Abstract
We describe the practice of ventilation and mortality rates in invasively ventilated normal-weight (18.5 ≤ BMI ≤ 24.9 kg/m2), overweight (25.0 ≤ BMI ≤ 29.9 kg/m2), and obese (BMI > 30 kg/m2) COVID-19 ARDS patients in a national, multicenter observational study, performed at 22 intensive care units in the Netherlands. The primary outcome was a combination of ventilation variables and parameters over the first four calendar days of ventilation, including tidal volume, positive end–expiratory pressure (PEEP), respiratory system compliance, and driving pressure in normal–weight, overweight, and obese patients. Secondary outcomes included the use of adjunctive treatments for refractory hypoxaemia and mortality rates. Between 1 March 2020 and 1 June 2020, 1122 patients were included in the study: 244 (21.3%) normal-weight patients, 531 (47.3%) overweight patients, and 324 (28.8%) obese patients. Most patients received a tidal volume < 8 mL/kg PBW; only on the first day was the tidal volume higher in obese patients. PEEP and driving pressure were higher, and compliance of the respiratory system was lower in obese patients on all four days. Adjunctive therapies for refractory hypoxemia were used equally in the three BMI groups. Adjusted mortality rates were not different between BMI categories. The findings of this study suggest that lung-protective ventilation with a lower tidal volume and prone positioning is similarly feasible in normal-weight, overweight, and obese patients with ARDS related to COVID-19. A patient’s BMI should not be used in decisions to forgo or proceed with invasive ventilation.
Keywords: coronavirus disease 2019, COVID-19, ARDS, body mass index, BMI, normal-weight overweight, obesity, obesity paradox, intensive care, critical care, artificial ventilation, mortality
1. Introduction
Several studies have implicated obesity as a risk factor for complications in COVID-19, such as the development of severe pneumonia and acute respiratory failure [1,2], the need for hospitalization [3], the need for intensive care unit (ICU) admission [3,4], and the need for invasive ventilation [5]. Multiple pathways by which obesity may affect outcomes in COVID-19 patients have been suggested, including underlying impairments in respiratory, cardiovascular, metabolic, and thrombotic pathways [1]. It is uncertain, though, whether obesity should be considered in the decision of who undergoes or will continue with treatment, including intubation and invasive ventilation.
Invasive ventilation can save the life of patients with acute respiratory distress syndrome (ARDS), but can also cause harm if not properly applied. Ventilatory interventions, such as lung-protective ventilation with a lower tidal volume [6] or a lower driving pressure [7], appropriate titration of positive end-expiratory pressure (PEEP) with recruitment maneuvers [8,9], and prone positioning [10] all have the potential to improve outcome of invasively ventilated patients. Obesity may hamper adequate use of these interventions and may increase the risk of severe atelectasis, but at the same time redistribute regional transpulmonary pressure, possibly reducing the potential negative effects of invasive ventilation in an inhomogeneous lung [11]. It is not clear how body mass index (BMI) affects ventilation practice and outcomes in patients with ARDS related to COVID-19.
In this secondary analysis of the “PRactice of Ventilation in COVID-19” (PRoVENT-COVID) study [12,13], we aimed to describe and compare ventilation management over the first 4 days of invasive ventilation in normal-weight, overweight and obese COVID-19 patients with ARDS. One secondary objective was to describe and compare clinical outcomes in the three BMI categories. The hypotheses tested were that ventilation practices differ between normal-weight, overweight, and obese patients with ARDS related to COVID-19, and that obese patients have a worse outcome compared to normal-weight and overweight patients.
2. Materials and Methods
2.1. Study Design
The PRoVENT-COVID study is an investigator-initiated, multicenter, observational cohort study undertaken at 22 ICUs during the first 3 months of the COVID-19 outbreak in the Netherlands [13]. The study protocol including a statistical analysis plan was prepublished [12]. A statistical analysis plan for the current analysis was published online before assessing the database of the PRoVENT-COVID study [14]. Study sites were recruited through direct contact by steering committee members of PRoVENT-COVID. Study coordinators contacted the local doctors, trained data collectors to assist local caregivers, and monitored the study according to the International Conference on Harmonization Good Clinical Practice Guidelines. The integrity and timely completion of data collection was ensured by the study coordinators.
2.2. Ethics
Ethical approval for this study (W20_157 # 20.171) was provided by the Ethical Committee of the Academic Medical Center (Chairperson C.L. van der Wilt) on 7 April 2020. The need for individual patient informed consent was waived given the observational design of the study.
2.3. Study Registration
The study was registered at clinicaltrials.gov (15 April 2020; study identifier NCT04346342).
2.4. Inclusion and Exclusion Criteria
The PRoVENT-COVID study had the following inclusion criteria: (1) age ≥ 18 years, (2) admitted to one of the participating ICUs, and (3) having received invasive ventilation for ARDS related to COVID-19. The study itself had no exclusion criteria; for the current analysis we excluded patients in whom BMI could not be calculated.
2.5. Collected Data, and Patient Classification
Demographics and data regarding premorbid diseases and medication were collected at baseline. In the first hour of invasive ventilation and every 8 h thereafter, at fixed time points in the first four calendar days, ventilator settings and parameters were collected. The driving pressure and mechanical power of ventilation were calculated as follows: driving pressure (in cm H2O) = peak pressure − positive end-expiratory pressure (PEEP); and mechanical power (in J/min) = 0.098 × tidal volume × respiratory rate × (peak pressure-0.5 × driving pressure). The categories of BMI were defined as underweight (BMI < 18.4 kg/m2), normal-weight (18.5 ≤ BMI ≤ 24.9 kg/m2), overweight (25.0 ≤ BMI ≤ 29.9 kg/m2), and obese (BMI > 30 kg/m2).
2.6. Endpoints
The primary outcome of this analysis was a combination of key ventilator settings and ventilation parameters during the first four calendar days of invasive ventilation, including tidal volume, PEEP, respiratory system compliance, and driving pressure. Secondary outcomes included the use of adjunctive treatments for refractory hypoxemia, including the use of alveolar recruitment maneuvers and prone positioning. We also collected adjunctive strategies including the use of neuromuscular blocking agents and extracorporeal membrane oxygenation. Other secondary outcomes included mortality at day 28, at ICU and hospital discharge, and at day 90, ventilator-free days and alive at day 28 (VFD-28) as defined before [15], and typical complications including venous thromboembolism, acute kidney injury, and the use of renal replacement therapy.
2.7. Statistical Analysis
Since the proportion of patients with underweight was very low (n = 3), underweight patients were excluded from the analyses. No statistical power calculation was conducted before the study, and sample size was based on available data. The amount of missing data was low for most of the variables and follow-up to day 90 was complete for 91% of patients.
Continuous variables are presented as medians (first quartile–third quartile) and categorical variables as numbers and percentages. The BMI groups were compared using Kruskal–Wallis test for continuous variables and Fisher exact tests for categorical variables.
Differences in ventilatory variables and laboratory tests between the BMI groups are visualized in cumulative distribution plots and boxplots at the start of ventilation and at day 1, 2, and 3. The effect of BMI categories on clinical outcomes was reported in Kaplan–Meier curves and compared with Log-rank tests. Further comparison between the groups was made with (shared-frailty) Cox proportional models with center as frailty. The proportional hazard assumption was assessed through Schoenfeld residuals.
To assess the impact of BMI categories on 28-day mortality, the following variables were included in the multivariable model based on clinical relevance and when a p < 0.20 was found in the univariable assessment: (1) ventilatory variables in the first day aggregated as the mean from a maximum of four assessments (tidal volume adjusted by predicted body weight (PBW) and respiratory system compliance and PEEP); (2) laboratory tests and vital signs on the first day aggregated as the mean from a maximum of four assessments (arterial pH, creatinine, heart rate, and mean arterial pressure); (3) organ support at the first day (use of vasopressor and cumulative fluid balance); (4) demographic characteristics (age, gender, body mass index, hypertension, heart failure, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, active hematological neoplasia, active solid neoplasia, use of angiotensin converting enzyme inhibitor, and use of angiotensin II receptor blocker); (5) severity of ARDS according to the Berlin definition [16]; and (6) use of rescue therapies at the first day of ventilation. Peak pressure and driving pressure were not considered due to collinearity with respiratory compliance, which was judged to be more clinically relevant in the model, and FiO2 was excluded due to association with PaO2/FiO2 and the severity of acute respiratory failure.
When predictors considered in the model were missing in less than 5% of the patients, these values were imputed by the median. All continuous variables were entered after standardization to improve the convergence of the model, and all effect estimates represent the increase in one standard deviation of the variable.
Two sensitivity analyses were performed. First, the impact of BMI categories on 28-day mortality was re-assessed within the mild, moderate, and severe categories of ARDS according to the Berlin definition [16]. In addition, obese categories class I (30.0 ≤ BMI ≤ 34.9 kg/m2), II (35.0 kg/m2 ≤ BMI ≤ 39.9 kg/m2), and III (BMI ≥ 40 kg/m2) were considered and assessed in this model.
All analyses were conducted in R v.4.0.2 (R Foundation, Vienna, Austria) and the significance level was set at 0.05.
3. Results
3.1. Participating ICUs and Patients Enrolled
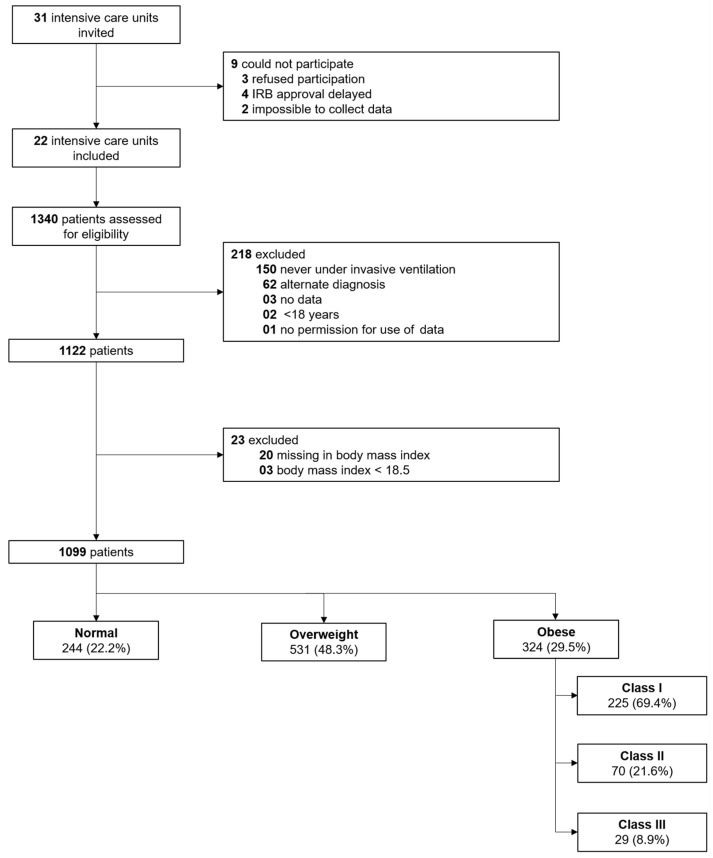
Between 1 March 2020 through 1 June 2020, 31 ICUs were invited for participation in PRoVENT-COVID, and 22 met inclusion criteria. Of 1340 individuals screened, 1122 were enrolled; the main reasons for exclusion were that they did not receive invasive ventilation or had an alternative diagnosis (Figure 1). Three (0.2%) patients were under-weight, 244 (21.3%) patients were normal-weight, 531 (47.3%) patients were overweight, and 324 (28.8%) patients were obese; in 20 patients BMI could not be calculated. Obese patients were younger, less often males, had more often a history of diabetes and less often chronic kidney injury (Table 1). Obese patients more often presented with severe ARDS, had a higher mean arterial pressure, and required less often inotropic agents on the first day of ventilation.
Figure 1.
Study profile. Follow-up to 90 days was complete in 996 patients. IRB: Institutional Review Board.
Table 1.
Baseline characteristics, vital signs, laboratory test results, and organ support on the first day of ventilation according to BMI category.
| Normal (n = 244) |
Overweight (n = 531) |
Obese (n = 324) |
p Value | |
|---|---|---|---|---|
| Age, years | 67.0 (60.0–73.0) | 66.0 (59.0–73.0) | 61.0 (53.0–70.0) | <0.001 |
| Male gender—no (%) | 186 (76.2) | 407 (76.6) | 209 (64.5) | <0.001 |
| Body mass index, kg/m2 | 23.9 (22.9–24.6) | 27.3 (26.2–28.5) | 32.9 (31.2–35.9) | <0.001 |
| Transferred under invasive ventilation | 51 (20.9) | 82 (15.4) | 59 (18.2) | 0.160 |
| Days between intubation and admission | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.583 |
| Use of non-invasive ventilation—no (%) | 15 (6.8) | 45 (9.4) | 24 (8.1) | 0.517 |
| Duration of non-invasive ventilation, hours | 5.5 (2.0–48.0) | 8.0 (2.0–15.1) | 7.5 (2.0–14.8) | 0.803 |
| Chest CT scan performed—no (%) | 83/232 (35.8) | 169/509 (33.2) | 103/314 (32.8) | 0.738 |
| Lung parenchyma affected—no (%) | 0.839 | |||
| 0% | 3/84 (3.6) | 6/172 (3.5) | 5/103 (4.9) | |
| 25% | 32/84 (38.1) | 51/172 (29.7) | 33/103 (32.0) | |
| 50% | 26/84 (31.0) | 52/172 (30.2) | 28/103 (27.2) | |
| 75% | 20/84 (23.8) | 53/172 (30.8) | 29/103 (28.2) | |
| 100% | 3/84 (3.6) | 10/172 (5.8) | 8/103 (7.8) | |
| Chest X-ray performed—no (%) | 127/149 (85.2) | 289/334 (86.5) | 175/204 (85.8) | 0.907 |
| Quadrants affected—no (%) | 0.068 | |||
| 1 | 14/126 (11.1) | 21/291 (7.2) | 7/173 (4.0) | |
| 2 | 24/126 (19.0) | 67/291 (23.0) | 48/173 (27.7) | |
| 3 | 37/126 (29.4) | 72/291 (24.7) | 55/173 (31.8) | |
| 4 | 51/126 (40.5) | 131/291 (45.0) | 63/173 (36.4) | |
| Severity of ARDS—no (%) | 0.028 | |||
| Mild | 24/239 (10.0) | 51/523 (9.7) | 29/318 (9.1) | |
| Moderate | 157/239 (65.7) | 326/523 (62.3) | 171/318 (53.8) | |
| Severe | 58/239 (24.3) | 146/523 (27.9) | 118/318 (37.1) | |
| Co-existing disorders—no (%) | ||||
| Hypertension | 74 (30.3) | 186 (35.0) | 114 (35.2) | 0.383 |
| Heart failure | 9 (3.7) | 31 (5.8) | 8 (2.5) | 0.058 |
| Diabetes | 41 (16.8) | 115 (21.7) | 90 (27.8) | 0.007 |
| Chronic kidney disease | 8 (3.3) | 31 (5.8) | 8 (2.5) | 0.045 |
| Baseline creatinine, µmol/L * | 77.0 (61.0–98.0) | 78.0 (64.0–97.0) | 76.0 (62.8–97.0) | 0.767 |
| Liver cirrhosis | 0 (0.0) | 2 (0.4) | 1 (0.3) | 0.999 |
| Chronic obstructive pulmonary disease | 20 (8.2) | 41 (7.7) | 24 (7.4) | 0.934 |
| Active hematological neoplasia | 5 (2.0) | 10 (1.9) | 1 (0.3) | 0.091 |
| Active solid neoplasia | 6 (2.5) | 14 (2.6) | 7 (2.2) | 0.967 |
| Neuromuscular disease | 2 (0.8) | 3 (0.6) | 3 (0.9) | 0.728 |
| Immunosuppression | 9 (3.7) | 8 (1.5) | 7 (2.2) | 0.165 |
| Previous medication—no (%) | ||||
| Systemic steroids | 10 (4.1) | 17 (3.2) | 11 (3.4) | 0.786 |
| Inhalation steroids | 21 (8.6) | 58 (10.9) | 45 (13.9) | 0.137 |
| Angiotensin converting enzyme inhibitor | 33 (13.5) | 93 (17.5) | 60 (18.5) | 0.250 |
| Angiotensin II receptor blocker | 24 (9.8) | 57 (10.7) | 44 (13.6) | 0.318 |
| Beta-blockers | 40 (16.4) | 98 (18.5) | 71 (21.9) | 0.235 |
| Insulin | 14 (5.7) | 38 (7.2) | 26 (8.0) | 0.590 |
| Metformin | 29 (11.9) | 77 (14.5) | 65 (20.1) | 0.021 |
| Statins | 70 (28.7) | 155 (29.2) | 100 (30.9) | 0.826 |
| Calcium channel blockers | 47 (19.3) | 79 (14.9) | 67 (20.7) | 0.067 |
| Vital signs at the day of start of ventilation | ||||
| Heart rate, bpm ** | 84.0 (71.5–97.0) | 84.0 (73.0–97.1) | 86.0 (76.9–98.0) | 0.130 |
| Mean arterial pressure, mmHg ** | 78.7 (73.0–86.0) | 80.0 (73.5–87.5) | 82.0 (75.7–89.5) | 0.002 |
| Laboratory tests at the day of start of ventilation | ||||
| pH ** | 7.36 (7.30–7.41) | 7.37 (7.32–7.41) | 7.36 (7.31–7.41) | 0.700 |
| Worst PaO2/FiO2, mmHg *** | 130.0 (101.0–166.9) | 125.0 (98.5–162.4) | 114.6 (87.6–146.0) | 0.001 |
| PaCO2, mmHg ** | 44.5 (39.5–51.3) | 44.3 (38.8–49.6) | 44.6 (39.8–51.0) | 0.356 |
| Lactate mmol/L ** | 1.2 (0.9–1.5) | 1.2 (1.0–1.4) | 1.1 (0.9–1.4) | 0.132 |
| Organ support at the day of start of ventilation—no (%) | ||||
| Continuous sedation | 231/243 (95.1) | 506/530 (95.5) | 316 (97.5) | 0.213 |
| Inotropic or vasopressor | 192/243 (79.0) | 412/530 (77.7) | 241 (74.4) | 0.377 |
| Vasopressor | 192/243 (79.0) | 412/530 (77.7) | 240 (74.1) | 0.328 |
| Inotropic | 18/243 (7.4) | 17/530 (3.2) | 10 (3.1) | 0.022 |
| Fluid balance, mL | 696.7 (29.0–1441.0) | 515.8 (7.3–1239.3) | 449.0 (-15.0–1299.9) | 0.171 |
| Urine output, mL | 692.5 (333.8–1116.2) | 647.5 (350.0–1145.0) | 705.0 (395.0–1115.0) | 0.500 |
| Ventilation support at the day of start of ventilation | ||||
| Assisted ventilation—no (%) | 73/243 (30.0) | 161/527 (30.6) | 84 (25.9) | 0.328 |
| Tidal volume, mL/kg PBW ** | 6.4 (5.9–6.9) | 6.4 (5.9–7.0) | 6.6 (5.9–7.5) | < 0.001 |
| PEEP, cmH2O ** | 12.0 (10.0–14.0) | 12.7 (11.0–14.5) | 14.0 (12.0–15.0) | < 0.001 |
| Peak pressure, cmH2O ** | 25.2 (22.8–28.9) | 26.6 (23.5–29.3) | 28.0 (25.3–31.0) | < 0.001 |
| Driving pressure, cmH2O ** | 13.0 (11.2–15.3) | 13.7 (12.0–16.0) | 14.5 (12.5–17.0) | < 0.001 |
| Mechanical power, J/min ** | 18.1 (14.7–21.6) | 18.2 (15.3–21.9) | 19.4 (15.8–23.5) | 0.014 |
| Compliance, mL/cmH2O ** | 36.2 (28.7–45.1) | 33.4 (26.8–41.1) | 31.9 (26.0–38.1) | < 0.001 |
| Total respiratory rate, mpm ** | 21.7 (19.3–24.0) | 21.7 (19.8–24.0) | 22.0 (19.2–24.0) | 0.921 |
| Minute ventilation, L/min | 9.8 (8.5–11.4) | 9.6 (8.4–11.2) | 9.7 (8.3–11.2) | 0.556 |
| Minute ventilation corrected, mL/kg/min PBW | 137.2 (122.3–157.5) | 138.3 (122.4–157.3) | 141.3 (125.7–164.1) | 0.053 |
| FiO2 ** | 0.54 (0.45–0.65) | 0.57 (0.47–0.66) | 0.60 (0.52–0.71) | <0.001 |
| etCO2, mmHg ** | 35.7 (32.0–40.7) | 36.5 (32.4–41.6) | 38.4 (34.6–43.8) | <0.001 |
| Rescue therapy at the day of start of ventilation—no (%) | ||||
| Prone positioning | 61/241 (25.3) | 161/522 (30.8) | 104/317 (32.8) | 0.142 |
| Duration, hours | 9.0 (6.0–14.0) | 8.0 (4.0–13.5) | 8.0 (3.1–13.0) | 0.138 |
| Recruitment maneuver | 3/197 (1.5) | 12/434 (2.8) | 5/268 (1.9) | 0.641 |
| ECMO | 1/241 (0.4) | 0/523 (0.0) | 3/318 (0.9) | 0.066 |
| Use of NMBA | 54/243 (22.2) | 154/529 (29.1) | 89 (27.5) | 0.128 |
| Duration, hours | 0.0 (0.0–0.0) | 0.0 (0.0–8.0) | 0.0 (0.0–8.0) | 0.182 |
Data are median (first quartile–third quartile) or No (%). Percentages may not total 100 because of rounding. CT: computed tomography; PEEP: positive end expiratory pressure; ECMO: extracorporeal membrane oxygenation; FiO2: inspired fraction of oxygen; PEEP: positive end-expiratory pressure; NMBA: neuromuscular blocking agent. * Most recent measurement in 24 h before intubation or at ICU admission under invasive ventilation. ** Aggregate as the mean of all values available at the first day of ventilation. *** Worst value of four available.
3.2. Ventilatory Support and Adjunctive Therapies
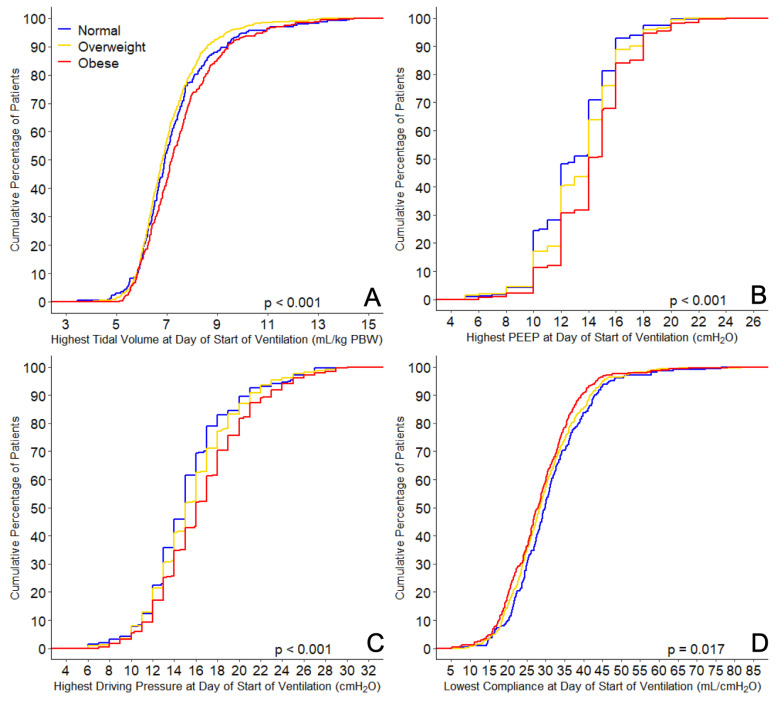
At the day of start of invasive ventilation, obese patients received ventilation with a slightly higher median tidal volume (6.4 (5.9–6.9) vs. 6.4 (5.9–7.0) vs. 6.6 (5.9–7.5) mL/kg PBW in normal-weight vs. overweight vs. obese patients (p < 0.001)) (Table 1 and Figure 2), a higher PEEP (12.0 (10.0–14.0) vs. 12.7 (11.0–14.5) vs. 14.0 (12.0–15.0) cm H2O (p < 0.001)), and a higher driving pressure (13.0 (11.2–15.3) vs. 13.7 (12.0–16.0) vs. 14.5 (12.5–17.0) cm H2O (p < 0.001)). Obese patients had a lower compliance of the respiratory system (36.2 (28.7–45.1) vs. 33.4 (26.8–41.1) vs. 31.9 (26.0–38.1) ml/cm H2O (p < 0.001)). Obese patients were ventilated with higher mechanical power and needed higher oxygen fractions. The four ventilatory variables did not change at successive days (Figures S1–S4), and the difference in tidal volume between the three BMI categories was no longer present at day 2, 3, and 4.
Figure 2.
Ventilation parameters. Cumulative frequency distribution of (A) tidal volume, (B) positive end-expiratory pressure (PEEP), (C) driving pressure, and (D) respiratory system compliance. p values calculated from Kruskal–Wallis tests.
In the first 4 days of ventilation, the use of alveolar recruitment maneuvers and prone positioning was not statistically different between the BMI categories (Table 2). Neuromuscular blocking agents were more often administered in obese patients.
Table 2.
Clinical outcomes according to BMI category.
| Normal (n = 244) |
Overweight (n = 531) |
Obese (n = 324) |
p Value | |
|---|---|---|---|---|
| 28-day mortality—no. (%) | 71/238 (29.8) | 162/525 (30.9) | 76/318 (23.9) | 0.082 |
| Ventilator-free days at day 28, days | 2.0 (0.0–18.0) | 0.0 (0.0–15.0) | 6.0 (0.0–17.0) | 0.088 |
| Duration of ventilation, days | 13.0 (7.0–23.0) | 15.0 (8.0–24.0) | 14.0 (9.0–22.8) | 0.206 |
| In survivors at day 28, days | 14.0 (8.0–27.0) | 16.0 (10.0–30.0) | 16.0 (10.0–26.0) | 0.192 |
| Tracheostomy—no (%) | 47/241 (19.5) | 86/527 (16.3) | 53/322 (16.5) | 0.517 |
| Thromboembolic complications—no (%) | 80 (32.8) | 146 (27.5) | 88 (27.2) | 0.258 |
| Pulmonary embolism | 64 (26.2) | 112 (21.1) | 70 (21.6) | 0.263 |
| Deep vein thrombosis | 12 (4.9) | 29 (5.5) | 14 (4.3) | 0.776 |
| Ischemic stroke | 9 (3.7) | 15 (2.8) | 6 (1.9) | 0.401 |
| Myocardial infarction | 5 (2.0) | 9 (1.7) | 2 (0.6) | 0.261 |
| Systemic arterial embolism | 2 (0.8) | 1 (0.2) | 1 (0.3) | 0.349 |
| Acute kidney injury—no (%) | 104 (42.6) | 235/529 (44.4) | 149/322 (46.3) | 0.689 |
| Need for RRT—no (%) | 40 (16.4) | 103 (19.4) | 58 (17.9) | 0.610 |
| Need of rescue therapy—no (%) * | 168/241 (69.7) | 393/526 (74.7) | 245/321 (76.3) | 0.191 |
| Prone positioning | 124/241 (51.5) | 300/527 (56.9) | 188/322 (58.4) | 0.232 |
| Recruitment maneuver | 9/200 (4.5) | 36/440 (8.2) | 18/271 (6.6) | 0.239 |
| Use of NMBA | 95 (38.9) | 266 (50.1) | 166 (51.2) | 0.006 |
| ECMO | 1/241 (0.4) | 6/525 (1.1) | 5/318 (1.6) | 0.415 |
| ICU length of stay, days | 14.0 (8.0–26.0) | 16.0 (9.0–26.0) | 15.0 (10.0–26.0) | 0.302 |
| In survivors, days | 15.5 (9.3–29.0) | 18.0 (11.0–31.0) | 17.0 (11.0–28.0) | 0.379 |
| Hospital length of stay, days | 23.0 (13.0–37.3) | 23.0 (13.0–36.0) | 25.0 (16.8–37.0) | 0.219 |
| In survivors, days | 29.0 (18.0–44.5) | 30.0 (20.0–45.8) | 29.0 (21.0–42.0) | 0.682 |
| ICU mortality—no (%) | 76/238 (31.9) | 186/519 (35.8) | 85/313 (27.2) | 0.034 |
| Hospital mortality—no (%) | 80/224 (35.7) | 191/490 (39.0) | 87/290 (30.0) | 0.041 |
| 90-day mortality—no (%) | 82/219 (37.4) | 201/492 (40.9) | 91/285 (31.9) | 0.046 |
Data are median (first quartile–third quartile) or No (%). Percentages may not total 100 because of rounding. RRT: renal replacement therapy; NMBA: neuromuscular blocking agent; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit. * assessed in the first four days of ventilation.
3.3. Patient Outcomes
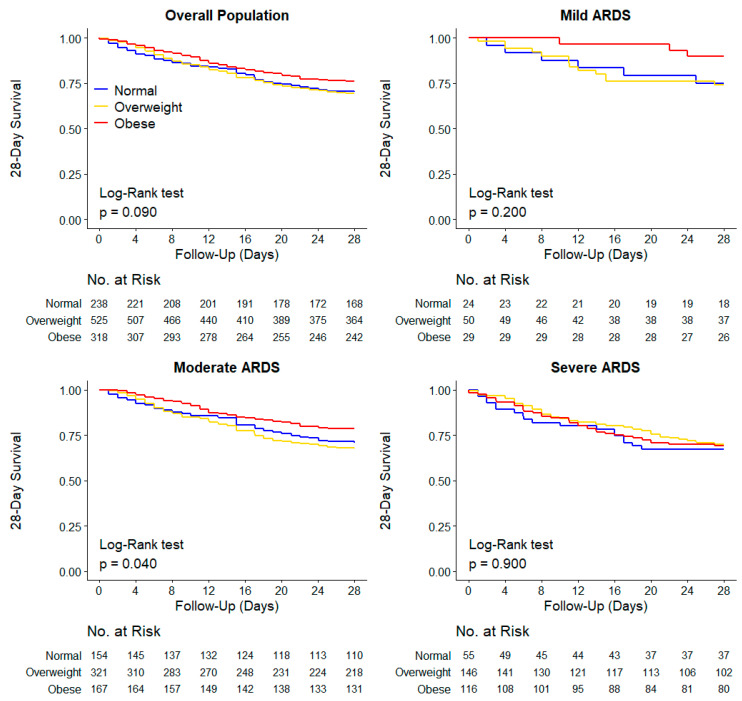
The 28-day mortality was similar in the three BMI categories (29.8% vs. 30.9% vs. 23.9% in normal-weight vs. overweight vs. obese patients (p = 0.082 for the Fisher exact test; p = 0.090 for the Log-rank test) (Figure 3). BMI category was not associated with 28-day mortality, neither in the unadjusted nor in the adjusted analyses (Table 3, Tables S1 and S2). Among secondary outcomes, ICU, hospital, and 90-day mortality were lower in obese patients in the unadjusted analysis (Table 2 and Figure S5), but not in the adjusted analysis (Table 3).
Figure 3.
Kaplan–Meier Curves for 28-day mortality in the overall population and groups according to ARDS severity. p values calculated from Log-rank test. Unadjusted and adjusted (shared-frailty) Cox proportional hazard models are shown in Tables S2–S4.
Table 3.
Adjusted effect of body mass index categories in 28-day, ICU, hospital, and 90-day mortality *.
| Effect Estimate (95% CI) |
p Value | |
|---|---|---|
| 28-day mortality | ||
| Body mass index category | ||
| Normal | 1 (Reference) | |
| Overweight | 1.10 (0.83 to 1.47) | 0.500 |
| Obese | 0.89 (0.63 to 1.25) | 0.510 |
| ICU mortality | ||
| Body mass index category | ||
| Normal | 1 (Reference) | |
| Overweight | OR, 1.39 (0.96 to 2.00) | 0.079 |
| Obese | OR, 1.07 (0.69 to 1.65) | 0.753 |
| Hospital mortality | ||
| Body mass index category | ||
| Normal | 1 (Reference) | |
| Overweight | OR, 1.38 (0.95 to 2.00) | 0.090 |
| Obese | OR, 1.05 (0.68 to 1.64) | 0.817 |
| 90-day mortality | ||
| Body mass index category | ||
| Normal | 1 (Reference) | |
| Overweight | HR, 1.16 (0.89 to 1.52) | 0.270 |
| Obese | HR, 1.00 (0.72 to 1.38) | 0.999 |
Continuous variables were included after standardization, and the hazard ratio represents the increase in one standard deviation of the variable. OR: odds ratio; HR: hazard ratio. * All models adjusted for age, hypertension, heart failure, diabetes, chronic obstructive pulmonary disease, use of angiotensin converting enzyme inhibitor, heart rate, mean arterial pressure, pH, PEEP, fluid balance, use of vasopressor, and prone positioning on the day of start of ventilation.
In the unadjusted analysis, 28-day mortality was lower in obese patients with moderate ARDS (p = 0.040 for the Log-Rank test) (Figure 3). However, there was no effect of BMI categories within each category of ARDS after multivariable adjustment in 28-day, ICU, hospital, or 90-day mortality (Table S3).
Baseline characteristics of the patients according to five BMI categories are shown in Table S4. On the first day of ventilation, tidal volume, PEEP, and driving pressure were higher, and compliance was lower, in more obese patients (Figure S6). The 28-day mortality was lower in patients with obesity class III in the unadjusted analysis (Table S5 and Figure S7). After adjustment for confounders, only the higher risk of 28-day mortality in patients with class II obesity and severe ARDS remained statistically significant (Table S6).
4. Discussion
We here describe the associations of BMI with ventilation parameters and outcomes in COVID-19 patients with ARDS who received invasive ventilation during the first 3 months of the outbreak in the Netherlands. First, while tidal volume was slightly higher in obese patients compared to overweight and normal-weight patients at the day of start of ventilation, still >70% of them received protective ventilation with a low tidal volume. Of note, the tidal volume differences were small and probably clinically irrelevant. The tidal volumes became equally large in the three BMI categories on successive days. Second, the applied PEEP and driving pressure were higher and compliance was lower in obese patients. These differences persisted over the successive days. Off all adjunctive treatments for refractory hypoxemia, only neuromuscular blocking agents were more often used in obese patients. Third, 28-day mortality was not different between the BMI groups, and although ICU, hospital, and 90-day mortality were lower in obese patients in the unadjusted analysis, but this was no longer the case in the adjusted analysis. While survival seemed better in obese patients with mild ARDS, this finding disappeared after adjustments. It is possible that mild ARDS was relatively over-diagnosed due to derecruitment, causing oxygenation impairments rather than that a patient really having ARDS.
Previous studies showed that obese patients often receive ventilation with a higher tidal volume [17,18,19] and higher PEEP [18,19], probably because clinicians frequently overestimate lung size by using actual body weight instead of PBW and because obese patients are more susceptible to atelectasis. Obese patients also receive ventilation with a higher plateau and peak pressures [18,19], probably to compensate for the decreased lung and chest wall compliance. In addition, obese patients frequently need a higher FiO2 and a higher minute volume because they consume more oxygen and produce more carbon dioxide [20,21]. We found similar differences between the BMI categories in our cohort of COVID-19 patients, but the effects of BMI on tidal volume were much less outspoken and disappeared over the successive days. One explanation for the latter finding could be that compliance with existing guidelines is much better in COVID-19 patients, either because care for the surge in COVID-19 patients had to be provided by hospital personnel who had less experience or confidence with setting a ventilator and thus followed the local guidelines more strictly, or because the use of ventilation with a lower tidal volume has improved in general over recent years. Nevertheless, the use of a correct tidal volume is important also in COVID-19 patients with ARDS [13].
Esophagus catheters were seldom used in this cohort of patients, probably because hospital personnel with little experience with setting a ventilator also had less knowledge of transpulmonary and transthoracic pressure measurements to adjust ventilator settings. It remains uncertain whether (adequate) use of esophagus pressure measurements would have altered the current findings [22].
Use of prone positioning was high, but not different between BMI categories. This was surprising as prone positioning in an obese patient could be more burdensome than in overweight or normal-weight patients. It is probable that the common use of the prone positioning is due to the high incidence of severe hypoxemia in COVID-19 patients with ARDS. Prone positioning has been indisputably shown to improve oxygenation in patients with ARDS [23]. Neuromuscular blocking agents were prescribed more often in obese patients. Obese patients may have more dyspnea because of a higher minute volume needed to compensate for the greater production of carbon dioxide. This may increase patient-ventilator asynchronies, for which clinicians could prescribe neuromuscular blocking agents.
The effects of BMI on mortality in the current study contrast with the findings of one meta-analysis showing a significant association between obesity and COVID-19 severity and outcome [24]. That meta-analysis, however, accepted studies that included patients other than those receiving invasive ventilation in the ICU; the impact of BMI on outcome could differ between ventilated and non-ventilated COVID-19 patients. We cannot exclude the possibility that decisions to forgo invasive ventilation were driven by factors that may impact outcome, including the presence of comorbidities and the health status before development of COVID-19 pneumonia. This may have resulted in BMI categories with comparable mortality rates. Nevertheless, in our cohort obese patients had diabetes more often. The incidence of chronic kidney injury, however, was lower in obese patients.
Our findings are also different from the results of two meta-analyses showing better outcomes in invasively ventilated obese patients compared to overweight or normal-weight patients [25,26]. In the meta-analysis of studies of invasively ventilated ICU patients [25], obese patients had a lower ICU, hospital, and long-term mortality. In the meta-analysis of studies of invasively ventilated ARDS patients [26], obesity and morbid obesity were more likely to result in a lower mortality. These meta-analyses, however, lacked adjustments for age, gender, comorbidities, and illness severity. In our cohort, differences in outcomes between BMI categories disappeared in the adjusted analyses [11].
One important limitation of the current analysis was that we could not use esophageal pressures to calculate pulmonary compliance; indeed, we could only report total respiratory system compliance. In an obese patient, an elevated plateau pressure may be related to an elevated transthoracic pressure and not necessarily an increase in transpulmonary pressure. This may explain, at least in part, the finding that there is no significant difference in mortality in severe ARDS (between obese and normal-weight) and even the important difference in favor of obese patients in mild and moderate ARDS. It may also explain the finding that obese patients received ventilation with a higher driving pressure and higher mechanical power and had a lower respiratory system compliance [22].
This analysis has other limitations, some of which have been mentioned before [13]. First, the collection of ventilation variables and adjunctive treatments was restricted to the first 4 days of ventilation, and we cannot exclude the possibility that ventilation practices and use of adjunctive treatments beyond the first days of invasive ventilation also had an effect on outcome. Follow-up to day 90 was not complete for all patients, but we missed follow-up at day 28 for only 30 patients, mainly because these patients were transferred to a non-participating hospital. Third, the models were not adjusted for laboratory test results such as D-dimers, which were not measured daily as part of standard care and were therefore not collected. However, patients with COVID-19 ARDS with increased D-dimer concentrations have higher mortality rates [27]. Nevertheless, one recent study showed that obese COVID-19 patients have lower D-dimer concentrations than non-obese COVID-19 patients [28].
The findings of this analysis extend our knowledge of ventilation practice in normal–weight, overweight, and obese patients with ARDS in general and in COVID-19 patients with ARDS in particular. Furthermore, they provide important information about the outcomes of invasively ventilated patients in the three BMI categories. The design of the study assured the completeness of data collection. The short timeframe within which data were gathered, only 3 months, avoided the effect of practice changes over time.
Our findings may have important suggestions for the clinical management of obese COVID-19 patients with ARDS. Despite understandable differences in ventilation management, it was noticeable that providing lung-protective ventilation with a lower tidal volume and prone positioning are very feasible strategies in obese patients with ARDS related to COVID-19. Both strategies have been proven to be very effective in patients with ARDS [6,10] and have been advised in obese patients with ARDS from another origin [29]; we recently showed that tidal volume size has an independent effect on outcome in COVID-19 patients with ARDS [13]. Most importantly, our findings suggest that the patient’s BMI should not be used in decisions to forgo or proceed with invasive ventilation in patients with ARDS related to COVID-19.
5. Conclusions
Comparable to normal-weight, overweight, and obese patients with ARDS from another origin, lung-protective ventilation with a lower tidal volume and prone positioning is very feasible in normal-weight, overweight, and obese patients with ARDS related to COVID-19. Obese patients with ARDS related to COVID-19 do not have a worse outcome compared to normal-weight and overweight patients; therefore, obesity should not be considered in the decision of who undergoes or will continue with intubation and invasive ventilation.
Acknowledgments
PRoVENT–COVID collaborators: J.P. van Akkeren; A.G. Algera; C.K. Algoe; R.B. van Amstel; O.L. Baur; P. van de Berg; D.C.J.J. Bergmans; D.I. van den Bersselaar; F.A. Bertens; A.J.G.H. Bindels; M.M. de Boer; S. den Boer; L.S. Boers; M. Bogerd; L.D.J. Bos; M. Botta; J.S. Breel; H. de Bruin; S. de Bruin; C.L. Bruna; L.A. Buiteman–Kruizinga; O. Cremer; R.M. Determann; W. Dieperink; D.A. Dongelmans; H.S. Franke; M.S. Galek Aldridge; M.J. de Graaff; L.A. Hagens; J.J. Haringman; N.F.L. Heijnen; S.Hiel; S.T. van der Heide; P.L.J. van der Heiden; L.L. Hoeijmakers; L. Hol; M.W. Hollmann; M.E. Hoogendoorn; J. Horn; R. van der Horst; E.L.K. Ie; D. Ivanov; N.P. Juffermans; E. Kho; E.S. de Klerk; A.W.M. Koopman; M. Koopmans; S. Kucukcelebi; M.A. Kuiper; D.W. de Lange; N. van Mourik; S.G. Nijbroek; M. Onrust; E.A.N. Oostdijk; F. Paulus; C.J. Pennartz; J. Pillay; L. Pisani; I.M. Purmer; T.C.D. Rettig; J.P Roozeman; M.T.U. Schuijt; M.J. Schultz; A. Serpa Neto; M.E. Sleeswijk; M.R. Smit; P.E. Spronk; W. Stilma; A.C. Strang; A.M. Tsonas; P.R Tuinman; C.M.A. Valk; F.L. Veen; A.P.J. Vlaar; L.I. Veldhuis; P. van Velzen; W.H. van der Ven; P. van Vliet; P. van der Voort; H.H. van der Wier; L. van Welie; H.J.F.T. Wesselink; B. van Wijk; T. Winters; W.Y. Wong; A.R.H. van Zanten.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/6/1176/s1, Figure S1: Cumulative Distribution Plots of Ventilatory Variables at the Second Day of Ventilation, Figure S2: Cumulative Distribution Plots of Ventilatory Variables at the Third Day of Ventilation, Figure S3: Cumulative Distribution Plots of Ventilatory Variables at the Fourth Day of Ventilation, Figure S4: Ventilatory Variables and Oxygenation in the First Four Days of Ventilation, Figure S5: Kaplan-Meier Curve for 90-Day Mortality in the Overall Population and According to ARDS Severity, Figure S6: Cumulative Distribution Plots of Ventilatory Variables and Oxygenation at the First Day of Ventilation, Figure S7: Kaplan-Meier Curve for 28-Day Mortality in the Overall Population and According to ARDS Severity, Table S1: Univariable Assessment of Factors Associated with 28-Day Mortality, Table S2: Multivariable Assessment of Factors Associated with 28-Day Mortality, Table S3: Adjusted Effect of Body Mass Index Categories in 28-Day, ICU, Hospital and 90-Day Mortality, Table S4: Baseline Characteristics of the Included Patients According to BMI Category, Table S5: Clinical Outcomes in the Included Patients According to BMI Category, Table S6: Adjusted Effect of Body Mass Index Categories in 28-Day Mortality.
Author Contributions
Conceptualization, R.S., M.J.S., W.K.L., E.R.v.S.-B., A.S.N. and F.P.; methodology, M.J.S., A.S.N. and F.P.; writing—original draft preparation, M.J.S. and A.S.N.; writing—review and editing, R.S., M.J.S., W.K.L., E.R.v.S.-B., A.S.N. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Amsterdam UMC, location AMC, Amsterdam, The Netherlands.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Amsterdam UMC, location AMC, Amsterdam, The Netherlands.
Informed Consent Statement
Need for individual patient informed consent was waived seen the observational design of the study.
Data Availability Statement
All data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sattar N., McInnes I.B., McMurray J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 2.Goodman K.E., Magder L.S., Baghdadi J.D., Pineles L., Levine A.R., Perencevich E.N., Harris A.D. Impact of Sex and Metabolic Comorbidities on COVID-19 Mortality Risk Across Age Groups: 66,646 Inpatients Across 613 U.S. Hospitals. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., Stachel A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Amato M.B., Meade M.O., Slutsky A.S., Brochard L., Costa E.L., Schoenfeld D.A., Stewart T.E., Briel M., Talmor D., Mercat A., et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 8.Constantin J.M., Jabaudon M., Lefrant J.Y., Jaber S., Quenot J.P., Langeron O., Ferrandière M., Grelon F., Seguin P., Ichai C., et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019;7:870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 9.Cavalcanti A.B., Suzumura É.A., Laranjeira L.N., Paisani D.M., Damiani L.P., Guimarães H.P., Romano E.R., Regenga M.M., Taniguchi L.N.T., Teixeira C., et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 11.Ball L., Serpa Neto A., Pelosi P. Obesity and survival in critically ill patients with acute respiratory distress syndrome: A paradox within the paradox. Crit. Care. 2017;21:114. doi: 10.1186/s13054-017-1682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boers N.S., Botta M., Tsonas A.M., Algera A.G., Pillay J., Dongelmans D.A., Horn J., Vlaar A.P.J., Hollmann M.W., Bos L.D.J., et al. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): Rationale and protocol for a national multicenter observational study in The Netherlands. Ann. Transl. Med. 2020;8:1251. doi: 10.21037/atm-20-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botta M., Tsonas A.M., Pillay J., Boers L.S., Algera A.G., Bos L.D.J., Dongelmans D.A., Hollmann M.W., Horn J., Vlaar A.P.J., et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir Med. 2020;9:139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [(accessed on 11 February 2021)]; Available online: https://sites.google.com/view/provent-covid/provent-covid.
- 15.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of Ventilator-Free Days in Critical Care Research. Am. J. Respir. Crit. Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien J.M., Jr., Welsh C.H., Fish R.H., Ancukiewicz M., Kramer A.M. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann. Intern. Med. 2004;140:338–345. doi: 10.7326/0003-4819-140-5-200403020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Anzueto A., Frutos-Vivar F., Esteban A., Bensalami N., Marks D., Raymondos K., Apezteguía C., Arabi Y., Hurtado J., González M., et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66:66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]
- 19.Kalra S.S., Siuba M., Panitchote A., Mireles-Cabodevila E., Chatburn R.L., Krishnan S., Duggal A. Higher Class of Obesity Is Associated With Delivery of Higher Tidal Volumes in Subjects With ARDS. Respir. Care. 2020;65:1519–1526. doi: 10.4187/respcare.07110. [DOI] [PubMed] [Google Scholar]
- 20.Kress J.P., Pohlman A.S., Alverdy J., Hall J.B. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am. J. Respir. Crit. Care Med. 1999;160:883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 21.De Jong A., Chanques G., Jaber S. Mechanical ventilation in obese ICU patients: From intubation to extubation. Crit. Care. 2017;21:63. doi: 10.1186/s13054-017-1641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jong A., Verzilli D., Jaber S. ARDS in Obese Patients: Specificities and Management. Crit. Care. 2019;23:74. doi: 10.1186/s13054-019-2374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guérin C., Albert R.K., Beitler J., Gattinoni L., Jaber S., Marini J.J., Munshi L., Papazian L., Pesenti A., Vieillard-Baron A., et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik P., Patel U., Patel K., Martin M., Shah C., Mehta D., Malik F.A., Sharma A. Obesity a predictor of outcomes of COVID-19 hospitalized patients-A systematic review and meta-analysis. J. Med. Virol. 2021;93:1188–1193. doi: 10.1002/jmv.26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Li Z., Yang T., Wang M., Xi X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS ONE. 2018;13:e0198669. doi: 10.1371/journal.pone.0198669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni Y.N., Luo J., Yu H., Wang Y.W., Hu Y.H., Liu D., Liang B.M., Liang Z.A. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit. Care. 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasselli G., Tonetti T., Protti A., Langer T., Girardis M., Bellani G., Laffey J., Carrafiello G., Carsana L., Rizzuto C., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostaghim A., Sinha P., Bielick C., Knudsen S., Beeram I., White L.F., Apovian C., Sagar M., Hochberg N.S. Clinical outcomes and inflammatory marker levels in patients with Covid-19 and obesity at an inner-city safety net hospital. PLoS ONE. 2020;15:e0243888. doi: 10.1371/journal.pone.0243888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Jong A., Wrigge H., Hedenstierna G., Gattinoni L., Chiumello D., Frat J.P., Ball L., Schetz M., Pickkers P., Jaber S. How to ventilate obese patients in the ICU. Intensive Care Med. 2020;46:2423–2435. doi: 10.1007/s00134-020-06286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request.