Abstract
For centuries, scientists have been intrigued by the origin of dioecy in plants, characterizing sex-specific development, uncovering cytological differences between the sexes, and developing theoretical models. Through the invention and continued improvements in genomic technologies, we have truly begun to unlock the genetic basis of dioecy in many species. Here we broadly review the advances in research on dioecy and sex chromosomes. We start by first discussing the early works that built the foundation for current studies and the advances in genome sequencing that have facilitated more-recent findings. We next discuss the analyses of sex chromosomes and sex-determination genes uncovered by genome sequencing. We synthesize these results to find some patterns are emerging, such as the role of duplications, the involvement of hormones in sex-determination, and support for the two-locus model for the origin of dioecy. Though across systems, there are also many novel insights into how sex chromosomes evolve, including different sex-determining genes and routes to suppressed recombination. We propose the future of research in plant sex chromosomes should involve interdisciplinary approaches, combining cutting-edge technologies with the classics to unravel the patterns that can be found across the hundreds of independent origins.
Keywords: dioecy, sex determination, seed plants, bryophytes, whole-genome sequencing, two-gene model
1. Introduction
Across land plants exists an amazing variety of strategies for sexual reproduction [1]. Species have independently evolved self-incompatibility loci [2], temporal variation in flower development [3,4], and spatial distancing of male and female organs on the same plant [5,6,7], among many others [1]. Perhaps the most extreme case is dioecy, where sex-specific structures develop on separate plants. In angiosperms, dioecy is rare, found in an estimated 5% of species, but has hundreds of independent origins across more than half of the families [5]. In the other land plant lineages, most species are dioecious, at approximately 65% of gymnosperms, 68% of liverworts, 57% of mosses, and 40% of hornworts (technically the term in bryophytes is dioicous because they are haploid when expressing gametic sex, but here we will use dioecious for simplicity) [8,9]. The frequency and phylogenetic breadth of dioecy across plants provides an unparalleled opportunity to examine the key forces involved in its repeated evolution.
Early models theorized how dioecy can evolve from a hermaphroditic ancestor [10,11,12], invoking the need for two-linked mutations: one that causes male-sterility and another female-sterility. Recombination within this region can result in offspring that are either hermaphroditic or sterile. Thus, selection is strong to suppress recombination in the region containing these two mutations, forming a sex chromosome pair. For dioecious species that express gametic sex in the diploid stage, like in seed plants, the sex chromosomes are referred to as XY or ZW depending on which is the karyotypically heterogametic sex [13,14]. In haploid-dominant plants, like bryophytes, dioecious species with genetic sex determination have UV sex chromosomes, with the inheritance of a U correlating with female gametic sex expression and a V with male [13,15]. Though some species have multiple sex chromosomes (e.g., XY1Y2 or U1U2V) [8,16,17,18], which can occur through structural changes like chromosomal fusions and fissions or through polyploidy. These differences in heterogamety and ploidy of sex chromosomes found across land plants are powerful for contrasting the evolutionary processes that impact these genomic regions, especially as the mechanisms of sex determination on sex chromosomes have now expanded beyond the classic two-locus model [19].
Here we review the recent advances in sex chromosome evolution across land plants. We start by covering a brief history of identifying dioecy and sex chromosomes, and the advances in genome sequencing that have made new discoveries possible. We next broadly review new findings in plant sex chromosomes, particularly focusing on how the sex-determining region (SDR) evolves, both with the diversity of genes that are involved in sex determination and other processes that shape these complex regions of the genome. We conclude with future directions in plant sex chromosome evolution research.
2. The History of Identifying Plant Sex Chromosomes
Analyses of dioecy and sex chromosomes start with the remarkable works of naturalists who, with a careful eye, characterize reproductive structures throughout development. Categorizing plants as dioecious can be traced back to Linnaeus’ Systema Naturae (1735), where angiosperms were classified by their floral characteristics, such as number of stamens and pistils, or by sexual condition [20]. Darwin even discussed the curiosities of dioecy in The Different Forms of Flowers of the Same Species (1877) [21]. In some species dioecy is easily observable. One example is hops, where female inflorescences develop the characteristic “cones” used in beer production, while males have a completely different floral architecture [22]. Another example is found in the classic dioecy model white campion (Silene latifolia), studied intensively since the 19th century [23], where a suite of sexually dimorphic traits is obvious at early stages of flower development. However, in other species dioecy can be more subtle. In garden asparagus, both sexes phenotypically appear similar in early stages of floral development, but ultimately the stamens degenerate in females and the ovary is non-functional in males [24]. In some species, like Solanum appendiculatum or kiwifruit, dioecy is even more cryptic, where females even produce pollen grains, but they are non-viable [25,26]. In non-flowering groups, like the mosses, early naturalists searched for the “hidden flowers” (reviewed in [27]), which are called antheridia and archegonia (male and female gametangia, respectively) to identify dioecious species. Antheridia are easily visible during their development, however, archegonia are more challenging to locate because they are largely enclosed in modified leaves [28,29]. It is also common in mosses to not develop gametangia [30,31,32] and disentangling individual (i.e., genetically distinct) plants from their densely grown patches can be challenging. As such, some of the first confirmations of dioecy in species like Ceratodon purpureus and Bryum argenteum were done by growing individuals from spores [30]. It is unequivocal that these kinds of taxonomic observations form the critical basis of our understanding of dioecy, in addition to other sexual systems (for databases in angiosperms see [5,33]).
Uncovering the genetic basis for sex determination began with early cytological analyses (reviewed in [34]). Dr. Nettie Stevens first discovered the correlation between the inheritance of a smaller chromosome in a meiotic pair (which we now know as the Y chromosome) with male gametic sex expression in mealworms [35]. Indeed, this clear heteromorphy between sex chromosomes was critical to their identification in further cytological studies. The first plant sex chromosomes were identified in the liverwort Sphaerocarpos donellii [36] and subsequently many other heteromorphic pairs were found in Humulus, Rumex, and Silene [16,37,38], among others [14]. However, in many plants the sex chromosomes are cytologically homomorphic, or nearly so, making identifying them through classical microscopy a challenge.
Dioecious species with sex chromosomes played a pivotal role in the modern synthesis, in particular with regard to the inheritance of sex. In the early 1900s, after the re-discovery of Mendel’s foundational work on pea plants [39,40], dioecious flowering plant Silene latifolia (formerly Melandrium album) became a cornerstone for understanding the genetic basis of sex and sex-linked traits. This is partly because it has such obvious flowers and a particularly large Y chromosome that is nearly 1.5 times the size of the X [41]. In fact, the first sex-linked gene in plants was discovered in S. latifolia (and the related species Silene dioica); the X-linked recessive lethal angustifolia mutation produced narrow leaves that were only found in XY male plants and never led to viable XX females [42,43,44]. Decades of irradiation studies in S. latifolia have been elegantly used to map deletions that lead to sex mutants [45,46,47]. Such large-scale sex chromosome irradiation experiments are still immensely useful today, and have been leveraged to map sex-determining genes on the Y chromosome in S. latifolia [48] and in garden asparagus [49,50].
Genomic approaches have unlocked other previously intractable analyses of plant sex chromosomes. Some of the first genome references for dioecious species include the liverwort Marchantia polymorpha [51], grape [52], papaya [53,54], and poplar [55], published only a few years after the first plant genome (Arabidopsis thaliana [56]). More than two decades later, reference genomes for over 50 dioecious species have been published (Table 1). Though there are many characteristics about sex chromosomes that have made them challenging to assemble. Due to suppressed recombination, natural selection is less effective in these regions [57,58] and they often accumulate repeats [59]. This makes assembly of large contigs using short reads improbable [60] because reads often do not span the entire repeat, causing these regions to collapse [61,62]. Linkage maps, which use recombination rates across the genome, can help pull low-contiguity assemblies into linkage groups [63], but very small sex-determining regions (SDR) (e.g., ~59 kilobases (Kb) in Morella rubra [64]) are hard to reliably identify and very large SDRs (e.g., >100 megabases (Mb) in Ceratodon purpureus [65]) are hard to put in linear order due to the inherent lack of recombination. The use of Bacterial Artificial Chromosomes (BACs) has helped to resolve some sex chromosome assemblies [66,67], but like linkage maps, this approach is labor intensive. Adding to assembly issues, sequencing the heterogametic sex in diploids can result in chimeric contigs that contain a mixture of the X and the Y (or Z and W), especially if there is low divergence between homologous regions, as is expected if suppressed recombination has recently evolved [68,69]. These issues with assembling sex chromosomes are compounded by the fact that plant genomes are overall inherently complex, with many species having high heterozygosity and abundant repeats genome-wide, in addition to frequent polyploidy [70]. Despite these complications, through much tenacity, a lot of headway has been made on plant sex chromosomes using these short-read assembly approaches.
Table 1.
Published dioecious nuclear genomes. The species listed here are dioecious, though for many others, closely related hermaphroditic or monoecious references may be available.
| Lineage | Family | Species | Sex Chromosome Type | Citation |
|---|---|---|---|---|
| Moss | Ditrichaceae | Ceratodon purpureus | UV | [65] |
| Moss | Pottiaceae | Syntrichia princeps | UV | [71] |
| Moss | Fontinalaceae | Fontinalis antipyretica | UV | [72] |
| Moss | Hylocomiaceae | Pleurozium schreberi | UV | [73] |
| Liverwort | Marchantiaceae | Marchantia polymorpha | UV | [74,75] |
| Liverwort | Marchantiaceae | Marchantia inflexa | UV | [76] |
| Gymnosperm | Ginkgoaceae | Ginkgo biloba | XY | [77,78] |
| Gymnosperm | Gnetaceae | Gnetum montanum | Possibly XY | [79] |
| Angiosperm | Amborellaceae | Amborella trichopoda | ZW | [80] |
| Angiosperm | Dioscoreaceae | Dioscorea alata | XY | [81] |
| Angiosperm | Dioscoreaceae | Dioscorea rotundata | ZW | [82] |
| Angiosperm | Asparagaceae | Asparagus officinalis | XY | [49,50] |
| Angiosperm | Arecaceae | Phoenix dactylifera | XY | [83] |
| Angiosperm | Vitaceae | Vitis arizonica | XY | [84] |
| Angiosperm | Vitaceae | Vitis amurensis | XY | [85] |
| Angiosperm | Vitaceae | Vitis riparia | XY | [86] |
| Angiosperm | Vitaceae | Vitis vinifera sylvestris | XY | [84] |
| Angiosperm | Vitaceae | Muscadinia rotundifolia | XY | [84] |
| Angiosperm | Euphorbiaceae | Mercurialis annua | XY | [87] |
| Angiosperm | Salicaceae | Populus alba | ZW | [88] |
| Angiosperm | Salicaceae | Populus deltoides | XY | [88] |
| Angiosperm | Salicaceae | Populus euphratica | XY | [89] |
| Angiosperm | Salicaceae | Populus ilicifolia | XY | [90] |
| Angiosperm | Salicaceae | Populus tremula | XY | [88] |
| Angiosperm | Salicaceae | Populus trichocarpa | XY | [91] |
| Angiosperm | Salicaceae | Salix brachista | Possibly ZW | [92] |
| Angiosperm | Salicaceae | Salix matsudana | Possibly ZW | [93] |
| Angiosperm | Salicaceae | Salix purpurea | ZW | [94] |
| Angiosperm | Salicaceae | Salix suchowensis | ZW | [95] |
| Angiosperm | Salicaceae | Salix viminalis | ZW | [69] |
| Angiosperm | Rosaceae | Fragaria x ananassa | ZW | [96] |
| Angiosperm | Moraceae | Ficus carica | XY | [97] |
| Angiosperm | Moraceae | Ficus erecta | Possibly XY | [98] |
| Angiosperm | Moraceae | Ficus hispida | XY | [99] |
| Angiosperm | Cannabaceae | Cannabis sativa | XY | [100] |
| Angiosperm | Cannabaceae | Humulus lupulus | XY | [101] |
| Angiosperm | Myricaceae | Morella rubra | ZW | [64] |
| Angiosperm | Myricaceae | Morus alba | XY | [102] |
| Angiosperm | Myricaceae | Morus notabilis | Possibly XY | [103] |
| Angiosperm | Anacardiaceae | Pistacia vera | ZW | [104] |
| Angiosperm | Caricaceae | Carica papaya | XY | [54,105] |
| Angiosperm | Polygonaceae | Rumex hastatulus | XY | [106] |
| Angiosperm | Amaranthaceae | Amaranthus palmeri | XY | [107,108] |
| Angiosperm | Amaranthaceae | Amaranthus tuberculatus | XY | [108] |
| Angiosperm | Amaranthaceae | Spinacia oleracea | XY | [109] |
| Angiosperm | Simmondsiaceae | Simmondsia chinensis | XY | [110] |
| Angiosperm | Ebenaceae | Diospyros lotus | XY | [111] |
| Angiosperm | Actinidiaceae | Actinidia chinensis | XY | [112] |
| Angiosperm | Actinidiaceae | Actinidia eriantha | XY | [113] |
| Angiosperm | Solanaceae | Solanum appendiculatum | XY | [114] |
More recently, long-read technologies, like PacBio (Menlo Park, CA, USA) and Oxford Nanopore (Oxford Science Park, Oxford, UK), have made phenomenal strides for assembling complex regions, like sex chromosomes. As the reads are on average 10–15 Kb, as opposed to 100–300 base pairs with short reads, they are better at spanning repeat regions [70,115]. Not to mention longer reads mean fewer pieces of the genomic puzzle need to be put together. Although depending on the size and complexity of the genome, even with long reads, the assembly may not be pulled into pseudomolecules and may still contain misjoins. However, in these cases, with the addition of chromatin conformation data, like Hi-C, which does not rely on linkage, genomes can now readily be assembled to chromosome-scale, including the sex chromosomes [116,117,118]. Indeed, the telomere-to-telomere, gapless assembly of a human X chromosome, including the centromeres, [119] represents the future (or really the present) for genome assembly.
The most-recent improvements in long-read technologies (e.g., PacBio HiFi), including the lower error rates, and novel computational tools for assembling these data (e.g., HiCanu and HiFiAsm [120,121]), mean phasing the sex chromosomes in the heterogametic sex may now be possible. Though there are also downsides to long-read technologies, the foremost is the requisite high-molecular weight DNA, which ideally comes from fresh, young, flash-frozen tissue. This inherently precludes the incredible taxonomic resources maintained in herbaria, as well as any other avenues that could cause DNA degradation. As such, one of the biggest bottlenecks for genomic studies of most taxa today is identifying viable (and properly permitted) tissue that can be used for the genome reference, gene annotation, and maintained for future studies.
Critical for analyses in sex chromosomes, is identifying the non-recombining SDR. Currently, a combination of both long and short-read technologies is best suited for high-quality assemblies that include the sex chromosome pair. Long reads are ideal for assembling genomes into fewer contigs, while short reads are still valuable for genome polishing (even with lower error rates in long reads; e.g., with Racon [122]), Hi-C data for additional genome scaffolding (e.g., with JUICER [117]), genome annotation (e.g., with BRAKER2 [123]), and identifying the SDR (reviewed in [124]), in addition to gene expression analyses [70]. In fact, genes annotated to the SDR that have sex-specific expression are strong candidates for being involved in sex determination.
3. Advances in Sex-Determination Gene Identification
3.1. Yam
Most species in the genus Dioscorea (Dioscoreaceae) are dioecious [125] and have XY sex chromosomes [81,126,127], suggesting dioecy may have evolved ~80 million years ago (MYA) [128]. In D. alata a recent genetic map uncovered a ~10 Mb male-specific region of the Y (MSY) [81]. However, in D. rotundata, data support a ZW system with a small SDR (~161 Kb) [82], suggesting a recent turnover in this species. A candidate list of floral genes has been developed in D. rotundata [129], but more in-depth analyses are needed to identify those involved in sex determination.
3.2. Asparagus
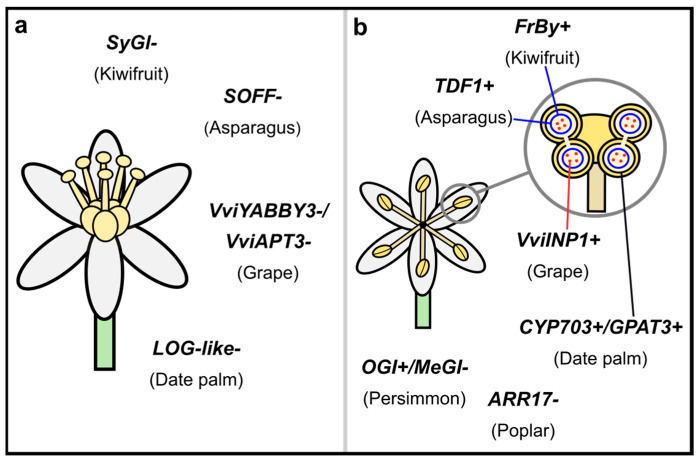
Several species of Asparagus (Asparagaceae) are dioecious including garden asparagus (A. officinalis) [130]. Asparagus officinalis has XY sex chromosomes, with a ~1 Mb MSY [49] that contains 13 genes with no homologs on the X (and only one X-specific gene), suggesting suppressed recombination is through a deletion on the X [49,50]. Two of the Y-linked genes have functionally been shown to be involved in the development of the sexes through gamma ray and Ethyl methanesulfonate (EMS) mutagenesis in XY males (Figure 1) [50]. Knockouts of Suppressor of Female Function (SOFF), which contains a DUF247 domain, develop hermaphroditic flowers with both functional anthers and ovules [50]. Knockouts of Tapetal Development and Function 1 (TDF1), an R2R3-MYB, make sterile individuals where neither functional carpels nor stamens develop [50]. Furthermore, knockouts of both SOFF and TDF1 develop functional ovaries, but non-functional anthers [50]. Together these results show that SOFF and TDF1 are the female and male-sterility genes, respectively, in A. officinalis. Further comparative analyses will uncover whether this sex-determination mechanism is shared across the other dioecious species in Asparagus or if other genes are involved.
Figure 1.
Recently discovered angiosperm sex-determination genes. Sex-determining genes recently identified that are involved with carpel development (a) include SyGl, SOFF, and LOG-like. When these genes are expressed (+) in males, it suppresses the function or development of the carpel. However, the lack of expression (-) in females allows for functional carpel development. In grapes, it is not yet known whether VviYABBY3 or VviAPT3 is the female-sterility gene. Several genes have also been identified for promoting stamen function (b). FrBy and TDF1 both promote tapetum development (in blue) and VviINP1 promotes pollen development (in red). It is unknown yet whether CYP703 or GPAT3 is the male-determining gene in date palm, but both are involved in pollen and/or anther development. In persimmon and poplar, a single gene is involved in sex determination (OGI and ARR17, respectively). When MeGI is expressed, flowers develop functional carpels, but not stamens. However, when the Y-linked OGI is expressed, it represses MeGI, resulting in functional stamens. Similarly, in poplars, ARR17 expression results in carpel production, but the lack of expression results in functional stamens.
3.3. Date Palm
In the genus Phoenix (Arecaceae), phylogenetic analyses of a MYB1 gene suggest the XY sex chromosomes may have an ancient origin, prior to the diversification of the species in this genus [131,132]. In the date palm, P. dactylifera, the MSY is ~13 Mb [133,134]. Comparative analyses across all 14 species of the genus identified three potential sex-determining genes [83]. Y-linked Cytochrome P450 (CYP703) and glycerol-3-phosphate acyltransferase 6-like (GPAT3) genes are expressed only in male flowers and are likely critical for pollen and/or anther development (Figure 1) [83]. The third gene, a Y-linked, Lonely Guy-like gene (LOG-like), which is involved in the activation of cytokinins, is also largely expressed in male flowers, and may have a role in suppressing carpel development [83]. While these genes seem like ideal candidates for sex determination, functional follow ups are necessary to validate these putative roles in Phoenix.
3.4. Grape
All wild species of Vitis (Vitaceae) are dioecious. However, like papaya (described below) domestic grapes have transitioned back to hermaphroditism [135,136]. Males are the heterogametic sex in Vitis and in V. vinifera sylvestris the MSY is small at ~150 Kb and contains 20 genes [137,138]. More recent analyses show grapes also support a two-gene model of sex determination (Figure 1). The gene inaperaturate pollen1 (VviINP1) likely plays a role in pollen aperture formation [139] and thus male fertility. Two strong candidates for the female-sterility gene are a YABBY3 gene (VviYABBY3) and an adenine phosphoribosyltransferase (APT) gene (VviAPT3) [84,140]. YABBY3 genes have been shown to play a role in flower and lateral organ development [141] and APT genes are involved in the cytokinin pathway and may be involved in suppressing carpel development [140,142]. However, functional follow-ups are necessary to confirm these roles in grapes.
3.5. Poplar
Nearly all species in Populus (Salicaceae) are dioecious [143,144] and across the genera, both XY (P. deltoides, P. euphratica, and P. tremula) and ZW (P. alba) sex chromosomes have been identified, suggesting at least one turnover event has occurred [145]. In P. tremula the MSY is ~1.5 Mb and contains a type-A cytokinin response regulator (RR), homologous to Arabidopsis RR 17 (ARR17), that is found in inverted repeats [88]. CRISPR knockouts of ARR17 in karyotypic females developed functional stamens and mostly did not develop carpels, whereas in karyotypic males, ARR17 knockouts showed no difference in development [88] (Figure 1). Some evidence suggests gene silencing of ARR17 in males is through RNA-directed DNA methylation, but this has not formally been tested [88]. In P. alba, the W also contains ARR17 that is lacking from the Z. This intriguing result highlights how a single gene can determine sex on both diploid sex chromosome types. Although, interestingly, within the same genus, there is recent evidence of two genes involved in sex determination. In P. deltoides one of the sex-determining genes is also related to ARR17, though they call it female-specifically expressed RESPONSE REGULATOR (FERR) [146]. The ~300 Kb MSY has a duplication of FERR that represses it (FERR-R), inhibiting carpel development. The second gene, a male-specific lncRNA (MSL), is likely involved in promoting male function [146].
3.6. Willow
The genus Salix is sister to poplars in the Salicaceae family and most species are also dioecious [144]. Salix purpurea and S. viminalis both have a ZW sex-determination system that share an evolutionary origin having arisen ~8.6 MYA [69,94]. The S. purpurea female-specific region of the W (FSW) is ~6.8 Mb and interestingly contains palindromic repeats, similar to those found in humans [94,147]. Within these repeats are five genes that may be associated with sex determination. The cytokinin RR is particularly of interest as this gene is homologous with the sex-determining ARR17 gene in poplar [88,94]. The S. viminalis FSW (~3.1 Mb) also contains ARR17, further supporting the putative role of this cytokinin-related gene in sex determination in willows and poplars, although this has not yet been confirmed with functional analyses in Salix [69]. Interestingly, >100 additional genes are found on the S. viminalis FSW, which show evidence of two strata. However, there is no evidence of chromosomal inversions involved in their capture into the SDR, suggesting instead the buildup of transposable elements may be involved in suppressing recombination [69]. Salix nigra, contrastingly, has XY sex chromosomes with a ~2 Mb MSY on a different chromosome than in the other Salix species examined, suggesting a translocation of the SDR (i.e., turnover) [148]. Though with current analyses it is unclear if RR is also sex-linked in this species [148]. Given the many turnovers and changes in heterogamety found in Salicaceae, often involving the same RR gene, a general model has been developed to explain this pattern [145]. Consistent with results described in Müller [88], in species with ZW sex chromosomes, RR acts as a dominant female promotor, but in XY systems RR duplicates target and repress RR by RNA-directed DNA methylation [145].
3.7. Strawberry
In Fragaria (Rosaceae) several species are dioecious, octoploids that are nested within a diploid, hermaphroditic clade [149], highlighting the role polyploidy can play in the evolution of dioecy [150]. Strawberries have ZW sex chromosomes that arose ~1 MYA [151]. In F. chiloensis the FSW is small at ~280 Kb [152], though in other Fragaria there is evidence the SDR is in different locations, suggesting either independent evolutions or translocations [153]. Recent evidence supports the latter, where the FSW has translocated at least twice among homeologous chromosomes, each time capturing more DNA into the region of suppressed recombination [154]. In F. virginiana ssp. virginiana, which has the smallest SDR, there are two genes, a GDP-mannose 3,5 epimerase 2 gene and a 60S ribosomal protein P0 [154]. These two genes are also located in the F. virginiana ssp. platypetala and F. chiloensis SDRs [154], although functional analyses will highlight whether they play a role in sex determination across these species.
3.8. Red Bayberry
In the genus Morella (Myricaceae), most species are dioecious, including M. rubra, the red bayberry [155]. Recent genome sequencing found M. rubra has ZW sex chromosomes with a ~59 Kb FSW that contains seven genes. Three of these have putative roles in flower development (MrCKA2, MrASP2, MrFT2) and two are related to hormones (MrCPS2, MrSAUR2; [64]). More functional work will help uncover which are involved in sex determination. All genes in the FSW have a paralogous copy on the same chromosome, suggesting gene duplication may have also played a role in the evolution of the sex chromosomes in this species [64].
3.9. Papaya
Papaya (Carica papaya) is the sole species in the genus Carica (Caricaceae) [156]. Across Caricaceae, 32 species are dioecious, two are trioecious, and one is monoecious [14]. Multiple lines of evidence suggested that sex chromosomes have evolved multiple times independently in Caricaceae, and sex chromosomes in Carica and Vasconcellea may have originated from the same ancestral autosomes after the divergence of these two genera [157,158]. Papaya is one of the two trioecious species, and sex determination of papaya is controlled by an XY system with two slightly different Y chromosomes, a MSY and a hermaphrodite-specific Yh [159]. The papaya MSY is 8.1 Mb [67,160] and two large inversions in the Y-linked region caused recombination suppression with the X and initiated sex chromosome evolution [67]. No hermaphrodite papayas have been found in wild populations and the Yh chromosome exhibits lower nucleotide diversity than the Y, suggesting that hermaphrodite papaya is likely a product of human domestication [136]. Several candidate genes showing functional and/or structural association with sex types were identified based on sequence comparison and gene expression analysis [161,162]. Further functional validation of candidate genes is still needed, although several independent studies point towards SVP (SHORT VEGETATIVE PHASE) as being involved in male flower development [163,164], though this putative gene does not have a sex-related function in other species.
3.10. Palmer Amaranth
Most species are monoecious in the genus Amaranthus (Amaranthaceae), however, dioecy is thought to have evolved multiple times independently [165]. The recent genome sequences of A. palmerii identified an XY sex chromosome system with a ~1.3–2 Mb MSY containing 121 gene models [107,108,166]. Amaranthus tuberculatus has a larger MSY (~4.6 Mb) with 147 genes [108]. Despite being in separate dioecious clades [165], two genes are found in the MSY of both species (Disintegrin and metalloproteinase domain-containing protein 9, ADAM9, and FLOWERING LOCUS T, FT) [108], making them candidates for sex determination or male-specific development.
3.11. Spinach
All three species of Spinacia (Amaranthaceae) are dioecious, and though S. oleracea and S. tetrandra diverged ~5.7 MYA, analyses of sex-linked homologs suggest suppressed recombination occurred after their divergence [167]. Recent analyses in S. oleracea have found the SDR to be between 10–19 Mb, with a 10 Mb MSY that has 210 genes [109,168]. These genes have been captured into the region of suppressed recombination through chromosomal inversions, making two strata of divergence between the X and the Y [109]. The 12 MSY genes with putative floral functions [109] and additional transcriptomic analyses of female and male flowers [169] have narrowed in potential sex-determining genes, though none so far are clear candidates.
3.12. Persimmon
Most species in Ebenaceae are dioecious including Diospyros [170]. Diospyros lotus has XY sex chromosomes with a ~1.3 Mb MSY [111]. Expression of an autosomal HD-Zip1 family gene, Male Growth Inhibitor (MeGI), results in the development of female flowers, with functional carpels, but not functional stamens. However, a Y-linked pseudogene, Oppressor of MeGI (OGI), encodes a small RNA that suppresses MeGI, resulting in male flowers [171] (Figure 1). Moreover, the male-determining role of OGI is stable in the hexaploid persimmon, D. kaki, which has both monoecious and female flowers [172,173]. These data, like in poplar, support that a single gene is involved in sex-determination in persimmons. This sex-determination system evolved through a recent whole-genome duplication, making two copies of MeGI. Functional analyses of these genes in tobacco suggests SiMeGI (sister copy of MeGI) may have maintained the original gene function, while MeGI neofunctionalized as a repressor of anther development [111]. A second duplication of MeGI resulted in the Y-linked OGI.
3.13. Kiwifruit
Most species in Actinidia (Actinidiaceae) are dioecious [174] and the sex chromosomes arose ~20 MYA [175]. Although kiwifruit is in a different family than persimmons, they are in the same order (Ericales), representing at least two independent origins of sex chromosomes. Actinidia chinensis var. chinensis have XY sex chromosomes and the MSY is ~0.8 Mb, containing 30 genes [176]. Two of these have been identified as sex determining, additionally supporting the two-locus model for the evolution of dioecy. One gene, a type-C cytokinin RR, suppresses ovary formation (SyGl), and the other has a fasciclin domain that contributes to tapetum degradation resulting in male fertility (FrBy) [175,176] (Figure 1). The function of these genes was validated through several approaches [176]. First, analyses of the genome of the hermaphroditic species, A. deliciosa, showed no evidence of a copy of SyGl, but did have FrBy [176]. This suggests either the loss of SyGl or the gain of FrBy caused the transition to hermaphroditism [176]. Moreover, knock-ins of FrBy into an XX female were hermaphroditic, with both functional carpels and stamens that produced fertile seeds after self-pollination [176]. Current work is in progress to also functionally validate SyGl [177].
3.14. Solanum
Dioecy evolved at least four times across the genus Solanum (Solanaceae) [178]. In S. appendiculatum, the XY system arose <4 MYA [179] and the MSY contains at least 20 genes [114]. Consistent with female flowers producing inaperturate pollen, many sex-biased and Y-linked genes are involved in pectin development [114], though more analyses will undoubtedly uncover genes involved in sex determination.
3.15. Amborella
Amborella trichopoda is a monotypic species in Amborellaceae that is sister to the rest of flowering plants [80,180]. Although the Amborella lineage diverged from the rest of angiosperms ~200 MYA [181], the ZW sex chromosomes are estimated to have diverged 9.5 to 14.5 MYA [182]. This recent origin of A. trichopoda sex chromosomes is consistent with the ancestral flower of all angiosperms being reconstructed as hermaphroditic [183]. The FSW is ~4 Mb and has ~150 genes [182], though which are involved in sex determination is unknown.
3.16. Maidenhair Tree
The dioecious gymnosperm Ginkgo biloba (Ginkgoaceae) [184] is a monotypic species. Two recent genomes suggest Ginkgo has an XY system [77,78] that arose ~14 MYA [77]. The MSY is ~27 Mb, with 241 genes, including 4 MADs-box genes expressed in staminate (male) cones [78]. Given the clear role MADs-box genes play in flower development in angiosperms [185], these genes are interesting candidates for sex-determination in Ginkgo as well.
3.17. Fire Moss
The moss Ceratodon purpureus (Ditrichaceae) UV sex chromosomes provide an interesting contrast to the XY/ZW systems in seed plants. The C. purpureus U and V are large with each >100 Mb and have >3400 annotated genes, totaling ~30% of the 360 Mb genome and ~12% of the gene content [65]. The moss sex chromosomes evolved at least 300 MYA in the ancestor to ~95% of extant mosses, making them among the oldest known sex chromosomes across Eukarya [65]. Compared to angiosperms, much less is known about the functions of genes in bryophytes, so narrowing in on candidate sex determiners is a challenge. However, some genes have been identified that are potentially of interest in sex-specific development. For example, the C. purpureus female-specific U chromosome contains an RWP-RK transcription factor [65], which are involved in egg cell formation across land plants [186,187] and in the same gene family as the MID mating-type loci in green algae [188]. Other notable genes on the C. purpureus U and V [65] are orthologs to the cis-acting sexual dimorphism switch found in Marchantia polymorpha (described below; [189]).
3.18. Common Liverwort
The liverwort M. polymorpha (Marchantiaceae) also has a UV sex-determination system with an ancestral origin [65,74]. The male-specific V is ~7.5 Mb and the female-specific U ~4.3 Mb, with 247 and 74 genes annotated, respectively [74,108], though the U has not been fully assembled, which may explain some of the difference in size. Like C. purpureus, it is unclear which genes on the U or V are involved in sex determination in M. polymorpha. However, intriguingly, an autosomal MYB transcription factor has a clear role in sex-specific development. Expression of FEMALE GAMETOPHYTE MYB (MpFGMYB) results in archegonia development, whereas expression of its cis-acting antisense gene suppresses MpFGMYB resulting in antheridia development and sperm production, though the sperm lack motility [189]. Several other dioecious bryophyte genomes have recently been published or are in progress [71,73,76,190], commencing an era for comparative analyses to uncover sex determination and further insights on sex chromosomes in this predominantly dioecious clade.
4. The Diversity of Proposed Mechanisms of Sex Determination
The plant sex chromosomes analyzed to date vary in age, size, and overall gene content, but what may be most striking is how many different genes have evolved to be the sex-determiners (Figure 1). This stands in contrast to animal systems where the same gene(s) have been shown to be involved in sex determination across many taxa (e.g., SRY/SOX3; DRMT1 [191]). For the genes identified in plants, some necessary similarities exist: they must be involved at some stage of sex-specific structure development (e.g., anther or carpel). Whether certain genes in these developmental pathways are more likely to evolve sex determination than others is unknown. Genes with broad-expression patterns seem to be unlikely candidates, as sex-linkage, and any subsequent molecular evolutionary consequences like protein evolution, may be deleterious to other functions. Although, duplications, whether by doubling of the whole genome or single genes, free genes from such constraints, allowing for neofunctionalization [150]. In fact a common theme in recent studies has been that duplications play a role in sex-determining genes (e.g., Asparagus, strawberry, persimmon, red bayberry, date palm, and kiwifruit [49,64,83,111,154,175]) or subsequent translocations to the SDR (e.g., Ceratodon [65]). Though not all the sex-determining genes in these systems show evidence of a recent duplication (e.g., Asparagus TDF1 [49]). In these latter cases, genes with tissue-specific or narrower expression may be more likely to evolve a sex-determining role.
Although several different genes have evolved to be sex-determining, in other dioecious species where they remain autosomal, they often instead show sex-biased expression, suggesting they play a conserved, sex-specific role or may be regulated by the sex-determining (or other sex-linked) genes [192]. For example, in kiwifruit, FrBy is the Y-linked, male-fertility gene, but TDF1 also shows male-biased expression [176], which makes sense given its role in tapetum development [50,193,194]. One pattern shared across many of these systems is the role many of these genes play in the cytokinin pathway (e.g., poplar, willow, date palm, and kiwifruit [69,83,88,175]), which is involved in floral development, particularly in the carpel and female gametophyte (reviewed in [195]). As we characterize the SDRs of more independent evolutions of dioecy, we will gain more insight on what genes are more likely to be involved, if any.
Another notable pattern emerging is the empirical support for the two-gene model for dioecy. In asparagus, kiwifruit, date palm, and grape [50,83,84,176], the SDRs have two genes involved in female and in male sterility (Figure 1). In-depth analyses in asparagus and kiwifruit have verified the Y-linked genes TDF1 and FrBy, respectively, promote male development and SOFF and SyGl, respectively, suppress female development [50,176,177]. In date palm, LOG-like is a strong candidate for being the female-sterility gene and both CYP703 and GPAT3 are candidates for promoting male development, whereas in grapes, VviYABBY3 and VviAPT3 are both candidates for female-sterility genes and VviINP1 for promoting male development. Though interestingly, in grapes, recombination between the tightly linked SDR on the X and the Y caused the transition back to hermaphroditism seen in domestic grapes [84]. The copy of VviYABBY3 found in hermaphroditic individuals more-closely resembles the female haplotype, rather than the male Y-linked copy, adding some additional weight to VviYABBY3 being the female-sterility gene [84]. It will be interesting if similar patterns of occasional recombination are involved in other transitions back to hermaphroditism (e.g., papaya) or if other processes like whole-genome duplications are involved [150]. In other systems, a single gene has been shown to be a sex-determining switch, like ARR17 in poplar and OGI in persimmon [88,171]. Though this result does not dispute the two-gene model, as the putatively ancestral hermaphroditic population had to first transition to gyno- or androdioecious [146,196].
Recent genome assemblies in dioecious plants have revealed more than sex-determining genes. Some studies have uncovered similar patterns in the evolution of the SDR that have been found in animal systems. The ancestral origins of sex chromosomes in the bryophytes more-closely resemble that of mammalian, bird, and some insect lineages [65,197,198,199], where the evolution happened early in the clade and remains shared among most taxa. This contrasts with most angiosperm sex chromosomes, which have more recent and independent origins (see examples above), though once they evolve many are also stable (e.g., Phoenix [83]). In other genera there is clear evidence of turnovers [82,88,145,154], where the sex-determining gene translocates to a new autosome, similar to what has been found in frogs and some fishes [200]. Neo-sex chromosomes have also been found [65,106,201], which can involve either part or an entire autosomal chromosome fusing to one or both sex chromosomes. Some theories have been developed on why some sex chromosomes are conserved while others turnover (reviewed in [200]) and closer examinations across plants may provide new insights.
Other similar patterns of gene gain to the sex chromosomes have been found between plant and animal sex chromosomes. Key to the movement of genes from the pseudoautosomal region to the SDR is suppressed recombination, a classic signature of which is evolutionary strata [202]. These strata occur when suites of genes are added to the region of suppressed recombination at the same time, causing them to have similar levels of divergence between gametologs (measured in synonymous substitutions, Ks). Multiple recombination-suppression events thus show a stepwise pattern of Ks along the SDR, with lower Ks for more-recent captures and higher Ks for older-captured genes. Indeed, evidence of evolutionary strata has been found in several plant species [69,87,109], suggesting plants may experience similar selective pressures that drive gene gain as animals [203]. The structural changes that cause suppressed recombination have classically been shown to be chromosomal inversions and several recent papers have found evidence in plants to also support this [109,204]. Some other striking convergent patterns, like palindromes, have been found in animal and plant sex chromosomes [94,147]. These palindromes consist of large inverted repeats and genes within these regions can undergo conversion [94,147]. Together, these results highlight that there are many dynamic patterns in sex chromosomes that are shared across these kingdoms.
However, there are just as many differences as there are similarities that have been found. Most often seen to date are differences in how suppressed recombination occurs. In some species, suppressed recombination can evolve before the SDR [106] with several evolving in close proximity to centromeres [53,66]. In other systems hemizygosity between the SDR caused by a deletion on the X suppresses recombination, rather than other structural changes like inversions [50,83,146]. In others suppressed recombination can occur without any structural changes at all, likely through the build-up of transposable elements (TEs) [69]. This latter result contradicts the often-thought pattern that TEs will build up after recombination has been suppressed on the SDR. Indeed, other characteristic patterns of degeneration and gene loss thought to affect sex-specific chromosomes, or at the very least the tempo of these processes, are questioned in several recent analyses [65,87,203]. Much of this is likely due to haploid gene expression [205], which occurs in gametophyte stages in plants and exposes genes to purifying selection. Together these results beg the question of whether the proposed linear model for the stages of sex chromosome evolution is overall applicable to plants or if these systems represent interesting exceptions to otherwise encompassing rules (see also [206]).
5. The Future of Plant Sex Chromosome Research
Combined, plants provide many independent tests for the evolution of sex chromosomes. While here we have focused on land plants, algae also provide other exciting, independent evolutions [15,207]. Although, despite the many recent publications, we have only just begun to uncover what plant sex chromosomes can illuminate. Assuming 5% of the 300,000 species of angiosperms are dioecious (using conservative numbers), only ~0.3% of these species have had their genomes sequenced to date, with an order of magnitude fewer in the other major clades (Table 1). Thus, one clear path moving forward is to increase the number and phylogenetic breadth of high-quality genome assemblies and annotations of dioecious species. While this has traditionally meant assembling a single exemplar genome for a species, the future of sex chromosome genomics should encompass pangenomes [208] that incorporate within-species variation, as well as closely-related, non-dioecious sister taxa that serve as outgroups. As sequencing technologies continue to improve, and the costs decrease, this becomes more tractable. Adding gene co-expression analyses will uncover downstream regulatory pathways [173,209] and whether these are more conserved than the sex-determining genes [192]. In addition to gene annotations, we should move to consistently annotate non-coding sequences, like small RNAs, [88,171,210] and uncover their targets to better understand their role in sex-specific development and sex determination. Moreover, as technologies like CRISPR improve, and protocols are established for more species, functional validations of these results will likely become standard [211]. These discoveries are all valuable for breeding programs of dioecious and closely related hermaphroditic crops. In fact, most of the species described in this review are economically important. There are also applications for controlling invasive species, like in palmer amaranth [108]. Additionally, from a conservation perspective, focusing on dioecious species is especially pressing, as the sexes often respond to stressors differently, meaning that due to climate change these species may be especially at risk for extinction [212].
In addition to comparative and functional genomics, a lot more interdisciplinary work in dioecy and sex chromosome research awaits. We need to focus on many classic (albeit also constantly improving) analyses rather than just the so-called “cutting-edge”. We need to fund more field work to identify new, potentially dioecious species and common-garden analyses to characterize development (e.g., [213]). We need better-supported, species-level phylogenies to infer the number of evolutions of dioecy, for example using Angiosperm353 [214] and GoFlag (Genealogy of Flagellate plants) [215] probe sets. We need more cytological analyses, to uncover how these chromosomes behave in the cell (e.g., [216,217]) or verifying in what tissues genes are expressed (e.g., [50]). Together, through these many approaches, we can discover a wealth of untapped knowledge to better understand the rules at play in these complex and dynamic regions of the genome in plants [15].
Acknowledgments
We thank three anonymous reviewers for helpful feedback that greatly improved this manuscript.
Author Contributions
Conceptualization: S.C., Q.Y., and A.H.; Writing—Original Draft Preparation: S.C., Q.Y., and A.H.; Writing—Review and Editing: S.C., Q.Y., and A.H.; Visualization: S.C.; Funding Acquisition: A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by start-up funds to A.H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cardoso J.C.F., Viana M.L., Matias R., Furtado M.T., Caetano A.P.D.S., Consolaro H., de Brito V.L.G. Towards a unified terminology for angiosperm reproductive systems. Acta Bot. Brasilica. 2018;32:329–348. doi: 10.1590/0102-33062018abb0124. [DOI] [Google Scholar]
- 2.de Nettancourt D. Incompatibility in Angiosperms. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 3.Cruden R.W. Temporal Dioecism: Systematic Breadth, Associated Traits, and Temporal Patterns. Bot. Gaz. 1988;149:1–15. doi: 10.1086/337684. [DOI] [Google Scholar]
- 4.Endress P.K. The evolution of floral biology in basal angiosperms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:411–421. doi: 10.1098/rstb.2009.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renner S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014;101:1588–1596. doi: 10.3732/ajb.1400196. [DOI] [PubMed] [Google Scholar]
- 6.Opedal Ø.H. Herkogamy, a Principal Functional Trait of Plant Reproductive Biology. Int. J. Plant Sci. 2018;179:677–687. doi: 10.1086/700314. [DOI] [Google Scholar]
- 7.Opedal Ø.H. Evolutionary Potential of Herkogamy. Elsevier Oceanogr. Ser. 2019:1–8. doi: 10.1002/9780470015902.a0028258. [DOI] [Google Scholar]
- 8.Renner S.S., Heinrichs J., Sousa A. The sex chromosomes of bryophytes: Recent insights, open questions, and reinvestigations of Frullania dilatata and Plagiochila asplenioides. J. Syst. Evolut. 2017;55:333–339. doi: 10.1111/jse.12266. [DOI] [Google Scholar]
- 9.Walas Ł., Mandryk W., Thomas P.A., Tyrała-Wierucka Ż., Iszkuło G. Sexual systems in gymnosperms: A review. Basic Appl. Ecol. 2018;31:1–9. doi: 10.1016/j.baae.2018.05.009. [DOI] [Google Scholar]
- 10.Storey W.B. GENETICS OF THE PAPAYA. J. Hered. 1953;44:70–78. doi: 10.1093/oxfordjournals.jhered.a106358. [DOI] [Google Scholar]
- 11.Westergaard M. The mechanism of sex determination in dioecious flowering plants. Adv. Genet. 1958;9:217–281. doi: 10.1016/s0065-2660(08)60163-7. [DOI] [PubMed] [Google Scholar]
- 12.Charlesworth B., Charlesworth D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 1978 doi: 10.1086/283342. [DOI] [Google Scholar]
- 13.Bachtrog D., Kirkpatrick M., Mank J.E., McDaniel S.F., Pires J.C., Rice W., Valenzuela N. Are all sex chromosomes created equal? Trends Genet. 2011;27:350–357. doi: 10.1016/j.tig.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Ming R., Bendahmane A., Renner S.S. Sex chromosomes in land plants. Annu. Rev. Plant Biol. 2011;62:485–514. doi: 10.1146/annurev-arplant-042110-103914. [DOI] [PubMed] [Google Scholar]
- 15.Carey S., Kollar L., McDaniel S. Does degeneration or genetic conflict shape gene content on UV sex chromosomes? EcoEvoRxiv. 2020 doi: 10.32942/osf.io/hs6w3. [DOI] [Google Scholar]
- 16.Winge Ö. On the nature of the sex chromosomes in Humulus. Hereditas. 1929;12:53–63. doi: 10.1111/j.1601-5223.1929.tb02497.x. [DOI] [Google Scholar]
- 17.Westergaard M. Aberrant Y chromosomes and sex expression in Melandrium album. Hereditas. 1946;32:419–443. doi: 10.1111/j.1601-5223.1946.tb02784.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith B.W. The Evolving Karyotype of Rumex hastatulus. Evolution. 1964;18:93–104. doi: 10.1111/j.1558-5646.1964.tb01573.x. [DOI] [Google Scholar]
- 19.Renner S.S. Pathways for making unisexual flowers and unisexual plants: Moving beyond the “two mutations linked on one chromosome” model. Am. J. Bot. 2016;103:587–589. doi: 10.3732/ajb.1600029. [DOI] [PubMed] [Google Scholar]
- 20.Linnaeus C. Systema Naturae. Volume 1. Holmiae; 1758. Impensis direct. Laurentii Salvii. [Google Scholar]
- 21.Darwin C. The Different Forms of Flowers on Plants of the Same Species. D. Appleton; New York, NY, USA: 1897. [Google Scholar]
- 22.Shephard H.L., Parker J.S., Darby P., Ainsworth C.C. Sexual development and sex chromosomes in hop. New Phytol. 2000;148:397–411. doi: 10.1046/j.1469-8137.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- 23.Schulz A.A.H. Bibliotheca Botanica. Schweizerbart Science Publishers; Stuttgart, Germany: 1890. Beiträge zur kenntnis der bestäubungseinrichtungen und geschlechtsvertheilung bei den pflanzen. [Google Scholar]
- 24.Caporali E., Carboni A., Galli M.G., Rossi G., Spada A., Marziani Longo G.P. Development of male and female flower in Asparagus officinalis. Search for point of transition from hermaphroditic to unisexual developmental pathway. Sex. Plant Reprod. 1994;7:239–249. doi: 10.1007/BF00232743. [DOI] [Google Scholar]
- 25.McNeilage M.A. Gender variation in Actinidia deliciosa, the kiwifruit. Sex. Plant Reprod. 1991;4:267–273. doi: 10.1007/BF00200546. [DOI] [Google Scholar]
- 26.Knapp S., Persson V., Blackmore S. Pollen morphology and functional dioecy in Solanum (Solanaceae) Plant Syst. Evol. 1998;210:113–139. doi: 10.1007/BF00984731. [DOI] [Google Scholar]
- 27.Reski R. Development, Genetics and Molecular Biology of Mosses. Bot. Acta. 1998;111:1–15. doi: 10.1111/j.1438-8677.1998.tb00670.x. [DOI] [Google Scholar]
- 28.Crandall-Stotler B.J., Bartholomew-Began S.E. Morphology of mosses (phylum Bryophyta) Flora of North America North of Mexico. 2007;27:3–13. [Google Scholar]
- 29.Landberg K., Pederson E.R.A., Viaene T., Bozorg B., Friml J., Jönsson H., Thelander M., Sundberg E. The moss Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiol. 2013;162:1406–1419. doi: 10.1104/pp.113.214023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchal É., Marchal E. Recherches expérimentales sur la sexualité des spores chez les Mousses dioïques. Mém. Cl. Sci. Collect. 8. 1906;1:1–50. [Google Scholar]
- 31.Johnson M.G., Shaw A.J. The effects of quantitative fecundity in the haploid stage on reproductive success and diploid fitness in the aquatic peat moss Sphagnum macrophyllum. Heredity. 2016;116:523–530. doi: 10.1038/hdy.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baughman J.T., Payton A.C., Paasch A.E., Fisher K.M., McDaniel S.F. Multiple factors influence population sex ratios in the Mojave Desert moss Syntrichia caninervis. Am. J. Bot. 2017;104:733–742. doi: 10.3732/ajb.1700045. [DOI] [PubMed] [Google Scholar]
- 33.Tree of Sex Consortium Tree of Sex: A database of sexual systems. Scientific Data. 2014;1 doi: 10.1038/sdata.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harkess A., Leebens-Mack J. A Century of Sex Determination in Flowering Plants. J. Hered. 2017;108:69–77. doi: 10.1093/jhered/esw060. [DOI] [PubMed] [Google Scholar]
- 35.Stevens N.M. Studies in Spermatogenesis. Carnegie Institution of Washington; Washington, DC, USA: 1905. [Google Scholar]
- 36.Allen C.E. A chromosome difference correlated with sex differences in sphaerocarpos. Science. 1917;46:466–467. doi: 10.1126/science.46.1193.466. [DOI] [PubMed] [Google Scholar]
- 37.Blackburn K.B. Sex Chromosomes in Plants. Nature. 1923;112:687–688. doi: 10.1038/112687c0. [DOI] [Google Scholar]
- 38.Kihara H., Ono T. Cytological Studies on Rumex L. II On the Relation of Chromosome Number and Sexes in Rumex acetosa L. Bot. Mag. Tokyo. 1923;37:147–149. doi: 10.15281/jplantres1887.37.438_147. [DOI] [Google Scholar]
- 39.Mendel G. Versuche uber pflanzen-hybriden. Verhandlungen des naturforschenden Vereins in Brunn fur. 1866;4:3–47. [Google Scholar]
- 40.Rheinberger H.-J. Re-discovering Mendel: The Case of Carl Correns. Sci. Educ. 2015;24:51–60. doi: 10.1007/s11191-013-9665-7. [DOI] [Google Scholar]
- 41.Vyskot B., Hobza R. The genomics of plant sex chromosomes. Plant Sci. 2015;236:126–135. doi: 10.1016/j.plantsci.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Baur E. Ein Fall von geschlechtsbegrenzter Vererbung beiMelandrium album. Z. Indukt. Abstamm. Vererbungsl. 1912;8:335–336. doi: 10.1007/BF01835499. [DOI] [Google Scholar]
- 43.Shull G.H. Sex-limited inheritance in Lychnis dioica L. Z. Indukt. Abstamm. Vererbungsl. 1914;12:265–302. doi: 10.1007/BF01837314. [DOI] [Google Scholar]
- 44.Winge Ö. On a Y-linked gene in Melandrium. Hereditas. 1927;9:274–284. doi: 10.1111/j.1601-5223.1927.tb03528.x. [DOI] [Google Scholar]
- 45.Farbos I., Veuskens J., Vyskot B., Oliveira M., Hinnisdaels S., Aghmir A., Mouras A., Negrutiu I. Sexual Dimorphism in White Campion: Deletion on the Y Chromosome Results in a Floral Asexual Phenotype. Genetics. 1999;151:1187–1196. doi: 10.1093/genetics/151.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lardon A., Georgiev S., Aghmir A., Le Merrer G., Negrutiu I. Sexual dimorphism in white campion: Complex control of carpel number is revealed by y chromosome deletions. Genetics. 1999;151 doi: 10.1093/genetics/151.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebel-Hardenack S., Hauser E., Law T.F., Schmid J., Grant S.R. Mapping of Sex Determination Loci on the White Campion (Silene latifolia) Y Chromosome Using Amplified Fragment Length Polymorphism. Genetics. 2002;160:717–725. doi: 10.1093/genetics/160.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kazama Y., Ishii K., Aonuma W., Ikeda T., Kawamoto H., Koizumi A., Filatov D.A., Chibalina M., Bergero R., Charlesworth D., et al. A new physical mapping approach refines the sex-determining gene positions on the Silene latifolia Y-chromosome. Sci. Rep. 2016;6:18917. doi: 10.1038/srep18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harkess A., Zhou J., Xu C., Bowers J.E., Van der Hulst R., Ayyampalayam S., Mercati F., Riccardi P., McKain M.R., Kakrana A., et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 2017;8:1279. doi: 10.1038/s41467-017-01064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harkess A., Huang K., van der Hulst R., Tissen B., Caplan J.L., Koppula A., Batish M., Meyers B.C., Leebens-Mack J. Sex Determination by Two Y-Linked Genes in Garden Asparagus. Plant Cell. 2020;32:1790–1796. doi: 10.1105/tpc.19.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamato K.T., Ishizaki K., Fujisawa M., Okada S., Nakayama S., Fujishita M., Bando H., Yodoya K., Hayashi K., Bando T., et al. Gene organization of the liverwort Y chromosome reveals distinct sex chromosome evolution in a haploid system. Proceedings of the National Academy of Sciences. 2007;104:6472–6477. doi: 10.1073/pnas.0609054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaillon O., Aury J.-M., Noel B., Policriti A., Clepet C., Casagrande A., Choisne N., Aubourg S., Vitulo N., Jubin C., et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463–467. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- 53.Yu Q., Hou S., Hobza R., Feltus F.A., Wang X., Jin W., Skelton R.L., Blas A., Lemke C., Saw J.H., et al. Chromosomal location and gene paucity of the male specific region on papaya Y chromosome. Mol. Genet. Genomics. 2007;278:177–185. doi: 10.1007/s00438-007-0243-z. [DOI] [PubMed] [Google Scholar]
- 54.Ming R., Hou S., Feng Y., Yu Q., Dionne-Laporte A., Saw J.H., Senin P., Wang W., Ly B.V., Lewis K.L.T., et al. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus) Nature. 2008;452:991–996. doi: 10.1038/nature06856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuskan G.A., Difazio S., Jansson S., Bohlmann J., Grigoriev I., Hellsten U., Putnam N., Ralph S., Rombauts S., Salamov A., et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 56.Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 57.Charlesworth B., Charlesworth D. The degeneration of Y chromosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:1563–1572. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bachtrog D. Y-chromosome evolution: Emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 2013;14:113–124. doi: 10.1038/nrg3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobza R., Kubat Z., Cegan R., Jesionek W., Vyskot B., Kejnovsky E. Impact of repetitive DNA on sex chromosome evolution in plants. Chromosome Res. 2015;23:561–570. doi: 10.1007/s10577-015-9496-2. [DOI] [PubMed] [Google Scholar]
- 60.Li S.-F., Zhang G.-J., Yuan J.-H., Deng C.-L., Gao W.-J. Repetitive sequences and epigenetic modification: Inseparable partners play important roles in the evolution of plant sex chromosomes. Planta. 2016;243:1083–1095. doi: 10.1007/s00425-016-2485-7. [DOI] [PubMed] [Google Scholar]
- 61.Green P. Whole-genome disassembly. Proc. Natl. Acad. Sci. USA. 2002;99:4143–4144. doi: 10.1073/pnas.082095999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alkan C., Sajjadian S., Eichler E.E. Limitations of next-generation genome sequence assembly. Nat. Methods. 2011;8:61–65. doi: 10.1038/nmeth.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fierst J.L. Using linkage maps to correct and scaffold de novo genome assemblies: Methods, challenges, and computational tools. Front. Genet. 2015;6:220. doi: 10.3389/fgene.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jia H.-M., Jia H.-J., Cai Q.-L., Wang Y., Zhao H.-B., Yang W.-F., Wang G.-Y., Li Y.-H., Zhan D.-L., Shen Y.-T., et al. The red bayberry genome and genetic basis of sex determination. Plant Biotechnol. J. 2019;17:397–409. doi: 10.1111/pbi.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carey S.B., Jenkins J., Lovell J.T., Maumus F., Sreedasyam A., Payton A.C., Shu S., Tiley G.P., Fernandez-Pozo N., Barry K., et al. The Ceratodon purpureus genome uncovers structurally complex, gene rich sex chromosomes. bioRxiv. 2020 doi: 10.1101/2020.07.03.163634. [DOI] [Google Scholar]
- 66.Pilkington S.M., Tahir J., Hilario E., Gardiner S.E., Chagné D., Catanach A., McCallum J., Jesson L., Fraser L.G., McNeilage M.A., et al. Genetic and cytological analyses reveal the recombination landscape of a partially differentiated plant sex chromosome in kiwifruit. BMC Plant Biol. 2019;19:172. doi: 10.1186/s12870-019-1766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J., Na J.-K., Yu Q., Gschwend A.R., Han J., Zeng F., Aryal R., VanBuren R., Murray J.E., Zhang W., et al. Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc. Natl. Acad. Sci. USA. 2012;109:13710–13715. doi: 10.1073/pnas.1207833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peichel C.L., McCann S.R., Ross J.A., Naftaly A.F.S., Urton J.R., Cech J.N., Grimwood J., Schmutz J., Myers R.M., Kingsley D.M., et al. Assembly of the threespine stickleback Y chromosome reveals convergent signatures of sex chromosome evolution. Genome Biol. 2020;21:177. doi: 10.1186/s13059-020-02097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almeida P., Proux-Wera E., Churcher A., Soler L., Dainat J., Pucholt P., Nordlund J., Martin T., Rönnberg-Wästljung A.-C., Nystedt B., et al. Genome assembly of the basket willow, Salix viminalis, reveals earliest stages of sex chromosome expansion. BMC Biol. 2020;18:78. doi: 10.1186/s12915-020-00808-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michael T.P., VanBuren R. Building near-complete plant genomes. Curr. Opin. Plant Biol. 2020;54:26–33. doi: 10.1016/j.pbi.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Silva A.T., Gao B., Fisher K.M., Mishler B.D., Ekwealor J.T.B., Stark L.R., Li X., Zhang D., Bowker M.A., Brinda J.C., et al. To dry perchance to live: Insights from the genome of the desiccation-tolerant biocrust moss Syntrichia caninervis. Plant J. 2020 doi: 10.1111/tpj.15116. [DOI] [PubMed] [Google Scholar]
- 72.Yu J., Li L., Wang S., Dong S., Chen Z., Patel N., Goffinet B., Liu H., Liu Y. Draft genome of the aquatic moss Fontinalis antipyretica (Fontinalaceae, Bryophyta) bioRxiv. 2020 doi: 10.46471/gigabyte.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pederson E.R.A., Warshan D., Rasmussen U. Genome Sequencing of Pleurozium schreberi: The Assembled and Annotated Draft Genome of a Pleurocarpous Feather Moss. G3. 2019;9:2791–2797. doi: 10.1534/g3.119.400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowman J.L., Kohchi T., Yamato K.T., Jenkins J., Shu S., Ishizaki K., Yamaoka S., Nishihama R., Nakamura Y., Berger F., et al. Insights into Land Plant Evolution Garnered from the Marchantia polymorpha Genome. Cell. 2017;171:287–304.e15. doi: 10.1016/j.cell.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 75.Montgomery S.A., Tanizawa Y., Galik B., Wang N., Ito T., Mochizuki T., Akimcheva S., Bowman J.L., Cognat V., Maréchal-Drouard L., et al. Chromatin Organization in Early Land Plants Reveals an Ancestral Association between H3K27me3, Transposons, and Constitutive Heterochromatin. Curr. Biol. 2020;30:573–588.e7. doi: 10.1016/j.cub.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marks R.A., Smith J.J., Cronk Q., Grassa C.J., McLetchie D.N. Genome of the tropical plant Marchantia inflexa: Implications for sex chromosome evolution and dehydration tolerance. Sci. Rep. 2019;9:8722. doi: 10.1038/s41598-019-45039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H., Zhang R., Yang X., Gu K.J., Chen W., Chang Y. Recent origin of an XX/XY sex-determination system in the ancient plant lineage Ginkgo biloba. bioRxiv. 2019 doi: 10.1101/517946. [DOI] [Google Scholar]
- 78.Liao Q., Du R., Gou J., Guo L., Shen H., Liu H. The genomic architecture of sex determining region and sex-related metabolic variation in Ginkgo biloba. The Plant. 2020 doi: 10.1111/tpj.15009. [DOI] [PubMed] [Google Scholar]
- 79.Wan T., Liu Z.-M., Li L.-F., Leitch A.R., Leitch I.J., Lohaus R., Liu Z.-J., Xin H.-P., Gong Y.-B., Liu Y., et al. A genome for gnetophytes and early evolution of seed plants. Nat. Plants. 2018;4:82–89. doi: 10.1038/s41477-017-0097-2. [DOI] [PubMed] [Google Scholar]
- 80.Amborella Genome Project The Amborella genome and the evolution of flowering plants. Science. 2013;342:1241089. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 81.Cormier F., Lawac F., Maledon E., Gravillon M.-C., Nudol E., Mournet P., Vignes H., Chaïr H., Arnau G. A reference high-density genetic map of greater yam (Dioscorea alata L.) Theor. Appl. Genet. 2019;132:1733–1744. doi: 10.1007/s00122-019-03311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamiru M., Natsume S., Takagi H., White B., Yaegashi H., Shimizu M., Yoshida K., Uemura A., Oikawa K., Abe A., et al. Genome sequencing of the staple food crop white Guinea yam enables the development of a molecular marker for sex determination. BMC Biol. 2017;15:86. doi: 10.1186/s12915-017-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torres M.F., Mathew L.S., Ahmed I., Al-Azwani I.K., Krueger R., Rivera-Nuñez D., Mohamoud Y.A., Clark A.G., Suhre K., Malek J.A. Genus-wide sequencing supports a two-locus model for sex-determination in Phoenix. Nat. Commun. 2018;9:3969. doi: 10.1038/s41467-018-06375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massonnet M., Cochetel N., Minio A., Vondras A.M., Lin J., Muyle A., Garcia J.F., Zhou Y., Delledonne M., Riaz S., et al. The genetic basis of sex determination in grapes. Nat. Commun. 2020;11:2902. doi: 10.1038/s41467-020-16700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Xin H., Fan P., Zhang J., Liu Y., Dong Y., Wang Z., Yang Y., Zhang Q., Ming R., et al. The genome of Shanputao (Vitis amurensis) provides a new insight into cold tolerance of grapevine. Plant J. 2020 doi: 10.1111/tpj.15127. [DOI] [PubMed] [Google Scholar]
- 86.Girollet N., Rubio B., Lopez-Roques C., Valière S., Ollat N., Bert P.-F. De novo phased assembly of the Vitis riparia grape genome. Sci. Data. 2019;6:127. doi: 10.1038/s41597-019-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veltsos P., Ridout K.E., Toups M.A., González-Martínez S.C., Muyle A., Emery O., Rastas P., Hudzieczek V., Hobza R., Vyskot B., et al. Early Sex-Chromosome Evolution in the Diploid Dioecious Plant Mercurialis annua. Genetics. 2019;212:815–835. doi: 10.1534/genetics.119.302045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Müller N.A., Kersten B., Leite Montalvão A.P., Mähler N., Bernhardsson C., Bräutigam K., Carracedo Lorenzo Z., Hoenicka H., Kumar V., Mader M., et al. A single gene underlies the dynamic evolution of poplar sex determination. Nat. Plants. 2020;6:630–637. doi: 10.1038/s41477-020-0672-9. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z., Chen Y., Zhang J., Ma X., Li Y., Li M., Wang D., Kang M., Wu H., Yang Y., et al. Improved genome assembly provides new insights into genome evolution in a desert poplar (Populus euphratica) Mol. Ecol. Resour. 2020;20 doi: 10.1111/1755-0998.13142. [DOI] [Google Scholar]
- 90.Chen Z., Ai F., Zhang J., Ma X., Yang W., Wang W., Su Y., Wang M., Yang Y., Mao K., et al. Survival in the Tropics despite isolation, inbreeding and asexual reproduction: Insights from the genome of the world’s southernmost poplar (Populus ilicifolia) Plant J. 2020;103:430–442. doi: 10.1111/tpj.14744. [DOI] [PubMed] [Google Scholar]
- 91.Hofmeister B.T., Denkena J., Colomé-Tatché M., Shahryary Y., Hazarika R., Grimwood J., Mamidi S., Jenkins J., Grabowski P.P., Sreedasyam A., et al. A genome assembly and the somatic genetic and epigenetic mutation rate in a wild long-lived perennial Populus trichocarpa. Genome Biol. 2020;21:259. doi: 10.1186/s13059-020-02162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J.-H., Huang Y., Brachi B., Yun Q.-Z., Zhang W., Lu W., Li H.-N., Li W.-Q., Sun X.-D., Wang G.-Y., et al. Genome-wide analysis of Cushion willow provides insights into alpine plant divergence in a biodiversity hotspot. Nat. Commun. 2019;10:5230. doi: 10.1038/s41467-019-13128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J., Yuan H., Li Y., Chen Y., Liu G., Ye M., Yu C., Lian B., Zhong F., Jiang Y., et al. Genome sequencing and phylogenetic analysis of allotetraploid Salix matsudana Koidz. Hortic. Res. 2020;7:201. doi: 10.1038/s41438-020-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou R., Macaya-Sanz D., Carlson C.H., Schmutz J., Jenkins J.W., Kudrna D., Sharma A., Sandor L., Shu S., Barry K., et al. A willow sex chromosome reveals convergent evolution of complex palindromic repeats. Genome Biol. 2020;21:38. doi: 10.1186/s13059-020-1952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai X., Hu Q., Cai Q., Feng K., Ye N., Tuskan G.A., Milne R., Chen Y., Wan Z., Wang Z., et al. The willow genome and divergent evolution from poplar after the common genome duplication. Cell Res. 2014;24:1274–1277. doi: 10.1038/cr.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edger P.P., Poorten T.J., VanBuren R., Hardigan M.A., Colle M., McKain M.R., Smith R.D., Teresi S.J., Nelson A.D.L., Wai C.M., et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019;51:541–547. doi: 10.1038/s41588-019-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mori K., Shirasawa K., Nogata H., Hirata C., Tashiro K., Habu T., Kim S., Himeno S., Kuhara S., Ikegami H. Corrigendum: Identification of RAN1 orthologue associated with sex determination through whole genome sequencing analysis in fig (Ficus carica L.) Sci. Rep. 2017;7:46784. doi: 10.1038/srep46784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shirasawa K., Yakushiji H., Nishimura R., Morita T., Jikumaru S., Ikegami H., Toyoda A., Hirakawa H., Isobe S. The Ficus erecta genome aids Ceratocystis canker resistance breeding in common fig (F. carica) Plant J. 2020;102:1313–1322. doi: 10.1111/tpj.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X., Wang G., Zhang S., Chen S., Wang Y., Wen P., Ma X., Shi Y., Qi R., Yang Y., et al. Genomes of the Banyan Tree and Pollinator Wasp Provide Insights into Fig-Wasp Coevolution. Cell. 2020;183:875–889.e17. doi: 10.1016/j.cell.2020.09.043. [DOI] [PubMed] [Google Scholar]
- 100.Gao S., Wang B., Xie S., Xu X., Zhang J., Pei L., Yu Y., Yang W., Zhang Y. A high-quality reference genome of wild Cannabis sativa. Hortic. Res. 2020;7:73. doi: 10.1038/s41438-020-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Padgitt-Cobb L.K., Kingan S.B., Wells J., Elser J., Kronmiller B., Moore D., Concepcion G., Peluso P., Rank D., Jaiswal P., et al. A phased, diploid assembly of the Cascade hop (Humulus lupulus) genome reveals patterns of selection and haplotype variation. bioRxiv. 2019:786145. doi: 10.1101/786145. [DOI] [Google Scholar]
- 102.Jiao F., Luo R., Dai X., Liu H., Yu G., Han S., Lu X., Su C., Chen Q., Song Q., et al. Chromosome-Level Reference Genome and Population Genomic Analysis Provide Insights into the Evolution and Improvement of Domesticated Mulberry (Morus alba) Mol. Plant. 2020;13:1001–1012. doi: 10.1016/j.molp.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 103.He N., Zhang C., Qi X., Zhao S., Tao Y., Yang G., Lee T.-H., Wang X., Cai Q., Li D., et al. Draft genome sequence of the mulberry tree Morus notabilis. Nat. Commun. 2013;4:2445. doi: 10.1038/ncomms3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng L., Tu X.-L., Dai H., Han F.-M., Lu B.-S., Wang M.-S., Nanaei H.A., Tajabadipour A., Mansouri M., Li X.-L., et al. Whole genomes and transcriptomes reveal adaptation and domestication of pistachio. Genome Biol. 2019;20:79. doi: 10.1186/s13059-019-1686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu Q., Tong E., Skelton R.L., Bowers J.E., Jones M.R., Murray J.E., Hou S., Guan P., Acob R.A., Luo M.-C., et al. A physical map of the papaya genome with integrated genetic map and genome sequence. BMC Genomics. 2009;10:371. doi: 10.1186/1471-2164-10-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rifkin J.L., Beaudry F.E.G., Humphries Z., Choudhury B.I., Barrett S.C.H., Wright S.I. Widespread Recombination Suppression Facilitates Plant Sex Chromosome Evolution. Mol. Biol. Evol. 2020 doi: 10.1093/molbev/msaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neves C.J., Matzrafi M., Thiele M., Lorant A., Mesgaran M.B., Stetter M.G. Male linked genomic regions determine sex in dioecious Amaranthus palmeri. bioRxiv. 2020 doi: 10.1101/2020.05.25.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montgomery J.S., Giacomini D.A., Weigel D., Tranel P.J. Male-specific Y-chromosomal regions in waterhemp (Amaranthus tuberculatus) and Palmer amaranth (Amaranthus palmeri) New Phytol. 2021;229:3522–3533. doi: 10.1111/nph.17108. [DOI] [PubMed] [Google Scholar]
- 109.She H., Liu Z., Xu Z., Zhang H., Cheng F., Wang X., Qian W. The female (XX) and male (YY) genomes provide insights into the sex determination mechanism in spinach. bioRxiv. 2020 doi: 10.1101/2020.11.23.393710. [DOI] [Google Scholar]
- 110.Sturtevant D., Lu S., Zhou Z.-W., Shen Y., Wang S., Song J.-M., Zhong J., Burks D.J., Yang Z.-Q., Yang Q.-Y., et al. The genome of jojoba (Simmondsia chinensis): A taxonomically isolated species that directs wax ester accumulation in its seeds. Sci. Adv. 2020;6:eaay3240. doi: 10.1126/sciadv.aay3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akagi T., Shirasawa K., Nagasaki H., Hirakawa H., Tao R., Comai L., Henry I.M. The persimmon genome reveals clues to the evolution of a lineage-specific sex determination system in plants. PLoS Genet. 2020;16:e1008566. doi: 10.1371/journal.pgen.1008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu H., Ma T., Kang M., Ai F., Zhang J., Dong G., Liu J. A high-quality Actinidia chinensis (kiwifruit) genome. Hortic. Res. 2019;6:117. doi: 10.1038/s41438-019-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang P., Shen R., He R., Yao X. The complete chloroplast genome sequence of Actinidia eriantha. Mitochondrial DNA B Resour. 2019;4:2114–2115. doi: 10.1080/23802359.2019.1623111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu M., Anderson G., Hahn M.W., Moyle L.C., Guerrero R.F. Inferring the genetic basis of sex determination from the genome of a dioecious nightshade. bioRxiv. 2020 doi: 10.1101/2020.07.23.218370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li F.-W., Harkess A. A guide to sequence your favorite plant genomes. Appl. Plant Sci. 2018;6:e1030. doi: 10.1002/aps3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burton J.N., Adey A., Patwardhan R.P., Qiu R., Kitzman J.O., Shendure J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 2013;31:1119–1125. doi: 10.1038/nbt.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Durand N.C., Shamim M.S., Machol I., Rao S.S.P., Huntley M.H., Lander E.S., Aiden E.L. Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell Syst. 2016;3:95–98. doi: 10.1016/j.cels.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghurye J., Rhie A., Walenz B.P., Schmitt A., Selvaraj S., Pop M., Phillippy A.M., Koren S. Integrating Hi-C links with assembly graphs for chromosome-scale assembly. PLoS Comput. Biol. 2019;15:e1007273. doi: 10.1371/journal.pcbi.1007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miga K.H., Koren S., Rhie A., Vollger M.R., Gershman A., Bzikadze A., Brooks S., Howe E., Porubsky D., Logsdon G.A., et al. Telomere-to-telomere assembly of a complete human X chromosome. Nature. 2020;585:79–84. doi: 10.1038/s41586-020-2547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nurk S., Walenz B.P., Rhie A., Vollger M.R., Logsdon G.A., Grothe R., Miga K.H., Eichler E.E., Phillippy A.M., Koren S. HiCanu: Accurate assembly of segmental duplications, satellites, and allelic variants from high-fidelity long reads. Genome Res. 2020;30:1291–1305. doi: 10.1101/gr.263566.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng H., Concepcion G.T., Feng X., Zhang H., Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods. 2021;18:170–175. doi: 10.1038/s41592-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vaser R., Sović I., Nagarajan N., Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoff K.J., Lomsadze A., Stanke M., Borodovsky M. BRAKER2: Incorporating protein homology information into gene prediction with GeneMark-EP and AUGUSTUS; Proceedings of the Plant and Animal Genomes XXVI; San Diego, CA, USA. 14 January 2018. [Google Scholar]
- 124.Palmer D.H., Rogers T.F., Dean R., Wright A.E. How to identify sex chromosomes and their turnover. Mol. Ecol. 2019;28:4709–4724. doi: 10.1111/mec.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin F.W. Sex ratio and sex determination in Dioscorea. J. Hered. 1966;57:95–99. doi: 10.1093/oxfordjournals.jhered.a107485. [DOI] [Google Scholar]
- 126.Terauchi R., Kahl G. Mapping of the Dioscorea tokoro genome: AFLP markers linked to sex. Genome. 1999;42:752–762. doi: 10.1139/g99-001. [DOI] [Google Scholar]
- 127.Sugihara Y., Darkwa K., Yaegashi H., Natsume S., Shimizu M., Abe A., Hirabuchi A., Ito K., Oikawa K., Tamiru-Oli M., et al. Genome analyses reveal the hybrid origin of the staple crop white Guinea yam (Dioscorea rotundata) Proc. Natl. Acad. Sci. USA. 2020 doi: 10.1073/pnas.2015830117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maurin O., Muasya A.M., Catalan P., Shongwe E.Z., Viruel J., Wilkin P., van der Bank M. Diversification into novel habitats in the Africa clade of Dioscorea (Dioscoreaceae): Erect habit and elephant’s foot tubers. BMC Evol. Biol. 2016;16:238. doi: 10.1186/s12862-016-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Girma G., Natsume S., Carluccio A.V., Takagi H., Matsumura H., Uemura A., Muranaka S., Takagi H., Stavolone L., Gedil M., et al. Identification of candidate flowering and sex genes in white Guinea yam (D. rotundata Poir.) by SuperSAGE transcriptome profiling. PLoS ONE. 2019;14:e0216912. doi: 10.1371/journal.pone.0216912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Norup M.F., Petersen G., Burrows S., Bouchenak-Khelladi Y., Leebens-Mack J., Pires J.C., Linder H.P., Seberg O. Evolution of Asparagus L. (Asparagaceae): Out-of-South-Africa and multiple origins of sexual dimorphism. Mol. Phylogenet. Evol. 2015;92:25–44. doi: 10.1016/j.ympev.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 131.Cherif E., Zehdi-Azouzi S., Crabos A., Castillo K., Chabrillange N., Pintaud J.-C., Salhi-Hannachi A., Glémin S., Aberlenc-Bertossi F. Evolution of sex chromosomes prior to speciation in the dioecious Phoenix species. J. Evol. Biol. 2016;29:1513–1522. doi: 10.1111/jeb.12887. [DOI] [PubMed] [Google Scholar]
- 132.Baker W.J., Couvreur T.L.P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. J. Biogeogr. 2013;40:274–285. doi: 10.1111/j.1365-2699.2012.02795.x. [DOI] [Google Scholar]
- 133.Al-Dous E.K., George B., Al-Mahmoud M.E., Al-Jaber M.Y., Wang H., Salameh Y.M., Al-Azwani E.K., Chaluvadi S., Pontaroli A.C., DeBarry J., et al. De novo genome sequencing and comparative genomics of date palm (Phoenix dactylifera) Nat. Biotechnol. 2011;29:521–527. doi: 10.1038/nbt.1860. [DOI] [PubMed] [Google Scholar]
- 134.Mathew L.S., Spannagl M., Al-Malki A., George B., Torres M.F., Al-Dous E.K., Al-Azwani E.K., Hussein E., Mathew S., Mayer K.F.X., et al. A first genetic map of date palm (Phoenix dactylifera) reveals long-range genome structure conservation in the palms. BMC Genomics. 2014;15:285. doi: 10.1186/1471-2164-15-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.This P., Lacombe T., Thomas M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006;22:511–519. doi: 10.1016/j.tig.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 136.VanBuren R., Zeng F., Chen C., Zhang J., Wai C.M., Han J., Aryal R., Gschwend A.R., Wang J., Na J.-K., et al. Origin and domestication of papaya Yh chromosome. Genome Res. 2015;25:524–533. doi: 10.1101/gr.183905.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fechter I., Hausmann L., Daum M., Sörensen T.R., Viehöver P., Weisshaar B., Töpfer R. Candidate genes within a 143 kb region of the flower sex locus in Vitis. Mol. Genet. Genomics. 2012;287:247–259. doi: 10.1007/s00438-012-0674-z. [DOI] [PubMed] [Google Scholar]
- 138.Picq S., Santoni S., Lacombe T., Latreille M., Weber A., Ardisson M., Ivorra S., Maghradze D., Arroyo-Garcia R., Chatelet P., et al. A small XY chromosomal region explains sex determination in wild dioecious V. vinifera and the reversal to hermaphroditism in domesticated grapevines. BMC Plant Biol. 2014;14:229. doi: 10.1186/s12870-014-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li P., Ben-Menni Schuler S., Reeder S.H., Wang R., Suárez Santiago V.N., Dobritsa A.A. INP1 involvement in pollen aperture formation is evolutionarily conserved and may require species-specific partners. J. Exp. Bot. 2018;69:983–996. doi: 10.1093/jxb/erx407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Badouin H., Velt A., Gindraud F., Flutre T., Dumas V., Vautrin S., Marande W., Corbi J., Sallet E., Ganofsky J., et al. The wild grape genome sequence provides insights into the transition from dioecy to hermaphroditism during grape domestication. Genome Biol. 2020;21:223. doi: 10.1186/s13059-020-02131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Q., Atkinson A., Otsuga D., Christensen T., Reynolds L., Drews G.N. The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development. 1999;126:2715–2726. doi: 10.1242/dev.126.12.2715. [DOI] [PubMed] [Google Scholar]
- 142.Coito J.L., Ramos M.J.N., Cunha J., Silva H.G., Amâncio S., Costa M.M.R., Rocheta M. VviAPRT3 and VviFSEX: Two Genes Involved in Sex Specification Able to Distinguish Different Flower Types in Vitis. Front. Plant Sci. 2017;8:98. doi: 10.3389/fpls.2017.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Slavov G.T., Zhelev P. Salient Biological Features, Systematics, and Genetic Variation of Populus. In: Jansson S., Bhalerao R., Groover A., editors. Genetics and Genomics of Populus. Springer; New York, NY, USA: 2010. pp. 15–38. [Google Scholar]
- 144.Cronk Q.C.B., Needham I., Rudall P.J. Evolution of Catkins: Inflorescence Morphology of Selected Salicaceae in an Evolutionary and Developmental Context. Front. Plant Sci. 2015;6:1030. doi: 10.3389/fpls.2015.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang W., Wang D., Li Y., Zhang Z., Tong S., Li M., Zhang X., Zhang L., Ren L., Ma X., et al. A general model to explain repeated turnovers of sex determination in the Salicaceae. Mol. Biol. Evol. 2020 doi: 10.1093/molbev/msaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xue L., Wu H., Chen Y., Li X., Hou J., Lu J., Wei S., Dai X., Olson M.S., Liu J., et al. Evidences for a role of two Y-specific genes in sex determination in Populus deltoides. Nat. Commun. 2020;11:5893. doi: 10.1038/s41467-020-19559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Skaletsky H., Kuroda-Kawaguchi T., Minx P.J., Cordum H.S., Hillier L., Brown L.G., Repping S., Pyntikova T., Ali J., Bieri T., et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 148.Sanderson B.J., Feng G., Hu N., Carlson C.H., Smart L.B., Keefover-Ring K., Yin T., Ma T., Liu J., DiFazio S.P., et al. Sex determination through X–Y heterogamety in Salix nigra. Heredity. 2021 doi: 10.1038/s41437-020-00397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tennessen J.A., Govindarajulu R., Ashman T.-L., Liston A. Evolutionary origins and dynamics of octoploid strawberry subgenomes revealed by dense targeted capture linkage maps. Genome Biol. Evol. 2014;6:3295–3313. doi: 10.1093/gbe/evu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ashman T.-L., Kwok A., Husband B.C. Revisiting the dioecy-polyploidy association: Alternate pathways and research opportunities. Cytogenet. Genome Res. 2013;140:241–255. doi: 10.1159/000353306. [DOI] [PubMed] [Google Scholar]
- 151.Njuguna W., Liston A., Cronn R., Ashman T.-L., Bassil N. Insights into phylogeny, sex function and age of Fragaria based on whole chloroplast genome sequencing. Mol. Phylogenet. Evol. 2013;66:17–29. doi: 10.1016/j.ympev.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 152.Tennessen J.A., Govindarajulu R., Liston A., Ashman T. Homomorphic ZW chromosomes in a wild strawberry show distinctive recombination heterogeneity but a small sex-determining region. New Phytol. 2016;211:1412–1423. doi: 10.1111/nph.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goldberg M.T., Spigler R.B., Ashman T.-L. Comparative genetic mapping points to different sex chromosomes in sibling species of wild strawberry (Fragaria) Genetics. 2010;186:1425–1433. doi: 10.1534/genetics.110.122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tennessen J.A., Wei N., Straub S.C.K., Govindarajulu R., Liston A., Ashman T.-L. Repeated translocation of a gene cassette drives sex-chromosome turnover in strawberries. PLoS Biol. 2018;16:e2006062. doi: 10.1371/journal.pbio.2006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilbur R.L. The myricaceae of the united states and canada: Genera, subgenera, and series. SIDA Contrib. Bot. 1994;16:93–107. [Google Scholar]
- 156.Carvalho F.A., Renner S.S. A dated phylogeny of the papaya family (Caricaceae) reveals the crop’s closest relatives and the family’s biogeographic history. Mol. Phylogenet. Evol. 2012;65:46–53. doi: 10.1016/j.ympev.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 157.Wu X., Wang J., Na J.-K., Yu Q., Moore R.C., Zee F., Huber S.C., Ming R. The origin of the non-recombining region of sex chromosomes in Carica and Vasconcellea. Plant J. 2010;63:801–810. doi: 10.1111/j.1365-313X.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 158.Iovene M., Yu Q., Ming R., Jiang J. Evidence for emergence of sex-determining gene(s) in a centromeric region in Vasconcellea parviflora. Genetics. 2015;199:413–421. doi: 10.1534/genetics.114.173021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu Z., Moore P.H., Ma H., Ackerman C.M., Ragiba M., Yu Q., Pearl H.M., Kim M.S., Charlton J.W., Stiles J.I., et al. A primitive Y chromosome in papaya marks incipient sex chromosome evolution. Nature. 2004;427:348–352. doi: 10.1038/nature02228. [DOI] [PubMed] [Google Scholar]
- 160.Na J.-K., Wang J., Murray J.E., Gschwend A.R., Zhang W., Yu Q., Navajas-Pérez R., Feltus F.A., Chen C., Kubat Z., et al. Construction of physical maps for the sex-specific regions of papaya sex chromosomes. BMC Genomics. 2012;13:176. doi: 10.1186/1471-2164-13-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Urasaki N., Tarora K., Shudo A., Ueno H., Tamaki M., Miyagi N., Adaniya S., Matsumura H. Digital transcriptome analysis of putative sex-determination genes in papaya (Carica papaya) PLoS ONE. 2012;7:e40904. doi: 10.1371/journal.pone.0040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ueno H., Urasaki N., Natsume S., Yoshida K., Tarora K., Shudo A., Terauchi R., Matsumura H. Genome sequence comparison reveals a candidate gene involved in male-hermaphrodite differentiation in papaya (Carica papaya) trees. Mol. Genet. Genomics. 2015;290:661–670. doi: 10.1007/s00438-014-0955-9. [DOI] [PubMed] [Google Scholar]
- 163.Lee C.-Y., Lin H.-J., Viswanath K.K., Lin C.-P., Chang B.C.-H., Chiu P.-H., Chiu C.-T., Wang R.-H., Chin S.-W., Chen F.-C. The development of functional mapping by three sex-related loci on the third whorl of different sex types of Carica papaya L. PLoS ONE. 2018;13:e0194605. doi: 10.1371/journal.pone.0194605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chae T., Harkess A., Moore R.C. Sex-linked gene expression and the reversion to hermaphroditism in Carica papaya L. (Caricaceae) bioRxiv. 2020 doi: 10.1101/2020.06.25.169623. [DOI] [Google Scholar]
- 165.Stetter M.G., Schmid K.J. Analysis of phylogenetic relationships and genome size evolution of the Amaranthus genus using GBS indicates the ancestors of an ancient crop. Mol. Phylogenet. Evol. 2017;109:80–92. doi: 10.1016/j.ympev.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 166.Murray M.J. The Genetics of Sex Determination in the Family Amaranthaceae. Genetics. 1940;25:409–431. doi: 10.1093/genetics/25.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Okazaki Y., Takahata S., Hirakawa H., Suzuki Y., Onodera Y. Molecular evidence for recent divergence of X- and Y-linked gene pairs in Spinacia oleracea L. PLoS ONE. 2019;14:e0214949. doi: 10.1371/journal.pone.0214949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu L., Ma X., Deng B., Yue J., Ming R. Construction of high-density genetic maps defined sex determination region of the Y chromosome in spinach. Mol. Genet. Genomics. 2020 doi: 10.1007/s00438-020-01723-4. [DOI] [PubMed] [Google Scholar]
- 169.Li N., Meng Z., Tao M., Wang Y., Zhang Y., Li S., Gao W., Deng C. Comparative transcriptome analysis of male and female flowers in Spinacia oleracea L. BMC Genomics. 2020;21:850. doi: 10.1186/s12864-020-07277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wallnöfer B. The Biology and Systematics of Ebenaceae: A Review. Ann. Nat. Hist. Mus. Wien Ser. B Bot. Zool. 2001;103:485–512. [Google Scholar]
- 171.Akagi T., Henry I.M., Tao R., Comai L. A Y-chromosome–encoded small RNA acts as a sex determinant in persimmons. Science. 2014;346:646–650. doi: 10.1126/science.1257225. [DOI] [PubMed] [Google Scholar]
- 172.Akagi T., Kawai T., Tao R. A male determinant gene in diploid dioecious Diospyros, OGI, is required for male flower production in monoecious individuals of Oriental persimmon (D. kaki) Sci. Hortic. 2016;213:243–251. doi: 10.1016/j.scienta.2016.10.046. [DOI] [Google Scholar]
- 173.Masuda K., Fujita N., Yang H.-W., Ushijima K., Kubo Y., Tao R., Akagi T. Molecular Mechanism Underlying Derepressed Male Production in Hexaploid Persimmon. Front. Plant Sci. 2020;11:567249. doi: 10.3389/fpls.2020.567249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ferguson A.R. Kiwifruit (actinidia) Genet. Resour. Temper. Fruit Nut. Cr. 1991;290:603–656. doi: 10.17660/ActaHortic.1991.290.14. [DOI] [Google Scholar]
- 175.Akagi T., Henry I.M., Ohtani H., Morimoto T., Beppu K., Kataoka I., Tao R. A Y-Encoded Suppressor of Feminization Arose via Lineage-Specific Duplication of a Cytokinin Response Regulator in Kiwifruit. Plant Cell. 2018;30:780–795. doi: 10.1105/tpc.17.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Akagi T., Pilkington S.M., Varkonyi-Gasic E., Henry I.M., Sugano S.S., Sonoda M., Firl A., McNeilage M.A., Douglas M.J., Wang T., et al. Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat. Plants. 2019;5:801–809. doi: 10.1038/s41477-019-0489-6. [DOI] [PubMed] [Google Scholar]
- 177.De Mori G., Zaina G., Franco-Orozco B., Testolin R., De Paoli E., Cipriani G. Targeted Mutagenesis of the Female-Suppressor SyGI Gene in Tetraploid Kiwifruit by CRISPR/CAS9. Plants. 2020;10:62. doi: 10.3390/plants10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anderson G.J., Anderson M.K.J., Patel N. The ecology, evolution, and biogeography of dioecy in the genus Solanum: With paradigms from the strong dioecy in Solanum polygamum, to the unsuspected and cryptic dioecy in Solanum conocarpum. Am. J. Bot. 2015;102:471–486. doi: 10.3732/ajb.1400486. [DOI] [PubMed] [Google Scholar]
- 179.Echeverría-Londoño S., Särkinen T., Fenton I.S., Purvis A., Knapp S. Dynamism and context-dependency in diversification of the megadiverse plant genus Solanum (Solanaceae) J. Syst. Evol. 2018 doi: 10.1111/jse.12638. [DOI] [Google Scholar]
- 180.One Thousand Plant Transcriptomes Initiative One thousand plant transcriptomes and the phylogenomics of green plants. Nature. 2019;574:679–685. doi: 10.1038/s41586-019-1693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li H.-T., Yi T.-S., Gao L.-M., Ma P.-F., Zhang T., Yang J.-B., Gitzendanner M.A., Fritsch P.W., Cai J., Luo Y., et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants. 2019;5:461–470. doi: 10.1038/s41477-019-0421-0. [DOI] [PubMed] [Google Scholar]
- 182.Käfer J., Bewick A., Andres-Robin A., Lapetoule G., Harkess A., Caïus J., Fogliani B., Gâteblé G., Ralph P., dePamphilis C.W., et al. A derived ZW chromosome system in Amborella trichopoda, the sister species to all other extant flowering plants. bioRxiv. 2020 doi: 10.1101/2020.12.21.423833. [DOI] [PubMed] [Google Scholar]
- 183.Sauquet H., von Balthazar M., Magallón S., Doyle J.A., Endress P.K., Bailes E.J., Barroso de Morais E., Bull-Hereñu K., Carrive L., Chartier M., et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017;8:16047. doi: 10.1038/ncomms16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Newcomer E.H. The Karyotype and Possible Sex Chromosomes of Ginkgo biloba. Am. J. Bot. 1954;41:542–545. doi: 10.1002/j.1537-2197.1954.tb14375.x. [DOI] [Google Scholar]
- 185.Theissen G., Becker A., Di Rosa A., Kanno A., Kim J.T., Münster T., Winter K.U., Saedler H. A short history of MADS-box genes in plants. Plant Mol. Biol. 2000;42:115–149. doi: 10.1023/A:1006332105728. [DOI] [PubMed] [Google Scholar]
- 186.Rövekamp M., Bowman J.L., Grossniklaus U. Marchantia MpRKD Regulates the Gametophyte-Sporophyte Transition by Keeping Egg Cells Quiescent in the Absence of Fertilization. Curr. Biol. 2016;26:1782–1789. doi: 10.1016/j.cub.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 187.Tedeschi F., Rizzo P., Rutten T., Altschmied L., Bäumlein H. RWP-RK domain-containing transcription factors control cell differentiation during female gametophyte development in Arabidopsis. New Phytol. 2017;213:1909–1924. doi: 10.1111/nph.14293. [DOI] [PubMed] [Google Scholar]
- 188.Ferris P., Olson B.J.S.C., De Hoff P.L., Douglass S., Casero D., Prochnik S., Geng S., Rai R., Grimwood J., Schmutz J., et al. Evolution of an expanded sex-determining locus in Volvox. Science. 2010;328:351–354. doi: 10.1126/science.1186222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hisanaga T., Okahashi K., Yamaoka S., Kajiwara T., Nishihama R., Shimamura M., Yamato K.T., Bowman J.L., Kohchi T., Nakajima K. A cis-acting bidirectional transcription switch controls sexual dimorphism in the liverwort. EMBO J. 2019;38 doi: 10.15252/embj.2018100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shaw A.J., Schmutz J., Devos N., Shu S., Carrell A.A., Weston D.J. Chapter Five—The Sphagnum Genome Project: A New Model for Ecological and Evolutionary Genomics. Adv. Bot. Res. 2016;78:167–187. doi: 10.1016/bs.abr.2016.01.003. [DOI] [Google Scholar]
- 191.Graves J.A.M. How to evolve new vertebrate sex determining genes. Dev. Dyn. 2013;242:354–359. doi: 10.1002/dvdy.23887. [DOI] [PubMed] [Google Scholar]
- 192.Feng G., Sanderson B.J., Keefover-Ring K., Liu J., Ma T., Yin T., Smart L.B., DiFazio S.P., Olson M.S. Pathways to sex determination in plants: How many roads lead to Rome? Curr. Opin. Plant Biol. 2020;54:61–68. doi: 10.1016/j.pbi.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 193.Zhu J., Chen H., Li H., Gao J.-F., Jiang H., Wang C., Guan Y.-F., Yang Z.-N. Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008;55:266–277. doi: 10.1111/j.1365-313X.2008.03500.x. [DOI] [PubMed] [Google Scholar]
- 194.Cai C.-F., Zhu J., Lou Y., Guo Z.-L., Xiong S.-X., Wang K., Yang Z.-N. The functional analysis of OsTDF1 reveals a conserved genetic pathway for tapetal development between rice and Arabidopsis. Sci. Bull. Fac. Agric. Kyushu Univ. 2015;60:1073–1082. doi: 10.1007/s11434-015-0810-3. [DOI] [Google Scholar]
- 195.Wybouw B., De Rybel B. Cytokinin—A developing story. Trends Plant Sci. 2019;24:177–185. doi: 10.1016/j.tplants.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 196.Charlesworth D. Plant sex determination and sex chromosomes. Heredity. 2002;88:94–101. doi: 10.1038/sj.hdy.6800016. [DOI] [PubMed] [Google Scholar]
- 197.Schatzkamer K., Kremitzki C.L., Graves T. Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome. 2008 doi: 10.1101/gr.7101908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Meisel R.P., Delclos P.J., Wexler J.R. The X chromosome of the German cockroach, Blattella germanica, is homologous to a fly X chromosome despite 400 million years divergence. BMC Biol. 2019;17:100. doi: 10.1186/s12915-019-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu L., Auer G., Peona V., Suh A., Deng Y., Feng S., Zhang G., Blom M.P.K., Christidis L., Prost S., et al. Dynamic evolutionary history and gene content of sex chromosomes across diverse songbirds. Nat. Ecol. Evol. 2019;3:834–844. doi: 10.1038/s41559-019-0850-1. [DOI] [PubMed] [Google Scholar]
- 200.Vicoso B. Molecular and evolutionary dynamics of animal sex-chromosome turnover. Nat. Ecol. Evol. 2019;3:1632–1641. doi: 10.1038/s41559-019-1050-8. [DOI] [PubMed] [Google Scholar]
- 201.Delgado C.L.R., Waters P.D., Gilbert C., Robinson T.J., Graves J.A.M. Physical mapping of the elephant X chromosome: Conservation of gene order over 105 million years. Chromosome Res. 2009;17:917–926. doi: 10.1007/s10577-009-9079-1. [DOI] [PubMed] [Google Scholar]
- 202.Lahn B.T., Page D.C. Four evolutionary strata on the human X chromosome. Science. 1999;286:964–967. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- 203.Bergero R., Charlesworth D. Preservation of the Y transcriptome in a 10-million-year-old plant sex chromosome system. Curr. Biol. 2011;21:1470–1474. doi: 10.1016/j.cub.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 204.Hooper D.M., Griffith S.C., Price T.D. Sex chromosome inversions enforce reproductive isolation across an avian hybrid zone. Mol. Ecol. 2019;28:1246–1262. doi: 10.1111/mec.14874. [DOI] [PubMed] [Google Scholar]
- 205.Chibalina M.V., Filatov D.A. Plant Y chromosome degeneration is retarded by haploid purifying selection. Curr. Biol. 2011;21:1475–1479. doi: 10.1016/j.cub.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 206.Furman B.L.S., Metzger D.C.H., Darolti I., Wright A.E., Sandkam B.A., Almeida P., Shu J.J., Mank J.E. Sex Chromosome Evolution: So Many Exceptions to the Rules. Genome Biol. Evol. 2020;12:750–763. doi: 10.1093/gbe/evaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Coelho S.M., Gueno J., Lipinska A.P., Cock J.M., Umen J.G. UV Chromosomes and Haploid Sexual Systems. Trends Plant Sci. 2018;23:794–807. doi: 10.1016/j.tplants.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hurgobin B., Edwards D. SNP Discovery Using a Pangenome: Has the Single Reference Approach Become Obsolete? Biology. 2017;6:21. doi: 10.3390/biology6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang H.-W., Akagi T., Kawakatsu T., Tao R. Gene networks orchestrated by MeGI: A single-factor mechanism underlying sex determination in persimmon. Plant J. 2019;98:97–111. doi: 10.1111/tpj.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mohanty J.N., Chand S.K., Joshi R.K. Multiple microRNAs Regulate the Floral Development and Sex Differentiation in the Dioecious Cucurbit Coccinia grandis (L.) Voigt. Plant Mol. Biol. Rep. 2019;37:111–128. doi: 10.1007/s11105-019-01143-8. [DOI] [Google Scholar]
- 211.Moradpour M., Abdulah S.N.A. CRISPR/dCas9 platforms in plants: Strategies and applications beyond genome editing. Plant Biotechnol. J. 2020;18:32–44. doi: 10.1111/pbi.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hultine K.R., Grady K.C., Wood T.E., Shuster S.M., Stella J.C., Whitham T.G. Climate change perils for dioecious plant species. Nat. Plants. 2016;2:16109. doi: 10.1038/nplants.2016.109. [DOI] [PubMed] [Google Scholar]
- 213.Martine C.T., Cantley J.T., Frawley E.S., Butler A.R., Jordon-Thaden I.E. New functionally dioecious bush tomato from northwestern Australia, Solanum ossicruentum, may utilize “trample burr” dispersal. PhytoKeys. 2016;63:19–29. doi: 10.3897/phytokeys.63.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Johnson M.G., Pokorny L., Dodsworth S., Botigué L.R., Cowan R.S., Devault A., Eiserhardt W.L., Epitawalage N., Forest F., Kim J.T., et al. A Universal Probe Set for Targeted Sequencing of 353 Nuclear Genes from Any Flowering Plant Designed Using k-Medoids Clustering. Syst. Biol. 2019;68:594–606. doi: 10.1093/sysbio/syy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Breinholt J.W., Carey S.B., Tiley G.P., Davis E.C., Endara L., McDaniel S.F., Neves L.G., Sessa E.B., Konrat M., Chantanaorrapint S., et al. A target enrichment probe set for resolving the flagellate land plant tree of life. Appl. Plant Sci. 2021 doi: 10.1002/aps3.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Easterling K.A., Pitra N.J., Jones R.J., Lopes L.G., Aquino J.R., Zhang D., Matthews P.D., Bass H.W. 3D Molecular Cytology of Hop (Humulus lupulus) Meiotic Chromosomes Reveals Non-disomic Pairing and Segregation, Aneuploidy, and Genomic Structural Variation. Front. Plant Sci. 2018;9:1501. doi: 10.3389/fpls.2018.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sousa A., Schubert V., Renner S.S. Centromere organization and UU/V sex chromosome behavior in a liverwort. Plant J. 2020 doi: 10.1111/tpj.15150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.