Abstract
Bloodstream infections (BSI) are a severe complication of antineoplastic chemotherapy or hematopoietic stem cell transplantation (HSCT), especially in the presence of antibiotic resistance (AR). A multinational, multicenter retrospective study in patients aged ≤ 18 years, treated with chemotherapy or HSCT from 2015 to 2017 was implemented to analyze AR among non-common skin commensals BSI. Risk factors associated with AR, intensive care unit (ICU) admission and mortality were analyzed by multilevel mixed effects or standard logistic regressions. A total of 1291 BSIs with 1379 strains were reported in 1031 patients. Among Gram-negatives more than 20% were resistant to ceftazidime, cefepime, piperacillin-tazobactam and ciprofloxacin while 9% was resistant to meropenem. Methicillin-resistance was observed in 17% of S. aureus and vancomycin resistance in 40% of E. faecium. Previous exposure to antibiotics, especially to carbapenems, was significantly associated with resistant Gram-negative BSI while previous colonization with methicillin-resistant S. aureus was associated with BSI due to this pathogen. Hematological malignancies, neutropenia and Gram-negatives resistant to >3 antibiotics were significantly associated with higher risk of ICU admission. Underlying disease in relapse/progression, previous exposure to antibiotics, and need of ICU admission were significantly associated with mortality. Center-level variation showed a greater impact on AR, while patient-level variation had more effect on ICU admission and mortality. Previous exposure to antibiotics or colonization by resistant pathogens can be the cause of AR BSI. Resistant Gram-negatives are significantly associated with ICU admission and mortality, with a significant role for the treating center too. The significant evidence of center-level variations on AR, ICU admission and mortality, stress the need for careful local antibiotic stewardship and infection control programs.
Keywords: antibiotic resistance, intensive care admission and mortality, bloodstream infections, pediatric patients, chemotherapy, allogeneic stem cell transplant
1. Introduction
Infections represent important complications in pediatric patients receiving antineoplastic chemotherapy, or allogeneic hematopoietic stem cell transplantation (HSCT). The introduction of empirical antibacterial therapy with the combination of an anti-pseudomonal beta-lactam and an aminoglycoside in febrile neutropenic cancer patients has significantly decreased mortality [1,2]. Following the results of a recent a meta-analysis [3] monotherapy with beta-lactams active against Gram-negatives (including P. aeruginosa) is now recommended for the management of febrile neutropenia in pediatric patients with cancer [4] for institutions where resistance rates in Gram-negative isolates are low. However, this approach requires careful epidemiologic surveillance and continual re-evaluation of empiric antibiotic regimens in light of evolving institutional microbial resistance patterns [4,5]. Antibiotic resistance is a worldwide problem, although geographic and institution-level differences are observed (https://atlas-surveillance.com/#/heatmap/resistance) (access on 4 March 2021). This phenomenon also affects pediatric patients receiving antineoplastic chemotherapy or allogeneic HSCT [6] who become at risk of receiving an inadequate initial empirical therapy of febrile neutropenia, with an increased likelihood of complicated clinical course [7,8,9,10,11,12,13]. Knowledge of the epidemiology of antibiotic resistant bacterial infections and their consequences in pediatric cancer and HSCT patients is therefore mandatory in order to identify the best management strategies.
Aims of the present study were to describe the proportion of antibiotic resistant non-common skin contaminants causing bloodstream infections (BSI) in pediatric patients receiving antineoplastic treatments or HSCT, to describe clinical risk factors associated with development of antibiotic resistance and to the risk of complicated clinical course, i.e., intensive care unit (ICU) admission and death.
2. Materials and Methods
The study was a retrospective chart review conducted in centers located in Australia (n = 1), Brazil (n = 1), Canada (n = 1), Chile (n = 1), Germany (n = 3), Italy (n = 4), Russian Federation (n = 1) and Switzerland (n = 3). The research ethics board approval was obtained at each site where it was required, according to local regulations.
Inclusion criteria were a BSI diagnosed between 1 January 2015 and 31 December 2017 in patients aged ≤ 18 years, with a disease treated with chemotherapy or allogeneic HSCT, and due to Gram-positive (S. aureus, E. faecalis and E. faecium, viridans streptococci) or Gram-negative rods or yeasts. Episodes due to common skin contaminants (https://www.cdc.gov/nhsn/XLS/Common-Skin-Contaminant-List-June-2011.xlsx) (access on 4 March 2021) were excluded in order to avoid the bias of possible cases of contaminated blood cultures erroneously classified as infections and consequently to know the real impact of antibiotic resistance among “true pathogens” causing BSI.
For each BSI episode, patient-level demographic and disease-related variables were age (years), sex, type of underlying disease and the possible presence of relapse/progression, reception of autologous or allogeneic HSCT and post-transplant phases (pre-engraftment, presence of acute or chronic graft versus host disease). The type of underlying disease was classified into three major groups: (1) hematologic malignancy (HM) including acute lymphoblastic leukemia, acute myeloid leukemia, non-Hodgkin lymphoma, hemophagocytic lymphohistiocytosis and other leukemias; (2) solid tumors (ST) including neuroblastoma, bone or soft tissue sarcoma, central nervous system tumor, Hodgkin disease and other solid tumors; (3) non-malignant diseases (NMD) receiving allogeneic HSCT (bone marrow failure, primary immunodeficiency, and inborn errors of metabolism). Episode-level variables were administration of antibiotics (prophylaxis or therapy) in the 30 days preceding the episode, bacterial infection or colonization by the same pathogen causing the BSI in the three months preceding the episode, presence of neutropenia (absolute neutrophil count (ANC) < 500/μL) at diagnosis of the episode, administration of anti-pseudomonal empirical therapy for febrile neutropenia (piperacillin/tazobactam, ceftazidime or cefepime, monotherapy or combined with an aminoglycoside) at the time of BSI, ICU admission for BSI, and death within 30 days from the episode.
For each isolated strain’s antibiotic, susceptibility to the following drugs was registered: S. aureus: methicillin, vancomycin, daptomycin, linezolid, tigecycline, ceftaroline or ceftobiprole; E. faecalis and E. faecium: ampicillin, vancomycin, teicoplanin, daptomycin, linezolid, tigecycline; viridans streptococci: ampicillin or penicillin; Gram-negatives: meropenem or other carbapenems, colistin, amikacin, gentamycin, tobramycin, ciprofloxacin, ceftazidime, cefepime, piperacillin-tazobactam, tigecycline, ceftolozane-tazobactam and ceftazidime-avibactam; Candida spp.: fluconazole, caspofungin and micafungin. Pathogens were recorded as susceptible or resistant according to the local microbiology laboratory classifications following EUCAST or CLSI methodologies and criteria [14,15], since the minimum inhibitory concentrations were not consistently available. In the case of the definition of intermediate or dose-dependent susceptibility, the strain was recorded as susceptible.
Data were collected at each center by trained personnel and registered in a secure web-based database using the Research Electronic Data Capture (REDCap) platform (www.project-redcap.org) (access on 4 March 2021) [16].
Statistical Analysis
Categorical variables were reported as absolute frequencies and percentages. Continuous data were reported as the median and interquartile range (IQR), due to their non-normal (Gaussian) distribution. Percentages of antibiotic-resistant infections by pathogen were calculated with a 95% confidence interval (CI) and reported with the robust estimator of variance allowing for intra-group correlation due to centers. The association between binary outcome variables (antibiotic resistant BSI, ICU admission or death) and independent variables was assessed by multilevel (three or two levels) mixed effects logistic regressions [17], or by standard logistic regression and reported in terms of the odds ratio (OR) and 95% CI. The three-level model had two random-effects equations, the first was a random intercept at the center level, and the second was a random intercept at the patient level (nested in the center level). Multivariable regressions for the likelihood of antibiotic resistance were focused on groups or single pathogens more representative in terms of the tested susceptibility and frequency of antibiotic resistance. The demographic and clinical characteristics of patients at the time of the BSI were entered into the multivariable models. For the likelihood of ICU admission and death, the antibiotic resistance of the Gram-negatives was also included considering their resistance to 1, 2–3 and 4–5 drugs among meropenem, amikacin, ciprofloxacin, ceftazidime, and piperacillin-tazobactam. A likelihood-ratio test (LR) was used to measure the effect of each predictor and to compare multilevel mixed effects logistic model versus standard logistic regression that was performed in case of a statistically insignificant LR test. All tests were two-tailed and a p value < 0.05 was considered statistically significant. All analyses were performed using Stata (StataCorp. Stata Statistical Software, Release 13.1, College Station, TX, USA, StataCorporation, 2013).
3. Results
A total of 1340 BSIs were registered, but 49 (3.6%) were not eligible since they occurred in patients > 18 years old (n = 18), or after December 31, 2017 (n = 24); age > 18 and year > 2017 (n = 1) or pathogen were not recorded (n = 6). The analysis was therefore performed on 1291 BSIs observed in 1031 patients, 840 (81.5%) with a single episode, 149 (14.4%) with two episodes and 42 (4.1%) with ≥ three episodes. BSIs were observed in 756 (58.6%) males and the median age at BSI was eight (IQR 3–13) years. The most frequent underlying condition was HM (n = 838, 64.9%), mainly acute lymphoblastic leukemia (n = 457, 54.5%), while in 320 (24.8%) BSIs followed HSCT, mainly allogeneic (83.1%). Supplementary Table S1 reports demographic and clinical characteristics at the time of BSI. Overall, 1210 (93.7%) episodes were single-agent (723 Gram-negatives, 402 Gram-positives and 85 fungi) and 81 (6.3%) polymicrobial (74 two-agent and 7 three-agents) for a total of 1379 strains. Among the strains recorded 1289 (93.5%) were bacteria, 831 (64.5%) Gram-negatives, 458 (35.5%) Gram-positives, and 90 (6.5%) fungi. A complete list of the isolated pathogens is available in Supplementary Table S2. E. coli (20.5%) was the most frequently isolated pathogen, followed by S. aureus (13.5%), K. pneumoniae (12.8%), viridans streptococci (12.0%) and P. aeruginosa (10.2%). A. baumannii complex (2.2%), S. maltophilia (2.5%), B. cepacia (0.5%), and anaerobes (0.1%) were rare. Candida (90.0%) was the most frequently isolated yeast genus and C. parapsilosis (30.0%) was the most frequently isolates species. No case of C. auris was registered.
3.1. Resistance to Anti-Infectives
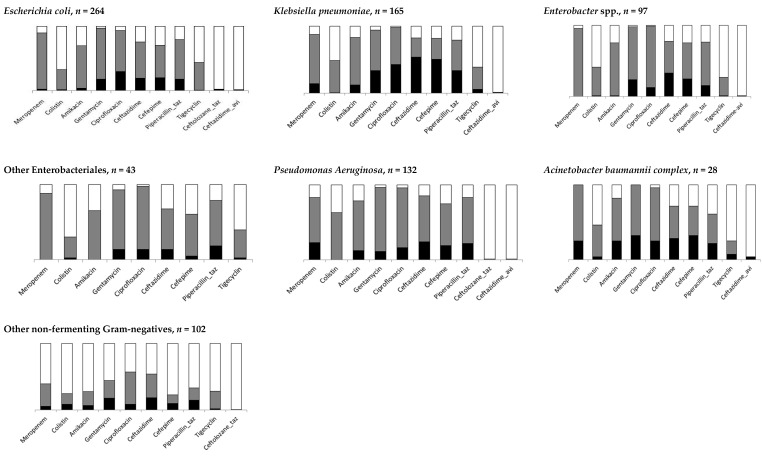
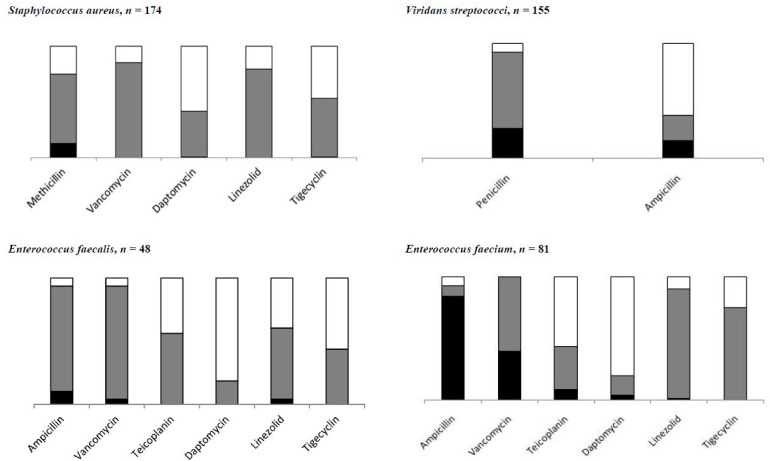
Table 1 summarizes the proportions of antibiotic-resistant Gram-negatives and Gram-positives, while data for specific pathogens and antibiotics are shown in Figure 1 and Figure 2 and detailed in Supplementary Table S3. Among Gram-negatives, the proportion of resistant strains was similar for ceftazidime, cefepime, and ciprofloxacin (29.5%, 25.8% and 25.5%, respectively) and a little lower for piperacillin-tazobactam (21.8%). The overall amikacin resistance was 7.5% but was higher for P. aeruginosa (15.4%), A. baumannii complex (30.4%) and K. pneumoniae (14.6%). Globally, meropenem resistance was 9.0%, with higher proportions for P. aeruginosa (27.3%), A. baumannii complex (25.0%), and K. pneumoniae (15.9%). Among the 489 Enterobacteriales tested, resistance was 5.9% (n = 29) (supplementary Table S3). As for Gram-positives, methicillin resistance was 16.8% for S. aureus (MRSA) without any resistance to vancomycin. Among enterococci, vancomycin resistance was 26.8%, but it was highest in E. faecium (39.5%). Among Candida strains, fluconazole resistance was 27.0% (95% CI 16.2–41.5). Data on yeast resistance to antifungal are detailed in Supplementary Table S3.
Table 1.
Proportions of strains resistant to the antibiotics most frequently used against Gram-negatives and for specific Gram-positives.
| Resistant, n | % Resistance (95% CI) | |
|---|---|---|
| Gram-negatives, n = 797 | ||
| Meropenem | 72 | 9.0 (3.7–20.5) |
| Amikacin | 60 | 7.5 (3.1–17.0) |
| Gentamycin | 173 | 21.7 (11.8–36.5) |
| Ceftazidime | 235 | 29.5 (14.2–51.4) |
| Cefepime | 206 | 25.8 (10.0–52.4) |
| Piperacillin-tazobactam | 174 | 21.8 (16.8–27.8) |
| Ciprofloxacin | 203 | 25.5 (14.2–41.4) |
| S.aureus, n = 131 | ||
| Methicillin | 22 | 16.8 (7.9–32.1) |
| Enterococci, n = 127 | ||
| Ampicillin | 73 | 57.5 (28.0–82.4) |
| Vancomycin | 34 | 26.8 (13.4–46.4) |
| Viridans streptococci | ||
| Penicillin, n = 143 | 40 | 28.0 (18.7–39.6) |
| Ampicillin, n = 58 | 24 | 41.4 (28.1–56.1) |
Figure 1.
Distribution of resistant antibiotic bacteremia by pathogen type (details in Supplementary Table S3) of Gram-negative isolates. Black box represents percentages of resistant bacteremia, gray box represents susceptible bacteremia, and white box for bacteremia not tested.
Figure 2.
Distribution of resistant antibiotic infections by pathogen type (details in Supplementary Table S3) of Gram-positive. Black box represents percentages of resistant antibiotic infections, gray box of susceptible antibiotic infections, and white box for antibiotic infections not tested.
Table 2 and Table 3 report on risk factors for Gram-negative and Gram-positive antibiotic resistant BSIs, respectively. Previous exposure to antibiotics or previous infectious episode due to the same Gram-negative were significantly associated with a higher risk of resistance to all antibiotics except gentamycin (Table 2). Previous exposure to carbapenems was significant for resistance for many antibiotics, and especially to meropenem. No effect was observed for previous colonization on the development of a resistant Gram-negative BSI, except for piperacillin-tazobactam. A center-level variation was observable for all antibiotics, while variation for patients nested within the center was observable for meropenem, gentamycin, ceftazidime, and piperacillin-tazobactam (Table 2). Conversely, among Gram-positives (Table 3), previous colonization was significantly associated with a higher risk of MRSA BSI. A significant effect of the treating center was observable for MRSA or ampicillin-resistant enterococci (Table 3). Supplementary Figures S1 and S2 show proportions of antibiotic-resistant strains stratified by treating center.
Table 2.
Multivariable logistic regression models for BSI due to antibiotic resistant Gram-negatives (n = 797).
| Odds Ratio (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Factors | Meropenem * | Amikacin ** | Gentamycin * | Ciprofloxacin ** | Ceftazidime * | Cefepime ** | Piperacillin-Tazobactam * |
| Sex, p-value | 0.768 | 0.981 | 0.327 | 0.204 | 0.316 | 0.296 | 0.013 |
| Male vs. female | 0.9 (0.4–1.9) | 1.0 (0.5–1.8) | 1.3 (0.7–2.3) | 1.3 (0.9–1.9) | 0.7 (0.4–1.3) | 1.3 (0.8–2.0) | 0.6 (0.3–0.9) |
| Age at bloodstream infections, years, p-value | 0.9 (0.8–1.1), 0.062 | 1.0 (0.9–1.1), 0.383 | 1.0 (0.9–1.1), 0.344 | 1.0 (1.0–1.1), 0.017 | 1.0 (0.9–1.1), 0.324 | 1.0 (0.9–1.1), 0.120 | 1.0 (0.9–1.1), 0.654 |
| Underlying disease, p-value | 0.038 | 0.015 | 0.706 | 0.061 | 0.195 | 0.438 | 0.476 |
| NMD vs. HM | 4.0 (1.1–14.0) | 3.5 (1.5–8.0) | 1.5 (0.6–3.7) | 2.0 (1.0–3.7) | 1.4 (0.5–3.5) | 1.2 (0.6–2.4) | 1.6 (0.7–3.5) |
| ST vs. HM | 0.8 (0.3–2.4) | 1.0 (0.4–2.4) | 1.0 (0.5–2.1) | 0.8 (0.5–1.4) | 0.6 (0.3–1.2) | 0.7 (0.4–1.3) | 1.2 (0.7–2.2) |
| Allogeneic stem cell transplant, p-value | 0.533 | 0.611 | 0.199 | 0.773 | 0.927 | 0.443 | 0.382 |
| Yes vs. no | 1.3 (0.5–3.3) | 0.8 (0.4–1.8) | 1.8 (0.9–3.7) | 0.9 (0.6–1.6) | 1.0 (0.5–2.1) | 0.8 (0.4–1.4) | 1.3 (0.7–2.4) |
| Relapse/ progression, p-value | 0.279 | 0.438 | 0.150 | 0.099 | 0.218 | 0.008 | 0.467 |
| Yes vs. no | 1.6 (0.7–3.9) | 1.3 (0.7–2.6) | 1.6 (0.8–3.0) | 1.4 (0.9–2.2) | 1.5 (0.8–2.9) | 2.0 (1.2–3.3) | 1.2 (0.7–2.1) |
| BSI, p-value | 0.768 | 0.298 | 0.668 | 0.392 | 0.216 | 0.280 | 0.028 |
| Single agent vs. polymicrobial | 0.8 (0.1–4.0) | 0.5 (0.1–1.8) | 0.8 (0.3–2.1) | 1.4 (0.6–3.4) | 0.5 (0.2–1.4) | 0.6 (0.2–1.5) | 0.4 (0.2–0.9) |
| Previous antibacterial exposure (prophylaxis/therapy)1, p-value | <0.001 | <0.001 | 0.138 | 0.008 | 0.009 | 0.215 | <0.001 |
| Standard regimen vs. none | 5.1 (1.5–17.4) | 4.5 (1.8–11.4) | 2.1 (1.1–4.2) | 1.7 (1.0–2.8) | 2.2 (1.1–4.6) | 1.7 (0.9–2.9) | 3.3 (1.7–6.3) |
| Carbapenems vs. none | 31.5 (5.1–193.4) | 7.3 (2.6–20.1) | 2.0 (0.8–4.7) | 2.5 (1.4–4.7) | 3.8 (1.4–10.2) | 1.9 (0.9–3.8) | 3.4 (1.5–7.4) |
| Fluoroquinolones/β-lactams/Combination 2/Others vs. none | 5.8 (1.3–25.7) | 2.2 (0.7–6.9) | 1.4 (0.6–3.3) | 2.1 (1.1–3.7) | 1.4 (0.6–3.5) | 1.4 (0.7–2.9) | 2.3 (1.1–4.9) |
| Neutropenia, p-value | 0.016 | 0.494 | 0.485 | 0.427 | 0.872 | 0.190 | 0.048 |
| Yes vs. no | 3.1 (1.1–8.9) | 1.3 (0.6–2.7) | 1.3 (0.7–2.4) | 1.2 (0.8–1.9) | 0.9 (0.5–1.8) | 1.4 (0.8–2.4) | 1.7 (1.0–3.0) |
| Previous colonization, p-value | 0.120 | 0.257 | 0.895 | 0.891 | 0.421 | 0.653 | 0.035 |
| No vs. yes | 2.2 (0.8–5.7) | 1.6 (0.7–3.5) | 1.0 (0.5–2.2) | 1.0 (0.6–1.8) | 1.4 (0.6–2.9) | 1.1 (0.6–2.0) | 2.0 (1.0–3.8) |
| Previous infection, p-value | 0.161 | 0.040 | 0.280 | <0.001 | <0.001 | <0.001 | 0.031 |
| No vs. yes | 1.9 (0.8–5.0) | 2.2 (1.1–4.6) | 1.5 (0.7–3.3) | 2.6 (1.5–4.4) | 3.9 (1.7–8.9) | 3.6 (1.8–6.9) | 2.0 (1.1–3.7) |
| Random effect, variance component, centre | 2.0 (0.4–9.1) | 1.6 (0.4–6.2) | 1.1 (0.3–3.7) | 1.8 (0.7–4.7) | 4.4 (1.3–14.6) | 8.4 (2.9–24.9) | 0.7 (0.2–2.6) |
| Random effect, variance component, patient | 1.8 (0.1–28.9) | NA | 2.9 (0.7–11.0) | NA | 2.7 (0.6–12.5) | NA | 1.3 (0.2–7.0) |
| LR test vs. logistic regression, p-value *** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 |
* Three-level mixed effects logistic regression with random effects for patients nested within centers. ** Two-level mixed effects logistic regression with random effects for centers. *** If p-value of likelihood-ratio test (LR) test comparing multilevel mixed effects logistic model versus standard logistic regression was not statistically significant, standard logistic regression was adopted. 1 Due to low numbers, Fluoroquinolones, β-lactams not active vs. P. aeruginosa, combination and other previous exposure were grouped together. 2 Combination of two or more of the following fluoroquinolone/β-lactams not active vs. P. aeruginosa/Standard regimen active vs. P. aeruginosa/carbapenem. NMD: Non-malignant disease receiving allogeneic stem cell transplant; HM: hematologic malignancy; ST: solid tumors; NA: not applicable.
Table 3.
Multivariable logistic regression models for models for BSI due to antibiotic resistant among Gram-positives.
| Odds Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Factors | Methicillin-Staphylococcus aureus, n = 131 * | Penicillin-Viridians Streptococci, n = 143 ** | Ampicillin-Viridians Streptococci, n = 58 ** | Ampicillin-Enterococcus (faecalis & faecium), n = 127 * | Vancomycin-Enterococcus(faecalis & faecium), n = 127 ** |
| Sex, p-value | 0.760 | 0.792 | 0.228 | 0.490 | 0.197 |
| Male vs. female | 1.2 (0.3–4.9) | 0.9 (0.4–2.0) | 0.4 (0.1–1.7) | 1.5 (0.5–4.5) | 1.9 (0.7–5.1) |
| Age at bloodstream infections, years, p-value | 1.0 (0.9–1.1), 0.868 | 0.9 (0.8–1.1), 0.107 | 0.9 (0.8–1.1), 0.118 | 1.0 (0.9–1.1), 0.790 | 1.0 (0.9–1.1), 0.790 |
| Underlying disease, p-value | 0.462 | 0.418 | 0.947 | 0.072 | 0.570 |
| NMD vs. HM | 1.4 (0.2–9.7) | 1.0 (0.1–9.8) | NA | 0.1 (0.0–1.2) | 0.4 (0.1–4.9) |
| ST vs. HM | 2.7 (0.5–13.6) | 0.4 (0.1–1.8) | 0.9 (0.1–5.8) | 0.4 (0.1–1.6) | 0.7 (0.2–2.9) |
| Allogeneic stem cell transplant, p-value | 0.324 | 0.693 | 0.461 | 0.046 | 0.796 |
| Yes vs. no | 2.9 (0.3–24.4) | 1.3 (0.4–4.6) | 2.2 (0.5–19.5) | 5.2 (0.9–29.2) | 1.2 (0.3–4.4) |
| Relapse/ progression, p-value | 0.278 | 0.890 | 0.498 | 0.282 | 0.550 |
| Yes vs. no | 0.3 (0.1–2.4) | 0.9 (0.3–2.7) | 0.5 (0.1–3.1) | 2.0 (0.6–7.4) | 1.3 (0.5–3.6) |
| BSI, p-value | 0.176 | 0.447 | 0.879 | 0.895 | 0.925 |
| Single agent vs. polymicrobial | 0.1 (0.0–2.4) | 0.6 (0.2–2.0) | 1.2 (0.2–8.5) | 1.1 (0.2–6.1) | 0.9 (0.2–4.8) |
| Previous antibacterial exposure (prophylaxis/therapy), p-value | 0.004 | ||||
| Yes vs. no | NA | NA | 7.7 (1.7–35.5) | NA | NA |
| Previous antibacterial exposure (prophylaxis/therapy) 1, p-value | 0.068 | 0.489 | 0.396 | 0.083 | |
| Standard regimen vs. no one | 6.4 (1.1–39.5) | 1.8 (0.6–5.6) | NA | 1.1 (0.2–7.1) | 3.2 (0.4–25.5) |
| Carbapenem vs. no one | NA | 1.6 (0.4–6.1) | NA | 1.3 (0.2–8.2) | 8.7 (0.8–90.7) |
| Fluoroquinolones/β-lactams/Combination 2/Others vs. no one | 4.5 (0.8–26.7) | 2.2 (0.7–6.7) | NA | 3.6 (0.5–24.5) | 2.5 (0.2–25.2) |
| Neutropenia, p-value | 0.656 | 0.840 | 0.617 | 0.047 | 0.048 |
| Yes vs. no | 0.7 (0.2–3.1) | 1.1 (0.3–4.4) | 0.6 (0.1–4.1) | 3.7 (0.9–14.5) | 3.5 (1.1–11.1) |
| Previous colonization, p-value | 0.013 | 0.414 | 0.918 | 0.346 | 0.073 |
| Yes vs. no | 6.7 (1.4–31.3) | 0.3 (0.1–4.6) | 0.9 (0.1–10.8) | 2.2 (0.4–11.9) | 2.6 (0.9–7.4) |
| Previous infection, p-value | 0.556 | 0.725 | 0.021 | 0.726 | |
| Yes vs. no | NA | 1.5 (0.4–6.4) | 0.6 (0.1–7.9) | 5.7 (1.1–28.8) | 0.8 (0.2–2.7) |
| Random effect, variance component, centre | 2.7 (0.3–22.7) | NA | NA | 1.5 (0.3–7.0) | NA |
| LR test vs. logistic regression, p-value *** | 0.0255 | 0.5326 | 1.000 | 0.0002 | 0.6495 |
* Two-level mixed effects logistic regression with random effects for centers. ** Standard logistic regression *** If p-value of likelihood-ratio test (LR) test comparing multilevel mixed effects logistic model versus standard logistic regression was not statistically significant, standard logistic regression was performed. 1 Due to low numbers, Fluoroquinolones, β-lactams not active vs. P. aeruginosa, combination and other previous exposure were grouped into one group. 2 Combination of two or more of the following fluoroquinolone/β-lactams not active vs. P. aeruginosa/Standard regimen active vs. P. aeruginosa/carbapenem. NMD: Non-malignant disease receiving allogeneic stem cell transplant; HM: hematologic malignancy; ST: solid tumors; NA: not applicable.
3.2. Admission in Intensive Care Unit and Mortality
Table 4 reports risk factors for ICU admission or death. A total of 171 (13.2%) episodes required ICU admission. HM had a greater risk of ICU admission as well as BSI in the presence of neutropenia, previous exposure to carbapenems and BSI due to Gram-negatives resistant to >3 antibiotics. Overall, death was reported for 99 (7.7%) episodes and in 67 (67.7%) it was attributed to BSI. The underlying disease in relapse/progression, a previous exposure to antibiotics (mainly carbapenems or combination therapy), and a need for ICU admission for BSI were significantly associated with mortality. Patient-level variation showed a greater impact on ICU admission and mortality than center-level variation.
Table 4.
Multivariable logistic regression models for ICU admission or mortality during BSI.
| Factors | Odds Ratio (95% Confidence Interval) | |
|---|---|---|
| ICU Admission * | Mortality * | |
| Sex, p-value | 0.302 | 0.724 |
| Male vs. female | 0.7 (0.4–1.3) | 0.9 (0.4–1.9) |
| Age at bloodstream infections, years, p-value | 1.0 (0.9–1.1), 0.210 | 0.9 (0.8–1.1), 0.068 |
| Underlying disease, p-value | 0.018 | 0.098 |
| NMD vs. HM | 0.8 (0.3–2.2) | 3.6 (1.0–13.4) |
| ST vs. HM | 0.3 (0.1–0.8) | 1.2 (0.4–3.3) |
| Relapse/ progression, p-valueYes vs. no | 0.2701.5 (0.7–2.9) | 0.0045.3 (1.7–16.5) |
| Allogeneic stem cell transplant phase, p-value | 0.5778 | 0.089 |
| Pre-engraftment vs. no allogenic-HSCT | 1.3 (0.5–3.0) | 0.8 (0.2–2.9) |
| Acute GvHD vs. no allogenic-HSCT | 2.1 (0.5–8.9) | 1.7 (0.3–10.8) |
| Chronic GvHD vs. no allogenic-HSCT | 1.8 (0.3–11.3) | 7.0 (0.9–51.6) |
| Post-engraftment vs. no allogenic-HSCT | 0.6 (0.1–2.1) | 4.2 (1.0–17.8) |
| Neutropenia, p-value | 0.023 | 0.327 |
| Yes vs. no | 2.5 (1.2–5.3) | 1.6 (0.6–4.1) |
| Previous antibacterial exposure (prophylaxis/therapy)1, p-value | 0.267 | 0.002 |
| Fluoroquinolones vs. no one/β-lactams | 1.4 (0.3–6.8) | 2.5 (0.3–21.0) |
| Standard regimen vs. no one/β-lactams | 1.4 (0.7–2.8) | 0.9 (0.3–2.7) |
| Carbapenem vs. no one/β-lactams | 2.8 (1.2–6.7) | 3.8 (1.1–13.5) |
| Combination 2 vs. no one/β-lactams | 1.1 (0.1–8.4) | 9.1 (1.1–77.8) |
| Others vs. no one/β-lactams | 2.0 (0.6–6.6) | 8.2 (1.6–41.9) |
| Previous colonization, p-value | 0.193 | 0.265 |
| Yes vs. no | 0.6 (0.2–1.3) | 1.8 (0.6–5.3) |
| Previous infection, p-value | 0.835 | 0.095 |
| Yes vs. no | 0.9 (0.4–2.0) | 2.7 (0.8–8.9) |
| BSI, p-value | 0.936 | 0.441 |
| Single agent vs. polymicrobial | 0.9 (0.3–2.8) | 0.5 (0.1–2.6) |
| Gram-negatives antibiotic resistance, p-value | <0.001 | 0.167 |
| Gram-negatives resistant to 1 antibiotic 3 vs. susceptible | 0.3 (0.1–0.8) | 3.4 (0.8–13.9) |
| Gram-negatives resistant to 2 or 3 antibiotics 3 vs. susceptible | 0.7 (0.3–1.9) | 3.7 (1.0–13.7) |
| Gram-negatives resistant to 4 or 5 antibiotics 3 vs. susceptible | 18.0 (3.7–87.2) | 4.5 (1.0–20.0) |
| Not applicable vs. susceptible | 0.8 (0.4–1.6) | 2.3 (0.8–6.5) |
| ICU for bloodstream infection, p-value Yes vs. no | NA | <0.001,44.4 (7.6–258.5) |
| Random effect, variance component, center | 1.8 (0.5–6.6) | 0.8 (0.2–5.9) |
| Random effect, variance component, patient | 4.4 (0.9–20.2) | 3.8 (0.7–19.7) |
| LR test vs. logistic regression, p-value ** | <0.0001 | 0.0172 |
* Three-level mixed effects logistic regression with random effects for patients nested within centers. ** If p-value of likelihood-ratio test (LR) test, comparing multilevel mixed effects logistic model versus standard logistic regression, was not statistically significant, standard logistic regression was performed. 1 β-lactams not active vs. P. aeruginosa was considered as reference group due to no observed events in this group. 2 Combination of two or more of the following fluoroquinolone/β-lactams not active vs. P. aeruginosa/Standard regimen active vs. P. aeruginosa/carbapenem. 3 Meropenem, amikacin, ciprofloxacin, ceftazidime and piperacillin-tazobactam. NMD: Non-malignant disease receiving allogeneic stem cell transplant; HM: Hematologic malignancy; ST: Solid tumors; NA: Not applicable.
4. Discussion
In this multicenter, multinational retrospective study, we collected 1291 BSIs due to non-common skin contaminants occurring in pediatric patients treated with chemotherapy or allogeneic HSCT to study proportions of resistant strains and risk factors for antibiotic resistance, ICU admission, or death.
Resistance to antibiotics was high among Gram-negatives being approximately 25% for ceftazidime and cefepime and near 20% for piperacillin-tazobactam. Resistance to meropenem was < 10%, but was higher for K. pneumoniae (15.9%), P. aeruginosa (27.3%), and A. baumannii complex (25.0%), the 3rd, 5th, and 9th most frequently reported pathogens, respectively. These proportions of resistant strains are worrisome since they regard the antibiotics generally recommended as monotherapy for empirical treatment of febrile neutropenia in pediatric patients [2,4], with the consequent non-negligible risk of treatment failure. Previous exposure to antibiotics, with a highest risk for carbapenems [18,19], was significantly associated with antibiotic-resistant Gram-negative BSI, as reported in adults [20]. Finally, a BSI due to Gram-negatives resistant to >3 antibiotics was significantly associated with ICU admission and death. It is noteworthy that a previous colonization or infection by the same pathogen did not affect ICU admission or death, perhaps since the choice of empirical therapy in case of febrile neutropenia in a colonized patient could have been guided by this information. The study also showed that about 25% of Gram-negatives were resistant to ciprofloxacin. This finding indicates the need for a rethink on the fluoroquinolone prophylaxis of febrile neutropenia, which, despite some effectiveness during chemotherapy courses (but not pre-engraftment neutropenia), has been associated with increased antibiotic resistance [21,22,23,24]. In this regard, it should be noted that in the most recent pediatric guidelines this procedure has received a weak recommendation about its use [25]. Multilevel mixed-effects logistic regressions showed an evident center-level variation on antibiotic resistance among Gram-negatives. Taken together, all of these observations emphasize the need for the establishment at the local level of antimicrobial stewardship and infection prevention and control programs [4,5,25,26,27], which could also have a favorable impact on the management of infectious episodes and their complications. S.aureus was the second most frequently reported pathogen, with MRSA detected in near 1/6 of cases; VRE represented near 40% of E.faecium strains, but was about 4% in E.faecalis, while penicillin/ampicillin resistance was frequent among viridans streptococci (28 and 41.4%, respectively). MRSA colonization was a significant risk factor for resistant BSI, similarly to what is generally observed for surgical site infections [28]. Finally, yeasts represented an infrequent cause of BSI in this patient population.
Conditions related to the underlying disease and its treatment also had a significant impact on ICU admission and mortality: HM was significantly associated with the risk of ICU admission, as well as a BSI developing during neutropenia alongside a relapsing/resistant disease or ICU admission was associated with an increased risk of death.
This study represents the largest available series on antimicrobial susceptibility of non-common skin-contaminant bacteria causing BSI in pediatric patients receiving chemotherapy or HSCT collected from different parts of the world and provides important information on the burden of antibiotic resistance in this patient population and its relationship with complicated clinical course, but it also has important limitations. The choice of not collecting data on BSI due to common skin contaminants permitted a better understanding of the phenomenon of antibiotic resistance and its consequences, but could have biased the results at least partially, especially after observations of infections due to multidrug resistant coagulase-negative staphylococci [29]. Moreover, we do not know if the carbapenem resistance we observed was due to carbapenemases (and if so, which ones) or other mechanisms. Knowledge of this aspect could have important implications since new antibiotics as ceftazidime-avibactam or meropenem-vaborbactam [30,31] are not effective against some carbapenemases frequently identified in pediatric patients [26], while ceftolozane-tazobactam could have some effectiveness against these strains [32]. Unfortunately, while some pediatric pharmacological data are available for ceftazidime-avibactam [33] and ceftolozane-tazobactam [34,35], they are scarce and fragmented, when not available at all, for meropenem-vaborbactam [36], cefiderocol [37], and cefepime-zidebactam [38], drugs that could be effective against bacteria resistant to the other antibiotics. We do not have data on the in vitro effectiveness of the new antibiotics in our patient population since they were tested in a negligible proportion of strains, if any, maybe because of scarce availability and/or the restriction or absence of authorizations in pediatrics. This is another limitation. Due to the multinational nature of the study, we were reliant on local antimicrobial cultures, susceptibility testing techniques, and reporting. While this variation could impact our results, the large number of episodes involved maintains the generalizability of the results.
Finally, this study showed the significant effect of local conditions on the development of antibiotic resistant BSI and unfavorable outcomes. Local antimicrobial stewardship and adherence to infection control programs are mandatory in order to reduce the spread of resistant pathogens and the unnecessary use of antibiotics, as well as studies on new antibiotics in order to quickly offer the best therapeutic strategies in children with underlying diseases that could expose the patient to severe infections and their complications.
Acknowledgments
Authors thank Lara I. Maissen and Noemi H. Steiner for their support in the data collection and Manuela Rescali for the support in the preparation of the paper.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/10/3/266/s1, Table S1: demographic and clinical characteristics at time of BSI, Table S2 reports a complete list of isolated pathogens, Table S3 reports data on yeast resistance of specific pathogens to antibiotics and antifungals; Figures S1 and S2 show proportions of antibiotic resistant strains stratified by treating center.
Author Contributions
All authors contributed to this article. Study design, results analysis and discussion, paper 1st draft and revision: E.C. Overall data quality control and statistical analysis: F.B. Study design, local data collection, overall quality control, results analysis and discussion, paper revision: A.M., P.K.A.A. Study design, results analysis, discussion, paper revision: R.A.A., F.C., M.E.S.d.P., A.H.G., G.M.H., T.L., A.S., L.S. Local data quality control and collection, paper revision: M.R.D., G.M., G.R., G.S., A.T. Data quality control and local collection: A.D., S.P., M.S., Y.T. Local data quality control and collection: E.A.I., M.L., M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was partially supported by grants from IRCCS Istituto Giannina Gaslini, Genoa—Italy, and Ministero della Salute, Ricerca corrente 2011, Ricerca scientifica corrente 2011 MSALRC11 DEL.30/12.
Institutional Review Board Statement
Research ethics board approval was obtained at each site where it was required, according to institutional or national regulations. Among sites that did require research ethics board approval, the need for informed consent was waived given the retrospective nature of the study. Data were anonymized according to REDCap procedures and European Union Data Protection Rules (https://ec.europa.eu/commission/priorities/justice-and-fundamental-rights/data-protection/2018-reform-eu-data-protection-rules_en).
Data Availability Statement
Data is contained within the article or supplementary material. They are stored in a REDCap database and can be available on request from the corresponding author. The data are not publicly available due to personal data protection rules.
Conflicts of Interest
Authors have no conflict of interest to declare for the present study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dupont H.L., Spink W.W. Infections Due to Gram-Negative Organisms: An Analysis Of 860 Patients with Bacteremia at the University of Minnesota Medical Center, 1958. Medicine. 1969;48:307–332. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mikulska M., Viscoli C., Orasch C., Livermore D.M., Averbuch D., Cordonnier C., Akova M. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J. Infect. 2014;68:321–331. doi: 10.1016/j.jinf.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Robinson P.D., Lehrnbecher T., Phillips R., Dupuis L.L., Sung L. Strategies for Empiric Management of Pediatric Fever and Neutropenia in Patients with Cancer and Hematopoietic Stem-Cell Transplantation Recipients: A Systematic Review of Randomized Trials. J. Clin. Oncol. 2016;34:2054–2060. doi: 10.1200/JCO.2015.65.8591. [DOI] [PubMed] [Google Scholar]
- 4.Lehrnbecher T., Robinson P., Fisher B., Alexander S., Ammann R.A., Beauchemin M., Carlesse F., Groll A.H., Haeusler G.M., Santolaya M., et al. Guideline for the Management of Fever and Neutropenia in Children with Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J. Clin. Oncol. 2017;35:2082–2094. doi: 10.1200/JCO.2016.71.7017. [DOI] [PubMed] [Google Scholar]
- 5.Castagnola E., Caviglia I., Pescetto L., Bagnasco F., Haupt R., Bandettini R. Antibiotic susceptibility of Gram-negatives isolated from bacteremia in children with cancer. Implications for empirical therapy of febrile neutropenia. Futur. Microbiol. 2015;10:357–365. doi: 10.2217/fmb.14.144. [DOI] [PubMed] [Google Scholar]
- 6.Tumbarello M., Viale P., Viscoli C., Trecarichi E.M., Tumietto F., Marchese A., Spanu T., Ambretti S., Ginocchio F., Cristini F., et al. Predictors of Mortality in Bloodstream Infections Caused by Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: Importance of Combination Therapy. Clin. Infect. Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 7.Shankar K., Radhakrishnan V., Vijayakumar V., Ramamoorthy J., Ganesan P., Dhanushkodi M., Ganesan T.S., Sagar T.G. Prevalence of multi-drug resistant organisms in stool of paediatric patients with acute leukaemia and correlation with blood culture positivity: A single institution experience. Pediatr. Blood Cancer. 2018;65:6740. doi: 10.1002/pbc.26740. [DOI] [PubMed] [Google Scholar]
- 8.Satlin M.J., Walsh T.J. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistantEnterococcus: Three major threats to hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2017;19:e12762. doi: 10.1111/tid.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Averbuch D., Tridello G., Hoek J., Mikulska M., Akan H., Segundo L.Y.S., Pabst T., Özçelik T., Klyasova G., Donnini I., et al. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin. Infect. Dis. 2017;65:1819–1828. doi: 10.1093/cid/cix646. [DOI] [PubMed] [Google Scholar]
- 10.Haeusler G.M., Mechinaud F., Daley A.J., Starr M., Shann F., Connell T.G., Bryant P.A., Donath S., Curtis N. Antibiotic-resistant Gram-negative Bacteremia in Pediatric Oncology Patients—Risk Factors and Outcomes. Pediatr. Infect. Dis. J. 2013;32:723–726. doi: 10.1097/INF.0b013e31828aebc8. [DOI] [PubMed] [Google Scholar]
- 11.Bodro M., Gudiol C., Vidal G.C., Tubau F., Contra A., Boix L., Domenech D.E., Calvo M., Carratalà J. Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support. Care Cancer. 2014;22:603–610. doi: 10.1007/s00520-013-2012-3. [DOI] [PubMed] [Google Scholar]
- 12.Girmenia C., Gitmo T., Rossolini G.M., Piciocchi A., Bertaina A., Pisapia G., Pastore D., Sica S., Severino A., Cudillo L., et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: A nationwide retrospective survey from Italy. Bone Marrow Transplant. 2014;50:282–288. doi: 10.1038/bmt.2014.231. [DOI] [PubMed] [Google Scholar]
- 13.Spychala Z.O., Wachowiak J., Kwiatkowska G.O., Gietka A., Baginska D.B., Semczuk K., Fangrat D.K., Czyzewski K., Dziedzic M., Wysocki M., et al. Prevalence, Epidemiology, Etiology, and Sensitivity of Invasive Bacterial Infections in Pediatric Patients Undergoing Oncological Treatment: A Multicenter Nationwide Study. Microb. Drug Resist. 2021;27:53–63. doi: 10.1089/mdr.2019.0393. [DOI] [PubMed] [Google Scholar]
- 14.EUCAST v 10.0 Recommendations The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.1; EUCAST. [(accessed on 4 March 2021)]; Available online: http://www.eucast.org.
- 15.The Clinical & Laboratory Standards Institute (CLSI) Global Laboratory Standards for a Healthier World. [(accessed on 4 March 2021)]; Available online: https://clsi.org.
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman A., Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models (Analytical Methods for Social Research) Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- 18.MacFadden D., Coburn B., Shah N., Robicsek A., Savage R., Elligsen M., Daneman N. Utility of prior cultures in predicting antibiotic resistance of bloodstream infections due to Gram-negative pathogens: A multicentre observational cohort study. Clin. Microbiol. Infect. 2018;24:493–499. doi: 10.1016/j.cmi.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Giacometti A., Siquini F.M., Cirioni O., Petroni S., Scalise G. Imipenem and meropenem induced resistance to beta-lactam antibiotics inPseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13:315–318. doi: 10.1007/BF01974609. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin M., Advincula M.R., Malczynski M., Qi C., Bolon M., Scheetz M.H. Correlations of Antibiotic Use and Carbapenem Resistance in Enterobacteriaceae. Antimicrob. Agents Chemother. 2013;57:5131–5133. doi: 10.1128/AAC.00607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander S., Fisher B.T., Gaur A.H., Dvorak C.C., Luna D.V., Dang H., Chen L., Green M., Nieder M.L., Fisher B., et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children with Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation. JAMA. 2018;320:995–1004. doi: 10.1001/jama.2018.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calitri C., Ruberto E., Castagnola E. Antibiotic prophylaxis in neutropenic children with acute leukemia: Do the presently available data really support this practice? Eur. J. Haematol. 2018;101:721–727. doi: 10.1111/ejh.13162. [DOI] [PubMed] [Google Scholar]
- 23.Ricci E., Mesini A., Bandettini R., Faraci M., Castagnola E. Antibacterial prophylaxis of febrile neutropenia is not effective in the pre-engraftment period in pediatric allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2020;22:3340. doi: 10.1111/tid.13340. [DOI] [PubMed] [Google Scholar]
- 24.Widjajanto P.H., Sumadiono S., Cloos J., Purwanto I., Sutaryo S., Veerman A.J. Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia. J. Blood Med. 2013;4:1–9. doi: 10.2147/JBM.S33906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrnbecher T., Fisher B.T., Phillips B., Alexander S., A Ammann R., Beauchemin M., Carlesse F., Castagnola E., Davis B.L., Dupuis L.L., et al. Guideline for Antibacterial Prophylaxis Administration in Pediatric Cancer and Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2020;71:226–236. doi: 10.1093/cid/ciz1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castagnola E., Tatarelli P., Mesini A., Baldelli I., La Masa D., Biassoni R., Bandettini R. Epidemiology of carbapenemase-producing Enterobacteriaceae in a pediatric hospital in a country with high endemicity. J. Infect. Public Health. 2019;12:270–274. doi: 10.1016/j.jiph.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Gyssens I.C., Kern W.V., Livermore D.M. The role of antibiotic stewardship in limiting antibacterial resistance among hematology patients. Haematology. 2013;98:1821–1825. doi: 10.3324/haematol.2013.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strymish J., Elliman B.W., Itani K.M.F., Williams S., Gupta K. A Clinical History of Methicillin-Resistant Staphylococcus aureus Is a Poor Predictor of Preoperative Colonization Status and Postoperative Infections. Infect. Control. Hosp. Epidemiol. 2012;33:1113–1117. doi: 10.1086/668026. [DOI] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Disease Control Surveillance Report. [(accessed on 4 March 2021)];Surveillance of Antimicrobial Consumption in Europe. Available online: http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-antibiotic-consumption-ESAC-report-2010-data.pdf.
- 30.Van Duin D., Bonomo R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016;63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giacobbe D.R., Mikulska M., Viscoli C. Recent advances in the pharmacological management of infections due to multidrug-resistant Gram-negative bacteria. Expert Rev. Clin. Pharmacol. 2018;11:1219–1236. doi: 10.1080/17512433.2018.1549487. [DOI] [PubMed] [Google Scholar]
- 32.Wi Y.M., Quaintance G.K.E., Schuetz A.N., Ko K.S., Peck K.R., Song J.H., Patel R. Activity of Ceftolozane-Tazobactam against Carbapenem-Resistant, Non-Carbapenemase-Producing Pseudomonas aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2017;62 doi: 10.1128/AAC.01970-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassetti M., Peghin M., Mesini A., Castagnola E. Optimal Management of Complicated Infections in the Pediatric Patient: The Role and Utility of Ceftazidime/Avibactam. Infect. Drug Resist. 2020;13:1763–1773. doi: 10.2147/IDR.S209264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley J.S., Ang J.Y., Arrieta A.C., Larson K.B., Rizk M.L., Caro L., Yang S., Yu B., Johnson M.G., Rhee E.G. Pharmacokinetics and Safety of Single Intravenous Doses of Ceftolozane/Tazobactam in Children with Proven or Suspected Gram-Negative Infection. Pediatr. Infect. Dis. J. 2018;37:1130–1136. doi: 10.1097/INF.0000000000002170. [DOI] [PubMed] [Google Scholar]
- 35.Larson K.B., Patel Y.T., Willavize S., Bradley J.S., Rhee E.G., Caro L., Rizk M.L. Ceftolozane-Tazobactam Population Pharmacokinetics and Dose Selection for Further Clinical Evaluation in Pediatric Patients with Complicated Urinary Tract or Complicated Intra-abdominal Infections. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02578-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanretty A.M., Kaur I., Evangelista A.T., Moore W.S., Enache A., Chopra A., Cies J.J. Pharmacokinetics of the Meropenem Component of Meropenem-Vaborbactam in the Treatment ofKPC-ProducingKlebsiella pneumoniaeBloodstream Infection in a Pediatric Patient. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018;38:e87–e91. doi: 10.1002/phar.2187. [DOI] [PubMed] [Google Scholar]
- 37.Alamarat Z.I., Babic J., Tran T.T., Wootton S.H., Dinh A.Q., Miller W.R., Hanson B., Wanger A., Gary J.L., Arias C.A., et al. Long-Term Compassionate Use of Cefiderocol To Treat Chronic Osteomyelitis Caused by Extensively Drug-Resistant Pseudomonas aeruginosa and Extended-Spectrum-β-Lactamase-Producing Klebsiella pneumoniae in a Pediatric Patient. Antimicrob. Agents Chemother. 2019;64 doi: 10.1128/AAC.01872-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jean S.S., International Society of Antimicrobial Chemotherapy (ISAC) Gould I.M., Lee W.S., Hsueh P.R. New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship. Drugs. 2019;79:705–714. doi: 10.1007/s40265-019-01112-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or supplementary material. They are stored in a REDCap database and can be available on request from the corresponding author. The data are not publicly available due to personal data protection rules.