Abstract
Schisandra chinensis has been widely used as a traditional herbal medicine to treat chronic coughs, fatigue, night sweats, and insomnia. Numerous bioactive components including lignans have been identified in this plant. Lignans with a dibenzocyclooctadiene moiety have been known to possess anti-cancer, anti-inflammatory, and hepatoprotective activity. Fragmentary studies have reported the ability of some lignans to modulate some cytochrome P450 (P450) enzymes. Herein, we investigated the drug interaction potential of six dibenzocyclooctadiene lignans (schisandrin, gomisin A, B, C, and N, and wuweizisu C) on nine P450 enzymes (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A) and six uridine 5′-diphosphoglucuronosyl transferase (UGT) enzymes (UGT1A1, 1A3, 1A4, 1A6, 1A9, and 2B7) using human liver microsomes. We found that lignans with one or two methylenedioxyphenyl groups inhibited CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1 activities in a time- and concentration-dependent like their CYP3A inhibition. In comparison, these lignans do not induce time-dependent inhibition of CYP1A2, CYP2A6, and CYP2D6. The time-dependent inhibition of gomisin A against CYP2C8, CYP2C19, and CYP3A4 was also elucidated using glutathione as a trapping reagent of reactive carbene metabolites given that gomisin A strongly inhibits these P450 enzymes in a time-dependent manner. A glutathione conjugate of gomisin A was generated in reactions with human recombinant CYP2C8, CYP2C19, and CYP3A4. This suggests that the time-dependent inhibition of gomisin A against CYP2C8, CYP2C9, and CYP3A4 is due to the production of carbene reactive metabolite. Six of the lignans we tested inhibited the activities of six UGT to a limited extent (IC50 > 15 μM). This information may aid the prediction of possible drug interactions between Schisandra lignans and any co-administered drugs which are mainly metabolized by P450s.
Keywords: Schisandra chinensis, lignans, cytochrome P450, uridine 5′-diphosphoglucuronosyl transferase, drug interaction
1. Introduction
Herb drug interactions (HDI) which result in serious adverse events have received significant attention with the increased use of alternative medicines as well as the widespread use of combination therapies for various diseases in recent years [1,2]. The underlying mechanisms of HDI mainly involve the modulation of cytochrome P450 (P450) and uridine 5′-diphosphoglucuronisyl transferase (UGTs) enzyme activities [2,3]. For example, Saint John’s Wort, a well-studied example of such an HDI, and hyperforin is thought to be the main component that modulates CYP3A and CYP2C9 enzymes [4]. Many well-known herbal medicines including ginseng [5,6], ginkgo [7], green tea [8], and Schisandra [9] have also been reported to result in pharmacokinetic drug interactions with clinical drugs. In a recent study, curcuma was found to significantly increase the urine metabolic ratio of dextromethorphan/dextrorphan by inhibiting CYP2D6 [10].
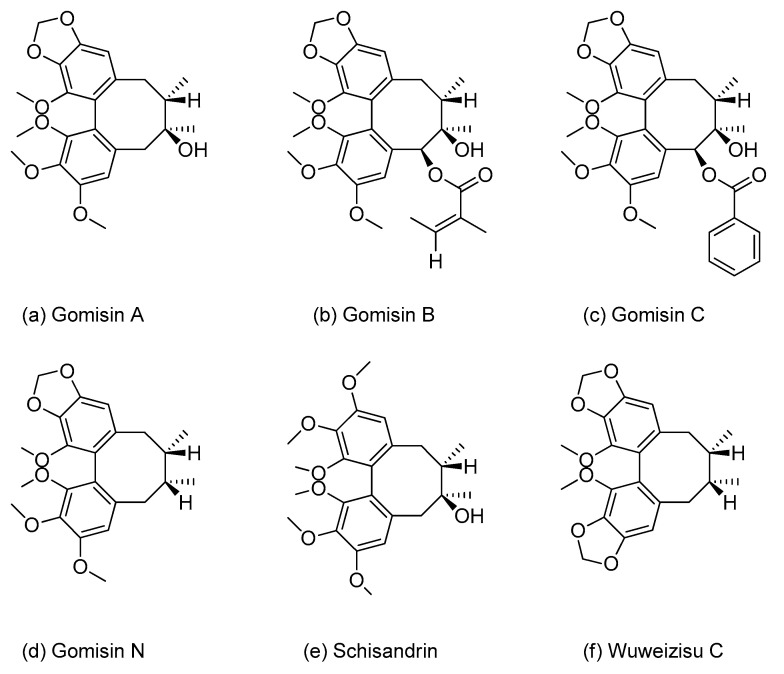
Schisandra chinensis Bailon (Schisandraceae), a climbing plant distributed in Asia (Korea, Japan, and China) [11] and its fruits, known as omija in Korea, have been extensively used in traditional herbal medicine to treat chronic coughs, enuresis, fatigue, night sweats, and insomnia [12]. In clinical settings, however, co-administration of Schisandra extracts or their components with other therapeutic drugs may lead to HDIs. For example, Schisandra extracts markedly increased the blood concentration of taclolimus by inhibiting the CYP3A enzyme in liver transplant patients [9]. Gomisin N, one of the most abundant lignans isolated from the fruits of S. chinensis, has also been shown to increase the oral bioavailability of drugs metabolized by CYP3A, including midazolam in rats [13]. Therefore, dibenzocyclooctadiene lignans, the major active components of S. chinensis, may account for some HDIs. The major lignans in the fruits of S. chinensis are gomisin A, -B, -C, and -N, as well as schisandrin and wuweizisu C (Figure 1), with schisandrin being the most abundant, accounting for 33−45% of the total lignans in omija (S. chinensis) fruits [14,15,16].
Figure 1.
Chemical structures of the six lignans: (a) gomisin A, (b) gomisin B, (c) gomisin C, (d) gomisin N, (e) schisandrin, and (f) wuweizisu C.
A few in vitro studies investigating the modulatory effects of individual dibenzocyclooctadiene lignans on P450 enzyme activities have been carried out. For example, previous studies found that gomisin A, -C, and -G inhibit CYP3A-mediated midazolam 1′-hydroxylation with IC50 values of 1.86 μM, 0.059 μM, and 0.19 μM in a recombinant CYP3A4 isoform, respectively [17,18], while schisandrin A inhibits CYP2C19-catalyzed omeprazole hydroxylation with an IC50 of 86.4 μM in recombinant CYP2C19 [19]. Moreover, schisandrin and gomisin A were found to inhibit recombinant CYP3A4 activity with IC50 values of 32.0 and 1.39 μM, respectively [20]. Iwata et al. (2004) also evaluated the effects of six lignans including schisandrin, gomisin A, and -N on P450 activities, however, their inhibitory effects were estimated for only five P450s (1A2, 2C9, 2C19, 2D6, and 3A) [21]. Recent studies have discussed the clinical significance of CYP2A6, CY2B6, CYP2C8, and CYP2E1, in addition to the five major P450s, with respect to drug interactions [22]. For example, clopidogrel [23] and piperine [24] were shown to cause a significant increase in the plasma levels of montelukast and chlorzoxazone via inhibition of CYP2C8 and CYP2E1, respectively. Cho et al. (2016) previously suggested that rifampin significantly enhances CYP2B6-mediated efavirenz 8-hydroxylation in humans by inducing CYP2B6 activity [25].
Compared to the studies on P450-mediated drug interaction with lignans, data on the inhibitory effects of lignans on UGT enzymes are rare, even though UGT inhibition is regarded as one of the most important factors for clinical HDIs [26]. The only relevant available data are on the inhibitory potential of schisandrin A and gomisin C against UGT activities [27].
Although some studies have investigated the inhibitory effects of several dibenzocyclooctadiene lignans on P450 and UGT, there are not enough published data to compare the inhibitory effects of individual lignans on P450 and UGT enzyme activities in human liver microsomes (HLMs). Thus, the goals of our study were three-fold: (1) to evaluate the inhibitory potential of six lignans on nine P450s and six UGTs in HLMs; (2) to investigate the time-dependent inhibition of six lignans on nine P450s in HLMs; and (3) to elucidate the time-dependent inhibition mechanism of gomisin A in recombinant P450s (rP450s).
2. Materials and Methods
2.1. Chemicals and Reagents
Acetaminophen, N-acetylserotonin (AS), amodiaquine, bupropion, chenodeoxycholic acid (CDCA), chlorzoxazone, N-desethylamodiaquine, dextromethorphan, dextrorphan, 6-hydroxybupropion, 7-hydroxycoumarin, estrone-β-D-glucuronide, naloxone, naloxone-β-D-glucuronide, phenacetin, trifluoperazine, trifluoperazine-β-D-glucuronide, trimipramine, alamethicin, uridine diphosphoglucuronic acid (UDPGA), nicotinamide adenine dinucleotide phosphate (NADP+), glucose-6-phosphate (G6P), glucose-6-phosphate dehydrogenase (G6PDH), and glutathione (GSH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-Acetylserotonin-β-D-glucuronide, coumarin, chenodeoxycholic acid-24-acyl-β-glucuronide, dehydronifedipine, diclofenac, 7-ethyl-10-hydroxy camptothecin (SN-38) glucuronide, 4-hydroxydiclofenac, 4-hydroxymephenytoin, 6-hydroxychlorzoxazone, 6β-hydroxytestosterone, midazolam, S-mephenytoin, mycophenolic acid (MPA), mycophenolic acid-β-D-glucuronide, nifedipine, and testosterone were purchased from Toronto Research Chemicals (Toronto, ON, Canada). We obtained 1′-hydroxymidazolam and 7-ethyl-10-hydroxy camptothecin (SN-38) from Cayman Chemical (Ann Arbor, MI, USA) and Santa Cruz Biotechnology (Dallas, TX, USA), respectively. Nicotinamide adenine dinucleotide phosphate (NADPH) was provided by Oriental Yeast (Tokyo, Japan). Pooled HLMs (XTreme 200, H2630, mixed gender) were supplied by XenoTech (Lenexa, KS, USA). We purchased rP450 isoforms (rCYP1A2, rCYP2A6, rCYP2B6, rCYP2C8, rCYP2C9, rCYP2C19, rCYP2D6, rCYP2E1, and rCYP3A4) from SPMED (Busan, Korea). All solvents used in the analyses were LC-MS grade (Fisher Scientific Co., Pittsburgh, PA, USA).
We isolated gomisin A, gomisin N, schisandrin, and wuweizisu C from fruits of Schisandra chinensis Bailon. The four compounds were purified and analyzed by high-performance liquid chromatography system (HPLC) to obtain 95% pure samples. Chemical structures of the isolated compounds were determined by NMR. All structures were consistent with previously published data [28]. Gomisin B (95%) and gomisin C (98%) were purchased from Toronto Research Chemical (Toronto, ON, Canada) and Sigma-Aldrich, respectively. We adopted the nomenclature of lignans from the recent review article by Opletal et al. (2004) [29].
2.2. Inhibitory Effects of Six Lignans against Human Cytochrome P450 Activity
The inhibitory potential of six lignans on nine P450 activities was evaluated as previously described with slight modifications [30]. Lignans were first dissolved in methanol. The final organic solvent concentration in the incubation media was ≤1.0% (v/v). The microsomal incubation was conducted using two cocktail sets containing non-interactive substrates: set A containing phenacetin, bupropion, amodiaquine, diclofenac, S-mephenytoin, and dextromethorphan as substrates for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6, respectively; and set B containing coumarin and chlorzoxazone as substrates for CYP2A6 and CYP2E1, and midazolam, nifedipine, and testosterone as substrates for CYP3A4 (Table 1). These substrates are known to be selective for each P450 isoforms. Incubation mixtures containing potassium phosphate buffer (0.1 M, pH 7.4), pooled HLMs (0.25 mg/mL), P450 probe substrate cocktails, and lignan (0~50 μM) were pre-incubated at 37 °C (5 min). Following pre-incubation, an NADPH generating system (1.3 mM NADP+, 3.3 mM G6P, 1 unit/mL G6PDH, and 3.3 mM MgCl2) was added to initiate the reaction, and further incubated for 10 min. The reaction was quenched with 50 μL of cold acetonitrile containing internal standard (IS; 7 nM trimipramine). After centrifugation at 14,000 rpm (5 min at 4 °C), aliquots of supernatants were filtered through a 0.2 μm membrane filter and were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). All microsomal incubations were performed in triplicate.
Table 1.
Optimized selected reaction monitoring (SRM) parameters for metabolites of the nine P450 substrates and internal standard (IS).
| P450 Enzyme |
Substrate | Concentration (μM) | Metabolite | SRM Transition (m/z) |
Polarity * | Collision Energy (eV) |
|---|---|---|---|---|---|---|
| 1A2 | Phenacetin | 20 | Acetaminophen | 152 > 110 | ESI+ | 25 |
| 2A6 | Coumarin | 1 | 7-Hydroxycoumarin | 163 > 107 | ESI+ | 17 |
| 2B6 | Bupropion | 3 | 6-Hydroxybupropion | 256 > 238 | ESI+ | 10 |
| 2C8 | Amodiaquine | 0.1 | N-Desethylamodiaquine | 328 > 283 | ESI+ | 13 |
| 2C9 | Diclofenac | 1 | 4-Hydroxydiclofenac | 312 > 231 | ESI+ | 15 |
| 2C19 | S-Mephenytoin | 40 | 4-Hydroxymethenytoin | 235 > 150 | ESI+ | 15 |
| 2D6 | Dextromethorphan | 2 | Dextrorphan | 258 > 157 | ESI+ | 30 |
| 2E1 | Chlorzoxazone | 5 | 6-Hydroxychlorzoxazone | 184 > 120 | ESI− | 18 |
| 3A4 | Midazolam | 0.1 | 1′-Hydroxymidazolam | 342 > 203 | ESI+ | 28 |
| Nifedipine | 0.2 | Dehydronifedipine | 345 > 284 | ESI+ | 30 | |
| Testosterone | 2 | 6β-Hydroxytestosterone | 305 > 269 | ESI+ | 15 | |
| IS | Trimipramine | 0.007 | - | 295 > 100 | ESI+ | 17 |
* ESI: Electrospray ionization (ESI) interface to generate protonated molecular ion [M+H]+ or deprotonated molecular ion [M-H]−.
For IC50 shift assay [31], each lignan (0~50 μM) was pre-incubated with HLMs in the presence of an NADPH generating system at 37 °C for 30 min. After pre-incubation, P450 probe substrate cocktails were added to start the reaction, and further incubated at 37 °C for 10 min. Incubation was terminated by the addition of 50 μL ice-cold acetonitrile containing IS. After centrifugation, aliquots of supernatants were measured by LC-MS/MS.
2.3. Inhibitory Effects of Gomisin A against Recombianat CYP2C8, CYP2C19, and CYP3A4 Activity
The incubation mixture consisted of 0.1 M potassium phosphate buffer (pH 7.4), 20 pmol/mL rCYP2C8, rCYP2C19, or rCYP3A4 enzyme, respective probe substrate and gomisin A (0~50 μM) in a final volume of 100 μL. After a 5 min pre-incubation period at 37 °C, reactions were initiated by the addition of an NADPH generating system, and further incubated for 10 min at 37 °C. For time-dependent inhibition studies, gomisin A (0~50 μM) was pre-incubated with rP450s in the presence of an NADPH generating system at 37 °C for 30 min. After pre-incubation, P450 probe substrates (0.1 μM amodiaquine for CYP2C8, 40 μM S-mephenytoin for CYP2C19 or 0.1 μM midazolam for CYP3A4) were added to start the reaction, and further incubated at 37 °C for 10 min. Other conditions remained as described above.
2.4. Inhibitory Effects of Six Lignans against Human Uridine-5-Diphosphoglucuronosyl Transferase Activity
The inhibitory potential of six lignans on six UGT activities was evaluated using the previously developed cocktail method with slight modifications [32]. The microsomal incubation was conducted using two cocktail sets containing non-interactive substrates: set A with SN-38, CDCA, and trifluoperazine as substrates for UGT1A1, UGT1A3, and UGT1A4, respectively; and set B containing AS, MPA, and naloxone as substrates for UGT1A6, UGT1A9, and UGT2B7 (Table 2). These substrates are known to be selective for each UGT isoforms. The incubation mixtures containing Tris buffer (0.1 M, pH 7.4), pooled HLMs (0.25 mg/mL), alamethicin (25 μg/mL), UGT probe substrate cocktails, and lignan (0~50 μM) were pre-incubated at 37 °C. After pre-incubation, 5 mM UDPGA was added to initiate the reaction, and further incubated for 60 min. The reaction was quenched with 50 μL of cold acetonitrile containing IS (350 nM estrone-β-D-glucuronide). After centrifugation, aliquots of supernatants were measured by LC-MS/MS.
Table 2.
Optimized selected reaction monitoring (SRM) parameters for the metabolites of the six UGT substrates and internal standard (IS).
| UGT Enzyme |
Substrate | Concentration (μM) | Metabolite | SRM Transition (m/z) |
Polarity | Collision Energy (eV) |
|---|---|---|---|---|---|---|
| 1A1 | SN-38 * | 0.5 | SN-38 glucuronide | 569 > 393 | ESI+ | 30 |
| 1A3 | Chenodeoxycholic acid | 2 | CDCA-24 glucuronide |
567 > 391 | ESI- | 20 |
| 1A4 | Trifluoperazine | 0.5 | TFP N-glucuronide | 584 > 408 | ESI+ | 30 |
| 1A6 | N-Acetylserotonin | 1 | N-SER glucuronide | 395 > 219 | ESI+ | 10 |
| 1A9 | Mycophenolic acid | 0.2 | MPA 7-O-glucuronide |
495 > 319 | ESI- | 25 |
| 2B7 | Naloxone | 0.2 | NX 3-glucuronide | 504 > 310 | ESI+ | 30 |
| IS | Estrone-β-D-glucuronide | 0.25 | 445 > 269 | ESI- | 35 |
* SN-38: 7-Ethyl-10-hydroxy camptothecin; SI: Electrospray ionization (ESI) interface to generate protonated molecular ion [M+H]+ or deprotonated molecular ion [M-H]−.
2.5. Characterization of Reactive Metabolites of Gomisin A in Recombinant P450 Isoforms
Gomisin A (50 μM) was incubated for 120 min at 37 °C with rP450s (20 pmol/mL) in 0.1 M phosphate buffer (pH 7.4) in the presence of 2 mM NADPH and 5 mM GSH. Control incubations in the absence of NADPH and GSH were conducted. Incubation samples were quenched with a half-fold volume of cold methanol. After centrifugation, supernatants were concentrated under vacuum and reconstituted with methanol (100 μL). Samples were analyzed by ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS).
2.6. LC-MS/MS Analysis
The IS and each P450- and UGT-isoform-specific metabolites were separated on a Kinetex XB-C18 column (100 × 2.1 mm, 2.6 μm, Phenomenex, Torrance, CA, USA) and analyzed using a Shimadzu LC-MS 8060 triple-quadrupole mass spectrometer (Shimadzu, Kyoto, Japan) equipped with a Nexera X2 ultra HPLC system (Shimadzu) coupled with an electrospray ionization (ESI) interface. The mobile phase was composed of water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B). Elution conditions for the analysis of metabolites of P450 probe substrates were set as 8% B for 0–0.5 min, 8%→60% B for 0.5–5 min, 60% B for 5–6 min, 60%→8% B for 6–6.1 min, and 8% B for 6.1–9 min [30], while that of UGT probe substrates was set as 0% B for 0–1 min, 50% B for 1–5 min, and 0% B for 5.1–8 min [32]. The flow rate was set to 0.2 mL/min. ESI was performed in negative-ion mode at −3500 V or in positive ion mode at 4000 V. For quantitation of each metabolite, the analysis was performed in selected reaction monitoring (SRM) with the precursor-to-product ion transition (Table 1 and Table 2).
To determine the GSH adducts produced by rP450s, a Vanquish UPLC system coupled with a QExactive Focus Orbitrap mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used. A Kinetex C18 column (100 × 2.1 mm, 2.6 μm) was used to separate the samples. The mobile phase was composed of water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B). The gradient elution was set as 10% B for 0–3 min, 10%→98% B for 3–10 min, 98%→10% B for 10–10.1 min, 10% B for 10.1–12 min. The flow rate was set to 0.2 mL/min. Data acquisition was carried out at m/z 100–800 with a resolution of 70,000 in the total ion scan mode, and MS/MS spectra were acquired at m/z 50–750 at a resolution of 17,500 in the daughter ion scan mode [33]. Parallel reaction monitoring (PRM) was also employed, and the PRM transition m/z 710.2576 was used for the detection of GSH conjugate [34]. Instrument settings were as follows: normalized collision energy (CE), 25 eV; capillary temperature, 320 °C; spray voltage, 3.5 kV; sheath gas flow rate, 40 arb; auxiliary gas flow rate, 10 arb; S-lens RF level, 50.0 V. Nitrogen was used for spray stabilization and as the collision gas in the C-trap.
2.7. Data Analysis
All results were acquired from three replicates in different microsomal incubations. Analytical data were processed by Shimadzu LabSolutions LC-MS software (Shimadzu, Kyoto, Japan) or Thermo Xcalibur software (Thermo Fisher Scientific Inc.). IC50 values were determined by nonlinear regression analysis using WinNonlin software (Pharsight, Mountain View, CA, USA).
3. Results and Discussion
3.1. Inhibition of Cytochrome P450 Activities by Six Lignans
The inhibitory potential of the six Omija lignans (gomisin A, -B, -C, and -N, as well as schisandrin, and wuweizisu C) against P450 enzyme activity was evaluated in HLMs (Table 3). Lignans with one methylenedioxyphenyl group such as gomisin A, -B, -C, and -N most strongly inhibited CYP3A activity with IC50 of 1.8–2.3, 0.28–0.42, 0.19–0.30, and 1.3–4.5 μM, respectively. The inhibitory potential (IC50 = 2.3 μM) of gomisin A on CYP3A-mediated testosterone hydroxylation was similar to previously published data (Ki = 1.01 μM) [35]. The inhibitory potential (IC50 = 4.5 μM) of gomisin N on CYP3A-mediated midazolam hydroxylation was also similar to the previously reported value (IC50 = 5.5 μM) [36]. The inhibition of eight other P450 isoforms was much lower (IC50 > 10 μM) than on CYP3A (IC50 ≤ 4.5 μM). Iwata et al. (2004) also reported weak inhibition (IC50 > 10 μM) of gomisin A, -B, -C, and -N on CYP1A2, CYP2C9, CYP2C19, and CYP2D6 [21]. Schisandrin without a methylenedioxyphenyl group showed moderate inhibition (IC50 ≤ 16 μM) on CYP1A2, CYP2B6, CYP2C19, CYP2E1, and CYP3A, while it showed weak inhibition (IC50 = 22.0 ~ 43.0 μM) on other P450s. We found that IC50 schisandrin inhibits CYP3A with IC50 values of 10.5–16.0 μM, slightly lower than the the 32.0 μM reported by Wan et al. (2010) [20]. This discrepancy could be due to differences in incubation conditions perhaps in the CYP3A probe substrates (midazolam and nifedipine versus fluorescent substrate) and/or the enzyme source (HLM vs rCYP3A4). Wuweizisu C with two methylenedioxyphenyl groups moderately inhibited CYP2B6, CYP2C9, CYP2C19, and CYP3A, while it weakly inhibited CYP1A2, CYP2A6, CYP2C8, CYP2D6, and CYP2E1 (IC50 > 20 μM).
Table 3.
Inhibitory effects of six lignans against nine cytochrome P450 enzymes. All incubations were performed in triplicate (n = 3).
| P450 Enzyme |
Probe Substrate |
IC50 (µM) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gomisin A | Gomisin B | Gomisin C | Gomisin N | Schisandrin | Wuweizisu C | ||||||||||||||
| RI * | TDI ** | IC50 Shift |
RI | TDI | IC50 Shift |
RI | TDI | IC50 Shift |
RI | TDI | IC50 Shiaft |
RI | TDI | IC50 Shift |
RI | TDI | IC50 Shift |
||
| 1A2 | Phenacetin | 37.4 | >50 | - | >50 | >50 | - | >50 | >50 | - | >50 | >50 | - | 4.0 | >50 | - | 25.6 | 22.4 | <1.5 |
| 2A6 | Coumarin | >50 | >50 | - | >50 | >50 | - | >50 | >50 | - | 38.2 | >50 | - | 40.9 | >50 | - | >50 | >50 | - |
| 2B6 | Bupropion | >50 | >50 | - | 42.6 | >50 | - | 33.1 | >50 | - | 38.3 | >50 | - | 14.7 | >50 | - | 2.9 | 1.4 | 2.1 |
| 2C8 | Amodiaquine | 29.3 | 2.8 | 10.5 | 16.5 | 4.9 | 3.4 | 10.9 | 5.8 | 1.9 | 31.7 | 9.9 | 3.2 | 22.0 | >50 | - | 21.0 | 14.6 | <1.5 |
| 2C9 | Diclofenac | 45.4 | 22.0 | 2.1 | 45.7 | >50 | - | >50 | >50 | - | 36.2 | 10.9 | 3.3 | 43.0 | >50 | - | 8.9 | 3.6 | 2.5 |
| 2C19 | S-Mephenytoin | 11.2 | 4.8 | 2.3 | >50 | 37.8 | - | 16.3 | 20.4 | - | 10.4 | 3.5 | 3.0 | 5.3 | 46.1 | - | 2.7 | 1.5 | 1.8 |
| 2D6 | Dextromethorphan | 45.7 | >50 | - | 45.5 | >50 | - | >50 | >50 | - | 42.0 | >50 | - | 40.2 | >50 | - | 20.3 | 28.2 | - |
| 2E1 | Chlorzoxazone | >50 | 15.6 | >3.2 | >50 | 20.6 | >2.4 | >50 | 24.4 | >2.0 | >50 | 23.6 | >2.1 | 4.2 | 36.0 | - | >50 | 25.2 | >2.0 |
| 3A | Midazolam | 3.1 | 1.2 | 2.6 | 0.42 | 0.12 | 3.5 | 0.30 | 0.10 | 3.0 | 4.5 | 1.7 | 2.7 | 10.5 | 35.0 | - | 25.9 | 2.5 | 10.4 |
| Nifedipine | 1.8 | 0.77 | 2.3 | 0.32 | 0.10 | 3.2 | 0.26 | 0.09 | 2.9 | 1.4 | 0.61 | 2.4 | 16.0 | 43.3 | - | 5.6 | 1.2 | 4.7 | |
| Testosterone | 2.3 | 0.77 | 3.0 | 0.28 | 0.09 | 3.1 | 0.19 | 0.09 | 2.1 | 1.3 | 0.55 | 2.4 | 5.8 | 20.6 | - | 3.6 | 1.2 | 3.0 | |
* RI: Reversible inhibition, ** TDI: Time-dependent inhibition.
All lignans tested weakly inhibited CYP2D6-mediated dextromethorphan O-demethylation and CYP2A6-mediated coumarin hydroxylation with IC50 values of over 20 μM and 38 μM, respectively. Lignans without a methylenedioxyphenyl group induced stronger inhibition of CYP1A2 and CYP2E1 activities (IC50 ≅ 4 μM) than lignans with a methylenedioxyphenyl group (IC50 > 25 μM). Gomisin B and gomisin C with a bulky angeloyl or benzoyl group at position 6 showed the strongest inhibitory effect on CYP3A-mediated testosterone hydroxylase activity (IC50 = 0.19 ~ 0.28 μM) similar to Iwata et al.’s findings (IC50 = 0.26 ~ 0.62 μM) [21], In comparison, gomisin A and gomisin N lacking this functional group showed moderate inhibition (IC50 = 1.3 ~ 4.5 μM). Similar inhibitory effects of these four gomisin compounds (gomisin A, -B, -C, and -N) on CYP3A were also demonstrated when assessed by determining midazolam hydroxylation and nifedipine oxidation activities as an index activity of CYP3A (IC50 = 0.26 ~ 0.42 μM). Iwata et al. (2004) also reported substrate independent inhibitory effects of these gomisin compounds on CYP3A activity [21]. Gomisin B and gomisin C with a bulky group at position 6 more strongly inhibited CYP2C8-mediated amodiaquine O-demethylase activity with IC50 values of 10.9 μM and 16.5 μM, respectively, compared to gomisin A and gomisin N lacking this functional group at this position (IC50 > 29 μM). Wuweizisu C containing two methylenedioxyphenyl groups induced the strongest inhibition of CYP2B6, CYP2C9, and CYP2C19 (IC50 = 2.7 ~ 8.9 μM) compared to the five other lignans. This suggests that the additional methylenedioxyphenyl group influences inhibition of these three P450s.
In addition, several P450 inhibitors including clopidogrel [37], furafylline [38], and ticlopidine [37] have been shown to be time-dependent inhibitors of P450. We investigated the effect of incubation time on the IC50 values of six lignans on nine P450s (Table 3). A test compound with an IC50 fold-shift decrease ≥ 1.5 is considered to be a time-dependent inhibitor as recommended by Awortwe et al. [39]. Previous studies have shown that gomisin A, -B, -C, and -N inhibit CYP3A activity in a time- and NADPH-dependent manner when co-incubated with HLMs or rP450s [21,35,36]. Our data confirm and elaborate on previous findings allowing us to characterize the time-dependent inhibition of CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1 by dibenzocyclooctadiene-based lignans for the first time. Similar to published data [21,35,36], we found that lignans with a methylenedioxyphenyl group inhibit CYP3A and CYP2E1 in a time-dependent manner with IC50 shift values > 2.0. Gomisin A, gomisin N, and wuweizisu C without a bulky angeloyl or benzoyl group showed time-dependent inhibition of CYP2C9-mediated diclofenac hydroxylase activity and CYP2C19-mediated mephenytoin hydroxylase activity with IC50 shift values > 1.8, whereas gomisin B and gomisin C containing a bulky angeloyl or benzoyl functional group showed time-independent inhibition. Unlike the other five lignans, wuweizisu C containing two methylenedioxyphenyl groups also displayed time-dependent inhibition of CYP2B6-catalyzed bupropion hydroxylase activity with an IC50 shift value of 2.1. Inactivation of CYP2C8 is only time-dependent when exposed to lignans with one methylenedioxyphenyl group. The inhibitory potential of schisandrin without a methylenedioxyphenyl group against nine P450s in HLMs pre-incubated in the presence of NADPH did not increase compared to the untreated HLMs. This suggests that the presence of the methylenedioxyphenyl group is a prerequisite for dibenzocyclooctadiene-based lignans to display time-dependent inhibition of P450s. Several studies have also reported the time-dependent inhibition of P450s by compounds containing dibenzocyclooctadiene group (i.e., paroxetine, noscapine, and gomisin C) [21,40,41]. None of the lignans tested showed time-dependent inhibition of CYP1A2, CYP2A6, or CYP2D6 enzymes.
3.2. Inhibitory Effects of Gomisin A against Recombinant CYP2C8, CYP2C19, and CYP3A4
Gomisin A displayed the strongest time-dependent inhibition of CYP2C8, CYP2C19, and CYP3A with IC50 values ≤ 5.0 μM. We further investigated the inhibitory effects of gomisin A against these three P450s using rP450s. Much like HLMs, gomisin A inhibited CYP2C8, CYP2C19, and CYP3A with IC50 values of 30.4 μM, 11.3 μM, and 1.51 μM, respectively, in the absence of an NADPH generating system (Table 3 and Table 4). The inhibitory potential of gomisin A on CYP3A-mediated midazolam hydroxylation was similar to the previously reported value (IC50 = 1.86 μM) [18]. Gomisin A showed time-dependent inhibition of CYP2C8-mediated amodiaquine demethylase activity, CYP2C19-mediated S-mephenytoin hydroxylase activity, and CYP3A-mediated midazolam hydroxylase activity with an IC50 shift of 9.2, 2.3, and 3.0, respectively. IC50 value shifts in rP450s were also similar to those in HLMs (Table 3 and Table 4).
Table 4.
Inhibitory effects of gomisin A against CYP2C8, CYP2C9, and CYP3A4 isoforms in human recombinant P450 isoforms.
| Recombinant P450 Enzyme | Probe Substrate | IC50 (μM) * | ||
|---|---|---|---|---|
| Gomisin A | ||||
| RI ** | TDI ** | IC50 shift | ||
| rCYP2C8 | Amodiaquine | 30.4 ± 8.1 | 3.32 ± 1.05 | 9.2 |
| rCYP2C19 | S-Mephenytoin | 11.3 ± 3.3 | 4.98 ± 0.49 | 2.3 |
| rCYP3A4 | Midazolam | 1.51 ± 0.20 | 0.51 ± 0.07 | 3.0 |
* Values represent the average ± S.E. of triplicate. ** RI: Reversible inhibition, TDI: Time-dependent inhibition.
3.3. Inhibition of UGT Enzyme Activities by Six Lignans
The inhibitory potential of the six lignans against six UGTs was evaluated using HLMs (Table 5). Gomisin C inhibited UGT1A1 and UGT1A3 activities with IC50 values of 24.0 µM and 15.0 µM, respectively, while it had negligible inhibition (IC50 > 50 μM) on the other UGTs. The inhibitory potential of gomisin C for UGT1A3 was similar to previously published data (IC50 = 12.5 μM) [27]. Gomisin B also inhibited UGT1A1 and UGT1A3 activities with IC50 values of 20.7 µM and 16.5 µM, respectively.
Table 5.
Inhibitory effects of six lignans against six uridine 5′-diphosphoglucuronosyl transferase (UGT) enzymes.
| UGT Enzyme | Substrate | IC50 (μM) * | |||||
|---|---|---|---|---|---|---|---|
| Gomisin A | Gomisin B | Gomisin C | Gomisin N | Schisandrin | Wuweizisu C | ||
| 1A1 | SN-38 ** | >50 | 20.7 | 24.0 | >50 | >50 | >50 |
| 1A3 | Chenodeoxycholic acid | >50 | 16.5 | 15.0 | 26.9 | >50 | >50 |
| 1A4 | Trifluoperazine | >50 | >50 | >50 | >50 | >50 | >50 |
| 1A6 | N-Acetylserotonin | >50 | >50 | >50 | >50 | >50 | >50 |
| 1A9 | Mycophenolic acid | >50 | >50 | >50 | >50 | >50 | >50 |
| 2B6 | Naloxone | >50 | >50 | >50 | >50 | >50 | >50 |
* Values represent the average in triplicate; ** SN-38: 7-Ethyl-10-hydroxy camptothecin.
3.4. Characterization of Reactive Metabolites of Gomisin A in Recombinant P450 Isoforms
Methylenedioxyphenyl compounds are converted to reactive intermediates known as carbene metabolites by P450-mediated metabolism. These carbene metabolites easily react with P450 to form a metabolite-intermediate complex (MIC). The formation of this MIC has been reported to play an important role in the time-dependent inhibition of P450 by methylenedioxyphenyl compounds [21,40,42,43]. Dibenzocyclooctadiene-based lignans also generate MIC because they contain a methylenedioxyphenyl group. Iwata et al. previously showed that gomisin C inactivates CYP3A4 by forming an MIC with CYP3A4 [21]. Gomisin N and P450-induced carbene reactive metabolite formation has also been demonstrated [33]. GSH can be used as trapping agent to identify carbene reactive metabolites because carbene is unstable and cannot be detected directly [44,45,46]. In this study, gomisin A showed time-dependent inhibition of CYP2C8-mediated amodiaquine demethylase activity with an IC50 shift of 10.5 and 9.2 in HLMs and rCYP2C8, respectively–the highest observed values in this study. In addition, we found that gomisin A displayed strong time-dependent inhibition of CYP2C8, CYP2C19, and CYP3A4 in HLMs and rP450s (IC50 < 5 µM).
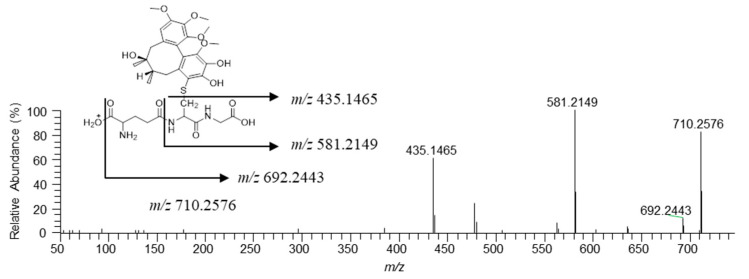
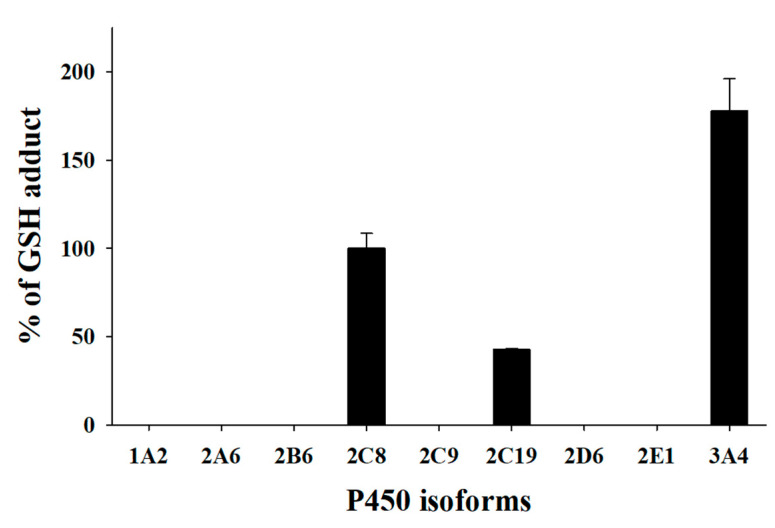
To elucidate the time-dependent inhibition mechanism of gomisin A against CYP2C8, CYP2C19, and CYP3A4, gomisin A was incubated with rP450s in the presence of NADPH and GSH. UPLC-HRMS analyses indicated that there was only one GSH conjugate ([M+H]+, m/z 710.2576, tR = 6.2 min) formed in rCYP2C8. UPLC-HRMS analyses of the peak responsible for this GSH conjugate displayed a protonated molecule [M+H]+ at m/z 710.2576 (mass error < 2 ppm), 293 Da higher than that of gomisin A. This suggests that gomisin A first loses [-CH2] before it is conjugated with one molecule of GSH [47]. The MS/MS spectrum of the GSH conjugate by fragmenting m/z 710.2576 through collision gave the characteristic daughter ions at m/z 692.2443, 581.2149, and 435.1485, suggesting the loss of a water molecule (−18 Da), the loss of a pyroglutamate residue (−129 Da) of GSH, and cleavage of the cysteinyl C-S bond (−275 Da) (Figure 2). The fragment ions observed from the loss of a pyroglutamate residue and cleavage of the cysteinyl C−S bond are the most typical ions found in GSH conjugates [33,44,48,49]. A further experiment was carried out to identify the P450 isoforms involved in the bioactivation of gomisin A using the PRM method. The results showed that CYP2C8, CYP2C19, and CYP3A4 were involved in the formation of carbene reactive metabolite of gomisin A (Figure 3).
Figure 2.
MS/MS spectrum of glutathione conjugate of gomisin A annotated with the proposed structures of fragment ions obtained by UPLC-HRMS analysis of the human recombinant CYP2C8 incubates of gomisin A in the presence of NADPH generating system and glutathion.
Figure 3.
Representative plots for the formation of the glutathione adduct of gomisin A by human recombinant cytochrome P450 enzymes (rP450s). The incubation system (100 μL) contained 0.1 M phosphate buffer solution (pH 7.4), 1 pmol P450 isoforms, 50 μM gomisin A, 2 mM NADPH, and 5 mM glutathione. Each data represent the mean ± SD of triplicate determinations.
3.5. Evaluation of Drug Interaction Potential of Six Lignans
In previous studies, Schisandra extracts and lignans were found to alter the pharmacokinetics of drugs which are substrates of CYP3A. Animal studies show that oral Wuzhi capsules (including 0.14 mg/g schisandrin, 0.09 mg/g gomisin A, 5.79 mg/g gomisin C, 0.63 mg/g schisanhenol, and 5.69 mg/g deoxyshisandrin) significantly increase blood tacrolimus concentration through CYP3A inhibition [50]. Wang et al. (2014) reported that in rats, Schisandra chinensis alcoholic extracts (containing 1.84% schisandrin, 1.54% gomisin A, 2.43% deoxyshisandrin, and 1.23% gomisin N) with tacrolimus exert a greater increase on tacrolimus’s Cmax and AUC values than when used alone, thereby indicating inhibition of CYP3A, a major tacrolimus-metabolizing enzyme [51]. Deoxyshisandrin, a CYP3A inhibitor, markedly increases plasma concentrations of midazolam in rats [52]. The magnitude of AUC variation for drugs that are predominantly biotransformed by CYP3A4 was estimated to increase by 22–321% in the presence of gomisin C, a strong CYP3A inhibitor [17].
In contrast to the extensive studies on drug interactions with CYP3A substrates, there is a paucity of data detailing drug interactions with other P450s. We predicted the clinical herb drug interaction risk induced by Schisandra lignans based on each of the inhibitory potential. Gomisin A inhibited CYP2C8 activity with an IC50 value of 2.8 μM, similar to CYP3A inhibition (IC50 = 0.77~1.2 μM) in a time- and concentration-dependent manner. Considering that gomisin A participates in the pharmacokinetic intervention of cyclophosphamide by blocking CYP3A-mediated metabolism and reducing chloroacetaldehyde production in rats [35], gomisin A may interact with CYP2C8 substrate drugs such as amodiaquine [53], paclitaxel [54], and repaglinide [55]. In rats, oral administration of gomisin N for 3 days also resulted in a significant increase in midazolam AUC values [13]. Gomisin N might also interact with CYP2C19 substrate drugs, such as clopidogrel [56] and omeprazole [57], because its CYP2C19 inhibitory potential (IC50 = 3.5 μM) is similar to CYP3A inhibition (IC50 = 1.7 μM). In vivo studies are required to determine the clinical relevance of potential herb drug interactions between typical doses of Schisandra extracts, including lignans, and CYP2C8 or CYP2C19 substrate drugs.
4. Conclusions
In conclusion, we report that lignans containing one or two methylenedioxyphenyl groups inhibit CYP2B6, CYP2C8, CYP2C9, CYP2C19, and/or CYP2E1 in a time- and concentration-dependent manner which is similar to CYP3A inhibition. We found that six lignans inhibit six UGTs to a limited extent (IC50 > 15 μM). Moreover, our data show that gomisin A inhibits CYP2C8, CYP2C19, and CYP3A4 enzymes in a time-dependent manner by forming carbene reactive metabolites in a similar fashion to gomisin C-induced inhibition of CYP3A4. Additionally, these lignans may result in clinically relevant pharmacokinetic interactions with other co-administered drugs biotransformed by CYP2B6, CYP2C8, CYP2C9, CYP2C19, and/or CYP2E1.
Author Contributions
D.S.J., and K.-H.L. conceived and designed the experiments; H.-J.S., S.-B.J., S.-E.K., G.-M.L., S.-Y.P., and Z.W. performed experiments; H.-J.S., and K.-H.L. analyzed the data; H.-J.S., D.S.J., and K.-H.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundations of Korea, Ministry of Science and ICT, Republic of Korea [NRF-2019R1A2C1008713].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this study have been included in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hao M., Zhao Y., Chen P., Huang H., Liu H., Jiang H., Zhang R., Wang H. Structure-Activity Relationship and Substrate-Dependent Phenomena in Effects of Ginsenosides on Activities of Drug-Metabolizing P450 Enzymes. PLoS ONE. 2008;3:e2697. doi: 10.1371/journal.pone.0002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parvez M.K., Rishi V. Herb-Drug Interactions and Hepatotoxicity. Curr. Drug Metab. 2019;20:275–282. doi: 10.2174/1389200220666190325141422. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed M.-E.F., Frye R.F. Effects of Herbal Supplements on Drug Glucuronidation. Review of Clinical, Animal, andIn VitroStudies. Planta Med. 2010;77:311–321. doi: 10.1055/s-0030-1250457. [DOI] [PubMed] [Google Scholar]
- 4.Adiwidjaja J., Boddy A.V., McLachlan A.J. Physiologically Based Pharmacokinetic Modelling of Hyperforin to Predict Drug Interactions with St John’s Wort. Clin. Pharmacokinet. 2019;58:911–926. doi: 10.1007/s40262-019-00736-6. [DOI] [PubMed] [Google Scholar]
- 5.Malati C.Y., Robertson S.M., Hunt J.D., Chairez C., Alfaro R.M., Kovacs J.A., Penzak S.R. Influence of Panax ginseng on cytochrome P450 (CYP)3A and P-glycoprotein (P-gp) activity in healthy participants. J. Clin. Pharmacol. 2012;52:932–939. doi: 10.1177/0091270011407194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderón M.M., Chairez C.L., Gordon L.A., Alfaro R.M., Kovacs J.A., Penzak S.R. Influence of Panax ginseng on the steady state pharmacokinetic profile of lopinavir-ritonavir in healthy volunteers. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2014;34:1151–1158. doi: 10.1002/phar.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan L., Tao G.-Y., Wang G., Chen Y., Zhang W., He Y.-J., Li Q., Lei H.-P., Jiang F., Hu D.-L., et al. Effects of Ginkgo biloba Extract Ingestion on the Pharmacokinetics of Talinolol in Healthy Chinese Volunteers. Ann. Pharmacother. 2009;43:944–949. doi: 10.1345/aph.1L656. [DOI] [PubMed] [Google Scholar]
- 8.Kim T.-E., Ha N., Kim Y., Kim H., Lee J.W., Jeon J.-Y., Kim M.-G. Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers. Drug Des. Dev. Ther. 2017;11:1409–1416. doi: 10.2147/DDDT.S130050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang W., Wang X., Xu X., Kong L. Effect of Schisandra sphenanthera extract on the concentration of tacrolimus in the blood of liver transplant patients. Int. J. Clin. Pharmacol. Ther. 2010;48:224–229. doi: 10.5414/CPP48224. [DOI] [PubMed] [Google Scholar]
- 10.Al-Jenoobi F.I., Al-Thukair A.A., Alam M.A., Abbas F.A., Al-Mohizea A.M., Alkharfy K.M., Al-Suwayeh S.A. Effect of Curcuma longa on CYP2D6- and CYP3A4-mediated metabolism of dextromethorphan in human liver microsomes and healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 2014;40:61–66. doi: 10.1007/s13318-014-0180-2. [DOI] [PubMed] [Google Scholar]
- 11.Szopa A., Ekiert R., Ekiert H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017;16:195–218. doi: 10.1007/s11101-016-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018;97:958–968. doi: 10.1016/j.biopha.2017.10.145. [DOI] [PubMed] [Google Scholar]
- 13.Li W.-L., Xin H.-W., Yu A.-R., Wu X.-C. In vivo effect of Schisandrin B on cytochrome P450 enzyme activity. Phytomedicine. 2013;20:760–765. doi: 10.1016/j.phymed.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y.-G., Yang B.-Y., Liang J., Wang J.-S., Kuang H.-X. Simultaneous quantification of five dibenzocyclooctadiene lignans in Schisandra chinensis by HPLC separation and fluorescence detection. Anal. Methods. 2014;6:5981. doi: 10.1039/C4AY00017J. [DOI] [Google Scholar]
- 15.Sun J., Jing S., Jiang R., Wang C., Zhang C., Chen J., Li H. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by D-galactose. Clin. Interv. Aging. 2018;13:829–841. doi: 10.2147/CIA.S163275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima K., Taguchi H., Ikeya Y., Endo T., Yosioka I. The Constituents of Schizandra chinensis BAILL. XIII. Quantitative Analysis of Lignans in the Fruits of Schizandra chinensis BAILL. by High Performance Liquid Chromatography. Yakugaku Zasshi. 1983;103:743–749. doi: 10.1248/yakushi1947.103.7_743. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J., Sun T., Wu J.-J., Cao Y.-F., Fang Z.-Z., Sun H.-Z., Zhu Z.-T., Yang K., Liu Y.-Z., Gonzalez F.J., et al. Inhibition of human CYP3A4 and CYP3A5 enzymes by gomisin C and gomisin G, two lignan analogs derived from Schisandra chinensis. Fitoterapia. 2017;119:26–31. doi: 10.1016/j.fitote.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J.-J., Ge G.-B., He Y.-Q., Wang P., Dai Z.-R., Ning J., Hu L.-H., Yang L. Gomisin A is a Novel Isoform-Specific Probe for the Selective Sensing of Human Cytochrome P450 3A4 in Liver Microsomes and Living Cells. AAPS J. 2015;18:134–145. doi: 10.1208/s12248-015-9827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L.M., Xu S.Y., Hu H.H., Zhou H., Jiang H.D., Yu L.S., Zeng S. Identification of CYP2C19 inhibitors from phytochemicals using the recombinant human enzyme model. Die Pharm. 2014;69:362–366. [PubMed] [Google Scholar]
- 20.Wan C.-K., Tse A., Yu Z.-L., Zhu G.-Y., Wang H., Fong D. Inhibition of cytochrome P450 3A4 activity by schisandrol A and gomisin A isolated from Fructus Schisandrae chinensis. Phytomedicine. 2010;17:702–705. doi: 10.1016/j.phymed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Iwata H., Tezuka Y., Kadota S., Hiratsuka A., Watabe T. Identification and Characterization of Potent Cyp3a4 Inhibitors in Schisandra Fruit Extract. Drug Metab. Dispos. 2004;32:1351–1358. doi: 10.1124/dmd.104.000646. [DOI] [PubMed] [Google Scholar]
- 22.Kim M.J., Kim H., Cha I.J., Park J.S., Shon J.H., Liu K.H., Shin J.G. High-throughput screening of inhibitory potential of nine cytochrome P450 enzymes in vitro using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:2651–2658. doi: 10.1002/rcm.2110. [DOI] [PubMed] [Google Scholar]
- 23.Itkonen M.K., Tornio A., Filppula A.M., Neuvonen M., Neuvonen P.J., Niemi M., Backman J.T. Clopidogrel but Not Prasugrel Significantly Inhibits the CYP2C8-Mediated Metabolism of Montelukast in Humans. Clin. Pharmacol. Ther. 2018;104:495–504. doi: 10.1002/cpt.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedada S.K., Boga P.K. Effect of piperine on CYP2E1 enzyme activity of chlorzoxazone in healthy volunteers. Xenobiotica. 2016;47:1035–1041. doi: 10.1080/00498254.2016.1241450. [DOI] [PubMed] [Google Scholar]
- 25.Cho D.-Y., Shen J.H., Lemler S.M., Skaar T.C., Li L., Blievernicht J., Zanger U.M., Kim K.-B., Shin J.-G., Flockhart D.A., et al. Rifampin enhances cytochrome P450 (CYP) 2B6-mediated efavirenz 8-hydroxylation in healthy volunteers. Drug Metab. Pharmacokinet. 2016;31:107–116. doi: 10.1016/j.dmpk.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Zhang L., Duan L.X., Wu J.J., Hu M., Liu Z.Q., Wang C.Y. Potential of herb-drug / herb interactions between substrates and inhibitors of UGTs derived from herbal medicines. Pharmacol. Res. 2019;150:104510. doi: 10.1016/j.phrs.2019.104510. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Cao Y.-F., Fang Z.-Z., Zhang Y.-Y., Hu C.-M., Sun X.-Y., Huang T., Zeng J., Fan X.-R., Hong M. Strong inhibition of deoxyschizandrin and schisantherin A toward UDP-glucuronosyltransferase (UGT) 1A3 indicating UGT inhibition-based herb–drug interaction. Fitoterapia. 2012;83:1415–1419. doi: 10.1016/j.fitote.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.M., Ryu B., Lee J.S., Choi J.-H., Jang D.S. Schisandrosides A–D, Dibenzocyclooctadiene Lignan Glucosides from the Roots of Schisandra chinensis. Chem. Pharm. Bull. 2015;63:746–751. doi: 10.1248/cpb.c15-00400. [DOI] [PubMed] [Google Scholar]
- 29.Opletal L., Sovova H., Bartlova M. Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;812:357–371. doi: 10.1016/S1570-0232(04)00646-4. [DOI] [PubMed] [Google Scholar]
- 30.Kim H.-J., Lee H., Ji H.-K., Lee T., Liu K.-H. Screening of ten cytochrome P450 enzyme activities with 12 probe substrates in human liver microsomes using cocktail incubation and liquid chromatography–tandem mass spectrometry. Biopharm. Drug Dispos. 2019;40:101–111. doi: 10.1002/bdd.2174. [DOI] [PubMed] [Google Scholar]
- 31.Perloff E.S., Mason A.K., Dehal S.S., Blanchard A.P., Morgan L., Ho T., Dandeneau A., Crocker R.M., Chandler C.M., Boily N., et al. Validation of cytochrome P450 time-dependent inhibition assays: A two-time point IC50 shift approach facilitates kinact assay design. Xenobiotica. 2009;39:99–112. doi: 10.1080/00498250802638155. [DOI] [PubMed] [Google Scholar]
- 32.Joo J., Lee B., Lee T., Liu K.-H. Screening of six UGT enzyme activities in human liver microsomes using liquid chromatography/triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2014;28:2405–2414. doi: 10.1002/rcm.7030. [DOI] [PubMed] [Google Scholar]
- 33.Feng S., Qiu B., Zou L., Liu K., Xu X., Zhu H. Schisandrin B elicits the Keap1-Nrf2 defense system via carbene reactive metabolite which is less harmful to mice liver. Drug Des. Dev. Ther. 2018;12:4033–4046. doi: 10.2147/DDDT.S176561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauniyar N. Parallel Reaction Monitoring: A Targeted Experiment Performed Using High Resolution and High Mass Accuracy Mass Spectrometry. Int. J. Mol. Sci. 2015;16:28566–28581. doi: 10.3390/ijms161226120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai J., Zhang F., Gao S., Chen L., Feng G., Yin J., Chen W. Time- and NADPH-Dependent Inhibition on CYP3A by Gomisin A and the Pharmacokinetic Interactions between Gomisin A and Cyclophosphamide in Rats. Molecules. 2017;22:1298. doi: 10.3390/molecules22081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W., Xin H., Su M., Xiong L. Inhibitory effects of schisandrin A and schisandrin B on CYP3A activity. Methods Find. Exp. Clin. Pharmacol. 2010;32:163–169. doi: 10.1358/mf.2010.32.3.1434161. [DOI] [PubMed] [Google Scholar]
- 37.Richter T., Mürdter T.E., Heinkele G., Pleiss J., Tatzel S., Schwab M., Eichelbaum M., Zanger U.M. Potent Mechanism-Based Inhibition of Human CYP2B6 by Clopidogrel and Ticlopidine. J. Pharmacol. Exp. Ther. 2003;308:189–197. doi: 10.1124/jpet.103.056127. [DOI] [PubMed] [Google Scholar]
- 38.Fairman D.A., Collins C., Chapple S. Progress Curve Analysis of CYP1A2 Inhibition: A More Informative Approach to the Assessment of Mechanism-Based Inactivation? Drug Metab. Dispos. 2007;35:2159–2165. doi: 10.1124/dmd.107.017236. [DOI] [PubMed] [Google Scholar]
- 39.Awortwe C., Manda V.K., Avonto C., Khan S.I., Khan I.A., Walker L.A., Bouic P.J., Rosenkranz B. In Vitro Evaluation of Reversible and Time-Dependent Inhibitory Effects of Kalanchoe crenata on CYP2C19 and CYP3A4 Activities. Drug Metab. Lett. 2015;9:48–62. doi: 10.2174/1872312809666150119110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertelsen K.M., Venkatakrishnan K., Von Moltke L.L., Obach R.S., Greenblatt D.J. Apparent Mechanism-based Inhibition of Human CYP2D6 in Vitro by Paroxetine: Comparison with Fluoxetine and Quinidine. Drug Metab. Dispos. 2003;31:289–293. doi: 10.1124/dmd.31.3.289. [DOI] [PubMed] [Google Scholar]
- 41.Fang Z.-Z., Zhang Y.-Y., Ge G.-B., Huo H., Liang S.-C., Yang L. Time-dependent inhibition (TDI) of CYP3A4 and CYP2C9 by noscapine potentially explains clinical noscapine-warfarin interaction. Br. J. Clin. Pharmacol. 2010;69:193–199. doi: 10.1111/j.1365-2125.2009.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers J.T., Davydova N.Y., Paragas E.M., Jones J.P., Davydov D.R. Kinetic mechanism of time-dependent inhibition of CYP2D6 by 3,4-methylenedioxymethamphetamine (MDMA): Functional heterogeneity of the enzyme and the reversibility of its inactivation. Biochem. Pharmacol. 2018;156:86–98. doi: 10.1016/j.bcp.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee P., Franklin M.R. Human cytochrome p450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab. Dispos. 2003;31:1391–1397. doi: 10.1124/dmd.31.11.1391. [DOI] [PubMed] [Google Scholar]
- 44.Ma S., Subramanian R. Detecting and characterizing reactive metabolites by liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2006;41:1121–1139. doi: 10.1002/jms.1098. [DOI] [PubMed] [Google Scholar]
- 45.Li F., Lu J., Ma X. Profiling the Reactive Metabolites of Xenobiotics Using Metabolomic Technologies. Chem. Res. Toxicol. 2011;24:744–751. doi: 10.1021/tx200033v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen B., Fitch W.L. Screening and characterization of reactive metabolites using glutathione ethyl ester in combination with Q-trap mass spectrometry. J. Mass Spectrom. 2009;44:90–100. doi: 10.1002/jms.1475. [DOI] [PubMed] [Google Scholar]
- 47.Fang Z.-Z., Krausz K.W., Li F., Cheng J., Tanaka N., Gonzalez F.J. Metabolic map and bioactivation of the anti-tumour drug noscapine. Br. J. Pharmacol. 2012;167:1271–1286. doi: 10.1111/j.1476-5381.2012.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng J., Ma L., Xin B., Olah T., Humphreys W.G., Zhu M. Screening and Identification of GSH-Trapped Reactive Metabolites Using Hybrid Triple Quadruple Linear Ion Trap Mass Spectrometry. Chem. Res. Toxicol. 2007;20:757–766. doi: 10.1021/tx600277y. [DOI] [PubMed] [Google Scholar]
- 49.Zhuo X., Huang X.S., Degnan A.P., Snyder L.B., Yang F., Huang H., Shu Y.-Z., Johnson B.M. Identification of Glutathione Conjugates of Acetylene-Containing Positive Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5. Drug Metab. Dispos. 2015;43:578–589. doi: 10.1124/dmd.114.061879. [DOI] [PubMed] [Google Scholar]
- 50.Wei H., Tao X., Di P., Yang Y., Li J., Qian X., Feng J., Chen W. Effects of Traditional Chinese Medicine Wuzhi Capsule on Pharmacokinetics of Tacrolimus in Rats. Drug Metab. Dispos. 2013;41:1398–1403. doi: 10.1124/dmd.112.050302. [DOI] [PubMed] [Google Scholar]
- 51.Wang B., Yang S., Hu J., Li Y. Multifaceted interaction of the traditional Chinese medicinal herb Schisandra chinensis with cytochrome P450-mediated drug metabolism in rats. J. Ethnopharmacol. 2014;155:1473–1482. doi: 10.1016/j.jep.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Li W.-L., Xin H.-W., Su M.-W. Inhibitory Effects of Continuous Ingestion of Schisandrin A on CYP3A in the Rat. Basic Clin. Pharmacol. Toxicol. 2011;110:187–192. doi: 10.1111/j.1742-7843.2011.00787.x. [DOI] [PubMed] [Google Scholar]
- 53.Li X.-Q., Björkman A., Andersson T.B., Ridderström M., Masimirembwa C.M. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: A new high affinity and turnover enzyme-specific probe substrate. J. Pharmacol. Exp. Ther. 2002;300:399–407. doi: 10.1124/jpet.300.2.399. [DOI] [PubMed] [Google Scholar]
- 54.Václavíková R., Horský S., Simek P., Gut I. Paclitaxel metabolism in rat and human liver microsomes is inhibited by phenolic antioxidants. Naunyn-Schmiedeberg Arch. Pharmacol. 2003;368:200–209. doi: 10.1007/s00210-003-0781-9. [DOI] [PubMed] [Google Scholar]
- 55.Bidstrup T.B., Bjørnsdottir I., Sidelmann U.G., Thomsen M.S., Hansen K.T. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br. J. Clin. Pharmacol. 2003;56:305–314. doi: 10.1046/j.0306-5251.2003.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim K.A., Park P.W., Hong S.J., Park J.-Y. The Effect of CYP2C19 Polymorphism on the Pharmacokinetics and Pharmacodynamics of Clopidogrel: A Possible Mechanism for Clopidogrel Resistance. Clin. Pharmacol. Ther. 2008;84:236–242. doi: 10.1038/clpt.2008.20. [DOI] [PubMed] [Google Scholar]
- 57.Andersson T., Cederberg C., Edvardsson G., Heggelund A., Lundborg P. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolizers of omeprazole. Clin. Pharmacol. Ther. 1990;47:79–85. doi: 10.1038/clpt.1990.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in this study have been included in this manuscript.