Abstract
The oral cancer constitutes 48% of head and neck cancer cases. Ninety percent of oral cancer cases are histologically diagnosed as oral squamous cell carcinomas (OSCCs). Despite new management strategies, the 5-year survival rate of oral cancer is still below 50% in most countries. Head and neck cancers are heterogeneous tumors, and this characteristic of them provides a challenge to treatment plan. Due to the poor outcomes in oral cancer, prevention is a necessity. In this review, a relevant English Literature search in PubMed, ScienceDirect, and Google Scholar from 2000 to mid-2018 was performed. All published articles related to oral cancer and its prevention were included. The risk factors of oral cancer and strategies of oral cancer prevention will be discussed.
Keywords: Early detection, HPV, mouth, neoplasm, prevention, tobacco
Introduction
Head and neck cancer is the sixth most common human cancer,[1,2] and the oral cancer constitutes 48% of head and neck cancer cases.[3] Ninety percent of oral cancer cases are histologically diagnosed as oral squamous cell carcinomas (OSCCs).[4] Despite new management strategies, the 5-year survival rate of oral cancer is still below 50% in most countries.[5] Head and neck cancers are heterogeneous tumors, and this characteristic of them provides a challenge to treatment plan.[6] Due to the poor outcomes in oral cancer, prevention is a necessity.[7] The development of OSCC is a multistep process which starts from some changes in the normal mucosa and continues until the development of invasive cancer and metastasis.[1] During this progress, the accumulation of multiple genetic and chromosomal alterations occurs.[1,4] Oral cancer is a multifactorial lesion and the risk factors include tobacco and alcohol, chronic inflammation, ultra violet (UV) radiation (for lip cancer), human papilloma virus (HPV) or Candida infections, immunosuppression, genetic predisposition, and diet.[8] Among them, tobacco use and alcohol consumption are considered as the main risk factors to develop malignancy in the oral cavity.[9] Oral inflammation is another suggested hypothesis for development of oral cancer due to involvement of several inflammation-related molecular pathways such as cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), p38a MAP kinase, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and STAT (signal transducer and activator of transcription).[10] Candida albicans genotype A strains have significantly been detected in oral cancer patients compared with non-cancer patients, and C. albicans has also been associated with leukoplakic lesions.[9] Immunosuppression has also been indicated as a risk factor for development of oral cancer in patients with renal transplant or bone marrow transplantation.[11] This review article aims to discuss the risk factors and some of new strategies for prevention of oral cancer.
Materials and Methods
A relevant English Literature search in PubMed, ScienceDirect, and Google Scholar from 2000 to mid-2018 was performed. All published articles related to oral cancer and its prevention were included in this review.
Risk factors
Tobacco, smokeless tobacco, and alcohol consumption
Tobacco smoking and alcohol consumption are considered as the main causal factors for oral cancer.[12] Besides, the World Health Organization (WHO) has labeled smokeless tobacco (SLT) as a carcinogenic agent although there is a controversy regarding the potential role of SLT in carcinogenesis. Naswar, a mixture of dried tobacco leaves, is kept in the buccal sulcus of the mouth and the active agents are absorbed through the oral mucosa. Naswar is cheap nicotine and is used in replacement therapy for people trying to quit smoking. The amount of carcinogenic agents differs among the different brands in the market. Thus, the risk of oral cancer associated with Naswar is challenging.[13] Cigarette smoke contains different compounds which have some effects on the gastrointestinal tract including oral cavity. Among them, nicotine is well known for its biological effects on the brain and other organs such as oral cavity.[14,15] Oral snuff and pipe tobacco also contain nicotine similar to cigarette tobacco.[15] The nicotine metabolites 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N′-nitrosonornicotine (NNN) have carcinogenic properties.[16] NNK and NNN bind to the nicotinic acetylcholine receptor to promote cell proliferation and create a microenvironment for tumor growth.[16] Alcohol alone has no association with cancer progression but synergistically functions with tobacco to develop cancer.[12] It is not clear how alcohol plays a role in oral carcinogenesis, but several mechanisms have been proposed. First, ethanol is metabolized into acetaldehyde, a known carcinogen. As acetaldehyde is a tumor promoter, the chronic consumption of alcohol promotes the development of oral cancer.[17] Second, alcohol contains some carcinogenic impurities such as polycyclic aromatic and nitrosamines. Finally, alcohol may contribute in solubilizing of other carcinogenic compounds that may increase the permeability of oral epithelium to these compounds and enhance the penetration of carcinogens into target tissues. The previous studies revealed that alcohol increases the permeability of oral mucosa that results in epithelial atrophy. Besides, alcohol decomposes the lipid composition of the epithelial cell membrane of oral mucosa, which facilities the penetration of carcinogens.[18] High levels of acetaldehyde production have been associated with certain Streptococcus species, Neisseria species, and other bacteria. Overgrowth of such bacteria has been demonstrated in smokers and heavy drinkers. These bacteria can metabolize ethanol to carcinogenic acetaldehyde and, therefore, are associated with the risk of head and neck squamous cell carcinoma (HNSCC).[19] Besides, Candida may also contribute to acetaldehyde production.[20]
HPV infection
HPV and human immunodefiency virus (HIV) are mainly involved viruses in the development of oral cancer.[20] HPV is a small virus containing a circular double-stranded DNA genome of approximately 8 kb. In head and neck area, HPV16 is the most common type associated with carcinogenesis, followed by HPV18. Regarding oropharyngeal squamous cell carcinoma (OPSCC), HPV infection is the main risk factor due to sexual behavior. The prevalence peak was found in older people especially in men showing a longer duration of infection at older ages.[21] In cases of oropharyngeal cancer, HPV positivity was mostly found in tonsils (94%), followed by base of the tongue (62%).[22,23] The possible mechanism is direct passage of pathogens like HPV from the mucosal lining of the tonsil and tonsillar crypts.[24] A previous published work indicated a high prevalence of genital HPV infection among U.S. men.[25] In addition, another study found the overall prevalence of oral HPV infection is 6.9% in the United States.[26] HPV16 has a higher risk for OPSCC.[22] E6 and E7 are the early proteins of HPV which have a key role in HPV-related OPSCC. E6 inhibits p53 and E7 binds to pRb, retinoblastoma protein.[27] It is suggested that some factors facilitate the oral infection of HPV.[28] For instance, it is believed that oral epithelial wound is a site for entry of the virus. Also, a previous study found a significant correlation between the number of extracted teeth and prevalence of oral HPV presence.[29] It is suggested that the basal layer of oral epithelium is infected by HPV as it happens for cervical epithelium.[28] It is also suggested that poor oral hygiene results in gingival inflammation which may help the penetration of HPV via the oral epithelial superficial layers to invade the basal layer.[28] HPV persistence can lead to a pre-malignant lesion which progresses to an invasive squamous cell carcinoma (SCC). Interestingly, it is proposed that smoking is associated with a higher risk for oral HPV infection. Although the exact mechanism of this association is not clear, it is believed that smoking induces pro-inflammatory and immunosuppressive effects that increase the risk of HPV oral infection.[30,31] HPV has been suggested as a critical etiological factor for OSCC in non-smokers and non-alcoholic drinkers as well.[32] In healthy control group, HPV positivity was detected in 12% of samples;[33] however, HPV positivity was demonstrated 2 to 3 times more in pre-cancerous lesions such as leukoplakia, erythroplakia, and oral submucous fibrosis, and 4.7 times more in oral cancer.[23] Regarding the oral site for distribution of HPV in cases of OSCC, tongue is the most prevalent site (50%) and the floor of mouth is the least prevalent site (26.8%).[34] In addition, HPV has been demonstrated as the etiology of some benign oral lesions including squamous papilloma and focal epithelial hyperplasia.[35] Interestingly, several studies have reported that HPV-related oral or oropharyngeal cancer have a better prognosis.[36] Prevention of cervical cancer associated with HPV16 and HPV18 infections has been done by vaccination of girls prior to sexual initiation which has an effective impact on oral and oropharyngeal cancer.[37]
The role of inflammation in cancer development
Chronic inflammation has an essential role in the development of some epithelial cancers such as oral and pharyngeal neoplasms.[38] Tumor microenvironment is connected to different steps of tumorigenesis and composed of different types of cells such as fibroblasts, myofibroblasts, adipose cells, immune and inflammatory cells, and extracellular matrix (ECM). ECM is composed of collagens, elastin, and proteoglycans/glycosaminoglycans. In pathological conditions, the biomechanical characteristics of ECM change and enhance cell migration which is essential for cancer development.[4] Besides, ECM is a reservoir for growth factors including fibroblast growth factors (FGFs), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), bone morphogenetic proteins (BMPs), and transforming growth factor beta (TGF-β).[39] Matrix metalloproteinases (MMPs) are necessary to degrade ECM.[40] For example, MMP2 and MMP9 degrade type IV collagen to facilitate invasion.[41] In HNSCC patients, the over-expression of MMP9 and MMP2 is associated with lymph node involvement.[42,43] A growing body of studies shows the overexpression of COX-2 in 80% of head and neck pre-malignant and malignant tissue samples. Besides, overexpression of COX-2 is associated with lymph node metastasis in head and neck cancer cases.[44] COX-2 induces carcinogenesis in murine models.[44,45]
Oral microbiome
Several cancers are linked to microorganisms such as HPV (cervical cancer, oropharyngeal cancer),[46] EBV (head and neck cancer),[47] hepatitis B and C virus (hepatocellular carcinoma),[48] Helicobacter pylori (gastroduodenal cancer),[49] and Porphyromonas gingivalis (orodigestive cancer).[50,51] More than 700 different bacterial species colonize the human oral cavity, called the oral microbiome.[52] The oral microbiota has a critical role in human health such as immune response, nutrient digestion, and carcinogen metabolism. Gingivitis and periodontitis are the most common inflammatory tooth structure conditions[53] and the main causative microorganism for periodontitis is Porphyromonas gingivalis. A previous study on OSCC showed that P. gingivalis interacts with oral cancer cells.[54] Another published work demonstrated that periodontitis promotes the development of oral leukoplakia in a dose-dependent manner.[55] Poor oral health is correlated to elevated risk of several diseases including cancers such as head and neck cancer, esophagus cancer, stomach cancer, and pancreas cancers. The mechanism is not clear, perhaps oral microbiome such as H. pylori causes chronic inflammation which in turn participates in cancer pathogenesis such as gastric cancer and oral cancer.[52,53,56,57,58]
Genetic predisposition
Genetic predisposition plays a critical role in the development of OSCC, especially tongue and buccal mucosa cancers.[4,59] It is suggested that in 29.5% of first-degree relatives of head and neck cancer patients, other cancers such as respiratory tract and upper orodigestive tract cancers can develop.[60] However, in terms of oral cancer, it is hard to determine genetic or familial disposition due to coexistence of risk factors such as smoking and alcohol. Some investigators believe that people who inherit the inability to metabolize carcinogens or pro-carcinogens are not able to repair DNA damage; therefore, they are susceptible to develop an oral malignancy. For example, in cases of tobacco-induced head and neck cancers, genetic polymorphisms of the P450 enzymes, and xenobiotic metabolizing enzymes (XMEs) which are responsible for tobacco carcinogen metabolism play an essential role in the genetic predisposition.[61] The inactivation of p53 and pRb tumor suppressor gene leads to the accumulation of genomic alterations, inhibition of the apoptotic signaling, and an increase of the telomerase activity.[24,62] Epithelial-mesenchymal transition (EMT) process contributes to cancer stemness and cancer metastasis.[63,64,65,66] Prior study demonstrated that areca nut extract or arecoline activates several EMT-related molecules, such as vimentin, in oral epithelial cells.[67]
Precancerous lesions and the field cancerization
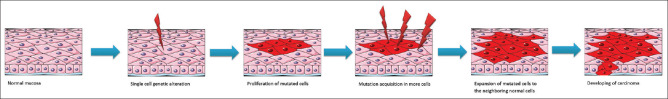
It is estimated that 50% of oral cancers develop from precursor lesions.[5] Therefore, early detection and proper management of the pre-malignant lesions play a critical role in preventive programs.[7] However, a prior published paper indicated that surgically treated pre-cancerous lesions cannot reduce the rate of malignant transformation perhaps due to field cancerization.[5] The concept of field cancerization, first coined by Slaughter et al. in 1953, explains the development of multiple primary tumors and recurrent local tumors. This term is a phenomenon by which molecular alterations develop in normal-appearing tissue and, overtime, form premalignant lesions which progress to dysplasia and finally to a frank cancer.[68] In the beginning, the oral epithelium exposed to a carcinogen may look like a normal mucosa.[3] Oral epithelial dysplasia is a precancerous change and has a high risk of progressing to oral cancer.[7,69] Oral premalignant lesions include leukoplakia, erythroplakia, submucous fibrosis, reverse smoking, lichen planus,[69,70] and discoid lupus erythematosus.[7] Tobacco smoking is one of the factors which increases the risk of malignant changes in oral epithelium. Increased proliferation rate of oral epithelium due to tobacco smoking occurs even after cessation of smoking which suggests that smoking involves field cancerization.[3] Loss of chromosomal material in 3p, 9q, and 17p has been reported as the early markers of carcinogenesis in dysplastic lesions.[71] Braakhuis et al. updated the model of field cancerization. According to their proposal, the stem cells of the proliferative basal layer are involved in carcinogenesis.[72] Van Houton et al. demonstrated the p53 gene-mutated cells called patches as an early event in oral cancer. They proposed that the altered cells acquire more mutations and spread laterally to replace the normal epithelial cells.[73] These cluster of cells show TP53 mutation especially in patients with multiple primary head and neck tumors.[72] Overtime, the accumulation of mutations within the fields results in invasive carcinoma with metastatic potential.[74] Lydiatt et al. found that clinically normal appearing oral mucosa surrounding cancer harbors early pre-malignant genetic alterations. The field of cancerization may extend from 4 mm to 7 cm.[75] Figure 1 summarizes the mechanism of genetic alteration and field cancerization to develop oral carcinoma.
Figure 1.
Schematic mechanism of genetic alteration and field cancerization to develop a carcinoma
Oral cancer prevention strategies
Probiotics
Recently published studies assessed microbial compositions in patients with OSCC. Interestingly, the microbial flora of patients was different from healthy individuals. It is suggested that some factors such as diet, age, and smoking have effects on the microbial composition and development of OSCC. It is assumed that cytokines and other secretory mediators of bacteria lead to malignant transformation. There are accumulating evidence to indicate a higher incidence of oral cancer in patients with poor oral hygiene.[76] Probiotics are live microorganisms which provide a health benefit on the host. The probiotic products mostly contain lactic acid bacteria and help host for the maintenance of intestinal microbial balance. Recent data on probiotic products show a protective effect against the carcinogenic activity of mediators of bacteria. Among them, Lactobacillus acidophilus and Lactobacillus casei have been known for reducing the level of cytokines and mediators of bacteria. Besides, consuming the probiotics significantly reduces Streptococcus mutans counts which prevents tooth decay, improves periodontitis, and is effective for the treatment of oral candidiasis.[76] Previous studies have shown that consumption of probiotics is useful for treatment of periodontitis and elimination of halitosis[76] as well as controlling oral candidiasis and hyposalivation in elderly patients.[77] Also, probiotics have potential for prevention of tumor formation and metastasis. A daily probiotic drink for 6 months enhances the clearance of HPV and cervical pre-malignant lesions.[78]
Chemoprevention
Chemoprevention is a promising plan to inhibit, suppress, or control the carcinogenesis. Regarding oral lesions, leukoplakia and postoperative oral cancer patients are the best target population for chemoprevention.[79] There are some natural products to prevent oral cancer such as polyphenols and strong antioxidants like vitamin C, E, and carotenoids.[2] Vitamin A, isotretinoin (13-cis retinoic acid), green tea extract, and some medicinal herbs have been used as chemo-preventive agents.[79] Curcumin, an active constituent of turmeric, is a polyphenol used as a spice or medicine in India.[80,81] Curcumin has anticancer effects in HNSCC due to anti-inflammatory effect via down-regulation of NF-κB and pro-apoptotic effects through up-regulation and activation of p53 and p21.[82,83] Besides, curcumin inhibits tumor growth via targeting VEGF, the most powerful angiogenic factor, and the EGFR.[82,84] Interestingly, curcumin inhibits HPV oncoprotein transcription via inhibiting AP-1 transcription factor and via interaction between curcumin and P53 binding site of E6 protein of HPV16.[85] Other sources of polyphenols are grape seed extract, green tea extract, cocoa extract, and coffee.[86,87,88] Previous epidemiological studies found an association between coffee consumption and reduced cancer incidence or mortality.[89] A previous study revealed that coffee consumption can reduce the risk of oral cancer.[90] Another meta-analysis found an association between reduced risk of oral cancer and tea consumption.[91] Hong et al. used isotretinoin to treat oral leukoplakia. They showed isotretinoin could decrease the size of the lesion and also reversed oral dysplastic changes,[92] even though isotretinoin could prevent recurrence of tumor in HNSCC patients in a dose-dependent manner.[82] In addition, isotretinoin in combination with interferon-α and α-tocopherol (vitamin E) has a great effect on oral pre-malignant lesions.[93] Luteolin, a flavonoid, found in vegetables like cabbage, celery, broccoli, and parsley, is absorbed by the oral epithelium and has anti-inflammatory effects by blocking the NF-κB pathway. Delivery of luteolin in nanoparticles inhibited the growth of HNSCC tumors in vitro and in vivo.[94] Lycopene, a natural compound in red-colored carotenoid especially in tomatoes, decreases the risk of cancer. Anti-cancer effect of lycopene acts by different mechanisms. For example, lycopene inhibits VEGF-mediated angiogenesis and also decreases plasma levels and activity of MMP-2 and MMP-9 to inhibit invasion and metastasis.[82] Pomegranate, the fruit of the tree Punica granatum, has anti-oxidant and anti-inflammatory effects. The polyphenol-rich fractions of pomegranate contain polyphenol which inhibits the growth of cancer cells such as breast cancer. Pomegranate decreases the expression levels of VEGF and hypoxia-inducible factor 1-alpha (HIF-1α).[95] Omega-3 fatty acids are also used for cancer prevention. Omega-3 fatty acids reduce production of cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF).[96] Resolvin D-series (RvDs) are endogenous lipid mediators derived from omega-3 fatty acids and have anti-cancer and analgesic effects on OSCC.[97] Diets containing vegetables like broccoli, cabbage, and cauliflower are associated with reduced risk of HNSCC. It is clear that broccoli extracts promote detoxication of chemical carcinogens found in tobacco smoke and the airborne pollutants acrolein and benzene.[98] Besides, salted food and some that contain preservatives develop head and neck cancer. Improper diet and nutrition including lack of vegetables, fruits, and vitamins are suggested to be another risk factor for oral cancer, as some food contain antioxidants which can inhibit DNA mutation and change in enzyme activity.[52,99] Mediterranean diet (MD), rich in monounsaturated fatty acids from olive oil, vegetables fruits, fish, low-fat dairy, moderate alcohol intake, and low red meat consumption, reduces the risk of developing many diseases including head and neck cancer due to α-tocopherol and phenol oils and being good sources of dietary fiber and antioxidants.[100] Pterostilbene, a pantropical genus of trees, inhibits DNA synthesis. Pterostilbene induces apoptosis of oral cancer cells via activation of caspase-3, -8, and -9. Iranian orthodox black tea extracts induces apoptosis in oral cancer cells in vitro.[2] H. pylori is a resident in the oral cavity especially in the developing countries. Eradication of H. pylori infection is difficult.[56,57,58] Sulforaphane, a compound obtained from cruciferous vegetables such as broccoli, Brussels sprouts, and cabbages, kills H. pylori which is recognized as the main cause of stomach cancer.[101,102]
The role of anti-inflammatory drugs on cancer prevention
Celecoxib (CXB), a selective COX-2 inhibitor, is approved for use in early cancer prevention in some lesions such as familial adenomatous polyposis. CXB has different anti-cancer progression molecular mechanisms including anti-angiogenesis activity via decreasing VEGF production and anti-EMT properties.[103] In murine model study, CXB inhibited EMT phenomenon in OSCC.[104] Aspirin, another anti-inflammatory drug, significantly reduces the risk of colorectal cancer.[105] A previous study on aspirin and its role in head and neck cancer found an association between the reduction of risk of cancer especially in people with low to moderate exposure to cigarette smoke or alcohol consumption.[106] Another study demonstrated an improved survival rate in patients with head and neck cancer who used aspirin after diagnosis.[107] However, a recent published work did not find any effect of aspirin on survival or recurrence of HNSCC cases.[108]
Cancer screening, early detection
Cancers detected at early stages can be treated more successfully. Delay in diagnosis has effect on cancer-related morbidity and mortality. Therefore, screening and early clinical diagnosis help to provide more safe and cheap treatments. Awareness of health care providers has a great impact on early diagnosis.[52,109,110] It is critical that oral health professionals understand the importance of oral screening examination for pre-malignant and malignant lesions.[110,111,112,113] Dentists are usually the first health care professionals who examine the oral cavity and, therefore, have the opportunity to screen oral cancer.[1,4,111,112,113,114] A visible precancerous lesion helps the practitioners in early detection and treatment.[115] Moreover, the mouth self-examination is another way to detect oral cancer at early stage.[116] Salivary biomarkers can also help in the diagnosis of potentially malignant and malignant disorders of the mouth. For example, Cyfra-21-1, tissue polypetide antigen [TPA], and cancer antigen CA-125 are tumor markers which are significantly increased in saliva of patients with OSCC.[117] Choline and pipecolate can be detected in the saliva of patients with OSCC even in early stages.[118] Altogether, the best diagnostic technique is experience and training. Salivary biomarkers help in early detection of oral cancer. Some other methods such as toluidine blue staining and lugol staining are helpful in the screening of pre-malignant and malignant lesions of the oral cavity. Besides, tissue biopsy and histopathological examination should remain the gold standard for oral cancer diagnosis.[119] Like the cervical cancer, the incidence of OPSCC in women is decreasing in the United States due to increased preventive health care such as screening.[120]
The role of biomarkers for oral cancer detection
Carcinogenesis is a multistep process, and recent studies on molecular biology have indicated the genetic basis of the process of carcinogenesis.[63,121] Secretory leukocyte protease inhibitor (SLPI) has broad anti-microbial and anti-inflammatory properties and has been considered as a potential diagnostic and prognostic biomarker in head and neck cancer. Besides, SLPI protects against head and neck cancer development. Salivary SLPI reduces transmission of HIV in the oral cavity and also protects against oral HPV infection. It is suggested that SLPI protects oral mucosa against proteolysis, epithelial tissue damage, and degradation in cases of prolonged epithelial cell exposure to carcinogens such as tobacco and alcohol. SLPI protein expression decreases the risk of lymph node metastases.[122] CD44, a cell surface transmembrane glycoprotein, is overexpressed in pre-malignant lesions of the larynx and stomach.[123,124] CD44 is found in body fluids such as saliva; a prior study suggested CD44 and total protein as reliable markers to test and screen increased risk of oral cancer.[125] Previous studies on OSCC and mucoepidermoid carcinoma, the most prevalent malignant salivary gland tumor, suggested CD44 as an effective head and neck cancer biomarker.[63,65] Zhong et al. detected telomerase activity in the saliva of the 75% of patients with OSCC. They suggested the telomerase detection as an assistant marker in the OSCC.[126] Different molecular biomarkers have been investigated regarding the heterogeneity of head and neck cancers[7,63,64,65] such as E-cadherin and VE-cadherin as the components of adherent junctions involved in EMT. Also DNA repair proteins like BRCA1/2 are linked to treatment outcomes.[121] BRCA1/2 genes are involved in DNA repair in cancers such as breast cancer and ovarian cancer. A recent published work revealed the BRCA1/2 mutations in salivary gland tumors.[121] In addition, miRNAs also have an impact on the head and neck cancer growth and metastasis.[6,7,127] Several recently published works demonstrated circular miRNAs as a non-invasive biomarker in early detection of different cancers.[6,84,128] Knowledge about biomarkers may help to predict prognosis and decrease the rate of mortality due to finding a new target therapy.[129] Table 1 gives a summary of risk factors and the prevention strategies of oral cancer.
Table 1.
Summary of risk factors and prevention strategies of oral cancer
| Risk factors | Prevention strategies |
|---|---|
| Tobacco, smokeless tobacco | Probiotics |
| Alcohol consumption | Chemoprevention |
| HPV infection | Anti-inflammatory drugs |
| Inflammation | Cancer screening |
| Oral microbiome | Early detection |
| Genetic predisposition | Using biomarkers |
| Precancerous lesions | |
| Field cancerization |
Conclusions
Several risk factors might be involved in the development of oral cancer. Among them, tobacco smoking, alcohol consumption, and HPV are the most studied risk factors. Besides, inflammation and genetic susceptibility are believed to play an essential role. Many studies have focused on oral cancer risk factors, early detection, and cancer treatments. Due to high morbidity and mortality rates of oral cancer, more effective strategies and treatment plans are needed. Surgery is the main treatment strategy for patients with oral cancer. Radiotherapy and chemotherapy are reserved for patients who are not able to tolerate surgery.[130] On the contrary, all the aforementioned treatment strategies have serious consequences such as emotional and physical disorders. Oral cancer prevention and early detection can reduce those serious consequences. Currently, the role of probiotics and natural products in the prevention and treatment of oral cancer has been studied, but further studies are required to evaluate the benefits of them in oral cancer prevention and treatment. Thus, the awareness of public and clinicians of the risk factors and early signs of oral cancer has a great impact on prevention. In addition, better understanding of molecular pathways in the development of oral cancer improves prevention and early detection. Much work has to be done to identify strategies to decrease morbidity and mortality rates of oral cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to thank Hamadan University of Medical Sciences for financial support.
References
- 1.Irani S. Metastasis to head and neck area: A 16-year retrospective study. Am J Otolaryngol. 2011;32:24–7. doi: 10.1016/j.amjoto.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Irani S. Herbal medicine and oral health: A review. J Int Oral Health. 2016;8:989–94. [Google Scholar]
- 3.Nakashima T, Tomita H, Hirata A, Ishida K, Hisamatsu K, Hatano Y, et al. Promotion of cell proliferation by the proto-oncogene DEK enhances oral squamous cell carcinogenesis through field cancerization. Cancer Med. 2017;6:2424–39. doi: 10.1002/cam4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent. 2016;6:265–71. doi: 10.4103/2231-0762.186805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sathiasekar AC, Mathew DG, Jaish Lal MS, Arul Prakash AA, Goma Kumar KU. Oral field cancerization and its clinical implications in the management in potentially malignant disorders. J Pharm Bioallied Sci. 2017;9(Suppl 1):S23–5. doi: 10.4103/jpbs.JPBS_109_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani S. miRNAs signature in head and neck squamous cell carcinoma metastasis: A literature review. J Dent (Shiraz, Iran) 2016;17:71–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Irani S. Pre-cancerous lesions in the oral and maxillofacial region: A literature review with special focus on etiopathogenesis. Iran J Pathol. 2016;11:303–22. [PMC free article] [PubMed] [Google Scholar]
- 8.Ram H, Sarkar J, Kumar H, Konwar R, Bhatt MLB, Mohammad S. Oral cancer: Risk factors and molecular pathogenesis. J Maxillofac Oral Surg. 2011;10:132–7. doi: 10.1007/s12663-011-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alnuaimi AD, Wiesenfeld D, O'Brien-Simpson NM, Reynolds EC, McCullough MJ. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015;51:139–45. doi: 10.1016/j.oraloncology.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Sarode GS, Sarode SC, Patil A, Anand R, Patil SG, Rao RS, et al. Inflammation and oral cancer: An update review on targeted therapies. J Contemp Dent Pract. 2015;16:595–602. doi: 10.5005/jp-journals-10024-1727. [DOI] [PubMed] [Google Scholar]
- 11.Jewett A, Head C, Cacalano NA. Emerging mechanisms of immunosuppression in oral cancers. J Dent Res. 2006;85:1061–73. doi: 10.1177/154405910608501201. [DOI] [PubMed] [Google Scholar]
- 12.Rock LD, Rosin MP, Zhang L, Chan B, Shariati B, Laronde DM. Characterization of epithelial oral dysplasia in non-smokers: First steps towards precision medicine. Oral Oncol. 2018;78:119–25. doi: 10.1016/j.oraloncology.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan Z, Dreger S, Shah SMH, Pohlabeln H, Khan S, Ullah Z, et al. Oral cancer via the bargain bin: The risk of oral cancer associated with a smokeless tobacco product (Naswar) PloS one. 2017;12:e0180445. doi: 10.1371/journal.pone.0180445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen K, Afroze S, Munshi MK, Guerrier M, Glaser SS. Mechanisms for nicotine in the development and progression of gastrointestinal cancers. Transl Gastroint Cancer. 2012;1:81–7. doi: 10.3978/j.issn.2224-4778.2011.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikh MN, Hanif S, Zia M, Qayyum Z. Effects of nicotine on an in vitro reconstituted model oral mucosa in terms of cytokine production. J Ayub Med Coll Abbottabad. 2011;23:80–4. [PubMed] [Google Scholar]
- 16.Xue J, Yang S, Seng S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers. 2014;6:1138–56. doi: 10.3390/cancers6021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan Z, Bisen PS. Oncoapoptotic signaling and deregulated target genes in cancers: Special reference to oral cancer. Biochim Biophys Acta. 2013;1836:123–45. doi: 10.1016/j.bbcan.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Feng L, Wang L. Effects of alcohol on the morphological and structural changes in oral mucosa. Pak J Med Sci. 2013;29:1046–9. doi: 10.12669/pjms.294.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes RB, Ahn J, Fan X, Peters BA, Ma Y, Yang L, et al. Association of oral microbiome with risk for incident head and neck squamous cell cancer. JAMA Oncol. 2018;4:358–65. doi: 10.1001/jamaoncol.2017.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neville B, Damm DD, Allen C, Chi A. Oral and Maxillofacial Pathology. 4th ed. Canada: Saunders Elsevier; 2016. [Google Scholar]
- 21.Shigeishi H, Sugiyama M. Risk factors for oral human papillomavirus infection in healthy individuals: A systematic review and meta-analysis. J Clin Med Res. 2016;8:721–9. doi: 10.14740/jocmr2545w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elrefaey S, Massaro M, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:299–309. [PMC free article] [PubMed] [Google Scholar]
- 23.Yete S, D'Souza W, Saranath D. High-risk human papillomavirus in oral cancer: Clinical implications. Oncology. 2018;94:133–41. doi: 10.1159/000485322. [DOI] [PubMed] [Google Scholar]
- 24.Taberna M, Mena M, Pavon MA, Alemany L, Gillison ML, Mesia R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol. 2017;28:2386–98. doi: 10.1093/annonc/mdx304. [DOI] [PubMed] [Google Scholar]
- 25.Han JJ, Beltran TH, Song JW, Klaric J, Choi YS. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National health and nutrition examination survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810–6. doi: 10.1001/jamaoncol.2016.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S, Dong Y. Human papillomavirus and oral squamous cell carcinoma: A review of HPV-positive oral squamous cell carcinoma and possible strategies for future. Curr Probl Cancer. 2017;41:323–7. doi: 10.1016/j.currproblcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Buitrago-Pérez Á, Garaulet G, Vázquez-Carballo A, Paramio JM, García-Escudero R. Molecular signature of HPV-induced carcinogenesis: pRb, p53 and gene expression profiling. Curr Genomics. 2009;10:26–34. doi: 10.2174/138920209787581235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalla Torre D, Burtscher D, Solder E, Rasse M, Puelacher W. The correlation between the quality of oral hygiene and oral HPV infection in adults: A prospective cross-sectional study. Clin Oral Investig. 2019;23:179–85. doi: 10.1007/s00784-018-2425-y. [DOI] [PubMed] [Google Scholar]
- 29.Bui TC, Markham CM, Ross MW, Mullen PD. Examining the association between oral health and oral HPV infection. Cancer Prev Res (Phila) 2013;6:917–24. doi: 10.1158/1940-6207.CAPR-13-0081. [DOI] [PubMed] [Google Scholar]
- 30.Sonawane K, Suk R, Chiao EY, Chhatwal J, Qiu P, Wilkin T, et al. Oral human papillomavirus infection: Differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714–24. doi: 10.7326/M17-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CC, Palefsky JM. Human papillomavirus-related oropharyngeal cancer in the HIV-infected population. Oral Dis. 2016;22(Suppl 1):98–106. doi: 10.1111/odi.12365. [DOI] [PubMed] [Google Scholar]
- 32.Vargas-Ferreira F, Nedel F, Etges A, Gomes AP, Furuse C, Tarquinio SB. Etiologic factors associated with oral squamous cell carcinoma in non-smokers and non-alcoholic drinkers: A brief approach. Braz Dent J. 2012;23:586–90. doi: 10.1590/s0103-64402012000500020. [DOI] [PubMed] [Google Scholar]
- 33.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):S59–66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Chocolatewala NM, Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: An Indian perspective. J Cancer Res Ther. 2009;5:71–7. doi: 10.4103/0973-1482.52788. [DOI] [PubMed] [Google Scholar]
- 35.Candotto V, Lauritano D, Nardone M, Baggi L, Arcuri C, Gatto R, et al. HPV infection in the oral cavity: Epidemiology, clinical manifestations and relationship with oral cancer. Oral Implantol (Rome) 2017;10:209–20. doi: 10.11138/orl/2017.10.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalavrezos N, Scully C. Mouth cancer for clinicians part 13: Life after mouth cancer treatment. Dent Update. 2016;43:672. doi: 10.12968/denu.2016.43.7.672. [DOI] [PubMed] [Google Scholar]
- 37.Nowosielska-Grygiel J, Owczarek K, Bielinska M, Waclawek M, Olszewski J. Analysis of risk factors for oral cavity and oropharynx cancer in the authors' own material. Otolaryngol Pol. 2017;71:23–8. doi: 10.5604/01.3001.0009.8411. [DOI] [PubMed] [Google Scholar]
- 38.Shivappa N, Hebert JR, Rosato V, Garavello W, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of oral and pharyngeal cancer in a large case-control study from Italy. Int J Cancer. 2017;141:471–9. doi: 10.1002/ijc.30711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31(Suppl 1):177–83. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 41.Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17:434. doi: 10.1186/s12885-017-3418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markwell SM, Weed SA. Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion. Cancers. 2015;7:382–406. doi: 10.3390/cancers7010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietruszewska W, Bojanowska-Pozniak K, Kobos J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An immunohistochemical study. Otolaryngol Pol. 2016;70:32–43. doi: 10.5604/00306657.1202546. [DOI] [PubMed] [Google Scholar]
- 44.Mohammad S, Ram H, Gupta PN, Husain N, Bhatt M. Overexpression of COX-2 in oral squamous cell carcinoma patients undergoing chemoradiotherapy. Nat J Maxillofac Surg. 2011;2:17–21. doi: 10.4103/0975-5950.85848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormick DL, Phillips JM, Horn TL, Johnson WD, Steele VE, Lubet RA. Overexpression of COX-2 in rat oral cancers and prevention of oral carcinogenesis in rats by selective and non-selective COX inhibitors. Cancer Prev Res (Philadelphia, Pa) 2010;3:73–81. doi: 10.1158/1940-6207.CAPR-09-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St Laurent J, Luckett R, Feldman S. HPV vaccination and the effects on rates of HPV-related cancers. Curr Probl Cancer. 2018;42:493–506. doi: 10.1016/j.currproblcancer.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes Q, Merhi M, Raza A, Inchakalody VP, Abdelouahab N, Zar Gul AR, et al. Role of epstein-barr virus in the pathogenesis of head and neck cancers and its potential as an immunotherapeutic target. Front Oncol. 2018;8:257. doi: 10.3389/fonc.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore MS, Bocour A, Tran OC, Qiao B, Schymura MJ, Laraque F, et al. Effect of hepatocellular carcinoma on mortality among individuals with Hepatitis B or Hepatitis C infection in New York City, 2001-2012. Open Forum Infect Dis. 2018;5:ofy144. doi: 10.1093/ofid/ofy144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinagre R, Vinagre IDF, Vilar ESA, Fecury AA, Martins LC. Helicobacter pylori infection and immune profile of patients with different gastroduodenal diseases. Arq Gastroenterol. 2018;55:122–7. doi: 10.1590/S0004-2803.201800000-21. [DOI] [PubMed] [Google Scholar]
- 50.Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16:131–45. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo BH, Kim DJ, Choi JI, Kim SJ, Park BS, Song JM, et al. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget. 2017;8:46981–92. doi: 10.18632/oncotarget.16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irani S. Oral Health and Related Factors: An Update. J Int Oral Health. 2016;8:140–4. [Google Scholar]
- 53.Irani S. Orofacial bacterial infectious diseases: An update. J Int Soc Prev Community Dent. 2017;7(Suppl 2):S61–7. doi: 10.4103/jispcd.JISPCD_290_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–15. doi: 10.4248/IJOS11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–9. [PMC free article] [PubMed] [Google Scholar]
- 56.Irani S, Monsef Esfahani A, Bidari Zerehpoush F. Detection of helicobacter pylori in oral lesions. J Dent Res Dent Clin Dent Prospects. 2013;7:230–7. doi: 10.5681/joddd.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Irani S, Monsef Esfahani A, Sabeti S, Bidari Zerehpoush F. Detection of helicobacter pylori in oral lichen planus and oral lichenoid reaction. Avicenna J Dent Res. 2014;6:e23213. [Google Scholar]
- 58.Monsef Esfahani A, Irani S, Sabeti S, Bidari Zerehpoush F. The possible role of helicobacter pylori in the development of Sjogren's syndrome and chronic sialadenitis. Avicenna J Dent Res. 2015;7:e23212. [Google Scholar]
- 59.Ali J, Sabiha B, Jan HU, Haider SA, Khan AA, Ali SS. Genetic etiology of oral cancer. Oral Oncol. 2017;70:23–8. doi: 10.1016/j.oraloncology.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Kim KM, Kim YM, Shim YS, Kim KH, Chang HS, Choi JO, et al. Epidemiologic survey of head and neck cancers in Korea. J Korean Med Sci. 2003;18:80–7. doi: 10.3346/jkms.2003.18.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12:458–63. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- 62.Shah A, Malik A, Garg A, Mair M, Nair S, Chaturvedi P. Oral sex and human papilloma virus-related head and neck squamous cell cancer: A review of the literature. Postgrad Med J. 2017;93:704–9. doi: 10.1136/postgradmedj-2016-134603. [DOI] [PubMed] [Google Scholar]
- 63.Irani S, Dehghan A. The expression and functional significance of vascular endothelial-cadherin, CD44, and vimentin in oral squamous cell carcinoma. J Int Soc Prev Community Dent. 2018;8:110–7. doi: 10.4103/jispcd.JISPCD_408_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irani S, Dehghan A. Expression of vascular endothelial-cadherin in mucoepidermoid carcinoma: Role in cancer development. J Int Soc Prev Community Dent. 2017;7:301–7. doi: 10.4103/jispcd.JISPCD_323_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irani S, Jafari B. Expression of vimentin and CD44 in mucoepidermoid carcinoma: A role in tumor growth. Indian J Dent Res. 2018;29:333–40. doi: 10.4103/ijdr.IJDR_184_17. [DOI] [PubMed] [Google Scholar]
- 66.Irani S, Salajegheh A, Smith RA, Lam AK. A review of the profile of endothelin axis in cancer and its management. Crit Rev Oncol Hematol. 2014;89:314–21. doi: 10.1016/j.critrevonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Wang TY, Peng CY, Lee SS, Chou MY, Yu CC, Chang YC. Acquisition cancer stemness, mesenchymal transdifferentiation, and chemoresistance properties by chronic exposure of oral epithelial cells to arecoline. Oncotarget. 2016;7:84072–81. doi: 10.18632/oncotarget.11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 69.Irani S, Monsef Esfahani A, Ghorbani A. Dysplastic change rate in cases of oral lichen planus: A retrospective study of 112 cases in an Iranian population. J Oral Maxillofac Pathol. 2016;20:395–9. doi: 10.4103/0973-029X.190911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Irani S. Squamous cell carcinoma arising in oral lichen planus. DJH. 2010;1:1–6. [Google Scholar]
- 71.Pitiyage G, Tilakaratne WM, Tavassoli M, Warnakulasuriya S. Molecular markers in oral epithelial dysplasia: Review. J Oral Pathol Med. 2009;38:737–52. doi: 10.1111/j.1600-0714.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 72.Braakhuis BJ, Leemans CR, Brakenhoff RH. A genetic progression model of oral cancer: Current evidence and clinical implications. J Oral Pathol Med. 2004;33:317–22. doi: 10.1111/j.1600-0714.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 73.van Houten VM, Tabor MP, van den Brekel MW, Kummer JA, Denkers F, Dijkstra J, et al. Mutated p53 as a molecular marker for the diagnosis of head and neck cancer. J Pathol. 2002;198:476–86. doi: 10.1002/path.1242. [DOI] [PubMed] [Google Scholar]
- 74.Mohan M, Jagannathan N. Oral field cancerization: An update on current concepts. Oncol Rev. 2014;8:244. doi: 10.4081/oncol.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lydiatt WM, Anderson PE, Bazzana T, Casale M, Hughes CJ, Huvos AG, et al. Molecular support for field cancerization in the head and neck. Cancer. 1998;82:1376–80. [PubMed] [Google Scholar]
- 76.Aghazadeh Z, Pouralibaba F, Yari Khosroushahi A. The prophylactic effect of Acetobacter syzygii probiotic species against squamous cell carcinoma. J Dent Res Dent Clin Dent Prospects. 2017;11:208–14. doi: 10.15171/joddd.2017.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hatakka K, Ahola AJ, Yli-Knuuttila H, Richardson M, Poussa T, Meurman JH, et al. Probiotics reduce the prevalence of oral candida in the elderly--a randomized controlled trial. J Dent Res. 2007;86:125–30. doi: 10.1177/154405910708600204. [DOI] [PubMed] [Google Scholar]
- 78.Verhoeven V, Renard N, Makar A, Van Royen P, Bogers JP, Lardon F, et al. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: A prospective controlled pilot study. Eur J Cancer Prev. 2013;22:46–51. doi: 10.1097/CEJ.0b013e328355ed23. [DOI] [PubMed] [Google Scholar]
- 79.Xie A, Liu J. Chemoprevention of oral cancer in leukoplakia patients: A systematic review and meta-analysis. J Pak Med Assoc. 2017;67:1415–9. [PubMed] [Google Scholar]
- 80.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 81.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149–72. doi: 10.1007/978-0-387-46401-5_5. [DOI] [PubMed] [Google Scholar]
- 82.Crooker K, Aliani R, Ananth M, Arnold L, Anant S, Thomas SM. A review of promising natural chemopreventive agents for head and neck cancer. Cancer Prev Res (Philadelphia, Pa) 2018;11:441–50. doi: 10.1158/1940-6207.CAPR-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maroof H, Irani S, Ariana A, Vider J, Gopalan V, Lam AK. Interactions of vascular endothelial growth factor and p53 with miR-195 in thyroid carcinoma: Possible therapeutic targets in aggressive thyroid cancers? Curr Cancer Drug Targets. 2018 doi: 10.2174/1568009618666180628154727. doi: 10.2174/1568009618666180628154727. [DOI] [PubMed] [Google Scholar]
- 85.Mahran RI, Hagras MM, Sun D, Brenner DE. Bringing curcumin to the clinic in cancer prevention: A review of strategies to enhance bioavailability and efficacy. AAPS J. 2017;19:54–81. doi: 10.1208/s12248-016-0003-2. [DOI] [PubMed] [Google Scholar]
- 86.Ramshankar V, Krishnamurthy A. Chemoprevention of oral cancer: Green tea experience. J Nat Sci Biol Med. 2014;5:3–7. doi: 10.4103/0976-9668.127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cádiz-Gurrea M, Borrás-Linares I, Lozano-Sánchez J, Joven J, Fernández-Arroyo S, Segura-Carretero A. Cocoa and grape seed byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins? Int J Mol Sci. 2017:18. doi: 10.3390/ijms18020376. doi: 10.3390/ijms18020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li YM, Peng J, Li LZ. Coffee consumption associated with reduced risk of oral cancer: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:381–9e1. doi: 10.1016/j.oooo.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Alicandro G, Tavani A, La Vecchia C. Coffee and cancer risk: A summary overview. Eur J Cancer Prev. 2017;26:424–32. doi: 10.1097/CEJ.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Wang X, Cui D. Association between coffee consumption and the risk of oral cancer: A meta-analysis of observational studies. Int J Clin Exp Med. 2015;8:11657–65. [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Yang Y, Zhang W, Wu W. Association of tea consumption and the risk of oral cancer: A meta-analysis. Oral Oncol. 2014;50:276–81. doi: 10.1016/j.oraloncology.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 92.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 93.Gorsky M, Epstein JB. The effect of retinoids on premalignant oral lesions: Focus on topical therapy. Cancer. 2002;95:1258–64. doi: 10.1002/cncr.10874. [DOI] [PubMed] [Google Scholar]
- 94.Majumdar D, Jung KH, Zhang H, Nannapaneni S, Wang X, Amin A, et al. Luteolin nanoparticle in chemoprevention-in vitro and in vivo anticancer activity. Cancer Prev Res (Philadelphia, Pa) 2014;7:65–73. doi: 10.1158/1940-6207.CAPR-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma P. Pomegranate for prevention and treatment of cancer: An update. Molecules. 2017;22 doi: 10.3390/molecules22010177. doi: 10.3390/molecules22010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang P, Henning SM, Schokrpur S, Wu L, Doan N, Said J, et al. Effect of dietary omega-3 fatty acids on tumor-associated macrophages and prostate cancer progression. Prostate. 2016;76:1293–302. doi: 10.1002/pros.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ye Y, Scheff NN, Bernabe D, Salvo E, Ono K, Liu C, et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology. 2018;139:182–93. doi: 10.1016/j.neuropharm.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 98.Bauman JE, Zang Y, Sen M, Li C, Wang L, Egner PA, et al. Prevention of carcinogen-induced oral cancer by sulforaphane. Cancer Prev Res (Philadelphia, Pa) 2016;9:547–57. doi: 10.1158/1940-6207.CAPR-15-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Filomeno M, Bosetti C, Garavello W, Levi F, Galeone C, Negri E, et al. The role of a Mediterranean diet on the risk of oral and pharyngeal cancer. Br J Cancer. 2014;111:981–6. doi: 10.1038/bjc.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giraldi L, Panic N, Cadoni G, Boccia S, Leoncini E. Association between Mediterranean diet and head and neck cancer: Results of a large case-control study in Italy. Eur J Cancer Prev. 2017;26:418–23. doi: 10.1097/CEJ.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 101.Fahey JW, Stephenson KK, Wallace AJ. Dietary amelioration of helicobacter infection. Nutr Res (New York, NY) 2015;35:461–73. doi: 10.1016/j.nutres.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hołubiuk Ł, Imiela J. Diet and Helicobacter pylori infection. Prz Gastroenterol. 2016;11:150–4. doi: 10.5114/pg.2016.61487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer.Implications for tumor angiogenesis and metastasis. Neoplasia (New York, NY) 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiang SL, Velmurugan BK, Chung CM, Lin SH, Wang ZH, Hua CH, et al. Preventive effect of celecoxib use against cancer progression and occurrence of oral squamous cell carcinoma. Sci Rep. 2017;7:6235. doi: 10.1038/s41598-017-06673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.García Rodríguez LA, Soriano-Gabarró M, Bromley S, Lanas A, Cea Soriano L. New use of low-dose aspirin and risk of colorectal cancer by stage at diagnosis: A nested case-control study in UK general practice. BMC Cancer. 2017;17:637. doi: 10.1186/s12885-017-3594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jayaprakash V, Rigual NR, Moysich KB, Loree TR, Nasca MA, Menezes RJ, et al. Chemoprevention of head and neck cancer with aspirin: A case-control study. Arch otolaryngol Head Neck Surg. 2006;132:1231–6. doi: 10.1001/archotol.132.11.1231. [DOI] [PubMed] [Google Scholar]
- 107.Rafei H, Catherine C, Han J, Kaffenberger TM, Maxwell JH. Aspirin use and survival in head and neck squamous cell cancer (HNC) patients. J Clin Oncol. 2017;35(15 Suppl):6042. [Google Scholar]
- 108.Kim SA, Roh JL. Aspirin use and head and neck cancer survival: An observational study of 11,623 person-years follow-up. Int J Clin Oncol. 2018;23:52–8. doi: 10.1007/s10147-017-1165-3. [DOI] [PubMed] [Google Scholar]
- 109.Galvao-Moreira LV, da Cruz M. Screening and early detection of oral cancer: Current controversies. Acta Odontol Scand. 2017;75:361–5. doi: 10.1080/00016357.2017.1316868. [DOI] [PubMed] [Google Scholar]
- 110.Irani S, Meschi M, Goodarzi A. Influence of education on oro-dental knowledge among school hygiene instructors. J Dent Res Dent Clin Dent Prospects. 2009;3:56–9. doi: 10.5681/joddd.2009.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Irani S. Metastasis to the oral soft tissues: A review of 412 cases. J Int Soc Prev Community Dent. 2016;6:393–401. doi: 10.4103/2231-0762.192935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Irani S. Metastasis to the Jawbones: A review of 453 cases. J Int Soc Prev Community Dent. 2017;7:71–81. doi: 10.4103/jispcd.JISPCD_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Irani S, Moshref M, Lotfi A. Metastasis of a gastric adenocarcinoma to the mandible: A case report. Oral Oncol Extra. 2004;40:85–7. [Google Scholar]
- 114.Irani S, Bidari-Zerehpoush F, Sabeti S. Prevalence of pathological entities in neck masses: A study of 1208 consecutive cases. Avicenna J Dent Res. 2016;8:e25614. [Google Scholar]
- 115.Mariño R, Haresaku S, McGrath R, Bailey D, McCullough M, Musolino R, et al. Oral cancer screening practices of oral health professionals in Australia. BMC Oral Health. 2017;17:151. doi: 10.1186/s12903-017-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jornet PL, Garcia FJ, Berdugo ML, Perez FP, Lopez AP. Mouth self-examination in a population at risk of oral cancer. Aust Dent J. 2015;60:59–64. doi: 10.1111/adj.12274. [DOI] [PubMed] [Google Scholar]
- 117.Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, Tu M, Garcia-Godoy F, Wong DT. Saliva diagnostics-Current views and directions. Exp Biol Med (Maywood, NJ) 2017;242:459–72. doi: 10.1177/1535370216681550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishikawa S, Sugimoto M, Kitabatake K, Sugano A, Nakamura M, Kaneko M, et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci Rep. 2016;6:31520. doi: 10.1038/srep31520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carreras-Torras C, Gay-Escoda C. Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Med Oral Patol Oral Cir Bucal. 2015;20:e305–15. doi: 10.4317/medoral.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–6. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Irani S, Bidari-Zerehpoush F. BRCA1/2 mutations in salivary pleomorphic adenoma and carcinoma-ex-pleomorphic adenoma. J Int Soc Prev Community Dent. 2017;7(Suppl 3):S155–62. doi: 10.4103/jispcd.JISPCD_184_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pierce Campbell CM, Giuliano AR, Torres BN, O'Keefe MT, Ingles DJ, Anderson RL, et al. Salivary secretory leukocyte protease inhibitor (SLPI) and head and neck cancer: The cancer prevention study II nutrition cohort. Oral Oncol. 2016;55:1–5. doi: 10.1016/j.oraloncology.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ioachim E, Assimakopoulos D, Goussia AC, Peschos D, Skevas A, Agnantis NJ. Glycoprotein CD44 expression in benign, premalignant and malignant epithelial lesions of the larynx: An immunohistochemical study including correlation with Rb, p53, Ki-67 and PCNA. Histol Histopathol. 1999;14:1113–8. doi: 10.14670/HH-14.1113. [DOI] [PubMed] [Google Scholar]
- 124.Tongtawee T, Wattanawongdon W, Simawaranon T, Kaewpitoon S, Kaengpenkae S, Jintabanditwong N, et al. Expression of cancer stem cell marker CD44 and its polymorphisms in patients with chronic gastritis, precancerous gastric lesion, and gastric cancer: A cross-sectional multicenter study in Thailand. Biomed Res Int. 2017;2017:4384823. doi: 10.1155/2017/4384823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pereira LHM, Reis IM, Reategui EP, Gordon C, Saint-Victor S, Duncan R, et al. Risk stratification system for oral cancer screening. Cancer Prev Res (Philadelphia, Pa) 2016;9:445–55. doi: 10.1158/1940-6207.CAPR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong LP, Chen GF, Xu ZF, Zhang X, Ping FY, Zhao SF. Detection of telomerase activity in saliva from oral squamous cell carcinoma patients. Int J Oral Maxillofac Surg. 2005;34:566–70. doi: 10.1016/j.ijom.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 127.Perisanidis C. Prevalence of EGFR tyrosine kinase domain mutations in head and neck squamous cell carcinoma: Cohort study and systematic review. In Vivo (Athens, Greece) 2017;31:23–34. doi: 10.21873/invivo.11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pal MK, Jaiswar SP, Dwivedi VN, Tripathi AK, Dwivedi A, Sankhwar P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol Med. 2015;12:328–41. doi: 10.7497/j.issn.2095-3941.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Polverini PJ, D'Silva NJ, Lei YL. Precision therapy of head and neck squamous cell carcinoma. J Dental Res. 2018;97:614–21. doi: 10.1177/0022034518769645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang SH, O'Sullivan B. Oral cancer: Current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18:e233–40. doi: 10.4317/medoral.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]