Abstract
Anti-Melanoma Differentiation-Associated gene 5 (MDA-5) Dermatomyositis (MDA5, DM) is a recently identified subtype of myositis characteristically associated with Rapidly Progressive Interstitial Lung Disease (RP-ILD) and unique cutaneous features. We reviewed PubMed, SCOPUS and Web of Science databases and selected 87 relevant articles after screening 1485 search results, aiming to gain a better understanding of the pathophysiology, clinical features, diagnosis, and treatment approaches of anti-MDA-5 DM described in the literature. The etiopathogenesis is speculatively linked to an unidentified viral trigger on the background of genetic predisposition culminating in an acquired type I interferonopathy. The clinical phenotype is highly varied in different ethnicities, with new clinical features having been recently described, expanding the spectrum of cases that should raise the suspicion of anti-MDA-5 DM. Unfortunately, the diagnosis is frequently missed despite excessive mortality, calling for wider awareness of suspect symptoms. RP ILD is the major determinant of survival, treatment being largely based on observational studies with recent insights into aggressive combined immunosuppression at the outset.
Keywords: Dermatomyositis, Myositis, Phenotype, Interstitial lung disease, Genetic predisposition, Autoantibodies, Immunosuppression
Introduction
Our understanding of Dermatomyositis (DM) as a disease entity with classic skin rashes and skeletal muscle involvement [1, 2] has transformed into a heterogeneous disease with a wide array of systemic manifestations. There are many faces of DM within both juvenile and adult subtypes, ranging from classic DM, clinically hypo- and amyopathic DM (CADM), and cancer associated DM, to the more recently described forms such as anti-melanoma differentiation-associated gene 5 (MDA5) DM with rapidly progressive (RP) Interstitial Lung Disease (ILD).
The clinical spectrum of the Immune-Mediated Inflammatory Myopathies (IMIM) is broad and is largely defined by the presence of characteristic myositis-specific and associated antibodies (MSA, MAA)[3]. Anti-MDA5 DM is of variable frequency ranging from 7 to 10% in European cohorts to 25% in the Japanese amongst adult DM [4, 5]. Similarly, in children, the prevalence is around 10–40% of juvenile DM cases [6, 7]. Anti-MDA5 DM is typically associated with unique and varied cutaneous features (e.g. cutaneous and oral ulcerations, painful palmar papules and macules) and RP ILD, often on a background of modest muscle involvement with an aggressive course and high early mortality. Initial reports of anti-MDA5 DM arose from Japan with the description of CADM with a novel autoantibody that immunoprecipitated a polypeptide of ∼ 140 kd labelled as the CADM-140 autoantigen [8]. The target antigen was later recognized as the Ribonucleic Acid (RNA) helicase encoded by the melanoma differentiation-associated gene 5, a protein involved in the innate immune response. These patients typically had RP ILD with a poor response to treatment. After initial suggestions of a milder disease in Caucasians, a biphenotypic spectrum was identified in cases reported from the United States.
Notably, the acute presentation of the disease with predominant lung involvement may result in a delay in diagnosis due to overlap of the clinical picture with other causes of acute pneumonitis such as infections, hypersensitivity pneumonitis, drug and toxin induced, among other causes. Emerging insights suggest a benefit in survival with early and aggressive treatment with a combination of immunosuppressants [9–11]. Most recently, a comparison with Coronavirus disease 2019 (COVID-19) has led to some fascinating insights into this enigmatic disease. Due to the rapidly changing paradigm due to new discoveries relevant to this rare condition, we reviewed the literature to discuss the salient features and summarize the current understanding of anti-MDA5 DM by collating evidence from around the globe.
Methodology
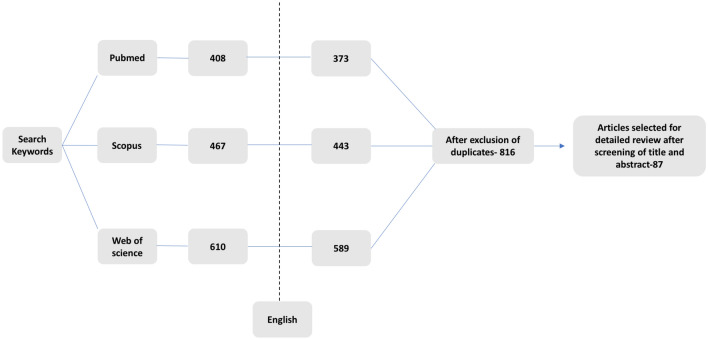
An electronic search strategy across three databases was performed as per Gasparyan et al. [12]. Articles available on MEDLINE, SCOPUS and Web of Science, published anytime till 26th November, 2020, were reviewed using the following search words: (“dermatomyositis” OR “DM”) AND (“MDA5” OR “anti-CADM-140” OR “Melanoma Differentiation Antigen” OR “RP ILD”), “Rapidly progressive Interstitial lung disease AND dermatomyositis”, “MDA5 AND malignancy”, “MDA5 AND treatment”. Of the 408, 467 and 589 articles obtained, respectively, in each database, articles in language other than English were excluded (Fig. 1). No restrictions were applied based on the age or method of detection of the anti-MDA5 antibody [13]. After an initial screening of titles and abstracts, relevant articles were retained. The qualifying full‐text publications and their citations were carefully reviewed to determine whether information on the topic of interest was included.
Fig. 1.
Flowchart of studies selected to inform the review
All these articles were subsequently filtered by two co-authors (PM and LG) to select only those that met the objectives of this review, resulting in 87 articles. Additional information pertaining to specific sections was obtained through an individualized search strategy: initial history and reporting of cases of CADM with high mortality, analogy between anti-MDA5 DM and COVID-19, and pathogenesis and genetics of DM.
History and pathogenesis
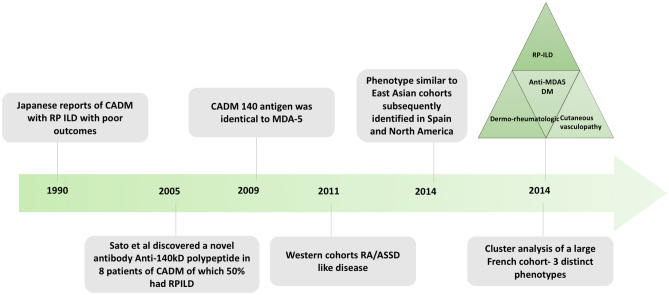
The initial reports of a subset of amyopathic DM with RP ILD from Japan in the early 1990s reported poor outcomes despite aggressive immunosuppression (Fig. 2) [14, 15]. In a seminal paper, Sato et al. reported a new antibody against a 140 kD polypeptide by immunoprecipitation and immunoblotting (against CADM 140 antigen) in 50–70% of CADM [13]. This opened the Pandora’s box for subsequent work on this new entity. The CADM 140 antigen was subsequently demonstrated to be identical to MDA5 described previously [16, 17]. MDA-5 is a Retinoic Acid Inducible Gene-1 (RIG-1)-like receptor coded by IFIH1 (interferon-induced helicase C domain-containing protein 1) gene that recognizes double stranded (ds) RNA of viruses and induces a type I interferon response through various intermediaries. The exact pathogenesis is not known and a role of infections and environmental factors superimposed on a genetic susceptibility has been proposed [18] (Fig. 3).
Fig. 2.
Timeline depicting the discovery and evolution of the disease phenotype of anti-MDA5 DM. CADM clinically amyopathic dermatomyositis, RP rapidly progressive, ILD interstitial lung disease, MDA5 melanoma differentiation-associated gene 5, RA rheumatoid arthritis, ASSD anti synthetase syndrome
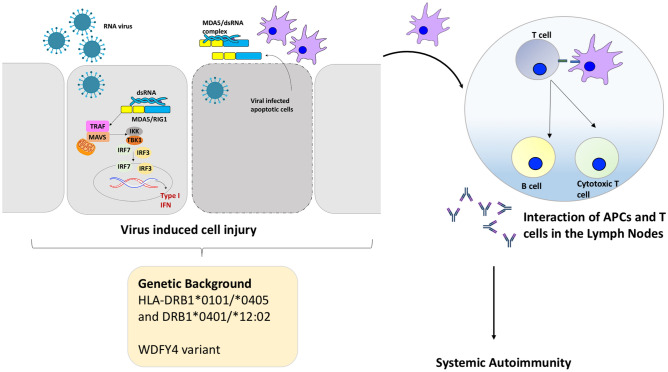
Fig. 3.
Proposed pathogenesis of anti-MDA5 DM. In individuals with a particular genetic factors (HLA or non-HLA), an unknown viral trigger can lead to sensing of the viral double stranded Ribonucleic Acid (dsRNA) by cytoplasmic Pattern Recognition Receptors (PRR) like Melanoma Differentiation-Associated gene 5 (MDA5)/Retinoic Acid Inducible Gene-1 (RIG-1). This in turn results in activation of mitochondrial antiviral signaling protein (MAVS) which in conjugation with TNF Receptor associated factors (TRAF) recruits Tank binding kinase-1 (TBK-1) and IBK kinase (IKK). These then result in phosphorylation and activation of transcription factors-Interferon Regulatory factors (IRF) 3 and 7. These translocate into the nucleus and trigger type-1 interferon production. Virus induced cell injury and lysis, may result in release of viral-MDA5 complexes/MDA5. These complexes can be recognized by antigen presenting cells (APCs) and with a subsequent activation of helper T cells and B cells, production of autoantibody against MDA5. Activated cells and autoantibodies enter the systemic circulation and encounter autoantigens resulting in a systemic autoimmune response
Infections
Viral infections are believed to induce autoimmunity and may be the eliciting event in the pathogenesis of myositis. RNA viruses such as the coxsackie and parvovirus B19 have been implicated in causation of DM in the past [19, 20]. MDA5 is a cytosolic viral RNA sensor that normally triggers an innate response and subsequent production of cytokines [interferon (IFN), tumor necrosis factor alpha (TNF), interleukin 1 (IL-1), IL-6, IL-18], activation of macrophages and helper T cells. Although, direct evidence of a specific viral trigger is lacking, it is hypothesized that RNA viruses may upregulate MDA5 expression in tissues, and the subsequent viral replication and cell lysis would release MDA5 or a viral-MDA5 complex with resultant autoantibody production against it (Figs. 2, 3). The autoimmune response may further perpetuate cell injury, exposure of self-antigens and subsequently maladaptive immune response resulting in disease [16, 21, 22]. Thus, anti-MDA5 antibodies can be a byproduct of the above process or may have a pathogenic role. The latter is supported by recent studies in which anti-MDA5 antibody concentrations correlate with the presence of RP ILD as well as relapses [23]. Furthermore, markers of macrophage activation like ferritin, IL-18 and sCD206 (marker of M2 polarization) correlate with severity of disease and poor prognosis entailing that macrophages and thus innate immunity plays an important role in the pathogenesis of disease.
A striking similarity in increasingly being recognized between severe COVID-19 and anti-MDA5 DM with a similar involvement of the lung, skin rashes, fever, fatigue and myalgia. Notably, similar blood cytokine profiles with elevated ferritin and C-reactive protein (CRP) supports the idea that severe COVID-19 may resemble a human model of anti-MDA DM [24–26]. The hyperinflammatory response triggered by COVID-19 in susceptible individuals culminates in widespread endothelial dysfunction, vasculopathy and thrombotic manifestations with a pathophysiologic overlap with anti-MDA5 DM [27]. The radiological picture of COVID-19 pneumonia is comparable to ILD in anti-MDA5 DM, with frequent presence of diffuse ground glass opacities (GGO) suggestive of peribronchovascular consolidations. Both these diseases might be treated with high-dose steroids, immunosuppressants and anti-cytokine therapy. A recent report identified antibodies against immunogenic epitopes with high sequence identity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients of DM, suggesting a role of latent viral infection and molecular mimicry in the pathogenesis of DM [28]. Recent reports of a high flare and admission rate among patients with myositis during the pandemic create the case to explore this speculative hypothesis [29–31].
Genetics
Human Leukocyte Antigen (HLA)-DRB1*0101/*0405 and DRB1*0401/*12:02 are associated with susceptibility to anti-MDA-5 DM in the Japanese and Han Chinese [32, 33]. Both these alleles contain a shared amino acid sequence QRRAAA at positions 70–74 of the beta1 subunit of the HLA DR molecule, suggesting that these may be important in antigenic peptide presentation and the production of anti-MDA5 antibodies. However, when a focused HLA analysis was conducted across 2582 Caucasian cases of IMIM across Europe through the Myositis Genetics Consortium (MYOGEN), no significant association was found between classic HLA alleles and anti-MDA5 DM [34].
A recent multicentric GWAS study conducted involving 592 patients of IMIM identified an association between a variant of WDFY4 and CADM. The splicing mutation resulted in increased expression of truncated variant of WDFY4 that showed increased interaction with pattern recognition receptors including MDA5 and resultant NF-kB overactivation and apoptosis. Since 70% of CADM in this study were anti-MDA5 positive with co-immunoprecipitation of sera from these individuals with truncated WDYF4 protein, this variant may play a role in the susceptibility to anti-MDA5 DM. This association was not significant when examined in the 21 European patients of CADM, although this could have possibly been due to lack of statistical power owing to smaller sample size [35].
Environmental
A detailed epidemiologic study from Japan revealed that cases of anti-MDA5 DM were clustered in the rural areas around the Kiso river with a link to detection of Coxsackie virus in the water with a seasonality from October to March [36, 37]. Similar case clustering has been reported around the Yangtze river in the Jiangsu province in China. These observations may explain a higher prevalence of this subtype of DM in South East Asia. A suggested role of environmental antigenic triggers to autoimmunity in the genetically predisposed is further consolidated by these studies, paving the way for larger epidemiological exploration. Following the eliciting trigger, a cascade of events leads on to a cytokine storm with predominant type 1 interferon signature as supported by the Interferon stimulated gene (ISG15) metric and MxA protein in muscle biopsies of patients [38].
The cytokine profile in patients with anti-MDA5 DM further supports an upregulated interferon axis (IFN-γ, IFN-α, and IFN-induced protein, IP-10) alongside other proinflammatory cytokines [39, 40]. A hierarchical cluster analysis of the cytokine profiles in DM revealed two distinct clusters, with predominance of IFN-related cytokines in cluster one, 75% of which was constituted by anti-MDA5 DM and strong correlation with cutaneous vasculitis [39]. It seems plausible that cytokine mediated microvascular injury may contribute to the unique cutaneous lesions in DM and, possibly, lung involvement.
Mutations in IFIH1 that code for MDA5 are also associated with Aicardi Goutier syndrome and other mendelian autoinflammatory interferonopathies such as STING-associated vasculopathy with onset in infancy (SAVI) and Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures (CANDLE). Interestingly, the latter also exhibit the interferon gene signature, and benefit from Janus kinase (JAK) inhibitors, suggesting another important overlap with anti-MDA5 DM [41]. Thus, it seems plausible that anti-MDA5 DM may represent an acquired form of the same type of condition [42, 43]. However, in a recent French study, no obvious pathogenic mutations were observed in genes associated with recognized monogenic interferonopathies in 21 anti-MDA5 JDM patients [40].
Clinical features
Clinical phenotype in adults with anti-MDA5 DM
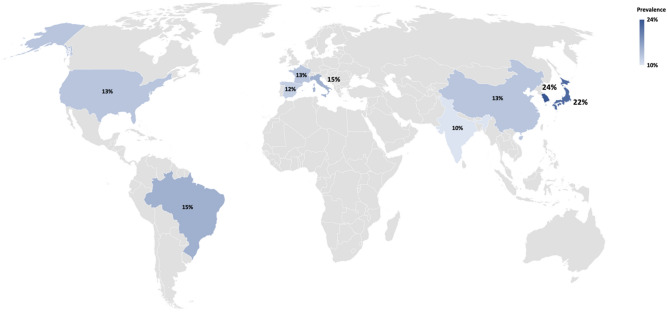
The original descriptions of anti-MDA5 DM from Asia were clinically amyopathic with cutaneous vasculopathy and RP ILD [44, 45]. Soon after that, data from different ethnic backgrounds emerged, and a clinically heterogeneous entity appears to be more likely to occur in the presence of anti-MDA5 antibodies (Tables 1, 2). Initial studies from Europe and North America described it as a mild Rheumatoid Arthritis/Anti-Synthetase Syndrome (ASSD) like disease with a prevalence of 7% amongst adult DM [46–48]. Subsequent studies from Spain and Pittsburgh cohorts depicted a prevalence of 15–20% among all DM, similar to Asian cohorts, with over 40–60% exhibiting RP ILD and poor survival [49]50 (Fig. 4). An unsupervised cluster analysis of 83 anti-MDA5 IMIM in a recently conducted multicentric French study yielded a revelation of a new phenotype—myopathy and cutaneous vasculopathy (27%, males, Raynaud’s phenomenon, skin ulcers, digital necrosis and calcinosis, early mortality of 4.5%, intermediate prognosis) apart from the aforementioned RP ILD (18%, RP ILD in over 90%, Mechanic’s hands, early mortality of 80%, poorest prognosis), and a dermo-rheumatologic syndrome (55%, typical skin rash, arthritis and ILD, early mortality of 0%, best prognosis) [51]. This suggests that a varied presentation and prognosis of anti-MDA5-positive IMIM may be encountered, subject to ethnicity and the clinical setting, calling for proportionate attention and monitoring on follow-up.
Table 1.
Frequency and demographic characteristics of anti-MD5 DM across various countries in adults and children
| Country | Year | Study design | N | Prevalence of anti MDA-5 (%) | Mean age (SD) | Women (%) |
|---|---|---|---|---|---|---|
| Japan [8] | 2005 | R | 42 | 19 | – | – |
| Japan [44] | 2012 | R | 79 DM | 22 | 55 (13) | 88 |
| Japan [102] | 2012 | R | 27 MDA5 DM | – | 48 (13) | 74 |
| China [9] | 2013 | P | 43 DM | 60 | 46 (13) | 40 |
| Korea [58] | 2010 | R | 38 DM | 24 | 46 (16) | 55 |
| Italy [47] | 2014 | R | 34 DM | 15 | 54 (13) | 60 |
| Spain [50] | 2013 | R | 117 DM | 12 | 47.8 (11) | 64 |
| France [51] | 2020 | R | 121 MDA5 DM | – | Median 49 (34–58) | 67 |
| USA (Stanford) [52] | 2011 | R | 77 | 13 | 51 (9) | 88 |
| USA (Pittsburgh) [49, 103] | 2016 | R | 122 DM | 13 | 43 (18.5) | 56 |
| USA (Hopkins) [46] | 2013 | R | 160 DM | 7 | 41.4 | – |
| India [104] | 2020 | P | 83 DM | 17 | – | 72.7 |
| Canada [75] (70% Asians) | 2019 | R | 21 MDA5 DM | – | 52 (21–69) | 57 |
| UK [105] | 2014 | R | 285 JDM | 7 | 6.3 (4–10) | – |
| USA, Canada [106] | 2020 | R | 453 JIIM | 7.7 | Median 8.7 | – |
| France [40] | 2020 | R | 64 JIIM | 20 | 9 (2–14) | 60 |
| India [104] | 2020 | P | 36 JDM | 11 | – | – |
MDA5 Melanoma Differentiation Associated gene 5, DM Dermatomyositis, P Prospective, R Retrospective, USA United Sates of America, UK United Kingdom, – data unavailable
Table 2.
Clinical features of anti-MDA5 DM described across various cohorts
| Cohort/country | Skin rash (%) | Skin ulcers (%) | ILD (%) | RP ILD (%) | CADM (%) | Arthritis (%) | Cancer (%) | Survival |
|---|---|---|---|---|---|---|---|---|
| Japan [8] | 50–75 | – | – | 50 | 100 | 50 | 0 | – |
| Japan [44] | 50–75 | 60 | 94 | 71 | 75 | – | 0 | 1 year, 60% |
| Japan [102] | – | – | – | 74 | 80 | – | 4 | 1 year, 68% (50% for those with RP ILD) |
| China [9] | 100 | 11 | 100 | 38 | 35 | – | – | 1 year, 40% |
| Korea [58] | – | – | 67 | 44 | 0 | – | 0 | – |
| Italy [47] | 40–80 | 20 | 60 | 20 | 100 | 0 | 0 | – |
| Spain [50] | – | 21 | 64 | 57 | 57 | – | 28 | 1 year, 70% |
| France [51] | 70 | 41 | 76 | 32 | 70 | 70 | 7 | Overall 70% (20% at 1 year with RP ILD) |
| USA (Stanford) [52] | 50–70 | 80 | 67 | 22 | 50 | 31 | – | – |
| USA (Pittsburgh) [49, 103] | 50–70 | 20 | 50 | 43.7 | 50 | 12 | – | 1 year, 70% |
| USA (Hopkins) [46] | 80–100 | 80 | 72 | 0 | 45 | 80 | – | Overall survival, 100% |
| India (adults) [104] | 80 | 20 | 60 | 40 | 0 | 60 | 0 | 1 year, 40% |
| India (children) [104] | 100 | 0 | 33 | 0 | 0 | 33 | 0 | 1 year, 100% |
| Canada [75] (70% Asians) | 100 | 42 | 100 | 38 | 57 | 53 | – | Overall survival, 80% (40% in those with RP ILD) |
| UK [105] | 70–80 | 50 | 10–19 | 0 | 12 | 86 | – | Overall survival, 100% |
| USA, Canada [106] | 60–97 | 30 | 25 | 5 | 0 | 90 | – | Overall Survival 97% |
| France [40] | 77 | 70 | 46 | 7 | – | 100 | – | Overall survival, 93% |
MDA5 melanoma differentiation-associated gene 5, DM dermatomyositis, USA United Sates of America, UK United Kingdom, ILD interstitial lung disease, RP rapidly progressive, – data unavailable
Fig. 4.
Prevalence of anti-MDA5 in adults and children with DM across various countries
Cutaneous
In addition to classic rashes of DM that occur in 60–70%, patients with anti-MDA5 DM may manifest cutaneous features suggestive of an underlying vasculopathy, such as cutaneous ulcers and palmar papules. The cutaneous ulcers associated with anti-MDA5 positive IMIM occur in 40–80% individuals, are deep and punched out, and commonly overly the digital pulp, knuckles, lateral nail folds, elbows and the knees, though they can also occur on the chest, back or arms [51–54].
Palmar papules are another unique feature of this subset of IMIM, reported over the palms, the palmar aspect of fingers, especially over the metacarpophalangeal and interphalangeal joints in up to 60% of patients. They are painful, with lesional biopsies suggestive of occlusive small and medium vessel vasculopathy with a type-I Interferon signature [52, 54, 55]. Tender gums and oral erosions are also characteristically described in this subset [52]. The other reported features include, eyelid edema, non-scarring alopecia (70–80%), mechanic’s hands (60%), oral ulcers (50%), psoriasiform rashes and panniculitis (20%) [52, 56] (Fig. 5).
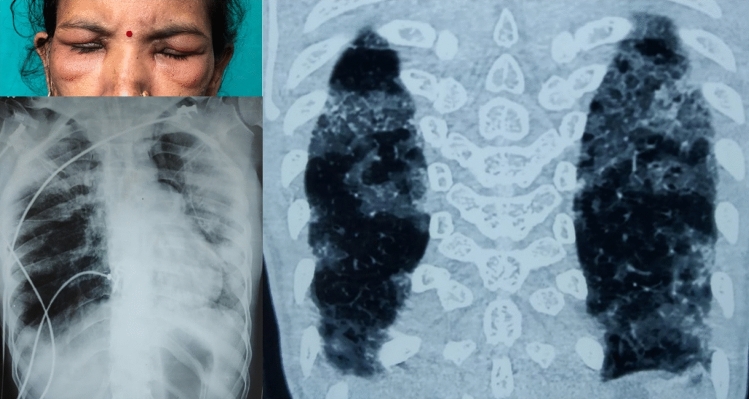
Fig. 5.
Periorbital edema in a patient with anti-MDA5 DM (top left). Chest radiograph (bottom left) showing patchy heterogeneous opacities involving both lung fields. Computed tomography (right) showing patchy consolidations with ground glass opacities involving both lung fields suggestive of an organizing pneumonia pattern
Joint involvement
Arthralgia and Arthritis are seen in 50–80% patients with anti-MDA5 DM. Notably the phenotype is similar to Rheumatoid Arthritis with a bilateral, symmetric distribution and small joint involvement. However, erosions are infrequent on conventional radiographs [46].
Lung
ILD is the most important clinical feature seen in 60–100% of patients, with a rapidly progressive phenotype reported in over 40% (20–75%) cases [44, 49, 51]. Anti-MDA5 DM has a 20 fold higher odds for development of RP ILD as compared to MDA5 negative DM and it is the most important factor predicting survival [9, 44, 49]. RP ILD presents with worsening dyspnea and cough, with radiographic deterioration causing hypoxia within 3 months from respiratory symptom onset [7, 51].
Muscle
Anti-MDA5 antibodies were originally described in a series of patients with CADM [8] with CADM occurring in over 80% of Japanese anti-MDA5 DM patients. However, in cohorts from the USA, most occurred in patients with classic DM, although the association with CADM was retained [8, 49, 57, 58].
Malignancy
Although most studies describe a protective effect of ILD and anti-MDA5 antibodies against cancer [4, 59, 60], malignancy has been described in 4–6% of patients with anti-MDA5 DM in Japanese and French studies. Contrary to previous reports, one Spanish anti-MDA5 DM study described a higher rate of cancer (4/14 cases, 28%) with risk of breast, ovarian and lung cancer.
Other systemic features
Fever, fatigue and constitutional symptoms are also common, occurring in 30–40% of these patients [44, 46, 50, 51].
Clinical phenotype in children with anti-MDA5 DM
The prevalence of anti-MDA5 DM in a large British cohort of JDM was 7% with a predominance of ulcerative skin disease, oral ulcers and arthritis, with ILD affecting 20% of individuals, none of them exhibiting the RP phenotype. CADM was seen in 12% of anti-MDA5 children, which was significantly greater than the anti-MDA5 antibody-negative group. Interestingly, the response to treatment was better than in the anti-MDA5-negative DM group, thus it may represent a milder subgroup in children. An increased association of ILD was seen with coexistent anti-Ro52 antibodies (70% vs 10% had ILD among patients with anti-MDA5 JDM) in an American cohort of JDM but RP ILD was uncommon [61]. Whereas, in East Asian cohorts over 1/3rd of JDM are constituted by anti-MDA5 DM with RP ILD seen in over half of them [7, 62, 63]. There were few anti-MDA5-positive JDM subjects in the Indian cohorts described, though the phenotype was akin to that described in the western literature [64]. A recent French multicenter study identified 13 patients with Juvenile myositis and positive anti-MDA5 antibodies, and this group was constituted equally by Juvenile Dermatomyositis (JDM) and Juvenile Overlap Myositis. They confirmed the presence of ulcerative skin disease and arthritis in frequencies similar to those previously reported; in addition, they identified that a lupus-like rash was present in half of the subjects, and aseptic abscesses in 20% [40].
Complications
Pneumomediastinum is a life-threatening complication reported in up to 15% of patients with anti-MDA5 DM and ILD, as compared to 2% in DM/PM. It is associated with a worse survival (mortality of 60% vs 37% in those without pneumomediastinum) especially in those ventilated using noninvasive pressure ventilation. Thus, in those requiring mechanical ventilation, invasive ventilation is suggested as soon pneumomediastinum is diagnosed. High-flow nasal oxygen merits further exploration in the setting of pneumomediastinum [65, 66]. The presence of cutaneous ulcers was associated with an increased odds (1.1–3.1) of pneumomediastinum in a multivariable analysis of a Chinese study that described predictors of pneumomediastinum in the setting of anti-MDA5 DM [67].
Another complication that is being reported more frequently with this type of DM is Macrophage Activation Syndrome (MAS) as described in case reports from Japan. The recent depictions of transaminitis in anti-MDA5 DM due to involvement of the liver may be a surrogate for extramedullary hemophagocytosis which merits further exploration [68, 69].
Diagnosis
A high index of suspicion for anti-MDA5DM should be considered in patients presenting with the following constellation of symptoms:
Middle-aged female, CADM, skin rash with acute onset of respiratory symptoms;
Middle-aged female with skin rash, arthritis and chronic respiratory symptoms;
Middle-aged male, classic DM rash, muscle weakness, Raynaud’s phenomenon with cutaneous ulceration;
Children with classic DM rash, ulcerative skin disease, ILD with or without muscle weakness.
The most important scenario in which a rapid diagnosis of anti-MDA5 DM has critical implications is the case of patients presenting with RP ILD. Acute interstitial pneumonitis can have a broad differential diagnosis even in the absence of obvious muscle involvement. Subtle rashes should be actively looked for as important clues to the otherwise elusive diagnosis. Although Rheumatologists may be better aware of this entity, it poses a significant diagnostic challenge for pulmonologists and intensivists who may be the primary contact physicians for such cases who rapidly deteriorate with high requirements of oxygen and intensive care support [70]. While ruling out other differentials such as viral pneumonitis, atypical bacterial pneumonia, and hypersensitivity pneumonitis, an asymptomatic elevation of muscle enzymes, cytoplasmic ANA pattern, and anti-MDA5 antibodies may be specifically looked for. In a recent multicenter French study, 36% of 47 IMIM admitted in the Intensive Care Unit with acute respiratory failure had no extrapulmonary feature (40% being anti-MDA5 DM). Timely identification is crucial for the reduction of mortality rates [71].
Anti-MDA5 antibody can be detected using IP (gold standard), line immunoassay or ELISA with a good agreement amongst the assays [5, 13, 72]. Associated Ro-52 antibodies are described in up to 60% of patients, and may pose a greater risk for RP ILD and cutaneous vasculopathy [48–50, 73, 74].
Screening for ILD should be done using HRCT of the chest, common findings being ground glass opacities (GGO), non-septal linear or plate-like opacities and interlobular septal thickening with absence of intralobular reticular opacities, bronchiectasis and honeycombing. The predominant patterns have been described as lower GGO and random GGO seen in over 80% of cases which corresponds to an organizing pneumonia type of ILD. The classic Atoll sign in patients with organizing pneumonia is nonspecific and may be seen in tuberculosis and fungal infections. Thus, a radiological picture suggestive of organizing pneumonia should a prompt consideration of a diagnosis of IMIM when work up for infections is negative. Elevated ferritin levels above 1000 ng/ml are predictive of lower lung predominant GGOs with an odds of 12 which in turn are predictive of a RP course [75, 76] (Fig. 5).
A European study described distinct histopathological features of anti-MDA5 DM as compared to classical DM. Absence of perifascicular fiber atrophy, characteristic vasculopathy, or important perivascular inflammation with lower IFN score was observed with overall minimal inflammatory changes on histopathology. Nitric oxide synthase 2-positive muscle fibers were seen only in this subset, which colocalized with markers of sarcolemmal regeneration and thus might have a protective effect [6, 77].
A recent study described the use of nail fold capillaroscopy to study the capillary architecture. Compared to ASSD, patients with anti-MDA5 DM showed greater scores for microhemorrhages and capillary disorganization representative of severe cutaneous vasculopathy and the scores further correlated with total lung fibrosis and ILD-related death [78].
Biomarkers
Numerous biomarkers have been evaluated to predict mortality and assess response to treatment (Table 3). Ferritin is widely available and elevated levels (> 1000 ng/ml) are predictive of extensive lung involvement and a poor prognosis. It can be used as a marker to initiate intensive immunosuppression and to judge the response to therapy similar to its role in MAS [79].
Table 3.
Biomarkers studied in anti-MDA5 DM
| Marker | Method | Inclusion | n | Marker for mortality | Decline with response | Correlation with relapse | Comment |
|---|---|---|---|---|---|---|---|
| Anti-MDA5 antibody [23] | ELISA | CADM or DM with RP ILD | 12 | – | Yes | Yes | PPV of reappearance or rise in index to > 50 for relapse 100%, NPV of negative result is 100% |
| Anti-MDA5 antibody [84] | ELISA, IP | CADM/DM with ILD | 15 | No | Yes | – | Decreased titers with treatment in 67% at 1 year |
| Ferritin [84] | ELISA | CADM/DM with ILD | 15 | No | Yes | – | Decreased to normal in 75% at 6 months |
| Ferritin [107] | CADM/DM with ILD | 39 | Yes | Yes | Yes | ||
| KL-6 [84] | ELISA | CADM/DM with ILD | 15 | No | Yes | – | Decreased to normal in 60% at 6 months |
| KL-6 [107] | CLEIA | CADM/DM with ILD | 39 | Yes | Yes | Yes | Level > 792 U/L predicted poor prognosis |
| Surfactant protein-D [84] | ELISA | CADM/DM with ILD | 15 | No | Yes | – | |
| Surfactant protein-D [108] | ELISA | MDA5 with ILD | 170 | Yes | – | – | |
| Serum neopterin [84] | ELISA | CADM/DM with ILD | 15 | No | – | – | Decreased to normal in 60% at 6 months |
| Serum neopterin [109] | ELISA | 48 | Yes | – | – | ||
| Serum CD 206 [110] | ELISA | MDA5 DM/CADM ILD | 33 | Yes | – | – | |
| Serum BAFF levels [111] | ELISA | MDA5 DM with ILD | 10 | – | Yes | – | |
| Type I interferon [112] | ELISA for Interferon alpha, IFN gene signature in PBMCs | MDA5 DM | 20 | – | Yes | – |
KL Krebs von den Lungen protein, BAFF B–cell activating factor, NPV negative predictive value, PPV positive predictive value, CADM clinically amyopathic dermatomyositis, DM dermatomyositis, ILD interstitial lung disease, ELISA enzyme linked immunosorbent assay, MDA5 melanoma differentiation-associated gene 5, – data unavailable
Declining titers of anti-MDA5 antibody was associated with survival and re-increase in the levels (by > 50 index from its level during remission) correlated with relapse of disease [23]. However, it is pertinent to note that although a mere absence or presence of antibody correlates with disease, titers of anti-MDA5 antibody at baseline did not show any correlation with severity of manifestations or death [49].
A recent Japanese study identified hepatic dysfunction disproportionate to muscle involvement (defined as disproportionate rise in alanine transaminase as compared to creatine phosphokinase levels) in anti-MDA5 DM. In 50 patients of DM, all 10 with anti-MDA5 DM had liver dysfunction and a subsequent liver biopsy done in four of them showed steatosis and ballooning of hepatocytes. The liver dysfunction correlated with KL-6 levels, but not with presence of ILD. Thus, hepatic dysfunction might represent an extramuscular involvement in this subset [80].
Four-gene interferon signature, serum interferon levels and interferon expression in cutaneous biopsies are elevated in both adults and children with anti-MDA5 IMIM and correlate with response to treatment [40].
Elevated ferritin (> 828 ng/ml), C-reactive protein (CRP, > 1 mg/dL), KL-6 (> 1000 ng/dL), decreased T helper subsets and serum CD 206 identify patients with a poor prognosis and at high risk for mortality. These markers return to normalcy 3–6 months following effective treatment and thus may be useful to guide treatment and monitor for relapses [81, 82].
Treatment
MDA5 DM presenting with RP ILD represents the most severe and difficult to treat form of the disease and mandates intensive immunosuppression. Pulse corticosteroids (15–20 mg/kg/dose) for 3–5 days followed by oral corticosteroids (1 mg/kg) along with immunosuppressants are preferred for induction (Table 4). There is a dearth of clinical studies evaluating the best line of treatment. The current evidence base largely stems from observational studies and case reports. Steroid monotherapy and standard immunosuppressants like methotrexate and azathioprine have shown poor results in RP ILD [14].
Table 4.
Treatment strategies for anti–MDA5 DM
| Drug/treatment | Type of study | n | Outcome |
|---|---|---|---|
| Dual therapy–CS + CYC/CNI | |||
| Gono et al. [102] | R | 8 | Overall survival, 75% |
| Muro et al. [113] | R | 11 | Overall survival, 72% |
| Upfront triple combination–CS + i.v. CYC + CNI | |||
| Gono et al. [102] | R | 12 | Overall survival, 41% |
| Kameda et al. [114] | P | 10 | 1 year survival, 50% |
| Matsuda et al. [88] | R | 8 | 1 year survival, 87.5% |
| Tsuji et al. [89] | P | 29 (15 from historical cohort) | 6 month survival, 89 vs 33% |
| Rituximaba | |||
| Ho so et al. [115] | R | 4 | 2 year survival,100% |
| Tokunaga et al. [116] | R | 2 | Overall survival, 0% |
| Basiliximabb | |||
| Zou et al. [117] | R | 4 | Overall survival, 75% |
| Plasma exchange (PE)a | |||
| Shirakshi et al. [94] | R | 8 of 13 received PE | 1 year survival, 60% vs 0 |
| Saito et al. [118] | R | 6 | 6 month survival, 77% |
| Abe et al. [93] | R | 6 of 10 received PE | 1 year survival, 100% vs 25% |
| Polymyxin B hemoperfusiona | |||
| Okabayashi et al. [119] | R | 14 | 3 month survival, 35.7% |
| Takada et al. [120] | R | 2 | 1 year survival, 50% |
| Case reports [121, 122] | 2 | Overall survival, 100% | |
| Tofacitinib | |||
| Kurasawa et al. [123] | R | 5 | Overall survival, 60% 6–month survival, 100% vs 78% |
| Chen et al. [43] | P | 18 (historical controls) | |
| Pirfenidone add–on | |||
| Li et al. [98] | P | 27 | 1–year survival, 73% vs 50%, subgroup analysis– no impact on survival in the acute ILD group |
| ECMO | |||
| Vuillard et al. [124] | R | 6 | Overall Survival, 0 |
| Hunag et al. | R | 3 | Survival, 100% (followed by lung transplant) |
CS corticosteroids, CYC cyclophosphamide, CNI calcineurin inhibitors, PE plasma exchange, R retrospective, P prospective, ECMO extra corporeal Membrane oxygenation
aRefractory to CS, CYC, CNI
bRefractory to CS, CNI
Recent retrospective series suggest benefit from upfront combination therapy [83–85]. Subsequently, in a prospective study of 29 anti-MDA5 DM Japanese patients with RP ILD, a combination of steroids, tacrolimus and cyclophosphamide (iv CYC) was administered upfront. The 6-month survival rates were significantly better for the combination group (89% vs 33%) as compared with a historical cohort which received step-up immunosuppression. A similar rate of serious infections was recorded in both groups, except for higher Cytomegalovirus (CMV) reactivation in the former (85% vs 35%) [86]. All the three deaths in the combination group were due to worsening ILD. Prophylaxis for pneumocystis carinii pneumonia was given to all, however, CMV prophylaxis was not. Although, there is lack of data for use of CMV prophylaxis in IMIM, there might be a rationale to adopt this measure in the setting of upfront combined immunosuppression. Patients with ILD and anti-MDA5 antibodies are at a higher risk of pneumocystis carinii pneumonia and prophylaxis should considered for these patients [61]. When the use of calcineurin inhibitors is not feasible, Mycophenolate Mofetil and Rituximab have been used, albeit unsuccessfully in some case reports [87, 88].
In refractory cases, plasma exchange, polymyxin B hemoperfusion and IVIG exhibit a variable response [89–91]. Of these, the best results have been seen with plasma exchange with improved 1-year survival up to 100% in some series. Plasma exchange has been used after unsuccessful combination therapy of immunosuppressants, ranging from 3 to 14 sessions per patient [92].
Another class of drugs that has been recently explored are the JAK inhibitors which may have specific benefits in this condition. JAK inhibitors directly interfere with interferon production, which may result in abrogation of the cytokine storm, in a quick and predictable manner unlike most other immunosuppressants which typically take 2–4 weeks to take effect. Lost time may result in a declining foothold on success of survival in a disorder of such severity. Tofacitinib use has led to an improved survival, reduction in ferritin levels and CT scores in case reports [43, 93]. Anakinra has been used successfully as well [94].
The antifibrotic pirfenidone yielded an insignificant effect on the survival in RP ILD in a prospective study albeit with suggested benefit in the subset with a subacute onset of ILD [95].
Extracorporeal Membrane Oxidation as a bridge therapy awaiting response to immunosuppressants or to a lung transplant may be used in equipped centers. There are 8 reported cases of lung transplant with an overall survival rate of 75% over 7 months to 12 years [96, 97].
Cutaneous vasculopathy also represents a severe form of the disease with requirement of immunosuppressants, IVIG along with vasodilators (Phosphodiesterase inhibitors and calcium channel blockers) and agents that improve peripheral circulation (Pentoxiphylline, at times hyperbaric oxygen) for which there is evidence in the form of anecdotal reports [98].
Prognosis
Older age (> 60 years), male gender, fever, elevated CRP more than 1 mg/dL and oxygen saturation less than 95% are associated with poor prognosis and mortality [99, 100] Sato et al. derived a prediction model in a large cohort of Japanese patients of myositis-ILD using a composite of age, CRP, oxygen saturation and anti-MDA5 antibodies for prediction of mortality. Another risk model, ‘FLAIR’, using Ferritin, LDH, anti-MDA5 Antibody, CT Imaging score, and RP ILD has been developed to predict survival in patients with CADM/ILD [101].
Conclusion
Anti-MDA5 DM is recently identified subtype of myositis characteristically associated with RP-ILD and unique cutaneous features. The etiopathogenesis is hypothetically linked to an unidentified viral trigger on the background of genetic predisposition culminating in an acquired type I interferonopathy. The clinical phenotype is highly varied in different ethnicities. The recent description of new cohorts of patients with a wider range of presenting clinical features has expanded the anti-MDA5 DM disease spectrum. Awareness of the condition and its potential manifestations is key to early detection and improved survival. RP ILD is the major determinant of survival, treatment being largely empiric and based on observational studies, with early and aggressive combined immunosuppression showing potential in recent reports.
Author contributions
PM and LG were involved in the conceptualization and methodology. PM was involved in writing of the original draft and LG and PMM were involved in manuscript review and editing.
Funding
PMM is supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC).
Declarations
Conflict of interest
PM and LG report no conflicts of interest/competing interests. PMM has received consulting/speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pankti Mehta, Email: drpankti.m@gmail.com.
Pedro M. Machado, Email: p.machado@ucl.ac.uk
Latika Gupta, Email: drlatikagupta@gmail.com.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 3.McHugh NJ, Tansley SL. Autoantibodies in myositis. Nature reviews. Rheumatology. 2018;14:290–302. doi: 10.1038/nrrheum.2018.56. [DOI] [PubMed] [Google Scholar]
- 4.Betteridge Z, Tansley S, Shaddick G, et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J Autoimmun. 2019;101:48–55. doi: 10.1016/j.jaut.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato S, Murakami A, Kuwajima A, et al. Clinical utility of an enzyme-linked immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PLoS ONE. 2016;11:e0154285. doi: 10.1371/journal.pone.0154285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasin SA, Schutz PW, Deakin CT, et al. Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: analysis by myositis autoantibody and pathological features. Neuropathol Appl Neurobiol. 2019;45:495–512. doi: 10.1111/nan.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi N, Takezaki S, Kobayashi I, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology. 2015;54:784–791. doi: 10.1093/rheumatology/keu385. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–1576. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res. 2013;65:1316–1324. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation–associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776–785. doi: 10.1016/j.jaad.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Moghadam-Kia S, Oddis CV, Aggarwal R. Anti-MDA5 antibody spectrum in western world. Curr Rheumatol Rep. 2018;20:78. doi: 10.1007/s11926-018-0798-1. [DOI] [PubMed] [Google Scholar]
- 12.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 13.Mahler M, Betteridge Z, Bentow C, et al. Comparison of three immunoassays for the detection of myositis specific antibodies. Front Immunol. 2019;10:848. doi: 10.3389/fimmu.2019.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokiyama K, Tagawa H, Yokota E, et al. Two cases of amyopathic dermatomyositis with fatal rapidly progressive interstitial pneumonitis. Ryumachi [Rheumatism] 1990;30:204–209. [PubMed] [Google Scholar]
- 15.Nanke Y, Tateisi M, Yamagata H, et al. A case of amyopathic dermatomyositis with rapidly progressive interstitial pneumonia. Ryumachi [Rheumatism] 2000;40:705–710. [PubMed] [Google Scholar]
- 16.Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193–2200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 17.Kang D, Gopalkrishnan RV, Wu Q, et al. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation–associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res. 2013;65:1316–1324. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 19.Christensen ML, Pachman LM, Schneiderman R, et al. Prevalence of coxsackie b virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986;29:1365–1370. doi: 10.1002/art.1780291109. [DOI] [PubMed] [Google Scholar]
- 20.Chevrel G, Calvet A, Belin V, Miossec P. Dermatomyositis associated with the presence of parvovirus B19 DNA in muscle. Rheumatology. 2000;39:1037–1039. doi: 10.1093/rheumatology/39.9.1037. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima R, Imura Y, Kobayashi S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology. 2010;49:433–440. doi: 10.1093/rheumatology/kep375. [DOI] [PubMed] [Google Scholar]
- 22.Dias Junior AG, Sampaio NG, Rehwinkel J. A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol. 2019;27:75–85. doi: 10.1016/j.tim.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176:395–402. doi: 10.1111/bjd.14882. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Du G, Zhang G, et al. Similarities and differences between severe COVID-19 pneumonia and anti-MDA-5-positive dermatomyositis-associated rapidly progressive interstitial lung diseases: a challenge for the future. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218594. [DOI] [PubMed] [Google Scholar]
- 25.Giannini M, Ohana M, Nespola B, et al. Similarities between COVID-19 and anti-MDA5 syndrome: what can we learn for better care? Eur Respir J. 2020 doi: 10.1183/13993003.01618-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Lorenzis E, Natalello G, Gigante L, et al. What can we learn from rapidly progressive interstitial lung disease related to anti-MDA5 dermatomyositis in the management of COVID-19? Autoimmun Rev. 2020;19:102666. doi: 10.1016/j.autrev.2020.102666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans—The Lancet Respiratory Medicine. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30243-5/fulltext. Accessed 12 Nov 2020 [DOI] [PMC free article] [PubMed]
- 28.Gupta L, Chinoy H. Monitoring disease activity and damage in adult and juvenile idiopathic inflammatory myopathy. Curr Opin Rheumatol. 2020;32:553–561. doi: 10.1097/BOR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 29.Gupta L, Misra DP, Agarwal V, et al. Response to: ‘telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort’ by Costa et al. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217953. [DOI] [PubMed] [Google Scholar]
- 30.Naveen R, Sundaram TG, Agarwal V, Gupta L. Teleconsultation experience with the idiopathic inflammatory myopathies: a prospective observational cohort study during the COVID-19 pandemic. Rheumatol Int. 2020 doi: 10.1007/s00296-020-04737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta L, Lilleker JB, Agarwal V, et al. COVID-19 and myositis—unique challenges for patients. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gono T, Kawaguchi Y, Kuwana M, et al. Brief report: association of HLA-DRB1*0101/*0405 with susceptibility to anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis in the Japanese population. Arthritis Rheum. 2012;64:3736–3740. doi: 10.1002/art.34657. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Wang Y, Kuwana M, et al. HLA-DRB1 alleles as genetic risk factors for the development of anti-MDA5 antibodies in patients with dermatomyositis. J Rheumatol. 2017;44:1389–1393. doi: 10.3899/jrheum.170165. [DOI] [PubMed] [Google Scholar]
- 34.Rothwell S, Chinoy H, Lamb JA, et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann Rheum Dis. 2019;78:996–1002. doi: 10.1136/annrheumdis-2019-215046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochi Y, Kamatani Y, Kondo Y, et al. Splicing variant of WDFY4 augments MDA5 signalling and the risk of clinically amyopathic dermatomyositis. Ann Rheum Dis. 2018;77:602–611. doi: 10.1136/annrheumdis-2017-212149. [DOI] [PubMed] [Google Scholar]
- 36.Muro Y, Sugiura K, Hoshino K, et al. Epidemiologic study of clinically amyopathic dermatomyositis and anti-melanoma differentiation-associated gene 5 antibodies in central Japan. Arthritis Res Ther. 2011;13:R214. doi: 10.1186/ar3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishina N, Sato S, Masui K, et al. Seasonal and residential clustering at disease onset of anti-MDA5-associated interstitial lung disease. RMD Open. 2020;6:e001202. doi: 10.1136/rmdopen-2020-001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun WC, Sun YC, Lin H, et al. Dysregulation of the type I interferon system in adult-onset clinically amyopathic dermatomyositis has a potential contribution to the development of interstitial lung disease. Br J Dermatol. 2012;167:1236–1244. doi: 10.1111/j.1365-2133.2012.11145.x. [DOI] [PubMed] [Google Scholar]
- 39.Bai J, Wu C, Zhong D, et al. Hierarchical cluster analysis of cytokine profiles reveals a cutaneous vasculitis-associated subgroup in dermatomyositis. Clin Rheumatol. 2020 doi: 10.1007/s10067-020-05339-2. [DOI] [PubMed] [Google Scholar]
- 40.Melki I, Devilliers H, Gitiaux C, et al. Anti-MDA5 juvenile idiopathic inflammatory myopathy: a specific subgroup defined by differentially enhanced interferon-α signalling. Rheumatology. 2020;59:1927–1937. doi: 10.1093/rheumatology/kez525. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Gunter-Rahman F, McGrath JA, et al. Expression of interferon-regulated genes in juvenile dermatomyositis versus Mendelian autoinflammatory interferonopathies. Arthritis Res Ther. 2020;22:69. doi: 10.1186/s13075-020-02160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jesus AA, Hou Y, Brooks S, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest. 2020;130:1669–1682. doi: 10.1172/JCI129301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med. 2019;381:291–293. doi: 10.1056/NEJMc1900045. [DOI] [PubMed] [Google Scholar]
- 44.Koga T, Fujikawa K, Horai Y, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology. 2012;51:1278–1284. doi: 10.1093/rheumatology/ker518. [DOI] [PubMed] [Google Scholar]
- 45.Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. 2010;49:1713–1719. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 46.Hall JC, Casciola-Rosen L, Samedy L-A, et al. Anti-melanoma differentiation–associated protein 5–associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. 2013;65:1307–1315. doi: 10.1002/acr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceribelli A, Fredi M, Taraborelli M, et al. Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin Exp Rheumatol. 2014;32(6):891–897. [PubMed] [Google Scholar]
- 48.Platteel ACM, Wevers BA, Lim J, et al. Frequencies and clinical associations of myositis-related antibodies in The Netherlands: a one-year survey of all Dutch patients. J Transl Autoimmun. 2019;2:100013. doi: 10.1016/j.jtauto.2019.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in north American patients with dermatomyositis. J Rheumatol. 2017;44:319–325. doi: 10.3899/jrheum.160682. [DOI] [PubMed] [Google Scholar]
- 50.Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, et al. Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. J Immunol Res. 2014;2014:290797. doi: 10.1155/2014/290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allenbach Y, Uzunhan Y, Toquet S, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody. Neurology. 2020;95:e70–e78. doi: 10.1212/WNL.0000000000009727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narang NS, Casciola-Rosen L, Li S, et al. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. 2015;67:667–672. doi: 10.1002/acr.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao H, Pan M, Kang Y, et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res. 2012;64:1602–1610. doi: 10.1002/acr.21728. [DOI] [PubMed] [Google Scholar]
- 55.Ono N, Kai K, Maruyama A, et al. The relationship between type 1 IFN and vasculopathy in anti-MDA5 antibody-positive dermatomyositis patients. Rheumatology. 2020;59:918. doi: 10.1093/rheumatology/keaa033. [DOI] [PubMed] [Google Scholar]
- 56.Rathore U, Haldule S, Gupta L. Psoriasiform rashes as the first manifestation of anti-MDA5 associated myositis. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa821. [DOI] [PubMed] [Google Scholar]
- 57.Fujikawa K, Kawakami A, Kaji K, et al. Association of distinct clinical subsets with myositis-specific autoantibodies towards anti-155/140-kDa polypeptides, anti-140-kDa polypeptides, and anti-aminoacyl tRNA synthetases in Japanese patients with dermatomyositis: a single-centre, cross-sectional study. Scand J Rheumatol. 2009;38:263–267. doi: 10.1080/03009740802687455. [DOI] [PubMed] [Google Scholar]
- 58.Kang EH, Nakashima R, Mimori T, et al. Myositis autoantibodies in Korean patients with inflammatory myositis: Anti-140-kDa polypeptide antibody is primarily associated with rapidly progressive interstitial lung disease independent of clinically amyopathic dermatomyositis. BMC Musculoskelet Disord. 2010;11:223. doi: 10.1186/1471-2474-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Xu L, Wu H, et al. Characteristics and predictors of malignancy in dermatomyositis: analysis of 239 patients from northern China. Oncol Lett. 2018;16:5960–5968. doi: 10.3892/ol.2018.9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoshino K, Muro Y, Sugiura K, et al. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology. 2010;49:1726–1733. doi: 10.1093/rheumatology/keq153. [DOI] [PubMed] [Google Scholar]
- 61.Sabbagh S, Pinal-Fernandez I, Kishi T, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis. 2019;78:988–995. doi: 10.1136/annrheumdis-2018-215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi I, Okura Y, Yamada M, et al. Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatri. 2011;158:675–677. doi: 10.1016/j.jpeds.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 63.Iwata N, Nakaseko H, Kohagura T, et al. Clinical subsets of juvenile dermatomyositis classified by myositis-specific autoantibodies: experience at a single center in Japan. Mod Rheumatol. 2019;29:802–807. doi: 10.1080/14397595.2018.1511025. [DOI] [PubMed] [Google Scholar]
- 64.Hussain A, Rawat A, Jindal AK, et al. Autoantibodies in children with juvenile dermatomyositis: a single centre experience from north-west India. Rheumatol Int. 2017;37:807–812. doi: 10.1007/s00296-017-3707-4. [DOI] [PubMed] [Google Scholar]
- 65.Zhou M, Ye Y, Yan N, et al. Noninvasive positive pressure ventilator deteriorates the outcome of pneumomediastinum in anti-MDA5 antibody-positive clinically amyopathic dermatomyositis. Clin Rheumatol. 2020;39:1919–1927. doi: 10.1007/s10067-019-04918-2. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi K, Yamaguchi A, Itai M, et al. Clinical features of patients with anti-melanoma differentiation-associated gene-5 antibody-positive dermatomyositis complicated by spontaneous pneumomediastinum. Clin Rheumatol. 2019;38:3443–3450. doi: 10.1007/s10067-019-04729-5. [DOI] [PubMed] [Google Scholar]
- 67.Ma X, Chen Z, Hu W, et al. Clinical and serological features of patients with dermatomyositis complicated by spontaneous pneumomediastinum. Clin Rheumatol. 2016;35:489–493. doi: 10.1007/s10067-015-3001-3. [DOI] [PubMed] [Google Scholar]
- 68.Kishida D, Sakaguchi N, Ueno K, et al. Macrophage activation syndrome in adult dermatomyositis: a case-based review. Rheumatol Int. 2020;40:1151–1162. doi: 10.1007/s00296-020-04590-9. [DOI] [PubMed] [Google Scholar]
- 69.Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune-associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244–246. doi: 10.1080/03009742.2019.1653493. [DOI] [PubMed] [Google Scholar]
- 70.Gupta L, Muhammed H, Naveen R, et al. Insights into the knowledge, attitude and practices for the treatment of idiopathic inflammatory myopathy from a cross-sectional cohort survey of physicians. Rheumatol Int. 2020;40:2047–2055. doi: 10.1007/s00296-020-04695-1. [DOI] [PubMed] [Google Scholar]
- 71.Vuillard C, Pineton de Chambrun M, de Prost N, et al. Clinical features and outcome of patients with acute respiratory failure revealing anti-synthetase or anti-MDA-5 dermato-pulmonary syndrome: a French multicenter retrospective study. Ann Intensive Care. 2018 doi: 10.1186/s13613-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gono T, Okazaki Y, Murakami A, Kuwana M. Improved quantification of a commercial enzyme-linked immunosorbent assay kit for measuring anti-MDA5 antibody. Mod Rheumatol. 2019;29:140–145. doi: 10.1080/14397595.2018.1452179. [DOI] [PubMed] [Google Scholar]
- 73.Xing X, Li A, Li C. Anti-Ro52 antibody is an independent risk factor for interstitial lung disease in dermatomyositis. Respir Med. 2020;172:106134. doi: 10.1016/j.rmed.2020.106134. [DOI] [PubMed] [Google Scholar]
- 74.Temmoku J, Sato S, Fujita Y, et al. Clinical significance of myositis-specific autoantibody profiles in Japanese patients with polymyositis/dermatomyositis. Medicine. 2019 doi: 10.1097/MD.0000000000015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanizawa K, Handa T, Nakashima R, et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med. 2011;105:1380–1387. doi: 10.1016/j.rmed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Zuo Y, Ye L, Liu M, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology. 2020;59:2829–2837. doi: 10.1093/rheumatology/keaa034. [DOI] [PubMed] [Google Scholar]
- 77.Allenbach Y, Leroux G, Suárez-Calvet X, et al. Dermatomyositis with or without anti-melanoma differentiation-associated gene 5 antibodies: common interferon signature but distinct NOS2 expression. Am J Pathol. 2016;186:691–700. doi: 10.1016/j.ajpath.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Wakura R, Matsuda S, Kotani T, et al. The comparison of nailfold videocapillaroscopy findings between anti-melanoma differentiation-associated gene 5 antibody and anti-aminoacyl tRNA synthetase antibody in patients with dermatomyositis complicated by interstitial lung disease. Sci Rep. 2020;10:15692. doi: 10.1038/s41598-020-72752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gono T, Kawaguchi Y, Ozeki E, et al. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol. 2011;21:223–227. doi: 10.1007/s10165-010-0371-x. [DOI] [PubMed] [Google Scholar]
- 80.Nagashima T, Kamata Y, Iwamoto M, et al. Liver dysfunction in anti-melanoma differentiation-associated gene 5 antibody-positive patients with dermatomyositis. Rheumatol Int. 2019;39:901–909. doi: 10.1007/s00296-019-04255-2. [DOI] [PubMed] [Google Scholar]
- 81.Nishioka A, Tsunoda S, Abe T, et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol. 2019;29:814–820. doi: 10.1080/14397595.2018.1548918. [DOI] [PubMed] [Google Scholar]
- 82.Chen F, Wang D, Shu X, et al. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int. 2012;32:3909–3915. doi: 10.1007/s00296-011-2323-y. [DOI] [PubMed] [Google Scholar]
- 83.Nara M, Komatsuda A, Omokawa A, et al. Serum interleukin 6 levels as a useful prognostic predictor of clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease. Mod Rheumatol. 2014;24:633–636. doi: 10.3109/14397595.2013.844390. [DOI] [PubMed] [Google Scholar]
- 84.Nakashima R, Hosono Y, Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus. 2016;25:925–933. doi: 10.1177/0961203316651748. [DOI] [PubMed] [Google Scholar]
- 85.Matsuda KM, Yoshizaki A, Kuzumi A, et al. Combined immunosuppressive therapy provides favorable prognosis and increased risk of cytomegalovirus reactivation in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. J Dermatol. 2020;47:483–489. doi: 10.1111/1346-8138.15274. [DOI] [PubMed] [Google Scholar]
- 86.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation–associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72:488–498. doi: 10.1002/art.41105. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi M, Aoki A, Asakawa K, et al. Cytokine profiles of amyopathic dermatomyositis with interstitial lung diseases treated with mycophenolate. Respirol Case Rep. 2017 doi: 10.1002/rcr2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mao M-M, Xia S, Guo B-P, et al. Ultra-low dose rituximab as add-on therapy in anti-MDA5-positive patients with polymyositis/dermatomyositis associated ILD. Respir Med. 2020;172:105983. doi: 10.1016/j.rmed.2020.105983. [DOI] [PubMed] [Google Scholar]
- 89.Mrosak J, Banasiak K, Edelheit B, et al. Polymyxin-B hemoperfusion as a novel treatment for rapidly progressive interstitial lung disease in a pediatric patient diagnosed with anti-MDA5 juvenile dermatomyositis. J Clin Rheumatol. 2019 doi: 10.1097/RHU.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 90.Abe Y, Kusaoi M, Tada K, et al. Successful treatment of anti-MDA5 antibody-positive refractory interstitial lung disease with plasma exchange therapy. Rheumatology. 2020;59:767–771. doi: 10.1093/rheumatology/kez357. [DOI] [PubMed] [Google Scholar]
- 91.Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa123. [DOI] [PubMed] [Google Scholar]
- 92.T S, M M, Y M, et al (2020) Anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease treated with therapeutic plasma exchange: a case series. J Clinical Apher. https://pubmed.ncbi.nlm.nih.gov/32823371/. Accessed 26 Oct 2020 [DOI] [PubMed]
- 93.Ishikawa Y, Kasuya T, Fujiwara M, Kita Y. Tofacitinib for recurrence of antimelanoma differentiation-associated gene 5 antibody-positive clinically amyopathic dermatomyositis after remission: a case report. Medicine. 2020;99:e21943. doi: 10.1097/MD.0000000000021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Groh M, Rogowska K, Monsarrat O, et al. Interleukin-1 receptor antagonist for refractory anti-MDA5 clinically amyopathic dermatomyopathy. Clin Exp Rheumatol. 2015;33:904–905. [PubMed] [Google Scholar]
- 95.Li T, Guo L, Chen Z, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep. 2016 doi: 10.1038/srep33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang K, Vinik O, Shojania K, et al. Clinical spectrum and therapeutics in Canadian patients with anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis: a case-based review. Rheumatol Int. 2019;39:1971–1981. doi: 10.1007/s00296-019-04398-2. [DOI] [PubMed] [Google Scholar]
- 97.Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, et al. Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum. 2020;50:776–790. doi: 10.1016/j.semarthrit.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeter J, Wolf EG, Richards M, Hill E. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy. J Clin Rheumatol. 2020;26:e266–e267. doi: 10.1097/RHU.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 99.Yamaguchi K, Yamaguchi A, Onuki Y, et al. Clinical features of dermatomyositis associated with anti-MDA5 antibodies by age. Mod Rheumatol. 2020 doi: 10.1080/14397595.2020.1740400. [DOI] [PubMed] [Google Scholar]
- 100.Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology. 2018;57:1212–1221. doi: 10.1093/rheumatology/key060. [DOI] [PubMed] [Google Scholar]
- 101.Lian X, Zou J, Guo Q, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the FLAIR model. Chest. 2020;158:1535–1545. doi: 10.1016/j.chest.2020.04.057. [DOI] [PubMed] [Google Scholar]
- 102.Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. 2012;51:1563–1570. doi: 10.1093/rheumatology/kes102. [DOI] [PubMed] [Google Scholar]
- 103.Moghadam-Kia S, Oddis CV, Sato S, et al. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in us patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res. 2016;68:689–694. doi: 10.1002/acr.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta P, Agarwal V, Gupta L. High early mortality in idiopathic inflammatory myopathies: results from the inception cohort at a tertiary care center in northern India. Rheumatology. 2021 doi: 10.1093/rheumatology/keab001. [DOI] [PubMed] [Google Scholar]
- 105.Tansley SL, Betteridge ZE, Gunawardena H, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16:R138. doi: 10.1186/ar4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mamyrova G, Kishi T, Shi M, et al. Anti-MDA5 autoantibodies associated with juvenile dermatomyositis constitute a distinct phenotype in north America. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye Y, Fu Q, Wang R, et al. Serum KL-6 level is a prognostic marker in patients with anti-MDA5 antibody-positive dermatomyositis associated with interstitial lung disease. J Clin Lab Anal. 2019;33:e22978. doi: 10.1002/jcla.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaieda S, Gono T, Masui K, et al. Evaluation of usefulness in surfactant protein D as a predictor of mortality in myositis-associated interstitial lung disease. PLoS ONE. 2020;15:e0234523. doi: 10.1371/journal.pone.0234523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng Q-L, Zhang Y-M, Liang L, et al. A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin Exp Immunol. 2020;199:314–325. doi: 10.1111/cei.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horiike Y, Suzuki Y, Fujisawa T, et al. Successful classification of macrophage-mannose receptor CD206 in severity of anti-MDA5 antibody positive dermatomyositis associated ILD. Rheumatology. 2019;58:2143–2152. doi: 10.1093/rheumatology/kez185. [DOI] [PubMed] [Google Scholar]
- 111.Matsushita T, Kobayashi T, Kano M, et al. Elevated serum B-cell activating factor levels in patients with dermatomyositis: association with interstitial lung disease. J Dermatol. 2019;46:1190–1196. doi: 10.1111/1346-8138.15117. [DOI] [PubMed] [Google Scholar]
- 112.Zhang SH, Zhao Y, Xie QB, et al. Aberrant activation of the type I interferon system may contribute to the pathogenesis of anti-melanoma differentiation-associated gene 5 dermatomyositis. Br J Dermatol. 2019;180:1090–1098. doi: 10.1111/bjd.16917. [DOI] [PubMed] [Google Scholar]
- 113.Muro Y, Sugiura K, Akiyama M. Limitations of a single-point evaluation of anti-MDA5 antibody, ferritin, and IL-18 in predicting the prognosis of interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Clin Rheumatol. 2013;32:395–398. doi: 10.1007/s10067-012-2142-x. [DOI] [PubMed] [Google Scholar]
- 114.Kameda H, Nagasawa H, Ogawa H, et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32:1719–1726. [PubMed] [Google Scholar]
- 115.So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983–1989. doi: 10.1007/s10067-018-4122-2. [DOI] [PubMed] [Google Scholar]
- 116.Tokunaga K, Hagino N. Dermatomyositis with rapidly progressive interstitial lung disease treated with rituximab: a report of 3 cases in Japan. Intern Med. 2017;56:1399–1403. doi: 10.2169/internalmedicine.56.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zou J, Li T, Huang X, et al. Basiliximab may improve the survival rate of rapidly progressive interstitial pneumonia in patients with clinically amyopathic dermatomyositis with anti-MDA5 antibody. Ann Rheum Dis. 2014;73:1591–1593. doi: 10.1136/annrheumdis-2014-205278. [DOI] [PubMed] [Google Scholar]
- 118.Saito T, Mizobuchi M, Miwa Y, et al. Anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease treated with therapeutic plasma exchange: a case series. J Clin Apher. 2020 doi: 10.1002/jca.21833. [DOI] [PubMed] [Google Scholar]
- 119.Okabayashi H, Ichiyasu H, Hirooka S, et al. Clinical effects of direct hemoperfusion using a polymyxin B-immobilized fiber column in clinically amyopathic dermatomyositis-associated rapidly progressive interstitial pneumonias. BMC Pulm Med. 2017;17:134. doi: 10.1186/s12890-017-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takada T, Aoki A, Asakawa K, et al. Serum cytokine profiles of patients with interstitial lung disease associated with anti-CADM-140/MDA5 antibody positive amyopathic dermatomyositis. Respir Med. 2015;109:1174–1180. doi: 10.1016/j.rmed.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 121.Ichiyasu H, Sakamoto Y, Yoshida C, et al. Rapidly progressive interstitial lung disease due to anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis complicated with cervical cancer: successful treatment with direct hemoperfusion using polymyxin B-immobilized fiber column therapy. Respir Med Case Rep. 2017;20:51–54. doi: 10.1016/j.rmcr.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Teruya A, Kawamura K, Ichikado K, et al. Successful polymyxin B hemoperfusion treatment associated with serial reduction of serum anti-CADM-140/MDA5 antibody levels in rapidly progressive interstitial lung disease with amyopathic dermatomyositis. Chest. 2013;144:1934–1936. doi: 10.1378/chest.13-0186. [DOI] [PubMed] [Google Scholar]
- 123.Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. 2018;57:2114–2119. doi: 10.1093/rheumatology/key188. [DOI] [PubMed] [Google Scholar]
- 124.Vuillard C, Pineton de Chambrun M, de Prost N, et al. Clinical features and outcome of patients with acute respiratory failure revealing anti-synthetase or anti-MDA-5 dermato-pulmonary syndrome: a French multicenter retrospective study. Ann Intensive Care. 2018;8:87. doi: 10.1186/s13613-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]