Abstract
Bronchopulmonary dysplasia (BPD) is a form of chronic lung disease that develops in neonates as a consequence of preterm birth, arrested fetal lung development, and inflammation. The incidence of BPD remains on the rise as a result of increasing survival of extremely preterm infants. Severe BPD contributes to significant health care costs and is associated with prolonged hospitalizations, respiratory infections, and neurodevelopmental deficits. In this study, we aimed to detect novel biomarkers of BPD severity. We collected tracheal aspirates (TAs) from preterm babies with mild/moderate (n = 8) and severe (n = 17) BPD, and we profiled the expression of 1048 miRNAs using a PCR array. Associations with biological pathways were determined with the Ingenuity Pathway Analysis (IPA) software. We found 31 miRNAs differentially expressed between the two disease groups (2-fold change, false discovery rate (FDR) < 0.05). Of these, 4 miRNAs displayed significantly higher expression levels, and 27 miRNAs had significantly lower expression levels in the severe BPD group when compared to the mild/moderate BPD group. IPA identified cell signaling and inflammation pathways associated with miRNA signatures. We conclude that TAs of extremely premature infants contain miRNA signatures associated with severe BPD. These may serve as potential biomarkers of disease severity in infants with BPD.
Keywords: bronchopulmonary dysplasia, prematurity, miRNA, biomarkers, tracheal aspirates
1. Introduction
Bronchopulmonary dysplasia (BPD) is a form of chronic lung disease that develops in neonates as a consequence of preterm birth and arrested fetal lung development [1]. BPD is associated with significant human and public health burdens, with reported cases varying significantly among centers worldwide [2]. The incidence of BPD remains on the rise, with recently stated global incidence ranging from 10% to up to 89% in one report [2,3]. The disease was first described by Northway and colleagues in 1967 in preterm infants receiving invasive mechanical ventilation with characteristic clinical, chest radiological, and pathologic findings [4].
The etiology and pathogenesis of BPD are complex and multifactorial. The contributing factors include genetic predisposition, epigenetic factors, arrest of lung development, chronic inflammation, mechanical ventilation, and oxygen toxicity [1,5]. In addition, several maternal health factors such as pre-eclampsia, obesity, gestational diabetes, and inflammation can also predispose to preterm birth and BPD development [6,7,8]. Severe BPD is particularly prevalent in extremely low birth weight infants, a fast-growing population. Fetal growth restriction and male gender are additional risk factors for severe BPD [5,9]. Neonates with severe BPD are at risk for adverse short- and long-term outcomes that are potentially life-long [10]. Preterm infants who are survivors of BPD also experience prolonged hospitalizations, increased incidence of respiratory infections, growth failure, and neurodevelopmental deficits [10].
Clinically, BPD presents as chronic respiratory insufficiency, with oxygen requirement with or without the need for positive pressure support [1]. Historically, the incidence and classification of BPD have evolved due to the improved survival of extremely low birth weight infants, advances in neonatal intensive care, the evolution in the modes of mechanical ventilation, and other therapies such as exogenous surfactant and corticosteroids [11,12]. While BPD was previously classified by the National Institute of Child Health and Human Development (NICHD) into three categories (mild, moderate, or severe), since 2019 the same committee began to use a staging system for the classification of BPD, with grades I, II, and III to reflect mild, moderate, and severe disease, respectively [13,14]. As opposed to the initial classification system that focused on chronicity and extent of respiratory support, the more recent classification attempts to classify BPD severity based on short- and long-term outcomes such as death, need for tracheostomy, and hospital readmission rates. While these systems incorporate clinical phenotyping into diagnosis, we consider that synergizing them with endotyping through omics markers should be the future direction.
In the past decade, studies have proliferated looking at different genomic, transcriptomic, epigenomic, metabolomics, and other biological biomarkers for the prediction of BPD and its severity [15,16,17,18]. Animal models of newborn hyperoxia have identified several pathways involved in the development of BPD, including the cyclooxygenase-2 (COX-2) [19,20], sirtuin-1 (SIRT1) [21], and Wnt signaling pathways [22]. To date, there are no validated biomarkers for predicting risk and disease severity in the neonatal period. The current consensus is that biomarker development could lead to early recognition, individualized care, and interventions that could prevent severe BPD in the most vulnerable group, subsequently improving short- and long-term outcomes. Particular biomarkers of interest examined in the present study are microRNAs (miRNAs) from tracheal aspirates (TAs). miRNAs are small noncoding molecules that are approximately 22 nucleotides long. They regulate gene expression post-transcriptionally by inhibiting the expression of target messenger RNAs [23]. A few individual miRNAs have been identified as key players of BPD pathogenesis in mouse models and clinical studies. A recent study identified miR-219-5p as a marker for severe BPD in preterm infants when compared to full-term neonates [24]. Another study using cord blood and venous blood from infants with BPD identified an association of decreased expression of miR-29 and intra-amniotic and elevated inflammatory markers [25].
The potential of TA miRNAs to predict disease severity in neonatal and pediatric lung disease is promising and warrants additional investigation. This is highlighted by the fact that TAs are obtained relatively easily from endotracheal tube suctioning in infants receiving invasive mechanical ventilation [26,27]. Moreover, TAs are a vital source of information to study molecular pathways related to the microenvironment of the developing lung and could not only provide a source of novel biomarkers for BPD diagnosis but also identify molecular events occurring during lung development in the context of the disease [26,28,29].
With this goal in mind, the aim of the present study was to analyze the expression profile of miRNAs present in TAs obtained from a cohort of neonates with mild/moderate and severe BPD. This study was conducted as part of a larger single-center prospective cohort study with some of the results reported by us previously [18]. While our prior analysis identified miRNA signatures of prematurity and BPD pathogenesis in TAs, the current study focuses on specific miRNAs associated with BPD severity, in order to help develop more precise diagnostic markers.
2. Materials and Methods
2.1. Patient Population
We prospectively enrolled 25 extremely preterm infants receiving invasive mechanical ventilation at the Penn State Health Children’s Hospital Neonatal Intensive Care Unit between 2013 and 2018. Enrolled patients were 7 days of life or older and at risk for developing mild, moderate, or severe BPD according to the NHLBI consensus conference classification [30]. In short, the severity of BPD was determined based on the degree of respiratory support and/or oxygen requirement at 28 days of life and 36 weeks postmenstrual age or at 56 days of life, whichever was earlier. Infants with major congenital malformations, chromosomal anomalies, and complex congenital heart defects were excluded from the study [31]. We also excluded infants with severe lung disease and signs of pulmonary hypertension in echocardiogram at or close to 36 weeks of GA. All protocols used in this study were approved by the Pennsylvania State University College of Medicine Institutional Review Board on 5 March 2013 under protocol ID PRAMS042136EP.
2.2. Tracheal Aspirate Collection
Following informed consent by the parents, we collected TAs from enrolled patients following routine suctioning. We also obtained pertinent clinical information from electronic medical records, such as sex, race/ethnicity, method of delivery, antenatal steroids, gestational age (GA) at birth, birth weight, GA at the time of sample collection, day of life, and fraction of inspired oxygen (FiO2) at the time of sample collection. Immediately after collection, TAs were transported to the laboratory and stored at −80 °C until analysis.
2.3. MicroRNA Purification
Starting from 500 µL of TAs, we purified miRNAs using the Norgen miRNA Purification Kit, after addition of a spike-in control (cel-mir-39, QIAGEN Inc., Germantown, MD, USA). The miRNA fraction was then quantified using a Nanodrop, and its purity was assessed with a Bioanalyzer at the Penn State College of Medicine Genome Sciences Core Facility. For miRNA profiling, a total of 250 ng of RNA was first retrotranscribed using the miScriptII RT kit (QIAGEN Inc., Germantown, MD, USA), following the manufacturer’s protocol.
2.4. MicroRNA Arrays
The expression of 1066 human miRNAs was quantified using the miScript miRNA PCR array kit MIHS-3216Z (QIAGEN Inc., Germantown, MD, USA) on a QuantStudio 12K Flex system (Life Technologies, Carlsbad, CA, USA). Following the PCR reaction, cycle threshold (Ct) values were extracted and normalized by global means. Differential expression was calculated using the 2−ΔΔCT method and a mild/moderate sample as a calibrator [32]. Fold changes in miRNA expression were obtained using the Bioconductor limma package on R [33]. miRNAs with undetectable expression (Ct values > 40) in more than 90% of samples of each experimental group were excluded from the analysis. Datasets (metadata, non-normalized, and normalized values) were uploaded to the Gene Expression Omnibus under project GSE165828.
2.5. Ingenuity Pathway Analysis
The Ingenuity Pathway Analysis (IPA) software (QIAGEN Inc., Germantown, MD, USA) was used to identify miRNA interaction networks, predicted target genes, and molecular functions based on prediction scores [34]. The IPA core analysis functionality was performed for both direct and indirect relationships using the Ingenuity Knowledge Base. The miRNAs of interest were analyzed using the microRNA target filter feature. Only experimentally observed or predicted with high confidence targets in humans were considered.
2.6. MicroRNA Expression Validation
The expression of six differentially expressed miRNAs between mild/moderate and severe BPD samples was validated using the All-in-One miRNA qRT-PCR Reagent Kits (GeneCopoeia Inc., Rockville, MD, USA), using selected primers (hsa-miR-205-3p, hsa-let-7i-5p, hsa-miR-1255b, hsa-miR-24-1-5p, hsa-miR-545-5p, and hsa-miR-628-5p) and starting from 6 ng of RNA template. Differential expression between groups was calculated with the 2−ΔΔCT method using hsa-miR-16-1-3p as a normalization control [32,35].
2.7. Statistical Analysis and Code Availability
For miRNA arrays, statistical analyses were performed using the Bioconductor limma package on R using the parametric empirical Bayes method [33]. Differential expression was defined as Benjamini–Hochberg false discovery rate (FDR) below 0.05. Heatmaps were generated with the nonnegative matrix factorization (NMF) package on R [36], and volcano plots were generated using the GraphPad Prism software. For miRNA validation assays, differences in miRNA expression between the two groups were determined by t-test using the GraphPad Prism software and considered statistically significant at p < 0.05.
3. Results
3.1. Patient Demographics
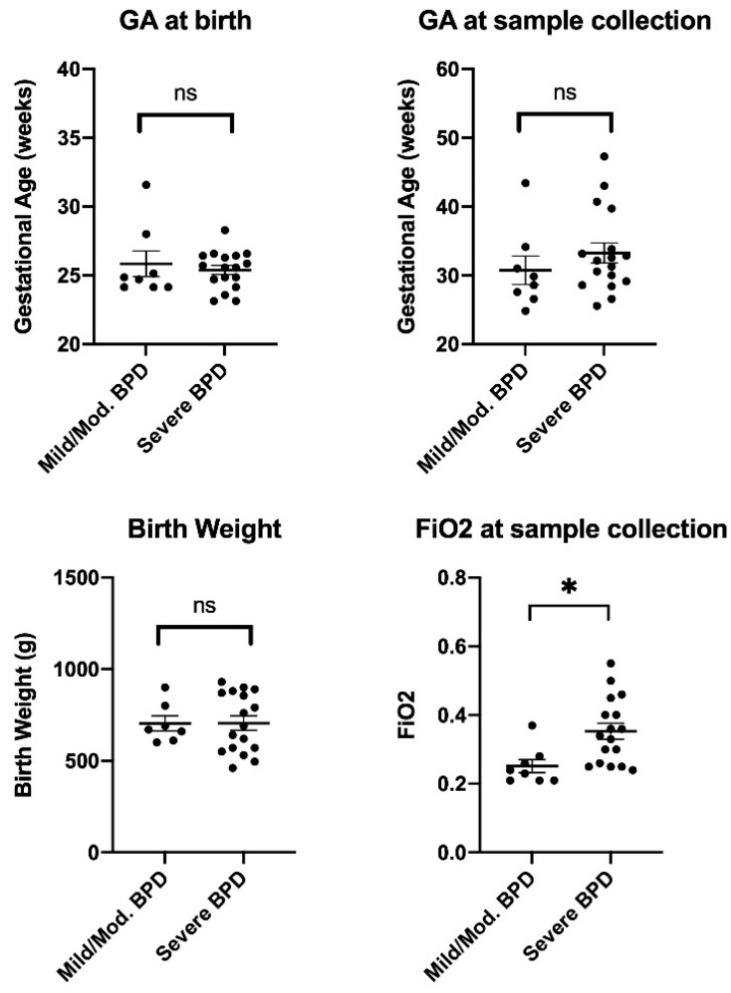
We obtained TAs from 25 mechanically ventilated preterm infants with mild/moderate BPD (n = 8) and severe BPD (n = 17). A summary of the patients’ clinical information is shown in Table 1, and individual data for main variables are visualized in Figure 1. As expected, FiO2 at the time of sample collection was significantly higher in the severe BPD group when compared to the mild/moderate BPD group (p < 0.05). No statistically significant differences were found for the rest of the measured variables, including GA at birth and at sample collection time, birth weight, day of life at sample collection time, male sex, and antenatal steroid exposure. Regarding the racial and ethnic composition of the patient cohort, there was a significant overrepresentation of Hispanic and mixed-race subjects in the severe BPD group when compared to the mild/moderate BPD group (p < 0.05).
Table 1.
Patient demographics at study enrollment.
| Characteristic | Mild/Moderate BPD (n = 8) |
Severe BPD (n = 17) |
p-Value |
|---|---|---|---|
| Gestational age (GA) at birth, weeks (mean ± SD) | 25.84 ± 2.64 | 25.39 ± 1.38 | 0.584 |
| GA at sample collection, weeks (mean ± SD) | 30.75 ± 5.86 | 33.26 ± 6.02 | 0.337 |
| Birth weight, grams (mean ± SD) | 704 ± 108 | 706 ± 162 | 0.981 |
| Day of life (mean ± SD) | 37 ± 31 | 60 ± 40 | 0.182 |
| Male sex, % (n) | 50 (4) | 47 (8) | >0.999 |
| FiO2 at sample collection (mean ± SD) | 0.25 ± 0.05 | 0.35 ± 0.10 | 0.010 |
| Antenatal steroids exposure, % (n) 1 | 75 (6) | 76 (13) | 0.289 |
| Delivered via C-section, % (n) | 75 (6) | 88 (15) | 0.289 |
| Racial/Ethnic Group, % (n) | |||
| Non-Hispanic White | 63 (5) | 53 (9) | 0.508 |
| Non-Hispanic Black | 25 (2) | 6 (1) | 0.289 |
| Hispanic | 0 (0) | 18 (3) | 0.008 |
| Asian | 13 (1) | 18 (3) | 0.070 |
| More than one race | 0 (0) | 6 (1) | 0.008 |
1 Any antenatal steroids exposure (1 or more doses received by mother before birth), values in bold indicate p = 0.05.
Figure 1.
Patient demographics. Gestational age (GA) at birth and at sample collection time, birth weight, and fraction of inspired oxygen (FiO2) in mild/moderate (Mild/Mod.) and severe bronchopulmonary dysplasia (BPD) infants enrolled. No differences are observed between groups for GA or birth weight parameters. The FiO2 at sample collection time is significantly higher in the severe BPD group when compared to the Mild/Mod. BPD group (unpaired t-test, * p = 0.0104).
3.2. MiRNA Expression in TAs
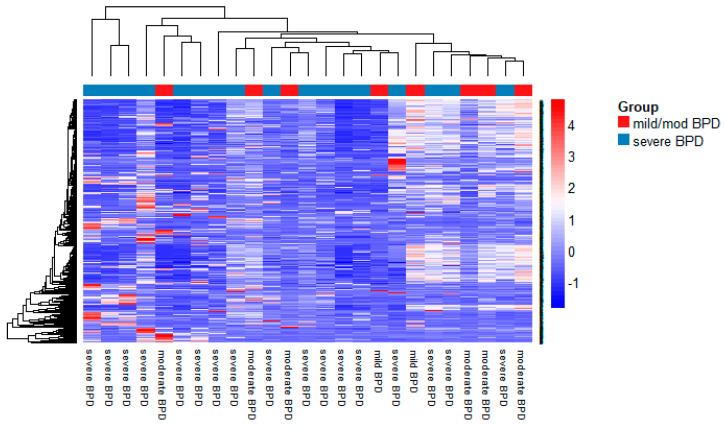
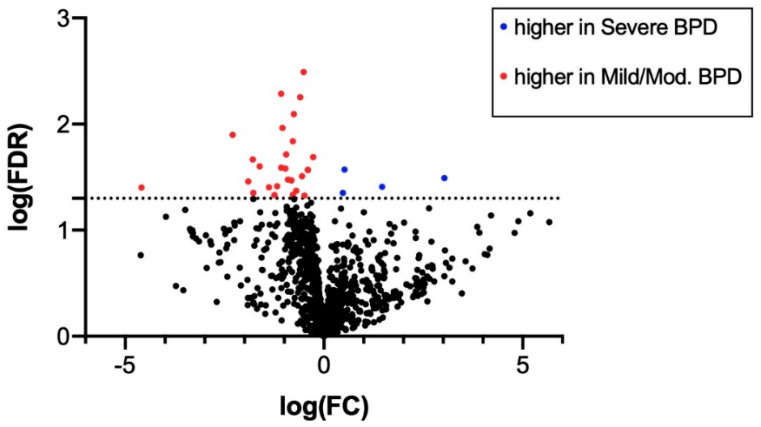
A heatmap of the expression of the 1048 miRNAs expressed in TA samples from mild/moderate and severe BPD infants is shown in Figure 2. A volcano plot indicating FDR vs. fold change (FC) of expression between severe BPD and mild/moderate BPD samples is also shown in Figure 3.
Figure 2.
Heatmap of expression of 1048 miRNAs in tracheal aspirates from mild/moderate (n = 8) and severe (n = 17) BPD infants (normalized by global mean), obtained by PCR arrays. Heatmap generated in R using the NMF package as described in Section 2.
Figure 3.
Volcano plot of miRNAs expressed in tracheal aspirates from mild/moderate (n = 8) and severe (n = 17) BPD infants. Red dots indicate miRNAs upregulated in mild/moderate BPD samples. Blue dots indicate miRNAs with higher expression in severe BPD samples. FC: fold change; FDR: false discovery rate. Dotted line indicates log(FDR) = 1.3, corresponding to FDR = 0.05. Volcano plot was generated in GraphPad Prism software as described in Section 2.
After accounting for FDR < 0.05, the PCR array analysis revealed differential expression of 31 miRNAs with |FC| > 2 between the mild/moderate and severe BPD samples (Table 2). Of these, 4 miRNAs had significantly higher expression in the severe BPD group (log FC > 0.3, FDR < 0.05) and 27 had significantly higher expression in the mild/moderate BPD group (log FC < −0.3, FDR < 0.05) (Table 2).
Table 2.
Differentially expressed miRNAs in severe vs. mild/moderate BPD tracheal aspirates (Tas).
| miRNA ID | log(Fold Change) | Average Expression | FDR |
|---|---|---|---|
| hsa-miR-628-5p | 3.031 | 2.823 | 0.032 |
| hsa-miR-185 | 1.463 | 1.591 | 0.039 |
| hsa-miR-545* | 0.516 | 0.361 | 0.027 |
| hsa-miR-378 | 0.479 | 0.607 | 0.044 |
| hsa-miR-3713 | −0.270 | 0.152 | 0.020 |
| hsa-miR-3151 | −0.404 | 0.414 | 0.027 |
| hsa-miR-1295 | −0.404 | 0.495 | 0.027 |
| hsa-miR-1286 | −0.488 | 0.487 | 0.047 |
| hsa-miR-380 | −0.509 | 0.318 | 0.003 |
| hsa-miR-15a* | −0.550 | 0.851 | 0.031 |
| hsa-miR-3175 | −0.599 | 0.427 | 0.006 |
| hsa-miR-493 | −0.698 | 1.030 | 0.042 |
| hsa-miR-3193 | −0.759 | 0.537 | 0.008 |
| hsa-miR-105* | −0.781 | 0.879 | 0.014 |
| hsa-miR-4300 | −0.784 | 1.019 | 0.046 |
| hsa-miR-631 | −0.815 | 0.756 | 0.034 |
| hsa-miR-2116* | −0.902 | 0.954 | 0.033 |
| hsa-miR-4304 | −0.951 | 0.986 | 0.019 |
| hsa-miR-3125 | −0.971 | 1.316 | 0.026 |
| hsa-miR-4303 | −1.046 | 1.158 | 0.011 |
| hsa-miR-1908 | −1.078 | 0.960 | 0.005 |
| hsa-miR-205* | −1.079 | 1.376 | 0.026 |
| hsa-miR-3674 | −1.176 | 1.101 | 0.039 |
| hsa-miR-615-3p | −1.243 | 1.649 | 0.047 |
| hsa-miR-4305 | −1.383 | 1.613 | 0.040 |
| hsa-let-7i* | −1.615 | 1.969 | 0.025 |
| hsa-miR-4330 | −1.776 | 1.918 | 0.045 |
| hsa-miR-1255b | −1.787 | 1.733 | 0.022 |
| hsa-miR-125b-1* | −1.905 | 2.199 | 0.035 |
| hsa-miR-24-1* | −2.300 | 1.153 | 0.013 |
| hsa-miR-646 | −4.590 | 5.529 | 0.040 |
3.3. Validation of Top Differentially Expressed miRNAs
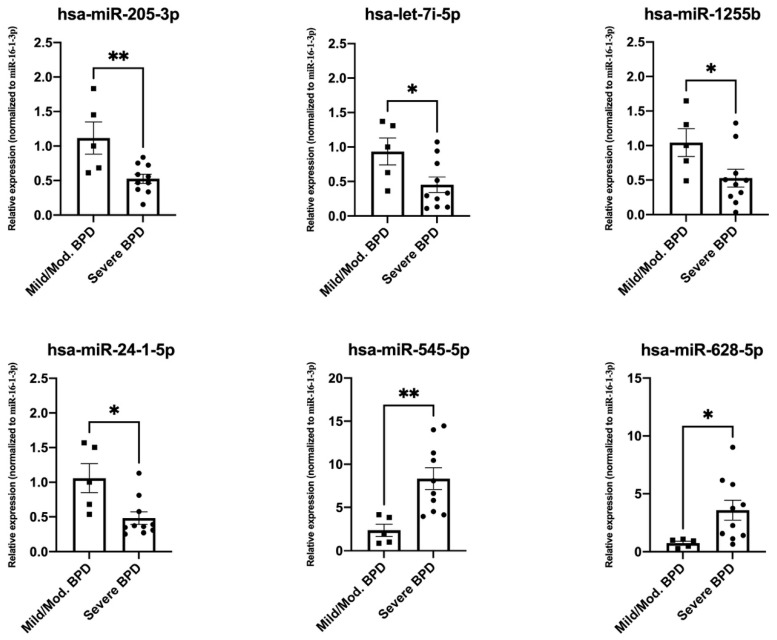
We conducted validation experiments for two randomly selected miRNAs with significantly higher expression in severe BPD (hsa-miR-628-5p, and hsa-miR-545*) and four miRNAs with significantly lower expression in severe BPD (hsa-miR-24-1*, hsa-miR-1255b, hsa-miR-205*, and hsa-let-7i*) by real-time PCR, using a subset of samples from mild/moderate BPD (n = 5) and severe BPD (n = 10) groups. Our results confirmed differential expression of all six miRNAs between the groups (p < 0.05) (Figure 4), validating the miRNA array results.
Figure 4.
Validation experiments by real-time PCR and specific primers for hsa-miR-205-3p, hsa-let-7i-5p, hsa-miR-1255b, hsa-miR-24-1-5p, hsa-miR-545-5p, and hsa-miR-628-5p in a subset of mild/moderate (Mild/Mod.) BPD samples (n = 5) and severe BPD samples (n = 10). Y axes indicate relative expression values after normalization to hsa-miR-16-1-3p, and a mild/moderate calibration sample using the 2−ΔΔCT method [32,35]. Significant differences were determined by t-test using the GraphPad software (* p < 0.05, ** p < 0.01).
3.4. Pathway Analysis
Ingenuity pathway analysis (IPA) of differentially expressed miRNAs in the two BPD groups indicated significant associations with molecular and cellular functions, including cell signaling, DNA replication, and cell cycle, as well as inflammatory response and disease, as summarized in Table 3.
Table 3.
IPA pathways associated with differentially expressed miRNAs.
| Top Pathways | p-Value |
|---|---|
| Top molecular and cellular functions | |
| Cell-to-cell signaling and interaction | 9.63 × 10−4–9.63 × 10−4 |
| Cellular function and maintenance | 9.63 × 10−4–9.63 × 10−4 |
| DNA replication, recombination, and repair | 1.39 × 10−2–1.39 × 10−2 |
| Cell death and survival | 1.86 × 10−2–1.86 × 10−2 |
| Cell cycle | 2.57 × 10−2–2.57 × 10−2 |
| Top diseases and disorders | |
| Inflammatory disease | 2.08 × 10−5–2.08 × 10−5 |
| Inflammatory response | 1.89 × 10−3–2.08 × 10−5 |
| Organismal injury and abnormalities | 3.97 × 10−2–2.08 × 10−5 |
| Renal and urological disease | 2.08 × 10−5–2.08 × 10−5 |
| Reproductive system disease | 3.97 × 10−2–2.01 × 10−4 |
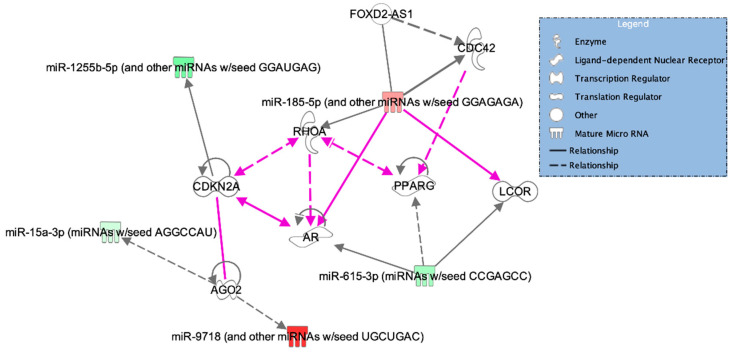
We also found that the top regulatory networks associated with the differentially expressed miRNAs included cell cycle; control of gene expression; cell signaling; and cellular function, assembly, and maintenance (Table 4). Of the 31 miRNAs identified in the array, 5 were associated with the most significant cellular and molecular networks found by IPA. These include three miRNAs with significantly lower expression in severe BPD when compared to mild/moderate BPD, namely miR-15* (mir-15a-3p), miR-615-3p, and miR-1255-5p, and two miRNAs with significantly higher expression in severe BPD, namely miR-185-5p (miR-185) and miR-9718 (which has the same seed sequence as miR-628-5p: UGCUGAC) (Table 4). A diagram of the identified networks, miRNA–gene target interactions, and connections among different networks based on experimental evidence is shown in Figure 5. The miRNA targets identified by IPA include cyclins, transcription factors, hormone receptors, and cell signaling enzymes (Table 4).
Table 4.
IPA core analysis top associated networks and molecules (miRNAs and target genes).
| Top Associated Networks | Molecules | Score |
|---|---|---|
| Cell cycle, gene expression, RNA post-transcriptional modification | AGO2, miR-15a-3p, miR-9718 | 6 |
| Cell-to-cell signaling and interaction, cellular assembly and organization, small molecule biochemistry | AR, LCOR, miR-615-3p, PPARG | 3 |
| Cell cycle, cellular function and maintenance, cell signaling | CDC42, FOXD2, RHOA, miR-185-5p | 3 |
| Cardiovascular disease, cardiovascular system development and function, cancer | CDKN2A, miR-1255b-5p | 3 |
AGO2: argonaut RISC (RNA-induced silencing complex) catalytic component 2; AR: androgen receptor; LCOR: ligand-dependent nuclear receptor corepressor; PPARG: peroxisome proliferator-activated receptor gamma; CDC42: cell division cycle 42; FOXD2-AS1: FOXD2 (forkhead box D2) adjacent opposite strand RNA 1; RHOA: Ras homolog family member A; CDKN2A: cyclin-dependent kinase inhibitor 2A.
Figure 5.
Graphical representation for the top 4 networks associated with differentially expressed miRNAs in severe BPD samples. Grey lines represent individual pathways, and pink lines indicate interactions among pathways. Full and dotted lines indicate direct and indirect relationships between molecules, respectively. miRNAs with higher expression in the severe BPD group are shown in shades of red, according to upregulation intensity. Similarly, miRNAs with lower expression in severe BPD are shown in shades of green. AGO2: argonaut RISC (RNA-induced silencing complex) catalytic component 2; CDKN2A: cyclin-dependent kinase inhibitor 2A; AR: androgen receptor; LCOR: ligand-dependent nuclear receptor corepressor; CDC42: cell division cycle 42; FOXD2-AS1: FOXD2 (forkhead box D2) adjacent opposite strand RNA 1; PPARG: peroxisome proliferator-activated receptor gamma; RHOA: Ras homolog family member A.
Next, to expand our analysis beyond experimentally validated interactions, we used the IPA miRNA target filter to identify predicted miRNA–mRNA target relationships, based on miRNA seed sequence foreseen interactions. Among the top networks associated with predicted target genes, we identified organismal injury and respiratory disease pathways, as well as cellular trafficking, immunological disease, and cell and tissue development gene networks (Table 5). Graphical representations for the top associated networks are also shown in Supplementary Figures S1 and S2.
Table 5.
IPA top associated networks based on miRNA–mRNA binding relationships.
| Top Associated Networks | Score |
|---|---|
| Respiratory disease, cancer, organismal injury and abnormalities | 37 |
| Cellular movement, immune cell trafficking, immunological disease | 35 |
| Cell-to-cell signaling and interaction, cellular assembly and organization, tissue development | 33 |
| Cell-to-cell signaling and interaction, cellular development, cellular growth and proliferation | 29 |
| Cellular movement, hematological system development and function, immune cell trafficking | 27 |
4. Discussion
Many studies have focused on the prevention of severe BPD, but there is a paucity of literature on the biological basis of this disease’s pathogenesis. There is also a lack of validated biomarkers for BPD severity and targeted pharmacological therapies to improve the disease burden [37]. The current recommendations include optimizing ventilator settings while focusing on nutrition and addressing comorbidities [38]. This mostly stems from the fact that the exact underlying mechanisms of severe BPD are uncertain. In the current study, we sought to identify the molecular signatures associated with BPD severity to uncover regulatory pathways and new biomarkers for severe BPD. Through analysis of TAs, a noninvasive method of sample collection in mechanically ventilated infants, we discovered specific miRNA profiles in age-matched preterm newborns with mild/moderate and severe BPD. Our analysis revealed 31 differentially expressed miRNAs between subject groups. Of these, 4 miRNAs displayed at least 2-fold higher expression and FDR < 0.05 in severe BPD infants, and 27 miRNAs had significantly lower expression in severe BPD infants than in TAs from mild/moderate BPD infants. We also found associations of these miRNAs with functional gene networks related to lung inflammation, respiratory disease, oxidative stress, and cellular development, indicating that specific regulatory pathways may contribute to fundamental mechanisms of BPD severity.
Of the four miRNAs with higher expression in severe BPD infants, two (hsa-miR-545* and miR-185) have been previously implicated in mechanisms of cell cycle arrest, a known effect of prolonged mechanical ventilation and hyperoxia [39,40]. Specifically, miR-545 has been shown to suppress cell proliferation through targeting of cyclins and cyclin-related kinases [41], and miR-185 has been associated with induction of G1 cell cycle arrest and promotion of necroptosis and apoptosis via receptor-interacting kinases and caspase activity in alveolar epithelial type II cells [42,43]. Moreover, the role of mir-185 in hyperoxia-induced DNA damage and lung epithelial cell death triggered by oxidative stress has been reported using both animal models and human cells [43,44]. Importantly, extracellular vesicles containing miR-185 are significantly elevated following hyperoxia-induced cell death in alveolar type II epithelial cells [43]. While we did not characterize the composition of TAs regarding extracellular vesicles, it is possible that the increased levels of miR-185 found in TAs from severe BPD infants (who are subjected to higher and more prolonged hyperoxia than mild/moderate BPD infants) could represent a signal of hyperoxia-induced epithelial cell damage via increased extracellular vesicle miR-185 cargo. Therefore, our results could provide clinical evidence of a mechanism previously described in lung disease models.
Two additional targets of miR-185 are the Rho GTPases, CDC42 and RHOA (Table 4), implicated in cell cycle, cellular function and maintenance, and cell signaling. By targeting these mRNAs, miR-185 has been shown to inhibit cell proliferation and migration in hepatic, colorectal, and lung cancer cell models [45,46,47,48]. Importantly, miR-185 can also interact with and target the long noncoding RNA FOXD2-AS1, known to inhibit cell proliferation, migration, and epithelial–mesenchymal transition (EMT) in glial cells and renal fibrosis models [49,50]. Regarding lung cells, Lei et al. demonstrated that miR-185 can reduce collagen V overexpression and EMT in pulmonary fibrosis [51]. While promotion of EMT in alveolar epithelial type II cells is known to alter normal alveolar development processes and contribute to BPD phenotypes, the effects of miR-185 in EMT processing during lung development and BPD progression have not been explored yet [52,53]. Finally, miR-185 has also been found to target the SOX9 gene and regulate Wnt signaling [54], a key regulator of lung organogenesis and development [55,56].
The remaining two miRNAs found overrepresented in TAs of severe BPD infants were miR-378 and miR-628-5p. With the exception of lung cancer [57], no studies to date have assessed the role of miR-378 in the lung. However, this small RNA has been implicated in several functions associated with BPD, including mitochondrial metabolism, autophagy, and oxidative stress responses in a variety of tissues [58,59]. On the other hand, miR-628-5p has been implicated in developmental functions such as embryonal implantation, as well as mechanisms of immune modulation, viral infection, and cell proliferation [60,61,62]. One study identified the fibroblast growth factor receptor 2 (FGFR2), the receptor for FGF10, as a target of miR-628-5p in ovarian cancer cells [63]. This gene has been identified as a key factor in early lung development [64], as well as alveolar epithelial type II cells homeostasis [65]. Interestingly, miR-628-5p has also been postulated as a potential biomarker for a variety of cancers in adults [66]. While no studies have yet assessed a direct role of this miRNA in lung development and disease, it could serve as a biomarker for severe BPD in neonates.
Our analysis identified 27 miRNAs with low expression in severe BPD when compared to mild/moderate BPD. Of these, three were implicated in the top significant pathways identified by IPA (hsa-miR-15a*, hsa-miR-1255b-5p, and hsa-miR-615-3p). Specifically, these miRNAs were implicated in cell cycle and cell signaling functions, as well as disease processes in extrapulmonary tissues (Table 4). Regarding miR-15a, an avian model of long-term hypoxia stress revealed that its levels are influenced by hypoxia-induced factor 1 (HIF-1) during lung development [67]. Hypoxic episodes are common stressors associated with BPD secondary to immature respiratory center drive. Both acute and chronic hypoxic events associated with reduced peripheral chemosensitivity and proinflammatory responses occur during the course of neonatal BPD and are associated with poorer neurodevelopmental outcomes [68]. In addition, miR-15a has been shown to inhibit lung fibrosis by targeting the yes-associated protein 1 (YAP1), a key downstream effector of the Hippo pathway [69]. Therefore, it is possible that lower expression of this miRNA in severe BPD infants is associated with fibrotic phenotypes in severe BPD. It is worthwhile to mention that the Hippo pathway is a powerful regulator of lung development, cell differentiation, and tissue regeneration and homeostasis, and it has been found dysregulated in lymphocytes of patients with BPD [70,71]. Therefore, the role of miR-15a and the Hippo pathway in mechanisms leading to BPD severity warrants further investigation.
We detected and validated the downregulation of miR-1255b-5p in TAs of severe BPD infants. This miRNA was previously noted to regulate vascular endothelial growth factor A (VEGFA) expression in liver cirrhosis and hepatocellular carcinoma [72]. Abnormal VEGF and disrupted angiogenesis are described in lung tissues of infants that died of severe BPD [73]. In addition, miR-1255b-5p is downregulated in hypoxia-induced lung A549 cells [74], as well as in models of high altitude retinopathy [75], and in colorectal cancer, where it also regulates telomerase activity and suppresses EMT [76]. Notably, hypoxic stress in the presence of hyperoxia results in the alteration of alveologenesis in mouse models of neonatal BPD [77].
Through IPA analysis, we found that the downregulated miRNA miR-615-3p directly targets the ligand-dependent nuclear receptor corepressor (LCOR) and androgen receptor (AR) transcripts and indirectly targets the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARG) (Table 4 and Figure 5). The AR is expressed in both male and female lung epithelial cells and can bind to DNA sequences to regulate the expression of cell differentiation genes [78,79]. Studies have also shown that the AR can mediate androgen-induced delays in fetal lung maturation [80,81,82]. For example, maternal treatment with dihydrotestosterone inhibits surfactant phospholipid production in the fetal lung, while treatment with the AR-selective antagonist flutamide enhances surfactant phospholipid production [83]. Thus, downregulation of miR-615-3p (a negative regulator of AR expression) in severe BPD could result in increased levels of AR, contributing to delays in lung maturation and surfactant expression. Similarly, PPARG contributes to homeostasis maintenance of epithelial–mesenchymal interactions, which are key players of lung organogenesis. Disruption of this process results in trans-differentiation of lung alveolar lipofibroblasts to myofibroblasts, contributing to the development of pulmonary fibrosis [84,85]. Importantly, PPARG agonists have been suggested as therapeutic targets for BPD due to their implications in the Wnt/β-catenin and TGF-β pathways [86]. Both pathways are induced by hypoxia, leading to decreased levels of PPARG and the subsequent differentiation of fibroblasts, leading to pulmonary fibrosis. Finally, because miR-615-3p is known to repress LCOR in macrophages [87], its downregulation in severe BPD may result in high LCOR levels, which also contribute to decreased PPARG. Overall, the miRNA signatures identified in severe BPD TAs can be associated with known mechanisms of lung inflammation, oxidative stress, developmental delay, and pulmonary fibrosis. More research is needed to explore their contributions to these mechanisms and their therapeutic potential.
The strengths of this study include the prospective nature of the design, using a cohort of preterm infants that is matched in age, sex, birth weight, method of delivery, and antenatal steroid exposure, and the identification of novel miRNA expression signatures associated with BPD phenotypes in TAs. An advantage of these samples is that they are readily available in intubated patients and may contain cellular makers that are very similar to those noted in the lungs. This study also excluded infants with pulmonary hypertension, which is a severe complication of BPD, hence providing more specificity to the signatures identified in the severe BPD cohort.
We have also identified the following study limitations: First, this was a very small but representative sample of preterm neonates admitted to a single center, which affects the generalizability of the findings. It is also possible that with such a small sample size, the differential miRNA expression could be specific to the patients studied. However, the study design was exploratory in nature, utilizing convenience sampling in a small cohort of preterm infants receiving invasive mechanical ventilation. Second, given that the samples were collected at a single time point of the disease process, we are unable to determine whether the observed miRNA signatures are representative of the disease status or are only present at a specific time in the course of the pathology. This limits our ability to determine if there is a predictive potential to these markers or whether they represent a reflection of ongoing molecular pathways inherent to the disease severity or a trigger for molecular events related to disease phenotypes. Additionally, some key clinical variables were not fully explored in this study due to the small sample size. For example, the influence of sex differences, birth weight, fetal growth restriction, and exposure to antenatal corticosteroids on miRNA profile changes was not directly evaluated. Third, given that we studied preterm neonates of different developmental trajectories, it is possible that the differences we noted in the miRNA expression may reflect changes in cell populations present in the TA [88,89]. Lastly, it is becoming evident in recent studies that the microbiota in TAs can affect miRNA expression [90,91]. Because pulmonary inflammation secondary to perinatal and postnatal infection has been implicated as a key contributor in the pathogenesis of BPD, changes in the TA microbiome may be reflected in the observed miRNA signatures. Despite these limitations, this study demonstrates that miRNA profiling in TAs of preterm neonates can be used successfully as a potential biomarker for BPD severity.
In summary, we have described miRNA expression signatures in TA samples obtained from extremely preterm infants with severe BPD and compared them to those of infants with mild to moderate BPD. We identified associations of these molecules with pathways involved in the disease phenotype, including lung inflammation, arrested cell division and development, and oxidative stress. We hypothesize that when identified in the early stages of the disease, these miRNAs can potentially predict the development of severe BPD and its associated phenotypes and hence could be a clinical tool for physicians to optimize ventilator settings, nutrition interventions, fluid balance, and aggressive treatment of infection. Future validation studies in larger cohorts of neonates at risk for severe BPD are needed to determine the effectiveness of these miRNA signatures as predictive and diagnostic tools for BPD severity, as well as to evaluate the significance of miRNA regulation in severe BPD progression.
Acknowledgments
The authors thank Susan DiAngelo and Melody Pham for assistance with sample and data collection and Neal Thomas for protocol and IRB development guidance. The authors thank the Pennsylvania State University College of Medicine Genome Sciences Core Facility and the University of North Carolina at Chapel Hill Biobehavioral Laboratory for real-time PCR equipment.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9059/9/3/257/s1, Figure S1: Respiratory disease, cancer, organismal injury and abnormalities network, Figure S2: Cellular movement, immune cell trafficking, immunological disease network.
Author Contributions
Conceptualization, R.S., C.N.O.-M. and P.S.; Data curation, D.T.M. and P.S.; Formal analysis, P.S.; Funding acquisition, R.S., C.N.O.-M. and P.S.; Investigation, R.S., C.N.O.-M., N.F. and P.S.; Methodology, R.S., C.N.O.-M., D.T.M., N.F., A.D., D.S. and P.S.; Project administration, R.S., C.N.O.-M. and P.S.; Resources, P.S.; Software, N.F.; Supervision, R.S., C.N.O.-M. and P.S.; Validation, D.T.M. and P.S.; Visualization, N.F. and P.S.; Writing—original draft, R.S., C.N.O.-M., N.F. and P.S.; Writing—review & editing, R.S., C.N.O.-M., N.F. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from Children’s Miracle Network (C.N.O-M., P.S.) 2013-2015, the Center for Research on Women and Newborn Health (R.S., P.S.) 2016-2017, and the Penn State College of Medicine Faculty Endowment Funds (R.S., C.N.O-M., P.S.) 2013-2020.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of The Pennsylvania State University College of Medicine (protocol code PRAMS042136EP approved on 2 May 2013).
Informed Consent Statement
Informed consent was obtained from parents of all subjects involved in the study and patient information has been de-identified accordingly.
Data Availability Statement
The data presented in this study are openly available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165828. The code used for data analysis in the current study can be found at the Silveyra lab repository, http://psilveyra.github.io/silveyralab/.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jobe A.J. The new BPD: An arrest of lung development. Pediatr. Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Siffel C., Kistler K.D., Lewis J.F., Sarda S.P. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: A systematic literature review. J. Mater. Fetal Neonat. Med. 2019;9:1–11. doi: 10.1080/14767058.2019.1646240. [DOI] [PubMed] [Google Scholar]
- 3.Narang I. Review series: What goes around, comes around: Childhood influences on later lung health? Long-term follow-up of infants with lung disease of prematurity. Chron. Respir. Dis. 2010;7:259–269. doi: 10.1177/1479972310375454. [DOI] [PubMed] [Google Scholar]
- 4.Northway W.H., Jr., Rosan R.C., Porter D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease: Bronchopulmonary dysplasia. N. Engl. J. Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 5.Sahni M., Bhandari V. Recent advances in understanding and management of bronchopulmonary dysplasia. F1000Research. 2020;9:F1000 Faculty Rev-703. doi: 10.12688/f1000research.25338.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmatz M., Madan J., Marino T., Davis J. Maternal obesity: The interplay between inflammation, mother and fetus. J. Perinatol. 2010;30:441–446. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- 7.Pelzer E., Gomez-Arango L.F., Barrett H.L., Nitert M.D. Review: Maternal health and the placental microbiome. Placenta. 2017;54:30–37. doi: 10.1016/j.placenta.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Viscardi R.M. Perinatal inflammation and lung injury. Semin. Fetal Neonat. Med. 2012;17:30–35. doi: 10.1016/j.siny.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Check J., Gotteiner N., Liu X., Su E., Porta N., Steinhorn R., Mestan K.K. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J. Perinatol. 2013;33:553–557. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sillers L., Alexiou S., Jensen E.A. Lifelong pulmonary sequelae of bronchopulmonary dysplasia. Curr. Op. Pediatr. 2020;32:252–260. doi: 10.1097/MOP.0000000000000884. [DOI] [PubMed] [Google Scholar]
- 11.Doyle L.W., Cheong J.L., Ehrenkranz R.A., Halliday H.L. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Coch. Database Syst. Rev. 2017;10:CD001145. doi: 10.1002/14651858.CD001145.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onland W., De Jaegere A.P., Offringa M., van Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Coch. Database Syst. Rev. 2017;1:CD010941. doi: 10.1002/14651858.CD010941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen E.A., Dysart K., Gantz M.G., McDonald S., Bamat N.A., Keszler M., Kirpalani H., Laughon M.M., Poindexter B.B., Duncan A.F. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 2019;200:751–759. doi: 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenkranz R.A., Walsh M.C., Vohr B.R., Jobe A.H., Wright L.L., Fanaroff A.A., Wrage L.A., Poole K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 15.Rivera L., Siddaiah R., Oji-Mmuo C., Silveyra G.R., Silveyra P. Biomarkers for bronchopulmonary dysplasia in the preterm infant. Front. Pediatr. 2016;4:33. doi: 10.3389/fped.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Been J.V., Debeer A., van Iwaarden J.F., Kloosterboer N., Passos V.L., Naulaers G., Zimmermann L.J. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr. Res. 2010;67:83–89. doi: 10.1203/PDR.0b013e3181c13276. [DOI] [PubMed] [Google Scholar]
- 17.Piersigilli F., Lam T.T., Vernocchi P., Quagliariello A., Putignani L., Aghai Z.H., Bhandari V. Identification of new biomarkers of bronchopulmonary dysplasia using metabolomics. Metabolomics. 2019;15:20. doi: 10.1007/s11306-019-1482-9. [DOI] [PubMed] [Google Scholar]
- 18.Oji-Mmuo C.N., Siddaiah R., Montes D.T., Pham M.A., Spear D., Donnelly A., Fuentes N., Imamura-Kawasawa Y., Howrylak J.A., Thomas N.J., et al. Tracheal aspirate transcriptomic and miRNA signatures of extreme premature birth with bronchopulmonary dysplasia. J. Perinatol. 2020 doi: 10.1038/s41372-020-00868-9. [DOI] [PubMed] [Google Scholar]
- 19.Choo-Wing R., Syed M.A., Harijith A., Bowen B., Pryhuber G., Janér C., Andersson S., Homer R.J., Bhandari V. Hyperoxia and interferon-γ-induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent pathway. Am. J. Respir. Cell Mol. Biol. 2013;48:749–757. doi: 10.1165/rcmb.2012-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britt R.D., Velten M., Tipple T.E., Nelin L.D., Rogers L.K. Cyclooxygenase-2 in newborn hyperoxic lung injury. Free Radic. Biol. Med. 2013;61:502–511. doi: 10.1016/j.freeradbiomed.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K., Dong W. SIRT1-Related Signaling Pathways and Their Association With Bronchopulmonary Dysplasia. Front. Med. 2021;8:181. doi: 10.3389/fmed.2021.595634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sucre J.M.S., Vickers K.C., Benjamin J.T., Plosa E.J., Jetter C.S., Cutrone A., Ransom M., Anderson Z., Sheng Q., Fensterheim B.A., et al. Hyperoxia injury in the developing lung is mediated by mesenchymal expression of Wnt5A. Am. J. Respir. Crit. Care Med. 2020;201:1249–1262. doi: 10.1164/rccm.201908-1513OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameis D., Khoshgoo N., Iwasiow B.M., Snarr P., Keijzer R. MicroRNAs in lung development and disease. Paediatr. Respir. Rev. 2017;22:38–43. doi: 10.1016/j.prrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Freeman A., Qiao L., Olave N., Rezonzew G., Gentle S., Halloran B., Pryhuber G.S., Gaggar A., Tipple T.E., Ambalavanan N., et al. MicroRNA 219-5p inhibits alveolarization by reducing platelet derived growth factor receptor-alpha. Respir. Res. 2021;22:57. doi: 10.1186/s12931-021-01654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlek L.R., Vudatala S., Bartlett C.W., Buhimschi I.A., Buhimschi C.S., Rogers L.K. MiR-29b is associated with perinatal inflammation in extremely preterm infants. Pediatr. Res. 2020 doi: 10.1038/s41390-020-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go H., Maeda H., Miyazaki K., Maeda R., Kume Y., Namba F., Momoi N., Hashimoto K., Otsuru S., Kawasaki Y. Extracellular vesicle miRNA-21 is a potential biomarker for predicting chronic lung disease in premature infants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;318:L845–L851. doi: 10.1152/ajplung.00166.2019. [DOI] [PubMed] [Google Scholar]
- 27.Lal C.V., Olave N., Travers C., Rezonzew G., Dolma K., Simpson A., Halloran B., Aghai Z., Das P., Sharma N. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight. 2018;3:e93994. doi: 10.1172/jci.insight.93994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W. Research progress of relationship between miRNA and neonatal lung injury. Int. J. Pediatr. 2018;45:520–523. [Google Scholar]
- 29.Sun Y.-F., Ma L., Gong X.-H., Hong W.-C., Cai C. Expression of microRNA-495-5p in preterm infants with bronchopulmonary dysplasia: A bioinformatics analysis. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:24–30. doi: 10.7499/j.issn.1008-8830.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEvoy C.T., Jain L., Schmidt B., Abman S., Bancalari E., Aschner J.L. Bronchopulmonary dysplasia: NHLBI Workshop on the primary prevention of chronic lung diseases. Annu. Am. Thorac. Soc. 2014;11:S146–S153. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jobe A.H., Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 32.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuentes N., Roy A., Mishra V., Cabello N., Silveyra P. Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol. Sex Differ. 2018;9:18. doi: 10.1186/s13293-018-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarzenbach H., da Silva A.M., Calin G., Pantel K. Data normalization strategies for MicroRNA quantification. Clin. Chem. 2015;61:1333–1342. doi: 10.1373/clinchem.2015.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaujoux R., Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinform. 2010;11:1–9. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abman S.H., Collaco J.M., Shepherd E.G., Keszler M., Cuevas-Guaman M., Welty S.E., Truog W.E., McGrath-Morrow S.A., Moore P.E., Rhein L.M., et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J. Pediatr. 2017;181:12–28. doi: 10.1016/j.jpeds.2016.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinney R.L., Shukla K., Daigle K., Zeigler J., Muller M., Keszler M. The BIT:S (Bronchopulmonary Dysplasia Interdisciplinary Team: Severe) initiative at women and infants hospital of rhode island. R. I. Med. J. 2019;102:22–25. [PubMed] [Google Scholar]
- 39.Londhe V.A., Sundar I.K., Lopez B., Maisonet T.M., Yu Y., Aghai Z.H., Rahman I. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr. Res. 2011;69:371–377. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroon A.A., Wang J., Kavanagh B.P., Kavanagh B., Huang Z., Kuliszewski M., van Goudoever J.B., Post M. Prolonged mechanical ventilation induces cell cycle arrest in newborn rat lung. PLoS ONE. 2011;6:e16910. doi: 10.1371/annotation/23c9512d-c429-4673-a640-35cc2e4c2c5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du B., Wang Z., Zhang X., Feng S., Wang G., He J., Zhang B. MicroRNA-545 suppresses cell proliferation by targeting cyclin D1 and CDK4 in lung cancer cells. PLoS ONE. 2014;9:e88022. doi: 10.1371/journal.pone.0088022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi Y., Forrest A.R., Maeno E., Hashimoto T., Daub C.O., Yasuda J. MiR-107 and MiR-185 can induce cell cycle arrest in human non small cell lung cancer cell lines. PLoS ONE. 2009;4:e6677. doi: 10.1371/journal.pone.0006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carnino J.M., Lee H., He X., Groot M., Jin Y. Extracellular vesicle-cargo miR-185-5p reflects type II alveolar cell death after oxidative stress. Cell Death Discov. 2020;6:82. doi: 10.1038/s41420-020-00317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D., Lee H., Cao Y., Dela Cruz C.S., Jin Y. miR-185 mediates lung epithelial cell death after oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L700–L710. doi: 10.1152/ajplung.00392.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q., Chen Y., Liu K. miR-185 inhibits cell migration and invasion of hepatocellular carcinoma through CDC42. Oncol. Lett. 2018;16:3101–3107. doi: 10.3892/ol.2018.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L., Zhang Y., Liu J., Yin W., Jin D., Wang D., Zhang W. miR-185 Inhibits the proliferation and invasion of non-small cell lung cancer by targeting KLF7. Oncol. Res. 2019;27:1015–1023. doi: 10.3727/096504018X15247341491655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W., Sun Z., Su L., Wang F., Jiang Y., Yu D., Zhang F., Liang W. miRNA-185 serves as a prognostic factor and suppresses migration and invasion through Wnt1 in colon cancer. Eur. J. Pharmacol. 2018;825:75–84. doi: 10.1016/j.ejphar.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Liu M., Lang N., Chen X., Tang Q., Liu S., Huang J., Zheng Y., Bi F. miR-185 targets RhoA and Cdc42 expression and inhibits the proliferation potential of human colorectal cells. Cancer Lett. 2011;301:151–160. doi: 10.1016/j.canlet.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Shen F., Chang H., Gao G., Zhang B., Li X., Jin B. Long noncoding RNA FOXD2-AS1 promotes glioma malignancy and tumorigenesis via targeting miR-185-5p/CCND2 axis. J. Cell Biochem. 2019;120:9324–9336. doi: 10.1002/jcb.28208. [DOI] [PubMed] [Google Scholar]
- 50.Xue R., Li Y., Li X., Ma J., An C., Ma Z. miR-185 affected the EMT, cell viability, and proliferation via DNMT1/MEG3 pathway in TGF-β1-induced renal fibrosis. Cell Biol. Int. 2019;43:1152–1162. doi: 10.1002/cbin.11046. [DOI] [PubMed] [Google Scholar]
- 51.Lei G.S., Kline H.L., Lee C.H., Wilkes D.S., Zhang C. Regulation of collagen V expression and epithelial-mesenchymal transition by mir-185 and mir-186 during idiopathic pulmonary fibrosis. Am. J. Pathol. 2016;186:2310–2316. doi: 10.1016/j.ajpath.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang H., Fu J., Xue X., Yao L., Qiao L., Hou A., Jin L., Xing Y. Epithelial-mesenchymal transitions in bronchopulmonary dysplasia of newborn rats. Pediatr. Pulmonol. 2014;49:1112–1123. doi: 10.1002/ppul.22969. [DOI] [PubMed] [Google Scholar]
- 53.Bartis D., Mise N., Mahida R.Y., Eickelberg O., Thickett D.R. Epithelial-mesenchymal transition in lung development and disease: Does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 54.Lei Z., Shi H., Li W., Yu D., Shen F., Yu X., Lu D., Sun C., Liao K. miR-185 inhibits non-small cell lung cancer cell proliferation and invasion through targeting of SOX9 and regulation of Wnt signaling. Mol. Med. Rep. 2018;17:1742–1752. doi: 10.3892/mmr.2017.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pongracz J.E., Stockley R.A. Wnt signalling in lung development and diseases. Respir. Res. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Langhe S.P., Reynolds S.D. Wnt signaling in lung organogenesis. Organogenesis. 2008;4:100–108. doi: 10.4161/org.4.2.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho C.S., Noor S.M., Nagoor N.H. mir-378 and mir-1827 regulate tumor invasion, migration and angiogenesis in human lung adenocarcinoma by targeting. J. Cancer. 2018;9:331–345. doi: 10.7150/jca.18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machado I.F., Teodoro J.S., Palmeira C.M., Rolo A.P. miR-378a: A new emerging microRNA in metabolism. Cell Mol. Life Sci. 2020;77:1947–1958. doi: 10.1007/s00018-019-03375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Li H. microRNA-378a regulates the reactive oxygen species (ROS)/phosphatidylinositol 3-kinases (PI3K)/AKT signaling pathway in human lens epithelial cells and cataract. Med. Sci. Monit. 2019;25:4314–4321. doi: 10.12659/MSM.916881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Revel A., Achache H., Stevens J., Smith Y., Reich R. MicroRNAs are associated with human embryo implantation defects. Hum. Reprod. 2011;26:2830–2840. doi: 10.1093/humrep/der255. [DOI] [PubMed] [Google Scholar]
- 61.Li D., Chen S., Zhang W., Zhang C., Sun T., Du Y., Ding R., Gao Y., Jin Y., Duan G. MicroRNA-628-5p facilitates enterovirus 71 infection by suppressing TRAF3 signaling. Cell Mol. Immunol. 2020 doi: 10.1038/s41423-020-0453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie P., Wang Y., Liao Y., Han Q., Qiu Z., Chen Y., Zuo X. MicroRNA-628-5p inhibits cell proliferation in glioma by targeting DDX59. J. Cell Biochem. 2019;120:17293–17302. doi: 10.1002/jcb.28991. [DOI] [PubMed] [Google Scholar]
- 63.Li M., Qian Z., Ma X., Lin X., You Y., Li Y., Chen T., Jiang H. MiR-628-5p decreases the tumorigenicity of epithelial ovarian cancer cells by targeting at FGFR2. Biochem. Biophys. Res. Commun. 2018;495:2085–2091. doi: 10.1016/j.bbrc.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 64.Arman E., Haffner-Krausz R., Gorivodsky M., Lonai P. Fgfr2 is required for limb outgrowth and lung-branching morphogenesis. Proc. Natl. Acad. Sci. USA. 1999;96:11895–11899. doi: 10.1073/pnas.96.21.11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimbori C., El Agha E. Good things come in 2s: Type 2 alveolar epithelial cells and fibroblast growth factor receptor 2. Am. J. Respir. Cell Mol. Biol. 2020;62:543–545. doi: 10.1165/rcmb.2020-0013ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rios-Colon L., Deep G., Kumar D. Emerging role of microRNA 628-5p as a novel biomarker for cancer and other diseases. Tumour Biol. 2019;41 doi: 10.1177/1010428319881344. [DOI] [PubMed] [Google Scholar]
- 67.Hao R., Hu X., Wu C., Li N. Hypoxia-induced miR-15a promotes mesenchymal ablation and adaptation to hypoxia during lung development in chicken. PLoS ONE. 2014;9:e98868. doi: 10.1371/journal.pone.0098868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin R.J., Di Fiore J.M., Walsh M.C. Hypoxic episodes in bronchopulmonary dysplasia. Clin. Perinatol. 2015;42:825–838. doi: 10.1016/j.clp.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y., Zhao X., Sun J., Su W., Zhang L., Li Y., Liu Y., Lu Y., Shan H., Liang H. YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019;26:1832–1844. doi: 10.1038/s41418-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhattacharya S., Mereness J., Baran A., Misra R., Peterson D., Ryan R., Reynolds A., Pryhuber G., Mariani T. Lymphocyte-specific biomarkers associated with preterm birth and bronchopulmonary dysplasia. Front. Immunol. 2021 doi: 10.3389/fimmu.2020.563473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahoney J.E., Mori M., Szymaniak A.D., Varelas X., Cardoso W.V. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Oliveira A., Castanhole-Nunes M.M.U., Biselli-Chicote P.M., Pavarino E.C., da Silva R., da Silva R.F., Goloni-Bertollo E.M. Differential expression of angiogenesis-related miRNAs and VEGFA in cirrhosis and hepatocellular carcinoma. Arch. Med. Sci. 2020;16:1150–1157. doi: 10.5114/aoms.2020.97967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatt A.J., Pryhuber G.S., Huyck H., Watkins R.H., Metlay L.A., Maniscalco W.M. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 74.Geng Y., Deng L., Su D., Xiao J., Ge D., Bao Y., Jing H. Identification of crucial microRNAs and genes in hypoxia-induced human lung adenocarcinoma cells. Onco. Targets Ther. 2016;9:4605–4616. doi: 10.2147/OTT.S103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su T., Gu C., Draga D., Zhou C., Lhamo T., Zheng Z., Qiu Q. Integrative analysis of miRNA-mRNA network in high altitude retinopathy by bioinformatics analysis. Biosci. Rep. 2021;41 doi: 10.1042/BSR20200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Bai J., Yin H., Long L., Zheng Z., Wang Q., Chen F., Yu X., Zhou Y. Exosomal miR-1255b-5p targets human telomerase reverse transcriptase in colorectal cancer cells to suppress epithelial-to-mesenchymal transition. Mol. Oncol. 2020;14:2589–2608. doi: 10.1002/1878-0261.12765. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Ratner V., Slinko S., Utkina-Sosunova I., Starkov A., Polin R.A., Ten V.S. Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology. 2009;95:299–305. doi: 10.1159/000178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boucher E., Provost P.R., Devillers A., Tremblay Y. Levels of dihydrotestosterone, testosterone, androstenedione, and estradiol in canalicular, saccular, and alveolar mouse lungs. Lung. 2010;188:229–233. doi: 10.1007/s00408-010-9231-x. [DOI] [PubMed] [Google Scholar]
- 79.Silveyra P. Chapter 9: Developmental lung disease. In: Hemnes A.R., editor. Gender, Sex Hormones and Respiratory Disease. A Comprehensive Guide. Sprenger; Berlin, Germany: 2016. p. 243. [DOI] [Google Scholar]
- 80.Dammann C.E., Ramadurai S.M., McCants D.D., Pham L.D., Nielsen H.C. Androgen regulation of signaling pathways in late fetal mouse lung development. Endocrinology. 2000;141:2923–2929. doi: 10.1210/endo.141.8.7615. [DOI] [PubMed] [Google Scholar]
- 81.Kimura Y., Suzuki T., Kaneko C., Darnel A.D., Akahira J., Ebina M., Nukiwa T., Sasano H. Expression of androgen receptor and 5alpha-reductase types 1 and 2 in early gestation fetal lung: A possible correlation with branching morphogenesis. Clin. Sci. Lond. 2003;105:709–713. doi: 10.1042/CS20030236. [DOI] [PubMed] [Google Scholar]
- 82.Volpe M.V., Ramadurai S.M., Mujahid S., Vong T., Brandao M., Wang K.T., Pham L.D., Nielsen H.C. Regulatory interactions between androgens, Hoxb5, and TGF β signaling in murine lung development. Biomed. Res. Int. 2013;2013:320249. doi: 10.1155/2013/320249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bresson E., Seaborn T., Côté M., Cormier G., Provost P.R., Piedboeuf B., Tremblay Y. Gene expression profile of androgen modulated genes in the murine fetal developing lung. Reprod. Biol. Endocrinol. 2010;8:2. doi: 10.1186/1477-7827-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rehan V.K., Torday J.S. PPARγ signaling mediates the evolution, development, homeostasis, and repair of the lung. PPAR Res. 2012;2012:289867. doi: 10.1155/2012/289867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehan V.K., Torday J.S. The lung alveolar lipofibroblast: An evolutionary strategy against neonatal hyperoxic lung injury. Antioxid. Redox Signal. 2014;21:1893–1904. doi: 10.1089/ars.2013.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lecarpentier Y., Gourrier E., Gobert V., Vallée A. Bronchopulmonary dysplasia: Crosstalk between PPARγ, WNT/β-catenin and TGF-β pathways; The potential therapeutic role of PPARγ agonists. Front. Pediatr. 2019;7:176. doi: 10.3389/fped.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang A., Zhang S., Li Z., Liang R., Ren S., Li J., Pu Y., Yang J. miR-615-3p promotes the phagocytic capacity of splenic macrophages by targeting ligand-dependent nuclear receptor corepressor in cirrhosis-related portal hypertension. Exp. Biol. Med. Maywood. 2011;236:672–680. doi: 10.1258/ebm.2011.010349. [DOI] [PubMed] [Google Scholar]
- 88.Pagel J., Twisselmann N., Rausch T.K., Waschina S., Hartz A., Steinbeis M., Olbertz J., Nagel K., Steinmetz A., Faust K., et al. Increased regulatory T cells precede the development of bronchopulmonary dysplasia in preterm infants. Front. Immunol. 2020;11:565257. doi: 10.3389/fimmu.2020.565257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eldredge L.C., Creasy R.S., Presnell S., Debley J.S., Juul S.E., Mayock D.E., Ziegler S.F. Infants with evolving bronchopulmonary dysplasia demonstrate monocyte-specific expression of IL-1 in tracheal aspirates. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;317:L49–L56. doi: 10.1152/ajplung.00060.2019. [DOI] [PubMed] [Google Scholar]
- 90.Permall D.L., Pasha A.B., Chen X.Q., Lu H.Y. The lung microbiome in neonates. Turk. J. Pediatr. 2019;61:821–830. doi: 10.24953/turkjped.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Tirone C., Pezza L., Paladini A., Tana M., Aurilia C., Lio A., D’Ippolito S., Tersigni C., Posteraro B., Sanguinetti M., et al. Gut and lung microbiota in preterm infants: Immunological modulation and implication in neonatal outcomes. Front. Immunol. 2019;10:2910. doi: 10.3389/fimmu.2019.02910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are openly available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165828. The code used for data analysis in the current study can be found at the Silveyra lab repository, http://psilveyra.github.io/silveyralab/.