Abstract
The phenolic composition, as well as the antioxidant and antimicrobial activities of two poorly investigated Achillea species, Achillea lingulata Waldst. and the endemic Achillea abrotanoides Vis., were studied. To obtain a more detailed phytochemical profile, four solvents with different polarities were used for the preparation of the plant extracts whose phenolic composition was analyzed using UHPLC-MS/MS (ultra-high performance liquid chromatography-tandem mass spectrometry). The results indicate that both of the investigated Achillea species are very rich in both phenolic acids and flavonoids, but that their profiles differ significantly. Chloroform extracts from both species had the highest yields and were the most chemically versatile. The majority of the examined extracts showed antimicrobial activity, while ethanolic extracts from both species were potent against all tested microorganisms. Furthermore, the antioxidant activity of the extracts was evaluated. It was found that the ethanolic extracts possessed the strongest antioxidant activities, although these extracts did not contain the highest amounts of detected phenolic compounds. In addition, several representatives of phenolic compounds were also assayed for these biological activities. Results suggest that ethanol is a sufficient solvent for the isolation of biologically active compounds from both Achillea species. Moreover, it was shown that the flavonoids naringenin and morin are mainly responsible for these antimicrobial activities, while caffeic, salicylic, chlorogenic, p-coumaric, p-hydroxybenzoic, and rosmarinic acid are responsible for the antioxidant activities of the Achillea extracts.
Keywords: Achillea lingulata Waldst, Achillea abrotanoides Vis, extraction, phenolic compounds, antioxidant activity, antimicrobial activity
1. Introduction
Selection of an appropriate solvent and extraction protocol is the key for successful isolation of biologically active compounds from medicinal plants. The extraction solvents are chosen according to their polarity, and therefore, the ability to isolate specific types of compounds with different structures and physicochemical properties. The solvents accepted for use in pharmaceutical formulations are water, ethanol, and glycerol [1]. The polarity of extraction solvents influences the extraction efficiency of phenolic compounds. Less polar solvents extract smaller amounts of phenolic compounds, and therefore, these extracts possess a lesser potential for scavenging free radicals [2]. Generally, highly hydroxylated aglycone forms of phenolic compounds are soluble in water, alcohols (ethanol, methanol), and their mixtures, while less polar and highly methoxylated aglycone forms are extracted into less polar solvents (ethyl acetate, acetone, chloroform), [3,4]. Since hydroxy groups of phenolic compounds contribute to antioxidant activity, more polar extracts generally possess higher antioxidant activities.
Even though the use of non-toxic solvents is more desirable, some phytochemicals with hydrophobic properties are necessary for extraction by non-polar solvents. Artemisinin could be mentioned as an example. It is a highly active antimalarial compound isolated from Artemisia annua that is extracted from the plant material using non-polar solvents, such as petrol ether and hexane. However, the use of hydrocarbon solvents is not environmentally friendly, and even after evaporation, the solvents could be still unintentionally present in the sample in trace amounts. However, the extraction of artemisinin with water is inefficient and ethanol extraction causes rapid degradation of the compound [5]. Another option for the extraction of non-polar analytes could be supercritical fluid extraction (SFE). The significant benefit of this type of extraction is the non-use of flammable and toxic solvents, which efficiently extract phenolic compounds [6], but also other bioactive molecules of interest [7] from different plant materials. The efficiency of SFE was tested on Achillea millefolium and resulted in an almost threefold increase in the concentration of total phenolic compounds in comparison with ethanolic extraction [8]. Ultrasound-assisted extraction is widely used for extracting compounds from plant material. This technique is based on the disruption of plant cells and the liberation of the compounds to the solvent under low temperatures, preventing the degradation of thermolabile natural metabolites [9]. It is very convenient for the isolation of phenolic compounds. Thus, these extracts may also have better antioxidant activity in comparison to the extracts from Soxhlet extraction and maceration [10]. Moreover, the use of ultrasound-assisted extraction reduces energy costs and extraction times [11].
The human population encounters different pathogens, including urinary tract infection pathogens. These infectious microorganisms are mainly Escherichia coli [12], Salmonella sp. [13], and Staphylococcus aureus, a foodborne [14], skin [15], and soft tissue infection pathogen [16]. Due to the extensive use of antibiotics, both E. coli [17], and S. aureus [18] are common pathogens that have developed multiple drug resistances. However, some antibiotics show higher activities when combined with medicinal plant extracts [19]. A rising number of pharmaceutical companies develop herbal remedies to be used as a replacement for or a supplement to conventional medicines [20], primarily as prevention against disorders. Some examples are members of the genus Carpobrotus [21] or Hedychium [22]. Another interest is the development of safer antioxidants from natural sources to substitute for synthetic antioxidants (BHT, BHA) with potential health risks [23].
Plants of the Asteraceae family have been widely used as traditional medicinal herbs since ancient times. They are the source of many compounds that possess antioxidant, antimicrobial, and anticancer properties [24]. Moreover, extracts of Asteraceae plants showed high efficiency in the treatment of diabetes, inflammations, etc. [25], and also in cardiovascular-related diseases [26]. This highly diverse family is mainly distributed in Europe and the northern hemisphere. In general, plants of the genus Achillea contain a broad spectrum of compounds with bioactive properties. They are reported as tonic, anti-inflammatory, anti-spasmodic, diaphoretic, diuretic, and emmenagogic agents and have been used for the treatment of hemorrhages, pneumonia, rheumatic pain, and wound healing [27]. Their protective and antioxidant activities are linked with their content of phenolics and flavonoids [28]. Furthermore, the chemistry of the Achillea species could be used for the chemotaxonomical description of this genus [29,30]. Moreover, Achillea millefolium L. is listed as a plant drug in the Czech Pharmacopoeia [31].
The present work is comprised of the identification and quantification of the compounds from the vegetative parts and inflorescences of Achillea lingulata Waldst. and A. abrotanoides Vis. (Figure 1). Achillea lingulata grows in the southeastern parts of Europe, with the main distribution in the Balkan Peninsula, Carpathians, and Belarus, while A. abrotanoides is endemic to the Balkan Peninsula. To isolate diverse compounds from these plants, four solvents with different polarities were used and all extracts were characterized by their phenolic composition and antioxidant and antimicrobial activities. To the best of our knowledge, there is little data about the phenolic composition and bioactive compounds in the roots [32] and aerial parts [33] of A. lingulata, and no data for A. abrotanoides.
Figure 1.
Inflorescences of the investigated Achillea species.
2. Results and Discussion
2.1. Extraction
The objective of the extraction process is to maximize the amount of target compounds and to obtain the highest biological activity of these extracts. The extraction yield and biological activity of the resulting extract were affected by both the extraction technique and the extraction solvent. Ultrasound-assisted extraction, or sonication, uses cavitation energy in the solvent that accelerates the dissolution and diffusion of the solute as well as the heat transfer, which improves the extraction efficiency. This extraction technique requires low solvent and energy consumption and allows for the reduction of extraction temperature and extraction time. Therefore, it is applicable for the extraction of thermolabile and unstable compounds [34].
Several factors should be considered in the selection of solvents, mainly selectivity, solubility, cost, and safety [35]. In general, alcohols, acetone, and water are used for the extraction of bioactive compounds from the plant material, but the selection is based on the properties of the compound of interest, as well as the plant material used [34].
Plant extracts were prepared through the sonication of dry plant material (vegetative part and inflorescence) in petrol ether, chloroform, ethanol, and water. The yields of the extracts are presented in Table 1, from which it can be seen that the chloroform extracts had the highest yields for both plant species and their investigated organs. Usually, this halogenated solvent with medium polarity can isolate a wide range of compounds from plant material and is commonly used for the non-targeted isolation and analysis of natural products [23]. However, due to its toxicity in long exposures, chloroform is not a suitable solvent for use in pharmaceutical preparations. Furthermore, water extracts also showed relatively high yields for both plants and their organs. On the contrary, petrol ether and ethanol revealed the lowest yields.
Table 1.
Yields (%) of obtained extracts of two Achillea species.
| Species | Plant Organ | Petrol Ether | Chloroform | Ethanol | Water |
|---|---|---|---|---|---|
| A. lingulata | inflorescence | 2.62 | 11.50 | 2.00 | 6.20 |
| vegetative part | 2.86 | 18.80 | 3.00 | 6.30 | |
| A. abrotanoides | inflorescence | 2.92 | 18.50 | 3.10 | 6.60 |
| vegetative part | 2.74 | 11.00 | 2.40 | 5.70 |
2.2. UHPLC-MS/MS Analysis of Phenolic Compounds
A UHPLC-MS/MS (ultra-high performance liquid chromatography-tandem mass spectrometry) analysis was used for the separation and identification of phenolic compounds in the extracts of these two Achillea species. This is the first report of the phenolic composition of aerial parts of both A. lingulata and A. abrotanoides. To the best of our knowledge, only reports on the composition of the roots [32], lignans [33,36], and volatile compounds [37,38,39,40] for A. lingulata are recorded.
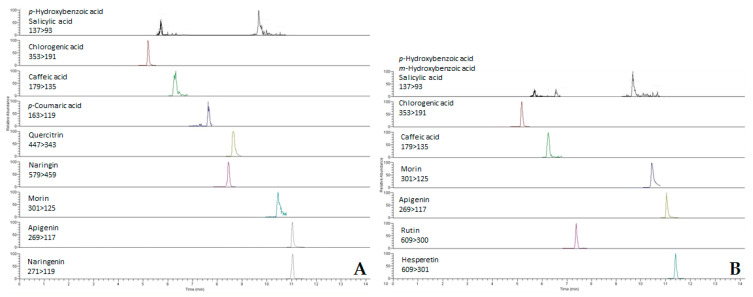
Among the 32 phenolic compounds analyzed, 21 phenolic acids and flavonoids were detected and quantified in the extracts of these two Achillea species (Table 2). In general, A. abrotanoides was found to be richer in phenolic compounds than A. lingulata. As an example, the (multiple reaction monitoring (MRM) chromatograms of ethanolic extracts of inflorescences of both investigated Achillea species are presented in Figure 2.
Table 2.
Phenolic composition (µmol/g) of the extracts of the two Achillea species.
| Compound | Plant Part | Achillea lingulata | Achillea abrotanoides | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PE 1 | CH 2 | ET 3 | W 4 | PE | CH | ET | W | ||
| pHBA 8 | INF 5 | 21.467 a
± 4.032 |
53.927 a ± 3.366 | 13.805 c
± 2.782 |
nd 7 | 25.150 a
± 3.008 |
205.073 b
± 8.626 |
24.831 d
± 0.217 |
nd |
| VEG 6 | nd | nd | 49.327 b
± 0.986 |
nd | 21.763 b
± 1.561 |
28.959 e
± 3.987 |
27.691 d
± 2.765 |
53.015 a
± 11.547 |
|
| mHBA 9 | INF | nd | nd | 1.308 e ± 0.274 |
1.108g
± 0.069 |
nd | nd | nd | 3.106 de
± 0.038 |
| VEG | nd | nd | 1.277 e ± 0.208 |
nd | nd | nd | nd | nd | |
| 23DHBA 10 | INF | nd | nd | nd | 2.196 g
± 0.203 |
nd | nd | nd | 0.550 e
± 0.032 |
| VEG | nd | nd | nd | nd | nd | 6.976 gh
± 0.495 |
nd | nd | |
| SA 11 | INF | 13.639 b ± 0.145 | 33.299 b ± 2.731 | 6.892 cd
± 0.774 |
nd | 16.580 c ± 0.094 | 58.153 c ± 1.777 |
15.362 ef
± 0.378 |
22.235 c
± 2.229 |
| VEG | nd | 52.029 a ± 8.987 | 9.627 c
± 0.431 |
4.332 f
± 0.010 |
22.375 ab
± 1.462 |
27.830 e
± 0.068 |
4.086 g
± 0.153 |
33.827 b
± 4.044 |
|
| ChA 12 | INF | nd | 0.909 d
± 0.056 |
0.026 d
± 0.002 |
6.055 e
± 0.077 |
nd | 13.772 f ± 0.484 |
0.129 h ± 0.010 |
2.884 de
± 0.221 |
| VEG | 0.691 b
± 0.072 |
0.600 d
± 0.008 |
0.020 d
± 0.001 |
4.066 f
± 0.431 |
4.224 d
± 0.290 |
1.032 hi
± 0.014 |
0.051 h
± 0.002 |
9.256 d
± 1.218 |
|
| CA 13 | INF | nd | 8.624 c
± 0.120 |
3.267 d
± 0.122 |
12.126 c ± 0.186 | nd | 16.049 f ± 2.010 |
2.917 gh
± 0.122 |
8.730 d
± 3.587 |
| VEG | nd | 12.908 c ± 0.264 | 3.185 d
± 0.285 |
9.182 d
± 0.462 |
nd | 8.653 gh
± 0.042 |
2.551 gh
± 0.009 |
11.276 d
± 0.776 |
|
| pCA 14 | INF | nd | nd | nd | nd | nd | 40.655 d ± 8.395 |
19.837 e
± 0.715 |
37.786 b
± 7.975 |
| VEG | nd | nd | nd | nd | nd | nd | 14.340 f
± 0.492 |
nd | |
| FA 15 | INF | nd | nd | nd | 0.848 h
± 0.004 |
nd | nd | nd | 0.981 e
± 0.091 |
| VEG | nd | nd | nd | 0.202 h
± 0.012 |
nd | nd | 24.756 d
± 1.377 |
0.219 e
± 0.025 |
|
| RA 16 | INF | nd | nd | nd | 54.359 a
± 0.282 |
nd | 207.473 b ± 17.557 |
nd | 26.866 c
± 0.513 |
| VEG | nd | nd | nd | nd | nd | nd | nd | nd | |
| Quercitrin | INF | nd | nd | nd | nd | nd | nd | 33.685 c
± 3.462 |
31.219 b
± 0.241 |
| VEG | nd | nd | nd | nd | nd | 0.590 i
± 0.033 |
nd | nd | |
| Naringin | INF | nd | nd | nd | nd | nd | nd | 35.326 c
± 2.285 |
nd |
| VEG | nd | nd | nd | nd | nd | 0.727 i
± 0.052 |
nd | nd | |
| Catechin | INF | nd | nd | nd | nd | nd | nd | nd | nd |
| VEG | nd | nd | nd | nd | nd | 0.894 i
± 0.047 |
nd | nd | |
| Morin | INF | nd | nd | 1.718d
± 0.321 |
nd | nd | nd | 23.889d
± 2.895 |
nd |
| VEG | nd | nd | 2.860 d
± 0.002 |
nd | nd | 1.175 hi
± 0.015 |
23.166 d
± 0.903 |
nd | |
| Apigenin | INF | nd | 38.268 b ± 3.845 | 2.785 d
± 0.646 |
nd | nd | 11.495 fg ± 0.170 |
0.637 h
± 0.076 |
31.647 b
± 0.913 |
| VEG | nd | nd | 165.688 a
± 9.680 |
nd | nd | 1.249 hi
± 0.027 |
112.010 a
± 6.564 |
nd | |
| Naringenin | INF | nd | nd | nd | nd | nd | 362.662 a
± 4.922 |
95.814 b
± 7.992 |
nd |
| VEG | nd | nd | nd | nd | nd | nd | nd | nd | |
| Kaempherol | INF | nd | nd | nd | nd | nd | nd | nd | nd |
| VEG | nd | nd | nd | nd | nd | 0.917 i
± 0.030 |
nd | nd | |
| Chrysin | INF | nd | nd | nd | nd | nd | nd | nd | nd |
| VEG | nd | nd | nd | nd | nd | 3.314 hi
± 0.186 |
nd | nd | |
| Pinocembrin | INF | nd | nd | nd | nd | nd | nd | nd | nd |
| VEG | nd | nd | nd | nd | nd | 1.510 hi ± 0.065 |
nd | nd | |
| Galangin | INF | nd | nd | nd | nd | nd | nd | nd | nd |
| VEG | nd | nd | nd | nd | nd | 1.048 hi
± 0.189 |
nd | nd | |
| Hesperetin | INF | nd | 9.895 c
± 1.297 |
0.253 d
± 0.060 |
nd | nd | nd | nd | nd |
| VEG | 1.408 b ±0.118 | 1.284 d
± 0.021 |
0.080 d
± 0.002 |
27.549 b ± 1.769 |
nd | nd | nd | nd | |
| Rutin | INF | nd | 11.910 c ± 1.883 | 0.294 d
± 0.011 |
nd | nd | nd | nd | nd |
| VEG | nd | 1.128 d
± 0.032 |
0.221 d
± 0.001 |
nd | nd | nd | nd | nd | |
1 Petrol ether, 2 chloroform, 3 ethanol, 4 water, 5 inflorescences, 6 vegetative part, 7 not detected, 8 p-hydroxybenzoic acid, 9 m-hydroybenzoic acid, 10 2,3-dihydroxybenzoic acid, 11 salicylic acid, 12 chlorogenic acid, 13 caffeic acid, 14 p-coumaric acid, 15 ferulic acid, 16 rosmarinic acid. The values within one row followed by the same letter do not differ significantly after factorial ANOVA post hoc Newman–Keuls analysis at a significance level of p < 0.01.
Figure 2.
MRM chromatograms of ethanolic extracts of inflorescences of A. abrotanoides (A) and A. lingulata (B).
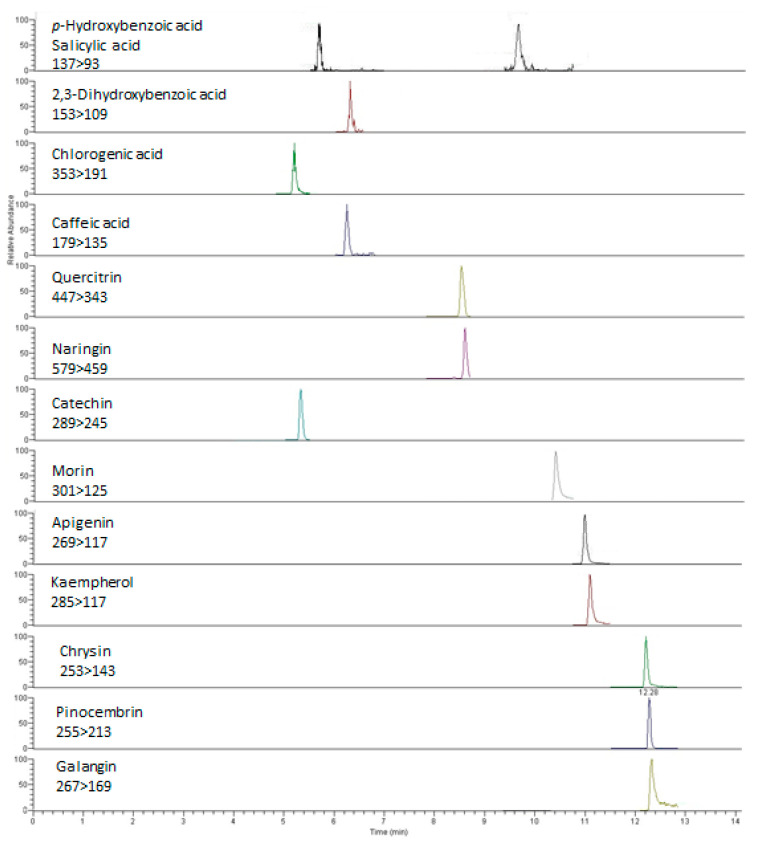
Chloroform as a solvent revealed the greatest diversity in phenolic profiles of both investigated plants. There were 7 phenolic acids and 9 flavonoids found in the vegetative part of A. abrotanoides, while 12 phenolic compounds in total were found in the extracts of A. lingulata. The highest concentration of phenolic compounds was detected in the chloroform extract of the inflorescence of A. abrotanoides (915.3 µmol/g), for which the MRM chromatograms are presented in Figure 3. As expected, the lowest content of phenolics was found in petrol ether extract of the vegetative part of A. lingulata (total concentration 2.1 µmol/g).
Figure 3.
MRM chromatograms of the chloroform extract of vegetative parts of A. abrotanoides.
Among hydroxybenzoic acids investigated, p-hydroxybenzoic acid was the most abundant in the extracts of A. abrotanoides (up to 205.073 ± 8.626 µmol/g), while the levels of salicylic acid were quite similar in all examined extracts of both species. Other hydroxybenzoic acids are mostly found in polar extracts, i.e., ethanolic and aqueous. In addition, the levels of chlorogenic and caffeic acids, cinnamic acids commonly found in plants, were relatively low in comparison with the levels of hydroxybenzoic acids (Table 2). On the contrary, significant levels of rosmarinic acid were found in the inflorescence of the endemic A. abrotanoides (up to 207.473 ± 17.557 µmol/g). Unexpectedly, p-coumaric acid was not a dominant phenolic acid in A. abrotanoides, and it was not detected in A. lingulata. However, this hydroxycinnamic acid was identified in many other yarrows, such as A. millefolium, A. distans, A. biserratae, and A. beibrestinii [41,42,43,44].
Furthermore, apigenin was found to be the most abundant flavonoid found in the vegetative parts of both Achillea species, with levels of up to 112.010 ± 6.564 µmol/g and 165.688 ± 9.680 µmol/g for A. abrotanoides and A. lingulata, respectively. Apigenin was suggested as the main flavonoid in other Achillea species, such as A. distans, A. ligustica, A. collina, A. millefolium, etc. [41,44,45,46,47]. Moreover, isoquercitrin and rutin were the main flavonoids in A. schurii in the study of Benedec et al. [48]. On the contrary, the inflorescences of the investigated Achillea species differ in their flavonoid profiles, i.e., A. abrotanoides was rich in naringenin (up to 362.662 ± 4.922 µmol/g), while the flowers of A. lingulata contained notable amounts of hesperetin and rutin (Table 2).
2.3. Antimicrobial Activity
All isolated extracts, together with the representatives of the phenolic compounds detected, were tested for antimicrobial activity using the diffusion method. The results are summarized in Table 3. This is the first report of antimicrobial activity for the aerial plant parts of A. abrotanoides, while the antimicrobial activity of A. lingulata aerial parts has been studied before [49]. However, this is the first attempt to compare different extraction solvents that isolate the phenolic compounds that might be responsible for the antimicrobial activities of A. lingulata and A. abrotanoides.
Table 3.
Antimicrobial potential of the extracts of two Achillea species and selected phenolic compounds.
| Species | Extract | Plant Part |
Salmonella
Abony |
Escherichia
Coli |
Enterococcus
Faecalis |
Staphylococcus
Aureus |
Candida
Albicans |
|---|---|---|---|---|---|---|---|
|
Achillea
lingulata |
PE 1 | INF 4 | 12.00 ± 1.00 | 15.33 ± 0.58 | 13.00 ± 0.00 | 11.67 ± 0.58 | 18.33 ± 2.52 * |
| VEG 5 | nd 6 | 12.00 ± 0.00 | 13.00 ± 0.00 | 12.33 ± 0.58 | 20.00 ± 2.00 * | ||
| CH 2 | INF | nd | 13.00 ± 0.00 * | 13.33 ± 1.53 | 13.33 ± 0.58 | 20.00 ± 2.00 * | |
| VEG | nd | 15.67 ± 1.15 * | 13.67 ± 0.58 | 15.00 ± 0.00 | 19.67 ± 0.58 * | ||
| ET 3 | INF | 15.33 ± 0.58 * | 13.00 ± 0.00 * | 16.67 ± 2.08 | 16.00 ± 0.00 | 25.33 ± 2.51 ** | |
| VEG | 12.33 ± 0.58 | 13.00 ± 1.73 * | 14.67 ± 0.58 | 12.67 ± 0.58 | 16.33 ± 0.58 * | ||
|
Achillea
abrotanoides |
PE | INF | 13.00 ± 0.00 | 14.33 ± 1.15 * | nd | nd | 20.00 ± 0.00 * |
| VEG | nd | nd | 12.33 ± 0.58 | 13.33 ± 1.53 | 18.00 ± 1.73 * | ||
| CH | INF | nd | 12.67 ± 1.15 * | 14.33 ± 1.15 | 13.33 ± 0.58 | 40.00 ± 0.00 ** | |
| VEG | nd | 14.00 ± 1.73 * | 14.00 ± 1.00 | 17.67 ± 0.58 | nd | ||
| ET | INF | 13.67 ± 0.58 | 14.00 ± 1.00 * | 26.00 ± 0.00 ** | 14.33 ± 0.58 | 21.67 ± 2.89 * | |
| VEG | 14.33 ± 1.15 * | 15.33 ± 1.15 * | 18.00 ± 1.00 * | 15.67 ± 0.58 | 24.00 ± 2.00 ** | ||
| Antibiotic/antimycotic | 17.00 ± 1.00 | 14.33 ± 2.08 | 19.33 ± 0.58 | 34.33 ± 2.08 | 19.67 ± 1.15 | ||
| Phenolic compound | p-Hydroxybenzoic acid | 12.33 ± 1.53 | 12.00 ± 0.00 | 12.67 ± 1.15 | nd | 18.67 ± 2.89 * | |
| Salicylic acid | 13.50 ± 0.71 | 13.00 ± 1.73 | 13.00 ± 1.73 | nd | 25.33 ± 0.58 ** | ||
| Chlorogenic acid | nd | nd | nd | nd | 14.33 ± 0.58 | ||
| Caffeic acid | 13.00 ± 1.41 | 15.00 ± 1.00 * | 12.00 ± 0.00 | nd | nd | ||
| p-Coumaric acid | 13.67 ± 0.58 | 11.33 ± 0.58 | 13.00 ± 0.00 | nd | 17.00 ± 0.00 * | ||
| Ferulic acid | nd | 14.67 ± 0.58 * | nd | nd | nd | ||
| Rosmarinic acid | nd | 13.33 ± 1.15 | 12.67 ± 1.15 | nd | 17.33 ± 1.15 * | ||
| Quercetin | nd | nd | 15.67 ± 1.53 | 13.67 ± 0.58 | 18.00 ± 1.73 * | ||
| Naringenin | nd | 12.33 ± 0.58 | 23.00 ± 1.00 ** | 27.33 ± 2.31 * | 22.33 ± 2.52 ** | ||
| Morin | 14.33 ± 0.58 * | 11.33 ± 0.58 | 18.00 ± 1.73 * | 12.00 ± 1.00 | 19.67 ± 2.52 * | ||
1 Petrol ether; 2 chloroform; 3 ethanol; 4 inflorescence; 5 vegetative part; 6 not detected; * equally as effective as the antibiotic/antimycotic; ** statistically significantly more effective than the antibiotic/antimycotic at significance level p < 0.05 after ANOVA post hoc Newman–Keuls analysis.
All four water extracts of both Achillea species showed no antimicrobial potential, whereas they contained significant amounts of phenolic compounds (Table 2). This result was also reported previously when aqueous extracts exhibited no antimicrobial activity [50], which might be attributed to the inefficient diffusability of aqueous solutions in the agar medium [51], as well as to the lesser ability of aqueous extracts to damage microbe cell walls [52]. However, ethanol extracts of both species showed strong antimicrobial effects against all tested microorganisms except for Staphylococcus aureus with an equal or even higher efficiency than the antibiotic ampicillin or the antimycotic nystatin that were used as positive controls (Table 3). In general, all inflorescence extracts of both species showed more potent antimicrobial activities than the same extracts from the vegetative parts. The inflorescence ethanolic extract of A. abrotanoides was significantly more effective against Enterococcus faecalis compared to the ampicillin. All extracts (except for the chloroform extract of the vegetative part of A. abrotanoides) exhibited the same or even more potent activity against Candida albicans than the antimycotic. In this case, the chloroform extract of A. abrotanoides inflorescence had two times greater an antimicrobial effect than nystatin. Moreover, E. coli was equally as susceptible to nearly all extracts as to the reference antibiotic.
According to the available literature, other Achillea species did not demonstrate such potent antifungal activity against Candida albicans. The inhibition zone of ethanol flower extracts of A. schurri was recorded to be only 6 mm [49], while significantly higher values were recorded for the A. lingulata ethanol extract and the A. abrotanoides chloroform extract (Table 3). A similar observation was recorded for A. millefolium by Maz et al. [53]. No activity of certain A. millefolium plants growing at an average altitude against E. coli was recorded and the activity was limited to Gram-positive bacteria, while the A. abrotanoides and A. lingulata extracts were as effective as the reference antibiotic.
In addition, several representatives of each class of phenolic compounds at a concentration of 0.1 mg/mL were also assayed for their antimicrobial activities (Table 3). Among them all, the flavonoid morin was the only compound that successfully inhibited the growth of all four microorganisms. This compound was found in the ethanolic extract of the inflorescences and vegetative parts of both investigated Achillea species. Moreover, the flavonon naringenin showed very high activity against E. faecalis, S. aureus, and C. albicans, with the inhibition zones being bigger than those of ampicillin and nystatin. High concentrations of naringenin were found in the chloroform and ethanolic extracts of A. abrotanoides. Therefore, the activity of these extracts might be explained by the presence of this phenolic compound. Representatives of both hydroxybenzoic and hydroxycinnamic acids revealed similar activities, lower than the activities of flavonoids (Table 3), which is in agreement with the literature data [54].
Antimicrobial activity of phenolic compounds might be explained by the modification of the permeability of cell membranes, the changes in various intracellular functions induced by the hydrogen binding of the phenolic compounds to enzymes, or by the modification of the cell wall rigidity [55,56,57]. Phenolic acids have been shown to disrupt membrane integrity, as they cause consequent leakage of essential intracellular constituents [58], while flavonoids may link to soluble proteins located outside the cells and with bacteria cell walls, thus promoting the formation of complexes [57,59].
2.4. Antioxidant Activity
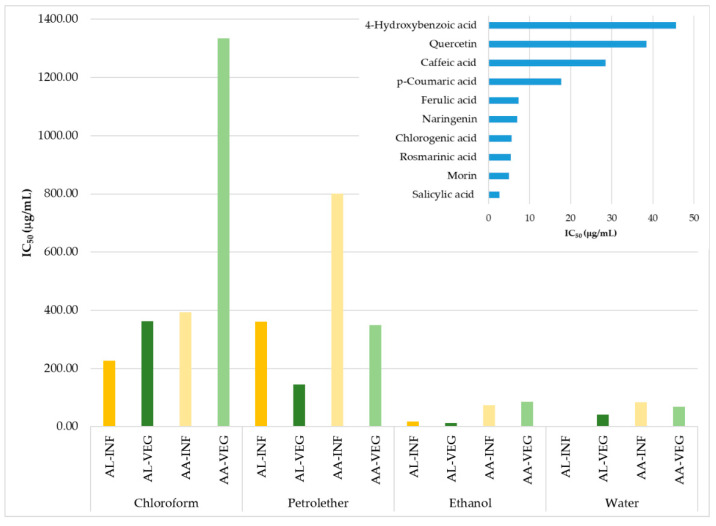
Antioxidant activity of the aerial plant parts of A. lingulata and A. abrotanoides has not been described previously. In this study, the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity of all the extracts was tested and expressed as an IC50 value, which represents the concentration of the extracts that scavenge 50% of the radicals. In addition, selected phenolic compounds were also assayed for their abilities to scavenge stable DPPH radicals. All results are presented in Figure 4. In general, all extracts of A. lingulata showed a stronger antioxidant potential compared to A. abrotanoides.
Figure 4.
Antioxidant activity of the extracts of two Achillea species and their selected phenolic compounds. AA = Achillea abrotanoides, AL = Achillea lingulata, INF = inflorescence, VEG = vegetative part.
The polar aqueous and ethanolic extracts of both investigated species had the lowest IC50 values. The most potent extract was the water inflorescence extract of Achillea lingulata, with an IC50 value of 1.52 µg/mL. This extract contained significant levels of rosmarinic acid, which also possesses strong antioxidant activity against stable DPPH radicals (IC50 5.47 µg/mL). Moreover, the activity of this extract could be attributed to other phenolic compounds that are not quantified due to the lack of standards. Ethanol extracts of the inflorescence, as well as the vegetative part of Achillea lingulata, also showed a high antioxidant capacity, with IC50 values of 18.14 and 12.73 µg/mL, respectively. These extracts were rich in p-hydroxybenzoic acid, but also the flavonoid apigenin. The antioxidant potential is decreased via a decrease in the polarity solvent used [60,61], and the chloroform extracts of A. abrotanoides had the lowest antioxidant potential and the highest IC50 values (Figure 4). On the contrary, chloroform extracts of the vegetative parts and the inflorescence were rich in the phenolic compounds detected. Therefore, its low ability to scavenge stable radicals could be explained by the fact that chloroform isolates a high amount of chlorophylls and other pigments from leaves. These compounds do not possess significant antiradical activities [62].
The antioxidant activity of the extracts was correlated with the concentrations of the selected phenolic compounds, and it was found that caffeic (R = 0.6256), salicylic (R = 0.6296), chlorogenic (R = 0.6394), p-coumaric (R = 0.6402), p-hydroxybenzoic (R = 0.7493), and rosmarinic acid (R = 0.9807) have very high positive correlations with the antioxidant activity. Therefore, it might be concluded that these acids are responsible for the antioxidant activity of the extracts that contain them.
DPPH radical scavenging potential has been previously recorded for other Achillea species [48,63], ranging from the low IC50 of 0.52 µg/mL, as recorded for the essential oils of A. pannonica [64], up to 1.172 µg/mL for extracts of A. eriophora [65]. For some extracts, such as water and ethanol extracts of A. lingulata and A. abrotanoides, reported IC50 values are even lower than those of some synthetic or natural antioxidants [50,66,67,68].
3. Materials and Methods
3.1. Plant Material
Samples of A. lingulata and A. abratonoides were collected during the flowering stage at Mt. Jahorina (June 2017) and Mt. Bjelašnica (July 2017), respectively (Figure 1). Specimens were deposited at the herbarium of the Department of Biology, Faculty of Science, the University of Sarajevo under the voucher no. LERP 355 and LERP 356. The inflorescence and the vegetative parts were separated and air-dried for 7 days at room temperature (23 °C) in a shaded, well-ventilated laboratory. Dried samples were finely powdered in the mill and stored at 4 °C until use.
3.2. Chemicals
Standards of phenolic acids and flavonoids (apigenin, 2,3-dihydroxybenzoic acid, caffeic acid, catechin, chlorogenic acid, chrysin, ferulic acid, galangin, gallic acid, hesperidin, m-hydroxybenzoic acid, p-hydroxybenzoic acid, 5-hydroxyferulic acid, kaempferol, methyl p-coumarate, morin, myricetin, naringenin, naringin, p-coumaric acid, pinocembrin, quercetin, quercitrin, rosmarinic acid, rutin, salicylic acid, salicylic acid glucoside, sinapic acid, syringic acid, trans-cinnamic acid, vanillic acid, p-coumaric acid-d6, and salicylic acid-d4) were purchased from Sigma-Aldrich (Steinheim, Germany).
The sodium molybdate dihydrate, sodium nitrite, sodium hydroxide, sodium carbonate, hydrogen peroxide, sodium ascorbate, dimethyl sulfoxide (DMSO), and bovine hemoglobin were also purchased from Sigma-Aldrich (Steinheim, Germany). The LC (liquid chromatography) grade methanol, analytical grade orthophosphoric acid, hydrochloric acid, aluminum chloride, sodium acetate, ethanol, and Folin–Ciocalteu reagent were purchased from Merck (Darmstadt, Germany). The DPPH (2,2-diphenyl-1-picrylhydrazyl) was obtained from Alfa-Aesar (Karlsruhe, Germany). All spectrophotometric data were acquired using a Jasco V-530 UV-Vis spectrophotometer (Jasco International Co., Ltd., Tokyo, Japan).
3.3. Preparation of the Extracts
For the inflorescence and vegetative aerial parts, 500 mg each of the powdered plant material was soaked in 12.5 mL of solvent (petrol ether, chloroform, ethanol, and water) and sonicated for 30 min at 23 °C. The supernatant was transferred into a new vial and the sediment was soaked again in the same solvent. Due to the high evaporation rate of petrol ether and chloroform, these extracts were evaporated to dryness and resuspended in DMSO.
3.4. UHPLC-MS/MS Analysis of the Extracts
Standard solutions of the target phenolic compounds were first prepared in methanol at 1 mM concentrations, and the solutions were gradually diluted in the mobile phase to the working concentrations that ranged from 0.01 to 50 µM. Quantification was performed by the isotope diluting method using p-coumaric acid-d6 and salicylic acid-d4.
UHPLC-MS/MS was performed on an UltiMate™ 3000 liquid chromatographic system consisting of binary pumps, an autosampler, and a column thermostat coupled to a TSQ Quantum Access Max triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic separation was performed on an Acquity BEHC18 (150 × 3.0 mm; 1.7 µm particle size) UHPLC column (Waters Corp., Milford, MA, USA) kept at 40 °C. The mobile phase consisted of 10 mM formic acid in water (component A) and acetonitrile (component B). Compounds were separated using a binary gradient starting at 5% B for 0.8 min, increasing to 10% B for 0.4 min with an isocratic run for 0.7 min, then at 15% B for 0.5 min with an isocratic run for 1.3 min, at 20% B for 0.3 min with an isocratic run for 1.2 min, at 25% B for 0.5 min, at 35% B for 2.3 min, at 70% B for 2.5 min, then at 100% B for 1 min with an isocratic run for 1 min, and then back to 5% B for 0.5 min. Finally, the equilibration to initial conditions took 3.3 min, with a total chromatographic run of 16 min. The flow rate was 0.4 mL/min and the injection volume was 10 µL.
All analytes were detected in negative electrospray ionization mode (ESI-). Multiple reaction monitoring (MRM) mode was used for their quantification. The spray voltage was 3 kV, the temperature of the ion transfer tube vaporizer was 320 °C, the sheath gas pressure was 45 psi, and the auxiliary gas pressure was 15 psi.
3.5. Determination of Antioxidant Activity
DPPH antioxidant activity was evaluated for all extracts and standards according to Meda et al. [69]. Briefly, 200 µL of sample solution was mixed with 50 µM of DPPH solution in ethanol, and the absorbance of the tested mixtures was measured at 517 nm after 30 min. Absolute ethanol was used to zero the spectrophotometer, DPPH solution was used as a blank sample, and different phenolic compounds (Figure 4) were used as a positive probe. Antioxidant activity was calculated as IC50, which is referred to as the concentration of extract that scavenges 50% of free DPPH radicals.
The radical-scavenging activities of the tested samples, expressed as a percentage of the inhibition of DPPH, were calculated according to the formula IC (%) = [(A0 − At)/A0] % 100, where A0 and At are the absorbance values of the blank sample and the test sample at 0 and 30 min, respectively. Four different concentrations of each sample were assayed. Percent inhibition after 30 min was plotted against concentration, and the equation for the line was used to obtain the IC50 value. A lower IC50 value indicates greater antioxidant activity.
3.6. Determination of Antimicrobial Activity
Agar well diffusion method was used to evaluate the antimicrobial activity of the plant extracts and standards according to National Committee for Clinical Laboratory Standards (NCCLS) [70,71]. Each well contained 100 µL of extract or standard. Bacterial strains used in the analysis included the Gram-positive bacteria E. faecalis ATCC® 19433TM and S. aureus subsp. aureus ATCC® 6538TM and the Gram-negative bacteria S. abony NCTC® 6017TM, E. coli ATCC® 8739TM, and the yeast C. albicans ATCC® 10231TM. Bacterial strains were used as a standardized inoculum of 5×105 CFU/mL using a McFarland standard [72].
Müller–Hinton and Sabouraud medium were used for the cultivation of the bacterial strains and yeast, respectively. Ampicillin was used as a positive standard for bacterial strains and nystatin for Candida albicans. Ethanol and DMSO were used as negative controls. The antimicrobial effect was expressed as a diameter of inhibition zone in mm reduced by the inhibition zone of the negative controls, if appropriate.
3.7. Statistical Analysis
All data were analyzed using the STATISTICA 10.0 software (Statsoft Inc.). Experimental results were presented in tables as the mean ± standard deviation of three independent replications. Data were subjected to variance analysis (ANOVA) and the Newman–Keuls post hoc test was carried out to identify significant differences between the extract types. Mean values with p < 0.05 were considered statistically significant. Pearson correlations were performed to observe the possible correlation between the phenolic profile, antioxidant capacity, and detected antimicrobial activity.
4. Conclusions
The phytochemical analysis of the aerial parts of two yarrow species, Achillea lingulata and the endemic Achillea abratonoides, was performed for the first time. The phenolic composition of four extracts with different polarities suggested that yields of chloroform extracts were the highest, and that they had the biggest diversity of phenolic compounds detected. In addition, ethanolic extracts revealed the strongest antioxidant activities and the ability to suppress the growth of selected microorganisms. Presented results suggest that both investigated Achillea species have strong potential for use in different pharmaceutical preparations.
Author Contributions
Conceptualization, E.K. and S.Ć.Z.; formal analysis, D.K., E.K., S.D., R.B.-G., A.P. and S.Ć.Z.; methodology, E.K. and S.Ć.Z.; writing–original draft, S.Ć.Z.; writing–review & editing, D.K., E.K. and S.Ć.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture of the Czech Republic (project No. RO0418: Sustainable systems and technologies, improving crop production for a higher quality of production of food, feed, and raw materials, under conditions of changing climate).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Sample Availability
Samples of the extracts are not available, but plant vouchers are deposited at the herbarium of the Department of Biology, Faculty of Science, the University of Sarajevo under the voucher no. LERP 355 and LERP 356.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grodowska K., Parczewski A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. Drug Res. 2010;67:3–12. [PubMed] [Google Scholar]
- 2.González-Montelongo R., Lobo M.G., González M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010;119:1030–1039. doi: 10.1016/j.foodchem.2009.08.012. [DOI] [Google Scholar]
- 3.Dorta E., Lobo M.G., Gonzalez M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temper-ature on their antioxidant properties. J. Food Sci. 2012;77:80–88. doi: 10.1111/j.1750-3841.2011.02477.x. [DOI] [PubMed] [Google Scholar]
- 4.Lafka T.-I., Sinanoglou V., Lazos E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007;104:1206–1214. doi: 10.1016/j.foodchem.2007.01.068. [DOI] [Google Scholar]
- 5.Lapkin A.A., Plucinski P.K., Cutler M. Comparative Assessment of Technologies for Extraction of Artemisinin. J. Nat. Prod. 2006;69:1653–1664. doi: 10.1021/np060375j. [DOI] [PubMed] [Google Scholar]
- 6.Tyśkiewicz K., Konkol M., Rój E. The Application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules. 2018;23:2625. doi: 10.3390/molecules23102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Essien S.O., Young B., Baroutian S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020;97:156–169. doi: 10.1016/j.tifs.2020.01.014. [DOI] [Google Scholar]
- 8.Villanueva-Bermejo D., Zahran F., Troconis D., Villalva M., Reglero G., Fornari T. Selective precipitation of phenolic compounds from Achillea millefolium L. extracts by supercritical anti-solvent technique. J. Supercrit. Fluids. 2017;120:52–58. doi: 10.1016/j.supflu.2016.10.011. [DOI] [Google Scholar]
- 9.Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Savic Gajic I., Savic I., Boskov I., Žerajić S., Markovic I., Gajic D. Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae Pseudoacaciae) flowers and comparison with conventional methods. Antioxidants. 2019;8:248. doi: 10.3390/antiox8080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savic I.M., Gajic I.M.S. Optimization of ultrasound-assisted extraction of polyphenols from wheatgrass (Triticum aestivum L.) J. Food Sci. Technol. 2020;57:2809–2818. doi: 10.1007/s13197-020-04312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paswan R., Park Y.W. Survivability of Salmonella and Escherichia coli O157:H7 pathogens and food safety concerns on com-mercial powder milk products. Dairy. 2020;1:189–201. doi: 10.3390/dairy1030014. [DOI] [Google Scholar]
- 13.Syahrul F., Wahyuni C.U., Notobroto H.B., Wasito E.B., Adi A.C., Dwirahmadi F. Transmission media of foodborne diseases as an index prediction of diarrheagenic Escherichia coli: Study at elementary school, Surabaya, Indonesia. Int. J. Environ. Res. Pub. Health. 2020;17:8227. doi: 10.3390/ijerph17218227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abril A.G., Villa T.G., Barros-Velázquez J., Cañas B., Sánchez-Pérez A., Calo-Mata P., Carrera M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins. 2020;12:537. doi: 10.3390/toxins12090537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higaki S., Kitagawa T., Kagoura M., Morohashi M., Yamagishi T. Predominant Staphylococcus aureus isolated from various skin diseases. J. Int. Med Res. 2000;28:187–190. doi: 10.1177/147323000002800404. [DOI] [PubMed] [Google Scholar]
- 16.Principi N., Argentiero A., Neglia C., Gramegna A., Esposito S. New antibiotics for the treatment of acute bacterial skin and soft tissue infections in pediatrics. Pharmaceuticals. 2020;13:333. doi: 10.3390/ph13110333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelbaum P.C. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin. Infect. Dis. 2007;45:S165–S170. doi: 10.1086/519474. [DOI] [PubMed] [Google Scholar]
- 18.Gregova G., Kmetova M., Kmet V., Venglovsky J., Feher A. Antibiotic resistance of Escherichia coli isolated from a poultry slaughterhouse. Ann. Agric. Environ. Med. 2012;19:75–77. [PubMed] [Google Scholar]
- 19.Darwish R.M., Aburjai T.A. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant in-hibitors on Escherichia coli. BMC Compl. Alternative Med. 2010;10:1–8. doi: 10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyakudya T., Tshabalala T., Dangarembizi R., Erlwanger K., Ndhlala A.R. The potential therapeutic value of medicinal plants in the management of metabolic disorders. Molecules. 2020;25:2669. doi: 10.3390/molecules25112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinyede K.A., Ekpo O.E., Oguntibeju O.O. Ethnopharmacology, therapeutic properties and nutritional potentials of Car-pobrotus edulis: A comprehensive review. Sci. Pharm. 2020;88:39. doi: 10.3390/scipharm88030039. [DOI] [Google Scholar]
- 22.Tavares W.R., Barreto M.D.C., Seca A.M.L. Uncharted source of medicinal products: The case of the Hedychium genus. Medicines. 2020;7:23. doi: 10.3390/medicines7050023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, isolation, and iden-tification of bioactive compounds from plant extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostić A.Ž., Janaćković P., Kolašinac S.M., Stevanović Z.P.D. Balkans’ Asteraceae species as a source of biologically active compounds for the pharmaceutical and food industry. Chem. Biodivers. 2020;17:2000097. doi: 10.1002/cbdv.202000097. [DOI] [PubMed] [Google Scholar]
- 25.Bessada S.M., Barreira J.C., Oliveira M.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crop. Prod. 2015;76:604–615. doi: 10.1016/j.indcrop.2015.07.073. [DOI] [Google Scholar]
- 26.Michel J., Rani N.Z.A., Husain K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020;11:852. doi: 10.3389/fphar.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross J. Combining Western Herbs and Chinese Medicine: Principles, Practice, and Materia Medica. Greenfields press; Seattle, WA, USA: 2003. pp. 165–182. [Google Scholar]
- 28.Saeidnia S., Gohari A., Mokhber-Dezfuli N., Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011;19:173–186. [PMC free article] [PubMed] [Google Scholar]
- 29.Radulovic N., Zlatković B., Palic R., Stojanovic G. Chemotaxonomic significance of the Balkan Achillea volatiles. Nat. Prod. Commun. 2007;2:453–474. doi: 10.1177/1934578X0700200417. [DOI] [Google Scholar]
- 30.Boskovic Z., Radulovic N., Stojanovic G. Essential oil composition of four Achillea species from the Balkans and its chemo-taxonomic significance. Chem. Nat. Compd. 2005;41:674–678. doi: 10.1007/s10600-006-0009-6. [DOI] [Google Scholar]
- 31.G.rada Publishing . Pharmacopoea Bohemica MMXVII. 1st ed. G.rada Publishing; Prague, Czech Republic: 2017. p. 4121. [Google Scholar]
- 32.Jovanović O., Radulović N., Palić R., Zlatković B. Root essential oil of Achillea lingulata Waldst. & Kit. (Asteraceae) J. Essent. Oil Res. 2010;22:336–339. doi: 10.1080/10412905.2010.9700340. [DOI] [Google Scholar]
- 33.Stojanovic G., Hashimoto T., Asakawa Y., Palić R. Chemical composition of the Achillea lingulata extract. Biochem. Syst. Ecol. 2005;33:207–210. doi: 10.1016/j.bse.2004.07.004. [DOI] [Google Scholar]
- 34.Zhang Q.W., Lin L.G., Ye W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong D.-H., Nguyen D.H., Ta N.T.A., Bui A.V., Do T.H., Nguyen H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019;2019:1–9. doi: 10.1155/2019/8178294. [DOI] [Google Scholar]
- 36.Trifunović S., Vajs V., Tešević V., Djoković D., Milosavljević S. Lignans from the plant species Achillea lingulata. J. Serbian Chem. Soc. 2003;68:277–280. doi: 10.2298/JSC0305277T. [DOI] [Google Scholar]
- 37.Chalchat J.C., Gorunovic M.S., Petrovic S.D., Zlatkovic V.V. Aromatic plants of Yugoslavia. II. Chemical composition of essential oils of three wild Achillea Species: A. clavenae L., A. collina Becker and A. lingulata W. et K. J. Essent. Oil Res. 2000;12:7–10. doi: 10.1080/10412905.2000.9712028. [DOI] [Google Scholar]
- 38.Stojanovic G., Palic R., Naskovic T., Dokovic D., Milosavljevic S. Volatile constituents of Achillea lingulata WK. J. Essent. Oil Res. 2001;13:378–379. doi: 10.1080/10412905.2001.9712239. [DOI] [Google Scholar]
- 39.Kovačević N.N., Ristić M.S., Tasić S.R., Menković N.R., Grubišić D.V., Đoković D.D. Comparative study of essential oil of three Achillea species from Serbia. J. Essent. Oil Res. 2005;17:57–60. doi: 10.1080/10412905.2005.9698830. [DOI] [Google Scholar]
- 40.Kundakovic T., Fokialakis N., Kovacevic N., Chinou I. Essential oil composition of Achillea lingulata and A. umbellata. Flavour Fragr. J. 2007;22:184–187. doi: 10.1002/ffj.1778. [DOI] [Google Scholar]
- 41.Popovici M., Vlase L., Oniga I., Tamas M. HPLC analyses on polyphenolic compounds from Achillea species. Farmacia. 2007;3:353–357. [Google Scholar]
- 42.Serdar G., Sökmen M., Demir E., Sökmen A., Bektaş E. Extraction of antioxidative principles of Achillea biserrata M. Bieb. and chromatographic analyses. Int. J. Second. Metab. 2015;2:3–15. doi: 10.21448/ijsm.240706. [DOI] [Google Scholar]
- 43.Bashi D.S., Mortazavi S.A., Rezaei K., Rajaei A., Karimkhani M.M. Optimization of ultrasound-assisted extraction of phenolic compounds from yarrow (Achillea beibrestinii) by response surface methodology. Food Sci. Biotechnol. 2012;21:1005–1011. doi: 10.1007/s10068-012-0131-0. [DOI] [Google Scholar]
- 44.Benedec D., Vlase L., Oniga I., Mot A.C., Damian G., Hanganu D., Duma M., Silaghi-Dumitrescu R. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules. 2013;18:8725–8739. doi: 10.3390/molecules18088725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuberoso C.I.G., Montoro P., Piacente S., Corona G., Deiana M., Dessì M.A., Pizza C., Cabras P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J. Pharm. Biomed. Anal. 2009;50:440–448. doi: 10.1016/j.jpba.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Karlova K. Accumulation of flavonoid compounds in flowering shoots of Achillea colllina Becker ex. Rchb. Alba during flower development. Hortic. Sci. 2006;33:158–162. doi: 10.17221/3756-HORTSCI. [DOI] [Google Scholar]
- 47.Lemmens-Gruber R., Marchart E., Rawnduzi P., Engel N., Benedek B., Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated Guinea-pig ilea. Arzneimittelforschung. 2006;56:582–588. doi: 10.1055/s-0031-1296755. [DOI] [PubMed] [Google Scholar]
- 48.Benedec D., Hanganu D., Oniga I., Filip L., Bischin C., Silaghi-Dumitrescu R., Tiperciuc B., Vlase L. Achillea schurii Flowers: Chemical, antioxidant, and antimicrobial investigations. Molecules. 2016;21:1050. doi: 10.3390/molecules21081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stojanović G., Radulović N., Hashimoto T., Palić R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005;101:185–190. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Candan F., Unlu M., Tepe B., Daferera D., Polissiou M., Sökmen A., Akpulat H. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003;87:215–220. doi: 10.1016/S0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 51.Mudzengi C.P., Murwira A., Tivapasi M., Murungweni C., Burumu J.V., Halimani T. Antibacterial activity of aqueous and methanol extracts of selected species used in livestock health management. Pharm. Biol. 2017;55:1054–1060. doi: 10.1080/13880209.2017.1287744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonelimali F.D., Lin J., Miao W., Xuan J., Charles F., Chen M., Hatab S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018;9:1639. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maz M., Mirdeilami S.Z., Pessarakli M. Essential oil composition and antibacterial activity of Achillea millefolium L. from different regions in North east of Iran. J. Med. Plant Res. 2013;7:1063–1069. doi: 10.5897/JMPR12.961. [DOI] [Google Scholar]
- 54.Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Anti-bacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikigai H., Nakae T., Hara Y., Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim. et Biophys. Acta (BBA) Biomembr. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-R. [DOI] [PubMed] [Google Scholar]
- 56.Taguri T., Tanaka T., Kouno I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006;29:2226–2235. doi: 10.1248/bpb.29.2226. [DOI] [PubMed] [Google Scholar]
- 57.Cushnie T.T., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 59.Tsuchiya H., Sato M., Miyazaki T., Fujiwara S., Tanigaki S., Ohyama M., Tanaka T., Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 60.Barchan A., Bakkali M., Arakrak A., Pagán R., Laglaoui A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:399–412. [Google Scholar]
- 61.Anwar F., Przybylski R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.) Acta Sci. Pol. Technol. Aliment. 2012;11:293–302. [PubMed] [Google Scholar]
- 62.Lanfer-Marquez U.M., Barros R.M., Sinnecker P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005;38:885–891. doi: 10.1016/j.foodres.2005.02.012. [DOI] [Google Scholar]
- 63.Gaweł-Bęben K., Strzępek-Gomółka M., Czop M., Sakipova Z., Głowniak K., Kukula-Koch W. Achillea millefolium L. and Achillea biebersteinii Afan. hydroglycolic extracts–bioactive ingredients for cosmetic use. Molecules. 2020;25:3368. doi: 10.3390/molecules25153368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bozin B., Mimica-Dukic N., Bogavac M., Suvajdzic L., Simin N., Samojlik I., Couladis M. Chemical composition, antiox-idant and antibacterial properties of Achillea collina Becker ex Heimerl s.l. and A. pannonica scheele essential oils. Molecules. 2008;13:2058–2068. doi: 10.3390/molecules13092058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gharibi S., Tabatabaei B.E.S., Saeidi G. Comparison of essential oil composition, flavonoid content and antioxidant activity in eight Achillea species. J. Essent. Oil Bear. Plants. 2015;18:1382–1394. doi: 10.1080/0972060X.2014.981600. [DOI] [Google Scholar]
- 66.Kazemi M. Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea mille-folium L., Anethum graveolens L., and Carum copticum L. essential oils. J. Herb. Med. 2015;5:217–222. doi: 10.1016/j.hermed.2015.09.001. [DOI] [Google Scholar]
- 67.Milutinovic M., Radovanovic N., Corovic M., Siler-Marinkovic S., Rajilic-Stojanovic M., Dimitrijevic-Brankovic S. Opti-misation of microwave-assisted extraction parameters for antioxidants from waste Achillea millefolium dust. Ind. Crop Prod. 2015;77:333–341. doi: 10.1016/j.indcrop.2015.09.007. [DOI] [Google Scholar]
- 68.Venditti A., Maggi F., Vittori S., Papa F., Serrilli A.M., Di Cecco M., Ciaschetti G., Mandrone M., Poli F., Bianco A. Antioxidant andα-glucosidase inhibitory activities of Achillea tenorii. Pharm. Biol. 2015;53:1505–1510. doi: 10.3109/13880209.2014.991833. [DOI] [PubMed] [Google Scholar]
- 69.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 70.Valgas C., De Souza S.M., Smania E.F.A., Smania A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007;38:369–380. doi: 10.1590/S1517-83822007000200034. [DOI] [Google Scholar]
- 71.Magaldi S., Mata-Essayag S., de Capriles C.H., Perez C., Colella M., Olaizola C., Ontiveros Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004;8:39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 72.McFarland J. The nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA. 1907;XLIX:1176–1178. doi: 10.1001/jama.1907.25320140022001f. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.