Abstract
Background:
There is limited information on the trends of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) co-infections in India – particularly from private health-care settings. We designed the present research to estimate the prevalence of HIV, HBV, and HCV over a period of 7 years and study the factors associated with them.
Materials and Methods:
The present study is a secondary data analysis of data from the laboratory records of 24,086 individuals who were tested over a period of 7 years (2009–2015). We estimated the proportion and 95% confidence intervals (CIs) for HIV, hepatitis B surface antigen (HBsAg), and HCV antibodies.
Results:
The overall seroprevalence of HIV was 0.35% (95% CI: 0.27%, 0.44%), HBsAg was 1.65% (95% CI: 1.48%, 1.82%), and HCV was 1.73% (95% CI: 1.56%, 1.90%). The prevalence of HIV among those who were more than 70 years of age was 0.14% (95% CI: 0.04%, 0.32%). The prevalence of HBsAg was highest in those aged 30–39 years (2.27%, 95% CI: 1.74%, 2.92%) (P = 0.008). The prevalence of HIV/HBsAg co-infection was 0.019% (95% CI: 0.005%, 0.050%), HIV/HCV co-infection was 0.005% (95% CI: 0.000, 0.027%), and HBsAg/HCV co-infection was 0.059% (95% CI: 0.030%, 0.102%). We did not encounter even a single case of all the three infections.
Conclusions:
HIV infection is relatively high in those who were aged 50 years of more; thus, they need to be included in the National AIDS Control Programme. HIV/HBV/HCV co-infections should be regularly monitored in surveillance programs, and antiretroviral therapy officers and counselors should be trained on the management of HIV in those who are co-infected.
Keywords: Elderly, epidemiology, hepatitis B virus, hepatitis C virus, human immunodeficiency virus
INTRODUCTION
India, the second most populous nation in the world, has a population of more than 1.2 billion. Reports indicate that about 2.3 million people are infected with human immunodeficiency virus (HIV), 50 million are infected with hepatitis B virus (HBV), and about 6 million are infected with hepatitis C virus (HCV).[1,2,3] The Indian subcontinent is classified as an intermediate HBV endemic (hepatitis B surface antigen [HBsAg] carriage 2%–7%) zone and has the second largest global pool of chronic HBV infections.[4] People at high risk for HIV infection are also likely to be at increased risk for other pathogens such as HBV and HCV, which share the route of transmission with HIV. There is a high degree of epidemiological similarity between these viruses in terms of routes of transmission, associated risk factors, and the presence of these viruses in various body fluids.[5,6,7]
Few studies conducted in India have shown the prevalence of co-infection of HBV with HIV to vary in different geographical areas from as low as 9% to as much as 30% and of HCV with HIV to vary from 2% to 8%.[8,9,10,11] Globally, the studies conducted on the prevalence of hepatitis viruses in patients infected with HIV have shown the rate of HIV and HBV/HCV co-infection to be around 12%–15%.[12,13,14] Expert guidelines developed in the USA and Europe recommend screening of all individuals infected with HIV for co-infection with HCV and HBV to help in the appropriate management of such patients. However, the authors have highlighted the need for such uniform guidelines in India.[15]
As there is an increased availability of antibiotics and antifungals, majority of the secondary infections associated with HIV have been taken care of. However, HBV and HCV infections are becoming a cause of concern for individuals infected with HIV. In addition, with the advent of antiretoviral therapy (ART), liver disease has emerged as a cause of morbidity and mortality in these patients at later stages. Preliminary screening of the associated hepatitis viruses in patients with HIV will help in proper management of the co-infection. There is limited information on the trends of these three infections in India – particularly in private health-care settings. Thus, we designed the present research to: (1) estimate the prevalence of HIV, HBV, and HCV over a period of 7 years; (2) study the association between these three infections; and (3) examine the association between demographic factors and these three infections at a private tertiary health-care center.
MATERIALS AND METHODS
The present study is a secondary data analysis of data from the laboratory records of 24,086 individuals who were tested over a period of 7 years (2009–2015).
Study site
The study was carried out at the Department of Immunology of a tertiary care hospital in Mumbai, Maharashtra, India. The tertiary hospital caters to patients from all socioeconomic strata. The hospital has facilities for general health checkups as well as specialty (such as pediatrics, dermatology, and surgery) and subspecialty clinics (such as hepatic and liver clinic and neuropsychiatry clinic).
Data
We used data from the laboratory records. We included the records of individuals who were 18 years or more at the time of testing. We used the following parameters: date of test, age, sex, inpatient/outpatient status, HIV antibody test results, HBsAg results, and anti-hepatitis C antibody test results. The HIV tests were done using the Vitros® (Ortho Clinical Diagnostics) Anti-HIV 1 + 2 reagent pack and Vitros® Anti-HIV 1 + 2 calibrator on the Vitros ECi/ECiQ® Immunodiagnostic System. This test uses an immunometric bridge technique, and the results were categorized according to the suggested cutoffs. The HBsAg test was done using the Vitros® HBsAg Reagent Pack and Vitros Immunodiagnostic Products HBsAg Calibrator on the Vitros ECi/ECiQ Immunodiagnostic Systems. This test also used the immunometric bridge technique, and the results were classified as positive/negative/retest based on the suggested cutoffs. The HCV antibody test was done using the Vitros Anti-HCV Reagent Pack and Vitros Immunodiagnostic Products Anti-HCV Calibrator on the Vitros ECi/ECiQ Immunodiagnostic Systems. As with the other two tests, this test used an immunometric technique, and the results were categorized based on the suggested cutoffs.
Statistical analysis
We calculated the means and standard deviations for linear variables and proportions for categorical variables. We estimated the overall proportion for positivity along with their 95% confidence intervals (CIs) for HIV, HBsAg, and anti-hepatitis C antibodies. We also estimated the proportions according to the following characteristics: age, sex, inpatient/outpatient status, and the year of test. The proportions were compared using the Chi-square test or Fisher's exact test for low expected cell counts. We also used a Chi-square test for trend changes in proportions across the 7 years. We then used logistic regression models to estimate the association between the explanatory variables and outcomes. We estimated the odds ratios (ORs) and their 95% CIs using multivariate logistic regression. P <0.05 was considered statistically significant.
The study was approved by the institutional ethics committee.
RESULTS
Demographics
The mean age of all the participants was 52.9 (16.5) years. Nearly 61% of the individuals included in the analyses were male and 39% were female. The mean age was statistically significantly higher in males compared with females (54.4 [16.1] vs. 50.5 [16.9], P < 0.001). There was a gradual increase in the number of tests from 2009 (2082) to 2015 (3892) [Figure 1]. Most of the patients were inpatients (94%); the mean age was statistically significantly higher in the inpatients compared with outdoor patients (53.3 [16.4] vs. 46.3 [17.6], P < 0.0001).
Figure 1.
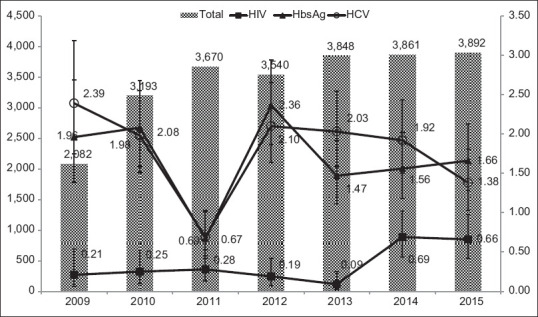
Graph showing the total number of individuals tested for human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C virus from 2009 to 2015, Mumbai, Maharashtra, India
Serological results
The overall seroprevalence of HIV was 0.35% (95% CI: 0.27%, 0.44%), that of HBsAg was 1.65% (95% CI: 1.48%, 1.82%), and that of HCV was 1.73% (95% CI: 1.56%, 1.90%).
HIV prevalence was significantly higher among those aged 40–49 years (0.93%, 95% CI: 0.64%, 1.31%) compared with other age groups. In our sample, we found that the prevalence of HIV among those who were more than 70 years of age was 0.14% (95% CI: 0.04%, 0.32%); all the cases were recorded in the years 2014 and 2015. The prevalence was statistically significantly higher in males compared with females (0.42% vs. 0.26%, P = 0.046). However, there were no significant differences in the prevalence of inpatient and outpatient individuals. The prevalence of HIV was low in 2009 (0.21%, 95% CI: 0.004%, 0.42%); however, it increased to 0.66% (95% CI: 0.39%, 0.92%). We found that the Chi-square for trend was statistically significant over these 7 years (P = 0.005). Table 1 and Figure 1 summarizes the prevalence of HIV according to all the characteristics.
Table 1.
The proportion of human immunodeficiency virus positivity according to age, sex, and site of test from 2009 to 2015, Mumbai, Maharashtra, India*
| Percentage (95% CI) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
| Age group | |||||||||
| 18-29 | 0.04 (0.00, 0.22) | 0.00 (0.00, 1.84) | 0.00 (0.00, 1.19) | 0.30 (0.01, 1.66) | 0.00 (0.00, 1.04) | 0.00 (0.00, 0.79) | 0.00 (0.00, 0.84) | 0.00 (0.00, 0.92) | 0.34 |
| 30-39 | 0.31 (0.13, 0.61) | 0.44 (0.01, 2.43) | 0.94 (0.20, 2.74) | 0.29 (0.01, 1.62) | 0.00 (0.00, 0.96) | 0.00 (0.00, 0.92) | 0.434 (0.05, 1.57) | 0.22 (0.01, 1.24) | 0.28 |
| 40-49 | 0.93 (0.64, 1.31) | 0.32 (0.08, 1.79) | 0.41 (0.05, 1.50) | 0.21 (0.01, 1.15) | 0.61 (0.12, 1.76) | 0.36 (0.04, 1.31) | 2.64 (1.45, 4.4) | 1.51 (0.69, 2.85) | 0.001 |
| 50-59 | 0.41 (0.26, 0.63) | 0.22 (0.06, 1.25) | 0.16 (0.04, 0.87) | 0.45 (0.12, 1.15) | 0.39 (0.08, 1.16) | 0.12 (0.00, 0.72) | 0.52 (0.14, 1.34) | 0.85 (0.34, 1.76) | 0.39 |
| 60-69 | 0.23 (0.11, 0.42) | 0.26 (0.07, 1.46) | 0.17 (0.01, 0.94) | 0.30 (0.03, 1.08) | 0.00 (0.00, 0.57) | 0.00 (0.00, 0.54) | 0.29 (0.03, 1.05) | 0.53 (0.14, 1.37) | 0.33 |
| >70 | 0.14 (0.04, 0.32) | 0.00 (0.00, 1.10) | 0.00 (0.00, 0.81) | 0.00 (0.00, 0.72) | 0.00 (0.00, 0.73) | 0.00 (0.00, 0.62) | 0.31 (0.03, 1.13) | 0.45 (0.09, 1.33) | 0.19 |
| P | <0.001 | 0.92 | 0.15 | 0.79 | 0.09 | 0.27 | <0.001 | 0.06 | |
| Sex | |||||||||
| Female | 0.26 (0.16, 0.39) | 0.00 (0.00, 0.51) | 0.28 (0.05, 0.82) | 0.32 (0.08, 0.83) | 0.08 (0.00, 0.45) | 0.06 (0.00, 0.37) | 0.35 (0.11, 0.82) | 0.54 (0.23, 1.07) | 0.09 |
| Male | 0.42 (0.32, 0.55) | 0.34 (0.09, 0.86) | 0.23 (0.06, 0.59) | 0.25 (0.08, 0.58) | 0.26 (0.08, 0.61) | 0.10 (0.01, 0.36) | 0.91 (0.54, 1.41) | 0.73 (0.41, 1.18) | <0.001 |
| P | 0.046 | 0.30 | >0.99 | 0.74 | 0.41 | >0.99 | 0.06 | 0.68 | |
| Site | |||||||||
| Outpatient | 0.41 (0.13, 0.96) | 0.00 (0.00, 7.87) | 1.07 (0.02, 5.84) | 1.34 (0.16, 4.76) | 0.00 (0.00, 2.67) | 0.00 (0.00, 6.60) | 0.49 (0.05, 1.76) | 0.00 (0.00, 1.10) | 0.29 |
| Inpatient | 0.35 (0.28, 0.45) | 0.21 (0.05, 0.55) | 0.22 (0.08, 0.48) | 0.22 (0.09, 0.47) | 0.20 (0.07, 0.43) | 0.08 (0.01, 0.25) | 0.71 (0.44, 1.07) | 0.72 (0.46, 1.07) | <0.001 |
| P | 0.75 | >0.99 | 0.11 | 0.06 | >0.99 | >0.99 | >0.99 | 0.16 | |
*The P value in the row for each variable (age, sex, and site) is the comparison of % across the groups, and the P value in the last column is the comparison of % across all years. CI=Confidence interval
The prevalence of HBsAg was highest in those aged 30–39 years (2.27%, 95% CI: 1.74%, 2.92%); the difference across the age groups was statistically significant (P = 0.008). Even though the prevalence of HBsAg was low in 2011 compared with other years, no trend was observed across these 7 years (Chi-square for trend, P = 0.55). The prevalence was statistically significantly higher in males compared with females (2.12% vs. 0.94%, P < 0.001). The prevalence was also statistically significantly higher in inpatients compared with outpatients (1.71% vs. 0.89%). Table 2 and Figure 1 summarizes the prevalence of HBsAg according to all the characteristics.
Table 2.
Table showing the proportion of Hepatitis B Surface Antigen positivity according to age, sex, and site of test from 2009-2015, Mumbai, India*
| Percentage (95% CI) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
| Age group | |||||||||
| 18-29 | 1.24 (0.84, 1.75) | 0.99 (0.12, 3.53) | 1.97 (0.72, 4.24) | 0.60 (0.07, 2.15) | 1.64 (0.61, 3.55) | 1.50 (0.60, 3.07) | 0.67 (0.13, 1.95) | 1.25 (0.40, 2.90) | 0.61 |
| 30-39 | 2.27 (1.74, 2.92) | 4.44 (2.15, 8.02) | 1.93 (0.71, 4.16) | 1.45 (0.47, 3.36) | 1.56 (0.57, 3.36) | 2.70 (1.35, 4.78) | 2.56 (1.33, 4.44) | 1.96 (0.90, 3.70) | 0.27 |
| 40-49 | 1.99 (1.56, 2.51) | 1.30 (0.35, 3.30) | 2.62 (1.40, 4.44) | 0.61 (0.12, 1.76) | 3.68 (2.23, 5.69) | 1.58 (0.72, 2.99) | 1.92 (0.96, 3.42) | 1.97 (1.02, 3.41) | 0.03 |
| 50-59 | 1.61 (1.29, 1.99) | 2.02 (0.93, 3.81) | 2.02 (1.08, 3.43) | 0.33 (0.06, 0.97) | 2.16 (1.26, 3.44) | 1.22 (0.58, 2.23) | 1.65 (0.88, 2.80) | 2.29 (1.38, 3.56) | 0.02 |
| 60-69 | 1.65 (1.30, 2.07) | 1.87 (0.75, 3.81) | 2.14 (1.14, 3.64) | 1.03 (0.41, 2.11) | 2.78 (1.68, 4.31) | 1.10 (0.48, 2.17) | 1.96 (1.08, 3.28) | 0.91 (0.36, 1.87) | 0.06 |
| >70 | 1.23 (0.90, 1.63) | 1.47 (0.48, 3.41) | 1.69 (0.73, 3.31) | 0.37 (0.04, 1.35) | 1.88 (0.90, 3.43) | 1.29 (0.56, 2.53) | 0.59 (0.16, 1.51) | 1.49 (0.72, 2.73) | 0.19 |
| P | 0.008 | 0.17 | 0.95 | 0.24 | 0.22 | 0.36 | 0.04 | 0.33 | |
| Sex | |||||||||
| Female | 0.94 (0.75, 1.16) | 0.41 (0.08, 1.19) | 1.37 (0.77, 2.26) | 0.48 (0.17, 1.05) | 1.80 (1.15, 2.70) | 0.97 (0.54, 1.60) | 0.61 (0.27, 1.15) | 0.79 (0.41, 1.39) | 0.003 |
| Male | 2.12 (1.88, 2.38) | 2.93 (2.03, 4.07) | 2.52 (1.84, 3.37) | 0.78 (0.44, 1.27) | 2.70 (2.04, 3.51) | 1.84 (1.30, 2.52) | 2.20 (1.62, 2.91) | 2.24 (1.67, 2.95) | <0.001 |
| P | <0.001 | <0.001 | 0.04 | 0.31 | 0.10 | 0.03 | <0.001 | 0.001 | |
| Site | |||||||||
| Outpatient | 0.89 (0.48, 1.52) | 0.00 (0.00, 7.70) | 1.72 (0.20, 6.08) | 0.00 (0.00, 1.97) | 1.66 (0.34, 4.79) | 2.94 (0.35, 10.22) | 0.61 (0.12, 1.79) | 0.80 (0.16, 2.32) | 0.18 |
| Inpatient | 1.71 (1.53, 1.89) | 2.01 (1.42, 2.76) | 2.10 (1.59, 2.71) | 0.71 (0.44, 1.07) | 2.39 (1.88, 3.00) | 1.45 (1.08, 1.90) | 1.71 (1.28, 2.21) | 1.76 (1.34, 2.26) | <0.001 |
| P | 0.02 | >0.99 | >0.99 | 0.63 | 0.80 | 0.27 | 0.08 | 0.20 | |
*The P value in the row for each variable (age, sex, and site) is the comparison of % across the groups, and the P value in the last column is the comparison of % across all years. CI=Confidence interval
Even though the prevalence of anti-HCV was higher among those aged 40–49 years (2.20%, 95% CI: 1.75%, 2.73%), the difference across age groups was not statistically significant (P = 0.08). Even though the prevalence of HCV was lower in 2011, we did not observe any trend over these 7 years (Chi-square for trend, P = 0.40). There were no statistically significant differences in the prevalence of HCV among males (1.81%, 95% CI: 1.59%, 2.05%) and females (1.62%, 95% CI: 1.36%, 1.90%) (P = 0.28). However, the prevalence was statistically significantly higher in outpatients compared with inpatients (3.84% vs. 1.63%, P < 0.001). Table 3 and Figure 1 summarizes the prevalence of anti HCV antibody according to all the characteristics.
Table 3.
Table showing the proportion of Hepatitis C antibody positivity according to age, sex, and site of test from 2009-2015, Mumbai, India*
| Percentage (95% CI) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
| Age group | |||||||||
| 18-29 | 1.14 (0.76, 1.66) | 0.51 (0.01, 2.81) | 1.67 (0.54, 3.85) | 2.35 (1.02, 4.59) | 0.87 (0.18, 2.52) | 0.23 (0.01, 1.32) | 1.76 (0.71, 3.60) | 0.54 (0.06, 1.94) | 0.07 |
| 30-39 | 1.66 (1.20, 2.22) | 1.77 (0.48, 4.48) | 2.40 (1.04, 4.67) | 0.28 (0.01, 1.55) | 1.80 (0.72, 3.68) | 2.50 (1.20, 4.54) | 1.54 (0.62, 3.15) | 1.35 (0.49, 2.93) | 0.29 |
| 40-49 | 2.20 (1.75, 2.73) | 3.53 (1.77, 6.24) | 3.26 (1.91, 5.17) | 0.38 (0.04, 1.37) | 2.46 (1.31, 4.18) | 2.96 (1.73, 4.69) | 1.57 (0.72, 2.97) | 1.81 (0.90, 3.22) | 0.01 |
| 50-59 | 1.79 (1.45, 2.18) | 3.41 (1.92, 5.57) | 1.86 (0.99, 3.16) | 0.94 (0.43, 1.77) | 1.96 (1.12, 3.16) | 1.57 (0.84, 2.68) | 2.40 (1.45, 3.73) | 1.31 (0.65, 2.33) | 0.03 |
| 60-69 | 1.72 (1.36, 2.13) | 2.84 (1.43, 5.04) | 1.09 (0.44, 2.24) | 0.27 (0.03, 0.99) | 3.06 (1.90, 4.64) | 2.05 (1.15, 3.36) | 1.25 (0.57, 2.36) | 1.92 (1.07, 3.14) | 0.001 |
| >70 | 1.64 (1.27, 2.10) | 0.92 (0.19, 2.69) | 1.83 (0.84, 3.45) | 0.36 (0.04, 1.29) | 1.70 (0.78, 3.20) | 2.62 (1.50, 4.22) | 2.71 (1.61, 4.26) | 0.90 (0.33, 1.96) | 0.008 |
| P | 0.08 | 0.051 | 0.20 | 0.01 | 0.27 | 0.01 | 0.37 | 0.36 | |
| Sex | |||||||||
| Female | 1.62 (1.36, 1.90) | 2.88 (1.79, 4.36) | 1.85 (1.15, 2.82) | 0.31 (0.08, 0.79) | 1.80 (1.14, 2.69) | 2.19 (1.51, 3.07) | 1.66 (1.07, 2.47) | 1.14 (0.66, 1.83) | <0.001 |
| Male | 1.81 (1.59, 2.05) | 2.08 (1.34, 3.08) | 2.05 (1.45, 2.81) | 0.92 (0.56, 1.42) | 2.28 (1.67, 3.03) | 1.90 (1.35, 2.58) | 2.09 (1.53, 2.79) | 1.53 (1.06, 2.13) | 0.018 |
| P | 0.28 | 0.28 | 0.79 | 0.04 | 0.35 | 0.54 | 0.36 | 0.32 | |
| Site | |||||||||
| Outpatient | 3.84 (2.75, 5.19) | 8.33 (1.75, 22.47) | 4.59 (1.26, 11.36) | 1.61 (0.19, 5.70) | 6.25 (2.73, 11.94) | 5.45 (1.13, 15.12) | 3.59 (1.86, 6.19) | 2.86 (1.24, 5.57) | 0.24 |
| Inpatient | 1.63 (1.47, 1.81) | 2.28 (1.65, 3.08) | 1.90 (1.43, 2.46) | 0.65 (0.41, 0.99) | 1.93 (1.47, 2.47) | 1.98 (1.54, 2.50) | 1.75 (1.33, 2.26) | 1.25 (0.91, 1.69) | <0.001 |
| P | <0.001 | 0.053 | 0.09 | 0.21 | 0.001 | 0.10 | 0.02 | 0.03 | |
*The P value in the row for each variable (age, sex, and site) is the comparison of % across the groups, and the P value in the last column is the comparison of % across all years. CI=Confidence interval
The prevalence of HIV and HBsAg co-infection in our population was 0.019% (95% CI: 0.005%, 0.050%). The prevalence of HIV and HCV co-infection was 0.005% (95% CI: 0.000, 0.027%). The prevalence of HBsAg and HCV co-infection was 0.059% (95% CI: 0.030%, 0.102%). We did not encounter even a single case of all the three infections.
Multivariate analyses
In the multivariate models, we found that the prevalence of HIV was higher in those aged 40–49 years and 50–59 years compared with those who were aged 18–29 years. We also found that males were significantly more likely to be HIV positive compared with females (OR: 1.7, 95% CI: 1.0, 2.8). Furthermore, HIV prevalence was significantly higher in 2014 and 2015 compared with 2009. The prevalence of HBsAg was significantly higher in those aged 30–39 years compared with those aged 18–29 years (OR: 1.8; 95% CI: 1.2, 2.8). In our sample, the prevalence of HBsAg was significantly higher in males and inpatients compared with females and outpatients. The prevalence of anti-HCV antibodies was significantly higher in those aged 40 years and more compared with those aged 18–29 years. However, we did not find any significant differences between the sexes and the type of patients (inpatient/outpatient). Table 4 summarizes the ORs and 95% CI for all these characteristics.
Table 4.
The odds ratio and their 95% confidence intervals to study the association between select characteristics and positivity for human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C virus
| Characteristics | OR (95% CI) | ||
|---|---|---|---|
| HIV | HbSAg | HCV | |
| Age | |||
| 18-29 | Baseline | Baseline | Baseline |
| 30-39 | 7.5 (0.9, 60.3) | 1.8 (1.2, 2.8)* | 1.5 (0.9, 2.5) |
| 40-49 | 22.7 (3.1, >100.0)* | 1.5 (0.97, 2.3) | 2.1 (1.4, 3.3)* |
| 50-59 | 9.8 (1.3, 73.1) | 1.2 (0.8, 1.7) | 1.7 (1.1, 2.7)* |
| 60-69 | 5.3 (0.7, 41.7) | 1.2 (0.8, 1.8) | 1.7 (1.1, 2.6)* |
| >70 | 3.0 (0.4, 26.1) | 0.8 (0.5, 1.3) | 1.6 (1.0, 2.5)* |
| Sex | |||
| Female | Baseline | Baseline | Baseline |
| Male | 1.7 (1.0, 2.8)* | 2.4 (1.8, 3.0)* | 1.1 (0.9, 1.4) |
| Site | |||
| Outpatient | Baseline | Baseline | Baseline |
| Inpatient | 1.1 (0.4, 2.7) | 1.9 (1.1, 3.3)* | 0.4 (0.3, 0.5) |
| Years | |||
| 2009 | Baseline | Baseline | Baseline |
| 2010 | 1.2 (0.3, 4.0) | 1.1 (0.7, 1.6) | 0.8 (0.5, 1.2) |
| 2011 | 1.3 (0.4, 4.3) | 0.3 (0.2, 0.6)* | 0.3 (0.2, 0.5)* |
| 2012 | 0.9 (0.3, 3.2) | 1.2 (0.8, 1.8) | 0.8 (0.6, 1.2) |
| 2013 | 0.4 (0.1, 1.9) | 0.8 (0.5, 1.2) | 0.9 (0.6, 1.3) |
| 2014 | 3.5 (1.2, 10.0)* | 0.8 (0.6, 1.3) | 0.7 (0.5, 1.1) |
| 2015 | 3.2 (1.1, 9.3) | 0.9 (0.6, 1.3) | 0.5 (0.3, 0.8)* |
*P<0.05. HbSAg=Hepatitis B surface antigen; HCV=Hepatitis C virus; HIV=Human immunodeficiency virus; OR=Odds ratio; CI=Confidence interval
DISCUSSION
Thus, we found that the HIV prevalence was <1% over this 7-year period, although the prevalence has increased in the last 2 years. The prevalence was highest among males and among those aged 40–49 years. Even though the prevalence of HbsAg and HCV was higher than HIV, we did not find a high proportion of co-infections in our population.
The national prevalence of HIV in India has been estimated to be 0.26%;[16] there has been a reduction in the prevalence of the past two decades. Shiradkar et al. found a high HIV prevalence in antenatal women – considered to be low-risk population – in the early 2000; however, they also reported that the prevalence had reduced by 2011.[17] The overall adult (15–49 years) prevalence in Maharashtra has steadily gone down from 2007 to 2015; it was estimated to be 0.60% (95% CI: 0.51%–0.71%) in 2007 and 0.37% (95% CI: 0.31%–0.44%) in 2015. Although we did find a reduction in the prevalence of HIV in our population from 2010 to 2013, there was a significant increase in 2014 and 2015.[18] Thus, apart from sentinel surveillance and program data, there is a need to monitor the trends reported by various individual researchers and private centers to understand the changes in the epidemic in the state.
As indicated earlier, the estimated prevalence of HIV in Maharashtra has been reported in the 15–49-year-old individuals. In our population, we found the HIV prevalence to be high even in those aged 50 years and more (including few cases of HIV in those aged 70 years or more). It has been reported that the proportion of HIV infections in those aged 50 years and more has increased recently.[19] HIV in these individuals requires specialized training and monitoring due to comorbidities, drug interactions, and inadequate CD4 response to treatment.[20] Thus, it is important to initiate regular surveillance and provide estimates for those above the age of 50 years as well. Furthermore, it is important to train the ART medical officers and counselors to address issues in HIV-infected individuals who are more than 50 years of age.
Knowledge of co-infections in HIV – particularly HBV and HCV – is important to plan management with ART in HIV-infected individuals. Indeed, higher levels of markers of systematic inflammation have been found in HIV/HCV co-infected individuals.[21,22] In a multicentric AIDS cohort study, it was observed that the liver-related mortality rate was 1.7/1000 person-years in HIV-seropositive patients, 0.8/1000 person-years in HBsAg-positive patients, and it was significantly higher in co-infected patients – 14.2/1000 person-years.[23] Furthermore, co-infection with hepatitis viruses may complicate management with ART by increasing the risk of drug-related hepatotoxicity and may also accelerate the progress of HIV infection.[24] Even though we found a low prevalence of co-infections in our population, a recent meta-analysis found the co-infection of HBV/HCV to be 1.89%, whereas other authors have found a low prevalence of HBV and HCV.[25,26] Furthermore, studies have shown that the risk of sexual transmission of HCV is low even in sexual partners; this may potentially explain the low prevalence of this infection in our population.[27,28] Nonetheless, there is a need to integrate the testing of these co-infections in all counseling and testing centers and include the management of co-infections in all training programs.
We did not have data on risk behaviors (such as sexual behavior and condom use) and other potential routes of transmission of HIV. Thus, we could account for these in our multivariate models – a potential limitation of our study. Another limitation is that many of those tested positive for either of these infections may have known about their status and may not have informed us about it; hence, we were not able to differentiate between newly identified infections and older infections. Despite these limitations, our data provide useful information on the trends of prevalence of the three infections, which is a useful contribution to the literature on the epidemiology of these infections in India and help in the design of the National AIDS Control Programme.
CONCLUSIONS
Our data suggest that the prevalence of HIV has increased in our study population in the recent years. Furthermore, HIV infection is relatively high in those who were aged 50 years of more; thus, they need to be included in the National AIDS Control Programme in India. Finally, HIV/HBV/HCV co-infections should be regularly monitored in surveillance programs, and ART officers and counselors should be trained on the management of HIV in those who are co-infected.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ray G. Current scenario of hepatitis B and its treatment in India. J Clin Transl Hepatol. 2017;5:277–96. doi: 10.14218/JCTH.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization – Country Office for India. Viral Hepatitis C treatment in India? 2016. [[Last accessed on 2018 Jan 02]]. Available from: http://wwwsearowhoint/india/mediacentre/events/2016/viral_hepatitis_c_treatment_in_indiapdfua=1 .
- 3.National AIDS Control Organization. Annual Report NACO 2016-17. New Delhi: Ministry of Health & Family Welfare, Government of India; 2017. [Google Scholar]
- 4.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.McCarron B, Thyagarajan SP. HIV and hepatotropic viruses: Interactions and treatments. Indian J Med Microbiol. 1998;16:4–11. [Google Scholar]
- 6.Rustgi VK, Hoofnagle JH, Gerin JL, Gelmann EP, Reichert CM, Cooper JN, et al. Hepatitis B virus infection in the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:795–7. doi: 10.7326/0003-4819-101-6-795. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Kumarasamy N, Solomon S, Flanigan TP, Hemalatha R, Thyagarajan SP, Mayer KH. Natural history of human immunodeficiency virus disease in Southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 9.Padmapriyadarsini C, Chandrabose J, Victor L, Hanna LE, Arunkumar N, Swaminathan S. Hepatitis B or hepatitis C co-infection in individuals infected with human immunodeficiency virus and effect of anti-tuberculosis drugs on liver function. J Postgrad Med. 2006;52:92–6. [PubMed] [Google Scholar]
- 10.Saravanan S, Velu V, Kumarasamy N, Nandakumar S, Murugavel KG, Balakrishnan P, et al. Coinfection of hepatitis B and hepatitis C virus in HIV-infected patients in south India. World J Gastroenterol. 2007;13:5015–20. doi: 10.3748/wjg.v13.i37.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tankhiwale SS, Khadase RK, Jalgoankar SV. Seroprevalence of anti-HCV and hepatitis B surface antigen in HIV infected patients. Indian J Med Microbiol. 2003;21:268–70. [PubMed] [Google Scholar]
- 12.Egah DZ, Banwat EB, Audu ES, Iya D, Mandong BM, Anele AA, et al. Hepatitis B surface antigen, hepatitis C and HIV antibodies in a low-risk blood donor group, Nigeria. East Mediterr Health J. 2007;13:961–6. [PubMed] [Google Scholar]
- 13.Rouet F, Chaix ML, Inwoley A, Anaky MF, Fassinou P, Kpozehouen A, et al. Frequent occurrence of chronic hepatitis B virus infection among West African HIV type-1-infected children. Clin Infect Dis. 2008;46:361–6. doi: 10.1086/525531. [DOI] [PubMed] [Google Scholar]
- 14.Telatela SP, Matee MI, Munubhi EK. Seroprevalence of hepatitis B and C viral co-infections among children infected with human immunodeficiency virus attending the paediatric HIV care and treatment center at Muhimbili National Hospital in Dar-es-Salaam, Tanzania. BMC Public Health. 2007;7:338. doi: 10.1186/1471-2458-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain M, Chakravarti A, Verma V, Bhalla P. Seroprevalence of hepatitis viruses in patients infected with the human immunodeficiency virus. Indian J Pathol Microbiol. 2009;52:17–9. doi: 10.4103/0377-4929.44955. [DOI] [PubMed] [Google Scholar]
- 16.Pandey A, Dhingra N, Kumar P, Sahu D, Reddy DCS, Narayan P, et al. Sustained progress, but no room for complacency: Results of 2015 HIV estimations in India. Indian J Med Res. 2017;146:83–96. doi: 10.4103/ijmr.IJMR_1658_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiradkar S, Mande S, Bapat G, Setia MS. Is it time to bring the “Parent” into the prevention of parent to child transmission programs in India.A study of trends over a 10-year period in a prevention of parent to child transmission clinic in India? Indian J Sex Transm Dis AIDS. 2016;37:58–64. doi: 10.4103/2589-0557.176211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National AIDS Control Organization. State Epidemiological Fact Sheets, Volume II West and South Regions. New Delhi: Ministry of Health & Family Welfare, Government of India; 2017. [Google Scholar]
- 19.Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: Evidence from estimates and survey data. AIDS. 2014;28(Suppl 4):S453–9. doi: 10.1097/QAD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453–72. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shmagel KV, Korolevskaya LB, Saidakova EV, Shmagel NG, Chereshnev VA, Margolis L, et al. HCV coinfection of the HIV-infected patients with discordant CD4(+) T-cell response to antiretroviral therapy leads to intense systemic inflammation. Dokl Biol Sci. 2017;477:244–7. doi: 10.1134/S0012496617060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shmagel KV, Saidakova EV, Shmagel NG, Korolevskaya LB, Chereshnev VA, Robinson J, et al. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med. 2016;17:581–9. doi: 10.1111/hiv.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 24.Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: A review. World J Gastroenterol. 2014;20:14598–614. doi: 10.3748/wjg.v20.i40.14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desikan P, Khan Z. Prevalence of hepatitis B and hepatitis C virus co-infection in India: A systematic review and meta-analysis. Indian J Med Microbiol. 2017;35:332–9. doi: 10.4103/ijmm.IJMM_17_257. [DOI] [PubMed] [Google Scholar]
- 26.da Motta LR, Adami AG, Sperhacke RD, Kato SK, Paganella MP, Pereira GFM, et al. Hepatitis B and C prevalence and risk factors among young men presenting to the Brazilian Army: A STROBE-compliant national survey-based cross-sectional observational study. Medicine (Baltimore) 2019;98:e16401. doi: 10.1097/MD.0000000000016401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tor J, Llibre JM, Carbonell M, Muga R, Ribera A, Soriano V, et al. Sexual transmission of hepatitis C virus and its relation with hepatitis B virus and HIV. BMJ. 1990;301:1130–3. doi: 10.1136/bmj.301.6761.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA. 1993;269:392–4. [PubMed] [Google Scholar]


