Abstract
Elizabethkingia anophelis is a multidrug-resistant pathogen. This study evaluated the antimicrobial activity of minocycline, tigecycline, ciprofloxacin, and levofloxacin using in vitro time-kill assays and in vivo zebrafish animal models. The E. anophelis strain ED853-49 was arbitrarily selected from a bacterial collection which was concomitantly susceptible to minocycline, tigecycline, ciprofloxacin, and levofloxacin. The antibacterial activities of single agents at 0.5–4 × minimum inhibitory concentration (MIC) and dual-agent combinations at 2 × MIC using time-kill assays were investigated. The therapeutic effects of antibiotics in E. anophelis-infected zebrafish were examined. Both minocycline and tigecycline demonstrated bacteriostatic effects but no bactericidal effect. Minocycline at concentrations ≥2 × MIC and tigecycline at concentrations ≥3 × MIC exhibited a long-standing inhibitory effect for 48 h. Bactericidal effects were observed at ciprofloxacin and levofloxacin concentrations of ≥3 × MIC within 24 h of initial inoculation. Rapid regrowth of E. anophelis occurred after the initial killing phase when ciprofloxacin was used, regardless of the concentration. Levofloxacin treatment at the concentration of ≥2 × MIC consistently resulted in the long-lasting and sustainable inhibition of bacterial growth for 48 h. The addition of minocycline or tigecycline weakened the killing effect of fluoroquinolones during the first 10 h. The minocycline-ciprofloxacin or minocycline–levofloxacin combinations achieved the lowest colony-forming unit counts at 48 h. Zebrafish treated with minocycline or a combination of minocycline and levofloxacin had the highest survival rate (70%). The results of these in vitro and in vivo studies suggest that the combination of minocycline and levofloxacin is the most effective therapy approach for E. anophelis infection.
Keywords: Elizabethkingia anophelis, minocycline, tigecycline, ciprofloxacin, levofloxacin, zebrafish
1. Introduction
The genus Elizabethkingia, which originated from Flavobacterium and Chryseobacterium, currently comprises six species: E. meningoseptica, E. miricola, E. anophelis, E. bruuniana, E. ursingii, and E. occulta [1]. Bacteria in this genus are aerobic, Gram-negative, glucose-nonfermenting, non-motile, and nonspore-forming bacilli [2]. These microorganisms, particularly E. anophelis, occasionally cause life-threatening infections in humans, such as meningitis, nosocomial pneumonia, bacteremia, catheter-related bloodstream infection, urinary tract infection, and biliary tract infection [3,4,5,6,7,8]. Outbreaks of severe E. anophelis infections, with an average case fatality rate of 24–60%, have been described in several countries, including Singapore [3], Hong Kong [4], South Korea [5], Taiwan [6,7], and the United States [8].
Numerous studies conducting susceptibility testing using minimum inhibitory concentration (MIC) methods have shown that E. anophelis is typically resistant to multiple antibiotics, including β-lactams, carbapenems, β-lactam/β-lactamase inhibitor combinations, aminoglycosides, and colistin [3,4,5,6,7,8]. Almost all E. anophelis isolates show susceptibility to minocycline, whereas a wide range of MIC levels have been reported for ciprofloxacin, levofloxacin, and tigecycline [5,6,7]. However, these MIC studies of E. anophelis have merely provided static in vitro information on the antimicrobial agents tested; they have not provided kinetic data on the bactericidal rate or activity dosage. At the time of writing this paper, no pharmacodynamic information of antimicrobial agents against E. anophelis is available.
The zebrafish (Danio rerio) is a tropical freshwater vertebrate that has similar organs and immune system to humans. Many studies have demonstrated that the zebrafish is an excellent and powerful animal model for infectious diseases because of their intact innate and adaptive immune [9]. Elizabethkingia species have been known as waterborne pathogens and could infect aquatic animals [10,11,12,13]. Therefore, zebrafish could be a potentially appropriate animal model for Elizabethkingia infections.
In this study, we used in vitro time-kill studies to evaluate the antimicrobial effects of minocycline, tigecycline, ciprofloxacin, and levofloxacin against E. anophelis, either singly or in combination. We also used zebrafish as an animal model to evaluate the in vivo antimicrobial effects of these tetracycline/glycylcycline and fluoroquinolones in zebrafish animal model with E. anophelis infections.
2. Materials and Methods
2.1. Study Setting and Ethics
The clinical microbiology database of E-Da Hospital, a 1000-bed university-affiliated medical center in Kaohsiung, Taiwan, was searched for routine cultures that yielded Elizabethkingia from 2005 to 2019. The collected Elizabethkingia isolates were stored as glycerol stocks at −80 °C until use. The precise species of the stored Elizabethkingia isolates was re-identified using 16S rRNA gene sequencing, as described in our previous study [6]. This study was approved by the Institutional Review Board of E-Da Hospital. This animal study followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of E-Da Hospital.
2.2. MIC Determination
The MICs of the antibiotics were determined using Sensititre 96-well broth microdilution panels in accordance with the manufacturer’s instructions (Thermo Fisher Scientific/Trek Diagnostics Systems, Oakwood Village, OH, USA). The breakpoints for susceptibility testing were appraised as per the interpretive standards for “other non-Enterobacteriaceae” from the CLSI guideline [14]. There are no interpretive criteria for tigecycline against “other non-Enterobacteriaceae” from the CLSI guideline. Therefore, the MICs of tigecycline were interpreted according to the Enterobacteriaceae susceptibility breakpoints of the US Food and Drug Administration (susceptible MIC ≤ 2 mg/L; intermediate MIC, 4 mg/L; resistant MIC ≥ 8 mg/L) [15].
2.3. Bacterial Strain
For in vitro time-kill studies and in vivo animal studies, the MIC results of all collected E. anophelis isolates were analyzed. Strain ED853-49 was arbitrarily selected from a collection of E. anophelis isolates that had demonstrated susceptibilities to minocycline, tigecycline, ciprofloxacin, and levofloxacin.
2.4. In Vitro Time-Kill Studies
The E. anophelis strain ED853-49 was cultured overnight at 35 °C in cation-adjusted Mueller–Hinton broth (CAMHB). The bacterial suspension was adjusted to a 1.7 McFarland standard (approximately 5 × 108 colony-forming unit (CFU)/mL). Then, 25 µL of the adjusted bacterial suspension was added to a flask with 25 mL of CAMHB (approximately 5 × 105 CFU/mL). To test the antimicrobial effect of a single antibiotic in time-kill studies, minocycline (Cyrusbioscience Inc., Taipei, Taiwan), tigecycline (Cyrusbioscience), ciprofloxacin (Sigma–Aldrich Inc., St. Louis, MO, USA), and levofloxacin (Sigma-Aldrich) at concentrations of 0.5 ×, 1 ×, 2 ×, 4 × MIC were added to the bacteria-containing flasks at 35 °C for 16–20 h. To investigate the synergistic and antagonistic effects of two antibiotics in time-kill studies, minocycline–ciprofloxacin, minocycline–levofloxacin, tigecycline–ciprofloxacin, and tigecycline–levofloxacin combinations were examined. Each antimicrobial agent at 2 × MIC was used for combination studies because the inhibitory activity against E. anophelis persisted for at least 12 h at concentrations equal to two or more times the MIC (shown later in the section of results) [16]. After an incubation time of 0, 2, 4, 6, 8, 10, 24, 30, and 48 h, 50 µL of bacterial suspension was added to 450 µL of PBS. The colony number was counted on Mueller–Hinton agar plates inoculated with 100-µL aliquots of 10-fold serially diluted bacterial suspension after 16–20 h of incubation at 35 °C. All experiments were repeated five times. The highest and lowest values were excluded. The average (mean) of the middle three values was calculated.
2.5. Analysis of Time-Kill Curves
The results of time-kill studies were analyzed using a previously reported method [16]. Antimicrobial agents were considered to be bactericidal if a CFU number decrease of ≥3 log10 compared with the initial inocula within 24 h [17]. Synergy of a given antimicrobial combination was defined as a CFU number decrease of ≥2 log10 compared with the most active single agent, and antagonism was defined as a ≥2 log10 increase in the CFU number compared with the most active single antibiotic [17].
2.6. Preparation of Bacteria for the Animal Study
The E. anophelis strain ED853-49 was overnight cultured in CAMHB at 37 °C. Then, the bacterial suspension was centrifuged at 10,000 g for 3 min. The supernatant of the centrifuged solution was removed, and the left cell pellet was re-suspended in 1 mL of 0.9% saline. These processes were repeated two times. The final bacterial suspension was adjusted to 7.5 × 109 CFU/mL by an OD600 spectrophotometer.
2.7. Antimicrobial Effects in Zebrafish with E. anophelis Infection
Approximately 7-month-old adult zebrafish of the wild-type AB-line strain were used in this study (G. Fish Animal Model Inc., Taipei, Taiwan). The care, housing, anesthetization, and euthanization of zebrafish were completed as in our previous study [18]. A total of 10 µL of bacterial solution (7.5 × 107 CFU) was injected into the peritoneal cavity of zebrafish as described previously [19]. Each group tested contained ten zebrafish. Antibiotics were given via intraperitoneal injection 2 h after bacterial injection as described previously for the peritonitis mouse model [20,21]. The dosages of single-agent and dual-agent therapy were as follows: Minocycline (Cyrusbioscience), 10 mg/kg every 12 h; tigecycline (Pfizer Inc., New York City, NY, USA), 25 mg/kg every 12 h; ciprofloxacin (Bayer AG, Leverkusen, Germany), 8 mg/kg every 12 h; and levofloxacin (Daiichi Sankyo Inc., Tokyo, Japan), 15 mg/kg every 24 h. The zebrafish were observed for a total of 72 h after antibiotic infections. Kaplan–Meier survival analysis was performed using SPSS version 18.0 (IBM, Armonk, NY, USA). A two-tailed p < 0.05 was considered statistically significant.
3. Results
3.1. Antimicrobial Susceptibility Determined Using MIC
The E. anophelis strain ED853-49 was resistant to piperacillin (MIC = 256 mg/L), piperacillin–tazobactam (MIC = 256/4 mg/L), ticarcillin–clavulanate (MIC = 256/4 mg/L), ceftazidime (MIC > 256 mg/L), cefepime (MIC > 256 mg/L), gentamicin (MIC > 256 mg/L), amikacin (MIC > 256 mg/L), and imipenem (MIC > 8 mg/L). By contrast, this strain was susceptible to minocycline (MIC = 0.25 mg/L), tigecycline (MIC = 0.5 mg/L), ciprofloxacin (MIC = 0.5 mg/L), and levofloxacin (MIC = 0.25 mg/L).
3.2. Time-Kill Studies of Single-Agent Therapy
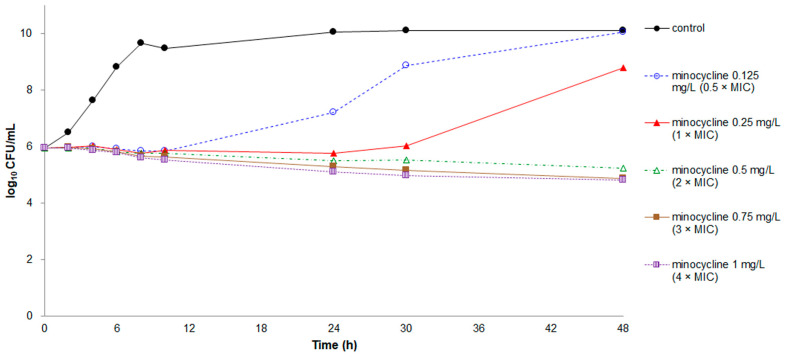
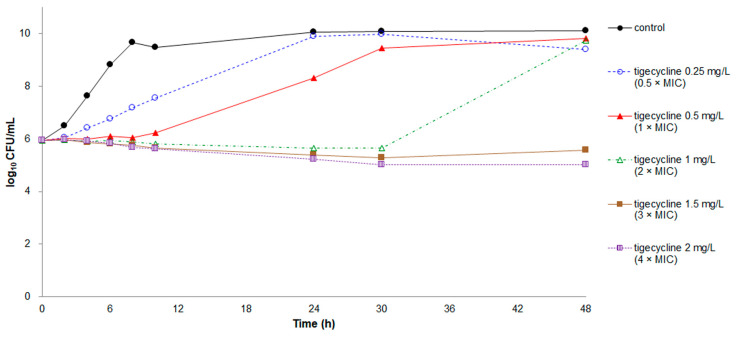
The growth patterns and time-kill curves of minocycline and tigecycline were similar (Figure 1 and Figure 2). Poor bacteriostatic effects and regrowth of bacteria were observed in minocycline at concentrations of 0.5–1 × MIC and in tigecycline at concentrations of 0.5–2 × MIC. Only minocycline at concentrations of 2–4 × MIC and tigecycline at concentrations of 3–4 × MIC constantly inhibited bacterial growth for 48 h. However, both minocycline and tigecycline exhibited no bactericidal effect at any concentration.
Figure 1.
Time-kill assays for minocycline against Elizabethkingia anophelis strain ED853-49. Data points represent the mean of three experiments. Minimum inhibitory concentration (MIC) of minocycline = 0.25 mg/L.
Figure 2.
Time-kill assays for tigecycline against E. anophelis strain ED853-49. Data points represent the mean of three experiments. MIC of tigecycline = 0.5 mg/L.
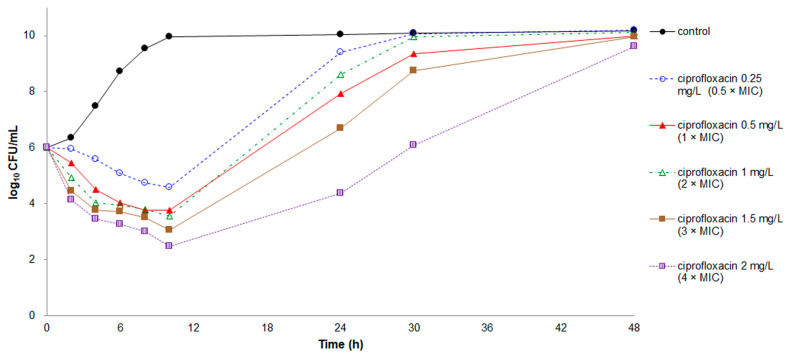
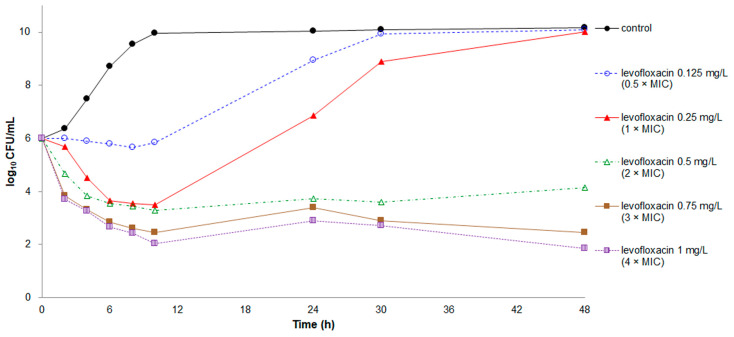
Rapid killing effects were recorded at all tested concentrations of ciprofloxacin and levofloxacin except for levofloxacin at the concentration of 0.5 × MIC (Figure 3 and Figure 4). Nevertheless, regrowth occurred in E. anophelis treated with ciprofloxacin, regardless of the concentration, and levofloxacin at concentrations of 0.5–1 × MIC. Both ciprofloxacin and levofloxacin at concentrations of 3 × and 4 × MIC demonstrated a bactericidal effect, with a CFU number decrease of ≥3 log10.
Figure 3.
Time-kill assays for ciprofloxacin against E. anophelis strain ED853-49. Data points represent the mean of three experiments. MIC of ciprofloxacin = 0.5 mg/L.
Figure 4.
Time-kill assays for levofloxacin against E. anophelis strain ED853-49. Data points represent the mean of three experiments. MIC of levofloxacin = 0.25 mg/L.
3.3. Time-Kill Studies of Dual-Agent Combinations
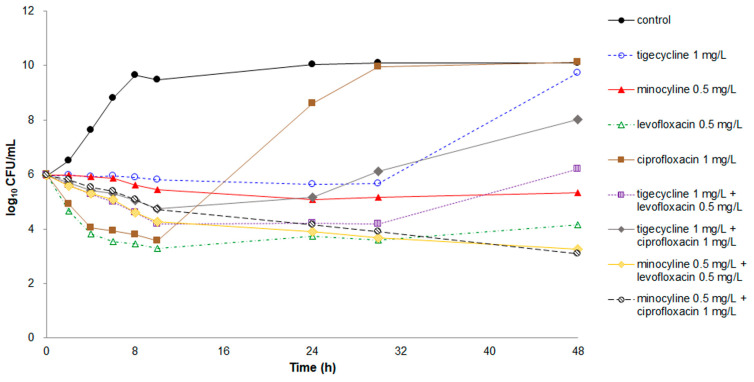
When minocycline or tigecycline was combined with either ciprofloxacin or levofloxacin (Figure 5), the killing ability of the antibiotics became weaker than that of ciprofloxacin or levofloxacin alone during the first 10 h. However, the reduction in killing activity was <2 log10 CFU. Regrowth of bacteria occurred after the 30-h inhibitory period for the tigecycline–ciprofloxacin and tigecycline–levofloxacin combinations. The CFU counts of the tigecycline–levofloxacin combination at 48 h were higher than that of levofloxacin alone.
Figure 5.
Time-kill assays for single and dual-agent combinations with 2× MIC against E. anophelis strain ED853-49. Data points represent the mean of three experiments. Minocycline: MIC, 0.25 mg/L; tigecycline: MIC, 0.5 mg/L; ciprofloxacin: MIC, 0.5 mg/L; and levofloxacin: MIC, 0.25 mg/L.
The minocycline–ciprofloxacin and minocycline–levofloxacin combinations achieved the lowest CFU counts at 48 h. The difference in CFU counts at 48 h for E. anophelis inoculated between the minocycline–ciprofloxacin combination and minocycline alone was 2.2 log10, meeting the criteria of synergy. Although a desirable synergistic effect was not observed, increased antimicrobial efficacy with a CFU number decrease of 0.9 log10 at 48 h was detected in the minocycline–levofloxacin combination compared with levofloxacin alone.
3.4. Therapeutic Effects of Antibiotics in the Zebrafish Animal Model
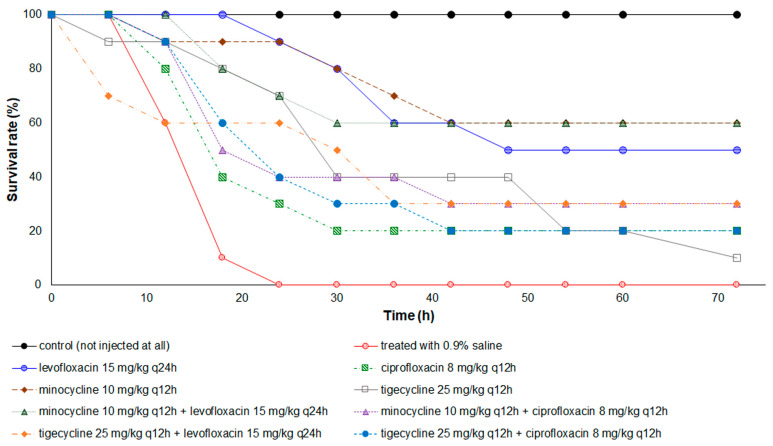
With an inoculum of E. anophelis via intraperitoneal administration, all zebrafish without antimicrobial treatment died within 24 h (Figure 6). Zebrafish treated with minocycline or minocycline-levofloxacin combination had the highest survival rate (70%) at the end point of 72 h, whereas those treated with tigecycline possessed the lowest survival rate (10%). The median survival time of zebrafish with minocycline or minocycline-levofloxacin combination therapy was >72 h, but for those with ciprofloxacin or minocycline-ciprofloxacin combination treatment, it was only 18 h. The Kaplan–Meier survival analysis revealed that zebrafish treated with all antibiotics, either singly or in combinations, had a significantly higher survival rate than the control group with saline injection. The survival time of the minocycline or minocycline–levofloxacin combination-treated group was significantly longer than that of the tigecycline-treated group (p = 0.035 and p = 0.049, respectively).
Figure 6.
Kaplan–Meier survival curves of the 72-h cumulative survival rate for zebrafish infected with E. anophelis strain ED853-49. All zebrafish treated with 0.9% saline died within 24 h. Zebrafish treated with antibiotic(s) exhibited a significantly higher survival rate than those treated with 0.9% saline (p < 0.005). The log rank test: Minocycline versus tigecycline, p = 0.035; levofloxacin + minocycline versus tigecycline, p = 0.049; and others, no significant difference.
4. Discussion
Minocycline, a semi-synthetic tetracycline, is primarily a bacteriostatic antimicrobial agent, and it exerts its antimicrobial activity through the inhibition of protein synthesis [22]. In antimicrobial susceptibility studies using MIC testing [3,4,5,6,7,8], minocycline has been demonstrated as the most active antibiotic against E. anophelis. Our study also demonstrated that monotherapy of minocycline exhibited an excellent therapeutic effect in healthy zebrafish infected with E. anophelis. However, minocycline expressed a prolonged but slight inhibitory effect rather than a bactericidal effect on E. anophelis, even at high concentrations. Because E. anophelis usually infects immunocompromised patients [3,4,5,6,7,8], the lack of rapid bacterial killing activity could be a critical problem in the clinical setting.
Tigecycline is an analogue of minocycline and has an extended spectrum to overcome resistance to tetracyclines [23]. In previous antimicrobial susceptibility studies using MIC testing, the susceptibility rate of E. anophelis to tigecycline ranged from 5.1% to 26.4% [7]. Although tigecycline is mainly considered to be a bacteriostatic antimicrobial agent [23], this new glycylcycline antibiotic exhibits bactericidal activity against some bacterial species, including Klebsiella pneumoniae, Acinetobacter baumannii, and Vibrio vulnificus [24,25,26]. However, our study revealed that tigecycline possessed only bacteriostatic activity against E. anophelis but no bactericidal effect, even at 4 × MIC. In contrast to the bacteriostatic effect of minocycline and tigecycline, both ciprofloxacin and levofloxacin demonstrated rapid bactericidal effects during the first 10 h after initial inoculation. This bactericidal effect is critical since rapid reduction of the bacterial burden in the beginning phase of infections could stabilize the septic condition of patients [27].
Previous studies using time-kill assays have demonstrated in vitro synergistic or antagonistic interactions between tetracycline/glycylcycline and fluoroquinolones against certain microorganisms. For example, the combination of tigecycline and ciprofloxacin was reported to have in vitro synergic effects against V. vulnificus, K. pneumoniae, and E. coli [28,29]. In vitro antagonism occurred in the tigecycline–levofloxacin combination against Brucella melitensis [30], but this antagonistic effect was not observed in the treatment of A. baumannii, K. pneumoniae, or other pathogens [31]. Another study revealed synergistic effects in the tigecycline–levofloxacin combination in the treatment of A. baumannii using both chequerboard and time-kill assays [32]. Moreover, the use tigecycline in combination with levofloxacin could produce postantibiotic effects along with enhancement of bactericidal activity and synergistic interaction against A. baumannii [33]. Consequently, combination therapy could be a potentially better choice for multidrug-resistant pathogens.
In the present study, the combination of minocycline or tigecycline with ciprofloxacin or levofloxacin markedly reduced the killing effects of ciprofloxacin and levofloxacin on E. anophelis in the first 10 h, although this decreased effect did not reach the criteria of antagonism. In addition, minocycline combined with ciprofloxacin or levofloxacin displayed additive bacterial killing activity on E. anophelis in the time-kill assays, although only the minocycline–ciprofloxacin combination achieved the criteria for synergy. However, the in vivo animal study revealed that the minocycline–levofloxacin combination exhibited a significantly higher survival rate than the minocycline–ciprofloxacin combination. Our time-kill study demonstrated that rapid regrowth of bacteria occurred after 10 h of killing effects in ciprofloxacin at the concentration of 2 × MIC, but this phenomenon was not observed in levofloxacin at 2 × MIC. This difference could explain why the minocycline–levofloxacin combination demonstrated the best therapeutic effect in zebrafish infected with E. anophelis.
Although this study provided valuable information about antimicrobial effects of minocycline, tigecycline, ciprofloxacin, and levofloxacin against E. anophelis, it has several limitations. First, only one strain of E. anophelis was examined because the time-kill assay is a very labor-intensive and time-consuming task. These results might not represent the antimicrobial effects of these four antibiotics against E. anophelis. Second, only four antibiotics were tested in this study. Other potentially effective antibiotics, such as rifampin, trimethoprim/sulfamethoxazole, and vancomycin, were not evaluated. Finally, zebrafish are non-mammal animals. Despite the similar organs and immune system to humans, further experiments using mammal animals might be necessary.
The results of this in vitro and in vivo study suggest that the combination of minocycline and levofloxacin is the most effective therapy for E. anophelis infection. Further clinical studies are warranted to delineate the antimicrobial effects of minocycline–levofloxacin combination against this life-threatening infection.
Author Contributions
Conceptualization, J.-N.L.; data curation, J.-N.L., C.-H.L., C.-H.Y. and Y.-H.H.; formal analysis, J.-N.L. and Y.-H.H.; funding acquisition, J.-N.L.; methodology, J.-N.L.; resources, C.-H.L.; supervision, J.-N.L.; validation, J.-N.L., C.-H.L., C.-H.Y. and Y.-H.H.; writing—original draft, J.-N.L. and C.-H.Y.; writing—review and editing, J.-N.L., C.-H.L., C.-H.Y. and Y.-H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants EDPJ108068/EDPJ109070 from E-Da Hospital and MOST 108-2314-B-214 -004/109-2314-B-214-006-MY2 from the Ministry of Science and Technology, Taiwan.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of E-Da Hospital (EMRP-107-139). This animal study followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of E-Da Hospital (IACUC-EDAH-108016).
Informed Consent Statement
The need for patient informed consent was waived by the Institutional Review Board of E-Da Hospital because the retrospective analysis of routine cultures posed no more than minimal risk of harm to the subjects.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin J.N., Lai C.H., Yang C.H., Huang Y.H. Elizabethkingia infections in humans: From genomics to clinics. Microorganisms. 2019;7:295. doi: 10.3390/microorganisms7090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson A.C., Gulvik C.A., Whitney A.M., Humrighouse B.W., Graziano J., Emery B., Bell M., Loparev V., Juieng P., Gartin J., et al. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. nov. Antonie Leeuwenhoek. 2018;111:55–72. doi: 10.1007/s10482-017-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teo J., Tan S.Y., Tay M., Ding Y., Kjelleberg S., Givskov M., Lin R.T., Yang L. First case of E anophelis outbreak in an intensive-care unit. Lancet. 2013;382:855–856. doi: 10.1016/S0140-6736(13)61858-9. [DOI] [PubMed] [Google Scholar]
- 4.Lau S.K., Chow W.N., Foo C.H., Curreem S.O., Lo G.C., Teng J.L., Chen J.H., Ng R.H., Wu A.K., Cheung I.Y., et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci. Rep. 2016;6:26045. doi: 10.1038/srep26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han M.S., Kim H., Lee Y., Kim M., Ku N.S., Choi J.Y., Yong D., Jeong S.H., Lee K., Chong Y. Relative prevalence and antimicrobial susceptibility of clinical isolates of Elizabethkingia species based on 16S rRNA gene sequencing. J. Clin. Microbiol. 2017;55:274–280. doi: 10.1128/JCM.01637-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J.N., Lai C.H., Yang C.H., Huang Y.H., Lin H.H. Clinical manifestations, molecular characteristics, antimicrobial susceptibility patterns and contributions of target gene mutation to fluoroquinolone resistance in Elizabethkingia anophelis. J. Antimicrob. Chemother. 2018;73:2497–2502. doi: 10.1093/jac/dky197. [DOI] [PubMed] [Google Scholar]
- 7.Lin J.N., Lai C.H., Yang C.H., Huang Y.H. Comparison of clinical manifestations, antimicrobial susceptibility patterns, and mutations of fluoroquinolone target genes between Elizabethkingia meningoseptica and Elizabethkingia anophelis isolated in Taiwan. J. Clin. Med. 2018;7:538. doi: 10.3390/jcm7120538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrin A., Larsonneur E., Nicholson A.C., Edwards D.J., Gundlach K.M., Whitney A.M., Gulvik C.A., Bell M.E., Rendueles O., Cury J., et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat. Commun. 2017;8:15483. doi: 10.1038/ncomms15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan C., Kim C.H. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Moore L.S.P., Owens D.S., Jepson A., Turton J.F., Ashworth S., Donaldson H., Holmes A.H. Waterborne Elizabethkingia meningoseptica in adult critical care. Emerg. Infect. Dis. 2016;22:9–17. doi: 10.3201/eid2201.150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yung C.F., Maiwald M., Loo L.H., Soong H.Y., Tan C.B., Lim P.K., Li L., Tan N.W., Chong C.Y., Tee N., et al. Elizabethkingia anophelis and association with tap water and handwashing, Singapore. Emerg. Infect. Dis. 2018;24:1730–1733. doi: 10.3201/eid2409.171843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs A., Chenia H.Y. Biofilm formation and adherence characteristics of an Elizabethkingia meningoseptica isolate from Oreochromis mossambicus. Ann. Clin. Microbiol. Antimicrob. 2011;10:16. doi: 10.1186/1476-0711-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu R., Yuan J., Meng Y., Wang Z., Gu Z. Pathogenic Elizabethkingia miricola infection in cultured black-spotted frogs, China, 2016. Emerg. Infect. Dis. 2017;23:2055–2059. doi: 10.3201/eid2312.170942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing, M100. 30th ed. CLSI; Wayne, PA, USA: 2020. [Google Scholar]
- 15.Kelesidis T., Karageorgopoulos D.E., Kelesidis I., Falagas M.E. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: A systematic review of the evidence from microbiological and clinical studies. J. Antimicrob. Chemother. 2008;62:895–904. doi: 10.1093/jac/dkn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko W.C., Chiang S.R., Lee H.C., Tang H.J., Wang Y.Y., Chuang Y.C. In vitro and in vivo activities of fluoroquinolones against Aeromonas hydrophila. Antimicrob. Agents Chemother. 2003;47:2217–2222. doi: 10.1128/AAC.47.7.2217-2222.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peck K.R., Kim M.J., Choi J.Y., Kim H.S., Kang C.-I., Cho Y.K., Park D.W., Lee H.J., Lee M.S., Ko K.S. In vitro time-kill studies of antimicrobial agents against blood isolates of imipenem-resistant Acinetobacter baumannii, including colistin- or tigecycline-resistant isolates. J. Med. Microbiol. 2012;61:353–360. doi: 10.1099/jmm.0.036939-0. [DOI] [PubMed] [Google Scholar]
- 18.Lin J.N., Chang L.L., Lai C.H., Lin K.J., Lin M.F., Yang C.H., Lin H.H., Chen Y.H. Development of an animal model for alcoholic liver disease in zebrafish. Zebrafish. 2015;12:271–280. doi: 10.1089/zeb.2014.1054. [DOI] [PubMed] [Google Scholar]
- 19.Saralahti A., Piippo H., Parikka M., Henriques-Normark B., Rämet M., Rounioja S. Adult zebrafish model for pneumococcal pathogenesis. Dev. Comp. Immunol. 2014;42:345–353. doi: 10.1016/j.dci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Tang H.J., Chang M.C., Ko W.C., Huang K.Y., Lee C.L., Chuang Y.C. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob. Agents Chemother. 2002;46:3580–3584. doi: 10.1128/AAC.46.11.3580-3584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko W.C., Lee H.C., Chiang S.R., Yan J.J., Wu J.J., Lu C.L., Chuang Y.C. In vitro and in vivo activity of meropenem and sulbactam against a multidrug-resistant Acinetobacter baumannii strain. J. Antimicrob. Chemother. 2004;53:393–395. doi: 10.1093/jac/dkh080. [DOI] [PubMed] [Google Scholar]
- 22.Greig S.L., Scott L.J. Intravenous minocycline: A review in Acinetobacter infections. Drugs. 2016;76:1467–1476. doi: 10.1007/s40265-016-0636-6. [DOI] [PubMed] [Google Scholar]
- 23.Noskin G.A. Tigecycline: A new glycylcycline for treatment of serious infections. Clin. Infect. Dis. 2005;41:S303–S314. doi: 10.1086/431672. [DOI] [PubMed] [Google Scholar]
- 24.Tessier P.R., Nicolau D.P. Tigecycline displays in vivo bactericidal activity against extended-spectrum-β-lactamase-producing Enterobacteriaceae after 72-hour exposure period. Antimicrob. Agents Chemother. 2013;57:640–642. doi: 10.1128/AAC.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozbek B., Mataraci E. In vitro effectiveness of colistin, tigecycline and levofloxacin alone and combined with clarithromycin and/or heparin as lock solutions against embedded Acinetobacter baumannii strains. J. Antimicrob. Chemother. 2013;68:827–830. doi: 10.1093/jac/dks472. [DOI] [PubMed] [Google Scholar]
- 26.Tang H.J., Chen C.C., Lai C.C., Zhang C.C., Weng T.C., Chiu Y.H., Toh H.S., Chiang S.R., Yu W.L., Ko W.C., et al. In vitro and in vivo antibacterial activity of tigecycline against Vibrio vulnificus. J. Microbiol. Immunol. Infect. 2018;51:76–81. doi: 10.1016/j.jmii.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Burrell A.R., McLaws M.L., Fullick M., Sullivan R.B., Sindhusake D. SEPSIS KILLS: Early intervention saves lives. Med. J. Aust. 2016;204:73. doi: 10.5694/mja15.00657. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.E., Kim H.K., Choi S.M., Yu Y., Kim U.J., Darboe K.S., Kang S.J., Park K.H., Kang G., Kim Y.R., et al. In vitro synergy and in vivo activity of tigecycline-ciprofloxacin combination therapy against Vibrio vulnificus sepsis. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00310-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yim H., Woo H., Song W., Park M.J., Kim H.S., Lee K.M., Hur J., Park M.-S. Time-kill synergy tests of tigecycline combined with imipenem, amikacin, and ciprofloxacin against clinical isolates of multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Ann. Clin. Lab. Sci. 2011;41:39–43. [PubMed] [Google Scholar]
- 30.Aliskan H., Can F., Demirbilek M., Colakoglu S., Kilic S., Arslan H. Determining in vitro synergistic activities of tigecycline with several other antibiotics against Brucella melitensis using checkerboard and time-kill assays. J. Chemother. Florence Italy. 2009;21:24–30. doi: 10.1179/joc.2009.21.1.24. [DOI] [PubMed] [Google Scholar]
- 31.Petersen P.J., Labthavikul P., Jones C.H., Bradford P.A. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J. Antimicrob. Chemother. 2006;57:573–576. doi: 10.1093/jac/dki477. [DOI] [PubMed] [Google Scholar]
- 32.Principe L., D’Arezzo S., Capone A., Petrosillo N., Visca P. In vitro activity of tigecycline in combination with various antimicrobials against multidrug resistant Acinetobacter baumannii. Ann. Clin. Microbiol. Antimicrob. 2009;8:18. doi: 10.1186/1476-0711-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozbek B., Sentürk A. Postantibiotic effects of tigecycline, colistin sulfate, and levofloxacin alone or tigecycline-colistin sulfate and tigecycline-levofloxacin combinations against Acinetobacter baumannii. Chemotherapy. 2010;56:466–471. doi: 10.1159/000321015. [DOI] [PubMed] [Google Scholar]