Abstract
Simple Summary
Genital leptospirosis is an important example of the neglected infectious zoonotic disease caused by Leptospira. The disease was just evaluated in bovine and domestic pig with important consequences for reproductive success. Recently, pathogenic Leptospira strains were also isolated and detected from reproductive system tissues collected from wild boar (Sus scrofa) free ranging in the Tuscany and Sardinia regions (Italy). This investigation aimed to understand this aspect in wild boar, describing the detection of intermediate Leptospira DNA belonging to Leptospira fainei for the first time in male reproductive organs of hunted wild boar. The obtained data shed significant light on this intermediate Leptospira species, because, other than circulating in wildlife, it can localize in testicles and epididymides of wild boar specimens. These findings add important information on genital leptospirosis epidemiology, especially among the wildlife that remains less investigated.
Abstract
Leptospirosis is a re-emerging and worldwide diffused zoonosis. Recently, the high importance of their epidemiology was explained by the intermediate Leptospira strains. Among these strains, Leptospira fainei was the first intermediate strain detected in domestic and wild swine. Wild boars (Sus scrofa) are well known as a reservoir, as well as all swine, for pathogenic Leptospira, but very little information is available concerning intermediate Leptospira infection. The investigation aim was to evaluate if intermediate Leptospira can infect the reproductive systems of wild boars hunted in the Tuscany region (Italy), as previously demonstrated for pathogenic ones. The reproductive system tissue (testicles, epididymides, uteri), and placentas and fetuses, were collected from 200 regularly hunted animals. Bacteriological examination and real-time PCR were performed to detect intermediate Leptospira DNA. Unfortunately, no isolates were obtained. Using real-time PCR, in six (3%) male organs (both testicles and epididymis), intermediate Leptospira DNA was found. The amplification of the 16S rRNA gene identified that all DNA obtained belong to Leptospira fainei. The results of this investigation highlighted for the first time the localization of Leptospira fainei in the male wild boar reproductive system, opening up a new avenue to further investigate.
Keywords: wild boar, intermediate Leptospira, Leptospira fainei, leptospirosis, reproductive system, testicles, epididymis, Italy, Tuscany region
1. Introduction
Leptospirosis is a re-emerging and neglected zoonoses diffused in all parts of the world. The disease is caused by bacteria belonging to the order Spirochaetales and the genus Leptospira [1,2,3]. Recently, due to a genomic approach and based on their clinical presentation, the Leptospira species were divided into three groups: pathogenic, which cause the most severe infection; intermediate, which cause a mild to severe infection; and saprophytic, which are naturally present in the environment and do not usually cause disease [3,4,5,6]. Nowadays, the Leptospira genus includes 64 different species, with 17 belonging to pathogenic group (Leptospira interrogans, Leptospira kirschneri, Leptospira noguchii, Leptospira borgpetersenii, Leptospira adleri, Leptospira alexanderi, Leptospira weilii, Leptospira santarosai, Leptospira kmetyi, Leptospira alstoni Leptospira mayottensis, Leptospira ellisii Leptospira barontonii, Leptospira dzianensis, Leptospira tipperaryensis, Leptospira gomenensis and Leptospira putramalaysiae), 21 to the intermediate group (Leptospira perolatii, Leptospira venezuelensis, Leptospira neocaledonica, Leptospira saintgironsiae, Leptospira haakeii, Leptospira hartskeelii, Leptospira licerasiae, Leptospira wolffii, Leptospira fainei, Leptospira broomii, Leptospira inadai, Leptospira andrefontaineae, Leptospira dzoumogneensis, Leptospira koniamboensis, Leptospira sarikeiensis, Leptospira johnsonii, Leptospira fluminis, Leptospira fletcheri, Leptospira semungkisensis, Leptospira langatensis and Leptospira selangorensis), and 26 to the saprophytic group (Leptospira idonii, Leptospira meyeri, Leptospira terpstrae, Leptospira biflexa, Leptospira levettiii, Leptospira brenneri, Leptospira harrisiae, Leptospira vanthielii, Leptospira yanagawae, Leptospira wolbachii, Leptospira bandrabouensis, Leptospira noumeaensis, Leptospira jelokensis, Leptospira bourretii, Leptospira kanakyensis, Leptospira kemamanensis, Leptospira mtsangambouensis, Leptospira bouyouniensis, Leptospira ellinghausenii, Leptospira congkakensis, Leptospira perdikensis, Leptospira montravelensis and Leptospira ognonensis, Leptospira ilyithenensis, Leptospira kobayashii, Leptospira ryugenii) [3,7].
Due to its phylogenetic position, between saprophytic and pathogenic, the intermediate Leptospira species could have a wide range of antigens in common with pathogenic serovars, and they may be responsible for mild or severe infections [8]. Among intermediate species, Leptospira fainei play an important role in the epidemiology of leptospirosis.
The intermediate Leptospira fainei serovar Hurstbridge was originally isolated, in 1994, from the kidneys and the uteri of domestic pigs bred in Australia [9], and, in the same year, was serologically detected in symptomatic and asymptomatic humans in Australia and the archipelagos of the Seychelles [10,11]. Later, leptospires belonging to this species were isolated from ill human patients with symptoms associable with leptospirosis in Denmark [12], France [13], Peru [14], India [5], and Ecuador [15].
Concerning animal Leptospira fainei infection, no isolates were obtained, but its DNA was detected in bats collected in Peru [16,17]. Consequently, within a leptospirosis survey, Leptospira fainei DNA was detected in wild boar (Sus scrofa) kidneys in Italy [18,19].
In Italy, as well as in all of Europe, wild boar is the most diffused wild ungulate [20,21]. This mammal is highly adaptable, and it can live in several habitats, especially near urban areas [20,22]. Wild boar is recognized as a reservoir of different Leptospira strains, especially for swine-adapted serogroups Tarassovi, Pomona and Bratislava [23,24,25,26,27,28,29,30]. In this animal species, as well as in all infected hosts, the main site of Leptospira localization is the kidney, with the consequent shedding of the bacteria in the environment via urine [1,2,31]. Moreover, a genital organ infection may occur a second time after bacteremia and/or renal localization [32,33,34]. However, recently, genital leptospirosis seems to be under consideration as a specific syndrome for bovine and swine [35]; indeed, genital leptospirosis has specific features, such as low systemic antibody titers, and chronic infection often associated in females with low fertility rates, abortion and perinatal mortality, and in males with testicular and epididymal infection [31,32,33,35,36,37,38]. In addition, sexual transmission was demonstrated [31,35]. In wild boar, genital infection was demonstrated, other than with the Leptospira DNA detection process undertaken in the reproductive system, through the isolation of the Leptospira borgpetersenii serogroup Ballum in aborted fetuses [39] and the isolation of Leptospira interrogans serovar Bratislava and Leptospira kirschneri serovar Grippotyphosa from testicles and epididymides [40].
This investigation aimed to evaluate the localization of intermediate Leptospira in the reproductive systems of male and female wild boar hunted in the Tuscany region (Italy).
2. Materials and Methods
2.1. Samples Collection
The samples employed in this investigation were the same as those previously reported [40], collected during the authorized hunting season 2018/2019 (October–January) according to the regional Italian hunting law (Regolamento di attuazione della legge regionale 12 gennaio 1994, n. 3 D.P.G.R. 48/R/2017—Regione Toscana). Briefly, from wild boar hunted in the Tuscany region, reproductive system tissue samples were collected, including testicles, epididymides, and uteri, as well as placentas and fetuses from pregnant wild boar. The fetuses from each pregnant wild boar were pooled and considered as a single sample. All organs were collected by veterinarians during the slaughtering activities, performed by hunters, minimizing all possible contamination [40]. Since sampling was conducted during the hunting season, the sample size could not be predicted beforehand. Moreover, for each specimen, sex and age were recorded. Age was determined after assessing the degree of tooth eruption and wear and tear of teeth of the lower jaw [41], subdividing them into young (under 12 months), subadult (between 12 and 24 months), and adult (over 24 months). No animals were specifically sacrificed for this study purpose.
2.2. Leptospira spp. Isolation
All organs were processed as previously reported [19,40]. Three distinct portions, approximately 1 cm3, of each tissue sample were homogenized into 5 mL of sterile phosphate-buffered saline (PBS) water using a Stomacher 400 Circulator (Seward LTD, West Sussex, United Kingdom). So, one milliliter of the homogenate was serially diluted in three tubes containing 5 mL of Ellinghausen–McCullough–Johnson–Harris (EMJH) media (Difco, Detroit, MI, USA), added with Leptospira enrichment EMJH (Difco) to perform the cultures. All tubes were then incubated at 30 ± 1 °C for 120 days and checked every 10 days through dark-field microscopy to evaluate possible bacterial growth. In case of contamination by other microorganisms, 2 mL from the contaminated tubes was filtered using 0.20 µm pore size filters and then sub-cultured in new EMJH media under the previously described conditions. Tubes were discarded if this procedure was not able to prevent further contamination.
2.3. Multiplex Real-Time PCR and Genotyping of Leptospira spp.
From each tissue samples, DNA was extracted using the Quick-DNA Plus Kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. After the first multiplex real-time PCR performed to detect the Leptospira genus (16S rRNA gene) and pathogenic species (lipL32 gene) [18], positive samples of 16S rRNA only were analyzed using a second multiplex real-time PCR. The second protocol is based on intermediate Leptospira (16S rRNA gene) and saprophytic Leptospira (23S rRNA gene) using specific probes for each gene target [4,42]. The real-time PCR assay was done using the Rotorgene Corbett 6000 (Corbett Research, Sidney, Australia) with the following thermal conditions: a holding stage of 95 °C for 5 min, 45 cycles of 95 °C for 15 sec, and 60 °C for 30 sec. Samples were considered positive with a Ct < 40 [4,42]. DNA extracted from the strains employed as live antigens for MAT was used as the positive control. Sterile DNase- and RNase-free distilled water was employed as a negative control.
Finally, Leptospira species were identified using a primer for the rrs2 gene from PCR-positive tissue samples [43]. The amplification of each target gene was performed using a HotStarTaq Master Mix Kit (Qiagen, Hilden, Germany). All amplicons were further sequenced (BMR Genomics, Padova, Italy) using the same amplification primer sets, and analyzed using BioEdit [44]. Phylogenetic analysis based on the rrs2 gene was performed using the maximum likelihood method based on the Tamura–Nei model using MEGA 10 software [45].
2.4. Statistical Analysis
The obtained data were analyzed using Chi-square (X2) and Fisher (F) tests. Statistical tests were used to evaluate the Leptospira infection ratio regarding sex (male or female), age (young, subadult, or adult) and pregnancy (pregnant or not) of the wild boar. Besides this, stratified analyses were performed considering all the possible parameter combinations (i.e., only males stratified by age). The statistical significance threshold was set at a p-value ≤ 0.05 [46].
3. Results
During the hunting season, 200 wild boar specimens were sampled. Of them, 85 were males and 115 females (35 were pregnant), subdivided into 92 adults, 31 subadults, and 77 young animals. Moreover, of these, 75 were sampled in Grosseto province, 58 in Pisa province, 55 in Siena province, and 12 in Livorno province. No macroscopic lesions in the genital organs were detected in the post-mortem examination. Furthermore, no gross lesions were detected in other organs, and the animals were considered to be healthy.
No isolates of intermediate Leptospira or saprophytic Leptospira were obtained during the four months of culture incubation. Furthermore, all samples were negative for saprophytic Leptospira using the molecular method. On the other hand, intermediate Leptospira DNA was detected in six male organs (3%). For each male, both the testicles and epididymis were assessed as positive via real-time PCR. Of these positive males, three were adult and three young (Table 1).
Table 1.
Provinces and age classes of Leptospira fainei-positive wild boar sampled in the Tuscany region.
| Samples | Province | Age Class | Leptospira Species |
|---|---|---|---|
| Wild Boar 22 | Livorno | Young | Leptospira fainei |
| Wild Boar 70 | Grosseto | Adult | Leptospira fainei |
| Wild Boar 92 | Pisa | Young | Leptospira fainei |
| Wild Boar 133 | Grosseto | Adult | Leptospira fainei |
| Wild Boar 144 | Pisa | Young | Leptospira fainei |
| Wild Boar 163 | Grosseto | Adult | Leptospira fainei |
Statistical difference was observed as regards sex, as intermediate Leptospira infection was shown to be significantly associated with males (p < 0.05). However, no statistical differences (p > 0.05) were recorded in the prevalence of intermediate Leptospira infection considering age and provinces.
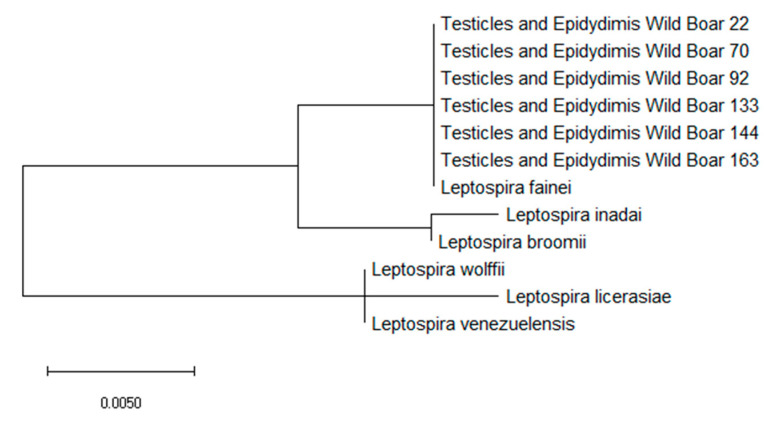
Concerning the characterization of PCR-positive samples, the amplification of 16s rRNA highlighted that intermediate Leptospira belonged to Leptospira fainei. The phylogenetic analysis identified a close relationship with this Leptospira species (Figure 1).
Figure 1.
Molecular phylogenetic analysis for the 16s rRNA gene of Leptospira fainei, Leptospira inadai, Leptospira broomii, Leptospira wolfii, Leptospira licerasiae, and Leptospira venezuelensis by the maximum likelihood method based on the Tamura–Nei model. The branch lengths of the tree measures the number of substitutions per site. The analysis involved 22 nucleotide sequences. There was a total of 438 positions in the final dataset.
4. Discussion
In this study, leptospiral infection of the wild boar reproductive system, caused by intermediate Leptospira, was evaluated for the first time. Unfortunately, no isolates were obtained, but the DNA of intermediate Leptospira was detected. In particular, only samples from males of different age classes scored positively. According to phylogenetic analysis, the intermediate Leptospira DNA detected belonged to Leptospira fainei. Although no referable macroscopic lesions were observed during a post-mortem examination, the results of this investigation suggest that infection by Leptospira fainei in the genital organs could occur in males of different age classes.
Samples (kidney, liver, serum and reproductive organs) from the same animals were previously analyzed for Leptospira spp., and the results are already published [19,40]. In particular, considering only the wild boar found to be positive for L. fainei in the present investigation, all livers were negative for Leptospira spp., all kidneys and reproductive organs were negative for pathogenic leptospires, and all sera scored negatively for MAT. The isolation of Leptospira from all the organs failed, except for “wild boar 22”, from which the L. interrogans serogroup Australis serovar Bratislava (ST 24) was isolated.
Previously, only one study focused on Leptospira infection in the reproductive systems of wild boar has been carried out [40], but this is the first time that Leptospira fainei has been detected in testicles and epididymides. Moreover, in all positive males, both testicles and epididymides scored positively for Leptospira, suggesting a dual localization in both organs. As previously supposed by the same authors, the presence of Leptospira, pathogenic or intermediate, in the epididymis could be transitory as a consequence of infection at the testicular level [40], although no detailed information is available.
As reported for bull and domestic boar, genital leptospirosis is always subclinical. Although during the acute phases the disease could cause mild and temporary orchitis, the damage to spermatozoa and the influence on sperm viability remain unknown [31,32,35,47]. The venereal transmission of pathogenic Leptospira could occur in swine, as well as in other animals like cattle, via infected semen that could carry the pathogen in the female uterus and oviduct [35,48,49,50]. Therefore, it is presumable that Leptospira fainei could be transmitted from males to females during coitus. However, no female reproductive organs scored positively. A possible explanation for this could be that this strain could not be adapted to the uterine environment of wild boar. Another possible hypothesis is that males could act as asymptomatic carriers, while females are susceptible to infection that evolves into clinical manifestations. This hypothesis seems to be supported by the fact that the detection of L. fainei DNA was statistically associated with males, but it is difficult to fully demonstrate due to the impossibility of detecting symptoms or reproductive disorders in wild animals. Nevertheless, it is important to stress that Leptospira fainei was initially isolated from the uteri of domestic pigs in Australia, suggesting a tropism for the female swine genital system, although information on the associated disease is scant [9]. The infection in the genital tract of sows could represent a risk for domestic animals due to the reproductive behavior of wild boar males that often mate with sow breeds in extensive or semi-extensive farms [51]. Finally, the obtained data (zero positivity among fetuses, placentas and pregnant uteri) suggest a low tropism of Leptospira fainei for wild boar fetuses and fetal annexes, but a vertical transmission cannot be excluded with certainty.
The different results obtained between cultural and molecular methods could be partially due to the medium employed. Indeed, nEMJH medium was used in this investigation, while in other studies aimed at isolating intermediate Leptospira, EMJH medium added with STAFF supplement containing five antimicrobials (sulfamethoxazole, trimethoprim, amphotericin B, fosfomycin, and 5-fluorouracil) was employed [3,4,5]. However, STAFF supplement makes the medium more selective, but not more suitable for Leptospira; it only helps to prevent contamination. Furthermore, L. fainei was isolated by other authors with conventional EMJH without modifications. In light of these considerations, although the use of the STAFF supplement could probably enhance the opportunities to isolate intermediate strains, the use of classical medium did not fully preclude the possibility of obtaining isolates; indeed, the same method allowed the authors to isolate pathogenic Leptospira strains from the same samples [19,40].
Only very few studies have highlighted Leptospira fainei infection in swine. In Italy, it was previously detected in the kidneys of wild boar collected in the Tuscany and, presumably, Liguria regions [18,19]. The prevalence of intermediate Leptospira recorded in this investigation (3%) was very similar to that reported in wild boar kidneys from the Tuscany and Liguria regions [18,19]. In particular, the kidneys and livers from the same animals analyzed in this investigation were previously tested by PCR; all livers were negative, while 9/287 (3.14%) of the kidneys were positive for intermediate Leptospira [19]. However, the animals that were positive at the kidney level were negative at the reproductive level and vice versa. The observed lack of correlation between Leptospira fainei infection in the kidney and genital tracts suggests two possible specific independent transmission routes for this intermediate strain: one venereal and one conventional via contaminated urines.
The impact of Leptospira fainei infection on the reproductive systems of male wild boar is of great interest because among neglected diseases such as leptospirosis, infection by intermediate strains is highly underestimated. Furthermore, it could potentially have an impact on population breeding. Indeed, in the natural ecosystem, control of genital leptospirosis and its impact on the reproductive performance of wildlife, as well as in wild boar, is very difficult to maintain.
Finally, Leptospira fainei infections in the reproductive systems of wild boar may also represent a risk for human health, especially for hunters. Based on the isolation and detection of Leptospira fainei infection in humans [5,12,13,15,16], the risk for hunters is represented by exposure to the pathogen during the slaughter activities; indeed, the reproductive systems are necessarily handled, commonly without the use of personal protective equipment.
5. Conclusions
In concluding, the results of this investigation suggest that the genital tracts of male wild boar could be a target for Leptospira fainei, as well as for different Leptospira interrogans and Leptospira kirschneri [40].
Genital Leptospira infection could be linked to reproductive complications, as recently evidenced in the domestic pig. It is likely that the wild boar is more resistant than selected breeding lineages, and this species is not exposed to intensive farming stress [40]. For these reasons, the impact of genital Leptospira infection on the reproductive performance might be lower than in domestic swine, as suggested by the high birth rates reported recently in Italy, as well in Tuscany [21,52].
Furthermore, the localization of Leptospira fainei in the male genital organs could represent a public health risk, mainly for the hunters and stakeholders that commonly handle these organs without personal protective equipment, increasing the contamination and infection.
Finally, the localization of Leptospira fainei suggests a new scenario within the epidemiology of leptospirosis in wild boar. More investigations must be carried out to understand the occurrence of intermediate leptospirosis in the reproductive system, the implications of genital leptospirosis for reproductive performance, and the possibility of venereal transmission from males to females and its consequences.
Author Contributions
Conceptualization, G.C., F.B., and F.F.; investigation, G.C. and F.B.; data curation, G.C., F.B., D.C., and F.F.; writing—original draft preparation, G.C., F.B., and F.F.; writing—review and editing, G.C., F.B., D.C., and F.F.; supervision, D.C. and F.F.; funding acquisition, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Pisa, grant number PRA_2018_56.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler B. Current Topics in Microbiology and Immunology. Springer; Cham, Switzerland: 2015. Leptospira and Leptospirosis. [Google Scholar]
- 3.Thibeaux R., Girault D., Bierque E., Soupé-Gilbert M.-E., Rettinger A., Douyère A., Meyer M., Iraola G., Picardeau M., Goarant C. Biodiversity of Environmental Leptospira: Improving Identification and Revisiting the Diagnosis. Front. Microbiol. 2018;9:816. doi: 10.3389/fmicb.2018.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barragan V., Chiriboga J., Miller E., Olivas S., Birdsell D., Hepp C., Hornstra H., Schupp J.M., Morales M., Gonzalez M., et al. High Leptospira Diversity in Animals and Humans Complicates the Search for Common Reservoirs of Human Disease in Rural Ecuador. PLoS Negl. Trop. Dis. 2016;10:e0004990. doi: 10.1371/journal.pntd.0004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balamurugan V., Gangadhar N.L., Mohandoss N., Thirumalesh S.R.A., Dhar M., Shome R., Krishnamoorthy P., Prabhudas K., Rahman H. Characterization of leptospira isolates from animals and humans: Phylogenetic analysis identifies the prevalence of intermediate species in India. Springerplus. 2013;2:362. doi: 10.1186/2193-1801-2-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerqueira G.M., Picardeau M. A century of Leptospira strain typing. Infect. Genet. Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Vincent A.T., Schiettekatte O., Goarant C., Neela V.K., Bernet E., Thibeaux R., Ismail N., Mohd Khalid M.K.N., Amran F., Masuzawa T., et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019;13:e0007270. doi: 10.1371/journal.pntd.0007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourhy P., Vray M., Picardeau M. Evaluation of an in-house ELISA using the intermediate species Leptospira fainei for diagnosis of leptospirosis. J. Med. Microbiol. 2013;62:822–827. doi: 10.1099/jmm.0.054304-0. [DOI] [PubMed] [Google Scholar]
- 9.Perolat P., Chappel R.J., Adler B., Baranton G., Bulach D.M., Billinghurst M.L., Letocart M., Merien F., Serrano M.S. Leptospira fainei sp. nov., isolated from pigs in Australia. Int. J. Syst. Bacteriol. 1998;48:851–858. doi: 10.1099/00207713-48-3-851. [DOI] [PubMed] [Google Scholar]
- 10.Chappel R.J., Khalik D.A., Adler B., Bulach D.M., Faine S., Perolat P., Vallance V. Serological titres to Leptospira fainei serovar hurstbridge in human sera in Australia. Epidemiol. Infect. 1998;121:473–475. doi: 10.1017/S095026889800137X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yersin C., Bovet P., Mérien F., Wong T., Panowsky J., Perolat P. Human leptospirosis in the Seychelles (Indian ocean): A population- based study. Am. J. Trop. Med. Hyg. 1998;59:933–940. doi: 10.4269/ajtmh.1998.59.933. [DOI] [PubMed] [Google Scholar]
- 12.Petersen A.M., Boye K., Blom J., Schlichting P., Krogfelt K.A. First isolation of Leptospira fainei serovar Hurstbridge from two human patients with Weil’s syndrome. J. Med. Microbiol. 2001;50:96–100. doi: 10.1099/0022-1317-50-1-96. [DOI] [PubMed] [Google Scholar]
- 13.Arzouni J.P., Parola P., La Scola B., Postic D., Brouqui P., Raoult D. Human infection caused by Leptospira fainei. Emerg. Infect. Dis. 2002;8:865–868. doi: 10.3201/eid0808.010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthias M.A., Ricaldi J.N., Cespedes M., Diaz M.M., Galloway R.L., Saito M., Steigerwalt A.G., Patra K.P., Ore C.V., Gotuzzo E., et al. Human Leptospirosis Caused by a New, Antigenically Unique Leptospira Associated with a Rattus Species Reservoir in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2008;2:e213. doi: 10.1371/journal.pntd.0000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiriboga J., Barragan V., Arroyo G., Sosa A., Birdsell D.N., España K., Mora A., Espín E., Mejía M.E., Morales M., et al. High Prevalence of Intermediate Leptospira spp. DNA in Febrile Humans from Urban and Rural Ecuador. Emerg. Infect. Dis. 2015;21:2141–2147. doi: 10.3201/eid2112.140659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganoza C.A., Matthias M.A., Saito M., Cespedes M., Gotuzzo E., Vinetz J.M. Asymptomatic Renal Colonization of Humans in the Peruvian Amazon by Leptospira. PLoS Negl. Trop. Dis. 2010;4:e612. doi: 10.1371/journal.pntd.0000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthias M.A., Mónica Díaz M., Campos K.J., Calderon M., Willig M.R., Pacheco V., Gotuzzo E., Gilman R.H., Vinetz J.M. Diversity of Bat-Associated Leptospira In The Peruvian Amazon Inferred By Bayesian Phylogenetic Analysis Of 16s Ribosomal Dna Sequences. Am. J. Trop. Med. Hyg. 2005;73:964–974. doi: 10.4269/ajtmh.2005.73.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cilia G., Bertelloni F., Mignone W., Spina S., Berio E., Razzuoli E., Vencia W., Franco V., Cecchi F., Bogi S., et al. Molecular detection of Leptospira spp. in wild boar (Sus scrofa) hunted in Liguria region (Italy) Comp. Immunol. Microbiol. Infect. Dis. 2020;68:101410. doi: 10.1016/j.cimid.2019.101410. [DOI] [PubMed] [Google Scholar]
- 19.Cilia G., Bertelloni F., Angelini M., Cerri D., Fratini F. Leptospira Survey in Wild Boar (Sus scrofa) Hunted in Tuscany, Central Italy. Pathogens. 2020;9:377. doi: 10.3390/pathogens9050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massei G., Kindberg J., Licoppe A., Gačić D., Šprem N., Kamler J., Baubet E., Hohmann U., Monaco A., Ozoliņš J., et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015;71:492–500. doi: 10.1002/ps.3965. [DOI] [PubMed] [Google Scholar]
- 21.Santilli F., Varuzza P. Factors affecting wild boar (Sus scrofa) abundance in southern Tuscany. Hystrix, Ital. J. Mammal. 2013;24:169–173. [Google Scholar]
- 22.Castillo-Contreras R., Carvalho J., Serrano E., Mentaberre G., Fernández-Aguilar X., Colom A., González-Crespo C., Lavín S., López-Olvera J.R. Urban wild boars prefer fragmented areas with food resources near natural corridors. Sci. Total Environ. 2018;615:282–288. doi: 10.1016/j.scitotenv.2017.09.277. [DOI] [PubMed] [Google Scholar]
- 23.Bertelloni F., Mazzei M., Cilia G., Forzan M., Felicioli A., Sagona S., Bandecchi P., Turchi B., Cerri D., Fratini F. Serological Survey on Bacterial and Viral Pathogens in Wild Boars Hunted in Tuscany. Ecohealth. 2020;17:85–93. doi: 10.1007/s10393-020-01475-y. [DOI] [PubMed] [Google Scholar]
- 24.Bertelloni F., Cilia G., Turchi B., Pinzauti P., Cerri D., Fratini F. Epidemiology of leptospirosis in North-Central Italy: Fifteen years of serological data (2002–2016) Comp. Immunol. Microbiol. Infect. Dis. 2019;65:14–22. doi: 10.1016/j.cimid.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Montagnaro S., Sasso S., De Martino L., Longo M., Iovane V., Ghiurmino G., Pisanelli G., Nava D., Baldi L., Pagnini U. Prevalence of Antibodies to Selected Viral and Bacterial Pathogens in Wild Boar (Sus scrofa) in Campania Region, Italy. J. Wildl. Dis. 2010;46:316–319. doi: 10.7589/0090-3558-46.1.316. [DOI] [PubMed] [Google Scholar]
- 26.San-Miguel Ayanz J.M., Garcia-Peña F.J., García-Lunar P., Ortega-Mora L.M., Ruano M.J., Álvarez-García G., Collantes-Fernández E. Seroprevalence of Leptospirosis, Brucellosis, and Q Fever in a Wild Red Deer (Cervus elaphus) Population Kept in a Fenced Reserve in Absence of Contact with Livestock. Vector-Borne Zoonotic Dis. 2017;17:692–697. doi: 10.1089/vbz.2016.2105. [DOI] [PubMed] [Google Scholar]
- 27.Boqvist S., Bergström K., Magnusson U. Prevalence of antibody to six Leptospira Servovars in Swedish wild boars. J. Wildl. Dis. 2012;48:492–496. doi: 10.7589/0090-3558-48.2.492. [DOI] [PubMed] [Google Scholar]
- 28.Vicente J., León-Vizcaíno L., Gortázar C., Cubero M.J., González M., Martín-Atance P. Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain. J. Wildl. Dis. 2002;38:649–652. doi: 10.7589/0090-3558-38.3.649. [DOI] [PubMed] [Google Scholar]
- 29.Żmudzki J., Jabłoński A., Nowak A., Zębek S., Arent Z., Bocian Ł., Pejsak Z. First overall report of Leptospira infections in wild boars in Poland. Acta Vet. Scand. 2016;58:3. doi: 10.1186/s13028-016-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vale-Goncalves H.M., Cabral J.A., Faria M.C., Nunes-Pereira M., Faria A.S., Veloso O., Vieira M.L., Paiva-Cardoso M.N. Prevalence of Leptospira antibodies in wild boars ( Sus scrofa ) from Northern Portugal: Risk factor analysis. Epidemiol. Infect. 2015;143:2126–2130. doi: 10.1017/S0950268814003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis W.A. Animal Leptospirosis. Curr. Top. Microbiol. Immunol. 2015;387:99–137. doi: 10.1007/978-3-662-45059-8_6. [DOI] [PubMed] [Google Scholar]
- 32.Ellis W.A., McParland P.J., Bryson D.G., Cassells J.A. Boars as carriers of leptospires of the Australis serogroup on farms with an abortion problem. Vet. Rec. 1986;118:563. doi: 10.1136/vr.118.20.563. [DOI] [PubMed] [Google Scholar]
- 33.Ellis W.A., Songer J.G., Montgomery J., Cassells J.A. Prevalence of Leptospira interrogans serovar hardjo in the genital and urinary tracts of non-pregnant cattle. Vet. Rec. 1986;118:11–13. doi: 10.1136/vr.118.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Ellis W.A., McParland P.J., Bryson D.G., Cassells J.A. Prevalence of Leptospira infection in aborted pigs in Northern Ireland. Vet. Rec. 1986;118:63–65. doi: 10.1136/vr.118.3.63. [DOI] [PubMed] [Google Scholar]
- 35.Loureiro A.P., Lilenbaum W. Genital bovine leptospirosis: A new look for an old disease. Theriogenology. 2020;141:41–47. doi: 10.1016/j.theriogenology.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Dhaliwal G.S., Murray R.D., Dobson H., Montgomery J., Ellis W.A., Baker J.R. Presence of antigen and antibodies in serum and genital discharges of heifers after experimental intrauterine inoculation with Leptospira interrogans serovar hardjo. Res. Vet. Sci. 1996;60:157–162. doi: 10.1016/S0034-5288(96)90011-9. [DOI] [PubMed] [Google Scholar]
- 37.Strutzberg-Minder K., Tschentscher A., Beyerbach M., Homuth M., Kreienbrock L. Passive surveillance of Leptospira infection in swine in Germany. Porc. Heal. Manag. 2018;4:10. doi: 10.1186/s40813-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis W.A., McParland P.J., Bryson D.G., Thiermann A.B., Montgomery J. Isolation of leptospires from the genital tract and kidneys of aborted sows. Vet. Rec. 1986;118:294–295. doi: 10.1136/vr.118.11.294. [DOI] [PubMed] [Google Scholar]
- 39.Brihuega B., Loffler S.G., Samartino L., Romero G., Auteri C., Martinez M. First Isolation of Leptospira borgpetersenii from Fetuses of Wild Boars (Sus scrofa) Electron. J. Biol. 2017;13:63–66. [Google Scholar]
- 40.Cilia G., Bertelloni F., Piredda I., Ponti M.N., Turchi B., Cantinle C., Parisi F., Pinzauti P., Armani A., Palmas B., et al. Presence of pathogenic Leptospira spp. in the reproductive system and fetuses of wild boars (Sus scrofa) in Italy. PLoS Negl. Trop. Dis. 2020;14:e0008982. doi: 10.1371/journal.pntd.0008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sáez-Royuela C., Gomariz R.P., Luis Tellería J. Age Determination of European Wild Boar. Wildl. Soc. Bull. 1989;17:326–329. [Google Scholar]
- 42.Bedir O., Kilic A., Atabek E., Kuskucu A.M., Turhan V., Basustaoglu A.C. Simultaneous detection and differentiation of pathogenic and nonpathogenic Leptospira spp. by multiplex real-time PCR (TaqMan) assay. Polish J. Microbiol. 2010;59:167–173. doi: 10.33073/pjm-2010-026. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N., Devi S.M., de los Á Valverde M., Vijayachari P., Machang’u R.S., Ellis W.A., Hartskeerl R.A. Multilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira species. Ann. Clin. Microbiol. Antimicrob. 2006;5:28. doi: 10.1186/1476-0711-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 45.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 47.Givens M.D. Review: Risks of disease transmission through semen in cattle. Animal. 2018;12:s165–s171. doi: 10.1017/S1751731118000708. [DOI] [PubMed] [Google Scholar]
- 48.Bielanski A., Surujballi O., Thomas E.G., Tanaka E. Sanitary status of oocytes and embryos collected from heifers experimentally exposed to Leptospira borgpetersenii serovar hardjobovis. Anim. Reprod. Sci. 1998;54:65–73. doi: 10.1016/S0378-4320(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 49.Lilenbaum W., Varges R., Brandão F.Z., Cortez A., de Souza S.O., Brandão P.E., Richtzenhain L.J., Vasconcellos S.A. Detection of Leptospira spp. in semen and vaginal fluids of goats and sheep by polymerase chain reaction. Theriogenology. 2008;69:837–842. doi: 10.1016/j.theriogenology.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Bolin C.A., Cassells J.A. Isolation of Leptospira interrogans serovars bratislava and hardjo from swine at slaughter. J. Vet. Diagn. Investig. 1992;4:87–89. doi: 10.1177/104063879200400121. [DOI] [PubMed] [Google Scholar]
- 51.Barlozzari G., Franco A., Macrì G., Lorenzetti S., Maggiori F., Dottarelli S., Maurelli M., Di Giannatale E., Tittarelli M., Battisti A., et al. First report of Brucella suis biovar 2 in a semi free-range pig farm, Italy. Vet. Ital. 2015;51:151–154. doi: 10.12834/VetIt.50.3384.1. [DOI] [PubMed] [Google Scholar]
- 52.Carnevali L., Pedrotti L., Riga F., Toso S. Banca Dati Ungulati: Status, distribuzione, consistenza, gestione e prelievo venatorio delle popolazioni di Ungulati in Italia. Rapporto 2001–2005. Biol. e Conserv. della Fauna. 2009;117:1–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.