Abstract
Background:
MECP2 Duplication syndrome (MDS) is a rare X-linked genomic disorder that is caused by interstitial chromosomal duplications at Xq28 encompassing the MECP2 gene. Although phenotypic features in MDS have been described, there is a limited understanding of the range of severity of these features, and how they evolve with age.
Methods:
The cross-sectional results of N = 69 participants (ages 6 months-33 years) enrolled in a natural history study of MDS are presented. Clinical severity was assessed using a clinician-report measure as well as a parent-report measure. Data was also gathered related to the top 3 concerns of parents as selected from the most salient symptoms related to MDS. The Child Health Questionnaire was also utilized to obtain parental reports of each child's quality of life to establish disease burden.
Results:
The results of linear regression from the clinician-reported measure show that overall clinical severity scores, motor dysfunction, and functional skills are significantly worse with increasing age. Top concerns rated by parents included lack of effective communication, abnormal walking/balance issues, constipation, and seizures. Higher levels of clinical severity were also related to lower physical health quality of life scores as reported by parents.
Conclusions:
The data suggest that increasing levels of clinical severity are noted with older age, and this is primarily attributable to motor dysfunction, and functional skills. The results provide an important foundation for creating an MDS-specific severity scale highlighting the most important domains to target for treatment trials and will help clinicians and researchers define clinically meaningful changes.
Keywords: gene duplication, pediatrics, phenotype
1. INTRODUCTION
MECP2 Duplication syndrome (MDS [MIM: 300260]) is a rare X-linked genomic disorder, primarily affecting males, that is caused by interstitial chromosomal duplications at Xq28 encompassing the MECP2 gene (Van Esch et al., 2005). This gene encodes methyl CpG binding protein 2 (MeCP2), a critical regulator of neuronal gene transcription that is required for normal brain maturation (Chahrour et al., 2008; Horvath & Monteggia, 2018). Loss of MeCP2 function (under-expression) is the primary cause of Rett Syndrome (RTT), a distinct neurodevelopmental disorder primarily affecting females exhibiting some symptom overlap with MDS. The core clinical phenotype of MDS is related to overexpression of MeCP2, and involves infantile hypotonia, epilepsy, global developmental delays, choreiform movements, progressive spasticity and recurrent respiratory infections (Friez et al., 2006; Ramocki et al., 2009; Van Esch, 2012). Our prior studies reveal considerable variability in severity of many symptom domains within MDS including ambulation, hand function, and non-verbal communication (Ramocki, Tavyev, & Peters, 2010) that could be related to gene duplication size and content (Peters et al., 2019). Developmental regression occurs in approximately half of the population with highly variable age of onset (Cutri-French et al., 2020; Peters et al., 2013a). A comprehensive picture of the natural history and severity of MDS is needed as disease-modifying therapies may be possible, as demonstrated by the recent report of phenotypic reversal after antisense oligonucleotide treatment (ASO) in an animal model of MDS (Sztainberg et al., 2015).
While several studies have characterized the frequency of phenotypic features in MDS, there is a more limited understanding of how these features evolve with age, or of the range of severity of these specific features. This becomes problematic when developing clinical outcome measures. In fact, translational (mouse to human clinical applications) studies often fail because of a lack of consensus regarding common measures, an inability of available measures to assess a broad functional range, limited standardization and floor effects, and/or an absence of direct-observation measures or validated biomarkers (Berry-Kravis et al., 2018; Gold, Krishnarajy, Ellaway, & Christodoulou, 2017; Kaufmann, Stallworth, Everman, & Skinner, 2016). Before consensus can be reached regarding the best possible outcomes/measures to target, however, a basic understanding of disease progression inclusive of ranges of severity and the breadth of phenotypes is required. In fact, the FDA's roadmap to patient-focused outcome measurement in clinical trials suggests that an understanding of the natural history/disease course as well as the impact of the disease is a crucial first step to outcome measure development. As such, in this paper, we present cross-sectional results of a natural history study in MDS showing clinical severity across a range of ages on a clinician-report measure as well as a parent report measure. We also present data related to parental reports of each child's quality of life to establish disease burden.
Understanding differences in how the most important features of MDS vary with age will assist with planning for clinical trials. More specifically, this approach will help with future instrument development by identifying the most salient domains for assessment in MDS, and possible items that would change over time.
2. METHODS
2.1. Participants
This cohort of patients is part of a broader longitudinal natural history study of Rett Syndrome, and Rett-Related disorders including MDS. The Rett Syndrome and Related Disorders natural history study consists of 14 sites around the United States. 69 participants, ranging between the ages of 6 months-33 years have enrolled in the study to date. For each participant, all data is gathered in person via direct clinical exam (by a child neurologist or geneticist), and via parent report. Participants with MDS are evaluated on a yearly basis. The data reported here reflect baseline evaluations from participants between May, 2016-November, 2019. Given the smaller number of participants who have participated in longitudinal follow-up to date, only baseline data is being reported at this time. The families of all participants provided written informed consent and all procedures performed in the studies were done in accordance with the ethical standards of the respective institutional research committees. Data within the consortium is routinely checked for compliance. The natural history study is registered with Clinicaltrials.gov: NCT02738281.
2.2. Scales
2.2.1. Motor behavioral assessment scale (MBA) – Clinician rating
The MBA has also been used to assess almost 2000 children, adolescents, and adults with RTT and related disorders (including MDS) who have been enrolled in the natural history study (Neul et al., 2014). These are scored during a clinical interview and in-person exam by a specialist once per year. Items are captured on a 5-point Likert scale. Lower total scores indicate milder disease severity (see supplementary files). A recent revision of the MBA (R-MBA) was created (Raspa et al., in press) using psychometric methods (exploratory and confirmatory factor analysis, item-response theory) utilizing 21 of the MBA items to create a five-factor model: (a) Motor dysfunction (e.g., bradykinesia, dystonia, scoliosis, hypertonia/rigidity), (b) Functional skills (e.g., hand clumsiness, regression of motor skills, regression of communication skills, feeding difficulties, etc.), (c) Social skills (poor eye gaze, lack of social interest, etc.), (d) Aberrant behavior (self-injury, aggression, etc.), and (e) Rett-specific behaviors (e.g., bruxism, breathing issues). Three additional individual (unfactored) items were also included to create the overall score; these included: Seizures, Truncal Rocking, and a derived item to assess overall hand stereotypies. In sum, the subscales contained 21 items with the three additional items included when calculating a total R-MBA score (Raspa et al., in press). The R-MBA was utilized for analyses in this study given that the revised resulting scale (Raspa et al., in press) is significantly improved both conceptually and statistically.
2.2.2. Parent rating of overall functioning
Parents are asked to rate their child's overall level of functioning over the past 6 months using an ordinal scale from 1 (Much improved) to 5 (Much worse). This rating is based on an overall opinion of function, inclusive of cognitive and physical/motor functioning as well as the impact of co-morbidities such as seizures.
2.2.3. Parental top concerns
Parents are asked to select their top three concerns for their child over the past 6 months from a list of options containing symptoms relevant to MDS (e.g., lack of effective communication, frequent infections, seizures, constipation, lack of effective chewing or swallowing, teeth grinding, problems with sleep, abnormal walking/balance issues, repetitive hand movement, poor weight gain, reflux, anxiety, and scoliosis) as well as the opportunity to select “Other” and freely text the concern not listed on the preexisting list.
2.2.4. Child health questionnaire—(parent-rating)
The child health questionnaire (CHQ) version that was utilized in this study is a parent-reported health-related quality of life measure. This measures 14 unique physical and psychosocial concepts and results in two overall summary scaled scores; a physical health score, and a psychosocial health score. Higher scores reflect a “better” quality of life. This measure has been used in previous studies of individuals with RTT (Barnes et al., 2015), as well as many studies of other genetic and metabolic rare diseases.
2.3. Cytogenetic and molecular analyses
All participants were required to have a documented MECP2 duplication for entry into the study, verified by the local sites by review of the original genetic analysis report. Genetic analyses were performed by a variety of laboratories depending upon where the participant was originally diagnosed (e.g., Baylor College of Medicine, University of Chicago, Signature Genomics, Greenwood Genetic Center, Athena, CHOP, Emory, Gene Dx, Duke, etc.). Most participants had their duplications detected via Array Comparative Genomic Hybridization (Array-CGH), while others (mostly older participants) had Multiplex-Ligation-dependent Probe Amplification (MLPA).
2.4. Analytic approach
Because prior studies have demonstrated the possibility of age-dependent changes in phenotypic features (Miguet et al., 2018; Peters, Hundley, Wilson, Carvalho, et al., 2013), simple linear regressions were performed to examine the degree to which age related to scores on the clinician-reported measure. The parent rating of overall functioning is an ordinal scale, and thus an analysis of variance (ANOVA) was conducted with parent rating as the independent variable, and age (which is a continuous variable) as the outcome variable. Parental top concerns are reported as weighted percentages. The weighting was calculated based on whether the parent rated their concern as first (1/1), second (2/3), or third-most important concern (1/3). To begin to gauge the impact of disease burden, a simple linear regression was performed to examine the degree to which overall R-MBA scores changed with ratings of parental quality of life for both physical health and social health scores. As a follow-up, correlations were examined between R-MBA subscales and the physical and social health quality of life scores.
3. RESULTS
3.1. Demographics
Sixty-nine participants have enrolled in this study to date (7 females and 62 males). The average age of participants at baseline was 9.45 years (SD = 7.78 years). All participants in this study lived at home. At baseline visit 28 participants had epilepsy. The average age of seizure onset was 8.96 years (SD = 5.83 years), and those who had seizures within the past 6 months were significantly older (n = 15.98 years; SD = 7.61 years) as compared to those without (n = 4.98 years; SD = 3.71 years) [F(1,68) = 63.85; p < 0.001]. Twenty-two percent (n = 15 of 69 total) of the participants had regressed at the time of their visit, and average age of regression was 10.38 years (SD = 7.72 years). Additional clinical characteristics of this population with regard to frequency of individual symptoms as well as duplication size have been reported elsewhere (Peters et al., 2019).
3.2. Clinician-reported measure
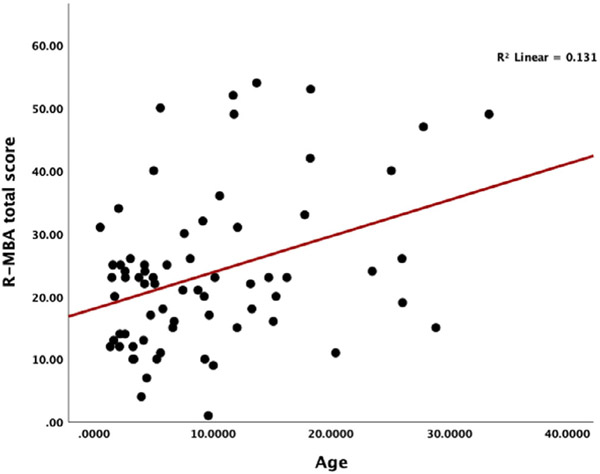
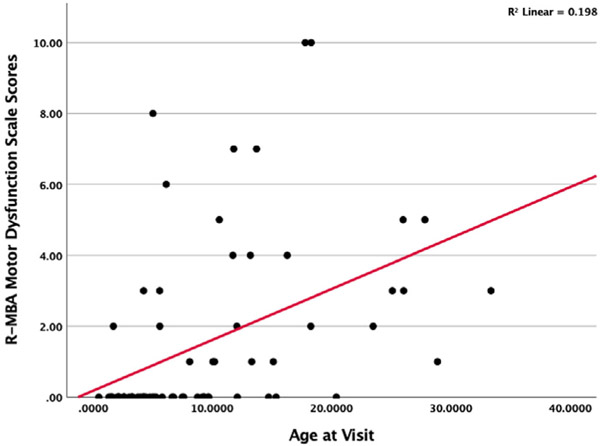
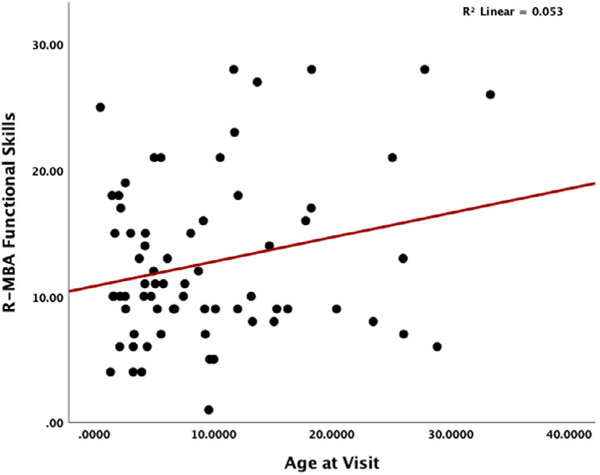
A simple linear regression was conducted to determine how overall R-MBA scores change by age [F(1,68) = 10.122; p = 0.002]; R2 = 0.13. Figure 1 displays a scatterplot of R-MBA scores by age and indicates increasing severity of scores with increasing age. Next, analyses were conducted examining differences in R-MBA subscales. Results revealed that Motor Dysfunction subscale (Figure 2) scores worsen with increasing age [F(1,68) = 16.53; p = 0.009]; R2 = 0.20. Scores on the Functional Skills (Figure 3) subscale also worsen with increasing age [F(1,68) = 3.77; p = 0.05]; R2 = 0.053. More specifically, older participants had total R-MBA, Motor Dysfunction, and Functional skills subscale scores that reflect increased severity; the increase in severity with age on the R-MBA is primarily accounted for by the Motor Dysfunction Scale and, to a lesser degree, the Functional Skills scale. Scores on the social skills F(1,68) = 1.75; p = 0.19], aberrant behavior F(1,68) = 2.34; p = 0.13], or Rett-like behaviors F(1,68) = 0.773; p = NS] scales did not significantly change with increasing age.
FIGURE 1.
Scatterplot of R-MBA scores by age
FIGURE 2.
Scatterplot of R-MBA motor dysfunction scale scores by age
FIGURE 3.
Scatterplot of R-MBA functional skills by age at visit
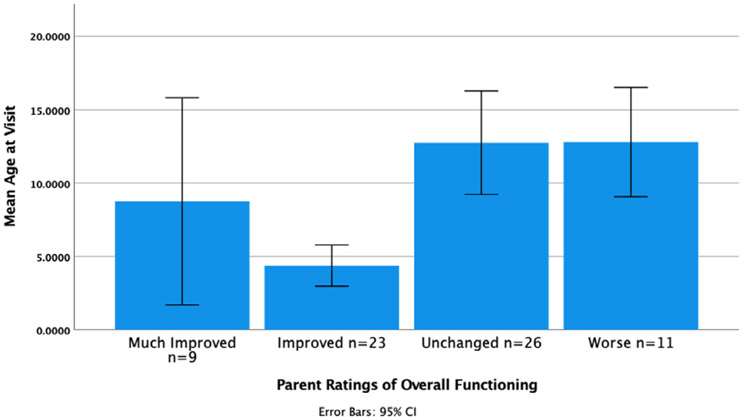
3.3. Parent rating of overall functioning
An ANOVA was conducted to examine how parent ratings of overall functioning change with the participant's age. The results revealed that parent ratings were related to age. Follow-up post-hoc tests using the Bonferroni correction revealed that parents of older children were significantly more likely to provide ratings that reflected either that their child's functioning was unchanged or worse [F(3,66) = 6.96; p = 0.000]; R2 = 0.21. The results are shown in Figure 4. An ANOVA was also conducted to examine the convergent validity of the parent ratings of functioning along with the clinician-reported measure (R-MBA), and results indicated that the parents who rated their children as “worse” had higher R-MBA scores (M = 26.8, SD = 15.6), as rated by clinicians as compared to those who rated their children as having improved (M = 19.03, SD = 8.79 [F = 3.87; p = 0.05)].
FIGURE 4.
Parent ratings of overall functioning by age
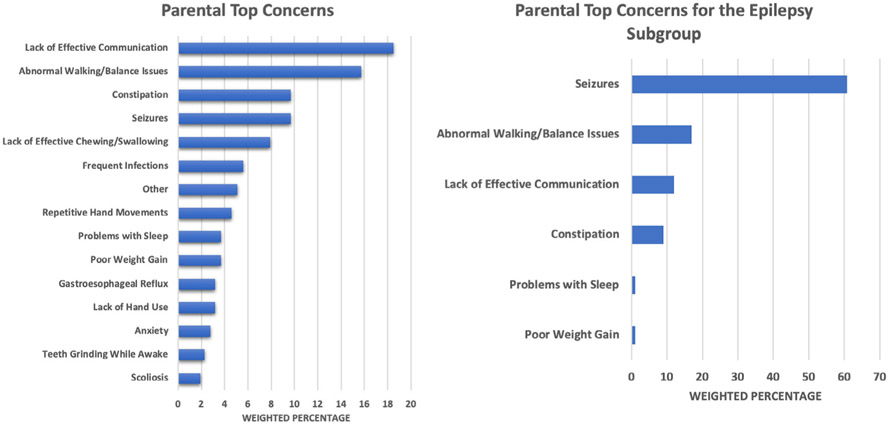
3.4. Parental top concerns
Figure 5 summarizes the results of parent-reported top concerns across the entire sample. The most commonly reported concern is lack of effective communication (18.5%). When examining the second most frequent concern, this was related to abnormal walking/balance issues (15.7%). A nearly equivalent number of parents rated constipation and seizures as the next concerns, followed by lack of effective chewing/swallowing, and frequent infections. Since prior studies have suggested that epilepsy significantly impacts the disease course/progression of MDS (Miguet et al., 2018), a more detailed analysis of parental top concerns was conducted for the subgroup (n = 28) of participants who had active epilepsy. These results are also shown in Figure 5. Of these participants, 17 listed seizures as their top concern, while three others listed seizures as a second most frequent concern. This was followed by abnormal walking/balance issues, lack of effective communication, and constipation.
FIGURE 5.
Parental top concerns and parental top concerns for the epilepsy subgroup
3.5. Health-related quality of life
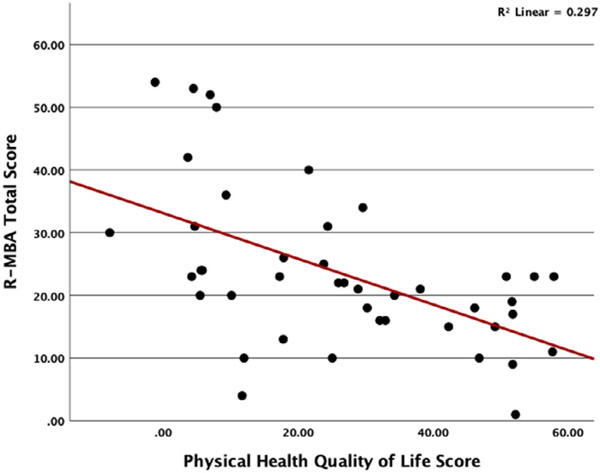
A simple linear regression was conducted to determine how parent ratings of their child's quality of life correspond to R-MBA scores. Results for the physical health scale revealed that higher R-MBA total score predicted lower physical health quality of life score [F(1,68) = 17.35; p = 0.000]; R2 = 0.30 (Figure 6). We did not find a significant relationship between R-MBA total score and social health quality of life score. Examining the correlations with R-MBA subscales revealed that better physical health quality of life scale scores are significantly correlated with lower severity on the Motor Dysfunction Scale (r = −0.31; p = 0.04), functional skills (r = −0.53; p = 0.000), social skills (r = −0.37; p = 0.015), and Rett-behaviors (r = −0.31; p = 0.042). A higher score on the Aberrant Behavior subscale is inversely correlated with lower social health quality of life scores (r = −0.31; p = 0.04).
FIGURE 6.
Scatterplot of R-MBA total score by physical health quality of life
4. DISCUSSION
This study utilized the R-MBA (Raspa et al., in press) as a first step toward examining how clinician ratings of severity in MDS change with age, and how clinical severity corresponds to parent-reported quality of life for the child. We also examined parental reports of top concerns as related to specific phenotypic features that are commonly reported in MDS. These data are critical in future planning for clinical trials in terms of establishing potential primary and secondary endpoints for treatments. The results of this study demonstrated that older participants with MDS have higher levels of clinical severity overall as measured by the R-MBA. When examining specific subscales, results indicated that older participants were more likely to have increased severity with regard to motor dysfunction which, in this study, reflected bradykinesia, dystonia, scoliosis, and/or hypertonia/rigidity. Infantile hypotonia has been consistently documented in studies of MDS (Lim, Downs, Wong, Ellaway, & Leonard, 2017; Miguet et al., 2018; Peters et al., 2019; Van Esch, 2012), although less has been written regarding changes in motor skills with advancing age. Recent studies do suggest, however, that spasticity and scoliosis are common in MDS (Miguet et al., 2018), and this current study reports the likelihood that these phenotypic features will emerge with advancing age. As is consistent with a previous study (Miguet et al., 2018), spasticity typically involved the lower limbs, and in those participants who were able to walk, gait abnormalities were noted. These observations mirror findings in animal models of MDS where mice develop a progressive neurologic phenotype that includes spasticity and seizures (Jiang et al., 2013; Na et al., 2012) as they age.
Older participants with MDS were also more likely to have increased severity with regard to Functional Skills (i.e., regression in motor skills, regression in communication skills, difficulties with chewing and swallowing, etc.). Our previous studies have shown there is a possibility of regression in MDS (Peters, Hundley, Wilson, Carvalho, et al., 2013) that occurs at a much older age as compared to RTT. Developmental regression is likely to coincide with the onset of epilepsy in MDS (Marafi et al., 2019), and both are increasingly likely as participants age (Marafi et al., 2019; Peters et al., 2019). A recent study indicated that epilepsy affected around 47% of participants with MDS, disproportionately affected those who were older, and it was treatment-refractory and consistent with epileptic encephalopathy in a high percentage of those cases (Marafi et al., 2019). Lennox–Gastaut Syndrome (LGS), a classic epileptic encephalopathy, was diagnosed in almost 25% of the sample. Further studies should be conducted to determine which participants with MDS are at increased risk for epilepsy, inclusive of epileptic encephalopathy, or whether it is likely that all individuals with MDS will acquire epilepsy as part of disease progression.
In addition to clinicians, parents and caregivers are important stakeholders in determining the most meaningful clinical symptoms and domains of clinical importance (Persaud, Desine, Blizinsky, & Bonham, 2019; Snure Beckman et al., 2019; Tsai, Scheimann, McCandless, Strong, & Bridges, 2018). As such, parental reports of clinical severity, as well as input regarding their top concerns were obtained as part of this study. Our results suggested that parental reports mirrored our findings from clinician ratings of overall severity in that parents of older participants were more likely to report increased severity. The results also suggested some convergent validity of the R-MBA with parents ratings. Results indicated that the top concerns as reported by parents, which reflected the most demanding symptoms as related to MDS that impacted daily life, mirrored some of the most common aspects of the phenotype. Parents expressed the most significant concerns as related to lack of effective communication, followed by abnormal walking/balance issues (inclusive of concerns about falling). The parental concerns related to movement mirror clinician ratings as related to Motor Dysfunction, and do indicate that changes to gait and coordination, especially as individuals age, are significant aspects of the phenotype in MDS. Additional significant concerns raised by parents included constipation, epilepsy, difficulties with chewing/swallowing, and frequent infections. It is important to note that top concerns seem to shift, depending upon whether or not a participant has epilepsy such that the presence or absence of epilepsy is a primary factor in determining day to day life and disease course. When a participant had epilepsy, this was listed as a top concern followed by abnormal walking/balance issues, lack of effective communication, and constipation. Future clinical trials in MDS will require standardized measures of clinical severity with content validity for MDS that can be applied by investigators across all study sites. Taken together, data from clinician ratings along with parental top concerns offer a starting point for determining the most salient domains and clinical features across the shifting age spectrum and a way to identify minimally clinically significant differences.
Our data also suggests that parental reports of a child's quality of life (QOL) are significantly impacted by clinical severity. This is most pronounced as related to physical health quality of life; a significant proportion of the variance in physical health QOL was reflected in R-MBA scores. Data suggest that there is a lesser impact on social QOL, which, for the scale utilized that in the current study, assessed externalizing problem behaviors. This is consistent with previous findings that suggest that aggression and self-injurious behaviors are less common in MDS as compared to other neurodevelopmental disorders (Peters et al., 2013). Rather, more commonly reported behavioral concerns in MDS are related to, for example, repetitive behaviors, sensory issues inclusive of hyposensitivity to pain, social withdrawal, and/or decreased eye contact (Peters, Hundley, Wilson, Warren, et al., 2013). These aspects of the behavioral phenotype in MDS are important to consider in light of the fact that some existing, standardized behavior rating scales could miss salient aspects of the phenotype in MDS.
The current study has some limitations that should be addressed in future work. First of all, the data presented are cross-sectional. There is a need for longitudinal studies that predict changes in clinical severity and disease progression in MDS. In particular, studies should focus on following younger cohorts over a longer period of time to better ascertain the onset of epilepsy and epileptic encephalopathy, motor dysfunction, and developmental regression. In addition, given the few numbers of females enrolled in the current study (n = 7) direct statistical comparisons were not made between overall clinical severity scores and parental top concerns of males vs. females. In future studies, however, it will be important to determine whether females with MDS have a different developmental trajectory and disease progression so as to better develop personalized interventions. Some prior studies suggest that females with MDS are as severely affected as their male counterparts (San Antonio-Arce et al., 2016), while others suggest that they could possibly have a milder disease course (Scott Schwoerer et al., 2014) possibly accounted for by differences in X-inactivation skewing.
In summary, this study extended prior research by examining age-dependent features of the phenotype in MDS, highlighting top concerns from parents and how these correspond to clinician ratings of severity, and also placing the significance of these concerns within the context of quality of life. These data provide an important foundation for creating an MDS-specific severity scale, highlighting the most important domains to target for treatment trials, and helping clinicians and researchers determine and define clinically meaningful changes for the purposes of clinical trials.
ACKNOWLEDGEMENTS
The investigators gratefully acknowledge the participants and families who took their time to contribute to this study. This project was funded by NIH R01HD084500 to SUP, and NIH U54HD061222 to AKP. The NIH Rare Diseases Clinical Research Network (RDCRN) Rett syndrome, MECP2 Duplication disorder, and Rett-related disorders Consortium (U54HD061222) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). The RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS, the NICHD, and NINDS.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD084500, U54HD061222
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest on the part of any investigators involved in this study.
DATA AVAILABILITY STATEMENT
Data Availability Statement: The de-identified data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Barnes KV, Coughlin FR, O'Leary HM, Bruck N, Bazin GA, Beinecke EB, … Kaufmann WE (2015). Anxiety-like behavior in Rett syndrome: Characteristics and assessment by anxiety scales. Journal of Neurodevelopmental Disorders, 7(1), 30. 10.1186/s11689-015-9127-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Lindemann L, Jønch AE, Apostol G, Bear MF, Carpenter RL, … Jacquemont S (2018). Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nature Reviews. Drug Discovery, 17(4), 280–299. 10.1038/nrd.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, & Zoghbi HY (2008). MeCP2, a key contributor to neurological disease, activates and represses transcription. Science, 320(5880), 1224–1229. 10.1126/science.1153252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutri-French C, Armstrong D, Saby J, Gorman C, Lane J, Fu C, … Marsh ED (2020). Comparison of Core features in four developmental encephalopathies in the Rett natural history study. Annals of Neurology. 10.1002/ana.25797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friez MJ, Jones JR, Clarkson K, Lubs H, Abuelo D, Bier JA, … Stevenson RE (2006). Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics, 118(6), e1687–e1695. [DOI] [PubMed] [Google Scholar]
- Gold WA, Krishnarajy R, Ellaway C, & Christodoulou J (2017). Rett syndrome: A genetic update and clinical review focusing on comorbidities. ACS Chemical Neuroscience, 9(2), 167–176. 10.1021/acschemneuro.7b00346 [DOI] [PubMed] [Google Scholar]
- Horvath PM, & Monteggia LM (2018). MeCP2 as an activator of gene expression. Trends in Neurosciences, 41(2), 72–74. 10.1016/j.tins.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, … Smirnakis SM (2013). Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. The Journal of Neuroscience, 33(50), 19518–19533. 10.1523/jneurosci.1745-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Stallworth JL, Everman DB, & Skinner SA (2016). Neurobiologically-based treatments in Rett syndrome: Opportunities and challenges. Expert Opinion on Orphan Drugs, 4(10), 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Z, Downs J, Wong K, Ellaway C, & Leonard H (2017). Expanding the clinical picture of the MECP2 duplication syndrome. Clinical Genetics, 91(4), 557–563. 10.1111/cge.12814 [DOI] [PubMed] [Google Scholar]
- Marafi D, Suter B, Schultz R, Glaze D, Pavlik VN, & Goldman AM (2019). Spectrum and time course of epilepsy and the associated cognitive decline in MECP2 duplication syndrome. Neurology, 92(2), e108–e114. 10.1212/wnl.0000000000006742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguet M, Faivre L, Amiel J, Nizon M, Touraine R, Prieur F, … El Chehadeh S (2018). Further delineation of the MECP2 duplication syndrome phenotype in 59 French male patients, with a particular focus on morphological and neurological features. Journal of Medical Genetics, 55(6), 359–371. 10.1136/jmedgenet-2017-104956 [DOI] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, & Monteggia LM (2012). A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. The Journal of Neuroscience, 32(9), 3109–3117. 10.1523/jneurosci.6000-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Lane JB, Lee HS, Geerts S, Barrish JO, Annese F, … Percy AK (2014). Developmental delay in Rett syndrome: Data from the natural history study. Journal of Neurodevelopmental Disorders, 6(1), 20. 10.1186/1866-1955-6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud A, Desine S, Blizinsky K, & Bonham VL (2019). A CRISPR focus on attitudes and beliefs toward somatic genome editing from stakeholders within the sickle cell disease community. Genetics in Medicine, 21(8), 1726–1734. 10.1038/s41436-018-0409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Fu C, Suter B, Marsh E, Benke TA, Skinner SA, … Percy AK (2019). Characterizing the phenotypic effect of Xq28 duplication size in MECP2 duplication syndrome. Clinical Genetics, 95 (5), 575–581. 10.1111/cge.13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Hundley RJ, Wilson AK, Carvalho CM, Lupski JR, & Ramocki MB (2013a). Brief report: Regression timing and associated features in MECP2 duplication syndrome. Journal of Autism and Developmental Disorders, 43(10), 2484–2490. 10.1007/s10803-013-1796-9 [DOI] [PubMed] [Google Scholar]
- Peters SU, Hundley RJ, Wilson AK, Warren Z, Vehorn A, Carvalho CM, … Ramocki MB (2013b). The behavioral phenotype in MECP2 duplication syndrome: A comparison with idiopathic autism. Autism Research, 6(1), 42–50. 10.1002/aur.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, … Zoghbi HY (2009). Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Annals of Neurology, 66(6), 771–782. 10.1002/ana.21715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Tavyev YJ, & Peters SU (2010). The MECP2 duplication syndrome. American Journal of Medical Genetics. Part A, 152a(5), 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspa M, Bann C, Gwaltney A, Benke TA, Fu C, Glaze DG, … Neul JL (in press). A psychometric evaluation of the motor-behavioral assessment scale for use as an outcome measure in Rett syndrome clinical trials. American Journal of Intellectual and Developmental Disabilities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Antonio-Arce V, Fenollar-Cortés M, Oancea Ionescu R, De Santos-Moreno T, Gallego-Merlo J, Illana Cámara FJ, & Cotarelo Pérez MC (2016). MECP2 duplications in symptomatic females: Report on 3 patients showing the broad phenotypic Spectrum. Child Neurology Open, 3, 2329048x16630673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Schwoerer J, Laffin J, Haun J, Raca G, Friez MJ, & Giampietro PF (2014). MECP2 duplication: Possible cause of severe phenotype in females. American Journal of Medical Genetics. Part A, 164a(4), 1029–1034. 10.1002/ajmg.a.36380 [DOI] [PubMed] [Google Scholar]
- Snure Beckman E, Deuitch N, Michie M, Allyse MA, Riggan KA, & Ormond KE (2019). Attitudes toward hypothetical uses of gene-editing Technologies in Parents of people with autosomal aneuploidies. CRISPR Journal, 2(5), 324–330. 10.1089/crispr.2019.0021 [DOI] [PubMed] [Google Scholar]
- Sztainberg Y, Chen HM, Swann JW, Hao S, Tang B, Wu Z, … Zoghbi HY (2015). Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature, 528 (7580), 123–126. 10.1038/nature16159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JH, Scheimann AO, McCandless SE, Strong TV, & Bridges JFP (2018). Caregiver priorities for endpoints to evaluate treatments for Prader-Willi syndrome: A best-worst scaling. Journal of Medical Economics, 21(12), 1230–1237. 10.1080/13696998.2018.1528980 [DOI] [PubMed] [Google Scholar]
- Van Esch H (2012). MECP2 duplication syndrome. Molecular Syndromology, 2(3-5), 128–136. 10.1159/000329580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, … Froyen G (2005). Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. American Journal of Human Genetics, 77 (3), 442–453. 10.1086/444549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Availability Statement: The de-identified data that support the findings of this study are available from the corresponding author upon reasonable request.