Abstract
Background—misinformation and mistrust often undermines community vaccine uptake, yet information in rural communities, especially of developing countries, is scarce. This study aimed to identify major challenges associated with coronavirus disease 2019 (COVID-19) vaccine clinical trials among healthcare workers and staff in Uganda. Methods—a rapid exploratory survey was conducted over 5 weeks among 260 respondents (66% male) from healthcare centers across the country using an online questionnaire. Twenty-seven questions assessed knowledge, confidence, and trust scores on COVID-19 vaccine clinical trials from participants in 46 districts in Uganda. Results—we found low levels of knowledge (i.e., confusing COVID-19 with Ebola) with males being more informed than females (OR = 1.5, 95% CI: 0.7–3.0), and mistrust associated with policy decisions to promote herbal treatments in Uganda and the rushed international clinical trials, highlighting challenges for the upcoming Oxford–AstraZeneca vaccinations. Knowledge, confidence and trust scores were higher among the least educated (certificate vs. bachelor degree holders). We also found a high level of skepticism and possible community resistance to DNA recombinant vaccines, such as the Oxford–AstraZeneca vaccine. Preference for herbal treatments (38/260; 14.6%, 95% CI: 10.7–19.3) currently being promoted by the Ugandan government raises major policy concerns. High fear and mistrust for COVID-19 vaccine clinical trials was more common among wealthier participants and more affluent regions of the country. Conclusion—our study found that knowledge, confidence, and trust in COVID-19 vaccines was low among healthcare workers in Uganda, especially those with higher wealth and educational status. There is a need to increase transparency and inclusive participation to address these issues before new trials of COVID-19 vaccines are initiated.
Keywords: COVID-19 clinical trials in resource poor countries, COVID-19, clinical trials in Africa, COVID-19 and medical workers, vaccines, COVAX
1. Introduction
Understanding community knowledge and trust has become increasingly important in the design of effective and ethical clinical trials. From 1991 to 2018, Africa contributed only 2.5% to the global total of clinical trials [1]. From a pharmacovigilance standpoint, the continent offers many potential advantages including genetic diversity and a large number of potential participants who are naïve to drug or vaccine products [1]. However, fear, distrust and suspicion are important barriers to trial participation [2,3]. Several factors contribute to skepticism regarding clinical trials and the products they test. Regulations and ethical guidelines to protect patients, while present in Egypt, South Africa, Uganda, and Ghana, are inadequate in many other African countries [1]. Additional factors causing fear and mistrust include a history of inadequate commitment and/or skill on the part of researchers and their staff, shortages of medical personnel, the failure of researchers to understand local culture, poor infrastructure, an absence of national regulatory requirements, and ineffective ethical counseling, community engagement and informed consent processes [1,2,3]. Inadequate human and/or financial resources contribute to the inability to build awareness regarding individual trials [1].
Misunderstanding also contributes to widespread myths and fears associated with infectious disease clinical trials. However, it is important to note that such fears are often related, in various ways, to a legacy of distrust due to past medical misconduct and unethical experimentation, which in some cases has led to major international lawsuits [4]. Fear of contracting infectious agents such as the Ebola virus from vaccines (EBOVAC) can also be compounded by psychological trauma following receipt of vaccines [2,3,4,5]. The media, advocacy groups, medical journals, and public information services can each shape how the population receives, analyses, and uses medical and health information. These groups, and social media, have contributed, sometimes inadvertently, to the dissemination of myths and misunderstandings without addressing emotional, psychosocial and ethical aspects of trials [2].
To ensure that clinical trials are implemented with the utmost adherence to humane and ethical standards, it is imperative that African research teams increase their human and financial resources, community engagement approaches, training and data collection tools [1,6]. High-quality clinical trials require collaboration with various stakeholders and awareness of the physical, emotional, psychosocial, and ethical needs of potential trial participants and their communities [2]. The emerging consensus is that communities should be inclusively involved in the design, implementation and monitoring and evaluation of such trials to increase trust, acceptability and to negotiate challenges as they arise [7]. Many African countries would benefit from improving their capacity to host clinical trials and investing in research collaborations [8]. A set of common ethical guidelines for the continent as a whole has been suggested as a way to improve both trust and research quality [1].
In April 2020, the World Health Organization (WHO), European Union (EU) and France launched the Coronavirus disease 2019 (COVID-19) Vaccines Global Access (COVAX) as a platform to accelerate development and manufacture of COVID-19 vaccines and regulate fair and equitable access of COVID-19 vaccines globally [9]. In December, Moderna and Pfizer/BioNTech vaccines had received ratification in the United States [10]. These mRNA vaccines appear to offer hope to the international community against COVID-19; however, the growth of skepticism continues to undermine vaccine confidence. In addition, increased reliance of local communities on alternative and complementary medicinal options to control infectious diseases furthermore complicates COVID-19 control [11,12]. For example, Echinacea, Cinchona, Curcuma longa, and Curcuma xanthorrhiza [12] have been recommended; however there is limited information on clinical assessment of their immunogenicity. The objective of the current study was to identify major challenges associated with prospective COVID-19 vaccine clinical trials in Uganda by understanding the perspective of healthcare workers, a group identified as crucial for COVID-19 community management [13]. It was important to assess their knowledge, confidence and trust level on COVID-19 vaccine trials in preparation for the Oxford University–AstraZeneca COVID-19 vaccine program planned to be launched in Uganda in March-April 2021.
2. Materials and Methods
2.1. Study Design
A descriptive cross-sectional study was conducted among healthcare workers in health facilities in Uganda from September 5th to October 7th 2020. During this period, COVID-19 national lockdown restrictions were just being lifted, and media reports emphasized the potential benefits of a COVID-19 vaccine. Data were collected using an online questionnaire to minimize printing and contact, consistent with COVID-19 precautionary measures [14,15]. Individuals working in a health facility (clinicians, nurses, pharmacists, laboratory personnel, and support staff) were targeted by using social media (Figure 1). Those who consented to participate in the study were included. Support staff were defined as persons working at the health facility involved in non-administrative activities at the time of the survey. Persons who declined to consent and those not working in a medical facility were excluded. Sample size was determined using the Krejcie and Morgan Table which allows us to determine the sample size of a given finite population at p ≤ 0.05 [16]. Since the medical professional population in Uganda was estimated to be 81,982 healthcare workers according to the Uganda Annual health sector performance report 2014/2015 [17], the sample size required was estimated at 390 participants, however 260 participants offered consent after responding to the online survey.
Figure 1.
Location of healthcare centers surveyed in the study area by region. In total, participants were based at medical facilities in 46 districts. In particular, 39% of participants came from the central region (101/260), 28% eastern region (72/260), 10% northern region (25/260), and 24% from the western region (62/260). Further individual district demographics indicated more males participated in the study (Supplement File S1).
2.2. Data Collection and Management
A semi-structured questionnaire was developed after a literature search to identify key areas of concern for community confidence in COVID-19 prevention measures. The questionnaire was divided into four sections—(1) sociodemographic characteristics (age, gender, marital status, educational level, occupation, and location of health facility); (2) knowledge about COVID-19 vaccines and vaccine trials; (3) confidence in vaccine trials, COVID-19 vaccines, the local medical community and government COVID-19 policies; and (4) trust-related questions. The questionnaire was reviewed and validated by 5 different experts in local and international universities with expertise on the topic and then uploaded using a Google form (via docs.google.com/forms (accessed on 9 March 2021)) for pretesting before data collection was conducted. Pretesting was then conducted amongst healthcare workers in selected private healthcare centers in Bushenyi and Mbarara districts (n = 30) and these were excluded from the analyzed dataset presented herein. Principal component analysis and further statistical analysis were conducted on the frequencies to check for consistency of responses and a Cranach’s α = 0.8 was considered acceptable.
Questions on knowledge involved binary responses (yes/no) while questions on confidence and trust were ranked using a Likert scale from 0–5, i.e., 0 = very low, 1 = low, 2 = not sure, 3 = moderate, 4 = high, 5 = very high. To reduce guessing responses and bias, the questions on knowledge, confidence and trust were presented randomly to each participant (Supplemental File S2).
The knowledge score was acquired by calculating scoring questions 7–10, 17 and 22. These were then expressed as an average count and converted to percentage and used for analysis. Knowledge questions were on SARS-CoV-2 virology, vaccine development, role of vaccines and research in clinical trials on COVID-19, fear on COVID-19 clinical trials, history of participation in COVID-19 clinical trials (since the government of Uganda is currently conducting preliminary studies), and having received communication on COVID-19 vaccine clinical trials (Supplemental File S1). Our hypothesis was that healthcare workers have a good knowledge on these basic clinical aspects since they have been identified as essential staff and are expected to be vaccinated first ahead of the general population.
The confidence score was acquired by summing the Likert scores on questions 16, 18–21 and 23, 25, 26 for which the average score was then expressed as a proportion and used for analysis. Questions asked ranged from ranking government commitment to develop a COVID-19 vaccine, ability of Ugandans to handle COVID-19 vaccine clinical trials, commitment of workmates to observe COVID-19 vaccine clinical trials and assess capacity of human resource at the health center to handle COVID-19 vaccine clinical trials.
The trust score was acquired by calculating the average score on questions 11–13, 15, and 24 in which the average score was expressed as a proportion. Questions ranged from the level of fear on COVID-19 vaccine clinical trials, level of suspicion, willingness to participate in COVID-19 clinical trials, and willingness to participate on a rushed COVID-19 vaccine clinical trial (Supplement File S2).
2.3. Statistical Analysis
Data were exported into STATGRAPHICS centurion CVI version 16.1.11 (StatPoint Tech., Inc., Warrenton, VA, USA) and descriptive statistics were conducted after conducting a D’Agostino-pearson omnibus normality test [18] (Supplementary File S3). For narrative description, Likert scores from 0–5 were transformed as follows—0 and 1 were coded for low, 2 and 3 for moderate, while 4 and 5 for high and presented as frequencies. Relationship models for knowledge, confidence, and trust using factorial analysis (FA) and standardized principal component (PC) were conducted followed by multivariable correlation analysis to assess the strength of the relationships. The observed trends in the FA were investigated using General linear Model (GLM) to determine the significant influential variables. All analyses were performed at 95% confidence level and p-values less than 0.05 were taken to be significant.
3. Results
3.1. Population Social Demographic Variables in Ugandan Healthcare Centers
A majority of study participants fell into the middle age category, were men, and had received a college education as shown in Table 1. Most were also either laboratory personnel (31%) or support staff (36%), while 13% were clinicians, 11% nurses and 9% pharmacists. Most preferred vaccines were inactivated vaccines (34.2%), however about 14.6% preferred herbal treatments and organics.
Table 1.
Statistic on sociodemographic variables in the study population.
| Parameter | Variable | Frequency (n = 260) | Percent | 95% CI |
|---|---|---|---|---|
| Age (years) | >45 | 23 | 8.8 | 5.8–12.8 |
| 25–45 | 166 | 63.8 | 57.9–69.5 | |
| <25 | 71 | 27.3 | 22.2–33.0 | |
| Gender | Female | 89 | 34.2 | 28.7–40.2 |
| Male | 171 | 65.8 | 59.8–71.4 | |
| Marital status | Married | 118 | 45.4 | 39.4–51.5 |
| Single | 142 | 54.6 | 48.5–60.6 | |
| Education level | Bachelors | 107 | 41.2 | 35.3–47.2 |
| Certificate | 26 | 10.0 | 6.8–14.1 | |
| Diploma | 47 | 18.1 | 19.8–23.1 | |
| None | 8 | 3.1 | 1.3–6.0 | |
| Postgraduate | 72 | 27.7 | 22.5–33.4 | |
| Occupation | Clinician | 34 | 13.1 | 9.4–17.8 |
| Laboratory personnel | 80 | 30.8 | 25.4–36.6 | |
| Nurse | 29 | 11.2 | 7.7–15.4 | |
| Pharmacist | 23 | 8.8 | 5.8–12.8 | |
| Support staff | 94 | 36.2 | 30.5–42.1 | |
| Location | Central | 101 | 38.8 | 33.1–44.9 |
| Eastern | 72 | 27.7 | 22.5–33.4 | |
| Northern | 25 | 9.6 | 6.5–13.7 | |
| Western | 62 | 23.8 | 19.0–29.3 | |
| Preferred COVID-19 vaccine type | DNA Recombinant vaccines | 41 | 15.8 | 11.7–20.6 |
| Herbal treatments | 38 | 14.6 | 10.7–19.3 | |
| Inactivated vaccines | 89 | 34.2 | 28.7–40.2 | |
| Live attenuated vaccines | 35 | 13.5 | 9.7–18.0 | |
| No vaccine | 57 | 21.9 | 17.2–27.3 | |
| Age (years) | Minimum | 18 | ||
| Maximum | 65 | |||
| Mean ± SEM | 31.8 ± 0.5 | |||
3.2. Influence of Sociodemographic Characteristics on the Knowledge Score, Confidence, and Trust for COVID-19 Vaccine Clinical Trials among the Healthcare Workers
Knowledge, confidence, and trust scores for COVID-19 vaccine clinical trials were generally low for all categories. Significant differences in knowledge, trust, and confidence were identified. Of interest, trust scores decreased with increasing education (p = 0.001), and confidence and trust levels were higher amongst occupational groups that required less education (Table 2). Confidence and trust levels varied by region, with the highest scores in facilities from eastern Uganda. Participants who preferred the herbal vaccine expressed a relatively higher knowledge on COVID-19 vaccine clinical trials as compared to those who are in favor of a live attenuated vaccine. Trust was also found to be highest in herbal treatments than all the other vaccine types presented in this study (p = 0.018).
Table 2.
Sociodemographic variables associations with knowledge, confidence and trust on COVID-19 vaccine clinical trials in Uganda.
| Parameter | Variable | N | Percentage Knowledge Score | Confidence Score | Trust Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | ANOVA F (p) Value | Median (Min-Max) |
Mean ± SEM | ANOVA F(p) Value | Median (Min-Max) |
Mean ± SEM | ANOVA F(p) Value | Median (Min-Max) |
|||
| Age | >45 | 23 | 44.2 ± 3.4 | 0.603 (0.548) |
50 (16.7–66.7) | 2.5 ± 0.2 | 0.2 (0.814) |
2.4 (0.9–4.3) | 2.4 ± 0.2 | 1.4 (0.246) |
2.6 (0.4–3.8) |
| 25–45 | 166 | 41.4 ± 1.2 | 33.3 (0.0–83.3) | 2.5 ± 0.1 | 2.5 (0.3–4.6) | 2.2 ± 0.1 | 2.0 (0.0–5.0) | ||||
| <25 | 71 | 43.4 ± 2.1 | 33.3 (0.0–83.3) | 2.6 ± 0.1 | 2.7 (0.8–4.4) | 2.4 ± 0.1 | 2.2 (0.4–4.8) | ||||
| Gender | Female | 89 | 39.3 ± 1.5 | 4.3 (0.039) |
33.3 (0.0–66.7) | 2.6 ± 0.1 | 2.1 (0.147) | 2.5 (0.9–4.6) | 2.3 ± 0.1 | 0.1 (0.747) | 2.2 (0.4–5.0) |
| Male | 171 | 43.7 ± 1.3 | 50.0 (0.0–83.3) | 2.5 ± 0.1 | 2.5 (0.3–4.4) | 2.3 ± 0.1 | 2.0 (0.0–4.8) | ||||
| Marital status | Married | 118 | 42.7 ± 1.5 | 0.2 (0.666) | 33.3 (16.7–83.3) | 2.6 ± 0.1 | 0.7 (0.413) | 2.5 (0.3–4.4) | 2.4 ± 0.1 | 4.1 (0.045) | 2.4 (0.4–5.0) |
| Single | 142 | 41.8 ± 1.4 | 33.3 (0.0–83.3) | 2.5 ± 0.1 | 2.5 (0.8–4.6) | 2.2 ± 0.1 | 2.0 (0.0–4.8) | ||||
| Education level | Bachelors | 107 | 41.0 ± 1.4 | 2.3 (0.63) |
33.3 (0.0–83.3) | 2.5 ± 0.1 | 1.4 (0.239) |
2.5 (0.6–4.1) | 2.2 ± 0.1 | 6.6 (0.001) |
2.0 (0.4–4.2) |
| Certificate | 26 | 46.2 ± 3.2 | 50.0 (16.7–66.7) | 2.8 ± 0.2 | 2.8 (1.0–4.6) | 2.9 ± 0.3 | 3.0 (0.4–5.0) | ||||
| Diploma | 47 | 46.1 ± 2.6 | 50.0 (16.7–83.3) | 2.5 ± 0.1 | 2.5 (1.0–4.4) | 2.4 ± 0.1 | 2.0 (0.6–4.8) | ||||
| None | 8 | 31.3 ± 6.6 | 33.3 (0.0–50) | 3.1 ± 0.4 | 3.1 (1.6–3.9) | 3.1 ± 0.4 | 3.0 (2.0–4.8) | ||||
| Postgraduate | 72 | 41.2 ± 1.9 | 33.3 (16.7–83.3) | 2.5 ± 0.1 | 2.5 (0.3–4.6) | 2.0 ± 0.1 | 2.0 (0.0–3.4) | ||||
| Occupation | Clinician | 34 | 39.7 ± 2.4 | 0.4 (0.825) |
33.3 (16.7–83.3) | 2.1 ± 0.1 | 3.5 (0.009) |
2.2 (0.3–3.6) | 2.1 ± 0.1 | 6.6 (0.001) |
2.0 (0.6–3.6) |
| Laboratory personnel | 80 | 42.5 ± 1.8 | 50.0 (0.0–83.3) | 2.5 ± 0.1 | 2.5 (0.8–4.3) | 1.9 ± 0.1 | 2.0 (0.0–4.4) | ||||
| Nurse | 29 | 42.5 ± 3.3 | 50.0 (16.7–83.3) | 2.5 ± 0.1 | 2.5 (1.0–4.1) | 2.5 ± 0.1 | 2.5 (1.2–5.0) | ||||
| Pharmacist | 23 | 44.9 ± 3.2 | 50.0 (16.7–83.3) | 2.4 ± 0.2 | 2.3 (0.9–4.6) | 2.2 ± 0.2 | 2.0 (1.0–4.0) | ||||
| Support staff | 94 | 42.0 ± 1.8 | 33.3 (0.0–83.3 | 2.7 ± 0.1 | 2.7 (0.6–4.6) | 2.6 ± 0.1 | 2.4 (0.4–4.8) | ||||
| Location | Central | 101 | 42.4 ± 1.5 | 1.8 (0.144) |
50 (16.7–83.3) | 2.5 ± 0.1 | 6.4 (0.001) |
2.5 (0.9–4.6) | 2.1 ± 0.1 | 10.8 (0.001) |
2.0 (0.4–4.4) |
| Eastern | 72 | 44.9 ± 1.9 | 50 (0.0–83.3) | 2.8 ± 0.1 | 2.9 (0.8–4.4) | 2.8 ± 0.1 | 2.8 (0.6–5.0) | ||||
| Northern | 25 | 36.7 ± 3.2 | 33.3 (16.7–83.3) | 2.2 ± 0.2 | 2.1 (0.9–3.4) | 2.4 ± 0.2 | 2.2 (1.2–3.8) | ||||
| Western | 62 | 40.9 ± 2.3 | 33.3 (0.0–83.3) | 2.3 ± 0.1 | 2.4 (0.3–4.1) | 2.0 ± 0.1 | 2.0 (0.0–3.8) | ||||
| Preferred COVID-19 vaccine | DRV | 41 | 42.3 ± 2.9 | 1.2 (0.319) |
33.3 (16.7–83.3) | 2.5 ± 0.1 | 1.0 (0.412) |
2.5 (0.6–4.1) | 2.2 ± 0.1 | 3.1 (0.018) |
2.0 (0.8–4.0) |
| HV | 38 | 46.9 ± 2.8 | 50 (16.6–83.3) | 2.7 ± 0.1 | 2.6 (1.3–4.3) | 2.5 ± 0.2 | 2.6 (0.8–4.6) | ||||
| IV | 89 | 41.0 ± 1.5 | 33.3 (0.0–83.3) | 2.5 ± 0.1 | 2.5 (0.8–4.6) | 2.1 ± 0.1 | 2.0 (0.0–4.2) | ||||
| LAV | 35 | 39.5 ± 2.5 | 33.3 (16.7–83.3) | 2.5 ± 0.1 | 2.6 (0.9–4.3) | 2.3 ± 0.2 | 2.2 (0.4–4.4) | ||||
| None | 57 | 42.2 ± 1.0 | 33.3 (0.0–83.3) | 2.4 ± 0.1 | 2.4 (0.3–4.6) | 2.6 ± 0.2 | 2.4 (0.6–5.0) | ||||
KEY: DRV = DNA Recombinant vaccines, HV = Herbal treatments, IV = Inactivated vaccines, LAV = Live attenuated vaccines. N = number of participants, SEM = Standard error mean, Min-Max = Minimum-Maximum values, ANOVA = Analysis of variance, P-probability value.
3.3. Descriptive Narrative on Knowledge, Trust and Confidence among Study Participants
A majority of participants (72.7%, 189/260) falsely stated that Ebola belongs to the coronavirus classification. In addition, a majority of the study participants were unaware of companies involved in COVID-19 vaccine development (65.4%, 170/260), did not think COVID-19 vaccines are necessary to stop the pandemic (87.3%, 227/260), expressed fear towards COVID-19 vaccines (70.8%, 184/260), had no experience on clinical trials (87.7%, 228/260), and had not received any information on the planned COVID-19 vaccine activity in Uganda (79.2%, 206/260). A logistic regression was performed to ascertain the effects of knowledge questions on the likelihood that a participant was a female. The logistic regression model was not statistically significant, χ2(7) = 9.622, p = 0.211. The model explained 3.2% (Nagelkerke, R2) of the variance in the gender and correctly classified 65.8% of cases. Males were more knowledgeable on coronavirus classification (OR = 1.5, 95% CI: 0.7–3.0) and companies involved in COVID-19 vaccine development (OR = 1.8, 95% CI: 1.0–3.2) as compared to their female counterparts (Table 3).
Table 3.
Descriptive narrative on knowledge, trust and confidence among study participants.
| Variables | Variable | Proportions by Gender | p Value | OR (95% CI) |
||
|---|---|---|---|---|---|---|
| Females | Males | Total | ||||
| Beta coronaviruses include the following except | Ebola | 67 (25.8) | 122 (46.9) | 189 (72.7) | 1 | |
| MERS | 9 (3.5) | 17 (6.5) | 26 (10.0) | 0.684 | 1.2 (0.5–2.9) | |
| SARS | 13 (5.0) | 32 (12.3) | 45 (17.3) | 0.316 | 1.5 (0.7–3.0) | |
| Do you know any company involved in COVID-19 vaccine development? | No | 65 (25.0) | 105 (40.4) | 170 (65.4) | 0.047 | 1 |
| Yes | 24 (9.2) | 66 (25.4) | 90 (34.6) | 1.8 (1.0–3.2) | ||
| Do you think breaking the COVID-19 circle involves vaccine development? | No | 12 (4.6) | 21 (8.1) | 33 (12.7) | 0.685 | 1 |
| Yes | 77 (29.6) | 150 (57.7) | 227 (87.3) | 1.2 (0.5–2.6) | ||
| Do you have fear about the COVID-19 vaccine? | No | 28 (10.8) | 48 (18.5) | 76 (29.2) | 0.310 | 1 |
| Yes | 61 (23.5) | 123 (47.3) | 184 (70.8) | 1.3 (0.8–2.4) | ||
| Have you ever participated in any clinical trial previously? | No | 79 (30.4) | 149 (57.3) | 228 (87.7) | 0.949 | 1 |
| Yes | 10 (3.8) | 22 (8.5) | 32 (12.3) | 1.0 (0.5–2.3) | ||
| I have received adequate communication on the COVID-19 vaccine trials in Uganda | No | 73 (28.1) | 133 (51.2) | 206 (79.2) | 0.569 | 1 |
| Yes | 16 (6.2) | 38 (14.6) | 54 (20.8) | 1.2 (0.6–2.4) | ||
| Variables | Frequencies on participants responses on COVID-19 | |||||
| High | Low | Moderate | ||||
| Confidence on COVID-19 vaccinations | ||||||
| I have been enlightened on WHO guidelines and stages for vaccine trials | 47 (18.1) | 120 (46.2) | 93 (35.8) | |||
| Rank the Ugandan government’s commitment to the development of a genuine COVID-19 vaccine and therapy? | 54 (20.8) | 101 (38.8) | 105 (40.4) | |||
| Confidence in the skills of Ugandans and their ability to handle the COVID-19 clinical trial? | 98 (37.7) | 44 (16.9) | 118 (45.4) | |||
| My workmates’ committment to COVID-19 control guidelines | 140 (53.8) | 18 (6.9) | 102 (39.2) | |||
| There are sufficient designated medical personnel handling COVID-19 cases at my workplace? | 93 (35.8) | 57 (21.9) | 110 (42.3) | |||
| My information about the planned COVID-19 vaccinations in Uganda | 41 (15.8) | 136 (52.3) | 83 (31.9) | |||
| Level of challenge posed by access to funding in vaccine development for Ugandan COVID-19 vaccine clinical trials | 145 (55.8) | 21 (8.1) | 94 (36.2) | |||
| My level of confidence in herbal COVID-19 treatments being promoted in Uganda | 51 (19.6) | 140 (53.8) | 69 (26.5) | |||
| Trust on COVID-19 vaccinations | ||||||
| Level of fear | 104 (40.0) | 69 (26.5) | 87 (33.5) | |||
| Level of suspicion | 118 (45.) | 58 (22.3) | 84 (32.3) | |||
| Willingness to participate in COVID-19 clinical trials | 60 (23.1) | 123 (47.3) | 77 (29.6) | |||
| Willingness to participate in a COVID-19 clinical trial | 41 (15.8) | 174 (66.9) | 45 (17.3) | |||
| Level of trust for the Ugandan national regulatory guidelines for clinical trials | 57 (21.9) | 86 (33.1) | 117 (45.0) | |||
KEY: OR = Odds ratios, 95% CI = confidence intervals.
A majority of study participants who expressed confidence on the COVID-19 clinical trials reported a high workmate commitment to implement COVID-19 control guidelines (140/260, 53.8%), regarded funding as a great challenge to Uganda’s personal investment in COVID-19 vaccines and therapies (145/260, 55.8%), and had a low level of confidence on Ugandan herbal COVID-19 vaccinations (140/260, 53.8%). In addition, a majority of participants (174/260, 66.9%) who expressed distrust for COVID-19 vaccinations for Uganda identified the rushed COVID-19 clinical trials being a major concern (Table 3).
3.4. Multivariate Analysis on COVID-19 Clinical Trials amongst Ugandans
From the GLM analysis in Table 4, the sociodemographic factors significantly explained the changes in the confidence (F = 2.74, p = 0.001) and trust (F = 5.30, p < 0.001), but not knowledge (F = 1.47, p = 0.117), with a variability accuracy of 2.65% for knowledge, 9.17% for confidence and 19.92% for trust.
Table 4.
Variable influence of the knowledge score, confidence, and trust.
| Source | SS | Df | MS | F-Ratio | p-Value | R-sq | R-sq (adj) |
|---|---|---|---|---|---|---|---|
| Knowledge | |||||||
| Model | 5589.81 | 15 | 372.654 | 1.47 | 0.117 | 8.29 | 2.65 |
| Residual | 61845.2 | 244 | 253.464 | ||||
| Total (Corr.) | 67435 | 259 | |||||
| Confidence | |||||||
| Model | 24.8141 | 15 | 1.654 | 2.74 | 0.001 | 14.43 | 9.17 |
| Residual | 147.151 | 244 | 0.603 | ||||
| Total (Corr.) | 171.965 | 259 | |||||
| Trust | |||||||
| Model | 59.5571 | 15 | 3.970 | 5.30 | <0.001 | 24.56 | 19.92 |
| Residual | 182.937 | 244 | 0.750 | ||||
| Total (Corr.) | 242.494 | 259 |
Note: Corr., Corrected; SS, Sum of Squares; MS, Mean Square; DF, Degree of freedom; R-sq., Correlation squared (accuracy); adj., adjusted.
Regression analysis (Table 5) showed that gender was the only significant influential variable (F = 8.49, p = 0.0039) for knowledge, while occupation (F = 3.02, p = 0.019) and region (F = 6.05, p = 0.001) were the significant influential variables for confidence. All sociodemographic variables except age group and gender (p > 0.05) were significant contributors to the variation in trust (marital status: F = 5.49, p = 0.02; education; F = 3.42; p = 0.01; occupation: F = 3.79; p = 0.005; region: F = 6.58; p < 0.001).
Table 5.
Regression model outcome summary and significance of predictor variables.
| Source | SS | Df | MS | F-Ratio | p-Value | Variance |
|---|---|---|---|---|---|---|
| Knowledge | ||||||
| Age group | 313.274 | 2 | 156.637 | 0.62 | 0.5399 | −2.105 |
| Gender | 2152.75 | 1 | 2152.750 | 8.49 | 0.0039 | 21.888 |
| Marital Status | 68.2873 | 1 | 68.287 | 0.27 | 0.6042 | −2.205 |
| Education | 1336.58 | 4 | 334.144 | 1.32 | 0.2637 | 1.948 |
| Occupation | 626.993 | 4 | 156.748 | 0.62 | 0.6498 | −2.392 |
| Region | 1054.89 | 3 | 351.630 | 1.39 | 0.2472 | 1.857 |
| Residual | 61845.2 | 244 | 253.464 | 253.464 | ||
| Total (corrected) | 67435 | 259 | ||||
| Confidence | ||||||
| Age group | 0.43659 | 2 | 0.218 | 0.36 | 0.697 | −0.008 |
| Gender | 0.39342 | 1 | 0.393 | 0.65 | 0.420 | −0.002 |
| Marital Status | 1.67448 | 1 | 1.674 | 2.78 | 0.097 | 0.013 |
| Education | 2.91125 | 4 | 0.728 | 1.21 | 0.309 | 0.003 |
| Occupation | 7.28699 | 4 | 1.822 | 3.02 | 0.019 | 0.030 |
| Region | 10.944 | 3 | 3.648 | 6.05 | 0.001 | 0.058 |
| Residual | 147.151 | 244 | 0.603 | 0.058 | ||
| Total (corrected) | 171.965 | 259 | ||||
| Trust | ||||||
| Age group | 2.44901 | 2 | 1.225 | 1.63 | 0.197 | 0.010 |
| Gender | 2.64235 | 1 | 2.642 | 3.52 | 0.062 | 0.022 |
| Marital Status | 4.11783 | 1 | 4.118 | 5.49 | 0.020 | 0.040 |
| Education | 10.252 | 4 | 2.563 | 3.42 | 0.010 | 0.044 |
| Occupation | 11.3622 | 4 | 2.841 | 3.79 | 0.005 | 0.052 |
| Region | 14.7894 | 3 | 4.930 | 6.58 | 0.000 | 0.079 |
| Residual | 182.937 | 244 | 0.750 | 0.750 | ||
| Total (corrected) | 242.494 | 259 |
Note: Corr., Corrected; SS, Sum of Squares; MS, Mean Square; DF, Degree of freedom; R-sq., Correlation squared (accuracy); adj., adjusted.
3.5. Relationship between Knowledge, Confidence, and Trust
Eigen analysis of the factor matrix for factorial analysis (FA) produced considerable variations at F2 explaining 84.4% of the cumulative variance in the component, but, with an eigenvalue ≥ 1.0. This left F1 (Eigen value = 1.68) with a cumulative variance of 56.06% as the model component that met the criteria (Table 6).
Table 6.
Descriptive characteristics and factor loading matrix of variables in factorial analysis (FA).
| Variables | Average ± SD | Factor 1 α | Factor 2 α | Estimated Communality | Specific Variance |
|---|---|---|---|---|---|
| Knowledge | 42.18 ± 16.14 | 0.557 | −0.828 | 0.636 | 0.364 |
| Confidence | 2.52 ± 0.81 | 0.815 | 0.341 | 0.674 | 0.326 |
| Trust | 2.29 ± 0.97 | 0.841 | 0.218 | 0.547 | 0.453 |
Note: F1 [Eigen value, 1.68169; cumulative %, 56.056], F2 [Eigen value, 0.849384; cumulative %, 84.369], α [factor loading matrix before rotation].
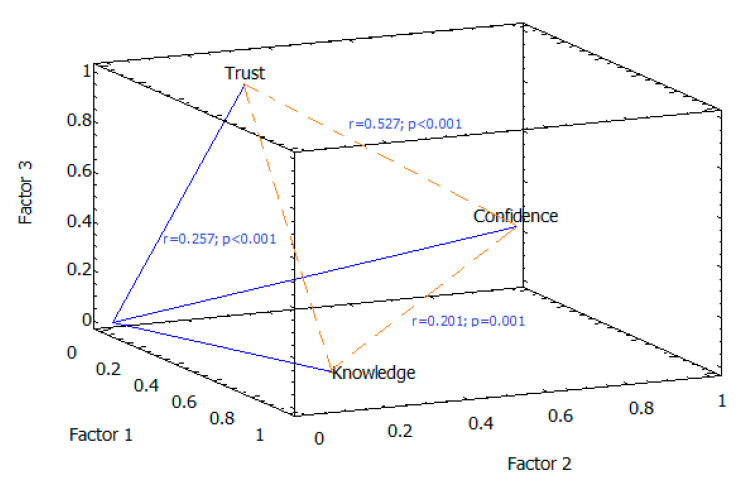
All three variables—knowledge (55.7%), confidence (81.5%), and trust (84.1%)—were responsible for the variability in factor (component) 1 on a positive multidirectional scale (Table 6), with a closer relationship between confidence and trust (r = 0.527; p < 0.001) than knowledge and confidence (r = 0.201; p = 0.001) or trust (r = 0.257; p < 0.001) (Figure 2).
Figure 2.
Factor loading components and correlation of variables. The strongest correlation was observed between confidence and trust with factor 2.
4. Discussion
We identified a low level of knowledge, confidence and trust of COVID-19 vaccine clinical trials amongst healthcare workers in Uganda. In particular, there were no differences in the knowledge, trust and confidence scores with age. These observations highlight mistrust in the community with regard to COVID-19 vaccine clinical trials in Uganda. These findings are in agreement with previous studies in Africa [1,2]. These circumstances signal possible problems for upcoming COVID-19 vaccinations in Uganda.
The majority of health workers in Uganda believe that the human resources designated to handle COVID-19 cases are inadequate; the health worker evaluation may contribute to antivaccine sentiments, in agreement with previous reports [1]. A previous nationwide study in Uganda showed that healthcare workers are six times more knowledgeable about COVID-19 than teachers (non-medical staff) [19], however a failure to replicate this self-reported knowledge on COVID-19 vaccine clinical trials raises major policy challenges. Our study also identified males as having a significantly higher knowledge score than females, thus identifying gender inequalities that parallel the disproportionate distribution of males and females in the healthcare profession. More educated people were reported to be more reluctant to accept vaccines [20] and this was in agreement with our study.
This is the first study from East Africa demonstrating widespread knowledge and trust gaps related to COVID-19 vaccines amongst healthcare workers. To increase vaccine uptake, community education to improve knowledge on COVID-19 vaccines has been promoted [20,21]. We found that the limited experience in clinical trials among Ugandans also contributes to reluctance and misinformation. This is important since Africa has generally limited experience with major vaccine clinical trials [22]. The Ugandan government’s decision to invest in parallel COVID-19 herbal treatments and therapeutic research through the Ministry of Health (to pursue vaccine research) and Busitema University (to pursue COVID therapies) continues to create further confusion [23,24]. The study also identified mistrust amongst Ugandans towards the herbal treatments, probably arising from the failed Madagascar COVID-Organics cocktail [25,26].
Discrepancies identified in a developing country like Uganda raise major challenges to vaccine distribution goals set by the WHO [9]. Equity education would help promote knowledge among healthcare workers since occupational status has a significant impact on knowledge level, i.e., bachelor’s degree holders are theoretically more knowledgeable than certificate holders [27]. This would also help build confidence in female patients since women talk more freely with other women, leading to more open discussions and helping improve vaccine uptake. The low productivity common in most healthcare centers of Uganda as a result of mistrust [28] only continues to precipitate the low confidence and trust on the planned COVID-19 vaccine clinical trials. This situation would be harmful and unproductive for Ugandans once COVID-19 vaccine uptake is low, since this would undermine the herd immunity offered through vaccination strategies.
Our study has a few limitations. This includes the gender disparities which were consistent with general conditions in the area, including access to education. Women in Uganda are more likely to be employed in nursing and other lower paying positions, leading to under-representation of females in managerial positions [29]. This online questionnaire required a smartphone and internet connectivity, which presented an economic and educational barrier to participation. Globally, there are more females in the healthcare profession than men [30], which suggests an alternate modality should be investigated for future surveys.
We found that the least educated, i.e., illiterate and certificate holders, had a higher confidence and trust level in the COVID-19 vaccine clinical trials than those who had a higher level of education. Findings in the study are in agreement with those from the Democratic Republic of Congo (DRC) in which doctors had a low (27.7%) acceptability for COVID-19 vaccines [31]. In France, healthcare workers were associated with increased vaccine acceptance [32], contrary to findings from Uganda and the DRC (resource-limited countries). The study identified knowledge as a barrier, if not well-nuanced and properly explained in the higher educated people—higher educated people have also more access to internet and hence to the misinformation as well as the real information. Of course, higher education is also associated with the need for greater demand for information about risks and benefits before consent to participate in a trial would be given [33]. This finding may indicate that insufficient information about COVID-19 clinical trials have been given to healthcare workers, and that health professionals do not feel consulted or adequately engaged in trial design and plans. These findings demonstrate challenges for the planned COVID-19 vaccinations in Uganda since medical staff are frontline workers in the global fight against the pandemic [13]. Support staff and nurses were more confident in the COVID-19 vaccine clinical trials than their senior counterparts. The skepticism identified amongst the educated and most professional healthcare workers re-emphasizes the need to increase transparency to encourage scientific and community scrutiny on COVID-19 vaccines [34].
Vaccine confidence was lowest in the central and western regions of Uganda and this was important since these are the highly developed regions of Uganda. Our research shows a lack of confidence by the relatively rich and more educated. In addition, the Ugandan herbal treatment is currently marred in a lot of secrecy, however, it is anticipated to be administered orally among COVID-19 patients in Uganda [23,24]. In this study, the largest proportion of Ugandans expressed skepticism against the Live Attenuated Vaccines (LAVs), and DNA Recombinant vaccines (DRVs). The Oxford–AstraZeneca vaccine is a viral vector, i.e., developed from an adenovirus to mimic SARS-CoV-2, thus making it a genetically modified organism and an example of a DRV [35]. Low confidence and trust levels against DRVs identified in this study would raise challenges once Uganda begins to use the Oxford–AstraZeneca vaccines as planned [36]. In addition, the Pfizer/BioNTech vaccines are messenger RNA vaccines—a new class of vaccines [37]—demonstrating a need for more studies in Uganda to promote already trusted and reliable vaccines. Since developing countries lack the capacity to develop vaccines, money spent on the herbal treatments and therapies may be better spent if invested into improved training and funding for basic institutional research to increase transparency and public confidence in scientific reports [1].
This study identifies major challenges to vaccine uptake in Uganda as well as regional differences in opinions. The high fear and mistrust against COVID-19 vaccines identified in this pilot survey were in agreement with previous reports from Liberia and Sierra Leone on EBOVAC studies [2,3]. The skepticism towards COVID-19 vaccines appears to be associated with the fact that vaccine manufacturers and scientists have been predominately from Europe and North America, raising suspicions of neocolonialism through medical research. This shows the need for well-structured clinical trials and drug development to be conducted in resource-poor countries as a strategy to address vaccine hesitancy.
5. Conclusions
Acceptance of COVID-19 vaccines in low-resource countries will probably be stymied by the fact that clinical trials have been conducted outside Africa. To address low trust in COVID-19, but also future pandemic vaccine clinical trials, it is important to situate clinical trials in Africa, led by respected African research institutes, with clear and transparent community engagement, legal and ethical protocols. Future studies should explore community perceptions of mRNA vaccines, since these are the leading vaccine candidates being deployed to control the COVID-19 pandemic despite their transportation logistical cold chain requirement challenge for African countries. Studies should also explore the scientific networks that have emerged around COVID-19 clinical trials and the influence of African researchers, including increasing trust and confidence in vaccines by healthcare workers. Enrolment in the study was stopped after failing to acquire more responses thus a further study involving more study participants would provide more robust data.
Acknowledgments
The work was supported by Taif University Researchers Supporting Program (project number: TURSP-2020/128), Taif University, Saudi Arabia.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-393X/9/3/253/s1, Supplementary File S1: Descriptive analysis showing location of study participants, Supplementary File S2: Identifying potential challenges in COVID-19 clinical trials in Uganda questionnaire. Supplementary File S3: Column statistics for normality testing on knowledge, confidence and trust scores.
Author Contributions
K.I.K., S.C.W. conceptualization, K.I.K. designed the study. G.E.-S.B., N.E.A.O., E.A., H.I.N., I.M.U., H.N., D.C.M., C.D.K., K.K., E.T.A., L.L., K.M., D.O., T.P., D.P.N., R.S., N.O.R., G.H.M., J.J.O., F.S., P.K., G.T. collected the data. E.A. and K.I.K. conducted statistical analysis, A.L., L.M.D., E.T.M., F.P.C., B.E.B., S.C.N.M., K.B., K.J.A., S.C.W. conducted statistical interpretation. K.I.K. drafted the manuscript, A.L., L.O.O., G.E.-S.B., N.E.A.O., E.A., H.I.N., I.M.U., L.M.D., E.T.M., H.N., F.P.C., B.E.B., D.C.M., C.D.K., K.K., E.T.A., L.L., K.M., S.C.N.M., D.O., T.P., D.P.N., R.S., N.O.R., G.H.M., K.B., J.J.O., F.S., P.K., G.T., K.J.A., S.C.W. critically revised it for important intellectual content, approved final version for publication and remain accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Zhejiang University Education Foundation Emergency Research Fund (SCW, KB and KIK); Global Challenges Research Fund and the University of Edinburgh.
Institutional Review Board Statement
This was acquired from Kampala International University Ethics Review Board and registered under with number Nr.UG-REC-023/201914. Consent to participate was acquired through online acceptance to participate in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are openly available in figshare at https://figshare.com/s/9f89827c45921ec5196a (accessed on 9 March 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Conradie A., Duys R., Forget P., Biccard B. Barriers to clinical research in Africa: A quantitative and qualitative survey of clinical researchers in 27 African countries. Br. J. Anaesth. 2018;121:813–821. doi: 10.1016/j.bja.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy S.B., Neaton J.D., Lane H.C., Kieh M.W.S., Massaquoi M.B.F., Touchette N.A., Nason M.C., Follmann D.A., Boley F.K., Johnson M.P., et al. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: Design, procedures, and challenges. Clin. Trials. 2016;13:49–56. doi: 10.1177/1740774515621037. [DOI] [PubMed] [Google Scholar]
- 3.Enria L., Lees S., Smout E., Mooney T., Tengbeh A.F., Leigh B., Greenwood B., Watson-Jones D., Larson H. Power, fairness and trust: Understanding and engaging with vaccine trial participants and communities in the setting up the EBOVAC-Salone vaccine trial in Sierra Leone. BMC Public Health. 2016;16:1–10. doi: 10.1186/s12889-016-3799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezeome E.R., Simon C. Ethical problems in conducting research in acute epidemics: The pfizer meningitis study in nigeria as an illustration. Dev. World Bioeth. 2010;10:1–10. doi: 10.1111/j.1471-8847.2008.00239.x. [DOI] [PubMed] [Google Scholar]
- 5.Erku D.A., Belachew S.A., Abrha S., Sinnollareddy M., Thomas J., Steadman K.J., Tesfaye W.H. When fear and misinformation go viral: Pharmacists’ role in deterring medication misinformation during the ’infodemic’ surrounding COVID-19. Res. Soc. Adm. Pharm. 2021;17:1954–1963. doi: 10.1016/j.sapharm.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folayan M.O., Allman D., Haire B., Yakubu A., Afolabi M.O., Cooper J. Considerations for community engage-ment when conducting clinical trials during infectious disease emergencies in West Africa. Dev. World Bioeth. 2019;19:96–105. doi: 10.1111/dewb.12215. [DOI] [PubMed] [Google Scholar]
- 7.Pratt B., de Vries J. Community engagement in global health research that advances health equity. Bioethics. 2018;32:454–463. doi: 10.1111/bioe.12465. [DOI] [PubMed] [Google Scholar]
- 8.Singh J.A. The Case for Why Africa Should Host COVID-19 Candidate Vaccine Trials. J. Infect. Dis. 2020;222:351–355. doi: 10.1093/infdis/jiaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seth B. COVAX Explained. [(accessed on 20 January 2021)];2020 Available online: https://www.gavi.org/vaccineswork/covax-explained.
- 10.Lederer K., Castaño D., Atria D.G., Oguin T.H., Wang S., Manzoni T.B., Muramatsu H., Hogan M.J., Amanat F., Cherubin P., et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity. 2020;53:1281–1295.e5. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathpati M.M., Payyappallimana U., Shankar D., Porter J.D. Population self-reliance in health’ and COVID-19: The need for a 4th tier in the health system. J. Ayurveda Integr. Med. 2020;8:3. doi: 10.1016/j.jaim.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19: A Review of Their Mechanisms, Pros and Cons. Evid.-Based Complement. Altern. Med. 2020;2020:1–12. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasozi K.I., Mujinya R., Bogere P., Ekou J., Zirintunda G., Ahimbisibwe S., Matama K., Ninsiima H.I., Echoru I., Ayikobua E.T., et al. Pandemic panic and anxiety in developing countries. Embracing One Health offers practical strategies in management of COVID-19 for Africa. Pan Afr. Med. J. 2020;35:3. doi: 10.11604/pamj.supp.2020.35.2.22637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasozi K.I., MacLeod E., Ssempijja F., Mahero M.W., Matama K., Musoke G.H., Bardosh K., Ssebuufu R., Wakoko-Studstil F., Echoru I., et al. Misconceptions on COVID-19 Risk Among Ugandan Men: Results From a Rapid Exploratory Survey, April 2020. Front. Public Health. 2020;8:416. doi: 10.3389/fpubh.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usman I.M., Ssempijja F., Ssebuufu R., Lemuel A.M., Archibong V.B., Ayikobua E.T., Aruwa J.O., Kembabazi S., Kegoye E.S., Ayuba J.T., et al. Community Drivers Affecting Adherence to WHO Guidelines Against COVID-19 Amongst Rural Ugandan Market Vendors. Front. Public Health. 2020;8:340. doi: 10.3389/fpubh.2020.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krejcie R.V., Morgan D.W. Determining Sample Size for Research Activities. Educ. Psychol. Meas. 1970;30:607–610. doi: 10.1177/001316447003000308. [DOI] [Google Scholar]
- 17.Omaswa F., Kadama P., Eriki P., Odedo R., Gidudu H.E. Brain to Brain Gain: Health Workforce Migration: A Case Study of General Practitioners in Uganda, WHO. [(accessed on 16 February 2021)];2017 Available online: https://www.who.int/workforcealliance/brain-drain-brain-gain/17-340Uganda-case-study2017-10-18.pdf.
- 18.D’Agostino R.B., Belanger A. A Suggestion for Using Powerful and Informative Tests of Normality. Am. Stat. 1990;44:316. doi: 10.2307/2684359. [DOI] [Google Scholar]
- 19.Ssebuufu R., Sikakulya F.K., Mambo S.B., Wasingya L., Nganza S.K., Ibrahim B., Kyamanywa P. Knowledge, Attitude, and Self-Reported Practice Toward Measures for Prevention of the Spread of COVID-19 Among Ugandans: A Nationwide Online Cross-Sectional Survey. Front. Public Health. 2020;8:618731. doi: 10.3389/fpubh.2020.618731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French J., Deshpande S., Evans W., Obregon R. Key Guidelines in Developing a Pre-Emptive COVID-19 Vaccination Uptake Promotion Strategy. Int. J. Environ. Res. Public Health. 2020;17:5893. doi: 10.3390/ijerph17165893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Vaccine Acceptance is the Next Hurdle. [(accessed on 20 January 2021)];2020 Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-acceptance-is-the-next-hurdle.
- 22.El-Sadr W. The Challenge of Minority Recruitment in Clinical Trials for AIDS. JAMA J. Am. Med. Assoc. 1992;267:954. doi: 10.1001/jama.1992.03480070070033. [DOI] [PubMed] [Google Scholar]
- 23.Natural Chemotherapeutics Research Institute Clinical Evaluation of Efficacy, Safety Immunogenicity and Reliability of UBV-01N- a Noval Natural Product- in Adult Patients Infected with SARS-CoV-2 (COVID-19), in Uganda. Published by the Ministry of Health Uganda. [(accessed on 31 December 2020)];2020 Available online: https://ncri.go.ug/covid-19/
- 24.Atukunda N. Uganda Turns to Herbs in Search for COVID-19 Cure, Daily Monitor. [(accessed on 31 December 2020)];2020 Available online: https://www.monitor.co.ug/uganda/news/national/uganda-turns-to-herbs-in-search-for-covid-19-cure-3238216.
- 25.Antananarivo R.I. Madagascar President’s Herbal Tonic Fails to Halt Covid-19 Spike. [(accessed on 25 December 2020)];BBC News. 2020 Aug 13; Available online: https://www.bbc.com/news/world-africa-53756752.
- 26.Felix T. Nigeria: Madagascar’s Herbal Drink Cannot Cure COVID-19. [(accessed on 25 December 2020)];2020 Available online: https://www.aa.com.tr/en/africa/nigeria-madagascars-herbal-drink-cannot-cure-covid-19/1915948.
- 27.Shannon G., Jansen M., Williams K., Cáceres C., Motta A., Odhiambo A., Eleveld A., Mannell J. Gender equality in science, medicine, and global health: Where are we at and why does it matter? Lancet. 2019;393:560–569. doi: 10.1016/S0140-6736(18)33135-0. [DOI] [PubMed] [Google Scholar]
- 28.Kiracho E.E. Increasing access to quality health care for the poor: Community perceptions on quality care in Uganda. Patient Prefer. Adherence. 2009;3:77–85. doi: 10.2147/PPA.S4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witter S., Namakula J., Wurie H., Chirwa Y., So S., Vong S., Ros B., Buzuzi S., Theobald S. The gendered health workforce: Mixed methods analysis from four fragile and post-conflict contexts. Health Policy Plan. 2017;32:v52–v62. doi: 10.1093/heapol/czx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinstry B. Are there too many female medical graduates? Yes. BMJ. 2008;336:748. doi: 10.1136/bmj.39505.491065.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nzaji M.K., Ngombe L.K., Mwamba G.N., Ndala D.B.B., Miema J.M., Lungoyo C.L., Mwimba B.L., Bene A.C.M., Musenga E.M. Acceptability of Vaccination Against COVID-19 Among Healthcare Workers in the Democratic Republic of the Congo. Pragmatic Obs. Res. 2020;11:103–109. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Detoc M., Bruel S., Frappe P., Tardy B., Botelho-Nevers E., Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38:7002–7006. doi: 10.1016/j.vaccine.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Medicine (US) Vaccine Safety Forum. Evans G., Bostrom A., Johnston R.B. Risk Communication and Vaccination: Summary of a Workshop. Washington (DC) National Academies Press; Washington, DC, USA: 1997. [(accessed on 18 February 2021)]. Influences on the Acceptability of Vaccine Risks. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233845/ [PubMed] [Google Scholar]
- 34.Mahase E. Covid-19: Vaccine trials need more transparency to enable scrutiny and earn public trust, say experts. BMJ. 2020;371:4042. doi: 10.1136/bmj.m4042. [DOI] [PubMed] [Google Scholar]
- 35.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asiimwe R. Uganda to Access Covid-19 Vaccine from AstraZeneca-Ministry of Health, Daily Monitor. [(accessed on 9 January 2021)];2021 Available online: https://www.monitor.co.ug/uganda/news/national/uganda-to-access-covid-19-vaccine-from-astrazeneca-ministry-of-health-3246104.
- 37.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are openly available in figshare at https://figshare.com/s/9f89827c45921ec5196a (accessed on 9 March 2021).