intestine and feeding on food residue from the digestive tract [44], producing complicated metabolites to regulate human physiological responses. Usually, DCs come into contact with intestinal flora through two different ways. The first model requires a special intestinal epithelial cell called the M cell. Commensal bacteria and other intact antigens discharged by M cells are captured by DCs in Peyer’s patches, which migrate to mesenteric lymph nodes (MLNs) and activate B cells in mesenteric lymph nodes, eventually resulting in IgA production [45,46,47]. Another model assumes that DCs directly sample symbiotic bacteria and/or other intact antigens in the lumen. The pioneering research supporting this model is the discovery that DCs extend dendrites into the lumen by penetrating the epithelium for tight connections [48].
DCs in the intestinal lamina propria (LP) of mice can be phenotypically divided into two major developmentally distinct populations, which are MHCII+ CD11chi CD103+ CD11b+ CX3CR1− M-CSFRlo DCs (CD103+ CD11b+ DCs) and MHCIIhi CD11chi CD103− CD11b+ CX3CR1+ M-CSFRhi DCs (CD103− CD11b+ DCs) [49]. Previous studies have shown that the DC assembly transports autoantigen from the ileum to the T-cell region of the mesenteric lymph node to maintain the immune response [50]. In addition, DCs are of the utmost importance in peripheral immune tolerance through the finely regulated DC and Foxp3+ Treg cell crosstalk, whereby Treg cells modulate DC phenotype and function. CD103+ DCs drive the differentiation of Foxp3+Treg cells from CD4+ T cells depending on retinoic acid (RA) through RALDH2 and TGF-β, with the assistance of integrin αvβ8 [51,52,53]. RALDH2, an enzyme expressed by cDCs in the gut and encoded by aldhla2, can produce RA from dietary vitamin A [54]. In the absence of CD103+ DCs, RALDH2 expression and Treg production were decreased, but Th1 response was enhanced [55] and the mechanisms for this change remain unclear. In colitis, the efficiency of producing Treg by MLN DCs is lower because of the specific loss of CD103+ DCs [56]. CTLA-4 expression in Treg cells down-/upregulates CD80/86 co-stimulatory molecules, which are significant for the activation of the immune response on DCs [51].
In some bacteria like L. reuteri and L. casei, IL-10 is also involved in inducing tolerogenic setting-predominated DC subtypes [53]. IL-10 also can inhibit multiple aspects of DC function including MHCI/II and CD80/CD86 co-stimulatory molecule expression and the release of pro-inflammatory cytokines [57]. In addition, IL-10 production by human DCs is triggered by Treg cells, which stimulate B7-H4 expression and render APCs immunosuppressive [58]. The immunomodulatory molecule, polysaccharide A (PSA), of B. fragilis mediates the conversion of CD4+ T cells into Foxp3+ Treg cells that produce IL-10 during commensal colonization and suppress the activation and proliferation of inflammatory effector T cells [59]. Endogenous biomolecules like adrenomedullin, hepatocyte growth factor, immunoglobulin-like transcript, NF-κB, placental growth factor, TGF, TNF, VEGF, and several possible molecular mechanisms or signal pathways exert a considerable tolerogenic influence on DC function [60,61].
Tolerogenic DCs (tol-DCs), which consist of naive immature DCs or alternatively activated semimature DCs induced by apoptotic cells or the regulatory cytokine milieu, play a pivotal role in immune tolerance [62]. Tol-DCs constitutively migrate throughout the periphery and the lymphatic system, presenting self-antigens in the absence of costimulatory molecules [63]. Meanwhile, DC plays a certain role in the immune tolerance of the human body to intestinal microorganisms, which is related to programmed death receptor 1 (PD-1). PD-1 is a member of the B7 family, and human or mouse PD-1 ligand (PD-L) 1 and PD-L2 are expressed on immature DCs, mature DCs, interferon (IFN)-treated monocytes, and follicular dendritic cells [64]. Binding of PD-L1 to PD-1 leads to inhibition of T cell receptor (TCR)-mediated lymphocyte proliferation and cytokine secretion [65]. Moreover, mice with PD-L1−/− developed autoimmune diseases, which indicated that peripheral tolerance was defective [66].
It is worth mentioning that unique tolerogenic properties are not only shaped by tissue-derived migratory CD103+ DCs, but also by resident lymph node (LN) stromal cells (SCs) [67]. A study has shown that mLN SCs are imprinted with a high Treg-inducing capacity soon after birth, and instruct LN-resident DCs (resDCs) to foster efficient Foxp3+ Treg induction in a Bmp2-dependent manner [68]. Bone morphogenetic protein (Bmp), a member of the TGF-β superfamily, has a synergistic effect with TGF-β on the induction of Foxp3+ iTreg [69]. These regulatory molecules or cells mentioned above contribute to the immunity tolerance caused by DCs.
4. Regulatory Relationship between the Gut and DCs
In most tissues, exposure to microbial products is sufficient to convert immature cDCs into mature cDCs, thereby producing an effective effector response. However, it is likely to be common that symbiotic bacteria expose their PAMPs in the healthy intestine. How the intestine can tolerate trillions of intestinal bacteria, initiate tolerance toward food antigens, and fight infections is the subject of an intense area of research. Recent advances have highlighted a fundamental role of mouse DCs in these functions.
Numerous studies have shown that exposure to PAMPs present on intestinal commensal bacteria promote DCs to express a unique molecular footprint so as to promote the differentiation of naive B2 cells into IgA, producing plasma cells with the help of RA and TGF-β [70,71]. IgA secreted by plasma cells effectively limits the penetration of commensal intestinal bacteria and opportunistic pathogens. Other studies have provided further evidence that stimulation of early bacterially exposed cells results in increased IL-10 secretion and the inhibition of DC differentiation through the MyD88 signaling pathway, leading to functional suppression [72].
Apart from the influence of intestinal flora, epithelial cells can also be affected by the condition of mucosal dendritic cells through the constitutive release of thymic stromal lymphopoietin (TSLP) and TGF-β. Commensal bacteria via microbe-associated molecular patterns (MAMPs) bind to TLRs on intestinal epithelium cells (IECs) and DCs, and upon activation of TLR signaling, IECs release TSLP and TGF-β [73]. TSLP and TGF-β cooperate to elicit the tolerogenic phenotype of DCs, as well as promoting the polarization of T cells toward a noninflammatory Th2 response [74,75]. Mincle, a Syk-kinase-coupled C-type lectin receptor, and Syk signaling couples the sensing of mucosa-associated bacteria by DCs in PPs with the production of IL-6 and IL-23, cytokines that regulate IL-22 and IL-17 production through T cells and innate lymphoid cells, thus promoting the intestinal immune barrier and limiting microbial translocation [76]. A common downstream signaling adaptor, caspase recruitment domain 9 (CARD9), is reported to regulate the gut mycrobial and bacterial landscape [77,78]. Based on the above, the explain for the general mechanism of DC immune tolerance is clear, which is of great importance in commensal intestinal bacteria colonization.
However, a study has demonstrated that the absence of CD103+ CD11b+ LP DCs, as well as reduced numbers of Th17 and Treg cells in the LP, does not significantly affect the composition of the steady-state enteric microflora [79]. This may demonstrate the importance of parental transmission and early bacterial explosion we mentioned before, which also deserves further study.
Germ-free (GF) mice showed delayed development of intestinal DC, indicating the important role of intestinal microbes in intestinal immunity [80,81]. Some researchers report that moDCs are able to mediate the responses of robust T helper cells (Th) 1 and Th17 upon stimulation by Escherichia coli Schaedler or Morganella morganii, whereas the probiotic Bacillus subtilis strain limits this effect [82]. Some studies indicate that lipid-regulated nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) plays a major role as a positive transcriptional regulator in human developmental DCs, mainly by controlling the genes involved in lipid metabolism and thereby indirectly modifying the immune phenotype [83]. Other researchers attached the importance of vitamin A to intestinal homeostasis, because cytokine-activated colonic epithelial cells trigger the secretion of distinct combinations of chemokines depending on the pro-inflammatory stimulus and are controlled by RA [84]. In addition, the outcome of moDC differentiation is able to accommodate to unique cellular microenvironments and remains remarkably plastic until the terminal differentiation of the moDCs ensues [82]. Bifidobacteria can significantly improve the antigen uptake and processing capacity of DCs in Crohn’s disease (CD) patients, which can partially solve the reduction of intestinal innate immunity and reduce the uncontrolled microbial growth in the intestinal tract of children with IBD [85]. Moreover, bifidobacteria are able to directly cause the production of DC maturation and cytokines, and activate the production and maturation of DCs [86], which in turn supports improved effector function of tumor-specific CD8+ T cells [87]. Researchers studied a symbiotic microorganism called Bifidobacterium longum subspecies infantis 35,624 and found that it can be recognized by mDCs and pDCs through different PRRs, inducing Foxp3T regulatory cells through different ways of maintaining intestinal homeostasis [88].
Intestinal bacteria have attracted extensive attention in the field of digestion, and it is worth mentioning that the role of enterovirus in intestinal homeostasis has gradually been recognized and verified by researchers. Researchers suggested that pretreatment with antiviral cocktail therapy can lead to severe colitis, and found that resident intestinal viruses played a protective role in gut inflammation through TLR3 and TLR7-mediated IFN-β secretion by pDCs [89]. On the other hand, several studies have shown that TLR3 agonist poly I:C pretreatment can improve the therapeutic effect of umbilical cord mesenchymal stem cells in dextran sulphate sodium (DSS)-induced colitis [90,91,92]. Importantly, there were disease-specific changes in the enterovirus in IBD, which is a significant expansion of the taxonomic richness of Caudovirales bacteriophages, and the viruses appear to be different, although the changes have been observed in both Crohn’s disease (CD) and ulcerative colitis (UC) [92]. In addition, during intestinal tumor therapy, DCs from the combination treatment group of radiotherapy and vancomycin significantly increased when compared with radiotherapy, which indicates the potential relationship between intestinal bacterial and DCs [93]. The summary of the regulatory relationship between gut and DC refers to Figure 2 and Box 2.
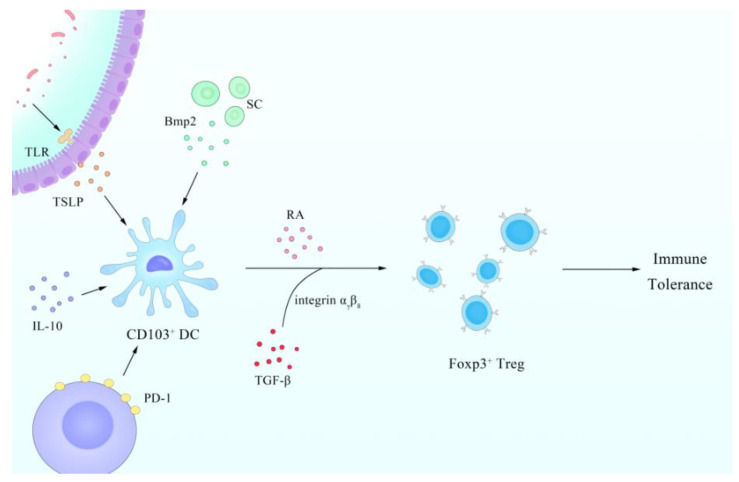
Figure 2.
Summary of the role of CD103+ DCs in intestinal immune tolerance. The immune tolerance in the intestine is mainly due to the Foxp3+ Treg. CD103+ DCs play a significant role in regulating Foxp3+ Treg, in which TGF-β and retinoic acid (RA) are important signaling molecules. IL-10, PD-1, and many others are also involved and have effects on DCs. In addition, on intestinal epithelium cells (IECs) and stromal cells (SCs) can promote the effect of CD103+ DCs after activation.
Box 2. Crosstalk and interaction between the gut and DCs.
DCs come into contact with intestinal flora within or without the assistance of microfold cells.
CD103+DCs help the establishment of immune tolerance through the effect of T cells.
IL-10, PD-1, and many other factors play roles in immune tolerance.
Differentiation and proliferation of DCs can be influenced by intestinal flora, epithelial cells, and viruses.
5. Intestinal DCs and IBD and Intestinal Tumors
IBD and DCs
IBD, including UC and CD, is a chronic inflammatory disease caused by microbial invasion or mucosal barrier damage, observed primarily in genetically susceptible populations. In recent years, tremendous investigation has proven the development of the pro-inflammatory effect of DC cells of mice on IBD, considering DCs as a potential target for the treatment of IBD [11,94].
A recent study suggested that the maladjustment of the Th1 response in the inflammatory colonic mucosa of IBD patients was caused by a change in PD-L1 expression in the mucosal mesenchymal stromal cell compartment, because increased PD-L1 expression inhibits Th1 cell activity in UC, whereas the loss of PD-L1 expression observed in CD leads to the persist existence of a Th1 inflammatory milieu [95]. Although the experiment focused on CD90+ stromal cells, the expression of PD-L1 in blood DCs did increase during CD. The detailed mechanism needs to be further studied. Similar studies have demonstrated the protective effect of PD-L1 mediated inhibitory signals during colon inflammation [96]. PD-L2, another ligand of PD-1, is expressed on DCs and through PD-L1 indirectly promotes the secretion of the proinflammatory cytokines TNF-α and IFN-γ, which are known to cause the pathogenesis of the disease and are involved in the progression of CD [97].
Furthermore, the role of pDCs in IBD has attracted the attention of scientists. Some researchers have demonstrated that the circulating pDCs in active IBD patients migrate to secondary lymphatic organs and inflammatory sites, secreting inflammatory cytokines such as IL-6, IL-8, and TNF-α [98,99]. It may lead to a pro-inflammatory phenotype in T cells (Th1), which helps to understand why the inflammation is perpetuated in IBD [100].
Experiments on mice showed that at homeostasis, pDCs can migrate to specific tissues, such as the lymph gland or gut, under the control of chemokine receptors including CCR5, CCR7 [101], CCR9 [102], and its corresponding ligands CCL19 (MIP-3β), CCL21, integrin α4β7, and CCL25 [103]. During the development of DSS-induced acute colitis in mice, the transcriptional expression level of CCL2, CCL3, CCL5, CCL7, CCL8, CCL25, CXCL9, CXCL10, and CXCL11 is increased [104]. These results suggest that pDCs may be chemokine-dependent and transported in the inflammatory laminae of the colon. However, through targeting the pDC-specific transcription factor TCF4 (E2-2) in experimental IBD caused by deficiency of Wiskott–Aldrich syndrome protein (WASP) or IL-10, some researchers proposed that pDCs do not play a major role in the pathogenesis of intestinal inflammation in IBD [105]. The differences regarding the roles of pDCs may be due to the use of pDC ablation systems and a genetic IBD model, which is T-cell-dependent, a different colitis model from T-cell-independent colitis induced by DSS [106]. Although the role of pDC in IBD may be controversial and deeper and more accurate studies are needed, the potential therapeutic value of pDCs in IBD cannot be completely ignored.
cDCs, another subset of DCs, may also play a role in the development of IBD. CD11c+ DCs, a subtype of cDCs mentioned above, were reduced by over 75% in the inflamed and uninflamed ilea in patients with CD compared to controls, as measured through multicolor tyramide fluorescent labeling with automated analysis, and these non-inflammatory areas showed no visible damage or inflammation, suggesting that the loss of DCs may be a precursor to subsequent damage [97]. In terms of health, the phenotypic DC subsets found in the intestinal mucosa maintain their tolerance and switch to a proinflammatory phenotype during infection or chronic IBD [107]. Dab2, a clathrin and cargo binding endocytic adaptor protein, modulates several cellular signaling pathways, including TGF-β [108], and has been linked to acute or chronic inflammation [109,110]. It was also found that Dab2 is mainly expressed in intestinal CD11b+ DCs, and the ablated expression of Dab2 in DC2.4 cells (murine immortalized dendritic cells)—measured using the CRISPR-CAS9 system—intensifies exacerbated experimental colitis [107]. In the presence of IL-15, all-trans RA induces the release of pro-inflammatory IL-12 and IL-23 by cDCs and promotes intestinal inflammation [111].
In contrast, some researchers found that CD103+ cDCs in patients with UC had acquired a potent ability to drive Th1/Th2/Th17 cell responses, which are associated with increased expression of pro-inflammatory cytokines [112]. The frequency of CD103+ cells among cDC1 and cDC2 subsets was lower in active IBD intestinal tissue compared to controls [113]. In mice, experiments show that the expression of p38α in CD103+ DCs regulated the balance between iTreg and Th1 differentiation in a TGF-β2-dependent manner [114]. A recent study revealed the further mechanism by which CD137, a potent costimulatory receptor for CD8+ T cells, can participate in activating TAK1 and subsequently stimulates the AMPK-PGC-1α axis to enhance expression of Aldh1a2, which encodes RALDH2 [115]. Furthermore, some studies revealed intestinal CD103+ CD11b- DCs could inhibit colitis through epithelial anti-inflammatory response induced by IFN-γ [116,117]. In addition, research shows that the frequency of αVβ8+ cDC2s, which are thought to induce Tregs, increased in Crohn’s patients compared to controls [118]. The evidence above shows the role of cDCs in IBD.
It can thus be seen that intestinal DCs may become a new treatment for patients with IBD and therefore warrant further study. For example, the latest studies proved that by transfecting primary rat bone marrow DCs with FasL plasmid into the peritoneum of IBD model rats, intestinal damage is reduced and the number of colon T cells, neutrophils, and pro-inflammatory Mφs decreased [119]. The summary of relationship between DC and IBD refers to Figure 3.
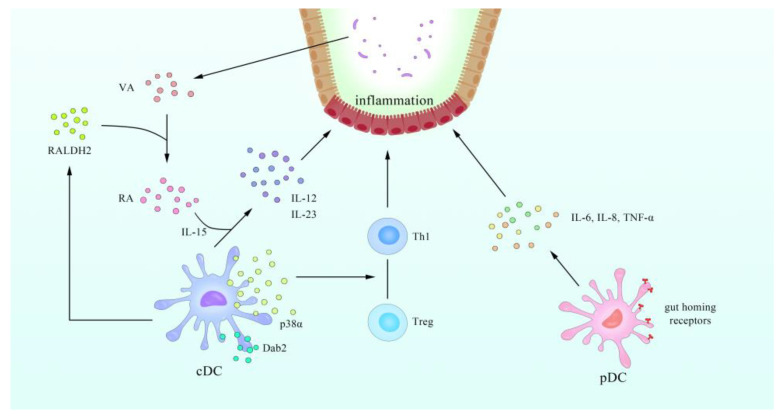
Figure 3.
A schematic overview of the regulatory relationship between DCs and inflammatory bowel disease (IBD). The lost integrity of the epithelial cell barrier and insufficient mucus layer facilitate bacterial translocation to subepithelial regions. Dab2 is expressed by cDCs. The expression of p38α in cDCs regulated the balance between iTreg and Th1 differentiation in a TGF-β2-dependent manner. RA, transforming from dietary vitamin A by RALDH2, induces the release of pro-inflammatory IL-12 and IL-23 by cDCs and promotes intestinal inflammation in the presence of IL-15. pDCs express gut homing receptors, which reside beneath the epithelial layer and are responsible for taking up luminal antigens and gaining the capacity to produce IL-6 and IL-8 TNF-α, which plays a key role in the establishment of IBD in model rats.5.2. Intestinal Tumors and DCs.
The relationship between DCs and tumors has been extensively studied, and the use of a “DC vaccine” has even been proposed to stimulate the entry of effector T cells into the tumor to suppress the tumor [120,121,122]. DC subsets are also considered to be predictive indictors of gastric cancer prognosis [123]. Some researchers suggested that a conserved DC regulatory program, which was related to the capture of cell-associated antigens in normal or excessive cell death, inhibited immune function and controlled the threshold of T-cell activation [124]. Inflammatory mediators and effector cells are important components of the tumor local microenvironment. In some types of cancer, chronic inflammation precedes malignant changes, and in others, carcinogenic changes trigger inflammatory microenvironments that promote tumor development [125,126].
Impaired numbers and functions of DCs have been widely observed in several types of cancer, including colon cancer, which may be related to tumor escape mechanisms that cancer cells use to evade host immune surveillance [127,128,129]. Tremendous studies have shown that the tumor microenvironment can act on human pDCs through immunosuppressive mediators (such as PGE2 and TGF-β [130]) or pDC regulatory receptors to inhibit or alter its functional activity, possibly leading to inhibition of IFN-α secretion or induction of Treg and preventing an effective anti-tumor response [131,132,133]. The IL-10 derived from tumors can also repress the antitumor immunity of human DCs [134]. In a murine breast cancer model, IL-10R was expressed at high levels on DCs, leading to the suppression of the anti-tumor cytokine IL-12 [121]. Another discovery was that the dysfunction of DCs may be related to lipid accumulation in human and mouse DCs caused by the up-regulation of scavenger receptor A, and thus DCs fail to effectively stimulate allogenic T cells or the presence of tumor-related antigens [135,136].
Another subset of DCs, inflammatory DCs (inf-DCs), is a subtype of moDCs described earlier, and can effectively capture and deliver tumor antigens [137,138]. Human inf-DCs are known to induce Th17 cells in inflammation and are one of the major sources of IL-17 [139]. IL-17R family members in inflammatory environments may play a role in the tumor-promoting effect of IL-23 [140]. Moreover, the barrier damage caused by the tumor triggers the inflammation caused by the tumor and promotes the growth of the tumor [141].
Inf-DCs can also produce reactive oxygen species (ROS) and directly mediate the anti-tumor response in mice [142]. Experiments on mice show that specific dynamic fluctuations in the expression of AT-rich sequence-binding protein 1 (Satb1) control the generation and immune stimulation activity of homeostasis DCs and inf-DCs, and the continuous overexpression of Satb1 transforms them into carcinogenic/pro-inflammatory cells, thus promoting malignant progression [143].
The binding of co-stimulatory molecules CD80/CD86 on DCs induces certain subsets of DCs to express functional indoleamine 2,3-dioxygenase (IDO), an enzyme that degrades essential amino acid tryptophan (Trp) into kynurenine (Kyn) [144]. IDO1-mediated tryptophan catabolism promotes local immune suppression in two ways [145]. The first is that tryptophan starvation limits T cell proliferation by weakening the T cell cycle mechanism, whereas the other one depends on the apoptosis of T cells caused by Kyn [146]. Wnt signaling networks in DCs that drive Treg responses have also been proven to be involved through several complicated signal axes, including molecules like β-catenin, RA, mTOR, IDO, and IL-10 [147].
In addition to the effect of DCs on tumors, γδT cells also have shown the ability to promote tumor progression by interfering with DC effector function, which can be mediated by bacteria through IL-1β and IL-23 [148]. As for the further mechanism, it is speculated that γδT cells suppress innate and adaptive immunity through the induction of immunosenescence, which significantly upregulated PD-L1 expression and resulted in other impaired phenotypic and functional features [134,149,150].
Another molecule mentioned above, PD-1, may play a vital role in tumor progression, as the antibody of PD-L1 can enhance the maturity of DCs and subvert the immunosuppressive state of the tumor microenvironment [151]. Anti-PD-1 cancer immunotherapy is based on the crosstalk between T cells and DCs, and the progress is licensed by IFN-γ and IL-12 [152].The summary of DC’s contact with intestinal tumor refers to Figure 4 and the contact with intestinal diseases refers to Box 3.
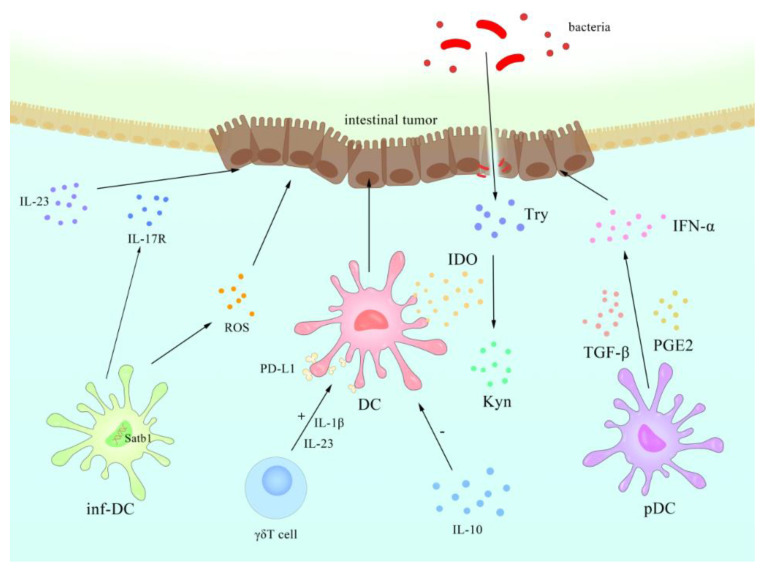
Figure 4.
Schematic representation of known regulatory mechanism between DCs and intestinal tumors. The ensuing intestinal flora are disturbed by the frequent use of antibiotics and/or diet, stimulating inflammation that is largely orchestrated by DCs. Their activation by products of pathogenic bacteria induces Try, which in turn causes changes in Kyn. In the tumor microenvironment, immunosuppressive mediators such as PGE2 and TGF-β can act on pDCs to inhibit or alter their functional activity, leading to inhibition of IFN-α secretion. The IL-10 derived from tumors can also repress the antitumor immunity of DCs. γδT cells can suppress innate and adaptive immunity by interfering with DC effector function, which can be mediated through IL-1β and IL-23, and significantly upregulate PD-L1 expression. The continuous overexpression of Satb1 transforms homeostasis DCs and inf-DCs into carcinogenic/pro-inflammatory cells. Inf-DCs can also produce DNA-damaging ROS and directly mediate the anti-tumor response.
Box 3. Diseases and DCs.
The detailed role of pDCs in IBD is controversial because of different experimental models.
cDCs are involved in the occurrence and development of IBD through several molecular processes like RA, IL-12, and IL-23 and influence the differentiation of T cells.
inf-DCs and PD-1 are vital to intestinal tumors through multiple immune factors.
6. Conclusions
The evidence above sufficiently demonstrates the importance of intestinal DCs, which may have huge potential for being applied in clinical treatments. DCs have several subgroups in different classification cases, including cDCs, pDCs, moDCs, and others, all of which participate in different regulatory networks. The regulatory relationship of DCs and intestinal microbiota provides a possible and easier way to access and influence DCs in the gut or even around the body. DCs are also involved in the immune tolerance to symbiotic bacterial colonization through Foxp3+ Treg cells, which require TGF-β, IL-10, RA, and many other biomolecules. Additionally, multiple molecules are combined in the complicated mechanism of regulating intestinal diseases such as IBD and intestinal tumors by DCs, which also makes it more difficult to achieve satisfactory therapeutic effects merely by altering several molecular targets. Collectively, DCs have shown large potential and warrant further and deeper studies from the prospective of intestinal physiology.
Acknowledgments
We appreciate Tao Xin from the Department of Applied Linguistics, School of Medical Humanities, Capital Medical University, for the excellent language expression assistance.
Abbreviations
| DC | dendritic cell |
| cDC | conventional DC |
| pDC | plasmacytoid dendritic DC |
| moDC | dendritic cell derived from monocytes |
| Mφs | macrophages |
| PAMP | pathogen associated molecular pattern |
| PRRs | pattern recognition receptors |
| TLR | toll-like receptor |
| RLR | retinoic acid-inducible gene I-like receptor |
| NLR | NOD-like receptor |
| NFIL3 | IL-3-regulated protein |
| LAG-3 | lymphocyte activation marker 3 |
| IBD | inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| GM-CSF | granulocyte/Mφ colony stimulating factor |
| SCFA | short chain fatty acid |
| TGF | transforming growth factor |
| PD-1 | programmed death receptor 1 |
| PD-L1 | programmed death receptor 1 ligand |
| IECs | intestinal epithelium cells |
| CARD9 | caspase recruitment domain 9 |
| Th | T helper cells |
| PPARγ | peroxisome proliferator-activated receptor γ |
| RA | retinoic acid |
| DSS | dextran sulphate sodium |
| WASP | Wiskott–Aldrich syndrome protein |
| Dab2 | disabled homolog 2 |
| inf-DCs | inflammatory DCs |
| ROS | reactive oxygen species |
| IDO | indoleamine 2,3-dioxygenase |
| Trp | tryptophan |
| Kyn | kynurenine |
| NF-κB | nuclear factor kappa B subunit |
| TGF | transforming growth factor |
| TNF, | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
| GF | germ free |
Author Contributions
Z.-J.Y., T.-T.W., and J.-D.X. wrote the manuscript; B.-Y.W., Y.-X.G., and F.-F.W. designed the illustrations; R.-X.H., H.-W.S., and X.L. analyzed the literature; J.-D.X. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by National Natural Science Foundation of China Grant (No. 81673671, No. 81274173) and Special National Key Research and Development Plan (No. 2016YFC1306305).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steinman R.M., Cohn Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussenzweig M.C., Steinman R.M., Unkeless J.C., Witmer M.D., Gutchinov B., Cohn Z.A. Studies of the cell surface of mouse dendritic cells and other leukocytes. J. Exp. Med. 1981;154:168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenzweig M.C., Steinman R.M., Witmer M.D., Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc. Natl. Acad. Sci. USA. 1982;79:161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudero-Hernandez C., Martin A., de Pedro Andres R., Fernandez-Salazar L., Garrote J.A., Bernardo D., Arranz E. Circulating Dendritic Cells from Celiac Disease Patients Display a Gut-Homing Profile and are Differentially Modulated by Different Gliadin-Derived Peptides. Mol. Nutr. Food Res. 2020;64:e1900989. doi: 10.1002/mnfr.201900989. [DOI] [PubMed] [Google Scholar]
- 5.Raki M., Beitnes A.C., Lundin K.E., Jahnsen J., Jahnsen F.L., Sollid L.M. Plasmacytoid dendritic cells are scarcely represented in the human gut mucosa and are not recruited to the celiac lesion. Mucosal Immunol. 2013;6:985–992. doi: 10.1038/mi.2012.136. [DOI] [PubMed] [Google Scholar]
- 6.Worbs T., Hammerschmidt S.I., Forster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 7.Gulden E., Chao C., Tai N., Pearson J.A., Peng J., Majewska-Szczepanik M., Zhou Z., Wong F.S., Wen L. TRIF deficiency protects non-obese diabetic mice from type 1 diabetes by modulating the gut microbiota and dendritic cells. J. Autoimmun. 2018;93:57–65. doi: 10.1016/j.jaut.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford B.M., Reizis B., Mabbott N.A. Oral Prion Disease Pathogenesis Is Impeded in the Specific Absence of CXCR5-Expressing Dendritic Cells. J. Virol. 2017;91:91. doi: 10.1128/JVI.00124-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B., Tian Q., Guo D., Lin W., Xie X., Bi H. Activated gammadelta T Cells Promote Dendritic Cell Maturation and Exacerbate the Development of Experimental Autoimmune Uveitis (EAU) in Mice. Immunol. Invest. 2020:1–20. doi: 10.1080/08820139.2020.1793775. [DOI] [PubMed] [Google Scholar]
- 10.Koyama M., Cheong M., Markey K.A., Gartlan K.H., Kuns R.D., Locke K.R., Lineburg K.E., Teal B.E., Leveque-El Mouttie L., Bunting M.D., et al. Donor colonic CD103+ dendritic cells determine the severity of acute graft-versus-host disease. J. Exp. Med. 2015;212:1303–1321. doi: 10.1084/jem.20150329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardo D., Chaparro M., Gisbert J.P. Human Intestinal Dendritic Cells in Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2018;62:e1700931. doi: 10.1002/mnfr.201700931. [DOI] [PubMed] [Google Scholar]
- 12.Audiger C., Fois A., Thomas A.L., Janssen E., Pelletier M., Lesage S. Merocytic Dendritic Cells Compose a Conventional Dendritic Cell Subset with Low Metabolic Activity. J. Immunol. 2020;205:121–132. doi: 10.4049/jimmunol.1900970. [DOI] [PubMed] [Google Scholar]
- 13.Solano-Galvez S.G., Tovar-Torres S.M., Tron-Gomez M.S., Weiser-Smeke A.E., Alvarez-Hernandez D.A., Franyuti-Kelly G.A., Tapia-Moreno M., Ibarra A., Gutierrez-Kobeh L., Vazquez-Lopez R. Human Dendritic Cells: Ontogeny and Their Subsets in Health and Disease. Med. Sci. 2018;6:88. doi: 10.3390/medsci6040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukhbaatar N., Hengstschlager M., Weichhart T. mTOR-Mediated Regulation of Dendritic Cell Differentiation and Function. Trends Immunol. 2016;37:778–789. doi: 10.1016/j.it.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collin M., McGovern N., Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilliams M., Ginhoux F., Jakubzick C., Naik S.H., Onai N., Schraml B.U., Segura E., Tussiwand R., Yona S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 18.Collin M., Bigley V. Human dendritic cell subsets: An update. Immunology. 2018;154:3–20. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granot T., Senda T., Carpenter D.J., Matsuoka N., Weiner J., Gordon C.L., Miron M., Kumar B.V., Griesemer A., Ho S.H., et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017;46:504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildner K., Edelson B.T., Purtha W.E., Diamond M., Matsushita H., Kohyama M., Calderon B., Schraml B.U., Unanue E.R., Diamond M.S., et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L., D’Amico A., Winkel K.D., Suter M., Lo D., Shortman K. RelB is essential for the development of myeloid-related CD8alpha- dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity. 1998;9:839–847. doi: 10.1016/S1074-7613(00)80649-4. [DOI] [PubMed] [Google Scholar]
- 22.Schiavoni G., Mattei F., Sestili P., Borghi P., Venditti M., Morse H.C., 3rd, Belardelli F., Gabriele L. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J. Exp. Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hacker C., Kirsch R.D., Ju X.S., Hieronymus T., Gust T.C., Kuhl C., Jorgas T., Kurz S.M., Rose-John S., Yokota Y., et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 24.Kashiwada M., Pham N.L., Pewe L.L., Harty J.T., Rothman P.B. NFIL3/E4BP4 is a key transcription factor for CD8alpha(+) dendritic cell development. Blood. 2011;117:6193–6197. doi: 10.1182/blood-2010-07-295873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seillet C., Jackson J.T., Markey K.A., Brady H.J., Hill G.R., Macdonald K.P., Nutt S.L., Belz G.T. CD8alpha+ DCs can be induced in the absence of transcription factors Id2, Nfil3, and Batf3. Blood. 2013;121:1574–1583. doi: 10.1182/blood-2012-07-445650. [DOI] [PubMed] [Google Scholar]
- 26.Guerriero A., Langmuir P.B., Spain L.M., Scott E.W. PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood. 2000;95:879–885. doi: 10.1182/blood.V95.3.879.003k13_879_885. [DOI] [PubMed] [Google Scholar]
- 27.Shortman K., Naik S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 28.Villadangos J.A., Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 29.Mahiddine K., Hassel C., Murat C., Girard M., Guerder S. Tissue-Specific Factors Differentially Regulate the Expression of Antigen-Processing Enzymes During Dendritic Cell Ontogeny. Front. Immunol. 2020;11:453. doi: 10.3389/fimmu.2020.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swiecki M., Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzionek A., Fuchs A., Schmidt P., Cremer S., Zysk M., Miltenyi S., Buck D.W., Schmitz J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 32.Matsui T., Connolly J.E., Michnevitz M., Chaussabel D., Yu C.I., Glaser C., Tindle S., Pypaert M., Freitas H., Piqueras B., et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J. Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues P.F., Alberti-Servera L., Eremin A., Grajales-Reyes G.E., Ivanek R., Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat. Immunol. 2018;19:711–722. doi: 10.1038/s41590-018-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Won H.Y., Lee J.Y., Ryu D., Kim H.T., Chang S.Y. The Role of Plasmacytoid Dendritic Cells in Gut Health. Immune Netw. 2019;19:e6. doi: 10.4110/in.2019.19.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villadangos J.A., Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Swiecki M., Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawai C.M., Sisirak V., Ghosh H.S., Hou E.Z., Ceribelli M., Staudt L.M., Reizis B. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J. Exp. Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tel J., van der Leun A.M., Figdor C.G., Torensma R., de Vries I.J. Harnessing human plasmacytoid dendritic cells as professional APCs. Cancer Immunol. Immunother. 2012;61:1279–1288. doi: 10.1007/s00262-012-1210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., Biagi E., Andersen M.H., Brigidi P., Odum N., et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivollier A., He J., Kole A., Valatas V., Kelsall B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanez A., Coetzee S.G., Olsson A., Muench D.E., Berman B.P., Hazelett D.J., Salomonis N., Grimes H.L., Goodridge H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity. 2017;47:890–902. doi: 10.1016/j.immuni.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakubzick C.V., Randolph G.J., Henson P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017;17:349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 43.Menezes S., Melandri D., Anselmi G., Perchet T., Loschko J., Dubrot J., Patel R., Gautier E.L., Hugues S., Longhi M.P., et al. The Heterogeneity of Ly6C(hi) Monocytes Controls Their Differentiation into iNOS(+) Macrophages or Monocyte-Derived Dendritic Cells. Immunity. 2016;45:1205–1218. doi: 10.1016/j.immuni.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Mach J., Hshieh T., Hsieh D., Grubbs N., Chervonsky A. Development of intestinal M cells. Immunol. Rev. 2005;206:177–189. doi: 10.1111/j.0105-2896.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 46.Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 47.Man A.L., Prieto-Garcia M.E., Nicoletti C. Improving M cell mediated transport across mucosal barriers: Do certain bacteria hold the keys? Immunology. 2004;113:15–22. doi: 10.1111/j.1365-2567.2004.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J.P., Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 49.Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kornete M., Piccirillo C.A. Functional crosstalk between dendritic cells and Foxp3(+) regulatory T cells in the maintenance of immune tolerance. Front. Immunol. 2012;3:165. doi: 10.3389/fimmu.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiokawa A., Kotaki R., Takano T., Nakajima-Adachi H., Hachimura S. Mesenteric lymph node CD11b(-) CD103(+) PD-L1(High) dendritic cells highly induce regulatory T cells. Immunology. 2017;152:52–64. doi: 10.1111/imm.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Stagg A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018;9:2883. doi: 10.3389/fimmu.2018.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boschetti G., Kanjarawi R., Bardel E., Collardeau-Frachon S., Duclaux-Loras R., Moro-Sibilot L., Almeras T., Flourie B., Nancey S., Kaiserlian D. Gut Inflammation in Mice Triggers Proliferation and Function of Mucosal Foxp3+ Regulatory T Cells but Impairs Their Conversion from CD4+ T Cells. J. Crohns Colitis. 2017;11:105–117. doi: 10.1093/ecco-jcc/jjw125. [DOI] [PubMed] [Google Scholar]
- 57.Mahnke K., Johnson T.S., Ring S., Enk A.H. Tolerogenic dendritic cells and regulatory T cells: A two-way relationship. J. Dermatol. Sci. 2007;46:159–167. doi: 10.1016/j.jdermsci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Kryczek I., Wei S., Zou L., Zhu G., Mottram P., Xu H., Chen L., Zou W. Cutting edge: Induction of B7-H4 on APCs through IL-10: Novel suppressive mode for regulatory T cells. J. Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 59.Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Svajger U., Rozman P. Induction of Tolerogenic Dendritic Cells by Endogenous Biomolecules: An Update. Front. Immunol. 2018;9:2482. doi: 10.3389/fimmu.2018.02482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steimle A., Frick J.S. Molecular Mechanisms of Induction of Tolerant and Tolerogenic Intestinal Dendritic Cells in Mice. J. Immunol. Res. 2016;2016:1958650. doi: 10.1155/2016/1958650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fucikova J., Palova-Jelinkova L., Bartunkova J., Spisek R. Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications. Front. Immunol. 2019;10:2393. doi: 10.3389/fimmu.2019.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horton C., Shanmugarajah K., Fairchild P.J. Harnessing the properties of dendritic cells in the pursuit of immunological tolerance. Biomed. J. 2017;40:80–93. doi: 10.1016/j.bj.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown J.A., Dorfman D.M., Ma F.R., Sullivan E.L., Munoz O., Wood C.R., Greenfield E.A., Freeman G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 65.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Latchman Y.E., Liang S.C., Wu Y., Chernova T., Sobel R.A., Klemm M., Kuchroo V.K., Freeman G.J., Sharpe A.H. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fletcher A.L., Acton S.E., Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat. Rev. Immunol. 2015;15:350–361. doi: 10.1038/nri3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pezoldt J., Pasztoi M., Zou M., Wiechers C., Beckstette M., Thierry G.R., Vafadarnejad E., Floess S., Arampatzi P., Buettner M., et al. Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes. Nat. Commun. 2018;9:3903. doi: 10.1038/s41467-018-06423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu L., Ma J., Wang X., Wang J., Zhang F., Yu J., He G., Xu B., Brand D.D., Horwitz D.A., et al. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3+ Treg. Eur. J. Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Massacand J.C., Kaiser P., Ernst B., Tardivel A., Burki K., Schneider P., Harris N.L. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS ONE. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakai F., Hosoya T., Ono-Ohmachi A., Ukibe K., Ogawa A., Moriya T., Kadooka Y., Shiozaki T., Nakagawa H., Nakayama Y., et al. Lactobacillus gasseri SBT2055 induces TGF-beta expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS ONE. 2014;9:e105370. doi: 10.1371/journal.pone.0105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davies J.M., Sheil B., Shanahan F. Bacterial signalling overrides cytokine signalling and modifies dendritic cell differentiation. Immunology. 2009;128:e805–e815. doi: 10.1111/j.1365-2567.2009.03086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weng M., Walker W.A. The role of gut microbiota in programming the immune phenotype. J. Dev. Orig. Health Dis. 2013;4:203–214. doi: 10.1017/S2040174412000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G.M., Nespoli A., Viale G., Allavena P., Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 75.Zeuthen L.H., Fink L.N., Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinez-Lopez M., Iborra S., Conde-Garrosa R., Mastrangelo A., Danne C., Mann E.R., Reid D.M., Gaboriau-Routhiau V., Chaparro M., Lorenzo M.P., et al. Microbiota Sensing by Mincle-Syk Axis in Dendritic Cells Regulates Interleukin-17 and -22 Production and Promotes Intestinal Barrier Integrity. Immunity. 2019;50:446–461. doi: 10.1016/j.immuni.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik A., Sharma D., Malireddi R.K.S., Guy C.S., Chang T.C., Olsen S.R., Neale G., Vogel P., Kanneganti T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity. 2018;49:515–530. doi: 10.1016/j.immuni.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamas B., Richard M.L., Leducq V., Pham H.P., Michel M.L., Da Costa G., Bridonneau C., Jegou S., Hoffmann T.W., Natividad J.M., et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welty N.E., Staley C., Ghilardi N., Sadowsky M.J., Igyarto B.Z., Kaplan D.H. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J. Exp. Med. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams A.M., Probert C.S., Stepankova R., Tlaskalova-Hogenova H., Phillips A., Bland P.W. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology. 2006;119:470–478. doi: 10.1111/j.1365-2567.2006.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bene K., Varga Z., Petrov V.O., Boyko N., Rajnavolgyi E. Gut Microbiota Species Can Provoke both Inflammatory and Tolerogenic Immune Responses in Human Dendritic Cells Mediated by Retinoic Acid Receptor Alpha Ligation. Front. Immunol. 2017;8:427. doi: 10.3389/fimmu.2017.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szatmari I., Torocsik D., Agostini M., Nagy T., Gurnell M., Barta E., Chatterjee K., Nagy L. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007;110:3271–3280. doi: 10.1182/blood-2007-06-096222. [DOI] [PubMed] [Google Scholar]
- 84.Chatterjee A., Gogolak P., Blottiere H.M., Rajnavolgyi E. The impact of ATRA on shaping human myeloid cell responses to epithelial cell-derived stimuli and on T-lymphocyte polarization. Mediat. Inflamm. 2015;2015:579830. doi: 10.1155/2015/579830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strisciuglio C., Miele E., Giugliano F.P., Vitale S., Andreozzi M., Vitale A., Catania M.R., Staiano A., Troncone R., Gianfrani C. Bifidobacteria Enhance Antigen Sampling and Processing by Dendritic Cells in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015;21:1491–1498. doi: 10.1097/MIB.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 86.Lopez P., Gueimonde M., Margolles A., Suarez A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int. J. Food Microbiol. 2010;138:157–165. doi: 10.1016/j.ijfoodmicro.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 87.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Konieczna P., Groeger D., Ziegler M., Frei R., Ferstl R., Shanahan F., Quigley E.M., Kiely B., Akdis C.A., O’Mahony L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 89.Yang J.Y., Kim M.S., Kim E., Cheon J.H., Lee Y.S., Kim Y., Lee S.H., Seo S.U., Shin S.H., Choi S.S., et al. Enteric Viruses Ameliorate Gut Inflammation via Toll-like Receptor 3 and Toll-like Receptor 7-Mediated Interferon-beta Production. Immunity. 2016;44:889–900. doi: 10.1016/j.immuni.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Zhao H.W., Yue Y.H., Han H., Chen X.L., Lu Y.G., Zheng J.M., Hou H.T., Lang X.M., He L.L., Hu Q.L., et al. Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis. World J. Gastroenterol. 2017;23:999–1009. doi: 10.3748/wjg.v23.i6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuenzalida P., Kurte M., Fernandez-O’ryan C., Ibanez C., Gauthier-Abeliuk M., Vega-Letter A.M., Gonzalez P., Irarrazabal C., Quezada N., Figueroa F., et al. Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy. 2016;18:630–641. doi: 10.1016/j.jcyt.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 92.Qiu Y., Guo J., Mao R., Chao K., Chen B.L., He Y., Zeng Z.R., Zhang S.H., Chen M.H. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. 2017;10:727–742. doi: 10.1038/mi.2016.78. [DOI] [PubMed] [Google Scholar]
- 93.Uribe-Herranz M., Rafail S., Beghi S., Gil-de-Gomez L., Verginadis I., Bittinger K., Pustylnikov S., Pierini S., Perales-Linares R., Blair I.A., et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2020;130:466–479. doi: 10.1172/JCI124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bates J., Diehl L. Dendritic cells in IBD pathogenesis: An area of therapeutic opportunity? J. Pathol. 2014;232:112–120. doi: 10.1002/path.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beswick E.J., Grim C., Singh A., Aguirre J.E., Tafoya M., Qiu S., Rogler G., McKee R., Samedi V., Ma T.Y., et al. Expression of Programmed Death-Ligand 1 by Human Colonic CD90(+) Stromal Cells Differs Between Ulcerative Colitis and Crohn’s Disease and Determines Their Capacity to Suppress Th1 Cells. Front. Immunol. 2018;9:1125. doi: 10.3389/fimmu.2018.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song M.Y., Hong C.P., Park S.J., Kim J.H., Yang B.G., Park Y., Kim S.W., Kim K.S., Lee J.Y., Lee S.W., et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut. 2015;64:260–271. doi: 10.1136/gutjnl-2014-307311. [DOI] [PubMed] [Google Scholar]
- 97.Faleiro R., Liu J., Karunarathne D., Edmundson A., Winterford C., Nguyen T.H., Simms L.A., Radford-Smith G., Wykes M. Crohn’s disease is facilitated by a disturbance of programmed death-1 ligand 2 on blood dendritic cells. Clin. Transl. Immunol. 2019;8:e01071. doi: 10.1002/cti2.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baumgart D.C., Metzke D., Schmitz J., Scheffold A., Sturm A., Wiedenmann B., Dignass A.U. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–236. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baumgart D.C., Metzke D., Guckelberger O., Pascher A., Grotzinger C., Przesdzing I., Dorffel Y., Schmitz J., Thomas S. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn’s disease and ulcerative colitis. Clin. Exp. Immunol. 2011;166:46–54. doi: 10.1111/j.1365-2249.2011.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 101.Seth S., Oberdorfer L., Hyde R., Hoff K., Thies V., Worbs T., Schmitz S., Forster R. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J. Immunol. 2011;186:3364–3372. doi: 10.4049/jimmunol.1002598. [DOI] [PubMed] [Google Scholar]
- 102.Wendland M., Czeloth N., Mach N., Malissen B., Kremmer E., Pabst O., Forster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl. Acad. Sci. USA. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lombardi V.C., Khaiboullina S.F. Plasmacytoid dendritic cells of the gut: Relevance to immunity and pathology. Clin. Immunol. 2014;153:165–177. doi: 10.1016/j.clim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arimura K., Takagi H., Uto T., Fukaya T., Nakamura T., Choijookhuu N., Hishikawa Y., Yamashita Y., Sato K. Crucial role of plasmacytoid dendritic cells in the development of acute colitis through the regulation of intestinal inflammation. Mucosal Immunol. 2017;10:957–970. doi: 10.1038/mi.2016.96. [DOI] [PubMed] [Google Scholar]
- 105.Sawai C.M., Serpas L., Neto A.G., Jang G., Rashidfarrokhi A., Kolbeck R., Sanjuan M.A., Reizis B., Sisirak V. Plasmacytoid Dendritic Cells Are Largely Dispensable for the Pathogenesis of Experimental Inflammatory Bowel Disease. Front. Immunol. 2018;9:2475. doi: 10.3389/fimmu.2018.02475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiesler P., Fuss I.J., Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Figliuolo da Paz V., Jamwal D.R., Gurney M., Midura-Kiela M., Harrison C.A., Cox C., Wilson J.M., Ghishan F.K., Kiela P.R. Rapid Downregulation of DAB2 by Toll-Like Receptor Activation Contributes to a Pro-Inflammatory Switch in Activated Dendritic Cells. Front. Immunol. 2019;10:304. doi: 10.3389/fimmu.2019.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hannigan A., Smith P., Kalna G., Lo Nigro C., Orange C., O’Brien D.I., Shah R., Syed N., Spender L.C., Herrera B., et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-beta from a tumor suppressor to a tumor promoter. J. Clin. Investig. 2010;120:2842–2857. doi: 10.1172/JCI36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adamson S.E., Griffiths R., Moravec R., Senthivinayagam S., Montgomery G., Chen W., Han J., Sharma P.R., Mullins G.R., Gorski S.A., et al. Disabled homolog 2 controls macrophage phenotypic polarization and adipose tissue inflammation. J. Clin. Investig. 2016;126:1311–1322. doi: 10.1172/JCI79590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adamson S.E., Polanowska-Grabowska R., Marqueen K., Griffiths R., Angdisen J., Breevoort S.R., Schulman I.G., Leitinger N. Deficiency of Dab2 (Disabled Homolog 2) in Myeloid Cells Exacerbates Inflammation in Liver and Atherosclerotic Plaques in LDLR (Low-Density Lipoprotein Receptor)-Null Mice-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2018;38:1020–1029. doi: 10.1161/ATVBAHA.117.310467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DePaolo R.W., Abadie V., Tang F., Fehlner-Peach H., Hall J.A., Wang W., Marietta E.V., Kasarda D.D., Waldmann T.A., Murray J.A., et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsuno H., Kayama H., Nishimura J., Sekido Y., Osawa H., Barman S., Ogino T., Takahashi H., Haraguchi N., Hata T., et al. CD103+ Dendritic Cell Function Is Altered in the Colons of Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2017;23:1524–1534. doi: 10.1097/MIB.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 113.Magnusson M.K., Brynjolfsson S.F., Dige A., Uronen-Hansson H., Borjesson L.G., Bengtsson J.L., Gudjonsson S., Ohman L., Agnholt J., Sjovall H., et al. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 2016;9:171–182. doi: 10.1038/mi.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang G., Wang Y., Chi H. Control of T cell fates and immune tolerance by p38alpha signaling in mucosal CD103+ dendritic cells. J. Immunol. 2013;191:650–659. doi: 10.4049/jimmunol.1300398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin J., Jung I.H., Moon S.H., Jeon S., Jeong S.J., Sonn S.K., Seo S., Lee M.N., Song E.J., Kweon H.Y., et al. CD137 Signaling Regulates Acute Colitis via RALDH2-Expressing CD11b(−)CD103(+) DCs. Cell Rep. 2020;30:4124–4136. doi: 10.1016/j.celrep.2020.02.103. [DOI] [PubMed] [Google Scholar]
- 116.Muzaki A.R., Tetlak P., Sheng J., Loh S.C., Setiagani Y.A., Poidinger M., Zolezzi F., Karjalainen K., Ruedl C. Intestinal CD103(+)CD11b(−) dendritic cells restrain colitis via IFN-gamma-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2016;9:336–351. doi: 10.1038/mi.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thelemann C., Eren R.O., Coutaz M., Brasseit J., Bouzourene H., Rosa M., Duval A., Lavanchy C., Mack V., Mueller C., et al. Interferon-gamma induces expression of MHC class II on intestinal epithelial cells and protects mice from colitis. PLoS ONE. 2014;9:e86844. doi: 10.1371/journal.pone.0086844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fenton T.M., Kelly A., Shuttleworth E.E., Smedley C., Atakilit A., Powrie F., Campbell S., Nishimura S.L., Sheppard D., Levison S., et al. Inflammatory cues enhance TGFbeta activation by distinct subsets of human intestinal dendritic cells via integrin alphavbeta8. Mucosal Immunol. 2017;10:624–634. doi: 10.1038/mi.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Jesus E.R., Isidro R.A., Cruz M.L., Marty H., Appleyard C.B. Adoptive Transfer of Dendritic Cells Expressing Fas Ligand Modulates Intestinal Inflammation in a Model of Inflammatory Bowel Disease. J. Clin. Cell. Immunol. 2016;7 doi: 10.4172/2155-9899.1000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalinski P., Muthuswamy R., Urban J. Dendritic cells in cancer immunotherapy: Vaccines and combination immunotherapies. Expert Rev. Vaccines. 2013;12:285–295. doi: 10.1586/erv.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DeVito N.C., Plebanek M.P., Theivanthiran B., Hanks B.A. Role of Tumor-Mediated Dendritic Cell Tolerization in Immune Evasion. Front. Immunol. 2019;10:2876. doi: 10.3389/fimmu.2019.02876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Plantinga M., de Haar C.G., Dunnebach E., van den Beemt D., Bloemenkamp K.W.M., Mokry M., Boelens J.J., Nierkens S. Cord-Blood-Stem-Cell-Derived Conventional Dendritic Cells Specifically Originate from CD115-Expressing Precursors. Cancers. 2019;11:181. doi: 10.3390/cancers11020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li F., Sun Y., Huang J., Xu W., Liu J., Yuan Z. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med. 2019;8:7330–7344. doi: 10.1002/cam4.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maier B., Leader A.M., Chen S.T., Tung N., Chang C., LeBerichel J., Chudnovskiy A., Maskey S., Walker L., Finnigan J.P., et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580:257–262. doi: 10.1038/s41586-020-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 126.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwaab T., Weiss J.E., Schned A.R., Barth R.J., Jr. Dendritic cell infiltration in colon cancer. J. Immunother. 2001;24:130–137. doi: 10.1097/00002371-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 128.Legitimo A., Consolini R., Failli A., Orsini G., Spisni R. Dendritic cell defects in the colorectal cancer. Hum. Vaccin. Immunother. 2014;10:3224–3235. doi: 10.4161/hv.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang M., Diao J., Cattral M.S. Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell. Mol. Life Sci. 2017;74:761–776. doi: 10.1007/s00018-016-2317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Terra M., Oberkampf M., Fayolle C., Rosenbaum P., Guillerey C., Dadaglio G., Leclerc C. Tumor-Derived TGFbeta Alters the Ability of Plasmacytoid Dendritic Cells to Respond to Innate Immune Signaling. Cancer Res. 2018;78:3014–3026. doi: 10.1158/0008-5472.CAN-17-2719. [DOI] [PubMed] [Google Scholar]
- 131.Demoulin S., Herfs M., Delvenne P., Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: Insight into the molecular mechanisms. J. Leukoc. Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 132.Gai X.D., Song Y., Li C., Lei Y.M., Yang B. Potential role of plasmacytoid dendritic cells for FOXP3+ regulatory T cell development in human colorectal cancer and tumor draining lymph node. Pathol. Res. Pract. 2013;209:774–778. doi: 10.1016/j.prp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 133.Huang X.M., Liu X.S., Lin X.K., Yu H., Sun J.Y., Liu X.K., Chen C., Jin H.L., Zhang G.E., Shi X.X., et al. Role of plasmacytoid dendritic cells and inducible costimulator-positive regulatory T cells in the immunosuppression microenvironment of gastric cancer. Cancer Sci. 2014;105:150–158. doi: 10.1111/cas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng G., Wang H.Y., Peng W., Kiniwa Y., Seo K.H., Wang R.F. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 135.Herber D.L., Cao W., Nefedova Y., Novitskiy S.V., Nagaraj S., Tyurin V.A., Corzo A., Cho H.I., Celis E., Lennox B., et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao F., Liu C., Guo J., Sun W., Xian L., Bai D., Liu H., Cheng Y., Li B., Cui J., et al. Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer. Sci. Rep. 2015;5:9613. doi: 10.1038/srep09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veglia F., Gabrilovich D.I. Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Segura E., Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 139.Segura E., Amigorena S. Identification of human inflammatory dendritic cells. Oncoimmunology. 2013;2:e23851. doi: 10.4161/onci.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Langowski J.L., Zhang X., Wu L., Mattson J.D., Chen T., Smith K., Basham B., McClanahan T., Kastelein R.A., Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 141.Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D., Taniguchi K., Yu G.Y., Osterreicher C.H., Hung K.E., et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pommier A., Audemard A., Durand A., Lengagne R., Delpoux A., Martin B., Douguet L., Le Campion A., Kato M., Avril M.F., et al. Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2013;110:13085–13090. doi: 10.1073/pnas.1300314110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tesone A.J., Rutkowski M.R., Brencicova E., Svoronos N., Perales-Puchalt A., Stephen T.L., Allegrezza M.J., Payne K.K., Nguyen J.M., Wickramasinghe J., et al. Satb1 Overexpression Drives Tumor-Promoting Activities in Cancer-Associated Dendritic Cells. Cell Rep. 2016;14:1774–1786. doi: 10.1016/j.celrep.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mellor A.L., Munn D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 145.Wculek S.K., Khouili S.C., Priego E., Heras-Murillo I., Sancho D. Metabolic Control of Dendritic Cell Functions: Digesting Information. Front. Immunol. 2019;10:775. doi: 10.3389/fimmu.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fallarino F., Grohmann U., Vacca C., Bianchi R., Orabona C., Spreca A., Fioretti M.C., Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 147.Suryawanshi A., Hussein M.S., Prasad P.D., Manicassamy S. Wnt Signaling Cascade in Dendritic Cells and Regulation of Anti-tumor Immunity. Front. Immunol. 2020;11:122. doi: 10.3389/fimmu.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fleming C., Morrissey S., Cai Y., Yan J. gammadelta T Cells: Unexpected Regulators of Cancer Development and Progression. Trends Cancer. 2017;3:561–570. doi: 10.1016/j.trecan.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ye J., Ma C., Hsueh E.C., Eickhoff C.S., Zhang Y., Varvares M.A., Hoft D.F., Peng G. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J. Immunol. 2013;190:2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun N.Y., Chen Y.L., Wu W.Y., Lin H.W., Chiang Y.C., Chang C.F., Tai Y.J., Hsu H.C., Chen C.A., Sun W.Z., et al. Blockade of PD-L1 Enhances Cancer Immunotherapy by Regulating Dendritic Cell Maturation and Macrophage Polarization. Cancers. 2019;11:1400. doi: 10.3390/cancers11091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garris C.S., Arlauckas S.P., Kohler R.H., Trefny M.P., Garren S., Piot C., Engblom C., Pfirschke C., Siwicki M., Gungabeesoon J., et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148–1161. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]