Abstract
Background:
Noise-induced hearing loss (NIHL) is one of the leading causes of acquired sensorineural hearing loss. However, molecular mechanisms responsible for its pathogenesis remain to be elucidated. Epigenetic changes, i.e. DNA methylation, histone and microRNA expression modifications may function as a link between noise exposure and hearing loss. Therefore, the aim of the present review was to assess whether epigenetic alterations may serve as biomarkers of noise exposure or early effect.
Materials and Methods:
A systematic review of studies available in Pubmed, Scopus, and ISI Web of Science databases was performed.
Results:
Noise exposure was able to induce alterations in DNA methylation levels in workers and animal models, resulting in expression changes of genes related to hearing loss and also to extra-auditory effects. Differently expressed microRNAs were determined in NIHL workers compared to noise-exposed subjects with normal hearing, supporting their possible role as biomarkers of effect. Acoustic trauma affected histon acethylation and methylation levels in animals, suggesting their influence in the pathogenesis of acute noise-induced damage and their role as targets for potential therapeutic treatments.
Conclusions:
Although preliminary data suggest a relationship between noise and epigenetic effects, the limited number of studies, their different methodologies and the lack of adequate characterization of acoustic insults prevent definite conclusions. In this context, further research aimed to define the epigenetic impact of workplace noise exposure and the role of such alterations in predicting hearing loss may be important for the adoption of correct risk assessment and management strategies in occupational settings.
Keywords: DNA methylation, histone modification, hearing loss, occupational exposure, microRNA
BACKGROUND
Noise-induced hearing loss (NIHL) represents one of the most common worldwide types of acquired sensorineural hearing loss besides the age-related form.[1,2] Among workplace hazards, occupational noise is one of the most common, affecting the health of exposed workers and potentially responsible for the occupational NIHL (ONIHL), which is a work-related medical condition characterised by a permanent sensorineural hearing loss due to an excessive exposure to high levels of noise in workplace settings.[3,4]
The rapid increase in the number of people exposed to the hazardous effects of noise in both industrialized and developing countries, the 7% to 21% prevalence of ONIHL among exposed workers,[5,6,7] together with the disabling consequences deriving from such disorder,[8] makes NIHL, and particularly ONIHL, a major public and occupational health problem.
The pathogenesis of NIHL relies on the complex interplay between genetic and environmental factors.[9] Susceptibility to the noise effects shows a relevant interindividual difference, meaning that an exposure to equal noise intensity levels may result in greatly different hearing impairments.[10,11,12] Long or repeated exposure to sounds at or >85 dB can cause hearing loss inducing the irreversible death or apoptosis of the specialized sensory hair epithelium of the basilar membrane of the organ of Corti in the cochlea, the inner ear part responsible for hearing.[13] Additionally, acute acoustic trauma, intended as a short exposure to intense impulse sounds of 100–150 dB, can cause hearing loss or impairment too.[14]
Although numerous studies revealed that multiple factors are responsible for the development of NIHL, including increased oxidative stress, reduced blood flow, disruption of calcium homeostasis, and mechanical trauma, the mechanisms by which the cochlea is damaged following noise exposure are not entirely clear.[15,16,17,18] The lack of comprehensive understanding of the causal molecular mechanisms underlying noise-induced damage, both in acute and chronic conditions of exposure, is responsible for the lack of established therapeutic strategies for prevention or amelioration of such disabling disorder and for the poor prognosis of the NIHL.[18] Therefore, due to the disease burden and disability associated with hearing impairment, NIHL deserves a high priority of research to develop innovative screening and therapies.
In this scenario, epigenetics, referred to as changes that alter gene transcription in the absence of direct alterations to the genetic sequence, may function as a link between noise exposure and hearing loss.[19] Alterations in DNA methylation, post-translational histone modifications, and changes in microRNAs expression, as the most used mechanisms able to initiate and sustain epigenetic information, have been tought to serve as stable non-invasive biomarkers of noise induced alterations.[20] Therefore, aim of the present review was to assess whether epigenetic changes may indicate noise exposure or occurrence of NIHL. This was finalized to gain insight into possible molecular mechanisms underlying noise exposure and to define possible early biological alterations that may act as suitable biomarkers of exposure, effect or susceptibility in exposed workers. Overall, from an occupational health perspective, this review may reveal a useful means to understand the relationship between noise exposure, auditory and extra-auditory related injuries. This may be important to extrapolate information to lead future reserch focused at the development of effective biological monitoring strategies, diagnostic possibilities, and potential treatments as well as to point out biological mechanisms and predictors of disease that may guide the adoption of the most effective preventive measures for exposed workers.
PROCEDURE
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) criteria were followed to perform a systematic literature search.[21] Studies addressing epigenetic effects induced by noise exposure, published until 15 March 2020, were identified by research on three principal scientific databases: PubMed, Scopus and ISIWeb of Science. A preliminary search was carried out for the terms “hearing or noise exposure” to assess the context of exposure and “epigenetic” as the investigation outcome, which were combined with the Boolean operator “AND”. The computerized search allowed to retrieve articles whose titles and abstracts were independently reviewed by two of the authors with the aim to select papers suitable for the review purposes. The following inclusion criteria were adopted: all types of human peer-reviewed research articles (i.e., cross-sectional, cohort, case-control studies) published in English, and exploring the noise exposure-epigenetic alterations relationship both in humans and in experimental animals. Exclusion criteria regarded reviews, case reports, conference papers, publications not focusing on the association between epigenetic effects and noise exposure, as well as all the papers published in languages other than English. The preliminary search retrieved 96, 147 and 102 references through PubMed, Scopus and ISI Web of Science databases, respectively, for a total of 345 articles [Figure 1]. After duplicate removal, 177 articles remained. Among those, studies that did not meet the inclusion criteria were excluded according to the following reasons: 161 because considered out of the topic from the title and abstract analysis; 11 as review articles, 1 as a conference paper and 1 as published in a language other than English. Indeed, three publications could be identified in this preliminary phase. Therefore, we extended our search including the following terms “hearing or noise exposure”, individually combined with “DNA methylation”, “microRNA” and “histone”, that allowed to include six additional papers. All full texts of the articles considered valuable for the aim of our review were obtained and a critical evaluation was performed. Overall, our search retrieved a total of nine publications for review.
Figure 1.
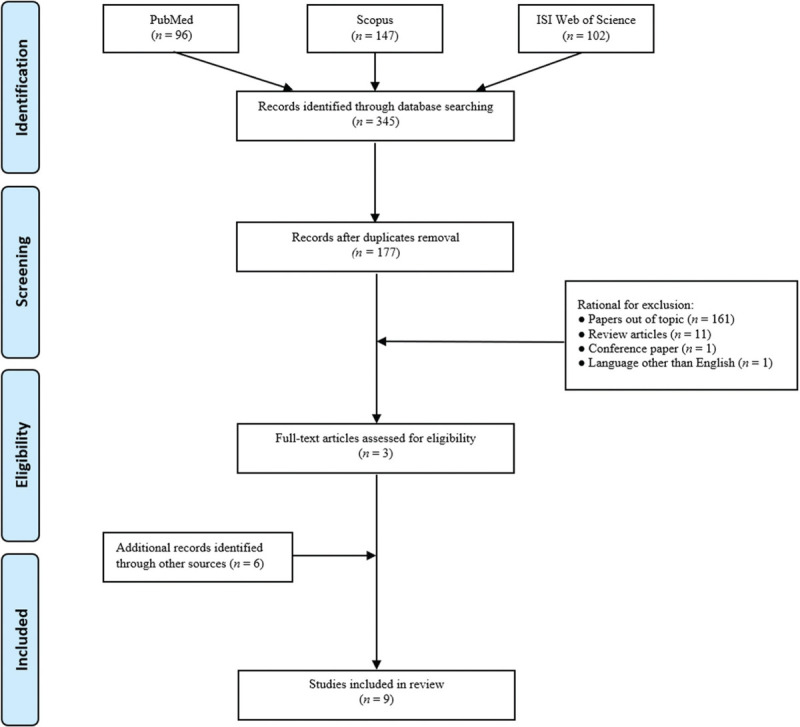
Flow diagram of literature search
EPIGENETIC EFFECTS INDUCED BY NOISE EXPOSURE
Noise exposure was reported to induce epigenetic effects, such as DNA methylation changes,[22,23] histone modifications,[24,25,26,27] as well as alterations in non-coding microRNAs (miRNAs),[28,29,30] both in occupational and experimental settings [Tables 123]. The following paragraphs will attempt to summarize such effects with the aim to point out currently available data and critical issues that require further research in order also to conceive epigenetic contribution to risk assessment and management strategies in noise exposure as well as NIHL.
Table 1.
Studies addressing DNA-methylation changes induced by noise exposure
| DNA methylation | ||||
|---|---|---|---|---|
|
| ||||
| Study location | Experimental settings | Study design | Results | References |
| HUMAN INVESTIGATION | ||||
| USA | Participants at the US Army explosive entry training sites (special operations and combat engineering 10-day course) (n. 34 healthy males; mean age: 30.79±4.57 years) | Noise exposure | ✓ Differently DNA methylated regions (n. 10) with corresponding gene expression changes (with the expected anticorrelation between DNA methylation and gene expression) were demonstrated in relation to chronic cumulative career blast exposures. | Wang et al.[22] |
| Subjects were divided in two groups according to the number of blast exposures reported (low: <40 vs high: >40) during their career in the military service. | ✓ A gain of methylation with a corresponding loss of gene expression was found in the promoter of the PAX8 gene (involved in thyroid function control) in high cumulative blast exposure group. | |||
| Exposure chronology during the 10-day training course: day 2: shotgun training; day 7: ~12 psi blast exposure; day 9: ~4 psi blast exposure. | ✓ No DNA methylation differences were detected between pre and post-acute blast exposures. | |||
| Biological analysis Blood sample for epigenetics and transcriptional studies were collected at the start (baseline) and at the end of the training course (pre- vs post-blast exposure). | ✓ DNA methylation analyses conducted in conjunction with reported symptoms of tinnitus in the low versus high blast incidents groups identified differently DNA methylated regions in KCNE1 and CYP2E1 genes (with loss of gene expression) which have been implicated in noise-related hearing loss. | |||
| IN VIVO STUDIES | ||||
| China | Male Wistar rats (n. 32) randomly divided into four groups. | Noise exposure | ✓ Following short term noise exposure, Comt gene DNA methylation was significantly increased in medulla oblongata. No additional significant changes could be detected in other genes and brain regions. | Guo et al.[23] |
| Rats were exposed to moderate intensity noise (70–75 dB with 20–4000 Hz) at night (7.00 PM − 7.00 AM) for 3 days (short-term exposure group:n. 8 rats) or for 21 days (long-term exposure group: n. 8 rats). Two control groups were exposed to 45 dB sound intensity during daytime for 3 (n. 8 rats) and 21 (n. 8 rats) days. | ✓ Following long-term noise exposure, Comt methylation in the inferior colliculus was significantly increased. The Mc2r gene displayed significantly decreased methylation in hippocampus. Global DNA methylation significantly increased in the medulla oblongata. | |||
| Biological analysis DNA methylation in the Bdnf, Comt, Crhr1, Mc2r and Snca genes was assessed in brain tissues: inferior colliculus, frontal lobe, hippocampus, medulla oblongata. Global DNA methylation was assessed through LINE-1 (LINE-1 UTR and ORF). | ✓ No observed differences in DNA methylation between short and long-term exposure. | |||
Bdnf, brain derived neurotrophic factor; Comt, catechol-O-methyltransferase; Crhr1, corticotropin-releasing hormone receptor 1; CYP2E1, cytochrome P450 family 2 subfamily E member 1; KCNE1, Isk-related family member 1; Mc2r, melanocortin 2 receptor; ORF, open reading frame; PAX8, pairedbox gene 8; psi, pounds per square inch; Snca, synuclein Alpha; UTR, untranslated region.
Table 2.
Studies addressing micro-RNA expression changes induced by noise exposure
| Micro-RNAs | ||||
|---|---|---|---|---|
|
| ||||
| Study location | Experimental settings | Study design | Results | References |
| HUMAN INVESTIGATIONS | ||||
| China | NIHL male patients (n. 23); Noise-exposed male individuals with normal hearing (n. 23); Non-noise exposed individuals with normal hearing (n. 23)Mean age of NIHL and noise-exposed subjects: 44.1 ± 5.1 years; Mean age of controls: 44.13±5.0 yearsNoise exposed subjects were employed in textile industry; non-noise exposed subjects were teachers in elementary and middle school. | Biological analysis | ✓ Compared with the noise-exposed controls, 73 miRNAs demonstrated at least 1.5-fold differential expression levels in the NIHL patients, of which 39 miRNAs were upregulated and 34 miRNAs were downregulated. | Ding et al. [28] |
| MiRNAs were extracted and purified from plasma samples of enrolled subjects (20 NIHL and noise-exposed subjects for the preliminary validation; all 46 NIHL and noise-exposed + 23 controls in the expanded validation). | ✓ Five miRNAs (let-7d-5p, miR-16-5p, miR-24-3p, miR-185-5p, miR-451a) were significantly upregulated, while miR-1915-3p was significantly downregulated in NIHL patients compared to the noise-exposed individuals with normal hearing. | |||
| RT-qPCR was performed to validate the expression levels of the candidate miRNAs screened from the microarray assay. Noise exposure Occupational noise exposure meant levels of noise exposure (Lex) are at least 85 dB (A) for a nominal 8-h working day. NIHL patients were exposed for 20.6 ± 6.5 years; Noise exposed subjects with normal hearing were exposed for 21.3 ± 6.5 years. Acute injury: acoustic trauma or extremely intense noise (>130 dB) was excluded. | ✓ RT-qPCR demonstrated that compared with the non-exposures, the plasma levels of miR-24, miR-185-5p and miR-451a were all significantly downregulated in the exposures while compared with the noise-exposed controls, miR-185-5p and miR-451a were slightly upregulated in the NIHL patients. | |||
| China | Male workers (n.10) with ONIHL and noise-exposed male individuals with normal hearing as controls (n. 10)Age range: 30–45 yearsThe industries of ONIHL workers included machinery (n. 8), food (n. 1) and chemical industry (n. 1). The industries of control subjects included machinery (n. 4), manufacturing (n. 4), energy (n. 1), and printing (n. 1). | Biological analysis | ✓ Three miRNAs upregulated (hsa-miR-3162-5p, hsa-miR-4484, hsa-miR-1229-5p) and 1 downregulated (hsa-miR-4652-3p) in ONIHL subjects compared to controls. | Li et al.[29] |
| MiRNAs were extracted and purified from blood serum of enrolled subjects. Microarray hybridization was conducted for profiling differentially expressed miRNAs between the two groups. RT-qPCR was performed to validate the expression levels of the candidate miRNAs screened from the microarray assay. Noise exposure Enrolled subjects had more than 1-year work experience in a noisy environment (no other details provided). | ✓ Significantly increased serum levels of miR-1229-5p in ONHIL group as compared to controls were confirmed by RT-qPCR. | |||
| IN VIVO STUDIES | ||||
| USA (2013) | Sprague Dawley male and female rats with normal hearing sensitivity − were exposed to acute noise (2 h exposure) and sacrificed 2 h (n. 4) or 1 day post-noise exposure (n. 8).Control animals (n. 12) received identical treatment without noise. | Biological analysis | ✓ ABR thresholds were measured before and at 2 h and 1 d post-noise exposure to determine the functional status of the cochlea. | Patel et al. [30] |
| MiRNA and mRNA gene array expression analysis was performed on the cochlear sensory epithelia sampled from euthanized animals. | ✓ Relative to pre-noise thresholds ABR threshold shifts of 47.12±4.3 dB and 32.3±6.2 dB (mean ±SD) at 2 h and 1 d post-noise exposure were reported, respectively. | |||
| Noise exposure | ✓ At 2 h post-noise exposure, only 1 gene, miR-331-5p, was significantly upregulated, but returned to a baseline level at 1 d post-noise exposure. | |||
| Broadband continuous noise (1–7 kHz) at 120 dB SPL for 2 h. | ✓ At 1 d post-noise exposure, 20 miRNAs (miRs 10a, 107, 124, 130b, 146b, 183, 186, 190b, 194, 200c, 30d, 30e, 325, 333, 339-3p, 381, 429, 532-3p, 674 and 99b) were significantly downregulated. | |||
| ✓ At 1 d post-noise exposure Taok1 mRNA, a predicted target of miR-183, was significantly upregulated (2.3 fold compared to normal) as demonstrated by the RT-qPCR. | ||||
ABR, auditory brainstem response; MiRNAs, microRNAs; NIHL, noise-induced hearing loss; ONIHL, occupational noise-induced hearing loss; RT-qPCR, Real Time quantitative Polymerase Chain Reaction; SD, standard deviation; SPL, sound pressure level.
Table 3.
Studies addressing histone modifications induced by noise exposure
| HISTONE MODIFICATIONS | ||||
|---|---|---|---|---|
|
| ||||
| Study location | Experimental settings | Study design | Results | References |
| IN VIVO STUDIES | ||||
| USA | Male CBA/J mice assigned to 3 experimental groups (n. 20 each group): the control group; the DMSO group, that was exposed to noise and received an intraperitoneal injection of DMSO (10%) 3 days before the exposure; the SAHA group, that was exposed to noise and received an intraperitoneal injection of SAHA (25 mg/kg) 3 days before noise exposure. | Noise exposure | ✓ Noise exposure resulted in PTS at 4, 8, 12, 24, 32 and 48 kHz, 2 weeks following exposure to noise. Compared with the DMSO-injected group, pre-treatment with the HDAC inhibitor, SAHA, significantly reduced PTS. | Wen et al.[24] |
| Mice were exposed to broadband noise with a frequency spectrum from 2 to 20 kHz for 2 h/day for 2 weeks at 110 dB SPL to induce a PTS with loss of cochlear OHCs. Control animals were not exposed to noise. | ✓ The expression of HDAC1 (2-fold) and HDAC4 (3 fold) increased 1 h following noise exposure compared to controls. The H3-AcK9 levels decreased 1 h following noise exposure. | |||
| Biological analysis The expression levels of H3K9ac and HDAC1 and HDAC4 were assessed by western blot analysis on cochlear tissue homogenates 1 h after exposure. | ✓ The number of OHCs loss decreased following SAHA pre-treatment compared to the DMSO pre-treatment. SAHA pre-treatment doubled the number of survival OHCs and attenuated cilia damage. | |||
| USA | Male CBA/J mice (max n.7 per group) were exposed to noise and cochlear tissues were analysed 1 h after the exposure completion | Noise exposure | ✓ Immunolabeling for H3K9ac decreased in the nuclei of OHCs (60%) and strial marginal cells of the cochlear basal turn 1 h after noise exposure compared to control mice. | Chen et al.[25] |
| Control group: mice not exposed to noise | Mice were exposed to broadband noise with a frequency spectrum from 2 to 20 kHz for 2 h at 98 dB SPL to induce a PTS with loss of OHCs. | ✓ Noise exposure increased HDAC1, 2, and 3 in cochlear tissues (organ of Corti, spiral ganglion cells and stria vascularis) 1 h after noise exposure compared to control mice. | ||
| Biological analysis | ✓ Blockade of HDAC1, 2, or 3 alone in cochlear tissues resulted not sufficient to reduce PTS. | |||
| The expression levels of H3K9ac was assessed by immunohistochemistry on cryosection 1 h after the exposure. The levels of HDAC1, 2, and 3 using paraffin-embedded cochlear sections from the mid-modiolar region were assessed 1 h after the exposure. Two week following noise exposure, treatment with SAHA (50 mg/kg intraperitoneal injection) was applied to the animals and noise-induced OHCs loss, PTS and H3K0ac distribution in the nuclei was assessed. | ✓ Treatment with SAHA significantly decreased noise-induced OHCs loss (40% at 4 mm, 50% at 4.5 mm, and 20% at 5 mm), and significantly attenuated PTS by about 20 dB at 16 kHz compared to vehicle-treated mice. After 1 h SAHA treatment there was an enrichment of H3K9ac at the nuclear periphery. | |||
| China | Male albino guinea pigs (n. 120) divided into 3 groups: control group (no noise exposure, n. 40); noise only group (n. 40); noise + SB (intraperitoneal injection at a dose of 600 mg/kg once per day on the 3 days before and after noise exposure, as well as 30 min before and 2 h after the noise exposure) group (n. 40). | Noise exposure | ✓ Noise exposure induced PTS in both the noise only group and the noise with SB group. However, the ABR threshold shifts in this latter group were significantly lower than those in the noise only group. | Yang et al.[26] |
| In the noise-exposed group: 10 animals underwent hearing test at 1 d before and 14 d after exposure and then were euthanized; 30 animals were euthanized at 2 h after noise exposure | Animals were exposed to 1/3-octave-wide narrowband noise, centered at 4kHz at 122 dB SPL for 3 h. | ✓ Noise exposure significantly decreased H3‑AcK9 expression, and increased HDAC1 expression, in the nuclei of OHCs, IHCs, and Hensen’s cells. SB treatment partially reversed these changes. | ||
| Biological analysis Cochlear tissues were collected for immunofluorescence staining to assess H3AcK9, HDAC1 and 3NT levels. Homogenized cochlear tissues were used for protein total extraction and western blot analysis | ✓ The 3-NT was significantly increased in OHCs in the noise only group compared to the control group. SB treatment significantly reduced the noise-induced increase of 3-NT | |||
| USA | Male CBA/J mice (max n.6 per group) were exposed to noise and cochlear tissues were analysed 1 h after the exposure completion | Noise exposure | ✓ Immunoreactivity of G9a protein increased in the nuclei of OHCs, IHCs, supporting cells of organ of Corti, the nuclei of SGNs and the nuclei of marginal cells in lateral wall tissues in the basal turn of the cochlea | Xiong et al. [27] |
| Control group: mice not exposed to noise | Mice were exposed to broadband noise with a frequency spectrum from 2 to 20 kHz for 2 h at 101 dB SPL to induce a PTS with loss of cochlear OHCs. | ✓ Noise exposure increased immunolabelling for H3K9me2 levels in nuclei of OHCs, strial marginal cells, and SGNs from the basal turn of the cochlea. | ||
| Biological analysis | ✓ Noise exposure reduced the expression of KCNQ4 in OHCs of the basal turn of the cochlea (75%). | |||
| The levels of the lysine dimethyltransferase G9a in cochlear cells were assessed by immunohistochemistry. Since a primary function of G9a is to demethylate lysine 9 of histone 3, levels of H3K9me2 in the cochlea 1 h after completion of noise exposure was assessed through immunolabelling. Expression levels of potassium channel genes KCNQ4 was assessed 1 h after noise exposure completion. | ✓ Treatment with BIX 01294 (a specific inhibitor of G9a prevented the noise-induced decrease of KCNQ4 immunolabelling in OHCs, the loss of OHCs and reduces auditory threshold shifts. | |||
3NT, 3-nitrotyrosine; DMSO, Dimethyl Sulfoxide; H3K9me2, histone H3 lysine 9 dimethylation; HDAC, histone deacetylases; IHCs, inner hair cells; KCNQ4, potassium voltage-gated channel subfamily Q member 4; OHCs, outer hair cells; PTS, permanent threshold shift; SAHA, Suberoylanilide hydroxamic acid; SB, sodium butyrate; SGNs, spiral ganglion neurons; SPL, sound pressure level.
DNA methylation
DNA methylation is recognized as an essential epigenetic mark that regulates chromatin structure and gene expression throughout the genome.[31] Understanding how noise exposures may affect DNA methylation patterns may help to elucidate the relationship between noise and its possible auditory and extra-auditory effects [Table 1].[23] In this context, Wang et al.[22] investigated DNA methylation and gene expression abnormalities induced by blast overpressure exposures in male “breachers”, military and law enforcement professionals subacutely and chronically exposed to repeated blasts as part of their job duties. Epigenetic effects were evaluated with respect to blast subacute exposures, intended as controlled, low-level blast exposures experienced during a 10-day explosive breaching course, and to the number of cumulative blast exposure events (< or >40) during the career in the military service. No different methylated regions could be identified comparing pre- and post-blast exposure blood DNA methylation patterns in subjects attending the above-mentioned training courses. When DNA methylation analysis was conducted considering cumulative exposure, 10 differently hyper-methylated regions resulted positively associated to the highest lifetime exposure with corresponding gene expression changes. Interestingly, hypermethylation localized in the promoter region of the antisense transcript within the paired box gene 8 (PAX8), resulted responsible for the control of the expression of thyroid-specific genes involved in the gland function as well as in sleep duration suggesting the possible epigenetic control of potential extra-auditory effects of noise.[32,33,34] DNA methylation and gene expression changes were also investigated in relation to the more frequently self-reported symptoms, particularly tinnitus. The authors identified genes with differential DNA methylation changes associated with symptoms of tinnitus in the high vs. low career breaching groups. Differently hypermethylated regions involved the potassium voltage-gated channel, Isk-related family, member 1 (KCNE1), and the cytochrome P450 family 2, subfamily E, member 1 (CYP2E1) genes, whose transcriptional expression resulted significantly reduced. Interestingly, both KCNE1 and CYP2E1 genes have been reported to be implicated in noise-related hearing loss in human genetics and animal investigations, respectively.[35,36,37,38,39,40]
DNA methylation changes were reported by Guo et al.[23] when exploring the effects of environmental noise exposure in vivo. They analysed the global DNA methylation levels in LINE-1 untranslated regions and the methylation of five selected genes expressed in different regions of the brain, i.e. the frontal lobe, hippocampus, inferior colliculus, and medulla oblongata, of male Wistar rats exposed to moderate intensity noise for 3 or 21 days, as short and long-term exposures, respectively. Following short term noise exposure, only the DNA methylation of the catechol-O-methyltransferase (Comt) gene was significantly increased in medulla oblongata. After long-term exposure, Comt methylation was significantly increased in the inferior colliculus, while the melanocortin 2 receptor (Mc2r) gene demonstrated a significantly decreased methylation in hippocampus. As far as the Comt gene is concerned, it codes for an enzyme involved in the dopaminergic circuit, in turn linked to cognitiveness, working memory and depression.[41] The hypermethylation of this gene in the inferior colliculus following a long-term noise exposure, which consequently leads to a reduced expression of the enzyme encoded by the same gene, could suggest a role of chronic noise exposure on certain brain functions, including memory and cognition, and in the increased risk of depression. Similarly, as regards Mc2r, receptor of the adrenocorticotropic hormone (ACTH) involved in the response to stressors,[42] the finding of hypomethylation of this gene in the hippocampus after chronic noise exposure, may be associated with memory deficit and cognition impairment. Global DNA methylation in LINE-1 significantly increased in the medulla oblongata, but not in the other brain regions examined at the same time point. However, the differences detected in the DNA methylation following long-term noise exposure, with respect to short-term one, have not been fully explained and no relation could be detected for specific genes, brain regions and functions.
Micro RNAs
MicroRNAs, small 20–22 nucleotide molecules, represent a new class of noncoding RNA genes able to regulate cellular functions by modulating mRNA expression levels.[13] Investigations on miRNA functions in the auditory system have been mainly focused on their roles in inner ear development [Table 2].[43,44,45,46,47] However, the role of miRNAs in noise-induced cochlear pathogenesis is yet to be established, and only preliminary data are available on the potential effects on miRNAs expression induced by noise exposure in humans [28,29] and animal models.[30]
A case-control study investigated the plasma miRNA profile of NIHL male patients, noise-exposed male individuals with normal hearing, both employed in a textile industry, and of an unexposed control group with normal hearing.[28] The authors found that the NIHL group showed 73 miRNAs with at least 1.5-fold differential expression levels compared to noise-exposed controls at the microarray analysis, suggesting a possible specific mRNA expression pattern indicative of a hearing loss occurrence. Among the miRNAs differently expressed in NIHL patients and noise-exposed controls, miR-16-5p, miR-24-3p, miR-185-5p, miR-451a, all molecules implicated in the regulation of oxidative stress responses, demonstrated a significant up-regulation in the first group. However, an additional validation analysis demonstrated that miR-24-3p, miR-185-5p, miR-451a were all significantly downregulated in both noise-exposed groups compared to unexposed controls. Interestingly, the findings of a downregulation of key miRNAs in noise exposed vs. unexposed subjects are in line with previous miRNA expression research of oxidative stress-related changes in cultured cells derived from the organ of Corti.[48] Excessive noise may generate, in the cochlea, an over-production of reactive oxygen species which may be responsible for changes in the expression of miRNAs involved in the oxidative stress response. Such miRNA expression pattern may therefore be involved in NIHL pathogenesis although the causal association and their role as possible early biomarkers of noise effects need further investigation. Importantly, the same additional verification confirmed that miR-185-5p and miR-451a were significantly upregulated in the NIHL group compared to the noise-exposed subjects as previously demonstrated. This may support the potential role of these two miRNAs as possible biomarkers of noise exposure and hearing loss damage.
Comparably, a different miRNA expression was detected in ONIHL workers compared to noise-exposed individuals with normal hearing enrolled as controls. In fact, seven differentially expressed miRNAs were determined between the two groups at the microarray analysis, with a >1.5 fold changed expression: three were upregulated (hsa-miR-3162-5p, hsa-miR-4484, hsa-miR- 1229-5p), and four were downregulated (hsa-miR-6752-3p, hsa-miR-6824-3p, hsa-miR-4769-3p, hsa-miR-4652-3p) in ONIHL subjects. The upregulation of the above-mentioned miRNAs was confirmed also using the real time quantitative polymerase chain reaction (RT-qPCR) as an additional analysis. However, this technique found a significant increase of serum expression only for miR-1229-5p in ONIHL group suggesting that such molecule could be implicated in hearing loss pathogenesis. The analyses of the predicted target genes of hsa-miR-1229-5p demonstrated that they were involved in a great number of biological processes and molecular functions, e.g. ion, nucleotide and ATP binding, transcription regulator and protein kinase activities. Among those involved genes, the mitogen-activated protein kinase (MAPK) signaling pathway has been determined, reported to function as a regulatory pathway in human genetic deafness and in the inner hair cell survival,[49] thus decreasing the susceptibility to noise-induced hearing loss in animals.[50] MiR-1229-5p may contribute to the pathogenesis of ONIHL producing a post-transcriptional repression of MAPK1 thus reducing a self-defensive mechanism against noise exposure. Overall, these findings may suggest serum miR-1229-5p level as a possible innovative biomarker for ONIHL prediction.
MiRNAs have been also implicated in responses to acoustic overstimulation. In this regard, Patel et al.[30] investigated the role of miRNAs in cochlear apoptotic pathogenesis after acoustic trauma in exposed Sprague Dawley rats. A significant auditory brainstem response (ABR) threshold shift and apoptotic activity, characterized by an increased percentage of cells with condensated nuclei and increased caspase-3 activity were demonstrated after the exposure to intense noise. Gene expression analysis of noise-traumatized cochleae revealed time-dependent transcriptional changes in the expression of miRNAs. Only miR-331-5p was significantly upregulated at 2 h post-noise exposure, while returned to a baseline level at 1 d post-noise exposure. At this time point, 20 miRNAs were significantly down-regulated with a fold change equal to or greater than 2.5, an alteration that was not evident at 2 h post-noise exposure. This may suggest that the noise-induced miRNA expression changes are time-specific and involve different sets of miRNAs at different time points. When target prediction analysis was performed to verify the possible involvement of potential regulators of the degenerative process of the cochlea following acoustic overstimulation, a significant upregulation of Taok1 mRNA, a target of miRNA-183, was demonstrated. In non-cochlear tissues, Taok1 has been associated with activation of mitogen-activated protein kinase pathway in response to stress and DNA damage[51,52,53] and apoptosis induction.[54] This may suggest its possible role in the regulation of cochlear response to acoustic trauma through the regulation of apoptotic pathways.
Histone modifications
Post-translational modifications of histones have emerged as key regulators of genomic activity without affecting DNA sequence.[27] Histone acetylation catalyzed by the activity of histone acetyltransferases (HATs), and deacetylation catalyzed by histone deacetylases (HDACs) are responsible for the activation and repression of gene transcription, respectively, influencing the interactions between histones and DNA.[55,56] Therefore, an imbalance in histone acetylation may be implicated in a wide range of transcriptional dysfunction, gene silencing and neurodegenerative disorders.[57,58,59]
Preliminary evidence in animal models is available concerning the role of HDACs in hearing impairment [Table 3].[24,25,26,27] Wen et al.[24], 1 h following an acute traumatic noise in a CBA/J mouse model, demonstrated a significant increase in the expression of HDAC1 and HDAC4, and a decrease in the levels of acetyl-histone H3 (Lys9) (H3K9ac) protein compared to unexposed controls. Significant hearing impairment was evident 2 weeks following noise exposure in mice with a reduction in the number of outer hair cells (OHCs). Interestingly, a pre-treatment with an HDAC inhibitor, the suberoylanilide hydroxamic acid (SAHA) significantly prevented such molecular and morphological alterations. This suggests a role for HDAC enzymes in NIHL. These results are in line with subsequent findings obtained by Chen et al.[25] who determined increased levels of HDAC1, HDAC2 and HDAC3 prevalently in the nuclei of cochlear cells in CBA/J mice following a traumatic noise exposure. As previously demonstrated, the levels of H3K9ac resulted significantly decreased in the nuclei of OHCs and marginal cells of the stria vascularis, suggesting that histone H3 tail epigenetic modification could be involved in noise-induced OHCs loss and NIHL. Inhibition of HDAC1, HDAC2, or HDAC3 with small-interfering RNAs decreased the expression of the target HDAC in OHCs, but did not ameliorate the permanent threshold shift (PTS) induced by the noise exposure. Conversely, the SAHA showed beneficial effects on OHCs loss, and reduced PTS. Comparably, Yang et al.[26] confirmed such previous results demonstrating that traumatic noise exposure significantly increased HDAC1 expression in the nuclei of OHCs and inner hair cells (IHCs) and decreased H3K9ac expression in guinea pig cochlear tissues. The administration of the HDAC inhibitor, sodium butyrate, partially recovered these effects limiting the effects on OHCs and the PTS. Additionally, the sodium butyrate treatment reduced the noise-induced oxidative stress, decreasing the 3-nytrotyrosine upregulation induced by the traumatic exposure suggesting an oto-protective role of the HDAC inhibitors by limiting the overproduction of reactive oxygen and nitrogen species. These findings suggest that histone acetylation is involved in the pathogenesis of noise-induced OHCs death and hearing loss. Although the exact molecular mechanisms underlying the protective effects of targeting histone deacetylases are still not understood, this treatment may characterize a strategy for protection against NIHL.
Apart from modifications in histone acetylation, also histone methylation has been investigated for a possible function in hearing loss development.[60,61] A traumatic noise exposure, able to affect OHCs and PTS in mice, resulted in the activation of the G9a, a major histone lysine methyltransferase responsible for histone H3 lysine 9 dimethylation (H3K9me2) in both OHCs and IHCs, some spiral ganglion neurons (SGNs) as well as marginal cells 1 hour after exposure.[27] Interestingly, the inhibition of G9a by treatment with BIX 01294 or G9a siRNA reduced noise-induced losses of OHCs and attenuated NIHL. These findings suggest that epigenetic modifications of H3K9me2 may play a role in NIHL and may function as a pharmacological target to prevent NIHL.
DISCUSSION AND CONCLUSION
This review represents the first attempt to comprehensively assess the possible role of epigenetic alterations in contributing to the pathogenesis of noise-induced injury, functioning both as potential biomarkers of early damage, possible effective diagnostic tools as well as potential target of treatments to prevent/ameliorate hearing loss. Although the number of available investigations on the topic is limited, some interesting issues for the discussion emerged from our revision.
Concerning acute to subacute noise exposure, no alterations in blood DNA methylation in military personnel could be detected following 10 day-blast exposure trainings (∼12 pounds per square inch.),[22] while an in vivo experiment demonstrated that moderate intensity noise (70–75 dB) for 3 days was able to increase the DNA methylation of the Comt gene in the medulla oblongata of exposed animals.[23] These latter results suggest that early epigenetic alterations may occur at even not so high levels of noise in a gene that has been reported to be involved with stress response, cognition, anxiety, that may be all factors influenced by the noise insult.[62,63,64,65] However, the different species susceptibility to noise injury, the diverse type of noise exposure in terms of duration and intensity, as well as the variable biological matrices analysed may all play a role in affecting results and need further investigation. Acoustic trauma induced by broadband continuous noise exposure to 98-122 dB SPL for 2 to 3 hours was able to induce an increased expression of the enzymes HDAC 1–4[24,25,26] involved into the deacetylation of the lysine 9 residue on histone 3, with a consequent reduction of the H3K9ac levels 1 h post-exposure. Additionally, a comparable noise exposure induced the activation of the methyltransferase G9a, responsible for the hypermethylation of the H3K9 site.[27] These preliminary data support the possible role of histone acetylation and methylation levels in the pathogenesis of acute noise-induced damage. This was further confirmed by the capacity of HDAC inhibitors to reduce NIHL, suggesting their possible role as potential therapeutic strategies for the prevention of hearing loss.
When the epigenetic impact of chronic, cumulative noise exposure along a professional career was assessed compared to shorter periods of exposure, some DNA methylation differences emerged.[22] In fact, several hypermethylated regions resulted associated with lifetime exposure compared to the absence of subacute alterations. It may be argued that DNA methylation effects, which represent highly stable, long-lasting effect indicators, may be more likely representative of cumulative noise exposures, with respect to acute or proximal events. Interestingly, changes in DNA methylation resulted also positively associated with noise-related symptoms reported by military and veterans, such as tinnitus. In line with these results, sub-chronic conditions of exposure have been demonstrated to induce different epigenetic alterations in animals, i.e. increased Comt and LINE-1, and decreased Mr2c methylation, compared to the previous described subacute effects.[23]
Moreover, possible time-dependent trends have been described in epigenetic effects. Patel et al.[30] demonstrated that a traumatic noise exposure caused a reversible up-regulation of a single miRNA and a significant down-regulation of a greater number of miRNAs, 2 hours and 1-day post-exposure, respectively. This temporal pattern of changes may be related to the progression of sensory cell degeneration post-noise insult.[66,67] The growth of the noise-induced lesion is expected to induce more cells to degenerate and consequently, more miRNAs to be differently expressed. Another possible factor influencing the temporal change in miRNA expression may be the differences in damaging triggers during the diverse phases of cochlear damage pathogenesis. Mechanical stress may function as an initial cause of cochlear effect, while other mechanisms of action, i.e. energy exhaustion,[68] oxidative stress,[69] and ionic imbalance,[70,71,72] may contribute to longer-term damage.
The gene expression analysis in relation to the epigenetic modifications may also be important to understand the pathogenesis of noise-induced effects. DNA methylation changes detected by Wang et al.[22] were associated with a decreased expression of KCNE1 and CYP2E1 genes, directly involved in auditory processing and sound perception. Such kind of analysis may also support the understanding of possible noise-induced extra-auditory effects as suggested by the reduced expression of the PAX8 gene in response to the increased DNA methylation in its promoter region, which may affect the control of the expression of thyroid-specific genes and thyroid development, as well as sleep patterns. Also changes in Comt and Mc2r DNA methylation may influence certain brain functions, including memory and cognition, as a possible outcome of chronic noise exposure.[23] Additionally, Patel et al.,[30] exploring the miRNA/mRNA target pairs showed that the down-regulation of miR-183 was associated with a Taok1 mRNA overexpression, responsible for the activation of the MAPK pathway potentially involved in cochlear apoptosis as a mechanism of noise damage. Overall, these data can assist the future development of target molecular therapeutic perspectives in reducing noise-induced cochlear damage.
Importantly, as suggested for plasma miR-1229-5p[29] and miR-185-5p and miR-451[28] in occupationally noise-exposed workers, the differences in microRNA and downstream gene expression alterations between ONIHL patients and noise-exposed subjects with normal hearing, may be important to understand the molecular responses to noise exposure and to define possible novel biomarkers to predict and eventually prevent its development and progression. However, to achieve more sustained conclusions in this regard, further research is necessary to prove the causal association between miRNA changes and noise exposure, and to determine whether these miRNAs may effectively function as biomarkers of exposure and/or early effect.
Finally, some limitations emerged from our revision should be stressed in order to plan methodologically adequate investigations able to provide more informative data. In this context, additional studies should include a greater number of subjects/animals to avoid statistical power limitations due to the small sample size. Most of the analysed investigations employed different kind of exposures. As the impact of noise depends upon the context and characteristics of the acoustic insult, including duration, intensity, intermittency, frequency, and predictability,[73,74,75,76] a future standardized epigenetic research approach should provide a precise description of noise characteristics in order to achieve more comparable and effective results. Additionally, most of the reviewed studies could only applied a candidate gene approach analysis, uneffective to identify all genes that are potentially affected by the exposure. Therefore, an extended analytical strategy, on suitable and routinary applicable biological matrices, should be pursued. Finally, a longitudinal methodological design, for both genetic and symptom-based studies, appears necessary to understand molecular alterations time-dependent trends and to verify their possible employment as early biomarkers for hearing damage, particularly in chronic occupational exposure settings.
In conclusion, additional studies are necessary to overcome the current gap in noise exposure-epigenetic changes relationship. From an occupational health perspective, to define the epigenetic impact of workplace noise exposure and the role of such alterations in predicting hearing loss development may be absolutely important for a correct evaluation of risks, according also to an individually tailored-based approach. Additionally, epigenetic data may provide future support to the health surveillance plans of chronically exposed workers, with the aim to detect early, maybe preclinical alterations, and therefore guiding the adoption/implementation of the most effective preventive and protective measures to avoid disabling hearing loss.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Concha-Barrientos M, Campbell-Lendrum D, Steenland K. Occupational noise: assessing the burden of disease from work-related hearing impairment at national and local levels. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Ding T, Yan A, Liu K. What is noise-induced hearing loss? Br J Hosp Med (Lond) 2019;80:525–9. doi: 10.12968/hmed.2019.80.9.525. [DOI] [PubMed] [Google Scholar]
- 3.Lie A, Skogstad M, Johnsen TS, Engdahl B, Tambs K. The prevalence of notched audiograms in a cross-sectional study of 12,055 railway workers. Ear Hear. 2015;36:86–92. doi: 10.1097/AUD.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M. The global burden of occupational noise-induced hearing loss. Am J Ind Med. 2005;48:446–58. doi: 10.1002/ajim.20223. [DOI] [PubMed] [Google Scholar]
- 5.Metidieri MM, Rodrigues HF, Filho FJ, Ferraz DP, Neto AF, Torres S. Noise-Induced Hearing Loss (NIHL): literature review with a focus on occupational medicine. Int Arch Otorhinolaryngol. 2013;17:208–12. doi: 10.7162/S1809-97772013000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Molen HF, de Vries SC, Stocks SJ, Warning J, Frings-Dresen MH. Incidence rates of occupational diseases in the Dutch construction sector, 2010-2014. Occup Environ Med. 2016;73:350–2. doi: 10.1136/oemed-2015-103429. [DOI] [PubMed] [Google Scholar]
- 7.Lie A, Skogstad M, Johannessen HA, Tynes T, Mehlum IS, Nordby KC, et al. Occupational noise exposure and hearing: a systematic review. Int Arch Occup Environ Health. 2016;89:351–72. doi: 10.1007/s00420-015-1083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Deafness and hearing loss. 2020. https://www.who.int/health-topics/hearing-loss . [Google Scholar]
- 9.Sliwinska-Kowalska M, Pawelczyk M. Contribution of genetic factors to noise-induced hearing loss: a human studies review. Mutat Res. 2013;752:61–5. doi: 10.1016/j.mrrev.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Pawelczyk M, Van Laer L, Fransen E, Rajkowska E, Konings A, Carlsson PI, et al. Analysis of gene polymorphisms associated with K ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann Hum Genet. 2009;73:411–21. doi: 10.1111/j.1469-1809.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 11.Konings A, Van Laer L, Van Camp G. Genetic studies on noise-induced hearing loss: a review. Ear Hear. 2009;30:151–9. doi: 10.1097/AUD.0b013e3181987080. [DOI] [PubMed] [Google Scholar]
- 12.Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miguel V, Cui JY, Daimiel L, Espinosa-Díez C, Fernández-Hernando C, Kavanagh TJ, et al. The role of MicroRNAs in environmental risk factors, noise-induced hearing loss, and mental stress. Antioxid Redox Signal. 2018;28:773–96. doi: 10.1089/ars.2017.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada T, Sano H, Nishio SY, Kitoh R, Ikezono T, Iwasaki S, et al. Differences between acoustic trauma and other types of acute noise-induced hearing loss in terms of treatment and hearing prognosis. Acta Otolaryngol. 2017;137:48–52. doi: 10.1080/00016489.2017.1297899. [DOI] [PubMed] [Google Scholar]
- 15.Hu BH, Henderson D, Yang WP. The impact of mitochondrial energetic dysfunction on apoptosis in outer hair cells of the cochlea following exposure to intense noise. Hear Res. 2008;236:11–21. doi: 10.1016/j.heares.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed Res Int. 2015;2015:617207. doi: 10.1155/2015/617207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129–37. doi: 10.1016/j.heares.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs. 2017;26:85–96. doi: 10.1080/13543784.2017.1269171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murgatroyd C, Wu Y, Bockmühl Y, Spengler D. The Janus face of DNA methylation in aging. Aging (Albany NY) 2010;2:107–10. doi: 10.18632/aging.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Wilson CM, Mendelev N, Ge Y, Galfalvy H, Elder G, et al. Acute and chronic molecular signatures and associated symptoms of blast exposure in military breachers. J Neurotrauma. 2019 doi: 10.1089/neu.2019.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L, Li PH, Li H, Colicino E, Colicino S, Wen Y, et al. Effects of environmental noise exposure on DNA methylation in the brain and metabolic health. Environ Res. 2017;153:73–82. doi: 10.1016/j.envres.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Wen LT, Wang J, Wang Y, Chen FQ. Association between histone deacetylases and the loss of cochlear hair cells: role of the former in noise-induced hearing loss. Int J Mol Med. 2015;36:534–40. doi: 10.3892/ijmm.2015.2236. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Hill K, Sha SH. Inhibitors of histone deacetylases attenuate noise-induced hearing loss. J Assoc Res Otolaryngol. 2016;17:289–302. doi: 10.1007/s10162-016-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang DH, Xie J, Liu K, Peng Z, Guo JY, Yu SK, et al. The histone deacetylase inhibitor sodium butyrate protects against noise-induced hearing loss in Guinea pigs. Neurosci Lett. 2017;660:140–6. doi: 10.1016/j.neulet.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Xiong H, Long H, Pan S, Lai R, Wang X, Zhu Y, et al. Inhibition of histone methyltransferase g9a attenuates noise-induced cochlear synaptopathy and hearing loss. J Assoc Res Otolaryngol. 2019;20:217–32. doi: 10.1007/s10162-019-00714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding L, Liu J, Shen HX, Pan LP, Liu QD, Zhang HD, et al. Analysis of plasma microRNA expression profiles in male textile workers with noise-induced hearing loss. Hear Res. 2016;333:275–82. doi: 10.1016/j.heares.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Li YH, Yang Y, Yan YT, Xu LW, Ma HY, Shao YX, et al. Analysis of serum microRNA expression in male workers with occupational noise-induced hearing loss. Braz J Med Biol Res. 2018;51:e6426. doi: 10.1590/1414-431X20176426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel M, Cai Q, Ding D, Salvi R, Hu Z, Hu BH. The miR-183/Taok1 target pair is implicated in cochlear responses to acoustic trauma. PLoS One. 2013;8:e58471. doi: 10.1371/journal.pone.0058471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb DJ, Hek K, Chen TH, Watson NF, Eiriksdottir G, Byrne EM, et al. Novel loci associated with usual sleep duration: the CHARGE Consortium Genome-Wide Association Study. Mol Psychiatry. 2015;20:1232–9. doi: 10.1038/mp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones SE, Tyrrell J, Wood AR, Beaumont RN, Ruth KS, Tuke MA, et al. Genome-wide association analyses in 128, 266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 2016;12:e1006125. doi: 10.1371/journal.pgen.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fechter LD, Gearhart C, Shirwany NA. Acrylonitrile potentiates noise-induced hearing loss in rat. J Assoc Res Otolaryngol. 2004;5:90–8. doi: 10.1007/s10162-003-4028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Laer L, Carlsson PI, Ottschytsch N, Bondeson ML, Konings A, Vandevelde A, et al. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum Mutat. 2006;27:786–95. doi: 10.1002/humu.20360. [DOI] [PubMed] [Google Scholar]
- 37.Pawełczyk M, Rajkowska E, Kotyło P, Dudarewicz A, Van Camp G, Śliwińska-Kowalska M. Analysis of inner ear potassium recycling genes as potential factors associated with tinnitus. Int J Occup Med Environ Health. 2012;25:356–64. doi: 10.2478/S13382-012-0061-3. [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishnan NA, Drescher MJ, Khan KM, Hatfield JS, Drescher DG. HCN1 and HCN2 proteins are expressed in cochlear hair cells: HCN1 can form a ternary complex with protocadherin 15 CD3 and F-actin-binding filamin A or can interact with HCN2. J Biol Chem. 2012;287:3762846. doi: 10.1074/jbc.M112.375832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YH, Holt JR. Functional contributions of HCN channels in the primary auditory neurons of the mouse inner ear. J Gen Physiol. 2013;142:207–23. doi: 10.1085/jgp.201311019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen H, Liu W, Geng Q, Li H, Lu M, Liang P, et al. Age-dependent up-regulation of hcn channels in spiral ganglion neurons coincide with hearing loss in mice. Front Aging Neurosci. 2018;10:353. doi: 10.3389/fnagi.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickinson D, Elvevåg B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridmanis D, Roga A, Klovins J. ACTH receptor (MC2R) specificity: what do we know about underlying molecular mechanisms? Front Endocrinol (Lausanne) 2017;8:13. doi: 10.3389/fendo.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, et al. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci USA. 2009;106:7915–20. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis MA, Quint E, Glazier AM, Fuchs H, De Angelis MH, Langford C, et al. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009;41:614–8. doi: 10.1038/ng.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frucht CS, Santos-Sacchi J, Navaratnam DS. MicroRNA181a plays a key role in hair cell regeneration in the avian auditory epithelium. Neurosci Lett. 2011;493:44–8. doi: 10.1016/j.neulet.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel M, Hu BH. MicroRNAs in inner ear biology and pathogenesis. Hear Res. 2012;287:6–14. doi: 10.1016/j.heares.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang W, et al. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res. 2010;1346:14–25. doi: 10.1016/j.brainres.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 49.Stamatiou GA, Stankovic KM. A comprehensive network and pathway analysis of human deafness genes. Otol Neurotol. 2013;34:961–70. doi: 10.1097/MAO.0b013e3182898272. [DOI] [PubMed] [Google Scholar]
- 50.Kurioka T, Matsunobu T, Satoh Y, Niwa K, Endo S, Fujioka M, et al. ERK2 mediates inner hair cell survival and decreases susceptibility to noise-induced hearing loss. Sci Rep. 2015;5:16839. doi: 10.1038/srep16839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutchison M, Berman KS, Cobb MH. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J Biol Chem. 1998;273:28625–32. doi: 10.1074/jbc.273.44.28625. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z, Hutchison M, Cobb MH. Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J Biol Chem. 1999;274:28803–7. doi: 10.1074/jbc.274.40.28803. [DOI] [PubMed] [Google Scholar]
- 53.Raman M, Earnest S, Zhang K, Zhao Y, Cobb MH. TAO kinases mediate activation of p38 in response to DNA damage. EMBO J. 2007;26:2005–14. doi: 10.1038/sj.emboj.7601668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu MF, Wang SG. Human TAO kinase 1 induces apoptosis in SH-SY5Y cells. Cell Biol Int. 2008;32:151–6. doi: 10.1016/j.cellbi.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Layman WS, Zuo J. Epigenetic regulation in the inner ear and its potential roles in development, protection, and regeneration. Front Cell Neurosci. 2015;8:446. doi: 10.3389/fncel.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu X, Wang L, Yu C, Yu D, Yu G. Histone acetylation modifiers in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci. 2015;9:226. doi: 10.3389/fncel.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol Pharmacol. 2010;77:621–32. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stilling RM, Fischer A. The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease. Neurobiol Learn Mem. 2011;96:19–26. doi: 10.1016/j.nlm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Gräff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- 60.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–91. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokochi T, Poduch K, Ryba T, Lu J, Hiratani I, Tachibana M, et al. G9a selectively represses a class of late-replicating genes at the nuclear periphery. Proc Natl Acad Sci USA. 2009;10:19363–8. doi: 10.1073/pnas.0906142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–6. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15:551–64. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- 64.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–23. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, et al. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011;31:6692–8. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002;16:62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- 67.Yang WP, Henderson D, Hu BH, Nicotera TM. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 200(196):69–76. doi: 10.1016/j.heares.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 68.Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: a review and tutorial. J Acoust Soc Am. 1985;78:833–60. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- 69.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 70.Konishi T, Salt AN, Hamrick PE. Effects of exposure to noise on ion movement in guinea pig cochlea. Hear Res. 1979;1:325–42. doi: 10.1016/0378-5955(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 71.Hsu CJ, Shau WY, Chen YS, Liu TC, Lin-Shiau SY. Activities of Na(+),K(+)-ATPase and Ca(2+)-ATPase in cochlear lateral wall after acoustic trauma. Hear Res. 2000;142:203–11. doi: 10.1016/s0378-5955(00)00020-4. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto H, Shi X, Nuttall AL. The influence of loud sound stress on expression of osmotic stress protein 94 in the murine inner ear. Neuroscience. 2009;158:1691–8. doi: 10.1016/j.neuroscience.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med. 2005;55:12–23. [PMC free article] [PubMed] [Google Scholar]
- 74.Hong J, Kim J, Lim C, Kim K, Lee S. The effects of long-term exposure to railway and road traffic noise on subjective sleep disturbance. J Acoust Soc Am. 2010;12:2829–35. doi: 10.1121/1.3493437. [DOI] [PubMed] [Google Scholar]
- 75.Kawada T. Noise and health−sleep disturbance in adults. J Occup Health. 2011;53:413–6. doi: 10.1539/joh.11-0071-ra. [DOI] [PubMed] [Google Scholar]
- 76.Salloum RH, Yurosko C, Santiago L, Sandridge SA, Kaltenbach JA. Induction of enhanced acoustic startle response by noise exposure: dependence on exposure conditions and testing parameters and possible relevance to hyperacusis. PLoS One. 2014;9:e111747. doi: 10.1371/journal.pone.0111747. [DOI] [PMC free article] [PubMed] [Google Scholar]


