Significance
Increasing crop field sizes and decreasing seminatural area is believed to lead to increased pest pressure and insecticide use due to earlier and frequent pest immigration and fewer natural enemies. However, after decades of research on how landscape simplification affects pest pressure in crops, there is conflicting evidence. We show that smaller fields surrounded by landscapes with 20 to 30% seminatural area had delayed and reduced pest immigration and spraying while producing the highest yields. These findings reveal a previously untested link sequence starting from agricultural intensification through pest immigration and dynamics, to insecticide use and yield. Moreover, fine temporal analyses provide unique understanding of how these effects change over time and potential explanation for the inconsistencies in the literature.
Keywords: landscape complexity, landscape composition, ecosystem disservices, pesticides, crop
Abstract
Agricultural systems have been continuously intensified to meet rising demand for agricultural products. However, there are increasing concerns that larger, more connected crop fields and loss of seminatural areas exacerbate pest pressure, but findings to date have been inconclusive. Even less is known about whether increased pest pressure results in measurable effects for farmers, such as increased insecticide use and decreased crop yield. Using extensive spatiotemporal data sampled every 2 to 3 d throughout five growing seasons in 373 cotton fields, we show that pests immigrated earlier and were more likely to occur in larger cotton fields embedded in landscapes with little seminatural area (<10%). Earlier pest immigration resulted in earlier spraying that was further linked to more sprays per season. Importantly, crop yield was the lowest in these intensified landscapes. Our results demonstrate that both environmental conservation and production objectives can be achieved in conventional agriculture by decreasing field sizes and maintaining seminatural vegetation in the surrounding landscapes.
There are increasing concerns that the rapid loss of seminatural areas and larger sizes and connectance of crop fields in the past several decades have made them more susceptible to pest outbreaks, thereby requiring greater insecticide uses (1–3). Meehan et al. (1) estimated that landscape simplification at the county scale was associated with increased insecticide application to 1.4 million hectares in a seven-state region of the United States and an associated increase in direct costs of between US$34 and US$103 million. Global market value of crop protection chemicals is projected to increase from US$50.62 billion in 2017 to US$68.82 billion by the end of 2025, mainly due to a growing demand for insecticides (4). This is an alarming issue, not only because of an increase in farm costs due to insecticide spraying but also because of increasing pest resistance and secondary incursion as well as increased insecticide exposure in surrounding areas negatively affecting air, soil, and water quality; biodiversity; ecosystem processes; and human health (5, 6).
There are several reasons why pest and insecticide pressure may be exacerbated in larger fields and in simple landscapes (low proportion of seminatural area). Simple landscapes can shorten the time and increase the frequency of pest immigration by increasing the amount and connectivity of crop resources to the pest, while simultaneously decreasing abundances of their natural enemies (7). Island biogeography theory (8) and the resource concentration hypothesis (9) set the framework for the mechanisms of increased pest pressure with increased field sizes, particularly if pests have large dispersal distances and high reproductive rate (10). In addition, natural enemies of crop pests may be restricted mainly to the edges of fields, thus limiting any effective biocontrol in the interior of large fields (10).
Despite this growing theoretical knowledge, it remains to be demonstrated in a real-world situation how local and landscape factors drive pest immigration and dynamics in crop fields. Management measures to reduce pest immigration might require lower investment and deliver better outcomes than managing for conservation biocontrol, but they have largely been overlooked (ref. 11, but see refs. 12 and 13). Previous studies that related landscape effects to pest pressure found inconsistent results (see syntheses in refs. 14 and 15). Moreover, the majority of these studies do not extend their findings to measures relevant to farmers, such as the consequences of pest management (insecticide spraying) and crop yield (16, 17). A handful of studies that measured landscape-dependent insecticide spraying did this at too coarse a scale (county rather than field data) to be directly applicable for farmers and decision making (1, 2, 18, 19). This is a major obstacle in the adoption of management recommendations, and therefore, it is essential to focus research on variables of interest and at relevant spatial and temporal scales in order to bridge the gap between field experiments, policy, and on the ground actions (16).
Here, we take advantage of a unique field-level crop, pest, insecticide, and yield dataset from 626 cotton field-season combinations on the Darling Downs, Queensland, Australia, studied from 2010 to 2015 (373 fields, some of which were sampled over several seasons, SI Appendix, Fig. S1). The data have high temporal resolution of the main cotton pest (mirids, Hemiptera: Heteroptera) sampling at two to three times per week throughout each crop season, allowing us to estimate the time of pest immigration and their dynamics in the field. The field-level observations allowed for the investigation of a link between agricultural intensification and insecticide use, while accounting for pest populations and estimating overall implications for yield. Furthermore, using data sampled over multiple seasons is uncommon (17), increases confidence in the results, and is important given inconsistencies in the previous findings over time (18).
Finally, the data were collected by an agronomic consultancy company, with no economic ties to insecticide sales (see Materials and Methods). This company is contracted by farmers to conduct surveys of pests and to provide recommendations on insecticide spraying based on industry standard economic thresholds [a pest population density at which control is recommended (20)]. These field-level decisions made according to the advice provided by the same company in our study minimized any possible effect of exogenous confounding variables which could have influenced insecticide use, such as the farmer’s knowledge, skills, income, perception of pest risk, and the possibility of more frequent insecticide applications at larger farms due to economies of scales (21).
Results and Discussion
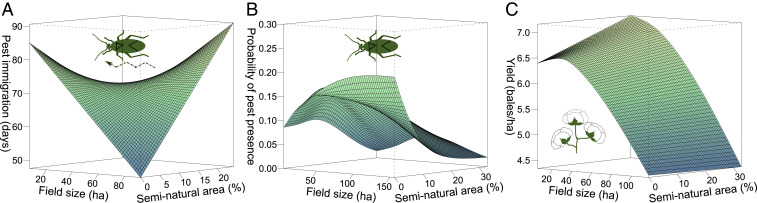
Our study provides empirical evidence for the commonly assumed, but rarely tested, hypothesis of delayed pest immigration into crop fields in less intensified landscapes. More specifically, we show that large fields embedded in simple landscapes are associated with the earliest mirid pest immigration (calculated as a number of days from crop planting to the time when first mirid is detected in the field; effective degrees of freedom [edf] = 3.00, F = 8.99, P < 0.001, R2 [adjusted] = 0.22, n = 218, Fig. 1A, see SI Appendix, Fig. S2 for the plot with SEs) and highest probability of pest presence throughout the season in unsprayed fields (edf = 10.36, F = 7.22, P < 0.001, R2 [adjusted] = 0.18, n = 20289, Fig. 1B and SI Appendix, Fig. S2).
Fig. 1.
Large fields embedded in simple landscapes have the earliest pest immigration, highest probability of pest presence, and lowest yield. Model predictions for the (A) pest immigration (time elapsed from crop planting to the first pest recorded), (B) probability of pest presence in unsprayed fields, and (C) yield in relationship to field sizes and proportion of seminatural areas. See SI Appendix, Fig. S2 for the plots with SEs.
First recorded presence of mirids in fields (Fig. 1A) is almost certainly due to immigration, instead of in situ reproduction or predation, because they don’t overwinter in cotton fields and because mirids were, in our study, detected before their predators in all but 1.9% of cases. Probability of pest presence later in the season (Fig. 1B) can partly be a result of predation, although mirids do not have any known natural enemies that can regulate their populations in cotton (20), and predators are likely to be negatively affected by a common use of broad-spectrum insecticides (19, 20). Furthermore, the mean proportion of nymphs over season was 0.83, indicating high postimmigration reproduction in cotton, but it did not change with agricultural intensification (field size, landscape complexity, landscape shape index [LSI], and connectivity) or time elapsed since the last spray.
Seminatural areas can reduce or delay pest immigration into cotton if pest reproduction is lower or mortality higher in these areas, or it can act as a temporary barrier to their dispersal from winter crops (19, 22). Indeed, native plants are found to have higher predator densities than crops in the same study region, while immature densities of mirids on native plants were only 5% of those in crops, indicating low reproduction on native plants (22). Furthermore, mirids feed on various crops, but the summer host crops other than cotton were rare in our study region (<1% cover for each crop). Thus, large, irrigated cotton fields were the main and easily encountered resource in summer. The importance of concentration of cotton crop resource is further confirmed by our finding that higher cotton edge density and disaggregation measured using LSI is associated with lower probability of pest presence (LSI: edf = 4.85, F = 0.03, P < 0.001, R2 (adjusted) = 0.23 SI Appendix, Fig. S4A), while the evidence for the effect of connectance of cotton crops on pest presence was weaker (edf = 1.89, F = 2.43, P = 0.06; SI Appendix, Fig. S4B).
We highlight two potential reasons for inconsistencies in the literature that reported both positive and negative or no effects of landscape simplification on pests (14, 15). This is in addition to the fact that numerous distantly related pest species are studied worldwide likely causing large variation in the findings. First, the effects of landscape simplification and field sizes on pests were curvilinear and interactive in our study, with stronger landscape effects in larger fields (Fig. 1 A and B, see also ref. 23). Similarly, pests immigrated faster, and the probability of pest presence increased quickly with field sizes when embedded in simple landscapes but remained low in complex landscapes (20 to 30% of seminatural area), virtually independent of field sizes. This means that measuring only one aspect of agricultural intensification might show strong or nonexistent effects depending on the value of another interacting variable. Moreover, this can have large implications for farmers’ decision making because it shows that the negative effects of increased field sizes (in terms of pest pressure) can be buffered by increasing landscape complexity and vice versa. Second, our study is in line with previously untested claims that the inconsistencies in the literature could potentially be related to the common low number of snapshot estimates or seasonal averages that may miss key temporal dynamics of the pests (15, 16). We show that the strength of the landscape and field size effects on pests varied drastically over season (SI Appendix, Fig. S5). For example, only approximately 3 mo after beginning of sampling did field sizes and landscapes show a strong effect on the probability of pest presence, while earlier in the season the effect was weaker.
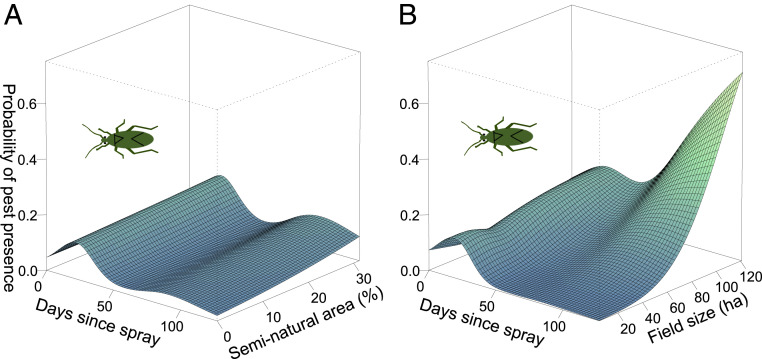
Next, we tested whether pest dynamics after insecticide spraying was dependent on field sizes and landscape complexity. We show that the probability of pest presence remained low after insecticide spraying only in smaller fields (edf = 10.60, F = 14.77, P < 0.001, Fig. 2B and SI Appendix, Fig. S3). Specifically, the probability of pest presence declined and was the lowest at ∼50 d after spraying, when it started to increase again only in larger fields (>40 ha). Although the interaction between landscape complexity and time since spraying was significant (but not in the reduced data set; see SI Appendix, Table S1), the probability of pest presence after insecticide spraying remained low (below 0.2) in all landscapes (edf = 3.01, F = 5.80, P < 0.001, R2 [adjusted] = 0.18, n = 15190, Fig. 2A and SI Appendix, Fig. S3). Thus, although our data from unsprayed fields indicate that both landscape complexity and field size affect pest immigration and probability of presence in crop fields, spraying seemed to have disturbed this process and only field sizes continued to have substantial effect on pests after spraying.
Fig. 2.
Landscape has little effect in sprayed fields, but pests stay lower for longer after spraying in smaller fields. Model predictions for the probability of pest presence over time after insecticide spraying in relationship to (A) proportion of seminatural area and (B) field sizes. See SI Appendix, Fig. S3 for the plots with SEs.
Spraying has been shown to diminish positive effects of landscape complexity on conservation biocontrol of other cotton pests (24), but this is unlikely to have had a strong effect in our study given the low estimated impact of natural enemies on mirids in cotton (19, 20). However, other landscape metrics, high aggregation, and low edge density of cotton fields (low LSI) were associated with the highest probability of pest presence after spraying (edf = 13.60, F = 8.14, P < 0.001, R2 [adjusted] = 0.22, n = 4587, SI Appendix, Fig. S4C), while connectance of cotton fields had no effect (SI Appendix, Fig. S4D). Thus, the size and spatial arrangement of cotton fields, rather than seminatural areas, appear to drive pest dynamics after spraying. Pests in sprayed fields could have been additionally influenced by the type of insecticide used (25), but we found no effect of our explanatory variables (field size and landscape complexity) on the impact rating of insecticides used in this study. The most common insecticides were 66.95% Dimethoate and 15.57% Fipronil, both highly effective against mirids (20, 25).
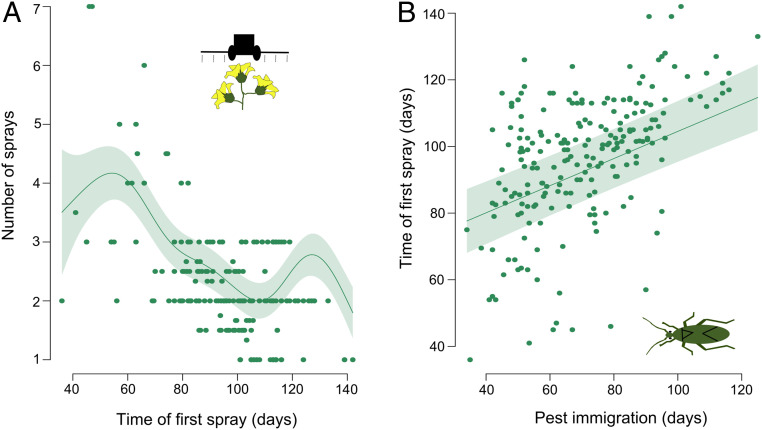
Since crop monitoring reports were communicated to growers, we tested whether farmers followed the advice provided by consultants. Indeed, we show that the time when mirid pests immigrated to the fields was strongly, linearly related to the time when the first insecticide sprays were applied (edf = 1, F = 80.40, P < 0.001, R2 [adjusted] = 0.64, n = 218, Fig. 3B). Moreover, we show that delayed spraying is negatively linked to the overall number of sprays in the season (edf = 6.94, F = 20.74, P < 0.001, R2 [adjusted] = 0.61, n = 218, Fig. 3A). Since estimated pest pressure and spraying decisions are closely linked in our study, these findings demonstrate the importance of delayed pest immigration for reducing overall insecticide use. Reducing insecticide use in cotton is important given that it is a heavily sprayed crop, accounting for over 18% of all insecticide use globally (26)
Fig. 3.
Early pest immigration results in early spraying that are further linked to more sprays per season. Model predictions showing (A) the relationship between the overall number of sprays in the season and the time when farmers start spraying and (B) the relationship between the time when first mirid pests immigrate and the time when farmers start spraying. Pest immigration is calculated as a number of days from crop planting to the time when first mirid is detected in the field.
Similarly, several studies using the same US county–level source (US Department of Agriculture) recently showed that insecticide use increased with average farm size (27) and with landscape simplification (1, 27, 28) but with inconsistency over years (18, 29). Larsen et al. (3) also showed increased insecticide use with increased field sizes. Demonstrated effects were presumably due to reduced costs of the insecticide purchased and its application in larger farms and for larger pest pressure, but these were typically not directly measured (but see refs. 1 and 18 where county-level pest abundances were measured). In contrast, Wu et al. (30) found that reduced insecticide use is associated with increased farm sizes in China, but, unlike in our study, their farmers operating larger enterprise farms were more knowledgeable and more skillful than those with smaller holdings.
The benefit of reduced pests and spraying would unlikely be a sufficient argument for reducing agricultural intensification if crop yields were also lower in less intensified fields. We found a positive effect of reducing agricultural intensification (reduced field sizes and increased landscape complexity) on yield, despite frequent spraying that is supposed to counteract the negative effects of pests. Specifically, the largest crop yields were achieved in smaller fields embedded in complex landscapes (edf = 3.54, F = 3.13, P = 0.03, R2 [adjusted] = 0.70, n = 154, Fig. 1C) after controlling for a strong linear effect of the number of irrigations (edf = 1, F = 141.33, P < 0.001) and curvilinear effect of nitrogen fertilization (edf = 6.80, F = 7.74, P < 0.001). Thus, smaller fields embedded in complex landscapes promoted not only delayed pest immigration and lower probability of pest presence but also highest yields. Similar to the effect on pest dynamics after spraying, the effect of field sizes on yield was much stronger than landscape effect (a maximum of less than one bale/ha increase with landscape complexity in small fields), while connectance of cotton fields had no effect on pests or yield. However, given the correlation between our measured field sizes and average field size in the surrounding farm as well as LSI (see Materials and Methods), our results partly reflect interplay between landscape configuration and composition, supporting previous findings (23, 31).
Although mirid pests might have caused some crop damage in intensified fields before they were sprayed, the relationship between agricultural intensification and yield could have additionally been influenced by the local and landscape effects on pollinators or secondary pests such as aphids and whiteflies (32, 33). These are less-mobile pests in comparison to mirids and likely suffer higher predation pressure in cotton. Cotton yield losses due to insect pests, weeds, and diseases can be around 30%, despite widespread chemical control (26). Thus, additional mechanisms could have affected yield as also suggested by our finding that, although the probability of mirid pest presence increased with aggregation of cotton fields, this effect did not translate to changes in yield.
Evidence for a relationship between agricultural intensification and crop yield has been inconsistent in the literature (15, 17), possibly because of numerous factors and different pest species influencing yield and a number of confounding effects (16). For example, Wu et al. (30) found that an increase in field sizes is associated with insignificant changes in crop yields, but this study did not control for any possible landscape effects. In the largest synthesis studies to date across various cropping systems, Karp et al. (15) and Martin et al. (31) found inconsistent, nonlinear or no landscape effects on yield, while Dainese et al. (32) showed that landscape simplification has indirect, negative effect on crop yield worldwide, mediated through biocontrol, but only in unsprayed fields. Our study shows consistent, cascading effects of agricultural intensification on pests, insecticide use, and yield. However, unlike above-mentioned syntheses, we investigated only one crop system and pest species, and caution should be taken when applying these findings for the management of pests that are poor dispersers or have low reproductive rate, as they may respond differently to these land-use metrics (10).
Our study provides an example of how to minimize insecticide spraying and its cost to farmers (direct costs of application, future costs of pesticide resistance, and secondary pest incursion due to reduced natural enemies), the environment (effect on biodiversity and ecosystem processes), and society (worker and food safety), while maximizing yield and profit. This is by reducing field sizes and increasing seminatural area in the surrounding landscapes. However, if new seminatural vegetation is to be established at the cost of cropland, the benefits should be calculated together with the cost of taking land out of production, and reducing field sizes should be considered as the first step. Importantly, in our study, these benefits did not come at the cost of establishment and opportunity for the existing farms, as no new landscape elements were established and existing seminatural areas were mostly located along field margins, rivers, or roadsides, which are largely inaccessible for agriculture. Thus, this study demonstrates the benefits that ecological intensification (sensu 17) can have even on high-input farming systems.
Overall, our findings provide support for the agroecological hypothesis that pests immigrate later in the season and stay at lower densities for longer (below the economic threshold for spraying), thereby reducing insecticide spraying in less-intensified landscapes (smaller fields and high proportion of seminatural area) while maximizing crop yield. This means that managing directly for reducing pest immigration through decreasing agricultural intensification may provide desirable economic and environmental outcomes, especially when biocontrol does not seem effective (11).
Materials and Methods
Study Sites.
Study sites were located in the Darling Downs region of the Southern Brigalow Belt, Queensland, Australia (SI Appendix, Fig. S1). The climate is subtropical with hot, dry summers and cold winters (climate zones map based on temperature and humidity, Australian Government, Bureau of Meteorology). The dominant crops in this area are cotton (Gossypium hirsutum L.), sorghum (Sorghum bicolor L. Moench), wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), and chickpea (Cicer arietinum L.). Seminatural areas consist of grasslands and remnant woody native vegetation, mainly as linear strips along roads and creeks. The woody remnant area is dominated by Eucalyptus sp. (tree layer) and Acacia sp. [shrub layer, (22)]. Cotton is mostly flood irrigated (one to five irrigation events per field), planted in September/October, and harvested after approximately 6 mo. Cotton planted in the area during the study period was transgenic Bt cotton [Bollgard II and Bollgard (3)], expressing insecticidal proteins which selectively control larvae of Helicoverpa sp. and other lepidoptera. Bt cotton does not affect other nonlepidopteran pests and their natural enemies (34).
Study System.
The wide-scale adoption of Bt cotton led to a reduction of insecticide use in Australia and worldwide because of no longer needing to spray to control Helicoverpa sp (35). However, pests that were previously controlled by sprays for Helicoverpa, have now become the primary pest (36) leading to a more recent rise in insecticide use (37). Following the introduction of Bt cotton in Australia, the main pests became mirids [Hemiptera: Heteroptera, mainly Creontiades dilutus (Stål) and occasionally Creontiades pacificus (Stål) and most insecticides are now applied to suppress this pest (25)]. Mirids attack growing tips of the cotton plants, flower buds, and the young, small bolls, causing shedding and deformation of these structures and reducing overall lint yield (20). Abundances of mirids in cotton are often the highest during the first 5 wk from the beginning of flowering (at about 800 degree days), and most control occurs during this time (25). Mirids can be found in other crops, such as soybeans, mung beans, pigeon pea, safflower, sunflowers, as well as on some weeds, and native plants (22, 20). However, Bianchi et al. (22) showed that mirids are more common in crops than on Australian native plants. Furthermore, our study region had less than 1% on average of each of other summer host crops during the study period. Green mirids develop from an egg through five nymphal stages to an adult in approximately 3 wk in the southeastern Queensland summer conditions (reviewed by ref. 38). Adults live for 3 to 4 wk, and females lay up to 80 eggs within plant tissue.
Data Collection.
Insect pest population information was sourced from crop monitoring reports (Meteora Agronomic Consulting, declared no conflict of interest: “We are an independent consultancy company with no economic benefits from insecticide chemical sales or any other means of insect control”). Pest sampling occurred across 373 cotton fields (SI Appendix, Fig. S1) during five cotton growing seasons (2010/2011 to 2014/2015). Some fields were sampled more than once, resulting in 626 field-season combinations. Mirid pests were sampled at a minimum of three sampling points and at least 5 m apart in a zig-zag pattern. The sampling area was at a minimum of 50 m from the field margin. Mirids were sampled using a “beat-sheet” method (25): Using a 1 m stick, plants were vigorously shaken above the sheet laid out on the ground and the resulting pests and their natural enemies (damsel bugs, big-eyed bugs, predatory shield bugs, and spiders) falling onto a sheet were counted. Samples were taken in this way from planting until harvest approximately two to three times per week. The sampling intensity did not differ with field sizes. Sampling was conducted by a private company, which had been employed by farmers to conduct arthropod sampling and, in addition, to provide recommendations on management actions, such as insecticide spraying, based on established economic thresholds for spraying. The company also collected data on the yield and crop management practices, including insecticide spraying reported by farmers. The study fields were geolocated and digitized using ArcGIS Desktop 10.5 (39). The proportion of seminatural habitats surrounding each field was measured in circles of 1,000 m diameter. A remotely sensed map of seminatural habitats was provided by the Queensland Department of Environment and Science (Queensland Land Use Mapping Program, March 2018) and corrected by ground-truthing. The proportion of seminatural area ranged from 0% (structurally simple landscapes) to 32.8% (structurally complex landscapes). Field sizes varied from 5.15 to 156.6 ha (median = 39.4 ha). The tested proportion of seminatural area was not strongly related to the field sizes (SI Appendix, Fig. S6). Relationship between field sizes and average field sizes in the surrounding farm was positive (Spearman's rank correlation = 0.61, p < 0001), indicating that our field size effect also partly reflects configurational heterogeneity of the surrounding landscape.
Statistical Analysis.
We used generalized additive mixed-effects models (GAMM) to assess any curvilinear and interactive effects of seminatural area (indicator of landscape complexity) and field size on the following: 1) mirid presence in unsprayed fields, 2) time from planting to when mirids start immigrating into cotton fields, and 3) yield. Additionally, we estimated the probability of mirid presence in the field over time after spraying by fitting curvilinear and interactive effects of time elapsed since the last spray with a percentage of the seminatural area and with field size. We analyzed pest presence, rather than abundance, because of the very low spraying thresholds (1.5 to 2 mirids per beat-sheet sample) and thus very low counts in our data, in which the majority of data consists of zeros and ones. Both adults and nymphs were counted, but for the purpose of the analysis of pest presence, they were merged because both adults and nymphs are considered when deciding to spray (25).
In the second step, to investigate whether local population processes (reproduction) affected our results, we analyzed whether the proportion of mirid nymphs changes in response to the above-described variables and using only a subset of the data when pests were present. To aid interpretation of landscape mechanisms linked to the spatial arrangement of the most common mirid host crops (cotton fields), we additionally calculated LSI and connectance of cotton fields using FRAGSTATS (version 4.2). The metrics were chosen based on their interpretation and low correlation with measured landscape complexity. Proportion of seminatural area was weakly correlated with LSI (Spearman’s rank correlation rho = −0.008) or connectance (Spearman’s rank correlation rho = 0.029). Connectance was not correlated with focal field sizes (Spearman’s rank correlation rho = 0.03), while LSI and field sizes were negatively correlated (Spearman’s rank correlation rho = −0.44), and this is why they were not used in the same model. We tested the curvilinear effects of LSI and connectance on pest presence, proportion of nymphs, and yield in the same models as described above but using a subset of 200 field season combinations for which we had appropriate data. LSI is a standardized measure of edge density adjusted for landscape size, but it can also be interpreted as a measure of patch disaggregation, so that as LSI increases, the patches become increasingly disaggregated (LSI ≥ 1). Connectance represents a percentage of the maximum possible connectance given to the number of patches and ranges between 0 and 100.
We verified that the time when farmers start spraying (as the response variable) is related to the time of mirid pest colonization (as an explanatory variable) and that the total number of sprays (as the response variable) is related to the time when farmers start spraying (as an explanatory variable) using generalized additive models. Seven data points with extreme values for times when farmers started spraying were excluded in the models for pest immigration, time of first spray, and number of sprays because they are likely errors in the data, but results from the analysis with the full dataset were similar (SI Appendix, Table S1). Additionally, since 15% of spraying recommendations referred to pests other than mirids, we tested all models after excluding these fields, but our conclusions remained unchanged. To test whether the use of insecticides with high versus moderate impact rating on beneficial arthropods (20) changes with landscape complexity, field sizes, or their interaction, we used logistic regression (GAMM) with Farm ID in the random structure.
We used the Bernoulli distribution with probit link function in the models for mirid presence. We chose the probit link function because these models had better convergence and lower Akaike information criterion than inferentially comparable models using logit or cloglog link functions. We used Beta distribution in the models for the proportion of nymphs and the Gaussian distribution for response variables measuring time of immigration and spraying, number of sprays, and yield. We used Gaussian distribution because these variables were averaged across years, as some but not all fields were sampled in more than one season. These models provided similar results to the models using Gamma distribution when used to predict on the response scale but are more robust and had better convergence.
To account for multiple sampling during and across the seasons in the models for mirid presence and proportion of nymphs in the fields, we used a standard moving average correlation structure of order (0, 2) and with Field ID nested in the season as grouping variables. Additionally, we estimated changes in the probability of mirid presence over the season by using a smoother (with cubic regression spline) for a continuous variable recording “Day in a season” (SI Appendix, Fig. S7). For specifying interactions, we used a tensor product smooth with cubic regression spline bases. Since cotton yield in Australia largely depends on availability of water and nutrients, we included the number of irrigation events and fertilizer amount as covariates in the model for yield.
We checked visually for any appreciable spatial or spatiotemporal residual autocorrelation using semivariogram and autocorrelation function graphs. If present, autocorrelation could indicate remaining spatially or temporally structured processes not captured by the variables in a model. Models for the number of insecticide sprays, pest immigration, yield, and time when insecticides were first sprayed showed evidence of some spatial autocorrelation and in some cases nonstationarity. In these models, we included a Gaussian process smooth with spherical correlation structure, in which the autocorrelation function is constrained to decline to zero at some point (40) (SI Appendix, Fig. S8). We found the best range parameter for Gaussian smoother based on the minimum of restricted maximum likelihood score (40) (SI Appendix, Fig. S9). We found no appreciable remaining autocorrelation and the assumption of stationarity could be retained in all cases (SI Appendix, Fig. S10). Additionally, to compare our results with more traditional ways of field selection, we selected only those fields that had no neighboring fields sampled within 1,000 m radius, resulting in 368 field-season combinations. We reran all models with this subset of the data and found similar results (note that interactive effects of time since sprayed and seminatural area on the probability of pest presence is nonsignificant in the reduced model, but the interpretation is similar given very weak effect in the original model, SI Appendix, Table S1). In the models using the Gaussian distribution, we used the exponential or identity variance function to enhance the model fit (homoscedasticity) when necessary. All explanatory variables were tested for curvilinear effects. All analyses were conducted in R (41) using packages mgcv (40) and gstat (42).
Supplementary Material
Acknowledgments
We thank Catherine Liddington and Matt Elmer for help in data management. Funding was provided by the Australian Cotton Research and Development Corporation for the project: “Keeping pest populations lower for longer: Connecting farms and natural systems.”
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. T.H.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018100118/-/DCSupplemental.
Data Availability
The anonymized data and R code data have been deposited in Figshare (https://figshare.com/articles/dataset/AI_pests_insecticides_yield_csv/12062370, https://figshare.com/articles/journal_contribution/R_code_for_all_models/12072105, and https://figshare.com/articles/journal_contribution/R_code_for_all_Figures/12072108). All other study data are included in the article and/or SI Appendix.
References
- 1.Meehan T. D., Werling B. P., Landis D. A., Gratton C., Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc. Natl. Acad. Sci. U.S.A. 108, 11500–11505 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W.-R., Grieneisen M., Chen H., Zhang M., Reduction of crop diversity does not drive insecticide use. J. Agric. Sci. 7, 1–16 (2015). [Google Scholar]
- 3.Larsen A. E., Noack F., Identifying the landscape drivers of agricultural insecticide use leveraging evidence from 100,000 fields. Proc. Natl. Acad. Sci. U.S.A. 114, 5473–5478 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortune Business Insights , “Crop protection chemicals market size, share and industry analysis by product type (herbicides, insecticides, fungicides), origin (synthetic chemicals, bio-based) and regional forecast 2018-2025 (Fortune Business Insights)” Fortune Business Insights (2020) https://www.fortunebusinessinsights.com/industry-reports/crop-protection-chemicals-market-100080. Accessed 18 March 2020.
- 5.Sexton S. E., Lei Z., Zilberman D., The economics of pesticides and pest control. Int. Rev. Environ. Resour. Econ. 1, 271–326 (2007). [Google Scholar]
- 6.Matthews G. A., Ed., “Spray drift, bystander, resident and worker exposure” in Pesticides, Health, Safety and the Environment, (Blackwell Publishing, Oxford, 2006), pp. 129–158. [Google Scholar]
- 7.Tscharntke T., et al., Landscape moderation of biodiversity patterns and processes–Eight hypotheses. Biol. Rev. Camb. Philos. Soc. 87, 661–685 (2012). [DOI] [PubMed] [Google Scholar]
- 8.MacArthur R. H., Wilson O., The Theory of Island Biogeography (Princeton University Press, Princeton, 1967). [Google Scholar]
- 9.Root R. B., Organization of a plant-arthropod association in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecol. Monogr. 43, 95–124 (1973). [Google Scholar]
- 10.Segoli M., Rosenheim J. A., Should increasing the field size of monocultural crops be expected to exacerbate pest damage? Agric. Ecosyst. Environ. 150, 38–44 (2012). [Google Scholar]
- 11.Shackleton C. M., et al., Unpacking Pandora’s box: Understanding and categorising ecosystem disservices for environmental management and human wellbeing. Ecosystems (N. Y.) 19, 587–600 (2016). [Google Scholar]
- 12.Macfadyen S., et al., Early-season movement dynamics of phytophagous pest and natural enemies across a native vegetation-crop ecotone. Agric. Ecosyst. Environ. 200, 110–118 (2015). [Google Scholar]
- 13.Avelino J., Romero-Gurdián A., Cruz-Cuellar H. F., Declerck F. A. J., Landscape context and scale differentially impact coffee leaf rust, coffee berry borer, and coffee root-knot nematodes. Ecol. Appl. 22, 584–596 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Veres A., Petit S., Conord C., Lavigne C., Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 166, 110–117 (2013). [Google Scholar]
- 15.Karp D. S., et al., Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. U.S.A. 115, E7863–E7870 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schellhorn N. A., Parry H. R., Macfadyen S., Wang Y., Zalucki M. P., Connecting scales: Achieving in-field pest control from areawide and landscape ecology studies. Insect Sci. 22, 35–51 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Kleijn D., et al., Ecological intensification: Bridging the gap between science and practice. Trends Ecol. Evol. 34, 154–166 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Larsen A. E., Agricultural landscape simplification does not consistently drive insecticide use. Proc. Natl. Acad. Sci. U.S.A. 110, 15330–15335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W., et al., Multidecadal, county-level analysis of the effects of land use, Bt cotton, and weather on cotton pests in China. Proc. Natl. Acad. Sci. U.S.A. 115, E7700–E7709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maas S., Redfern R., “Cotton pest management guide 2017-18” (Tech. Rep. Toowoomba, Qld, Australia Greenmount Press, 2017).
- 21.Waterfield G., Zilberman D., Pest management in food systems: An economic perspective. Annu. Rev. Environ. Resour. 37, 223–245 (2012). [Google Scholar]
- 22.Bianchi F. J. J. A., Schellhorn N. A., Cunningham S. A., Habitat functionality for the ecosystem service of pest control: Reproduction and feeding sites of pests and natural enemies. Agric. For. Entomol. 15, 12–23 (2012). [Google Scholar]
- 23.Sirami C., et al., Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. U.S.A. 116, 16442–16447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagic V., et al., Biocontrol in insecticide sprayed crops does not benefit from semi-natural habitats and recovers slowly after spraying. J. Appl. Ecol. 56, 2176–2185 (2019). [Google Scholar]
- 25.Whitehouse M. E. A., IPM of mirids in Australian cotton: Why and when pest managers spray for mirids. Agric. Syst. 104, 30–41 (2011). [Google Scholar]
- 26.Deguine J.-P., Ferron P., Russell D., Sustainable pest management for cotton production: A review. Agron. Sustain. Dev. 28, 113–137 (2008). [Google Scholar]
- 27.Meehan T. D., Gratton C., A landscape view of agricultural insecticide use across the conterminous US from 1997 through 2012. PLoS One 11, e0166724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meehan T. D., Gratton C., A consistent positive association between landscape simplification and insecticide use across the Midwestern US from 1997 through 2012. Environ. Res. Lett. 10, 114001 (2015). [Google Scholar]
- 29.Larsen A. E., Gaines S. D., Deschênes O., Spatiotemporal variation in the relationship between landscape simplification and insecticide use. Ecol. Appl. 25, 1976–1983 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Wu Y., et al., Policy distortions, farm size, and the overuse of agricultural chemicals in China. Proc. Natl. Acad. Sci. U.S.A. 115, 7010–7015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin E. A., et al., The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 22, 1083–1094 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Dainese M., et al., A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 5, eaax0121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagic V., Marcora A., Howie L., Additive and interactive effects of pollination and biological pest control on crop yield. J. Appl. Ecol. 56, 2528–2535 (2019). [Google Scholar]
- 34.Whitehouse M. E. A., et al., Target and nontarget effects of novel “triple-stacked” Bt-transgenic cotton 1: Canopy arthropod communities. Environ. Entomol. 43, 218–241 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Wilson L. J., Mensah R. K., Fitt G. P., “Implementing integrated pest management in Australian cotton” in Insect Pest Management: Field and Protected Crops, Horowitz A. R., Ishaaya I., Eds. (Springer, Berlin, 2004), pp. 97–118. [Google Scholar]
- 36.Lu Y., et al., Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328, 1151–1154 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Ferrigno S., Guadagnini R., Tyrell K., Is Cotton Conquering its Chemical Addiction? A Review of Pesticide Used in Global Cotton Production (UK Pesticide Action Network, 2017). [Google Scholar]
- 38.McColl S. A., Khan M., Umina P. A., Review of the biology and control of Creontiades dilutus (Stål) (Hemiptera: Miridae). Aust. J. Entomol. 50, 107–117 (2011). [Google Scholar]
- 39.ESRI , ArcGIS Desktop: Release 10 (Environmental Systems Research Institute, Redlands, 2011). [Google Scholar]
- 40.Wood S. N., Generalized Additive Models: An Introduction with R (Chapman and Hall/CRC, ed. 2, 2017). [Google Scholar]
- 41.R Development Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 42.Gräler B., Pebesma E., Heuvelink G., Spatio-Temporal Interpolation using gstat. R J. 8, 204–218 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized data and R code data have been deposited in Figshare (https://figshare.com/articles/dataset/AI_pests_insecticides_yield_csv/12062370, https://figshare.com/articles/journal_contribution/R_code_for_all_models/12072105, and https://figshare.com/articles/journal_contribution/R_code_for_all_Figures/12072108). All other study data are included in the article and/or SI Appendix.