Abstract
Rationale: Nasopharyngeal administration of live virulence-attenuated Streptococcus pneumoniae strains is a potential novel preventative strategy. One target for creating reduced virulence S. pneumoniae strains is the capsule, but loss of the capsule reduces the duration of S. pneumoniae colonisation in mice which could impair protective efficacy against subsequent infection. Objectives: To assess protective efficacy of nasopharyngeal administration of unencapsulated S. pneumoniae strains in murine infection models. Methods: Strains containing cps locus deletions combined with the S. pneumoniae virulence factors psaA (reduces colonisation) or proABC (no effect on colonisation) were constructed and their virulence phenotypes and ability to prevent recolonisation or invasive infection assessed using mouse infection models. Serological responses to colonisation were compared between strains using ELISAs, immunoblots and 254 S. pneumoniae protein antigen array. Measurements and Main Results: The ∆cps/piaA and ∆cps/proABC strains were strongly attenuated in virulence in both invasive infection models and had a reduced ability to colonise the nasopharynx. ELISAs, immunoblots and protein arrays showed colonisation with either strain stimulated weaker serological responses than the wild type strain. Mice previously colonised with these strains were protected against septicaemic pneumonia but, unlike mice colonised with the wild type strain, not against S. pneumoniae recolonisation. Conclusions: Colonisation with the ∆cps/piaA and ∆cps/proABC strains prevented subsequent septicaemia, but in contrast, to published data for encapsulated double mutant strains they did not prevent recolonisation with S. pneumoniae. These data suggest targeting the cps locus is a less effective option for creating live attenuated strains that prevent S. pneumoniae infections.
Keywords: Streptococcus pneumoniae, capsule, vaccine, psaA, proABC, colonisation, immunity
1. Introduction
Streptococcus pneumoniae is the dominant bacterial pathogen causing acute lung infections in adults, responsible for up to 40 to 50% of community acquired pneumonia [1,2] and 25% of exacerbations of COPD [3]. However, existing S. pneumoniae vaccines have significant drawbacks. The pneumococcal polysaccharide vaccine (PPV) used in adult risk groups protects against invasive infection (septicaemia) but only has a limited efficacy for preventing S. pneumoniae lung infections [4]. The conjugated polysaccharide vaccine (PCV) used routinely prevents pneumonia in children and also in adults [5], as well as herd immunity due to preventing nasopharyngeal colonisation in children [6]. However, PCV vaccination is expensive and only protects against the limited number of capsular serotypes contained within the vaccine [5,7]. As a high proportion of adult disease is caused by non-vaccine serotypes [5] and the prevalence of these is increasing due to serotype replacement in response to vaccination of infants [8,9], PCV vaccination of adults is not cost-effective [10] and the benefits of herd immunity are being lost [7]. New strategies for the prevention of S. pneumoniae infections are required that possess broad serotype coverage and are able to induce pulmonary mucosal as well as systemic immunity.
A high proportion of adults have effective naturally acquired immunity against S. pneumoniae [11,12], which extensive human and mouse data suggest develops in response to natural S. pneumoniae colonisation of the nasopharynx. Colonisation induces antibody and CD4+ Th17 responses to protein antigens and antibody to capsular antigen, preventing recolonisation of the nasopharynx, pneumonia and sepsis [13,14,15,16,17,18,19,20,21,22,23,24]. These responses are likely to contribute to the decrease in rates of S. pneumoniae in older children and adults compared to infants [12]. Importantly, data obtained using experimental human colonisation with S. pneumoniae have demonstrated that adaptive immunity to S. pneumoniae is boosted by recolonisation events [24,25]. Hence, a potential strategy for preventing S. pneumoniae infections in adults would be deliberate nasopharyngeal administration of immunizing S. pneumoniae strains genetically modified to have markedly reduced virulence. However, to be successful this preventative approach requires live S. pneumoniae strains that cannot cause serious infections in susceptible subjects but are still able to colonise the nasopharynx and effectively boost adaptive immunity [13,16].
Recently, we have published pre-clinical data confirming the potential of this approach using two encapsulated mutant strains containing deletions of two loci encoding protein virulence factors (∆fhs/piaA or ∆proABC/piaA) [23]. In murine models both strains colonised the nasopharynx at a similar density at seven days as the wild type strains. Double mutant strains were used to prevent a single reversion event restoring full virulence of the strain, essential for maintaining safety for strains that may be deliberately administered to humans. Previous publications have shown prior exposure to other attenuated strains also induce protective immunity [15,26,27,28]. A major determinant of S. pneumoniae virulence is the capsule and unencapsulated mutants very rarely cause invasive disease. Hence, preventing capsule expression could be a particularly safe strategy for making virulence attenuated strains able to stimulate protective immunity. However, we have previously shown that in mice colonisation-induced immunity to S. pneumoniae is dependent on the duration of colonisation [13,14,26,29]. As unencapsulated strains have a shorter duration of colonisation of the nasopharynx in mice these data suggest loss of the capsule could have a negative effect on colonisation-induced protection against subsequent S. pneumoniae infection.
Here, we describe the design of genetically engineered S. pneumoniae strains carrying a deletion of the cps locus and genes encoding one of two other protein virulence factors, PsaA [30,31] or ProABC [23,32]. To clarify the utility of targeting the capsule locus for making live attenuated S. pneumoniae vaccine strains, the immune response to nasopharyngeal administration of these strains and their protective efficacy against pneumonia with septicaemia and colonisation were tested in mouse models of infection.
2. Methods
2.1. Bacterial Methods and Construction of the Deletion Mutant Strains
S. pneumoniae strains were cultured at 37 °C and 5% CO2 on either solid Columbia agar supplemented with 5% horse blood (SLS) or in Todd–Hewitt broth supplemented with 0.5% yeast extract (THY). Spectinomycin (150 µg mL−1) and kanamycin (250 µg mL−1) were added to blood agar plates when needed. Growth of strains was measured in THY broth by measuring OD595 at regular intervals of 0.5 h using a TECAN Spark® plate reader. Working stocks of bacterial cultures in THY (OD580 0.4–0.5) were stored at −80 °C with 10% glycerol. All mutant strains were constructed in the BHN418 capsular serotype 6B clinical S. pneumoniae isolate using overlap extension PCR as previously described [29] and the primers shown in Table S1. The constructs were transformed into S. pneumoniae by homologous recombination and allelic replacement using competence stimulating peptides (CSP-1, CSP-2) and previously described standard protocols [33].
2.2. C3b Complement Binding Assays
Complement factor C3b deposition on S. pneumoniae was assessed using an established flow cytometry assay after incubation in human serum. C3b was measured using a fluorescein isothiocyanate (FITC)-conjugated polyclonal anti-human C3 antibody (ICN-Cappel, Canada) [34]. Pooled serum from unvaccinated human volunteers stored as single-use aliquots at −70 °C was used as the source of complement.
2.3. Immunofluorescence Microscopy
The reactivity of the anti-pneumococcal antiserum (Statens Serum Institute, Denmark) against pneumococci was determined using a fluorescence-based antibody protocol [35]. Briefly, fresh bacterial cultures grown at an OD595 of 0.2–0.3 were incubated with a 1/500 dilution of the serotype 6 antiserum for 30 min and this was followed by incubation with a 1/500 dilution of an anti-rabbit Alexa Fluor 546 antibody (Abcam, UK). A 1/10,000 dilution of DAPI (Biolegend, San Diego, CA, USA) was used as counterstains in order visualize DNA. Samples were examined using a compact confocal laser scanning microscope Zeiss LSM 800.
2.4. Immunological Assays
Immunoblots of S. pneumoniae lysates and whole cell ELISAs were performed as previously described [13,36]. Briefly, immunoblotting was performed using serum from S. pneumoniae colonised mice (1:200), which was detected with a 1/10,000 dilution of goat anti-mouse IgG-HRP (Abcam, UK), following probing of 10 µL aliquots of concentrated bacterial lysates from three different S. pneumoniae strains (6B, D39 and TIGR4), prepared from normalized cultures grown to OD595 0.3 and repetitive cycles of boiling and freezing. Lysates were probed with sera obtained day 28 after two episodes of colonisation with 6B, ∆cps/psaA, ∆cps/proABC, or sham colonised in order to detect IgG responses. Whole cell ELISAs were performed as previously described [13,36] incubating plates with dilutions of mouse serum for 1 h at room temperature and using HRP-conjugated goat anti mouse IgG or goat anti-mouse IgA (Abcam, UK), incubation with 50 µL TMB chromogen solution (Thermo Fisher Scientific, Waltham, MA, USA) and measuring absorbance at 450 nm using a VersaMax microplate reader.
2.5. Protein Microarray Assays
The protein microarray contained 254 proteins selected based on conservation from an original panel of >600 S. pneumoniae strains [23,37,38]. Exons were amplified from genomic DNA (strain TIGR4) and cloned into a T7 expression vector. Proteins were expressed incubating the plasmids for 16h in an E. coli-based in vitro transcription/translation (IVTT) reaction (Thermofisher, Waltham, MA, USA). Proteins were tested for expression by Western blot using antibodies against N-terminal poly-histidine (His) and printed onto nitrocellulose coated glass AVID slides (Grace Bio-Labs, Bend, OR, USA) using an Omni Grid 100 microarray printer (Genomic Solutions, Ann Arbor, MI, USA). Arrays were probed with mouse serum samples diluted 1:25 in protein array blocking buffer (Maine Manufacturing, Sanford, ME, USA) and supplemented with E. coli lysate. Images were acquired and analysed using an ArrayCAM® Imaging System from Grace Bio-Labs.
2.6. Mouse Experimental Models
Animal procedures were approved by the local ethical review process and conducted in accordance with the UK national guidelines for animal use and care under project license (PPL70/6510). Pneumonia, sepsis and colonisation models were performed as previously described [11,13,20,22,36] using group sizes of 5+ 4–8 weeks old CD1 mice and inocula sizes for each infection model of: pneumonia, intranasal inhalation 107 CFU in 50 µL PBS; sepsis, intraperitoneal injection of 5 × 106 CFU in 100 µL PBS; colonisation, intranasal inhalation of 107 CFU in 10 µL PBS. To obtain target organ CFU mice were sacrificed after 24–28 h (pneumonia/ sepsis models) or 7 days (colonisation model) using a lethal dose of pentobarbital. For the protection studies mice were colonised on day 0 and day 14 before serum collection or challenge with wild type S. pneumoniae on day 28+.
2.7. RNA Samples and Sequencing
RNA for RNA-seq were extracted from double mutant strains cultured to an OD600 0.4–0.5 using Mirvana RNA kit (Applied biosystems, Foster City, CA, USA) with an additional physical lysis step using 0.1 mm glass beads (MP Biomedicals, Irvine, CA, USA), treated with Turbo DNase (Applied biosystems, Foster City, CA, USA) and deleted of ribosomal RNA using Ribo-Zero Magnetic Kit Bacteria (Illumina, San Diego, CA, USA) before preparation of sequencing libraries using the KAPA RNA HyperPrep kit (Roche Diagnostics, Basel, Switzerland) and 8 cycles of amplification. Libraries were multiplexed to 24 samples per run and single-end sequenced with the NextSeq 500 desktop sequencer (Illumina, San Diego, CA, USA) using a 75 cycle high-output kit. The RNA sequencing data was mapped and quantified to the S. pneumoniae transcriptome 6B BHN418 reference using the Salmon algorithm. Downstream analyses were performed within the R statistical computing framework. The data were integrated into a matrix of raw counts using the TXimport package. The data were then normalised using the DEseq2 package using the rlog method which was used for differential gene expression. This analysis used a p-value cut-off of 0.01 and a cut-off of 1.5 log-fold-change to be considered significantly differentially expressed.
2.8. Statistical Analyses
Animal data are presented as median of log10 CFU/mL (IQR) recovered from target organs after infection with wild type, ∆cps/psaA, ∆cps/proABC or PBS controls. p-Values (* < 0.05, ** < 0.01, *** < 0.001) were obtained using Kruskal–Wallis tests with Dunn’s post hoc test comparing groups to the wild type 6B strain (for virulence models), the PBS sham-colonised (for colonisation then challenge data). Quantitative results are expressed as means ± S.D or median with interquartile range for animal experiments. Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). p-Values < 0.05 (95% confidence) were considered statistically significant.
3. Results
3.1. Design and Characterisation of Unencapsulated Mutant Strains
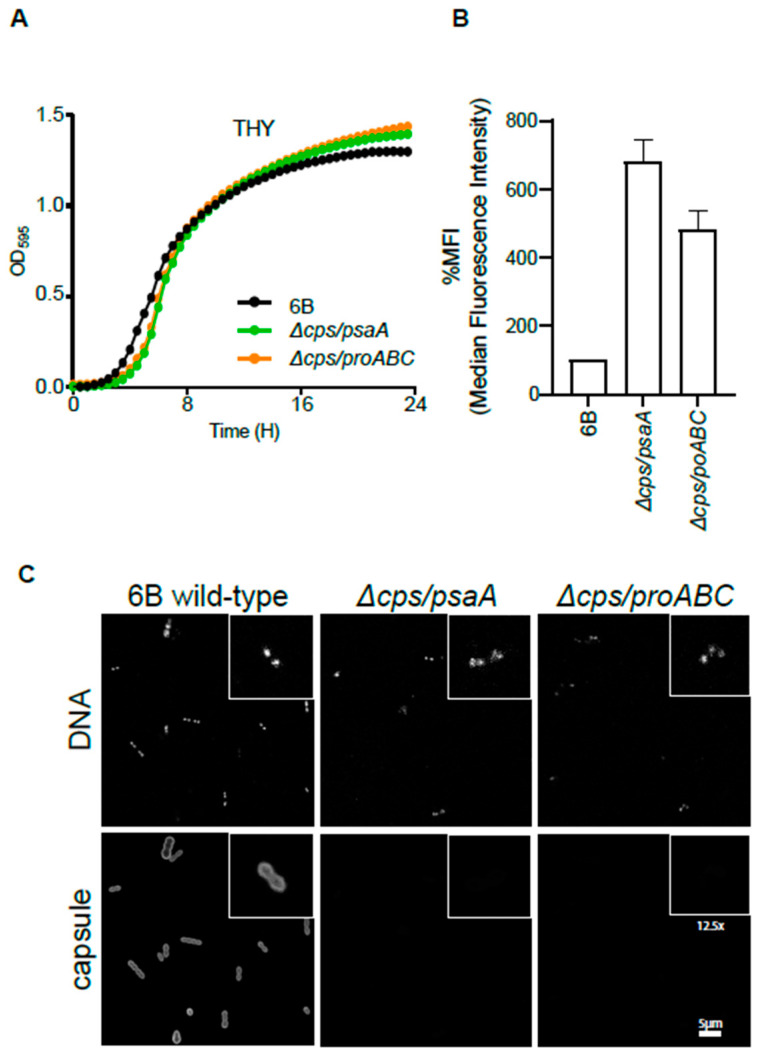
To test the potential efficacy of unencapsulated S. pneumoniae strains for preventing subsequent infections, a mutant strain containing complete deletion of the cps locus was constructed in the BHN418 capsular serotype 6B strain background. This strain was selected as it has low virulence in humans and is used in the Liverpool Experimental Human Pneumococcal Carriage model (EHPC) [24]. To minimise the chance of revertants leading to recovery of virulence when used in human studies, double mutant strains were created combining deletion of the cps locus with a deletion of either the psaA [39] or proABC [23,32] virulence genes to make the unencapsulated ∆cps/psaA and ∆cps/proABC strains. Loss of psaA is known to reduce murine nasopharyngeal colonisation, whereas loss of proABC has no effect [23,32]. Both mutants were stable after multiple rounds of growth without antibiotic selective pressure (data not shown) and showed no growth defect in complete media compared to the wild type strain (Figure 1A). As expected from previous publications, both unencapsulated strains were considerably more sensitive to complement recognition than the wild type parental strain (Figure 1B). Loss of the capsule in both strains was also confirmed by immunofluorescence (Figure 1C).
Figure 1.
Phenotypic characterization of double unencapsulated mutants ∆cps/psaA and ∆cps/proABC. (A) Growth of wild type and unencapsulated strains ∆cps/psaA and ∆cps/proABC in THY assessed by measuring OD595 for a 24 h period. (B) Effect of the S. pneumoniae capsule on C3b deposition. Median fluorescence intensity (MFI) of C3b deposition measured using flow cytometry on the wild type 6B and unencapsulated mutants when incubated in 25% human serum. Error bars represent SDs and asterisks represent the differences between wild type and the unencapsulated mutant strain. For both mutant strains, p is < 0.0001 (one way ANOVA) compared to the wild type strain. (C) Fluorescent microscopy of wild type and unencapsulated double mutant strains following incubation with 4′,6-diamidino-2-phenylindole (DAPI) (binds to DNA to identify bacterial cells, top panels) or pneumococcal antiserum labelled with Alexa fluor 546 (recognizes serotype 6 capsule, bottom panels). The scale bar (bottom right) represents 5 µm and the inserts show a 12.5 higher magnification of selected bacteria.
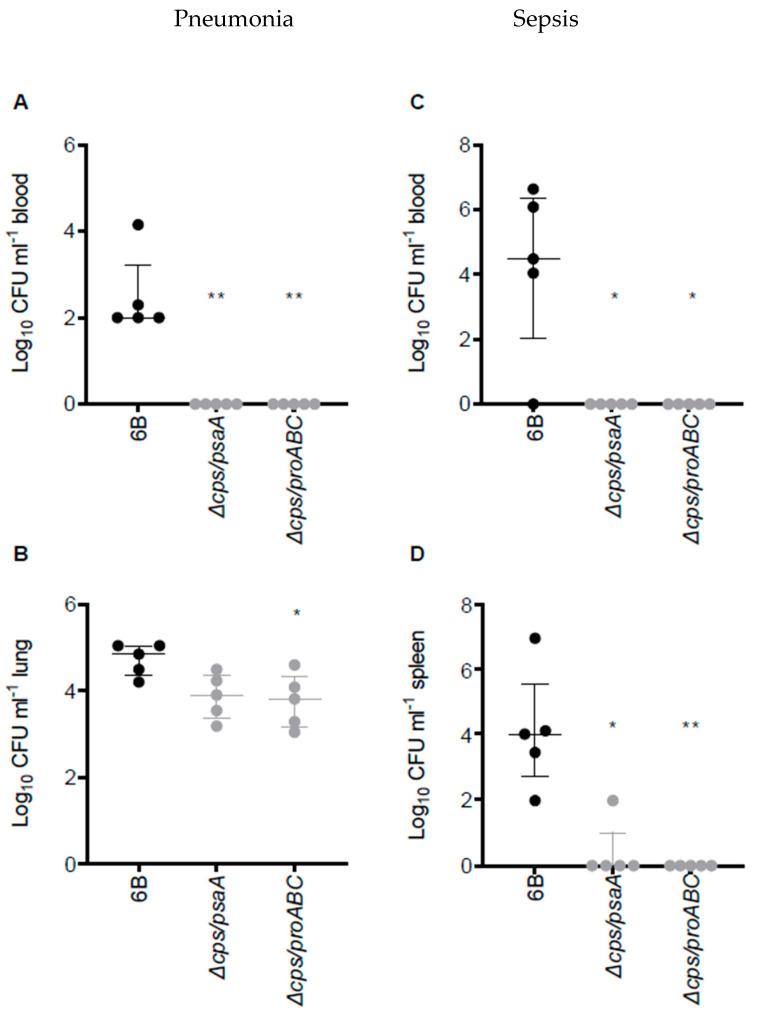
3.2. Virulence of ∆cps/psaA and ∆cps/proABC Strains
The virulence phenotype of ∆cps/psaA and ∆cps/proABC strains were assessed in an established CD1 mouse model of pneumonia and septicaemia (Figure 2). As expected, for both mutant strains almost no colony-forming units (CFU) were recovered in either model from blood or spleen, indicating that bacteria were unable to spread from lungs to blood or rapidly cleared when they reached the blood. Both the ∆cps/psaA and ∆cps/proABC mutant strains had lower lung CFU than the wild type 6B strain but this was only statistically significant for the ∆cps/proABC mutant (Figure 2B).
Figure 2.
Virulence of double unencapsulated mutant strains in murine pneumonia and sepsis models. (A,B) Pneumonia model; CFU obtained from blood (A) and lung (B) of CD1 mice 28 h post intranasal inoculation with 1 × 107 CFU of wild type 6B or double mutant S. pneumoniae strains. (C) and (D) Sepsis model; CFU in blood (C) or spleen (D) of CD1 mice 24 h post intraperitoneal inoculation with 5 × 106 CFU of wild type 6B or double mutant S. pneumoniae strains. Each symbol represents CFU data from a single mouse (black symbols, wild type S. pneumoniae infection, grey mutant S. pneumoniae strain infection), horizontal bars represent median values, error bars represent interquartile range and asterisks represent statistical significance compared to the wild type strain (Kruskal–Wallis with Dunn’s post hoc test to identify significant differences between groups, * p < 0.05; ** p < 0.01).
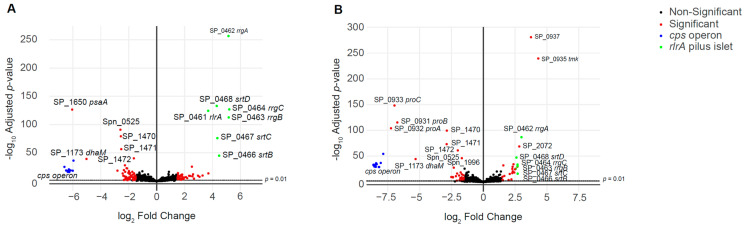
3.3. RNA-seq Analysis of the Unencapsulated Double Mutant Strains
To ensure there had been no unexpected effects of the mutations that could compromise safety if the double mutant strains were used in the EHPC model, RNA-seq was used to provide an overview of the effects of the mutations on pneumococcal gene expression. For this analysis, RNA was isolated from mid log-phase cultures in THY of the wild type, ∆cps/psaA and ∆cps/proABC strains. RNA-seq identified a relatively low number of significantly up- or down-regulation of multiple genes in the double mutant strains (Figure 3, Table S2). Using a log2 cut-off of 1.5, 46 and 28 genes were up-regulated in ∆cps/psaA and ∆cps/proABC, respectively. Several of the upregulated genes showed increased expression in both mutant strains, suggesting they could be a response to loss of the cps locus. Table 1 is showing the most important changes found. These included genes encoding an alcohol dehydrogenase XylB (Spn_00124), the adjacent transcriptional regulator and cation efflux system protein (Spn_00125, Spn_00126), MutT (Spn_00677), AliA (Spn_00914, just downstream of the capsule operon) and interestingly the rlrA islet locus which encodes expression of the pilus (Spn_01010-15; rrgA, rrgB, rrgC, srtB, srtC and srtD) (Table 1). Upregulated genes specific to the ∆cps/psaA strain included those that encoded proteins involved in type II fatty acid biosynthesis (Spn_00963, fabM, a enoyl-CoA hydratase/isomerase; Spn_00967, a trans-2-enoyl-ACP reductase II; and Spn_00968_fabD, a malonyl CoA-acyl carrier protein transacylase), along with the Blp bacteriocin loci (Spn_0599-600, blpUO; and Spn_01098-1100, blpIJK; and Spn_01108_BlpX, Spn_01111) (Table 1). For the ∆cps/proABC mutant there was increased expression of an operon predicted to encode a fructose phospostransferase transporter system (PTS) (Spn_02091_tkt, Spn_02092_alsE, Spn_02093_fruA_2, Spn_02094_manP, Spn_02095_hrsA, Spn_02096_ptsN, Spn_02097_licR_2) (Table 1). Importantly, the double mutant strains did not have increased expression of the well-described virulence factors such as Ply, PspA and PspC (Figure 3).
Figure 3.
Volcano plots showing differential expression of genes in the live attenuated strains. Log2 ratio gene expression levels for the mutant strains (A) ∆cps/psaA and (B) ∆cps/proABC compared to the wild type strain. Fold change expression is represented in the X-axis, with negative values indicating genes that are under-expressed in the mutant versus the wild type, while positive fold change values indicate genes that are over-expressed in the mutant versus the wild type. The Y-axis shows the p values (as -log10 values) for differentially expressed genes. Genes belonging to cps locus are shown in blue, while those belonging to the pilus are shown in green, other genes that are differentially expressed are represented in red and all other genes are represented in black. Differential gene expression and statistical significance were analysed using the DESeq2 method. The dashed line above the x axis marks the significance threshold of p = 0.01. A full list of the genes showing differential expression with their expression levels is shown in Table S2, with selected genes of interest shown in Table 1.
Table 1.
RNA-seq data. Selected S. pneumoniae genes and operons showing statistically significant differential expression between the double mutant strains and the wild type 6B strain when cultured to mid-log growth phase in THY broth. Gene numbers for both BHN418 and the TIGR4 strain are given, along with gene names where these have been described. Data are presented as log2 fold change (either for a single gene or mean and SD values for all genes in an operon) and include only genes with >1.5 log2 differences. Genes or operons marked in bold are deleted in the mutant strains (cps locus, Spn_00899-913; psaA, Spn_02120; proABC, Spn_01479-81).
| BHN418 Gene Number and Name or Operon Function | TIGR4 Gene Number (If Known) |
∆cps/psaA | ∆cps/proABC |
|---|---|---|---|
| Upregulated genes | |||
| Spn_00124_xylB | SP_1855 | 2.956 | 1.538 |
| Spn_00125_6 regulator/cation efflux | SP_1856-7 | 3.493 (0.293) | 2.099 (0.159 |
| Spn_00599-00 Blp bacteriocin | SP_0041- | 1.838 (0.127) | |
| Spn_00677_mutT | SP_0119 | 1.533 | 1.502 |
| Spn_00914_aliA | SP_0366 | 2.520 | 2.677 |
| Spn_00963-68 fatty acid synthesis | SP_0415-419-420 | 1.623 (0.153) | |
| Spn_01010-15_rlrA islet | SP_0462-68 | 4.766 (0.438) | 2.706 (0.157) |
| Spn_01098-00 Blp bacteriocin | SP_0531-33 | 2.168 (0.510) | |
| Spn_01108_blpX | SP_0544 | 2.561 | |
| Spn_01111 Blp bacteriocin | SP_0547 | 1.506 | |
| Spn_02091-97 fructose PTS | SP_1615-21 | 2.354 (0.083) | |
| Downregulated genes | |||
| Spn_00121-22 Galactose metabolism | SP_1852-53 | −2.058 (0.134) | −1.690 (0.006) |
| Spn_00617_bgaC | SP_0060 | −1.698 | |
| Spn_00618-21 sugar PTS | SP_0060-64 | −2.158 (0.238) | −2.273 (0.317) |
| Spn_00641-42 glycerol ABC transporter | SP_0091-92 | −2.344 (0.125) | −1.669 (0.183) |
| Spn_00899-913 cps locus | SP_0343-65 | −6.304 (0.164) | −8.385 (0.220) |
| Spn_01042-46 Endo-beta-N-acetylglucosaminidase | SP_0498 | −1.771 (0.331) | |
| Spn_01479-81_proBAC | SP_0931-33 | −6.992 (0.247) | |
| Spn_01723-26_lacGEFT | SP_1184-87 | −1.978 (0.281) | −1.831 (0.023) |
| Spn_02120_psaA | SP_1650 | −6.048 | |
The total number of downregulated genes were 64 and 46 in ∆cps/psaA and ∆cps/proABC, respectively. As expected, RNA transcripts from the cps locus (both strains), psaA (∆cps/psaA strain), or proABC operon (∆cps/proABC strain) were barely detected in RNA from the mutant strains. Other genes showing reduced expression included several genes involved in carbohydrate metabolism, including those encoding a sugar PTS (Spn_00618-621, PTS-EIIB, PTS-EIIC, manZ_2 and PTS_EII_2), GalT and GalK (Spn_00121-122) involved in galactose metabolism, the ABC-type glycerol-3-phosphate transport system (Spn_00641-642, the permease and substrate-binding protein of CUT1) [40] and the Lac operon II (Spn_01723-726, lacGEFT). Endo-beta-N-acetylglucosaminidase was significantly decreased only in ∆cps/psaA (Spn_01042-46) and bgaC in ∆cps/proABC (Spn_00617).
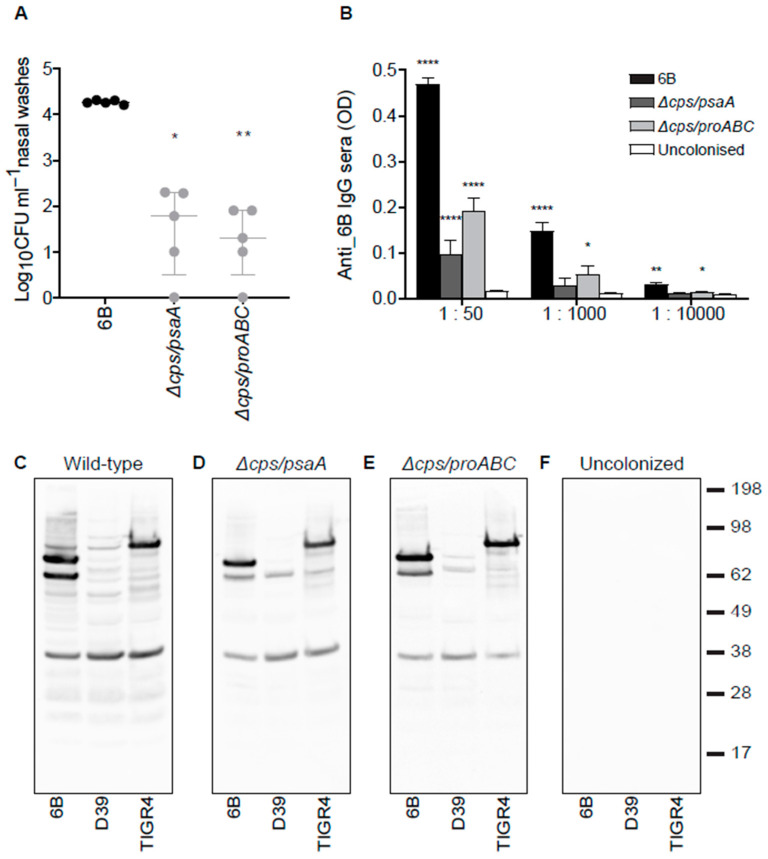
3.4. Nasopharyngeal Colonisation by Double Mutant Strains and Subsequent Serological Responses
The ability of the ∆cps/psaA and ∆cps/proABC strains to colonise the nasopharynx was assessed in an established mouse model of colonisation. Both double mutant strains had a significant defect in colonising ability, with nasal wash CFU two log10 lower than that for the wild type strain 7 days after inoculation (Figure 4A). Despite this, serum recovered 21 days after two episodes of colonisation with the double mutant strains demonstrated significant serum whole cell ELISA IgG responses to the homologous 6B strain (Figure 4B). These were markedly lower than the ELISA titres induced by colonisation with the wild type 6B S. pneumoniae strain, which potentially could reflect loss of anti-capsular IgG responses. Hence, the anti-protein antigen responses were specifically assessed using immunoblots against whole cell lysates of three different strains of S. pneumoniae. These confirmed serum IgG from colonised mice recognised multiple protein bands in S. pneumoniae 6B, TIGR4 and D39 strains lysates with similar band patterns for different serum samples and different target strains, suggesting the major protein targets were generally conserved (Figure 4C). The immunoblots demonstrated fewer protein bands were recognised by serum from mice colonised with the ∆cps/psaA and ∆cps/proABC strains compared to serum from mice colonised with the wild type strain. These data suggested there was a weaker serological response to colonisation with the ∆cps/psaA and ∆cps/proABC strains compared to colonisation with the wild type strain.
Figure 4.
Wild type 6B and the unencapsulated double mutant strains induce a systemic antibody response after nasopharynx colonisation. (A) Colonisation model; nasal wash CFU 7 days post colonisation of CD1 mice with 1 × 107 CFU of wild type 6B or the double mutant S. pneumoniae strains. (B) Whole-cell enzyme-linked immunosorbent assay (ELISA) anti-6B immunoglobulin (Ig)G responses in mouse sera 28 days post-colonisation with the corresponding strain 6B (black bars), ∆cps/psaA mutant (dark grey bars), ∆cps/proABC mutant (light grey bars) compared with uncolonised controls (white bars). N = 5 for each group and the data analysed using Kruskal-Wallis with Dunn’s post hoc test to identify significant differences between selected groups; *, p < 0.05; **, p < 0.01 ****, p < 0.0001. (C–F) IgG immunoblots for whole-cell lysates of three different S. pneumoniae strains (6B, D39 and TIGR4) probed with sera obtained 28 days after two episodes of colonisation with 6B (C), ∆csp/psaA (D), ∆cps/proABC (E) strains, or from PBS sham colonized mice (F).
3.5. Identification of Protein Antigens Recognised by Serological Responses to Colonisation Using Protein Microarrays
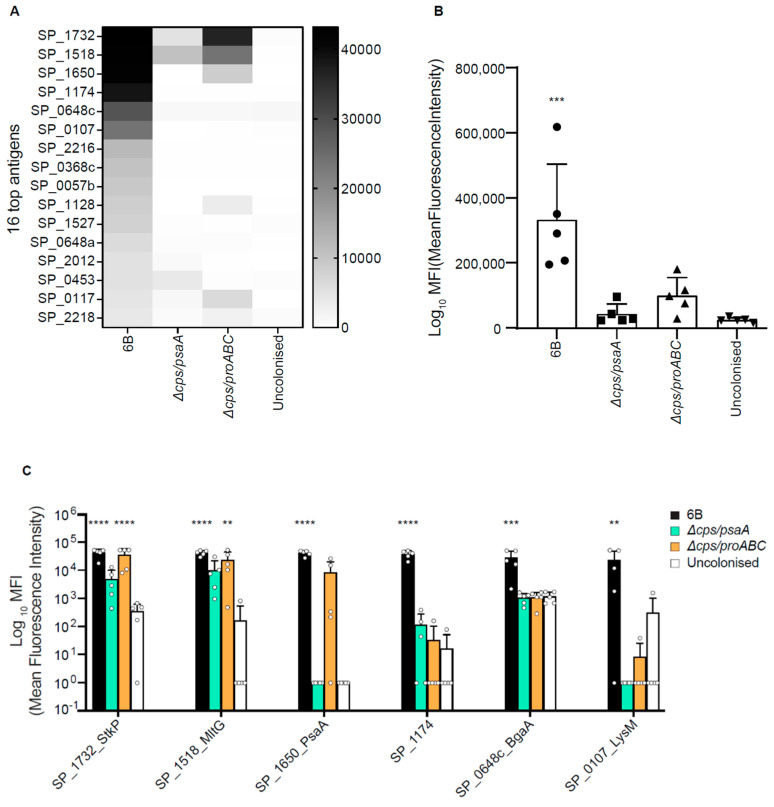
A protein microarray containing 254 major conserved S. pneumoniae proteins recognised by naturally acquired antibody found in human sera [37,41] was used to identify which protein antigens were recognised by IgG in sera from mice colonised with the wild type, ∆cps/psaA or ∆cps/proABC strains. The list of protein antigens on the array is reported in the Supplementary materials (Table S3). Comparison of the IgG-specific signal for the top 16 antigens after incubation with sera from colonized mice showed greater IgG response for the group of mice colonised with wild type 6B (Figure 5A). For the pooled data to the top 30 antigens responses for each individual mouse, only the serum from wild type colonised mice showed significantly greater IgG responses to mock colonised mice (p < 0.001) (Figure 5B). In addition, comparing the results for the most abundant protein antigens individually, IgG in sera from 6B colonized group showed statistically significant responses to the serine/threonine protease StkP (SP_1732), the transglycosidase MltG (SP_1518), PsaA (SP1650), SP_1174, BgaA (SP_0648) and the LysM domain protein (SP_0107). In contrast, sera from mice colonised with the ∆cps/proABC mutant showed significant increases in IgG just to StkP (SP_1732) and MltG (SP_1518) and sera from ∆cps/psaA colonised mice did not show any significant differences to specific antigens compared to sera from the control uncolonised group (Figure 5C). These data confirm that colonisation with the ∆cps/proABC and particularly the ∆cps/psaA strain induced weaker anti-protein antigen responses compared to colonisation with the wild type strain.
Figure 5.
Identification of the protein antigens recognized by IgG in serum from mice colonised with wild type and double mutant S. pneumoniae strains. Antigen specific IgG binding data obtained by probing a protein array containing 254 S. pneumoniae protein antigens with sera from mice colonised twice with the 6B, ∆cps/psaA, or ∆cps/proABC strains. (A) Heatmap of mean IgG binding levels to the top 16 proteins recognised by IgG in colonised mouse sera (n = 5 mice). (B) Aggregated MFI for IgG binding to the 30 antigens with the highest level of IgG binding in serum from mice colonised twice with the 6B, ∆cps/psaA, or ∆cps/proABC strains, or sham colonized with PBS. Each symbol represents data from 1 mouse, bars represent mean and error bars SD. Asterisks represent statistical significance compared to the uncolonised group (Kruskal-Wallis with Dunn’s multiple comparisons posthoc test; ***, p < 0.001). (C) Binding results in sera from mice colonised twice with the 6B strain, ∆cps/psaA, or ∆cps/proABC strains, or sham colonised to the 6 antigens with the highest level of IgG binding in serum obtained from wild type colonized mice. Each symbol represents data from 1 mouse, bars represent mean and error bars SD. Asterisks represent statistical significance compared to the uncolonised group (two-way ANOVA with Dunnett’s for multiple comparisons, **, p < 0.01; ***, p < 0.001, ****, p < 0.0001).
3.6. Double Mutant Colonisation Protects against Bacteraemia during Pneumonia Challenge
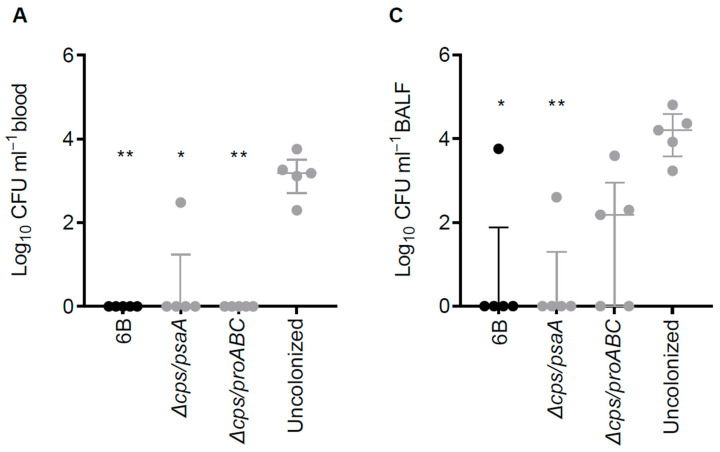
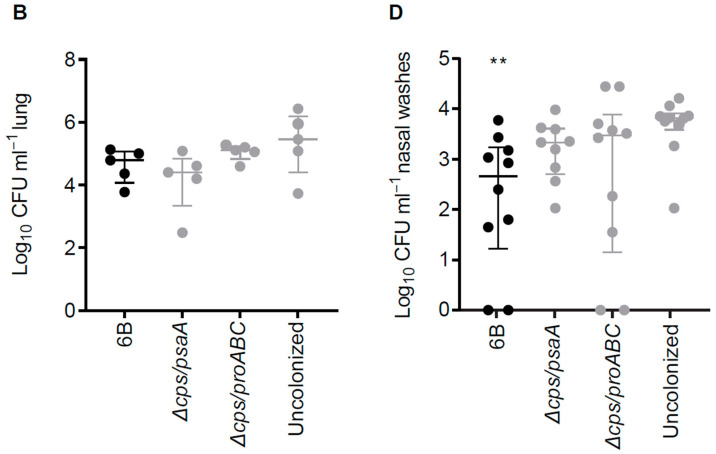
The protective efficacy of prior colonisation with the unencapsulated double mutant strains was assessed by two colonisation episodes followed by challenge with the 6B wild type strain 30 days later using pneumonia and colonisation models. Mice previously colonised twice with either wild type or the double mutant pneumococcal strains were totally protected against septicaemia (Figure 6A) after wild type S. pneumoniae challenge. In contrast, mice colonised with the wild type, ∆cps/psaA and ∆cps/proABC strains did not show a significant reduction of bacterial CFU within the lung after pneumonia challenge (Figure 6B), although the number of CFU recovered from BALF was significantly reduced after colonisation with the ∆cps/psaA mutant compared to uncolonised group (Figure 6C). Protection against nasopharyngeal colonisation challenge with the wild type 6B was assessed by using nasal wash CFU 7 days after inoculation. Prior colonisation with the wild type strains reduced nasal wash CFU by about one log10 compared to sham colonised control mice when recolonised with the 6B strain (Figure 6D). However, neither the ∆cps/psaA or ∆cps/proABC strains reduced nasal wash CFU compared to sham colonised control mice, showing that in contrast to the data for double mutant encapsulated virulence attenuated strains [23] previous colonisation with unencapsulated strains had no protective efficacy against subsequent recolonisation.
Figure 6.
Rechallenge of mice previously colonised with 6B and the double mutant attenuated strains using wild type 6B and pneumonia and colonisation models. (A–C) Target organ CFU for sham-colonised, 6B colonised, or double mutant strain colonised CD1 mice challenged 30 days after colonisation by intranasal inoculation of 1 × 107 CFU wild type 6B S. pneumoniae. (A) Blood, (B) lung homogenate and (C) BALF S. pneumoniae CFU (log10 ml−1) 24 hours following the pneumonia challenge. (D) Nasal wash CFU 7 days after intranasal recolonisation challenge of CD1 mice with 1 × 107 CFU of the S. pneumoniae 6B strain 42 days after two episodes of colonisation with the wild type 6B or double mutant strains. Each symbol represents data from a single mouse, horizontal bars represent median values, error bars represent interquartile range and asterisks represent statistical significance compared to sham colonised group (Kruskall–Wallis with Dunn’s post hoc test; *, p < 0.05; **, p < 0.01).
4. Discussion
S. pneumoniae nasopharyngeal colonisation is an immunising event and almost all adult humans have important levels of protective immunity against S. pneumoniae [42,43,44,45]. Deliberately administering immunizing S. pneumoniae to the nasopharynx could boost existing adaptive immune mechanisms that prevent nasopharyngeal colonisation (the precursor for all invasive infections), lung infection and septicaemia [20,24] as well as alveolar macrophage mediated innate immunity [46,47]. We have recently published pre-clinical data showing the feasibility of this approach using double mutant encapsulated S. pneumoniae strains [23] and have now extended these data to evaluate the protective efficacy of unencapsulated double mutant strains. The results show that both of our unencapsulated strains were highly attenuated in virulence, although unlike the double mutant strains targeting protein virulence determinants [23], they also had an impaired ability to colonise the nasopharynx. Although colonisation with either mutant stimulated an antibody response this was weaker than the response to colonisation with the wild type strain. Furthermore, although mice previously colonized with either mutant were protected against pneumonia with septicaemia, they were not protected against recolonisation with S. pneumoniae.
The S. pneumoniae capsule inhibits complement mediated opsonophagocytosis and is largely essential for systemic virulence of S. pneumoniae [34,39], so is an attractive target for making attenuated S. pneumoniae strains for use as vaccines. As any attenuated strains intended for use in humans will need to contain mutations of two separate virulence determinants to ensure safety, deletions of the cps locus were combined with deletion of either psaA, a previously described virulence gene that encodes the lipoprotein component of the dominant S. pneumoniae manganese uptake ABC transporter [48], or proABC, required for proline biosynthesis and recently shown to be important for virulence [23,49]. As the ∆proABC does not affect colonisation [23] and we have previously linked loss of the cps locus to reduced colonisation [13,14,22], the reduced nasopharyngeal colonisation with the ∆cps/proABC strain was likely to be due to loss of the capsule. RNA-seq showed limited changes in gene transcription between the ∆cps/psaA, ∆cps/proABC and wild type strains suggesting these mutations were unlikely to have caused unexpected effects that might compromise their safe use. The exception was that loss of the cps locus was associated with increase expression of the genome islet that encodes the S. pneumoniae pilus which might be predicted to increase adhesion of S. pneumoniae to epithelial surfaces [50,51]. However, the reduced levels of colonisation with the unencapsulated strains indicate the increased expression of the pilus islet had little functional significance.
Whole cell ELISA data and immunoblots against bacterial lysates suggested that colonisation with wild type S. pneumoniae induced stronger antibody responses to protein antigens than colonisation with the unencapsulated mutant strains. These data were confirmed by the results of probing the S. pneumoniae protein antigen array, which demonstrated that colonisation with the ∆cps/psaA or ∆cps/proABC strains induced statistically significant increases in IgG to none or only two protein antigens, respectively. In contrast, colonisation with the wild-type strain induced IgG to six protein antigens including well recognised protective antigens such as SktP and PsaA. Although the protein array contains only 254 of the most immunogenic protein antigens in human data [37,41] and therefore will miss responses to several other protein antigens not included on the array, these results collectively demonstrate there is a weaker adaptive immune response to the ∆cps/psaA and ∆cps/proABC strains. This could be the case because the mutation has reduced antigen expression, with the most obvious example being PsaA the gene for which is deleted in the ∆cps/psaA mutant. However, of the 6 antigens recognized after colonisation with the wild type strain, only SP_0648 and psaA showed significant reduced expression by the ∆cps/psaA mutant strain (log2 fold change, −1.694 and −6.048 respectively) and none for the ∆cps/proABC strain. The most likely reason for the overall reduced immune response to the ∆cps/psaA and ∆cps/proABC strains is their reduced density of colonisation of the nasopharynx, as our previous data have shown that a lower level of nasopharyngeal colonisation results in a weaker immune response [13,14].
Despite the reduced immune responses to nasal administration of the double unencapsulated mutant strains, this still provided strong protection against subsequent septicaemia caused by pneumonia re-challenge. Presumably, even the weaker antibody response to protein antigens induced by colonisation with the ∆cps/psaA and ∆cps/proABC strains is adequate to control septicaemia; another possibility is that T cell mediated immunity (not assessed here) was unaffected by the capsule mutation. However, the latter seems less likely as in mouse models prevention of recolonisation is thought to largely depend on T cell mediated immunity [21] and colonisation with the ∆cps/psaA and ∆cps/proABC strains did not prevent recolonisation with wild type S. pneumoniae unlike colonisation with the wild type or encapsulated mutant strains (as shown by our previous data) [23]. Overall, these results combined with our previous observations [13,22] suggest that although a lower level of colonisation with mutant S. pneumoniae strains can induce enough anti-protein antibody to prevent septicaemia, prevention of recolonisation needs a higher level of immunity induced by a more sustained initial colonisation of the nasopharynx by the attenuated strains. Whether this is due to reduced antibody responses in nasal wash or sera after colonisation with the unencapsulated strains, or potential effects on the strength of cellular responses will require further investigation. Other limitations of this study include only assessing antibody responses to 254 protein antigens which is likely to have missed important antibody responses and lack of data on CD4 cellular responses or on protection against pneumonia caused by a heterologous strain.
5. Conclusions
To summarise, we have previously shown that colonisation of the nasopharynx with mutant attenuated strains could be a cost-effective strategy to support current vaccination programmes that can overcome some of the limitations of vaccines based on capsular antigens [23]. Nasal administration of live attenuated S. pneumoniae strains could improve prevention of pneumonia in adults with considerable benefits for morbidity and mortality. However, here we demonstrate that although colonisation with unencapsulated strains can prevent septicaemia, it does not prevent subsequent recolonisation with wild type S. pneumoniae and therefore is likely to have limited effects on immunity to S. pneumoniae in the respiratory tract. These data provide further information on the parameters affecting the success or otherwise of a strategy using virulence attenuated S. pneumoniae strains to prevent future infections and suggest targeting the cps locus is a less attractive approach for this strategy.
Acknowledgments
The authors would like to thank the Pathogens Genomic Unit, an initiative established by grants from the Medical Research Council and the UCL/UCLH, for carrying the RNA sequencing 6B strain BHN418 was a gift from Prof Birgitta Henriques Normark (Karolinska Institute).
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-393X/9/3/261/s1, Table S1: Pneumococcal strain, plasmids and primers used in this study. Table S2: RNA-seq data. Table S3: Protein array whole dataset.
Author Contributions
E.R.-S. contributed to conceiving, designing, conducting and analysing experiments, design of the study and writing of the manuscript. G.E., J.A.G.-A., R.R.d.A., R.N., P.F. and K.K.A.T. contributed to conducting and analysing experiments. J.S.B., D.M.F., R.S.H. and S.B.G. contributed to conceiving and designing the study. D.G., R.S.H., P.F. contributed to designing and analysing experiments. J.S.B. contributed to designing and analysing experiments and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
E.R.-S., J.S.B., D.M.F., S.B.G. and R.S.H. were supported by MRC grant R/N02687X/1 and GE and JSB by MR/R001871/1. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme.
Institutional Review Board Statement
Animal procedures were approved by the local ethical review process and conducted in accordance with the UK national guidelines for animal use and care under project li-cense (PPL70/6510).
Data Availability Statement
Raw RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress, accessed on 14 March 2021) under accession number E-MTAB-10076.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lim W.S., Macfarlane J.T., Boswell T.C., Harrison T.G., Rose D., Leinonen M., Saikku P. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: Implications for management guidelines. Thorax. 2001;56:296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melegaro A., Edmunds W.J., Pebody R., Miller E., George R. The current burden of pneumococcal disease in England and Wales. J. Infect. 2006;52:37–48. doi: 10.1016/j.jinf.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Garcha D.S., Thurston S.J., Patel A.R.C., Mackay A.J., Goldring J.J.P., Donaldson G.C., McHugh T.D., Wedzicha J.A. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax. 2012;67:1075–1080. doi: 10.1136/thoraxjnl-2012-201924. [DOI] [PubMed] [Google Scholar]
- 4.Moberley S., Holden J., Tatham D.P., Andrews R.M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013;2013:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonten M.J., Huijts S.M., Bolkenbaas M., Webber C., Patterson S., Gault S., Van Werkhoven C.H., Van Deursen A.M., Sanders E.A., Verheij T.J., et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 6.Abdullahi O., Karani A., Tigoi C.C., Mugo D., Kungu S., Wanjiru E., Jomo J., Musyimi R., Lipsitch M., Scott J.A.G. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS ONE. 2012;7:e30787. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E., Andrews N.J., Waight P.A., Slack M.P., George R.C. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: An observational cohort study. Lancet Infect. Dis. 2011;11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 8.Balsells E., Guillot L., Nair H., Kyaw M.H. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0177113. doi: 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradshaw J.L., Pipkins H.R., Keller L.E., Pendarvis J.K., McDaniel L.S. Mucosal infections and invasive potential of nonencapsulated Streptococcus pneumoniae are enhanced by oligopeptide binding proteins AliC and AliD. mBio. 2018;9 doi: 10.1128/mBio.02097-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lochen A., Croucher N.J., Anderson R.M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 2020;10:18977. doi: 10.1038/s41598-020-75691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson R., Cohen J.M., Reglinski M., José R.J., Chan W.Y., Marshall H., De Vogel C., Gordon S., Goldblatt D., Petersen F.C., et al. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog. 2017;13:e1006137. doi: 10.1371/journal.ppat.1006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsitch M., Whitney C.G., Zell E., Kaijalainen T., Dagan R., Malley R. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med. 2005;2:e15. doi: 10.1371/journal.pmed.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J.M., Wilson R., Shah P., Baxendale H.E., Brown J.S. Lack of cross-protection against invasive pneumonia caused by heterologous strains following murine Streptococcus pneumoniae nasopharyngeal colonisation despite whole cell ELISAs showing significant cross-reactive IgG. Vaccine. 2013;31:2328–2332. doi: 10.1016/j.vaccine.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J.M., Chimalapati S., de Vogel C., van Belkum A., Baxendale H.E., Brown J.S. Contributions of capsule, lipoproteins and duration of colonisation towards the protective immunity of prior Streptococcus pneumoniae nasopharyngeal colonisation. Vaccine. 2012;30:4453–4459. doi: 10.1016/j.vaccine.2012.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche A.M., King S.J., Weiser J.N. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect. Immun. 2007;75:2469–2475. doi: 10.1128/IAI.01972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright A.K.A., Ferreira D.M., Gritzfeld J.F., Wright A.D., Armitage K., Jambo K.C., Bate E., El Batrawy S., Collins A., Gordon S.B. Human nasal challenge with Streptococcus pneumoniae is immunising in the absence of carriage. PLoS Pathog. 2012;8:e1002622. doi: 10.1371/journal.ppat.1002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright A.K.A., Bangert M., Gritzfeld J.F., Ferreira D.M., Jambo K.C., Wright A.D., Collins A.M., Gordon S.B. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog. 2013;9:e1003274. doi: 10.1371/journal.ppat.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David S.C., Laan Z., Minhas V., Chen A.Y., Davies J., Hirst T.R., McColl S.R., Alsharifi M., Paton J.C. Enhanced safety and immunogenicity of a pneumococcal surface antigen A mutant whole-cell inactivated pneumococcal vaccine. Immunol. Cell Biol. 2019;97:726–739. doi: 10.1111/imcb.12257. [DOI] [PubMed] [Google Scholar]
- 19.McCool T.L., Cate T.R., Moy G., Weiser J.N. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson R., Cohen J.M., Jose R.J., de Vogel C., Baxendale H., Brown J.S. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015;8:627–639. doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z., Clarke T.B., Weiser J.N. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Investig. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J.M., Khandavilli S., Camberlein E., Hyams C., Baxendale H.E., Brown J.S. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS ONE. 2011;6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos-Sevillano E., Ercoli G., Felgner P., De Assis R.R., Nakajima R., Goldblatt D., Heyderman R.S., Gordon S.B., Ferreira D.M., Brown J.S. Preclinical development of virulence attenuated Streptococcus pneumoniae strains able to enhance protective immunity against pneumococcal infection. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202011-4161LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira D.M., Neill D.R., Bangert M., Gritzfeld J.F., Green N., Wright A.K.A., Pennington S.H., Moreno L.B., Moreno A.T., Miyaji E.N., et al. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am. J. Respir. Crit. Care Med. 2013;187:855–864. doi: 10.1164/rccm.201212-2277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jochems S.P., De Ruiter K., Solórzano C., Voskamp A., Mitsi E., Nikolaou E., Carniel B.F., Pojar S., German E.L., Reiné J., et al. Innate and adaptive nasal mucosal immune responses following experimental human pneumococcal colonization. J. Clin. Investig. 2019;129:4523–4538. doi: 10.1172/JCI128865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chimalapati S., Cohen J., Camberlein E., Durmort C., Baxendale H., De Vogel C., Van Belkum A., Brown J.S. Infection with conditionally virulent Streptococcus pneumoniae ∆pab strains induces antibody to conserved protein antigens but does not protect against systemic infection with heterologous strains. Infect. Immun. 2011;79:4965–4976. doi: 10.1128/IAI.05923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.J., Seon S.H., Luong T.T., Ghosh P., Pyo S., Rhee D.K. Immunization with attenuated non-transformable pneumococcal pep27 and comD mutant provides serotype-independent protection against pneumococcal infection. Vaccine. 2019;37:90–98. doi: 10.1016/j.vaccine.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Rosch J.W., Iverson A.R., Humann J., Mann B., Gao G., Vogel P., Mina M., Murrah K.A., Perez A.C., Swords W.E., et al. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol. Med. 2014;6:141–154. doi: 10.1002/emmm.201202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chimalapati S., Cohen J.M., Camberlein E., Macdonald N., Durmort C., Vernet T., Hermans P.W.M., Mitchell T., Brown J.S. Effects of deletion of the Streptococcus pneumoniae lipoprotein diacylglyceryl transferase gene lgt on ABC transporter function and on growth in vivo. PLoS ONE. 2012;7:e41393. doi: 10.1371/journal.pone.0041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry A.M., Paton J.C. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 1996;64:5255–5262. doi: 10.1128/IAI.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng H.J., McEwan A.G., Paton J.C., Jennings M.P. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Opijnen T., Camilli A. A fine scale phenotype-genotype virulence map of a bacterial pathogen. Genome Res. 2012;22:2541–2551. doi: 10.1101/gr.137430.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havarstein L.S., Coomaraswamy G., Morrison D.A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyams C., Camberlein E., Cohen J.M., Bax K., Brown J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reglinski M., Ercoli G., Plumptre C., Kay E., Petersen F.C., Paton J.C., Wren B.W., Brown J.S. A recombinant conjugated pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. NPJ Vaccines. 2018;3:53. doi: 10.1038/s41541-018-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan W.-Y., Entwisle C., Ercoli G., Ramos-Sevillano E., McIlgorm A., Cecchini P., Bailey C., Lam O., Whiting G., Green N., et al. A Novel, multiple-antigen pneumococcal vaccine protects against lethal Streptococcus pneumoniae challenge. Infect. Immun. 2019;87 doi: 10.1128/IAI.00846-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croucher N.J., Campo J.J., Le T.Q., Liang X., Bentley S.D., Hanage W.P., Lipsitch M. Diverse evolutionary patterns of pneumococcal antigens identified by pangenome-wide immunological screening. Proc. Natl. Acad. Sci. USA. 2017;114:E357–E366. doi: 10.1073/pnas.1613937114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ercoli G., Ramos-Sevillano E., Nakajima R., de Assis R.R., Jasinskas A., Goldblatt D., Felgner P., Weckbecker G., Brown J. The influence of B cell depletion therapy on naturally acquired immunity to Streptococcus pneumoniae. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.611661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks L.R.K., Mias G.I. Streptococcus pneumoniae’s virulence and host immunity: Aging, diagnostics, and prevention. Front. Immunol. 2018;9:1366. doi: 10.3389/fimmu.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bidossi A., Mulas L., Decorosi F., Colomba L., Ricci S., Pozzi G., Deutscher J., Viti C., Oggioni M.R. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS ONE. 2012;7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campo J.J., Le T.Q., Pablo J.V., Hung C., Teng A.A., Tettelin H., Tate A., Hanage W.P., Alderson M.R., Liang X., et al. Panproteome-wide analysis of antibody responses to whole cell pneumococcal vaccination. Elife. 2018;7:7. doi: 10.7554/eLife.37015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldblatt D., Hussain M., Andrews N., Ashton L., Virta C., Melegaro A., Pebody R., George R., Soininen A., Edmunds J., et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: A longitudinal household study. J. Infect. Dis. 2005;192:387–393. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 43.Granat S.M., Ollgren J., Herva E., Mia Z., Auranen K., Makela P.H. Epidemiological evidence for serotype-independent acquired immunity to pneumococcal carriage. J. Infect. Dis. 2009;200:99–106. doi: 10.1086/599364. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger D.M., Dagan R., Givon-Lavi N., Regev-Yochay G., Malley R., Lipsitch M. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J. Infect. Dis. 2008;197:1511–1518. doi: 10.1086/587941. [DOI] [PubMed] [Google Scholar]
- 45.Cobey S., Lipsitch M. Niche and neutral effects of acquired immunity permit coexistence of pneumococcal serotypes. Science. 2012;335:1376–1380. doi: 10.1126/science.1215947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown J.S. Improving Pulmonary Immunity to Bacterial Pathogens through Streptococcus pneumoniae Colonization of the Nasopharynx. Am. J. Respir. Crit. Care Med. 2020;201:268–270. doi: 10.1164/rccm.201910-2047ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsi E., Carniel B., Reiné J., Rylance J., Zaidi S., Soares-Schanoski A., Connor V., Collins A.M., Schlitzer A., Nikolaou E., et al. Nasal pneumococcal density is associated with microaspiration and heightened human alveolar macrophage responsiveness to bacterial pathogens. Am. J. Respir. Crit. Care Med. 2020;201:335–347. doi: 10.1164/rccm.201903-0607OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown J.S., Gilliland S.M., Holden D.W. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 2001;40:572–585. doi: 10.1046/j.1365-2958.2001.02414.x. [DOI] [PubMed] [Google Scholar]
- 49.Belitsky B.R., Brill J., Bremer E., Sonenshein A.L. Multiple genes for the last step of proline biosynthesis in Bacillus subtilis. J. Bacteriol. 2001;183:4389–4392. doi: 10.1128/JB.183.14.4389-4392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basset A., Trzcinski K., Hermos C., O’Brien K.L., Reid R., Santosham M., McAdam A.J., Lipsitch M., Malley R. Association of the pneumococcal pilus with certain capsular serotypes but not with increased virulence. J. Clin. Microbiol. 2007;45:1684–1689. doi: 10.1128/JCM.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LeMieux J., Hava D.L., Basset A., Camilli A. RrgA and RrgB are components of a multisubunit pilus encoded by the Streptococcus pneumoniae rlrA pathogenicity islet. Infect. Immun. 2006;74:2453–2456. doi: 10.1128/IAI.74.4.2453-2456.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress, accessed on 14 March 2021) under accession number E-MTAB-10076.