Abstract
Seeking useful biological agents for mycotoxin detoxification has achieved success in the last twenty years thanks to the participation of many multidisciplinary teams. We have recently witnessed discoveries in the fields of bacterial genetics (inclusive of next-generation sequencing), protein encoding, and bioinformatics that have helped to shape the latest perception of how microorganisms/mycotoxins/environmental factors intertwine and interact, so the road is opened for new breakthroughs. Analysis of literature data related to the biological control of mycotoxins indicates the ability of yeast, bacteria, fungi and enzymes to degrade or adsorb mycotoxins, which increases the safety and quality of susceptible crops, animal feed and, ultimately, food of animal origin (milk, meat and eggs) by preventing the presence of residues. Microbial detoxification (transformation and adsorption) is becoming a trustworthy strategy that leaves no or less toxic compounds and contributes to food security. This review summarizes the data and highlights the importance and prospects of these methods.
Keywords: biodetoxification of mycotoxins, detoxifying microorganisms, detoxifying enzymes
1. Introduction
Mycotoxins are secondary metabolites synthesized by an array of fungal genera, usually Fusarium, Penicillium and Aspergillus. They are natural contaminants which commonly occur in food and feed and pose a threat to animal and human health. These hazards contaminate agricultural commodities either directly or they reach animal tissues, milk and eggs through a “carry-over” mechanism after feeding animals with contaminated feedstuffs [1,2]. From regulatory and food safety viewpoints, the most significant and prevailing types of mycotoxins are aflatoxins (AFs), zearalenone (ZEA), fumonisins (FUMs), trichothecenes (TCT) (deoxynivalenol (DON), T-2 toxin (T-2) and HT-2 toxin (HT-2)), ochratoxins (OTA), ergot alkaloids (EAs), patulin and citrinin. If these substances are present in a particularly high amount in feed and food, or in lower dosages but over a long period of time, they can cause a variety of adverse effects, from acute to chronic, both in humans and animals (Table 1).
Table 1.
Common mycotoxins, their main producers and toxic effects.
| Mycotoxin | Main Producing Fungi | Toxic Effects | Source |
|---|---|---|---|
| Aflatoxins |
Aspergillus flavus, A. parasiticus,
A. aflatoxiformans |
Hepatotoxicity, carcinogenicity, immunosuppression | [3] |
| Ochratoxins | Aspegillus ochraceus, Penicillium verrucosum, A. carbonarius, A. niger | Nephrotoxicity, hepatotoxicity, carcinogenicity, teratogenicity, and immunosuppression |
[4] |
| Deoxynivalenol |
Fusarium. graminearum (Giberella zeae),
F. culmorum, F. sporotrichioides, F. tricinctum, F. Roseum, F. acuminatum |
Gastrointestinal toxicity, immunodepression |
[5] |
| Zearalenone |
Fusarium. graminearum (Giberella zeae), F. culmorum,F. sporotrichioides, F. verticillioides (F. moniliforme), F. semitectum, F. equiseti and F. oxysporum |
Reproduction toxicity | [6] |
| Fumonisins | Fusarium verticillioides, F. proliferatum | Carcinogenicity, hepatotoxicity | [7] |
The economic ravages induced by mycotoxins are based on increased veterinary and human health care costs, decreased livestock production, expenses of contaminated food and feed disposal, research investments and implementation of different mitigation measures to reduce the severity of mycotoxin problems, and even the possibility of fatal outcomes [8]. The World Health Organization (WHO)—International Agency for Research on Cancer (IARC) evaluated the carcinogenic potential of AFs, OTA, TCT, ZEA, and FUMs [9,10]. Escola et al. [11] emphasized that mycotoxin occurrence above the detectable levels worldwide was up to 60–80% and that even low presence should not be neglected as common mycotoxin mixtures, due to their synergism, could induce combined adverse health effects. Based on their detrimental effects on humans and animals, mycotoxin limits in several food and feed commodities have been prescribed by different national and international regulations. Nevertheless, as noted by Mastanjevic et al. [12], certain updates on legislation have to be made to provide for the health of animals and, subsequently, humans.
Mycotoxin presence in feed and food is an issue of growing concern worldwide, especially as the planet is facing the emerging effects of climate change [1]. Based on the results of the investigation of milk samples routinely used for human consumption, Mehta et al. [13] suggested a need for steps to be taken to control potential contamination of animal feed and thus to mitigate mycotoxins concentration in milk. A similar conclusion was raised by Souza et al. [14] who established good analytical methods in order to contribute to suppression of the transfer of unwanted compounds from feed to milk. The importance of laboratory control and the improvement of monitoring tests are emphasized within the work of Moradi et al. [15] considering T-2 toxin detection, or Su et al. [16] in relation to the level of DON contamination of barley and the possibility to sort the grain into different classes accordingly.
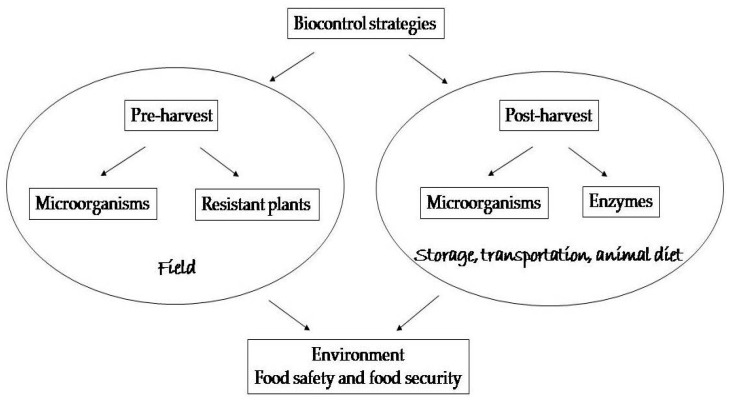
During the decades-long struggle with mycotoxin problems, many treatments have been tried: from physical, through chemical to biological. For instance, Horkey et al. [17] concluded after their investigation of effects of fungicides on mycotoxin occurrence in barley that they are partially successful and able to suppress some toxins, while not others. However, it is not just grains that are affected. The green plant mass is also at risk, as well as silage. That is why reliable solutions are being sought for these matrices as well [18,19]. Various methods have been implemented to ensure decontamination of affected commodities or to diminish the exposition to mycotoxins, but not all strategies are suitable for different purposes [20,21]. The approach based on biological agents is very promising in terms of efficiency and specificity, with the positive impact on the environment, food safety and food security. Therefore, the aim of this paper is to summarize the main achievements in this viewpoint as schematically presented in the Figure 1.
Figure 1.
A schematic presentation of the biological strategies.
2. Pre-Harvest Biological Control
Pre-harvest biological control is based on in-field strategies aimed to limit contamination levels in crops intended for human and animal consumption. In general, these systems are based on prevention and meant to evade the occurrence of contamination, and to influence the predisposing factors that favor the synthesis of mycotoxins. The use of biological control agents is an up-to-date pre-harvest concept to control mycotoxin production [22].
2.1. Use of Microorganisms
Use of biofungicides is an approach which involves application of different microorganisms, microbial antagonists or competitors that can provide suppression of toxic fungi. This method is practiced by application of selected microbes on plants in the flowering phase to limit or completely eradicate the growth of toxigenic fungi [23]. Some microbials, as some strains of Bacillus subtilis can inhibit endophytic growth phase of toxin producers. Bacteria such as Bacillus and Pseudomonas and fungi belonging to the genus Trichoderma are the most promising biocontrol agents which act against a vast array of plant pathogens in an environmentally friendly manner [24,25].
In the literature, there are examples of fungal strains for biocontrol. Dorner and Cole [26] demonstrated that the usage of atoxigenic strains of Aspergillus flavus and Aspergillus parasiticus in soil management procedures considerably decreased aflatoxin contamination. An international collaboration between researchers gathered around the goal of solving the aflatoxin problem in Africa “gave birth” to a product called Aflasafe, which was a mixture of four atoxigenic isolates of A. flavus. Aflasafe strains are able to compete with toxin producers for the colonization of plant residues in soil and organic matter and now exist in multiple variants and combinations. Implementation of such products contribute to the transition from toxigenic to atoxigenic populations of Aspergillus, although the total amount of these fungi in the environment is not affected [27,28].
According to Cleveland et al. [29] treatment of soil with nontoxic Fusarium verticillioides was useful in eliminating strains that produce fumonisin and inhibited them to synthesize this toxin. Luongo et al. [30] also showed lower presence and activity of toxigenic F. proliferatum and F. verticillioides in corn residues by implementation of non-pathogenic Fusarium fungi. Sarrocco and Vannacci [27] described useful preharvest application of beneficial fungi in field, which afterwards resulted in a good management and prevention of accumulation of mycotoxins during storage. Later, Sarrocco et al. [31] examined the history of implementation of non-aflatoxigenic isolates of Aspergillus flavus aimed for prevention of aflatoxin contamination of corn and also provided an overview of the prospective usage of competitive filamentous fungi beneficial in counteracting Fusarium head blight in wheat and alleviating Fusaria toxin synthesis. Their analysis focused on the exploitation of fungi that could compete for nutrients and space (competitive exploitation) and/or fight pathogens (intervening competition). The application of such useful isolates in the field, according to their conclusion, could be a valid approach in preventing the risks associated with mycotoxin pollution of these two basic cereal plants.
The potential of fungal competitors to beat mycotoxigenic strains is, nevertheless, related to environmental conditions during their interactions. A potential limitation of the use of atoxigenic strains for the biocontrol of unwanted fungi is the risk of sexual recombination between toxigenic lines and biocontrol strains, which can lead to the emergence of hyper virulent toxigenic strains [32]. The effectiveness of mycotoxin biocontrol agents is dependent on crucial capability to infest the target substrate and to be beneficial in various surroundings, in the field or during storage, without affecting the quality of the commodity [33]. At the preharvest stage, a metagenomic approach focused on studying crop-related communities (such as those on fruits which occur naturally in field) could be helpful in detecting those beneficial isolates that could be combined and used as the agents aimed at counteracting the mycotoxin accumulation during storage [27].
2.2. Use of Genetically Resistant Plants
Crop damage by insects is often one of the major etiological factors in enabling toxigenic fungal infestation of plants, as these herbivores create injuries on the corn kernels and act as a vector for some varieties of fungal spores [34]. For that reason, besides agrotechnical measures, a biological strategy to plant the sorts of cereals which would be less susceptible to injuries by fungi and insects has been developed. Fungal genetics has revealed the responsible genes, pathways of mycotoxin synthesis, in particular of aflatoxins and the trichothecenes, as well as the mode of regulation of this secondary metabolism [35,36]. The development of plants resistant to the accumulation of toxins is intensively promoted in regions with wide commercialization of genetically modified crops. The success was achieved by incorporating the Bt gene into maize hybrids for the purpose of protection against insect attacks [37]. In several research trials, transgenic Bt corn has been demonstrated to decrease the accumulation of usual mycotoxins compared to non-Bt isolines. This corn contains a gene from the soil bacterium Bacillus thuringiensis responsible for the synthesis of a protein delta-endotoxin which is toxic to frequent Lepidoptera insect pests. The obtained results demonstrate the success of indirect control of vermin attacks, which are common causes of mycotoxin contamination [38].
Nevertheless, as emphasized by Munkvold [39], development of genetic resistance to Aspergillus flavus, Gibberella zeae and Fusarium spp. (particularly F. verticillioides) in corn is a high priority. He also stated that Bt maize is efficient in the reduction of fumonisin occurrence, but is less successful in minimizing deoxynivalenol presence. This discrepancy mirrors various pathogen and disease models as deoxynivalenol is associated with Gibberella ear rot, whereas fumonisin synthesis is related to Fusarium ear rot, and the incidence of Gibberella ear rot is not as strongly affected by insect damage as is fumonisin formation. The total benefit of reducing fumonisins and aflatoxins by Bt corn in the United States is estimated at USD 23 million annually [37], while the new evaluation of the decrease in aflatoxin accumulation due to planting Bt corn reaches USD 120 to USD 167 million per year in over 16 states on average [38].
Continuous attention is being devoted to the uprising of transgenic plants which show resistance against various diseases. Fungal and mycotoxin counteraction strategies, as part of the plant-disease management via genetic engineering, are being pursued intensively in three basic ways: (a) detracting infestation by the pathogen, (b) inserting detoxifying genes, or (c) minimizing mycotoxin accumulation by influencing the biosynthetic pathway [39]. This is considered to be the latest approach to reduce the dependency on harmful synthetic fungicides. The current need is to identify genes across species to encourage the search for variation against biotic stress. During the last twenty years, remarkable efforts have been made towards implementation of genetic engineering in plant-disease management. Additionally, various molecular methods have appeared to unravel multiple plant-pathogen combinations and connected prospect genes responsible for disease resistance. Such genes have been recognized and estimated in crop improvement programs by transformation [40]. Observing recent events, which have resulted in new active resistance genes, it is motivating for emerging approaches to develop new specific resistance genes by gene modification [41].
3. Post-Harvest Biological Control
Although infestation by toxigenic fungi and mycotoxin synthesis are inevitable under certain environmental circumstances, their prevention is the preferred goal. Therefore, appropriate pre-harvest practices and initially good quality of cereals represents the first combat line, but post-harvest control systems are essential to diminish the final contamination of various agricultural products. A number of strategies are available for the mycotoxin degradation and/or fungal inactivation. The main advantages of biological control are that it proved to be more effective, specific, irreversible and environmentally friendly [22]. The main biological methods based on the use of microbiological agents and enzymes in food and feed will be further discussed.
3.1. Use of Microorganisms
Biodetoxification is a relatively new strategy for mycotoxins reduction via nonpathogenic microbes or their enzymes via catabolic processes. These germs not only lead to reduction or suppression of toxins to no or less toxic compounds, but are also considered as basically safe as they provide useful end products through the mechanisms of biodegradation or bioadsorption. The antagonistic outcome of probiotics on toxigenic fungi arises from competition for the living space and nutrients that are necessary for growth, metabolism, the parasitism and parasitic function on pathogen fungi by forming a biofilm and also making a defensive response during the release of free oxygen radicals [42]. The use of different microorganisms (bacteria, yeast and fungi) for the control of common mycotoxins have been summarized by Taheur et al. [43] and presented here within the Table 2.
Table 2.
The use of microorganisms (bacteria, yeast and fungi) for the control of common mycotoxins (Adapted from Taheur et al. [43]).
| Mycotoxins | Microorganisms |
|---|---|
| Aflatoxins | Lactobacillus plantarum LOCK 0945, L. brevis LOCK 0944, L. paracasei LOCK 0920, L. kefiri, Bacillus pumilus, Bacillus subtilis ANSB060, Kazachstania servazzii, Acetobacter syzygii, Rhodococcus erythropolis, Pseudomonas putida, Mycobacterium fluoranthenivorans sp. nov. DSM 44556T, Streptomyces lividans TK 24, Saccharomyces cerevisiae, Pichia anomala, Fusarium aurantiacum strain NRRL-B-184, Pseudomonas putida, Mycobacterium fluoranthenivorans sp. nov. DSM 44556T, Streptomyces lividans TK 24, Flavobacterium aurantiacum |
| Ochratoxin A | L. acidophilus VM 20, L. bulgaricus, L. helveticus, L. rhamnosus GG, B. lichniformis, B. subtilis, Bifidobacterium animalis VM 12, Brevibacterium, Cupriavidus basilensis ŐR16, Pediococcus parvulus, B. amyloliquefaciens ASAG1, S. cerevisiae O11, S. bayanus, Yarrowia lipolytica |
| Zearalenone | B. licheniformis CK1, B. pumilus ES-21, B. subtilis, L. mucosae lm4208, L. rhamnosus, P. otitidis TH-N1, Rhodococcus, Lysinibacillus sp., Geobacillus and Tepidimicrobium |
| Trichothecenes (DON, T-2/HT-2) |
Nocardioides and Devosia, Lactobacillus sakei KTU05-6, Pediococcus acidilactici KTU05-7, Pediococcus pentosaceus KTU05-8, KTU05-09 and KTU05-10, Eggerthella sp. DII-9 |
3.1.1. Bacteria
Development of bacteria capable for biotransformation of mycotoxins into nontoxic metabolites, which exert its function within the intestinal tract prior to the resorption of the mycotoxins, began in the 1980s. The first were Flavobacterium aurantiacum with the capacity to detoxify aflatoxins; Phenylobacterium immobile proved to degrade ochratoxin A and Gliocladium roseum which detoxified zearalenone via ring opening with subsequent decarboxylation [44]. Detoxification of aflatoxin B1 by Enterococcus faecium is a consequence of the mycotoxin adherence to the bacterial cell wall components, a modus that has been further set up through various studies. Bacterial cell wall peptidoglycans and polysaccharides were demonstrated to be constituents responsible for mycotoxin adsorption by the aid of microorganisms [45].
Considering effects of trichothecenes, it is well known that the 12,13-epoxide ring is in charge of their toxic activity, so the removal of this epoxide group causes a significant loss of toxicity [46]. Eubacterium BBSH 797 was the first isolated individual bacterial strain which was capable of biotransforming the epoxide group of trichothecenes. This strain, which originates from bovine rumen fluid, by its epoxidase enzymatically reduced deoxynivalenol (DON) to the non-toxic metabolite de-epoxy-deoxynivalenol (DOM-1). It was the first microorganism applied as a mycotoxin deactivating additive in feed. Regarding the microorganism DSM 11798 Genus nov. species nov. (BBSH 797) product, EFSA (European Food Safety Authority) delivered a positive opinion on its safety for the target animals (pigs and avian species), consumer, user and the environment, when used under the proposed conditions [47,48]. The appropriate implementing regulations were established in 2016 and 2017 [49,50]. Aerobic oxidation and epimerization of DON at the C3 group performed by multiple soil microorganisms, mainly belonging to the Gram-negative Devosia genus, was reviewed by Hassan and Zhou [51]. A novel bacterium Eggerthella sp. DII-9 was isolated by Gao et al. [52] from chicken intestines, who also determined its ability to biotransform DON, HT-2, T-2 triol and T-2 tetraol.
Several researchers have demonstrated the biodetoxification of mycotoxins using probiotic lactic acid bacteria [53,54,55,56]. Probiotics can remove these contaminants by biodegradation or bioadsorption pathways. Biodegradation is irreversible and of longer duration compared to bioadsorption, but it can modify toxin structure and also result in unwanted metabolites (e.g., aflatoxicol from aflatoxin B1), which could be detrimental for the host. Bioadsorption assumes quick direct binding of toxin which might be simply released and depends on the bacterial affinity toward toxin [57]. Bacillus and Brevibacterium species have been studied for degradation of different mycotoxins: aflatoxin, zearalenone, deoxynivalenol, ochratoxin and patulin. These mycotoxins could be also adsorbed by lactic acid bacteria of Lactobacillus, Bifidobacterium and Lactococcus strains, but in a different adsorption range [53,58].
3.1.2. Yeast
As a way of biological control, probiotic yeasts or products that contain yeast cell wall have also been implemented to defeat mycotoxins. A variety of yeast strains proved to be effective in transformation of toxins to non-toxic or at least less-toxic products, while some of them suppress the development of filamentous fungi. The utilization of yeasts in different technological procedures may have a direct inhibitory effect on the synthesis of toxins by certain fungi, whereas several species possess the ability to accumulate mycotoxins from agricultural products, thereby successfully detoxifying them [59].
The advantage of these microorganisms is that they have mere nutritional needs and are able to settle on dry surfaces over longer periods of time, as well as that they tolerate various pesticides used in the post-harvest conditions [60]. Contrary to many mycelial fungi, yeasts mostly do not produce allergenic spores or mycotoxins and they are also not capable of synthesizing antibiotic metabolites, which can be produced by bacterial antagonists [61,62]. Additionally, they can rise fast on affordable substrates in fermenters and are therefore convenient for production in large amounts [63]. Utilization of yeasts is harmless to humans, animals, host plants or the environment, and it is unlikely that the target organisms will generate resistance [64].
Four strains of yeasts: Saccharomyces cerevisiae AUMC 3875, Pichia anomala AUMC 2674, Pichia guilliermondii AUMC 2663 and Candida krusei AUMC 8161 were chosen by Zohri and Abdel-Kareem [65] as agents for biocontrol of growth and production of mycotoxins by 11 different toxigenic fungal isolates. In their experiment, Candida krusei AUMC 8161 absolutely prevented the development and production of toxins of all 11 investigated toxigenic isolates. Pichia anomala AUMC 2674 fully suppressed the development and synthesis of toxins originating from six mold isolates and extremely decreased the growth as well as the production of toxins from other experimental toxigenic fungi. Pichia guilliermondii AUMC 2663 greatly diminished the production and growth of toxins synthesized by 11 toxigenic fungi. Saccharomyces cerevisiae AUMC 3875 absolutely prevented development of five fungal isolates and greatly decreased the growth of other molds.
Saccharomyces cerevisiae is considered to be a probiotic yeast which can, according to Liu et al. [66], remarkably decompose deoxynivalenol (DON) and decrease the extent of lactate dehydrogenase (LDH) release in cells stimulated by DON. Success in alleviating the effects of ochratoxin A and aflatoxin B1 by utilization of yeast Saccharomyces cerevisiae cell wall in chicken diets has been recently reported by Mendieta et al. [67]. Efficacy of this yeast to remove patulin in fermented foods by physical adsorption has also been proven [68]. Kluyveromyces marxianus were used to bind aflatoxin B1, ochratoxin A and zearalenone, while authors demonstrated that mycotoxins can be bound especially by the Candida utilis cell [69]. In a different trial, the yeast Yarrowia lipolytica reduced the quantity of ochratoxin A to approximately half of the starting concentration applied in the culture [70]. More than 50% degradation of patulin by Rhodotorula mucilaginosa (R. mucilaginosa JM19) indicates the usefulness of this yeast in foods and raw materials [71].
3.1.3. Fungi
Concerning the fungi and their detoxifying abilities, it was demonstrated that those species capable of synthesizing mycotoxins could often also degrade them. Therefore, the application of nontoxigenic strains of A. parasiticus and A. flavus on plants (maize, peanuts, pistachio and cotton) has achieved exceptional results in the elimination of aflatoxins. This is due to the fact that these fungi commonly have the ability of degradation and probably conversion and utilization of degradation products [72]. Usage of high dosages of non-toxigenic inoculants in the soil around developing crops provides competition with toxigenic strains for infestation sites on the growing plant. This methodology also brings positive effects during storage as competitive elimination in the field transforms into a reduced risk of toxin presence in the storehouses and transportation. In this way, less toxin-producers move into the storage and the applied biocontrol agents persist on the crop until its final use [73].
There are also other fungi, like Rhizopus, Trichoderma, Clonostachys and Penicillium spp., that might fit for mycotoxin biocontrol [73]. It was demonstrated by Hackbart et al. [74] that Rhizopus oryzae and Trichoderma reesei reduce aflatoxins AFB1, AFB2, AFG1, AFG2 and AFM1. Trichoderma strains have also exerted considerable antibiosis and parasitism ability, making them suitable to be used as mycoparasites against toxigenic Fusarium isolates for preventing their growth by forming coils around the Fusarium hyphae and penetrating it [75]. As with other biocontrol methods, the concept is to have such formulation able to oppose the mycotoxin-producing strains and make a toxin-free product. Non-toxigenic Fusarium verticillioides appeared to be a promising species against fumonisin-forming Fusarium strains, but at the same time regrettably it is a plant pathogen [76].
In vitro experiments with inoculation of Microsphaerosis species on maize and wheat grains reduced production of Gibberella zeae ascospore by 73%, while also in another trial, under glass house conditions, with Phoma betae inoculation on wheat ears the prevalence of Fusarium head blight decreased up to 60% [77]. As summarized by Vankatesh and Keller [78], there are other fungi with mycotoxin transforming properties based on different mechanisms. Fungi Clonostachys rosea has been shown to synthesize lactonase, a zearalenone-specific enzyme which catalyzes the hydrolysis of the lactone ring followed by spontaneous decarboxylation [79]. Conversion of zearalenone into ZOM-1, characterized by the opening of the ring structure at the ketone group positioned at C6′, reported to be provided by Trichosporon mycotoxinivorans [80].
There are some doubts and even counter arguments considering the usage of some fungi for the wide control of mycotoxins. For instance, non-aflatoxigenic Aspergillus AF36 was officially applied for biological control treatments to alleviate aflatoxin problems in the USA, but it also produced cyclopiazonic acid (α-CPA), which is a proven inhibitor of ATP-ase enzyme and possessed the ability to impair physiological muscle function (contractions and relaxations). Consequently, instead of AF36 other non-aflatoxigenic strains, unable to synthesize α-CPA, are currently in use as agents for biocontrol [81]. Available data show that A. flavus strains can produce a multitude of different metabolites with unrevealed toxicological outcome, such as aflavinine, aspertoxin, aflatrem, kojic acid, leporin C, paspalinine and sterigmaticystin [82]. There is also an evidence that a nontoxigenic strain can transform into a toxigenic one through sexual reproduction. Therefore, it is necessary to have complete insight into the action of the agents and all safety issues must be considered before their usage.
3.2. Use of Enzymes
Significant efforts have been recently invested to find enzymes able to degrade and metabolize mycotoxins and thus provide adequate biotransformation solution to the mycotoxicology problems. Such biotechnological methods, which are highly specific, generate harmless products, and preferably lead to total detoxification while acting environmentally friendly, are a primary goal. The main conversion paths are hydroxylation, hydrogenation, hydrolysis, oxidation, esterification, glucuronidation and glycosylation, de-epoxidation, methylation, sulfation, demethylation and deamination [83], which depends on the type and nature of the mycotoxin. Many promising solutions have been reported targeting aflatoxins, fumonisins and ochratoxins [84,85,86,87,88,89,90,91]. Deoxynivalenol (DON) due to its widespread (globally the most commonly detected agricultural mycotoxin) and its chemical nature (small polar moiety), appeared to be the most difficult target to develop agents able to irreversibly bind it. This made it a challenging task for numerous innovative investigations designed to discover feasible and sustainable biological degrading solutions [92]. The mechanism of the enzyme action towards zearalenone (ZEN) has been studied in detail by several scientific teams [93,94] and developed detoxification strategies are intended to disrupt its estrogenic activity. The prevalent ZEN detoxifying mode described so far is cleavage of lactone ring, which is catalyzed by esterases. The reaction is irreversible since the resulting hydroxyketones spontaneously decarboxylate [95]. Ferrara et al. [96] have shown that a function-driven methodology which involves metagenomic analysis represents a potent researching tool aimed to reveal novel enzymes powerful in degradation of mycotoxins. They discovered the role of two new carboxylesterase genes belonging to Dysgonamonadaceae bacterium and Peptococcaceae bacterium assumed to be involved in fumonisin degradation. Enzymes for the control of common mycotoxins, accompanied with their producers, as summarized by Loi et al. [95] are given within the Table 3.
Table 3.
Enzymes for the control of common mycotoxins (Adapted from Loi et al. [95]).
| Mycotoxin | Enzyme | Producer |
|---|---|---|
| Aflatoxin | Aflatoxin oxidase enzyme (AFO) (EC 1.1) | Armillariella tabescens |
| Peroxidase (EC 1.11.1.7) | Horseradish (Armoracia rusticana) | |
| Laccase (EC 1.10.3.2) | Trametes versicolor (Sigma-Aldrich, St. Louis, MO, USA) | |
| Laccase (EC 1.10.3.2) | Streptomyces coelicor | |
| F420H2-dependent reductases (E.C. 1.5.8) | Mycobacterium smegmatis | |
| Mn peroxidase (EC 1.11.1.7) | Pleurotus ostreatus | |
| Aflatoxin degradation enzyme | Pleurotus ostreatus | |
| Myxobacteria aflatoxin degrading enzyme (MADE) | Myxococcus fulvus ANSM068 | |
| Laccase (lac2) (EC 1.10.3.2) | Pleurotus pulmonarius (ITEM 17144) | |
| Ery4 | Pleurotus eryngii (PS419) | |
| Fumonisin | Carboxylesterase and aminotransferase (E.C. 3.1.1, E.C. 2.6.1) |
Sphingomonas sp. ATCC55552 |
| Carboxylesterase B and aminotransferase (E.C. 3.1.1, E.C. 2.6.1/FJ426269.1) |
Sphingopyxis sp. MTA144 | |
| Fumonisin esterase (E.C. 3.1.1.87) | Sphingopyxis sp. MTA144 | |
| Trichothecenes | Cytochrome P450 system (Ddna + Kdx + KdR) (E.C. 1.14 AB744215.1 AB744217.1) (DON; NIV) |
Sphingomonas sp. strain KSM1 |
| UDP-glycosyltransferase (AC006282) | Arabidopsis thaliana | |
| Zearalenone | Laccase (EC 1.10.3.2) | Trametes versicolor (Sigma-Aldrich, USA) |
| laccase (EC 1.10.3.2) | Streptomyces coelicolor | |
| Lactono hydrolase (E.C. 3.1.1) | Clonostachys rosea | |
| 2cys-peroxiredoxin (EC 1.11.1.15) | Acinetobacter sp. SM04 | |
| Ochratoxin | Carboxypeptidase A: CPA (EC 3.4.24) | Bovine pancreas |
| Carboxypeptidase Y: CPY (EC 3.4.16) | Saccharomyces cerevisiae | |
| Lipase (EC 3.1) Protease A (EC 3.4) Amidase 2 (EC 3.5) |
Aspergillus niger |
As usually several mycotoxins simultaneously contaminate commodities, Lyagin and Efremenko [97] suggested development of biocontrol agents which contain several efficient enzymes. To select proper enzymes for such combinations precisely, both a thorough understanding of catalytic processes and proper analysis of enzyme properties are required. Useful solutions for this purpose would be enzymes that can degrade several mycotoxins at the same time, but only a limited number can answer this task. There are cytochromes (able to modify aflatoxins, trichothecenes and sterigmatocystin), aflatoxin oxidase AFO (working on aflatoxins and sterigmatocystin) and aldo-keto reductase AKR18A1 (performing reduction of trichothecenes and zearalenone) among them. In addition, there are numerous mycotoxins (like sterigmatocystin and ergot alkaloids) which can be detoxified by a very limited number of enzymes, if there is such possibility at all, while also more than a half of enzymes (lyases, isomerases, ligases and translocases) are unable or unknown to be able to modify mycotoxins [97].
Both from technological and economic points of view, a use of enzymes is beneficial. Reduced effectiveness due to matrix influence could be present. The physicochemical properties of food (fat content, moisture, acidity, texture) significantly affect the outcome of the detoxifying process. Furthermore, inhibitory components could be present in raw materials and the possibility to have masked forms of mycotoxins may restrict their bioavailability for the enzymatic catalysis. Such circumstances might require pretreatments, additional finance and time, which must be carefully considered when establishing industrial utilization [95]. The use of biological agents as feed additives could also be quite limited. To make it widely applicable more understanding is needed about the conversion procedures, the toxicological characteristics of the products obtained by transformation, the influence of the conversion on nutritional value of feed and on animal safety. Such feed additive must be harmless and stable in the digestive tract of animals [98]. An appropriate technological prescription of enzyme application is necessary in order to preserve its efficiency.
It is also important to have proper legislation, both for food and feed, which foresees the possibility of detoxifying treatments. The European Community set the regulation on food enzymes in 2008 [99]. This regulation covers “enzymes added to food to perform a technological function in the manufacture, processing, preparation, treatment, packaging, transport or storage of such food, including enzymes used as processing aids”. All enzymes included in this regulation are considered as processing aids, excepting invertase and lysozyme, which belong to the group of additives. Eligibility criteria for detoxifying treatments, including biotransformation, have been established for materials intended for animal nutrition in 2015 [100]. EU Regulations have been established regarding enzyme fumonisin esterase as feed additive for pigs and poultry [101,102,103] and EFSA gave its scientific opinion in 2020 [104] on safety and efficacy of fumonisin esterase from Komagataella phaffii DSM 32159 as a feed additive for all animal species, in accordance with Regulation (EC) No 1831/20031 which establishes the rules governing the community authorization of additives for use in animal nutrition [105].
4. Conclusions
Biological control agents should be affordable to food and feed producers and composed in a way that makes products easy and safe to handle. The efficacy might be enhanced by the selection of more efficient strains of microorganisms, gene manipulations, combination of more ingredients and inclusion of other synergistically acting bio-products. The biocontrol of mycotoxins is an approach with a bright future, although it will not be self-sufficient. It should be implemented in connection with good agricultural practices and coupled with good postharvest management, especially sorting and suitable storage. Most questions concerning safety, sustainability and the impact on the ecosystem of biological strategies are asked from stakeholders in industry, academia and local governments, and uppermost from the consumers. Therefore, many tests should be conducted and the results evaluated with the aim to eliminate any suspicions on possible adverse effects on plant, animal and human health and the environment. Without a doubt, before making the final choice of method, all issues must be resolved and a complete risk assessment carried out.
Acknowledgments
This study was conducted under the research contract with the Ministry of Education, Science and Technological Development, Republic of Serbia No. 451-03-9/2021-14/200030.
Author Contributions
Conceptualization, K.N. and K.H.; data curation K.M.; writing—original draft preparation, K.N.; writing—review and editing, K.H.; supervision, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
An approach based on biological agents to control mycotoxicological problems is very promising, but not all strategies are suitable for different purposes. This paper gives a summary of various possibilities in order to contribute to decision-making on solutions in practice and to point out possibilities for further research and improvement.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nešić K. Mycotoxins—Climate impact and steps to prevention based on prediction. Acta Vet. 2018;68:1–15. doi: 10.2478/acve-2018-0001. [DOI] [Google Scholar]
- 2.Emmanuel K.T., Els V.P., Bart H., Evelyne D., Els V.H., Els D. Carry-over of some Fusarium mycotoxins in tissues and eggs of chickens fed experimentally mycotoxin-contaminated diets. Food Chem. Toxicol. 2020;145:111715. doi: 10.1016/j.fct.2020.111715. [DOI] [PubMed] [Google Scholar]
- 3.Benkerroum N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health. 2020;17:423. doi: 10.3390/ijerph17020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heussner A.H., Bingle L.E.H. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins. 2015;7:4253–4282. doi: 10.3390/toxins7104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EFSA Scientific Opinion Risks to Human and Animal Health Related to the pre sence of deoxyni vale nol and its acetyla ted and modified forms in food and feed. EFSA J. 2017;15:4718. doi: 10.2903/j.efsa.2017.4718. [DOI] [Google Scholar]
- 6.Mahato D.K., Devi S., Pandhi S., Sharma B., Maurya K.K., Mishra S., Dhawan K., Selvakumar R., Kamle M., Mishra A., et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins. 2021;13:92. doi: 10.3390/toxins13020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voss K.A., Riley R.T. Fumonisin Toxicity and Mechanism of Action: Overview and Current Perspectives. Food Saf. 2013;1:2013006. doi: 10.14252/foodsafetyfscj.2013006. [DOI] [Google Scholar]
- 8.Milićević D., Nešić K., Jakšić S. Mycotoxin Contamination of the Food Supply Chain—Implications for One Health Programme. Procedia Food Sci. 2015;5:187–190. doi: 10.1016/j.profoo.2015.09.053. [DOI] [Google Scholar]
- 9.WHO—IARC Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and My-Cotoxins. Monographs on the Evaluation of Carcinogenic Risks to Humans 1993, 56. [(accessed on 18 January 2021)]; Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Naturally-Occurring-Substances-Food-Items-And-Constituents-Heterocyclic-Aromatic-Amines-And-Mycotoxins-1993.
- 10.WHO—IARC Fumonisin B1. Monographs on the Evaluation of Carcinogenic Risks to Humans 2002, 82. [(accessed on 18 January 2021)]; Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Traditional-Herbal-Medicines-Some-Mycotoxins-Naphthalene-And-Styrene-2002.
- 11.Eskola M., Kos G., Elliott C.T., HajšLová J., Mayar S., Krska R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25% Crit. Rev. Food Sci. Nutr. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- 12.Mastanjević K., Lukinac J., Jukić M., Šarkanj B., Krstanović V., Mastanjević K. Multi-(myco)toxins in Malting and Brewing By-Products. Toxins. 2019;11:30. doi: 10.3390/toxins11010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta R., Shetty S.A., Young M.F., Ryan P.B., Rangiah K. Quantification of aflatoxin and ochratoxin contamination in animal milk using UHPLC-MS/SRM method: A small-scale study. J. Food Sci. Technol. 2021;58:1–12. doi: 10.1007/s13197-021-04986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souza R., Fernández P., Muela A., Cesio M.V., Heinzen H., Pareja L. Development of a Methodology for the Simultaneous Analysis of Multiclass Contaminants in Milk. Food Anal. Methods. 2021;14:1–12. doi: 10.1007/s12161-020-01953-7. [DOI] [PubMed] [Google Scholar]
- 15.Moradi M., Azizi-Lalabadi M., Motamedi P., Sadeghi E. Electrochemical determination of T 2 toxin by graphite/polyacrylonitrile nanofiber electrode. Food Sci. Nutr. 2021;9:1171–1179. doi: 10.1002/fsn3.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su W.-H., Yang C., Dong Y.H., Johnson R., Page R., Szinyei T., Hirsch C.D., Steffenson B.J. Hyperspectral imaging and improved feature variable selection for automated determination of deoxynivalenol in various genetic lines of barley kernels for resistance screening. Food Chem. 2021;343:128507. doi: 10.1016/j.foodchem.2020.128507. [DOI] [PubMed] [Google Scholar]
- 17.Horky P., Skalickova S., Caslavova I., Deering A.J., Nevrkla P., Slama P., Trojan V., Skladanka J. Effect of fungicidal treatment and storage condition on content of selected mycotoxins in barley. Kvasny Prumysl. 2018;64:212–216. doi: 10.18832/kp201827. [DOI] [Google Scholar]
- 18.Skladanka J., Adam V., Zitka O., Mlejnkova V., Kalhotka L., Horky P., Konecna K., Hodulikova L., Knotova D., Balabanova M., et al. Comparison of Biogenic Amines and Mycotoxins in Alfalfa and Red Clover Fodder Depending on Additives. Int. J. Environ. Res. Public Health. 2017;14:418. doi: 10.3390/ijerph14040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaičiulienė G., Bakutis B., Jovaišienė J., Falkauskas R., Gerulis G., Baliukonienė V. Origanum vulgare and Thymus vulgaris Extract Usability to Improve Silage Hygienic Quality and Reduce Mycotoxin Concentrations. J. Microbiol. Biotechnol. 2020;30:1149–1155. doi: 10.4014/jmb.2003.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nešić K., Ivanović S., Nešić V. Fusarial Toxins: Secondary Metabolites of Fusarium Fungi. Rev. Environ. Contam. Toxicol. 2014;228:101–120. doi: 10.1007/978-3-319-01619-1_5. [DOI] [PubMed] [Google Scholar]
- 21.Conte G., Fontanelli M., Galli F., Cotrozzi L., Pagni L., Pellegrini E. Mycotoxins in Feed and Food and the Role of Ozone in Their Detoxification and Degradation: An Update. Toxins. 2020;12:486. doi: 10.3390/toxins12080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods. 2020;9:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouany J. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed. Sci. Technol. 2007;137:342–362. doi: 10.1016/j.anifeedsci.2007.06.009. [DOI] [Google Scholar]
- 24.Bhattacharjee R., Dey U. An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr. J. Microbiol. Res. 2014;8:1749–1762. doi: 10.5897/ajmr2013.6356. [DOI] [Google Scholar]
- 25.Mukhopadhyay R., Kumar D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest Control. 2020;30:133. doi: 10.1186/s41938-020-00333-x. [DOI] [Google Scholar]
- 26.Dorner J.W., Cole R.J. Effect of application of nontoxigenic strains of Aspergillus flavus and A. parasiticus on subsequent aflatoxin contamination of peanuts in storage. J. Stored Prod. Res. 2002;38:329–339. doi: 10.1016/S0022-474X(01)00035-2. [DOI] [Google Scholar]
- 27.Sarrocco S., Vannacci G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop. Prot. 2018;110:160–170. doi: 10.1016/j.cropro.2017.11.013. [DOI] [Google Scholar]
- 28.Senghor L.A., Ortega-Beltran A., Atehnkeng J., Callicott K.A., Cotty P.J., Bandyopadhyay R. The Atoxigenic Biocontrol Product Aflasafe SN01 Is a Valuable Tool to Mitigate Aflatoxin Contamination of Both Maize and Groundnut Cultivated in Senegal. Plant Dis. 2020;104:510–520. doi: 10.1094/PDIS-03-19-0575-RE. [DOI] [PubMed] [Google Scholar]
- 29.Cleveland T.E., Dowd P.F., Desjardins A.E., Bhatnagar D., Cotty P.J. United States Department of Agriculture—Agricultural Research Service research on pre-harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manag. Sci. 2003;59:629–642. doi: 10.1002/ps.724. [DOI] [PubMed] [Google Scholar]
- 30.Luongo L., Galli M., Corazza L., Meekes E., Haas L.D., Van Der Plas C.L., Köhl J. Potential of fungal antagonists for biocontrol of Fusarium spp. in wheat and maize through competition in crop debris. Biocontrol Sci. Technol. 2005;15:229–242. doi: 10.1080/09583150400016852. [DOI] [Google Scholar]
- 31.Sarrocco S., Mauro A., Battilani P. Use of Competitive Filamentous Fungi as an Alternative Approach for Mycotoxin Risk Reduction in Staple Cereals: State of Art and Future Perspectives. Toxins. 2019;11:701. doi: 10.3390/toxins11120701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagot V., Okoth S., De Boevre M., De Saeger S. Biocontrol of Aspergillus and Fusarium Mycotoxins in Africa: Benefits and Limitations. Toxins. 2019;11:109. doi: 10.3390/toxins11020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacon C.W., Yates I.E., Hinton D.M., Meredith F. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 2001;109:325–332. doi: 10.1289/ehp.01109s2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowd P.F. Insect Management to Facilitate Preharvest Mycotoxin Management. J. Toxicol. Toxin Rev. 2003;22:327–350. doi: 10.1081/TXR-120024097. [DOI] [Google Scholar]
- 35.Yu J.-H., Keller N. Regulation of Secondary Metabolism in Filamentous Fungi. Annu. Rev. Phytopathol. 2005;43:437–458. doi: 10.1146/annurev.phyto.43.040204.140214. [DOI] [PubMed] [Google Scholar]
- 36.Bhatnagar D., Rajasekaran K., Payne G.A., Brown R.I., Yu J., Cleveland T.E. The ‘omics’ tools: Genomics, proteomics, metabolomics and their potential for solving the aflatoxin contamination problem. World Mycotoxin J. 2008;1:3–12. doi: 10.3920/WMJ2008.x001. [DOI] [Google Scholar]
- 37.Wu F. Mycotoxin Reduction in Bt Corn: Potential Economic, Health, and Regulatory Impacts. Transgenic Res. 2006;15:277–289. doi: 10.1007/s11248-005-5237-1. [DOI] [PubMed] [Google Scholar]
- 38.Yu J., Hennessy D.A., Wu F. The Impact of Bt Corn on Aflatoxin-Related Insurance Claims in the United States. Sci. Rep. 2020;10:10046. doi: 10.1038/s41598-020-66955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munkvold G.P. Cultural and geneticapproaches tomanagingmycotoxins inmaize. Annu. Rev. Phytopathol. 2003;41:99–116. doi: 10.1146/annurev.phyto.41.052002.095510. [DOI] [PubMed] [Google Scholar]
- 40.Saharan V., Jain D., Pareek S., Pal A., Kumaraswamy R.V., Jakhar S.K., Singh M. Viral, Fungal and Bacterial Disease Resistance in Transgenic Plants. In: Al-Khayri J., Jain S., Johnson D., editors. Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits. Springer; Cham, Switzerland: 2016. pp. 627–656. [DOI] [Google Scholar]
- 41.Krattinger S.G., Keller B. Molecular genetics and evolution of disease resistance in cereals. New Phytol. 2016;212:320–332. doi: 10.1111/nph.14097. [DOI] [PubMed] [Google Scholar]
- 42.Afshar P., Shokrzadeh M., Raeisi S.N., Ghorbani-HasanSaraei A., Nasiraii L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon. 2020;178:50–58. doi: 10.1016/j.toxicon.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Taheur F.B., Kouidhi B., Al Qurashi Y.M.A., Salah-Abbès J.B., Chaieb K. Review: Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon. 2019;160:12–22. doi: 10.1016/j.toxicon.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Schatzmayr G., Zehner F., Täubel M., Schatzmayr D., Klimitsch A., Loibner A.P., Binder E.M. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 2006;50:543–551. doi: 10.1002/mnfr.200500181. [DOI] [PubMed] [Google Scholar]
- 45.Umesha S., Manukumar H.M.G., Chandrasekhar B., Shivakumara P., Kumar J.S., Raghava S., Avinash P., Shirin M., Bharathi T.R., Rajini S.B., et al. Aflatoxins and food pathogens: Impact of biologically active aflatoxins and their control strategies. J. Sci. Food Agric. 2017;97:1698–1707. doi: 10.1002/jsfa.8144. [DOI] [PubMed] [Google Scholar]
- 46.Foroud N.A., Baines D., Gagkaeva T.Y., Thakor N., Badea A., Steiner B., Bürstmayr M., Bürstmayr H. Trichothecenes in Cereal Grains—An Update. Toxins. 2019;11:634. doi: 10.3390/toxins11110634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Scientific Opinion on the safety and efficacy of micro-organism DSM 11798 when used as a technological feed additive for pigs. EFSA J. 2013;11:3203. doi: 10.2903/j.efsa.2013.3203. [DOI] [Google Scholar]
- 48.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Rychen G., Aquilina G., Azimonti G., Bampidis V., Bastos M.D.L., Bories G., Chesson A., Cocconcelli P.S., Flachowsky G., et al. Safety and efficacy of microorganism DSM 11798 as a technological additive for all avian species. EFSA J. 2017;15:e04676. doi: 10.2903/j.efsa.2017.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Commission Implementing Regulation (EU) No 1016/2013 of 23 October 2013 Concerning the Authorisation of a Preparation of a Micro-organism Strain DSM 11798 of the Coriobacteriaceae Family as a Feed Additive for Pigs. [(accessed on 6 January 2021)]; Available online: http://data.europa.eu/eli/reg_impl/2013/1016/oj.
- 50.Commission Implementing Regulation (EU) 2017/930 of 31 May 2017 Concerning the Authorisation of a Preparation of a Mi-Croorganism Strain DSM 11798 of the Coriobacteriaceae Family as a Feed Additive for All Avian Species and Amending Imple-menting Regulation (EU) No 1016/2013. [(accessed on 6 January 2021)]; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0930&from=EN.
- 51.Hassan Y.I., Zhou T. Addressing the mycotoxin deoxynivalenol contamination with soil-derived bacterial and enzymatic transformations targeting the C3 carbon. World Mycotoxin J. 2018;11:101–112. doi: 10.3920/WMJ2017.2259. [DOI] [Google Scholar]
- 52.Gao X., Mu P., Wen J., Sun Y., Chen Q., Deng Y. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 2018;112:310–319. doi: 10.1016/j.fct.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 53.Luo Y., Liu X., Li J. Updating techniques on controlling mycotoxins—A review. Food Control. 2018;89:123–132. doi: 10.1016/j.foodcont.2018.01.016. [DOI] [Google Scholar]
- 54.Adebo O.A., Kayitesi E., Njobeh P.B. Reduction of Mycotoxins during Fermentation of Whole Grain Sorghum to Whole Grain Ting (a Southern African Food) Toxins. 2019;11:180. doi: 10.3390/toxins11030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muhialdin B.J., Saari N., Meor Hussin A.S. Review on the Biological Detoxification of Mycotoxins Using Lactic Acid Bacteria to Enhance the Sustainability of Foods Supply. Molecules. 2020;25:2655. doi: 10.3390/molecules25112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perczak A., Goliński P., Bryła M., Waśkiewicz A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arch. Ind. Hyg. Toxicol. 2018;69:32–45. doi: 10.2478/aiht-2018-69-3051. [DOI] [PubMed] [Google Scholar]
- 57.Solis-Cruz B., Hernandez-Patlan D., Hargis B., Tellez G. Control of Aflatoxicosis in Poultry Using Probiotics and Polymers. In: Njobeh P.B., Stepman F., editors. Mycotoxins—Impact and Management Strategies. IntechOpen; London, UK: 2019. [Google Scholar]
- 58.Juodeikiene G., Bartkiene E., Cernauskas D., Cizeikiene D., Zadeike D., Lele V., Bartkevics V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT Food Sci. Technol. 2018;89:307–314. doi: 10.1016/j.lwt.2017.10.061. [DOI] [Google Scholar]
- 59.Pfliegler W.P., Pusztahelyi T., Pócsi I. Mycotoxins—Prevention and decontamination by yeasts. J. Basic Microbiol. 2015;55:805–818. doi: 10.1002/jobm.201400833. [DOI] [PubMed] [Google Scholar]
- 60.El-Tarabily K.A., Sivasithamparam K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience. 2006;47:25–35. doi: 10.1007/S10267-005-0268-2. [DOI] [Google Scholar]
- 61.Tilocca B., Balmas V., Hassan Z.U., Jaoua S., Migheli Q. A proteomic investigation of Aspergillus carbonarius exposed to yeast volatilome or to its major component 2-phenylethanol reveals major shifts in fungal metabolism. Int. J. Food Microbiol. 2019;306:108265. doi: 10.1016/j.ijfoodmicro.2019.108265. [DOI] [PubMed] [Google Scholar]
- 62.Farbo M.G., Urgeghe P.P., Fiori S., Marcello A., Oggiano S., Balmas V., Hassan Z.U., Jaoua S., Migheli Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018;284:1–10. doi: 10.1016/j.ijfoodmicro.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Druvefors U. Ph.D. Thesis. Swedish University of Agricultural Sciences; Uppsala, Sweden: 2004. Yeast Biocontrol of Grain Spoilage Mold. [Google Scholar]
- 64.Díaz M.A., Pereyra M.M., Picón-Montenegro E., Meinhardt F., Dib J.F. Killer Yeasts for the Biological Control of Postharvest Fungal Crop Diseases. Microorganisms. 2020;8:1680. doi: 10.3390/microorganisms8111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zohri A.A., Abdel-Kareem M.M. Four strains of yeasts: As effective biocontrol agents against both growth and mycotoxins formation by selected 11 toxigenic fungi. GARJM. 2018;7:132–135. [Google Scholar]
- 66.Liu Y., Chang J., Wang P., Yin Q., Huang W., Liu C., Bai X., Zhu Q., Gao T., Zhou P. Effects of Saccharomyces cerevisiae on alleviating cytotoxicity of porcine jejunal epithelia cells induced by deoxynivalenol. AMB Express. 2019;9:137. doi: 10.1186/s13568-019-0863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendieta C.R., Gómez G.V., Del Río J.C.G., Cuevas A.C., Arce J.M., Ávila E.G. Effect of the Addition of Saccharomyces Cerevisiae Yeast Cell Walls to Diets with Mycotoxins on the Performance and Immune Responses of Broilers. J. Poult. Sci. 2018;55:38–46. doi: 10.2141/jpsa.0170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z., Li M., Wu C., Peng B. Physical adsorption of patulin by Saccharomyces cerevisiae during fermentation. J. Food Sci. Technol. 2019;56:2326–2331. doi: 10.1007/s13197-019-03681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jakopović Ž., Čiča K.H., Mrvčić J., Pucić I., Čanak I., Frece J., Pleadin J., Stanzer D., Zjalic S., Markov K. Properties and Fermentation Activity of Industrial Yeasts Saccharomyces cerevisiae, S. uvarum, Candida utilis and Kluyveromyces marxianus Exposed to AFB1, OTA and ZEA. Food Technol. Biotechnol. 2018;56:208–217. doi: 10.17113/ftb.56.02.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Q., Wang J., Zhang H., Li C., Zhang X. Ochratoxin A is degraded by Yarrowia lipolytica and generates non-toxic degradation products. World Mycotoxin J. 2016;9:269–278. doi: 10.3920/WMJ2015.1911. [DOI] [Google Scholar]
- 71.Li X., Tang H., Yang C., Meng X., Liu B. Detoxification of mycotoxin patulin by the yeast Rhodotorula mucilaginosa. Food Control. 2019;96:47–52. doi: 10.1016/j.foodcont.2018.08.029. [DOI] [Google Scholar]
- 72.Horn B.W., Dorner J.W. Effect of nontoxigenic Aspergillus flavus and A. parasiticus on aflatoxin contamination of wounded peanut seeds inoculated with agricultural soil containing natural fungal populations. Biocontrol. Sci. Technol. 2009;19:249–262. doi: 10.1080/09583150802696541. [DOI] [Google Scholar]
- 73.Alberts J.F., Lilly M., Rheeder J.P., Burger H.-M., Shephard G.S., Gelderblom W.C.A. Technological and community-based methods to reduce mycotoxin exposure. Food Control. 2017;73:101–109. doi: 10.1016/j.foodcont.2016.05.029. [DOI] [Google Scholar]
- 74.Hackbart H.C.S., Machado A.R., Christ-Ribeiro A., Prietto L., Badiale-Furlong E. Reduction of aflatoxins by Rhizopus oryzae and Trichoderma reesei. Mycotoxin Res. 2014;30:141–149. doi: 10.1007/s12550-014-0202-6. [DOI] [PubMed] [Google Scholar]
- 75.Błaszczyk L., Basińska-Barczak A., Ćwiek-Kupczyńska H., Gromadzka K., Popiel D., Stępień Ł. Suppressive Effect of Trichoderma spp. on Toxigenic Fusarium Species. Pol. J. Microbiol. 2017;66:85–100. doi: 10.5604/17331331.1234996. [DOI] [PubMed] [Google Scholar]
- 76.Wagacha J., Muthomi J. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008;124:1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Pirgozliev S.R., Edwards S.G., Hare M.C., Jenkinson P. Strategies for the Control of Fusarium Head Blight in Cereals. Eur. J. Plant Pathol. 2003;109:731–742. doi: 10.1023/A:1026034509247. [DOI] [Google Scholar]
- 78.Venkatesh N., Keller N.P. Mycotoxins in Conversation with Bacteria and Fungi. Front. Microbiol. 2019;10:403. doi: 10.3389/fmicb.2019.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Utermark J., Karlovsky P. Role of Zearalenone Lactonase in Protection of Gliocladium roseum from Fungitoxic Effects of the Mycotoxin Zearalenone. Appl. Environ. Microbiol. 2006;73:637–642. doi: 10.1128/AEM.01440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vekiru E., Hametner C., Mitterbauer R., Rechthaler J., Adam G., Schatzmayr G., Krska R., Schuhmacher R. Cleavage of Zearalenone by Trichosporon mycotoxinivorans to a Novel Nonestrogenic Metabolite. Appl. Environ. Microbiol. 2010;76:2353–2359. doi: 10.1128/AEM.01438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbas H., Zablotowicz R., Horn B., Phillips N., Johnson B., Jin X., Abel C. Comparison of major biocontrol strains of non-aflatoxigenicAspergillus flavusfor the reduction of aflatoxins and cyclopiazonic acid in maize. Food Addit. Contam. Part A. 2011;28 Pt A:198–208. doi: 10.1080/19440049.2010.544680. [DOI] [PubMed] [Google Scholar]
- 82.Okoth S., De Boevre M., Vidal A., Diana Di Mavungu J., Landschoot S., Kyallo M., Njuguna J., Harvey J., De Saeger S. Genetic and Toxigenic Variability within Aspergillus flavus Population Isolated from Maize in Two Diverse Environments in Kenya. Front. Microbiol. 2018;9:57. doi: 10.3389/fmicb.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li P., Su R., Yin R., Lai D., Wang M., Liu Y., Zhou L. Detoxification of Mycotoxins through Biotransformation. Toxins. 2020;12:121. doi: 10.3390/toxins12020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abrunhosa L., Paterson R.R., Venâncio A. Biodegradation of Ochratoxin A for Food and Feed Decontamination. Toxins. 2010;2:1078–1099. doi: 10.3390/toxins2051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burgess K.M., Renaud J.B., McDowell T., Sumarah M.W. Mechanistic Insight into the Biosynthesis and Detoxification of Fumonisin Mycotoxins. ACS Chem. Biol. 2016;11:2618–2625. doi: 10.1021/acschembio.6b00438. [DOI] [PubMed] [Google Scholar]
- 86.Chang X., Wu Z., Wu S., Dai Y., Sun C. Degradation of ochratoxin A byBacillus amyloliquefaciensASAG1. Food Addit. Contam. Part A. 2015;32:564–571. doi: 10.1080/19440049.2014.991948. [DOI] [PubMed] [Google Scholar]
- 87.Dobritzsch D., Wang H., Schneider G., Yu S. Structural and functional characterization of ochratoxinase, a novel mycotoxin-degrading enzyme. Biochem. J. 2014;462:441–452. doi: 10.1042/BJ20140382. [DOI] [PubMed] [Google Scholar]
- 88.Hartinger D., Schwartz H., Hametner C., Schatzmayr G., Haltrich D., Moll W.-D. Enzyme characteristics of aminotransferase FumI of Sphingopyxis sp. MTA144 for deamination of hydrolyzed fumonisin B1. Appl. Microbiol. Biotechnol. 2011;91:757–768. doi: 10.1007/s00253-011-3248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heinl S., Hartinger D., Thamhesl M., Vekiru E., Krska R., Schatzmayr G., Moll W.D., Grabherr R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010;145:120–129. doi: 10.1016/j.jbiotec.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Taylor M.C., Jackson C.J., Tattersall D.B., French N., Peat T.S., Newman J., Briggs L.J., Lapalikar G.V., Campbell P.M., Scott C., et al. Identification and characterization of two families of F420H2-dependent reductases from Mycobacteria that catalyse aflatoxin degradation. Mol. Microbiol. 2010;78:561–575. doi: 10.1111/j.1365-2958.2010.07356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J., Ogata M., Hirai H., Kawagishi H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2010;314:164–169. doi: 10.1111/j.1574-6968.2010.02158.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J., Qin X., Guo Y., Zhang Q., Ma Q., Ji C., Zhao L. Enzymatic degradation of deoxynivalenol by a novel bacterium, Pelagibacterium halotolerans ANSP101. Food Chem. Toxicol. 2020;140:111276. doi: 10.1016/j.fct.2020.111276. [DOI] [PubMed] [Google Scholar]
- 93.Peng W., Ko T.-P., Yang Y., Zheng Y., Chen C.-C., Zhu Z., Huang C.-H., Zeng Y.-F., Huang J.-W., Wang A.H.-J., et al. Crystal structure and substrate-binding mode of the mycoestrogen-detoxifying lactonase ZHD from Clonostachys rosea. RSC Adv. 2014;4:62321–62325. doi: 10.1039/C4RA12111B. [DOI] [Google Scholar]
- 94.Vekiru E., Frühauf S., Hametner C., Schatzmayr G., Krska R., Moll W.D., Schuhmacher R. Isolation and characterisation of enzymatic zearalenone hydrolysis reaction products. World Mycotoxin J. 2016;9:353–363. doi: 10.3920/WMJ2015.2005. [DOI] [Google Scholar]
- 95.Loi M., Fanelli F., Liuzzi V.C., Logrieco A.F., Mulè G. Mycotoxin Biotransformation by Native and Commercial Enzymes: Present and Future Perspectives. Toxins. 2017;9:111. doi: 10.3390/toxins9040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrara M., Haidukowski M., D’Imperio M., Parente A., De Angelis E., Monaci L., Logrieco A.F., Mulè G. New insight into microbial degradation of mycotoxins during anaerobic digestion. Waste Manag. 2020;119:215–225. doi: 10.1016/j.wasman.2020.09.048. [DOI] [PubMed] [Google Scholar]
- 97.Lyagin I., Efremenko E. Enzymes for Detoxification of Various Mycotoxins: Origins and Mechanisms of Catalytic Action. Molecules. 2019;24:2362. doi: 10.3390/molecules24132362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Čolović R., Puvača N., Cheli F., Avantaggiato G., Greco D., Đuragić O., Kos J., Pinotti L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins. 2019;11:617. doi: 10.3390/toxins11110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Regulation (EC) No 1332/2008 of the European Parliament and of the Council of 16 December 2008 on Food Enzymes and Amending Council Directive 83/417/EEC, Council Regulation (EC) No 1493/1999, Directive 2000/13/EC, Council Directive 2001/112/EC and Regulation (EC) No 258/97. [(accessed on 13 January 2021)]; Available online: http://data.europa.eu/eli/reg/2008/1332/oj.
- 100.Commission Regulation 2015/786/EU Defining Acceptability Criteria for Detoxification Processes Applied to Products In-tended for Animal Feed as Provided for in Directive 2002/32/EC of the European Parliament and of the Council. [(accessed on 13 January 2021)]; Available online: http://data.europa.eu/eli/reg/2015/786/oj.
- 101.Commission Implementing Regulation (EU) No 1115/2014 of 21 October 2014 concerning the Authorisation of a Preparation of Fumonisin Esterase Produced by Komagataella pastoris (DSM 26643) as a Feed Additive for Pigs. [(accessed on 14 January 2021)]; Available online: http://data.europa.eu/eli/reg_impl/2014/1115/oj.
- 102.Commission Implementing Regulation (EU) 2017/913 of 29 May 2017 Concerning the Authorisation of a Preparation of Fumonisin Esterase Produced by Komagataella pastoris (DSM 26643) as a Feed Additive for All Avian Species. [(accessed on 14 January 2021)]; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0913&from=CS.
- 103.Commission Implementing Regulation (EU) 2018/1568 of 18 October 2018 Concerning the Authorisation of a Preparation of Fumonisin Esterase Produced by Komagataella Phaffii (DSM 32159) as a Feed Additive for All Pigs and All Poultry Species. [(accessed on 14 January 2021)]; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1568&from=EN.
- 104.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and Efficacy of Fumonisin Esterase from Komagataella Phaffii DSM 32159 as a Feed Additive for All Animal Species. EFSA J. 2020;18:e06207. doi: 10.2903/j.efsa.2020.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. [(accessed on 27 January 2021)]; OJEU 2003, L 268, 29–43. Available online: http://data.europa.eu/eli/reg/2003/1831/oj.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.