Graphical abstract

Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; CCDC, Chinese Centre for Disease Control and Prevention; WHO, World Health Organization; ARDS, acute respiratory distress syndrome; NHC, National Health Commission; MERS, Middle East respiratory syndrome; H1N1, Influenza A virus subtype H1N1; RSV, Respiratory Syncytial Virus infection; H5N1, avian influenza virus; LDT, long-distance transport; FLI, flu-like illnesses; WAO, World Allergy Organisation; COPD, chronic obstructive pulmonary diseases; CDC, Centers for Disease Control and Prevention; GINA, Global Initiative for Asthma; AAAAI, American Academy of Allergy, Asthma & Immunology; ERS, European Respiratory Society; ACE-2, angiotensin-converting enzyme 2; IgE, Immunoglobulin E; STaMPS, Simulator of Timing and Magnitude of Pollen Season; WRF, Weather Research Forecasting; CMAQ, Community Multiscale Air Quality; CESM, Community Earth System Model
Keywords: Bioaerosols, Airborne pollen, Virus, COVID-19, Allergic rhinitis, Asthma
Abstract
The fast spread of SARS-CoV-2 presented a worldwide challenge to public health, economy, and educational system, affecting wellbeing of human society. With high transmission rates, there are increasing evidences of COVID-19 spread via bioaerosols from an infected person. The current review was conducted to examine airborne pollen impact on COVID-19 transmission and to identify the major gaps for post-pandemic research. The study used all key terms to identify revenant literature and observation were collated for the current research. Based on existing literature, there is a potential association between pollen bioaerosols and COVID-19. There are few studies focusing the impact of airborne pollen on SARS-CoV-2, which could be useful to advance future research. Allergic rhinitis and asthma patients were found to have pre-modified immune activation, which could help to provide protection against COVID-19. However, does airborne pollen acts as a potent carrier for SARS-CoV-2 transport, dispersal and its proliferation still require multidisciplinary research. Further, a clear conclusion cannot be drawn due to limited evidence and hence more research is needed to show how pollen bioaerosols could affect virus survivals. The small but growing literature review focuses on searching for every possible answer to provide additional security layers to overcome near future corona-like infectious diseases.
1. Introduction
On 31st December, pneumonia-like cases of unknown etiology were reported in Wuhan, China associated with Huanan Seafood Market. The causative agent identified behind the pneumonia-like cases was subsequently named Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) by the Chinese Centre for Disease Control and Prevention (CCDC) as reported by Lei et al. (2020). On 30th January 2020, with a surge in the number of coronavirus cases, the World Health Organization (WHO) announced the outbreak of SARS-CoV-2 as Public Health Emergency of International Concern posing various countries at high risk (Sohrabi et al., 2020). The global spreading of coronavirus diseases in 2019 caused by SARS-CoV-2 presented an unprecedented challenge to the international public health, trade, economy and educational system affecting human society's welfare (Lippi, Henry, Bovo, & Sanchis-Gomar, 2020). On the contrary, the outbreak has posed a huge positive impact on the pollution level, wildlife and nature (Biswal, Singh, Singh, Ravindra, & Mor, 2020; Biswal, Singh, Singh, Kesarkar et al., 2020; Mor et al., 2021). As all human activities came to a halt, a reduction in the level of particulate matter in the air, lowering NO2 and CO2 emissions, was reported (Habib, Xia, Fareed, & Hashmi, 2020; Ravindra, Singh, Biswal, Singh, & Mor, 2021; Wang & Li, 2021). By 10th January 2021, the pandemic of COVID-19, as labeled by WHO, has reported more than 11.1 crores cases of COVID-19 globally, with more than a 2.5 million deaths and 87 million recoveries spontaneously (WHO Report, 2020).
The high transmission rate of coronavirus and vaccines unavailability led governments to enforce social distancing, compulsory use of facial masks and related measures. Thus, the basic purpose of maintaining social distancing (at least 1.8 m) is to slow down/minimize and eventually prevent the spread of novel coronavirus by reducing its possibility of transfer among infected persons to non-infected persons (CDC, 2007; Jin et al., 2020). Initially, scientists tried to solicit factors such as temperature, relative humidity and sunlight to explain novel coronavirus spread mainly in countries experiencing cold climates. However, meteorological variables do not completely explain disease occurrence variation (Liu, Zhi, & Ying, 2020; Liu, Zhou et al., 2020; Wu et al., 2020). The exact pathogenesis of coronavirus is not decoded yet, but it is well-known that the virus has the ability to spread via dispersal of bioaerosols from an infected person.
Airborne pollen constitute a significant fraction of bioaerosols and serve as carriers for various bacteria and viruses (Card, Pearson, & Clover, 2007). They are greatly influenced and triggered by meteorological factors (such as temperature, relative humidity, rainfall and wind, etc.) (Beggs, 2004) and jointly can explain the effect on COVID-19 (Hoogeveen, 2020; Hoogeveen, van Gorp, & Hoogeveen, 2020). As highlighted by Hoogeveen et al. (2020), the ability of SARS-CoV-2 to persist in the environment can cause eventual exposure to pollen bioaerosols, which can further alter the transmission rate and seasonality of coronavirus. The overview of airborne pollen effect, meteorological parameters on COVID-19 has been summarized in Fig. 1 . This review explains that there is a possibility of the pollen-virus complex may diffuse into the atmosphere and can be transported to long-distances from their original site. This could be one of the possible reasons for the rapid spread of COVID-19.
Fig. 1.
Overview summarizing the effect of pollen aerosols along with meteorological parameters on COVID-19.
Contrary to this, studies have found that pollen bio-aerosols are one of the independent factors involved in reducing or inhibiting flu-like epidemics incidences since 1889 and COVID-19. Typically, airborne pollen are considered major triggers of respiratory allergies causing allergic rhinitis (hay fever/pollinosis) and asthma. And theoretically, allergic rhinitis and asthmatic patients should have increased severity and susceptibility towards COVID-19, but surprisingly an unexpected low prevalence of asthmatic and allergic rhinitis is shown in existing studies. This suggests that pollen bio-aerosols may lead to pre-modified immune activation, which seems to protect against COVID-19. Hence, this review examines the existing scientific evidence to better understand the linkage between the pollen bioaerosols, COVID-19, meteorological parameters and anticipated risk in the severity of allergic rhinitis and asthma.
2. COVID-19, bio-aerosols and its spread
Among different symptoms showed by COVID-19 patients includes sore throat, cough, diarrhea, fever, loss of taste and smell, breathlessness accompanied with fatigue; with extreme conditions resulting in respiratory failure, multiple-organ failure, acute respiratory distress syndrome (ARDS), pneumonia, and even death (Huang et al., 2020; Jiang et al., 2020). On 18th February 2020, the National Health Commission (NHC) of People’s Republic of China published guidelines for effective diagnosis and treatment of COVID-19 infection (NHC, 2020). With a major focus on the transmission route, the guidelines show that apart from droplets produced by an infected person, aerosols transmission was considered one of the major routes of coronavirus transmission (Wang & Du, 2020). Aerosols are the suspension of fine solid or liquid particles (industrial dust particles, soil particles, bacteria, pollen grains and other components) dispersed or suspended in the air. In contrast, bioaerosols are solid and liquid airborne particles of biological origin (pollen, fungi, bacteria).
It is well-known that SARS-CoV-2 can spread via the dispersion of bio-aerosols by an infected person. Viruses exerted by an infected person upon speaking, sneezing, or coughing get dissolve with the aerosols suspended in the atmosphere and become bio-aerosols (Guzman, 2020). The bio-aerosol particles generally range from 0.3 to 100 μm in diameter, whereas primary concerns remain on a respirable fraction of size 1.0−10 μm. Generally, bio-aerosols in the size range of 1.0–5.0 μm persist in the ambient air, whereas heavier/ larger particles tend to settle down on surfaces. Whereas the particle size of a droplet of saliva released by a human upon speaking, sneezing, wheezing, or coughing generally varies between 1–5 mm and tend to spread in the vicinity of around 1–2 m from the source of infection as shown in Fig. 2 (Adhikari et al., 2019; Jones & Brosseau, 2015; Tellier, Li, Cowling, & Tang, 2019; Velraj & Haghighat, 2020). Moreover, both the aerosols and bioaerosols could travel hundreds/thousands of meters or more (Killingley and Nguyen-Van-Tam, 2013).
Fig. 2.
Modes of transmission and dispersal of COVID-19.
Varieties of the bacterial pathogens, namely Bordetella pertussis, Neisseria meningitides and Bacillus anthracis, are known to be transmitted through bioaerosols collapsing respiratory system (GBD, 2013). Some of the pathogenic fungi, such as Fusarium moniliforme, Aspergillus fumigatus and Mucorales sp, are also known to be transmitted through bioaerosols and caused various respiratory abnormalities, hypersensitivity and system organ infection (Jung, Lee, Lee, Kim, & Lee, 2009). Similarly, bio-aerosols have proven to be a transmission route in spreading the Middle East respiratory syndrome (MERS), H1N1, SARS-CoV and some other diseases (Li, Huang, Yu, Wong, & Qian, 2005; Zayas et al., 2012; Zhang et al., 2013). Airborne pollen are considered a major vector for various bacteria and viruses (Card et al., 2007; Verreault, Moineau, & Duchaine, 2008). More than 40 viruses belonging to 16 genera are identified as pollen-transmitted and the majority of them belong to Ilarvirus, Nepovirus, Potyvirus and Sobemovirus genera (Bhat & Rao, 2020; Card et al., 2007). However, understanding the transmission route in response to pathogens is an urgent need for public health.
3. Airborne pollen and virus interaction
From anther dehiscence to release in the atmosphere, airborne pollen come in contact with various atmospheric particles (Knox et al., 1997; Sedghy et al., 2017). Airborne pollen constitute a major fraction of bioaerosols and serve as carriers for various pathogens, including viruses (Jones & Harrison, 2004; Shiller, Lebas, Horner, Pearson, & Clover, 2010; West, 2014). This will affect the prevalence and occurrence of diseases linked to pollen exposure. Distribution patterns of pollen grains have suggested a relationship between pollen source areas and pollen transport factors such as ocean currents and wind patterns (Hooghiemstra, Lézine, Leroy, Dupont, & Marret, 2006). Due to regional climate change, pollen grains are dispersed and transported from one place to another, exposing the people to new and novel allergens to which they are not exposed previously (Poole et al., 2019). Regional and local wind patterns are considered critical factors associated with the spread of airborne pollen and diseases in the following ways: they influence the dispersal pattern and behavior of disease vector; affect the human susceptibility of diseases; their correlation with vector abundance (Parham, Christiansen-Jucht, Pople, & Michael, 2011). Chen et al. (2010) suggested that highly pathogenic avian influenza virus (H5N1) and influenza virus may be subjected to long-distance transport (LDT) due to Asian dust storms.
The introduction of a tracer transport approach integrated with models to predict infectious disease prevalence provides better insights on disease transport. This approach mainly works for the diseases that are transported along long-distances environmentally. These include infectious diseases caused by fungi (Prank, Kenaley, Bergstrom, Acevedo, & Mahowald, 2019), Aspergilli and Coccidioides (Sprigg et al., 2014) and pollen-borne viruses (Duhl et al., 2013; Zhang et al., 2014). To motivate discussion of the interplay of pollen and disease spread, various exemplars representing key long-range transmission: Zhang et al. (2014) integrate the STaMPS (Simulator of Timing and Magnitude of Pollen Season) pollen transport model into the WRF (Weather Research Forecasting) and CMAQ (Community Multiscale Air Quality) regional air-quality modeling system to simulate the variation of temporal-spatial patterns for different species of pollen under several meteorological parameters. Similarly, CESM (Community Earth System Model) is used to model the emission and long-range transport of fungal spores, the most relevant vector for the spread of crop diseases. Several studies examine the emission pattern and long-range transport of airborne pollen, leading to the spread of infectious disease. A survey conducted by Ye, Fu, Mao, and Shang (2016) showed that children who suffered from Respiratory Syncytial Virus infection (RSV) in China were promoted by particle-based transport.
Similarly, Selcuk, Gormus, and Guven (2021) examined the potential impact of wind speed changes on COVID-19 and reported a positive correlation between wind speed and crowd in cities with high COVID-19 transmission rates. This could be one of the possible reasons for the rapid spread of COVID-19. However, there are multiple studies events known where spores or the fungi spreading diseases from one continent to another via the atmospheric pathway, even crossing oceans, have been confirmed (Brown & Hovmøller, 2002). Therefore, it is possible that airborne pollen might behave as an effective carrier for viruses, including SARS-CoV-2 transport, dispersal and its proliferation.
In order to apprehend the actual biological relevance of virus-pollen dispersion, it is also necessary not only to study the physical transport but also to understand the changes that occur in biological effectivity during the period of dispersal. In the vicinity of pollen a question arises that does pollen type affect virus attachment? Moreover, how much virus can attach to single pollen grain, viability, and viral load required for transferring infection is still unanswered. Airborne pollen releases sticky cytoplasm containing moisture, which behaves as a perfect carrier for the virus and its viability. There are four possible processes known for pollen-particle interaction: sticking of particles during another dehiscence, pollen-particle coagulation; dry and wet scavenging of pollen-particles; co-deposition of pollen and particle (Visez et al., 2020). Particles are defined as any small bits of material or droplets, either organic or non-organic, viable or non-viable, that can become airborne (Salvaggio, 1994). Pollen-particles complexes are well-documented in the outdoor environment but highly-complex to examine (Behrendt et al., 1991). For instance, pollen allergens from grass were shown to bind diesel exhaust particles in vitro experiments (Knox et al., 1997; Namork, Johansen, & Løvik, 2006). Similarly, Ormstad (2000) found soot particles carried Birch pollen on their surface. In addition, the release of proteins by pollen grains has been shown to be enhanced in the presence of particles on their surface (Behrendt, Becker, Friedrichs, Darsow, & Tomingas, 1992; 1997). Particles adhered to pollen grains could interact with allergens and uptake proteins from pollen grains, thus favoring their dispersal in the respirable fraction of atmospheric aerosols (Solomon, 2002). Similarly, viruses have the potential to be absorbed on the pollen outer surface, i.e., exine and may remain in the atmosphere for hours, weeks, or days. During a study, Singh et al. (2010) examined pollen-virus association and suggested that there is a possibility that the virus is not only attached to the surface but instead present inside the pollen grain.
It is well- known that pollen undergo physical and biochemical modification while traveling in the lower troposphere (Sénéchal et al., 2015). During transport, pollen, spores, or fungi are subjected to harsh environmental conditions, including sunlight and subfreezing temperatures, which reduces their viability and time to persist in the environment and thus limiting the distances a pathogen or viruses can be transmitted in a viable state. However, a perfect example has been provided by Schueler, Schlünzen, and Scholz (2005) and Maddison and Manners (1973), where both examined that spectrum of UV radiation has detrimental effects on the viability of pollen and spores are crucial for gene transport patterns. Although, the magnitude of pollen-virus interaction is poorly understood and the extent of pollen pollution caused by viruses is substantially undetermined. The complexity of the coronavirus with aspects of bioaerosol transmission still requires further examination.
4. Role of airborne pollen and weather conditions in explaining COVID-19
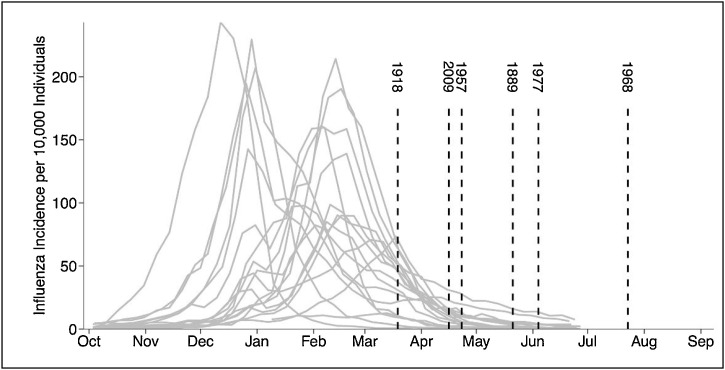
Virologists observed different circulation patterns of influenza or flu-like illnesses (FLI) that prevails in winters and “leave in May” in the Northern hemisphere while emerging in the Southern hemisphere and return in next autumn or winter (Moriyama, Hugentobler, & Iwasaki, 2020). Every flu-like epidemic since 1889 occurred at the tail-end of every flu-season in the Northern hemisphere, as shown in Fig. 3 (Fox, Miller, & Meyers, 2017). Moreover, Fox et al. (2017) revealed that most flu-like epidemics occur in multiple waves, while the initial wave is typically short-lived. This suggests that coronavirus is also subjected to similar multi-seasonal waves (Kissler, Tedijanto, Goldstein, Grad, & Lipsitch, 2020). The dispersal or distribution pattern followed by COVID-19 is consistent with earlier flu-like behavior incidences (Fox et al., 2017; Sajadi et al., 2020). Various studies conducted tried to explain the inconsistent association of flu-like illness with meteorological parameters, i.e., sunlight including UV radiation, humidity and temperature (Schuit et al., 2020; Shaman, Goldstein, & Lipsitch, 2011; Chong et al., 2020). Similarly, numerous studies across the world aimed to exemplify the impact of different weather parameters on COVID-19 cases. An exclusive reflection of some studies on each parameter is shown in Table 1 . Although inconsistent correlation is witnessed among COVID-19 and temperature (Ahmadi, Sharifi, Dorosti, Ghoushchi, & Ghanbari, 2020; Briz-Redón & Serrano-Aroca, 2020; Hoang & Tran, 2021; Iqbal et al., 2020; Shahzad et al., 2020) and equally inconsistent among COVID-19 and humidity (Ahmadi et al., 2020; Selcuk et al., 2021; Shi, Dong et al., 2020; Shi, Gao et al., 2020; Yuan et al., 2020). Selcuk et al. (2021) reported a positive correlation between precipitation and COVID-19 transmission for each average inch per day of rainfall and an increase of about 56 cases/day was observed. Contrary to this, a study conducted by Ahmadi et al. (2020) and Tosepu et al. (2020) reported a non-significant correlation between rainfall and COVID-19 cases, respectively. Very limited studies are available that have shown the relationship between wind and COVID-19 transmission. Selcuk et al. (2021) examined the influence of wind on the transmission of COVID-19 and reported a positive correlation between wind speed and crowd in cities with high COVID-19 transmission rates. Whereas another study carried out in Iran by Ahmadi et al. (2020) showed extremely opposite results. Therefore, none of the environmental factors thoroughly explains the association of meteorological parameters with COVID-19.
Fig. 3.
Historical flu-like epidemics emerged at the tail-end of every flu-season. Where grey curves indicate the occurrence of different flu-seasons of that year and vertical dash-lines depict the emergence of historical pandemics from 1889-2015 in the Northern hemisphere. (Image taken from- Fox et al., 2017).
Table 1.
Effect of various meteorological parameters on COVID-19 cases.
| Meteorological parameter | Country | Key findings | References |
|---|---|---|---|
| Temperature | China | Asymmetric nexus between temperature and COVID-19 in 10 affected provinces, three shows positive, two negatives and five mixed trends | Shahzad et al. (2020) |
| Iran | No significant correlation between temperature and COVID-19 outbreak | Ahmadi et al. (2020) | |
| China (Wuhan) | No significance of an increase in temperature is observed to slow down the COVID-19 cases | Iqbal et al. (2020) | |
| Korea | Significantly non-linear relationship between temperature and COVID‐19 cases | Hoang and Tran (2021) | |
| Spain | Insignificant impact between temperature on COVID-19 transmission | Briz-Redón and Serrano-Aroca (2020) | |
| ″Belt and Road” countries | Non-linear correlation between temperature and COVID-19 in 127 countries | Yuan et al. (2020) | |
| Relative humidity | Turkey | Negative correlation between humidity and number of COVID-19 cases (81 provinces) | Selcuk et al. (2021) |
| Iran | Reverse relationship between humidity and COVID-19 | Ahmadi et al. (2020) | |
| ″Belt and Road” countries | Non-linear correlation between relative humidity and COVID-19 in 127 countries | Yuan et al. (2020) | |
| China | No significant relationship between COVID-19 and absolute humidity | Shi, Dong et al. (2020a) and Shi, Gao et al. (2020b) | |
| Precipitation | Italy | Positive correlation between precipitation and COVID-19 transmission | Sobral, Duarte, da Penha Sobral, Marinho, and de Souza Melo (2020) |
| Jakarta (Indonesia) | Rainfall was not significantly correlated with COVID-19 | Tosepu et al. (2020) | |
| Iran | No correlation between rainfall and COVID-19 cases | Ahmadi et al. (2020) | |
| Wind speed | Turkey | Shows a positive correlation between wind and COVID-19 cases | Selcuk et al. (2021) |
| Iran | Significant inverse relationship between wind and COVID-19 infection rate | Ahmadi et al. (2020) |
Pollen bioaerosols present in the environment depend upon the surrounding vegetation and weather conditions. Nonetheless, meteorological parameters are associated with flower maturating, pollen ripening, and dispersion (Poole et al., 2019). Variations in climatic parameters influence pollen grains production, affecting their occurrence, duration and concentration in the atmosphere. Increased CO2 concentration and temperature are the major factors directly linked to increased pollen production, increased duration of pollen seasons, changes in pollen morphology and increased allergenicity of pollen. Pollen distribution greatly depends on weather conditions such as wind pressure, wind speed, wind direction, precipitation, humidity etc. and also on the micro and macro topography that exist within the area (Beggs, 2004). Factors such as sunlightand temperature trigger the flower and pollen maturation while the rain has a contrasting effect, i.e., lowers the formation of pollen bio-aerosol (Gatz & Dingle, 1963; Rahman et al., 2019; Sousa et al., 2008). Whereas relative humidity has a detrimental effect on pollen (Pacini, Guarnieri, & Nepi, 2006), it provides additional information that why flu-like illness is higher in the rainy season in tropical countries than in the rest of the year.
Dutch researchers recognized pollen bio-aerosols as one of the independent/discrete factors involved in reducing, waning or inhibiting flu-like illness incidences in the Netherlands over the time-period of 2016–2019 (Hoogeveen, 2020). They considered pollen as an anti-viral substance having anti-influenza effects. The study reports a highly significant inverse correlation between allergic pollen concentration and flu-like incidences, including COVID-19. Further, when the threshold value of allergenic pollen grains exceeds 100grains/m3 per week, it marks the onset and ending of moderate flu-like epidemics lifecycles, including COVID-19 and therefore might be used as a predictor. The indirect explanation is based on the fact that pollen bioaerosols are influenced and triggered by meteorological factors and jointly can explain the inverse effect on the seasonality of FLI. Moreover, pollen bio-aerosols and UV radiation lead to immune-activation, which seems to protect against flu-like pandemics.
A study conducted by Hoogeveen et al. (2020) tested Pollen Flu Seasonality theory (2016–2020) based on changes in pollen concentration, allergic pollen, FLI incidences and solar radiation threshold. They found a highly inverse correlation of flu-like incidences with an average threshold of solar radiation threshold of 510 J/cm2, 610 total pollen/m3, and 120 allergenic pollen/m3 every week. Moreover, the persistent observation over past years showed that passing these pollen thresholds in week 10 ± 5 and week 33 ± 2 each year corresponds to the peak of flu-like epidemic and the beginning of the new flu season, respectively. While the solar radiation was a co-inhibitor of FLI and temperature marks no effect. Therefore, this explains that pollen grains, solar radiation and humidity effects flu-like pandemics, including COVID-19.
5. COVID-19, allergic rhinitis and asthma
Pollen are considered as one of the primary triggers of respiratory allergies, causing 'pollinosis'. Allergic rhinitis (Hay fever/Pollinosis) has been recognized by the World Allergy Organisation (Pawankar, Holgate, Canonica, Lockey, & Blaiss, 2013) as one of the global health issues. It affects about 10–30 % of the worldwide population (Pawankar, 2014). Allergic rhinitis can start in early childhood and persist throughout life or can later develop risk factors for exacerbation of asthma and chronic obstructive pulmonary diseases (COPD) in susceptible individuals (Cecchi et al., 2010). Whereas coronaviruses are well-known respiratory pathogens and SARS-CoV-2 is also identified to infect the upper and lower respiratory tract. COVID-19 exhibits various distinct clinical manifestations varying from asymptomatic to symptomatic, i.e., mild and severe or uncontrolled disease. The US Centers for Disease Control and Prevention (CDC) listed asthma as one of the risk factors for novel coronavirus illness, given those respiratory pathogen are known to cause serious illness in patients with chronic airways diseases, but allergies are not classified as a risk factor.
However, Global Initiative for Asthma (GINA) provided a guideline on special allergy care during COVID-19. In contrast, the American Academy of Allergy, Asthma & Immunology (AAAAI), European Respiratory Society (ERS) and other prestigious national societies recommend continuing the usual treatment for asthma patients to prevent further exacerbations (Morais-Almeida et al., 2020). Interestingly, asthma and pre-existing respiratory allergies have not been identified as substantial risk factors among coronavirus patients (Shi, Dong et al., 2020; Shi, Gao et al., 2020). Patients with allergic diseases do not seem to develop any distinct symptoms or an increased risk of severe disease. Moreover, allergic diseases are underreported as a co-morbid condition among coronavirus patients (Dong et al., 2020; Zhang et al., 2020).
Consequently, multiple consistent studies have identified that asthma or allergic rhinitis do not seem to increase the risk against SARS-CoV-2 as shown in Table 2 . Surprisingly, despite the prevalence of 4.2 % and 9.7 % asthma and allergic rhinitis were not listed as co-morbidities in Wuhan, China, with 140 hospitalized COVID-19 patients (Zhang et al., 2020). Another study conducted in China revealed no physician-diagnosed asthma among 1590 COVID-19 patients (Guan et al., 2020). Similar findings were also reported by Carli, Cecchi, Stebbing, Parronchi, and Farsi (2020) in Italy, where a low prevalence of asthmatic patients admitted among COVID-19 patients, i.e., 3 out of 275 individuals, only one requiring ICU. These reports were theoretically conflicting with the well-known pattern of viral infections, which exacerbate asthma and other allergic diseases.
Table 2.
Summary of studies in relation to allergic rhinitis, asthma and COVID-19.
| Country | No. of COVID-19 cases | Key findings | Reference |
|---|---|---|---|
| China | 1590 | No asthmatic patient involved among 1590 COVID-19 patients | Guan et al. (2020) |
| Wuhan, China | 140 | No patients were found to have asthma or allergic rhinitis | Zhang et al. (2020) |
| New York, USA | 737 | Pre-existing eosinophils found in asthmatic patients, protective against COVID-19 with decreased mortality | Ferastraoaru et al. (2021) |
| China | 138 | With a prevalence of 1.1 %–2.9 %, chronic obstructive pulmonary diseases (COPD) are less common in COVID‐19 cases | Wang et al. (2020) |
| Prato, Italy | 275 | Very low prevalence of asthmatics patients among admitted COVID‐19 patients | Carli et al. (2020) |
| China | 2143 | No asthmatic patient among pediatric cases of COVID-19 | Dong, Mo et al. (2020) |
| China | 110 | Ratio of asthma and allergic rhinitis were far lower than those of domestic morbidity among COVID-19 patients. | Shi, Dong et al. (2020a) and Shi, Gao et al. (2020b) |
| Italy | 12,055 | allergic children have higher counts of eosinophils and no symptom of dyspnea than COVID-19 patient | Licari et al. (2020) |
Therefore, there arises a question that what could be the possible features of allergic diseases or asthmatic patients that are found related to reduced potential for the severity of COVID-19 disease.
Pollen grains are known to be allergenic (Basak et al., 2019; D’Amato et al., 2007; Schmidt, 2016) and plays a vital role in immune system activation (Janeway et al., 1999; Plötz et al., 2004). They are considered anti-viral substances and have also shown anti-influenza effects (Hoogeveen, 2020; Lee et al., 2016). Elucidating this, Licari et al. (2020) showed that allergic children have higher counts of eosinophils and no dyspnea symptoms than COVID-19 patients, where eosinophils are disease-fighting cells that help to recover from certain viral infections, including COVID-19 (Lindsley, Schwartz, & Rothenberg, 2020). Moreover, patients medicated with systematic steroids or patients on the oral dosage of corticosteroids (allergy treatments) may have increased susceptibility or severity to COVID-19 due to pre-modified immune response with evidence of cytokine-storm formation (Brough et al., 2020). However, direct evidence is still lacking, but some observations provide indirect evidence.
A study conducted by Jackson et al. (2020) found that allergic and allergen exposure have an inverse relation with angiotensin-converting enzyme 2 (ACE-2) expression, where COVID-19 requires this receptor to enter the host body (Ou et al., 2020; Wan, Shang, Graham, Baric, & Li, 2020). Furthermore, histamine and Immunoglobulin E (IgE) serum levels are found to be elevated in allergic rhinitis and asthmatic patients, which can down-regulate certain anti-viral responses, i.e., hyper-inflammation that mark the severity of various respiratory diseases, including COVID-19 (Carli et al., 2020). Like various other respiratory diseases, coronavirus might worsen asthmatic symptoms in severe or uncontrolled patients but owing to Th2-skewed immunity (pertaining to the allergic and interferon-mediated response), which drives anti-immune response may protect against COVID-19 diseases (Liu, Zhi et al., 2020; Liu, Zhou et al., 2020). Certain types of immune-responses, accumulation of eosinophils, lower ACE-2 receptors expression, Th-2 skewed immunity and elevated histamine, immunoglobin E (IgE) serum levels and systematic steroids are the possible features of allergic diseases or asthma patients that are found associated with reduced potential for the severity of COVID-19 as shown in Fig. 4 . Collectively, they indicate that allergic rhinitis and asthma may not be aggravating factors towards novel coronavirus. These observations were quite interesting and contrasting according to the theoretical knowledge, where allergic diseases and asthma are known to increase with viral infections. This inverse correlation might support the idea that the triggering of immune responses by airborne pollen (i.e., hay fever) makes it difficult for COVID-19 to penetrate a new host. Moreover, this gives substantial sense to the idea that an activated-immune system seems to protect against COVID-19 viruses. Although studies are limited but based on literature review, there is currently no clear evidence of elevated risk or fatal outcome of allergic rhinitis or asthmatic patients due to COVID-19.
Fig. 4.
Possible features of allergic diseases or asthma patients that are found associated with reduced potential for the severity of COVID-19.
6. Direction for future research
The current study examines the relationship between airborne pollen, meteorology and COVID-19. Based on the existing evidence, the study identifies the following gaps and challenges covered below:
Firstly, very few studies are available that have proved a highly significant inverse correlation between allergic pollen concentration, UV light, humidity and flu-like incidences, including COVID-19. But, the magnitude of pollen count differs at various geographic locations in response to meteorological variables. So, identifying whether the same inverse correlation between pollen count, meteorological parameters including UV light, temperature, humidity and COVID-19 also prevails in other countries at a large scale are not precisely estimable. The central question that pollen aerosols inhibit or increases the SARS-CoV-2 propagation and seasonality still remains unsolved.
Secondly, airborne pollen and spore counts are limited, especially long duration time series are completely hard to find. Continuous aerobiological monitoring is required to collect pollen and spores so that long-time-series data can be developed and compared with flu-like incidences. Thirdly, pollen allergic diseases and the mechanisms of respiratory disease disorders caused by them are highly complex and somewhere shown to protect against SARS-CoV-2. But the relationship between pollen allergies and COVID-19 patients is hard to be modeled or quantified due to inadequate patient data. Further work must understand how pollen aerosols provide a pre-active immune system for the low prevalence of allergic diseases in COVID-19 patients.
Interdisciplinary research and collaboration need to be promoted to better understand the mechanism underlying the linkage between pollen aerosols and these viruses. Further, there is a need to understand the impact of the pollution-pollen-virus complex on COVID-19 survivability and transmission at the global hotspots of ambient and indoor air pollution including the impact of air pollution reduction interventions (Singh, Ravindra et al., 2020; Singh, Biswal et al., 2020; Ravindra, Kaur-Sidhu et al., 2021). The complexity of the coronavirus with aspects of bioaerosol transmission still requires further examination. Moreover, extensive research is needed for determining projected variations in human health.
7. Conclusion
The recent COVID-19 outbreak led to a global health emergency affecting millions of people worldwide. It is unambiguously representing a major environmental, socio-economic threat faced by the human community. SARS-CoV-2 has the ability to spread through the dispersal of bioaerosols from an infected person. There is a possibility that airborne pollen can serve as an effective carrier for SARS-CoV-2. But this raised several important questions due to the highly complex nature of coronavirus and its association with airborne pollen. It is quite difficult to assess the impact of pollen bioaerosol and weather parameters on COVID-19 but the seasonality of previous flu-like pandemics and recent studies, indicates that airborne pollen is an anti-viral substance having anti-influenza effects and can act as an inhibiting factor towards COVID-19. However, future research is required to understand how pollen bioaerosols affect viruses altogether. Moreover, the clinical observations collectively suggest that there is some probable mechanism of the impaired anti-viral response in allergic rhinitis and asthma patients. During the course, observations were found surprisingly and theoretically conflicting with the well-known paradigm, where asthma and allergic disease worsen with viral infections. However, it is quite difficult to draw a conclusion based on the association between allergic rhinitis, asthma and COVID-19, but it provides hints and references for future research.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
RK and SM would like to thank the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India, New Delhi, India for providing the funding to carry out this research work under project entitled “Impact of climate change on air quality including pollen and their relation with symptoms and control among patients with chronic respiratory diseases” sanctioned via DST/CCP/NHH/111/2017(G) dated 24/03/2017. AG is a DST-INSPIRE Fellow and would also like to thank the DST, to provide financial support to pursue Ph.D. research work. The authors would also like to acknowledge the Department of Biotechnology (DBT) and British Council, India for providing the funding as this study is linked with the Newton Bhabha Fund Ph.D. Placement Programme 2019-20.
References
- Adhikari U., Chabrelie A., Weir M., Boehnke K., McKenzie E., Ikner L.…Rose J. A case study evaluating the risk of infection from middle eastern respiratory syndrome coronavirus (MERS‐CoV) in a hospital setting through bioaerosols. Risk Analysis. 2019;39(12):2608–2624. doi: 10.1111/risa.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M., Sharifi A., Dorosti S., Ghoushchi S.J., Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Science of the Total Environment. 2020:138705. doi: 10.1016/j.scitotenv.2020.138705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak T., Chakraborty A., Bhattacharya K. Identification of airborne pollen allergens from two avenue trees of India. International Journal of Environmental Health Research. 2019;29(4):414–429. doi: 10.1080/09603123.2018.1546836. [DOI] [PubMed] [Google Scholar]
- Beggs P.J. Impacts of climate change on aeroallergens: Past and future. Clinical & Experimental Allergy. 2004;34(10):1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- Behrendt H., Friedrich K.H., Kainka-Stänicke E., Darsow U., Becker W.M., Tomingas R. New trends in allergy III. Springer; Berlin, Heidelberg: 1991. Allergens and pollutants in the air—a complex interaction; pp. 467–478. [Google Scholar]
- Behrendt H., Becker W.M., Friedrichs K.H., Darsow U., Tomingas R. Interaction between aeroallergens and airborne particulate matter. International Archives of Allergy and Immunology. 1992;99(2–4):425–428. doi: 10.1159/000236303. [DOI] [PubMed] [Google Scholar]
- Behrendt H., Becker W.M., Fritzsche C., Sliwa-Tomczok W., Tomczok J., Friedrichs K.H., et al. Air pollution and allergy: Experimental studies on modulation of allergen release from pollen by air pollutants. International Archives of Allergy and Immunology. 1997;113(1–3):69–74. doi: 10.1159/000237511. [DOI] [PubMed] [Google Scholar]
- Bhat A.I., Rao G.P. Characterization of plant viruses. Humana; New York, NY: 2020. Virus transmission through pollen; pp. 61–64. [Google Scholar]
- Biswal A., Singh V., Singh S., Kesarkar A.P., Ravindra K., Sokhi R.S.…Mor S. COVID-19 lockdown induced changes in NO 2 levels across India observed by multi-satellite and surface observations. Atmospheric Chemistry and Physics Discussions. 2020:1–28. [Google Scholar]
- Biswal A., Singh T., Singh V., Ravindra K., Mor S. COVID-19 lockdown and its impact on tropospheric NO2 concentrations over India using satellite-based data. Heliyon. 2020;6(9):e04764. doi: 10.1016/j.heliyon.2020.e04764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz-Redón Á., Serrano-Aroca Á. A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain. Science of the Total Environment. 2020;728:138811. doi: 10.1016/j.scitotenv.2020.138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough H.A., Kalayci O., Sediva A., Untersmayr E., Munblit D., Rodriguez del Rio P.…Galli E. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics–The 2020 COVID‐19 pandemic: A statement from the EAACI‐section on pediatrics. Pediatric Allergy and Immunology. 2020;31(5):442–448. doi: 10.1111/pai.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.K., Hovmøller M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science. 2002;297(5581):537–541. doi: 10.1126/science.1072678. [DOI] [PubMed] [Google Scholar]
- Card S.D., Pearson M.N., Clover G.R.G. Plant pathogens transmitted by pollen. Australasian Plant Pathology. 2007;36(5):455–461. [Google Scholar]
- Carli G., Cecchi L., Stebbing J., Parronchi P., Farsi A. Is asthma protective against COVID‐19? Allergy. 2020;76(3):866–868. doi: 10.1111/all.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi L., d’Amato G., Ayres J.G., Galan C., Forastiere F., Forsberg B.…Dahl R. Projections of the effects of climate change on allergic asthma: The contribution of aerobiology. Allergy. 2010;65(9):1073–1081. doi: 10.1111/j.1398-9995.2010.02423.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2007. Interim pre-pandemic planning guidance: Community strategy for pandemic influenza mitigation in the United States-early, targeted, layered use of nonpharmaceutical interventions.http://www.pandemicflu.gov/plan/community/community_mitigation.pdf [Google Scholar]
- Centers for Disease Control and Prevention (U.S.). Coronavirus Disease 2019 (COVID-19)/People Who Need Extra Precautions/People Who Are At Higher Risk/People with Moderate to Severe Asthma: www.cdc.gov/coronavirus/2019-ncov/specific-groups/asthma.html.
- Chen P.S., Tsai F.T., Lin C.K., Yang C.Y., Chan C.C., Young C.Y., et al. Ambient influenza and avian influenza virus during dust storm days and background days. Environmental Health Perspectives. 2010;118(9):1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K.C., Liang J., Jia K.M., Kobayashi N., Wang M.H., Wei L.…Sumi A. Latitudes mediate the association between influenza activity and meteorological factors: A nationwide modelling analysis in 45 Japanese prefectures from 2000 to 2018. Science of the Total Environment. 2020;703:134727. doi: 10.1016/j.scitotenv.2019.134727. [DOI] [PubMed] [Google Scholar]
- D’Amato G., Cecchi L., Bonini S., Nunes C., Annesi‐Maesano I., Behrendt H., Liccardi G., Popov T., Van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62(9):976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- Dong X., Cao Y.Y., Lu X.X., Zhang J.J., Du H., Yan Y.Q.…Gao Y.D. Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Mo X.I., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145(6):e20200702. [Google Scholar]
- Duhl T.R., Zhang R., Guenther A., Chung S.H., Salam M.T., House J.M., Flagan R.C., Avol E.L., Gilliland F.D., Lamb B.K., VanReken T.M. The Simulator of the Timing and Magnitude of Pollen Season (STaMPS) model: a pollen production model for regional emission and transport modeling. Geoscientific Model Development Discussions. 2013;6(2):2325–2368. [Google Scholar]
- Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G.…Ramesh M. Eosinophilia in asthma patients is protective against severe COVID-19 illness. The Journal of Allergy and Clinical Immunology: In Practice. 2021;9(3):1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.J., Miller J.C., Meyers L.A. Seasonality in risk of pandemic influenza emergence. PLoS Computational Biology. 2017;13(10):e1005749. doi: 10.1371/journal.pcbi.1005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz D.F., Dingle A.N. Washout of ragweed pollen by rainfall. Journal of Geophysical Research. 1963;68(12):3641–3648. [Google Scholar]
- GBD Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2013;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Asthma. Recommendations for Inhaled Asthma Controller Medications:ginasthma.org/recommendations-for-inhaled-asthma-controller-medications/.
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. European Respiratory Journal. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M. 2020. Bioaerosol size effect in COVID-19 transmission. [Google Scholar]
- Habib Y., Xia E., Fareed Z., Hashmi S.H. Time–frequency co-movement between COVID-19, crude oil prices, and atmospheric CO 2 emissions: Fresh global insights from partial and multiple coherence approach. Environment, Development and Sustainability. 2020:1–21. doi: 10.1007/s10668-020-01031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T., Tran T.T.A. Ambient air pollution, meteorology, and COVID‐19 infection in Korea. Journal of Medical Virology. 2021;93(2):878–885. doi: 10.1002/jmv.26325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen M.J. Pollen likely seasonal factor in inhibiting flu-like epidemics. A Dutch study into the inverse relation between pollen counts, hay fever and flu-like incidence 2016–2019. Science of the Total Environment. 2020:138543. doi: 10.1016/j.scitotenv.2020.138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen M.J., van Gorp E.C., Hoogeveen E.K. Can pollen explain the seasonality of flu-like illnesses in the Netherlands? Science of the Total Environment. 2020;755:143182. doi: 10.1016/j.scitotenv.2020.143182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghiemstra H., Lézine A.M., Leroy S.A., Dupont L., Marret F. Late Quaternary palynology in marine sediments: a synthesis of the understanding of pollen distribution patterns in the NW African setting. Quaternary International. 2006;148(1):29–44. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N., Fareed Z., Shahzad F., He X., Shahzad U., Lina M. The nexus between COVID-19, temperature and exchange rate in Wuhan city: New findings from partial and multiple wavelet coherence. Science of the Total Environment. 2020;729:138916. doi: 10.1016/j.scitotenv.2020.138916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A.…Ober C. Association of respiratory allergy, asthma and expression of the SARS-CoV-2 receptor, ACE2. Journal of Allergy and Clinical Immunology. 2020;146(1):203–206. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Capra J.D., Travers P., Walport M. 1999. Immunobiology: The immune system in health and disease. (No. 577.27 JAN) [Google Scholar]
- Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) Journal of General Internal Medicine. 2020:1–5. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q., Han Y. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military Medical Research. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Brosseau L.M. Aerosol transmission of infectious disease. Journal of Occupational and Environmental Medicine. 2015;57(5):501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- Jones A.M., Harrison R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Science of the Total Environment. 2004;326(1–3):151–180. doi: 10.1016/j.scitotenv.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Jung J.H., Lee J.E., Lee C.H., Kim S.S., Lee B.U. Treatment of fungal bioaerosols by a high-temperature, short-time process in a continuous-flow system. Applied and Environmental Microbiology. 2009;75(9):2742–2749. doi: 10.1128/AEM.01790-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingley B., Nguyen-Van-Tam J. Routes of influenza transmission. Influenza and Other Respiratory Viruses. 2013;7:42–51. doi: 10.1111/irv.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R.B., Suphioglu C., Taylor P., Desai R., Watson H.C., Peng J.L., et al. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: Implications for asthma and air pollution. Clinical & Experimental Allergy. 1997;27(3):246–251. [PubMed] [Google Scholar]
- Lee I.K., Hwang B.S., Kim D.W., Kim J.Y., Woo E.E., Lee Y.J.…Yun B.S. Characterization of neuraminidase inhibitors in Korean Papaver rhoeas bee pollen contributing to anti-influenza activities in vitro. Planta Medica. 2016;82(06):524–529. doi: 10.1055/s-0041-111631. [DOI] [PubMed] [Google Scholar]
- Lei Z., Cao H., Jie Y., Huang Z., Guo X., Chen J., Peng L., Cao H., Dai X., Liu J., Li X. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Medicine and Infectious Disease. 2020:101664. doi: 10.1016/j.tmaid.2020.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Licari A., Votto M., Brambilla I., Castagnoli R., Piccotti E., Olcese R.…Luigi Marseglia G. Allergy and asthma in children and adolescents during the COVID outbreak: what we know and how we could prevent allergy and asthma flares? Allergy. 2020;75(9):2402–2405. doi: 10.1111/all.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. Journal of Allergy and Clinical Immunology. 2020;146(1):1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Henry B.M., Bovo C., Sanchis-Gomar F. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID-19) Diagnosis. 2020;7(2):85–90. doi: 10.1515/dx-2020-0041. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhi Y., Ying S. COVID-19 and asthma: Reflection during the pandemic. Clinical Reviews in Allergy & Immunology. 2020;59:78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhou J., Yao J., Zhang X., Li L., Xu X., He X., Wang B., Fu S., Niu T., Yan J. Impact of meteorological factors on the COVID-19 transmission: A multi-city study in China. Science of the Total Environment. 2020:138513. doi: 10.1016/j.scitotenv.2020.138513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison A.C., Manners J.G. Lethal effects of artificial ultraviolet radiation on cereal rust uredospores. Transactions of the British Mycological Society. 1973;60(3):471–494. [Google Scholar]
- Mor S., Kumar S., Singh T., Dogra S., Pandey V., Ravindra K. Impact of COVID-19 lockdown on air quality in Chandigarh, India: understanding the emission sources during controlled anthropogenic activities. Chemosphere. 2021;263:127978. doi: 10.1016/j.chemosphere.2020.127978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Almeida M., Aguiar R., Martin B., Ansotegui I.J., Ebisawa M., Arruda L.K., Caminati M., Canonica G.W., Carr T., Chupp G., Corren J. COVID-19, asthma, and biologic therapies: What we need to know. World Allergy Organization Journal. 2020:100126. doi: 10.1016/j.waojou.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annual Review of Virology. 2020;7 doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- Namork E., Johansen B.V., Løvik M. Detection of allergens adsorbed to ambient air particles collected in four European cities. Toxicology Letters. 2006;165(1):71–78. doi: 10.1016/j.toxlet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ormstad H. Suspended particulate matter in indoor air: Adjuvants and allergen carriers. Toxicology. 2000;152(1–3):53–68. doi: 10.1016/s0300-483x(00)00292-4. [DOI] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L.…Xiang Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications. 2020;11(1):1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini E., Guarnieri M., Nepi M. Pollen carbohydrates and water content during development, presentation, and dispersal: A short review. Protoplasma. 2006;228(1–3):73. doi: 10.1007/s00709-006-0169-z. [DOI] [PubMed] [Google Scholar]
- Parham P.E., Christiansen-Jucht C., Pople D., Michael E. Understanding and modelling the impact of climate change on infectious diseases–progress and future challenges. Climate Change—Socioeconomic Effects, InTech, Rijeka. 2011:43–66. [Google Scholar]
- Pawankar Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organization Journal. 2014;7(2014):12. doi: 10.1186/1939-4551-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawankar R., Holgate S.T., Canonica G.W., Lockey R.F., Blaiss M.S. World Allergy Organization; 2013. WAO white book on allergy 2013 update. [Google Scholar]
- Plötz S.G., Traidl-Hoffmann C., Feussner I., Kasche A., Feser A., Ring J.…Behrendt H. Chemotaxis and activation of human peripheral blood eosinophils induced by pollen-associated lipid mediators. Journal of Allergy and Clinical Immunology. 2004;113(6):1152–1160. doi: 10.1016/j.jaci.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Poole J.A., Barnes C.S., Demain J.G., Bernstein J.A., Padukudru M.A., Sheehan W.J., et al. Impact of weather and climate change with indoor and outdoor air quality in asthma: A Work Group Report of the AAAAI Environmental Exposure and Respiratory Health Committee. The Journal of Allergy and Clinical Immunology. 2019;143(5):1702–1710. doi: 10.1016/j.jaci.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prank M., Kenaley S.C., Bergstrom G.C., Acevedo M., Mahowald N.M. Climate change impacts the spread potential of wheat stem rust, a significant crop disease. Environmental Research Letters. 2019;14(12):124053. [Google Scholar]
- Rahman A., Luo C., Khan M.H.R., Ke J., Thilakanayaka V., Kumar S. Influence of atmospheric PM2. 5, PM10, O3, CO, NO2, SO2, and meteorological factors on the concentration of airborne pollen in Guangzhou, China. Atmospheric Environment. 2019;212:290–304. [Google Scholar]
- Ravindra K., Kaur-Sidhu M., Mor S., Chakma J., Pillarisetti A. Impact of the COVID-19 pandemic on clean fuel programmes in India and ensuring sustainability for household energy needs. Environment international. 2021;147:106335. doi: 10.1016/j.envint.2020.106335. [DOI] [PubMed] [Google Scholar]
- Ravindra K., Singh T., Biswal A., Singh V., Mor S. Impact of COVID-19 lockdown on ambient air quality in megacities of India and implication for air pollution control strategies. Environmental Science and Pollution Research. 2021:1–12. doi: 10.1007/s11356-020-11808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. 2020. Temperature and latitude analysis to predict potential spread and seasonality for COVID-19. Available at SSRN 3550308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaggio J.E. Inhaled particles and respiratory disease. Journal of Allergy and Clinical Immunology. 1994;94(2):304–309. [PubMed] [Google Scholar]
- Schmidt C.W. 2016. Pollen overload: Seasonal allergies in a changing climate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler S., Schlünzen K.H., Scholz F. Viability and sunlight sensitivity of oak pollen and its implications for pollen-mediated gene flow. Trees. 2005;19(2):154–161. [Google Scholar]
- Schuit M., Gardner S., Wood S., Bower K., Williams G., Freeburger D., et al. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. The Journal of Infectious Diseases. 2020;221(3):372–378. doi: 10.1093/infdis/jiz582. [DOI] [PubMed] [Google Scholar]
- Sedghy F., Sankian M., Moghadam M., Ghasemi Z., Mahmoudi M., Varasteh A.R. Impact of traffic-related air pollution on the expression of Platanus orientalis pollen allergens. International Journal of Biometeorology. 2017;61(1):1–9. doi: 10.1007/s00484-016-1186-z. [DOI] [PubMed] [Google Scholar]
- Selcuk M., Gormus S., Guven M. Impact of weather parameters and population density on the COVID-19 transmission: Evidence from 81 provinces of Turkey. Earth Systems and Environment. 2021:1–14. doi: 10.1007/s41748-020-00197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal H., Visez N., Charpin D., Shahali Y., Peltre G., Biolley J.P., Lhuissier F., Couderc R., Yamada O., Malrat-Domenge A., Pham-Thi N. A review of the effects of major atmospheric pollutants on pollen grains, pollen content, and allergenicity. The Scientific World Journal. 2015;2015 doi: 10.1155/2015/940243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad F., Shahzad U., Fareed Z., Iqbal N., Hashmi S.H., Ahmad F. Asymmetric nexus between temperature and COVID-19 in the top ten affected provinces of China: A current application of quantile-on-quantile approach. Science of the Total Environment. 2020;736:139115. doi: 10.1016/j.scitotenv.2020.139115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Goldstein E., Lipsitch M. Absolute humidity and pandemic versus epidemic influenza. American Journal of Epidemiology. 2011;173(2):127–135. doi: 10.1093/aje/kwq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P., Dong Y., Yan H., Li X., Zhao C., Liu W.…Xi S. The impact of temperature and absolute humidity on the coronavirus disease 2019 (COVID-19) outbreak-evidence from China. MedRxiv. 2020 doi: 10.1101/2020.03.22.20038919. [DOI] [Google Scholar]
- Shi W., Gao Z., Ding Y., Zhu T., Zhang W., Xu Y. Clinical characteristics of COVID‐19 patients combined with allergy. Allergy. 2020;75(9):2405–2408. doi: 10.1111/all.14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiller J.B., Lebas B.S., Horner M., Pearson M.N., Clover G.R. Sensitive detection of viruses in pollen using conventional and real‐time reverse transcription‐polymerase chain reaction. Journal of Phytopathology. 2010;158(11–12):758–763. [Google Scholar]
- Singh T., Biswal A., Mor S., Ravindra K., Singh V., Mor S. A high-resolution emission inventory of air pollutants from primary crop residue burning over Northern India based on VIIRS thermal anomalies. Environmental Pollution. 2020;266:115132. doi: 10.1016/j.envpol.2020.115132. [DOI] [PubMed] [Google Scholar]
- Singh R., Levitt A.L., Rajotte E.G., Holmes E.C., Ostiguy N., Lipkin W.I.…Cox-Foster D.L. RNA viruses in hymenopteran pollinators: Evidence of inter-taxa virus transmission via pollen and potential impact on non-apis hymenopteran species. PLoS One. 2010;5(12):e14357. doi: 10.1371/journal.pone.0014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T., Ravindra K., Sreekanth V., Gupta P., Sembhi H., Tripathi S.N., Mor S. Climatological trends in satellite-derived aerosol optical depth over North India and its relationship with crop residue burning: rural-urban contrast. Science of The Total Environment. 2020;748:140963. doi: 10.1016/j.scitotenv.2020.140963. [DOI] [PubMed] [Google Scholar]
- Sobral M.F.F., Duarte G.B., da Penha Sobral A.I.G., Marinho M.L.M., de Souza Melo A. Association between climate variables and global transmission oF SARS-CoV-2. Science of The Total Environment. 2020;729:138997. doi: 10.1016/j.scitotenv.2020.138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A.…Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) International Journal of Surgery. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon W.R. Airborne pollen: A brief life. Journal of Allergy and Clinical Immunology. 2002;109(6):895–900. doi: 10.1067/mai.2002.125556. [DOI] [PubMed] [Google Scholar]
- Sousa S.I.V., Martins F.G., Pereira M.C., Alvim-Ferraz M.C.M., Ribeiro H., Oliveira M., et al. Influence of atmospheric ozone, PM10 and meteorological factors on the concentration of airborne pollen and fungal spores. Atmospheric Environment. 2008;42(32):7452–7464. [Google Scholar]
- Sprigg W.A., Nickovic S., Galgiani J.N., Pejanovic G., Petkovic S., Vujadinovic M.…El-Askary H. Regional dust storm modeling for health services: The case of valley fever. Aeolian Research. 2014;14:53–73. [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infectious Diseases. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Health Commission of the People’s Republic of China. https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf. [DOI] [PMC free article] [PubMed]
- Tosepu R., Gunawan J., Effendy D.S., Lestari H., Bahar H., Asfian P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Science of the Total Environment. 2020:138436. doi: 10.1016/j.scitotenv.2020.138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velraj R., Haghighat F. The contribution of dry indoor built environment on the spread of Coronavirus: Data from various Indian states. Sustainable Cities and Society. 2020;62:102371. doi: 10.1016/j.scs.2020.102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiology and Molecular Biology Reviews. 2008;72(3):413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visez N., Ivanovsky A., Roose A., Gosselin S., Sénéchal H., Poncet P., et al. Atmospheric particulate matter adhesion onto pollen: A review. Aerobiologia. 2020;36(1):49–62. [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. Journal of Virology. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Du G. COVID-19 may transmit through aerosol. Irish Journal of Medical Science (1971-) 2020:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J.…Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Li S. Nonlinear impact of COVID-19 on pollutions–evidence from Wuhan, New York, Milan, Madrid, Bandra, London, Tokyo and Mexico city. Sustainable Cities and Society. 2021;65:102629. doi: 10.1016/j.scs.2020.102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.S. eLS Wiley, Ltd; Chichester, UK: 2014. Plant pathogen dispersal. [DOI] [Google Scholar]
- World Health Organization . 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ Available at: [Google Scholar]
- Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Science of the Total Environment. 2020:139051. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Fu J.F., Mao J.H., Shang S.Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environmental Science and Pollution Research. 2016;23(20):20178–20185. doi: 10.1007/s11356-016-7228-6. [DOI] [PubMed] [Google Scholar]
- Yuan J., Wu Y., Jing W., Liu J., Du M., Wang Y., et al. Non-linear correlation between daily new cases of COVID-19 and meteorological factors in 127 countries. Environmental Research. 2020;193:110521. doi: 10.1016/j.envres.2020.110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas G., Chiang M.C., Wong E., MacDonald F., Lange C.F., Senthilselvan A., et al. Cough aerosol in healthy participants: Fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulmonary Medicine. 2012;12(1):1–12. doi: 10.1186/1471-2466-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li X., Ma R., Li X., Zhou Y., Dong H.…Wei B. Airborne spread and infection of a novel swine-origin influenza A (H1N1) virus. Virology Journal. 2013;10(1):1–7. doi: 10.1186/1743-422X-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q.…Gao Y.D. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang R., Duhl T., Salam M.T., House J.M., Flagan R.C., Avol E.L., Gilliland F.D., Guenther A., Chung S.H., Lamb B.K., VanReken T.M. Development of a regional-scale pollen emission and transport modeling framework for investigating the impact of climate change on allergic airway disease. Biogeosciences. 2014;11(6):1461–1478. doi: 10.5194/bgd-10-3977-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]