Abstract
Nematodes are an important cause of disease and loss of performance in horses. Changes in the parasitic fauna of horses have occurred in the past few decades, making cyathostomins the major parasites in adult horses, while large strongyles have become less prevalent. Parascaris spp. remains the most important parasite infecting foals and weanlings. Anthelmintic resistance is highly prevalent in cyathostomins and Parascaris spp. worldwide and it must be factored into treatment decisions. To assess anthelmintic efficacy in Northern Italy, we sampled 215 horses from 17 sport and horse-breeding farms. Fecal egg count reduction tests (FECRT) were used to assess anthelmintic efficacy. Copromicroscopic analysis was performed using MiniFLOTAC before treatment with fenbendazole, pyrantel pamoate or ivermectin, and repeated 14 days post-treatment. Strongyle-type eggs were detected in 66.91% of horses (CI95% 61.40–73.79%), while Parascaris spp. was detected in 2.79% (CI95% 1.94–5.95%). Reduced efficacy against cyathostomins was observed for fenbendazole in 55.56% of the treated animals (CI95% 41.18–69.06%), and for pyrantel pamoate in 75% of animals (CI95% 30.06–95.44%). Ground-based actions must be set in place to promote the uptake of state-of-the-art worm control plans that will prevent clinical disease while minimizing the selection pressure of resistant parasites.
Keywords: strongyles, drug use, drug resistance, equine nematodes
1. Introduction
Resistance is a mechanism determined according to genetic base, defined as the ability of a parasite within a population to survive treatments that are generally effective against the same species and stage of infection [1]. Today, anthelmintic resistance in horses is a worldwide phenomenon involving all major equine parasites [2] which has arisen as a clinical and economic issue [3]. Three major categories of broad-spectrum anthelmintics are used worldwide for anthelmintic treatments in horses, namely imidazothiazoles/tetrahydropyrimidines, benzimidazoles and macrocyclic lactones [4]. Regular interval treatment protocols aiming to control migrating strongyles have contributed since the 1960s to greatly reducing the prevalence of Strongylus spp. [5], as a result making cyathostomin nematodes the most prevalent parasites in adult horses worldwide [6]. Among others, strategic treatment of all horses without previous assessment of Faecal Egg Count (FEC) has helped to trigger the emergence of anthelmintic resistance [7]. The first indications of resistance were reported for phenothiazine against cyathostomin nematodes in 1960 [8], and nowadays, resistance to all three broad-spectrum anthelmintics has been reported in ruminants, horses and companion animals [2,9,10]. While resistance to benzimidazoles and tetrahydropyrimidines has been increasing since the early 1990s, reaching 100% prevalence in some countries [11,12,13], macrocyclic lactones showed full efficacy until the past few years, when cases of resistance started to be reported [14,15,16,17]. The evidence of a widespread resistance against broad-spectrum anthelmintic drugs represents an important issue. Larval cyathostominosis is caused by the synchronous emergence of encysted small strongyle larvae, which, despite being rarely observed, causes severe intestinal syndromes in both foals and adult horses [18]. In fact, even though foals are generally more susceptible due to their lower immune response, the susceptibility can be lifelong and adult horses could experience clinical symptoms of infection [18]. Animal management influences the risk of infection. Previous studies have observed a higher level of infection in horses from stud farms and facilities with high traffic of animals [19], compared to stallions, which often graze alone, and animals predominantly stabled (i.e., riding schools, boarding stables), where the risk of infection is reduced [20].
The fecal egg count reduction test (FECRT) is recognized as the gold standard for defining the anthelmintic susceptibility of both Parascaris spp. and cyathostomin infections in the field [2]. We therefore used FECRT with the aim of assessing the occurrence and diffusion of anthelmintic efficacy in stud and performance horses in a previously uninvestigated area in Northern Italy.
2. Materials and Methods
A questionnaire was administered to the farm manager/veterinarian prior to enrollment to establish history of anthelmintic drug usage. Specifically, we collected information on frequency of treatment, active principles used as well as treatment criteria (selective vs. strategic vs. symptomatology-based treatment) (Table 1).
Table 1.
Anthelmintic drug usage (treatment criteria, frequency and active principle) is reported for each facility (N = number of facilities) enrolled in the study.
| Treatment Method | Facilities (N) |
|---|---|
| Strategic | 13 |
| Strategic + symptomatology | 4 |
| Selective | 0 |
| Frequency | |
| Every 3 months | 2 |
| Every 4 months | 4 |
| Every 6 months | 11 |
| Active Principle | |
| Ivermectin | 13 |
| Fenbendazole | 3 |
| Pyrantel Pamoate | 1 |
Only facilities for which detailed information on anthelmintic drug usage was available for the 3 years prior to the beginning of the study were included. Eligible facilities must have used the same anthelmintic product for 2 consecutive treatments to be enrolled. From each facility, 25% of the horses present (minimum enrolled horses n = 2, maximum enrolled horses n = 39, proportionally divided by age class) were subjected to FECRT [21,22] to evaluate anthelmintic efficacy. Sampled horses did not receive treatment within 8 weeks prior to FECRT. In the period between April and October, feces were collected from the rectum of each horse, preserved airtight at +4 °C and analyzed at the Department of Veterinary Sciences, University of Turin Italy within 48 h from collection. Full ethical and institutional approval was given by the Department of Veterinary Sciences, University of Turin (Italy). Quantitative copromicroscopic analysis was performed using MiniFLOTAC (sensitivity 5 EPG) [23] with zinc sulfate floatation solution (specific gravity at 75 °C: 1.366–1.394). For each horse, fecal egg count (FEC) was performed just before treatment (T0) and again 14 days later (T14). The egg count reduction (FECR) was calculated according to the following formula:
To establish treatment efficacy, we used the cutoff values (mean percent reduction in FEC) suggested for each drug category by Nielsen et al. [1]. Horses were treated with fenbendazole (FBZ), pyrantel pamoate (PYR) or ivermectin (IVM) with dosages recommended by manufacturers. The anthelmintic drug used for FECRT was the same used in each facility for the previous 2 treatments. To ensure the correct administration of treatment, horses were individually weighed and veterinary practitioners administered the treatment.
Coprocultures were performed on all positive samples at T0 and repeated, if positive, at T14 post-treatment. Individual samples (10 g) were incubated in Petri dishes for 10 days in a stove at 23–25 °C to obtain infective L3 larvae. The larvae were isolated using the Baermann technique and 10 larvae per sample were identified to larval type by morphology [24,25,26,27]. Total genomic DNA of L3 larvae was extracted using the GeneElute™ Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich International GmbH, Buchs, Sankt Gallen, Switzerland) and amplified using primers for the beta-tubulin codon 200, as described by von Samson-Himmelsrjerna [28]. Primer combination for the benzimidazole-sensitive allelic variant CN24FS/CN30R was used in parallel with the primer pair CN25FR/CN30R to differentiate the codon 200 TTC/TAC polymorphism in the small-strongyle beta-tubulin gene. Positive (DNA extracted from resistant strongyle larvae) and negative controls were included in each PCR reaction and all necessary measures were taken to minimize the risk of contamination. Samples of L3 from animals treated with FBZ were tested by PCR individually, while samples of animals from farms using anthelmintic products other than FBZ were homogeneously pooled by farm and drug used, prior to PCR.
Statistical analysis was performed using R3.4.4 [29]. Chi-square tests, (X2) confidence intervals at 95% and odds ratio (OR) and generalized linear models (GLM) were used to assess the influence of management practices and horse individual characteristics on parasite load and reduced efficacy. Differences were considered significant at p < 0.05.
3. Results
Seventeen facilities were enrolled in the study program, namely 2 stud farms, 1 racecourse and 14 livery yards. All selected facilities treated all horses on a strategic basis, with varying frequency between 3 and 6 months. The most commonly used drug was IVM (n = 166 horses from 13 facilities), followed by FBZ (n = 45 horses from 3 facilities) and PYR (n = 4 horses from 1 facility). A total of 215 horses were sampled (mean n = 13 subjects/farm, sd = 12). The horses were 38% geldings, 13% stallions and 49% mares and were distributed in three age classes (n = 7 < 1 years old, n = 35 1 ≤ x > 3 years, n = 173 ≥ 3 years old). FEC at T0 detected intestinal strongyle eggs in 66.91% of the tested animals (CI95% 61.40–73.79%). Infection in each positive facility ranged from 16.67% (CI95% 4.70–44.8%) to 100% (CI95% 34.24–100%) while all horses from one recreational center tested negative (Table 2).
Table 2.
For each of the 17 facilities enrolled in the study, we report the total number of sampled subjects, the number of subjects positive for strongyle eggs at T0, prevalence of infection, strongyle fecal egg count at T0 (mean egg per gram value, EPG), Parascaris spp. egg count (mean egg per gram value, EPG) and type of treatment (fenbendazole (FBZ), pyrantel pamoate (PYR) or ivermectin (IVM)).
| Facility | Total Sampled | Positive | Prevalence (IC95%) | Strongyles FEC (Mean EPG) |
Parascaris Egg (Mean EPG) | Anthelmintic Treatment |
|---|---|---|---|---|---|---|
| Stud farm 1 | 8 | 8 | 100.00% (67.56–100) | 1538 | IVM | |
| Stud farm 2 | 28 | 26 | 92.86% (77.35–98.02) | 620 | FBZ | |
| Racecourse | 11 | 8 | 72.73% (43.44–90.25) | 745 | 36 | IVM |
| Livery yard 1 | 21 | 11 | 52.38% (32.37–71.66) | 265 | IVM | |
| Livery yard 2 | 2 | 2 | 100.00% (34.24–100) | 250 | IVM | |
| Livery yard 3 | 37 | 30 | 81.08% (65.80–90.52) | 525 | 980 | IVM |
| Livery yard 4 | 10 | 2 | 20.00% (5.67–50.98) | 90 | IVM | |
| Livery yard 5 | 4 | 4 | 100.00% (51.01–100) | 225 | PYR | |
| Livery yard 6 | 10 | 5 | 50.00% (23.66–76.34) | 140 | IVM | |
| Livery yard 7 | 11 | 6 | 54.55% (28.01–78.73) | 255 | 9 | FBZ |
| Livery yard 8 | 7 | 3 | 42.86% (15.82–74.95) | 100 | IVM | |
| Livery yard 9 | 39 | 29 | 74.36% (58.92–85.43) | 340 | IVM | |
| Livery yard 10 | 6 | 6 | 100.00% (60.9–100) | 1615 | FBZ | |
| Livery yard 11 | 12 | 2 | 16.67% (4.70–44.80) | 50 | 50 | IVM |
| Livery yard 12 | 2 | 2 | 100.00% (34.24–100) | 750 | IVM | |
| Livery yard 13 | 2 | 2 | 100.00% (34.24–100) | 1250 | IVM | |
| Livery yard 14 | 5 | 0 | 0.00% (0.00–43.45) | 0 | IVM |
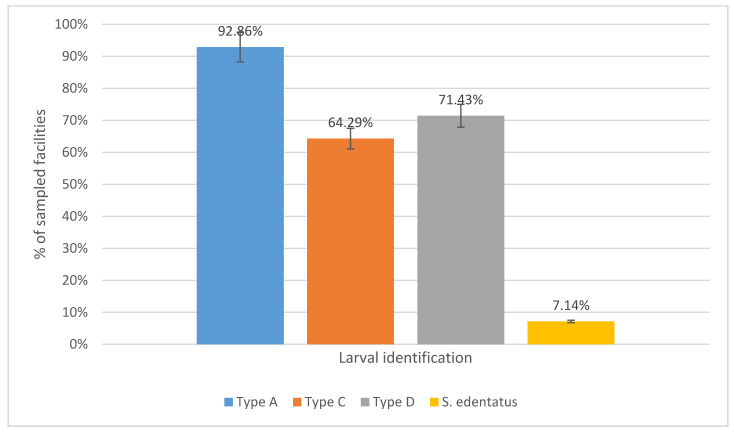
No significant differences were detected among subjects of different age classes (p > 0.05), while management was shown to highly influence parasite load. Horses housed exclusively on paddocks showed greater FEC than those housed in boxes (X2 = 12.35, p < 0.05, OR = 2.77) or on pastures (X2 = 4.89, p < 0.05, OR = 1.41). Frequent (once or more per week) manure removal was significantly associated with lower FEC (GLM, p < 0.05). The mean EPG value for strongyle eggs at T0 was 515 (min = 0, max = 1615, sd 497; Table 2). Parascaris spp. eggs were detected in six subjects (p = 2.79%; CI95% 1.94–5.95%) from four facilities (1 racecourse, 1 breeding center and 2 recreational facilities). As expected, animals under 1 year of age were significantly more infected with Parascaris spp. compared to older age categories (X2 = 4.98; p < 0.05) and Parascaris spp. was detected in 100% of the facilities where foals were present. Parascaris spp. FECRT results showed a 100% reduction in all infected animals. In animals treated with PYR, the efficacy of treatment against strongyles yielded an FECR lower than the expected cutoff [1] in 75% of animals (CI95% 30.06–95.44%), while full efficacy (100% strongyle FECR) was observed in animals treated with IVM. Among the 45 animals treated with FBZ, 55.56% (CI95% 41.18–69.06, 25/45) showed an FECR for strongyle eggs that was lower than 90%. Reduced efficacy was detected only in animals from one of the three facilities where FBZ was used, namely in stud farm 2, while FECRT from the other two livery yards using FBZ was above the 99% expected cutoff value for efficacy. High treatment frequency was highly associated with reduced efficacy. Reduced efficacy was detected only in stud farm 2, which strategically treated animals every 3 months (GLM, p < 0.05). PCR for the beta-tubulin codon 200, differentiating the codon TTC/TAC polymorphism, carried out on FBZ-resistant horses from stud farm 2, showed the presence of the resistant homozygote variant (RR) in 20 subjects, while the remaining five showed the heterozygote variant (Rr). PCR of pooled samples allowed determination of the presence of the resistance allele R in all samples from all studied facilities. Coproculture of positive T14 samples allowed the identification of three different cyathostomin larval types, namely type A (including Cylicocyclus nassatus (NAS), Cylicostephanus goldi (GLD), Cyathostomum catinatum (CAT), Cylicostephanus longibursatus (LNG), Cylicocyclus insigne (INS) and Coronocyclus coronatus (COR)), type C (including Cyathostomum pateratum (PAT) and Cylicostephanus calicatus (CAL)), and type D including Cylicocyclus ashworthi (ASH), while Strongylus edentatus (EDN) was detected in one individual sample. For each of the identified larval types at T0, we reported the distribution among sampled facilities (Table 3) and summarized the proportion of infected positive facilities (Figure 1).
Table 3.
Numbers of individuals within each larval type and strongyles in each of the enrolled facilities at T0. In each facility, for every positive horse, we morphologically identified 10 L3 specimens.
| Facility | Type D | Type C | Type A | S. edentatus |
|---|---|---|---|---|
| Stud farm 1 | 0 | 7 | 73 | 0 |
| Stud farm 2 | 21 | 34 | 205 | 0 |
| Racecourse | 7 | 2 | 68 | 4 |
| Livery yard 1 | 5 | 0 | 105 | 0 |
| Livery yard 2 | 1 | 0 | 19 | 0 |
| Livery yard 3 | 0 | 60 | 240 | 0 |
| Livery yard 4 | 2 | 2 | 16 | 0 |
| Livery yard 5 | 3 | 0 | 26 | 0 |
| Livery yard 6 | 10 | 5 | 35 | 0 |
| Livery yard 7 | 0 | 0 | 60 | 0 |
| Livery yard 8 | 2 | 6 | 24 | 0 |
| Livery yard 9 | 0 | 0 | 290 | 0 |
| Livery yard 10 | 3 | 5 | 54 | 0 |
| Livery yard 11 | 0 | 0 | 18 | 0 |
| Livery yard 12 | 0 | 0 | 20 | 0 |
| Livery yard 13 | 1 | 1 | 18 | 0 |
| Livery yard 14 | 0 | 0 | 0 | 0 |
Figure 1.
Total number (%) of facilities in which each of the identified larval types or S. edentatus were reported.
4. Discussion
The management of equine intestinal nematodes has always been a challenge for veterinarians worldwide. Following the rise in anthelmintic resistance and the lack of new treatment options, gold-standard strategies for parasite control have evolved from being drug-centered to relying on environmental management practices (e.g., pasture hygiene, regular monitoring of FEC), and on the implementation of selective treatment strategies to reduce selection pressure for resistance [2]. Guidelines for parasite control elaborated by veterinary parasitologists and equine practitioners worldwide are useful tools to mitigate the impact of anthelmintic resistance (i.e., European Scientific Counsel Companion Animal Parasites, American Association Equine Practitioners) [30,31]. Despite the resonance of growing resistance, owner uptake of evidence-based anthelmintic treatment protocols is still low and owners are reluctant to implement more sustainable practices for parasite control [16,32,33,34]. All enrolled facilities practiced calendar-based anthelmintic treatments without previous assessment of FEC.
IVM was the most frequently used anthelmintic product, as it was used by 12 recreational facilities and by one breeding center. Parascaris spp. showed no resistance, regardless of the type of anthelmintic used. For strongyles, FECRT evidenced no resistance phenomena in any of the facilities where macrocyclic lactones were used. In the literature, recent studies detected reduced efficacy of IVM in horses from the UK [16], Italy [16,35], the Netherlands [35], Belgium [35] and Finland [36]. FBZ was used in two livery yards and in one stud farm. In this last facility, the mean strongyle FECR value was 70.24%. PYR, used only in one of the enrolled facilities, showed values of strongyle FECR lower than the threshold limit for resistance in 75% of the horses. Previous studies detected resistance and/or suspected resistance in cyathostomins from Italian horse yards against PYR and FBZ [16,37]. In Europe, benzimidazole resistance was widely detected in horses from Ukraine [38], the UK [16,39], Germany [16], Switzerland [40], Sweden [41], Denmark [42] and the Slovak Republic [43], while resistance to PYR has been previously reported in Norway [12], Sweden [44], Denmark [45], the UK [16], Finland [36] and Germany [16].
Molecular determination of FBZ resistance-related alleles allowed determination of the presence of resistance allelic variants in all enrolled facilities, including those where FBZ had not been used for at least 3 years. Once acquired, resistance is permanent, even in populations of cyathostomins that remain unexposed to anthelmintic treatment for decades [46]. This finding underlines the importance of molecular-based approaches for early diagnosis of resistance [28], as extensively reported in the literature [47]. FBZ resistance was directly associated with frequency of treatment: horses treated every 3 months were more likely to host resistant strongyles (GLM, p < 0.05).
Our study accurately demonstrated the reduced efficacy of FBZ and PYR against strongyles, although some limitations are present. As previously reported from other countries [35], horse facilities investigated in this study tended to host a limited number of animals. Even though this clearly depicts the reality of the majority of the horse farms in Northern Italy, further studies are needed in order to investigate the occurrence of anthelmintic resistance in larger groups of horses.
We observed higher prevalence of strongyle eggs in horses housed in paddocks if compared to those housed in boxes and pasture. Access to grass (either pasture or paddock) has been only weakly associated with cyathostomin infection [18] and individually housed horses are reported to be at lower risk of infection. We underline the importance of housing and pasture hygiene in maintaining a lower burden of infection [48,49,50]. Moreover, recent studies have shown the possibility for equine cyathostomins to develop to infective larvae on straw bedding [51], so more studies are required to further investigate the burden of different management practices.
Given the increasing importance of anthelmintic-resistant cyathostomins in horse management, it will be essential to promote the uptake of sustainable practices among veterinary practitioners, farm managers and horse owners in order to improve the collaboration between them and to establish an integrated parasitic control plan based on the correct use of anthelmintic drugs, adequate pasture hygiene and periodic FEC analysis.
Acknowledgments
All authors would like to acknowledge the valuable help of veterinarians and owners for their invaluable support during sampling procedures. We are thankful to the anonymous reviewers, who greatly improved the initial version of the manuscript with their careful and insightful comments and suggestions.
Author Contributions
Conceptualization, E.F. and S.Z.; Methodology, E.B.; Formal Analysis, S.Z.; Investigation, F.L. and F.O.; Data Curation, F.L. and F.O.; Writing—Original Draft Preparation, S.Z., E.B.; Writing—Review and Editing, S.Z., F.L., F.O., E.B. and E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) under the program “Dipartimenti di Eccellenza ex L.232/2016” at the Department of Veterinary Science, University of Turin.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Consiglio di Dipartimento, Dipartimento di Scienze Veterinarie, Università degli Studi di Torino.
Informed Consent Statement
Informed consent was obtained from all the owners of all subjects involved in the study.
Data Availability Statement
All data are available in the text.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nielsen M.K., Mittel L., Grice A., Erskine M., Graves E., Wendy V., Tully R.C., French D.D., Bowman R., Kaplan R.M., et al. Internal Parasite Control Guidelines. American Association of Equine Practitioners AAEP; Lexington, KY, USA: 2019. [Google Scholar]
- 2.Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: Does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Raza A., Qamar A.G., Hayat K., Ashraf S., Williams A.R. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology. 2018;146:425–437. doi: 10.1017/S0031182018001786. [DOI] [PubMed] [Google Scholar]
- 4.Tzelos T., Morgan E.R., Easton S., Hodgkinson J.E., Matthews J.B. A survey of the level of horse owner uptake of evidence-based anthelmintic treatment protocols for equine helminth control in the UK. Vet. Parasitol. 2019;274:108926. doi: 10.1016/j.vetpar.2019.108926. [DOI] [PubMed] [Google Scholar]
- 5.Drudge J.H., Lyons E.T. Control of internal parasites of the horse. J. Am. Vet. Med. Assoc. 1966;148:378–383. [PubMed] [Google Scholar]
- 6.Leathwick D.M., Sauermann C.W., Nielsen M.K. Managing anthelmintic resistance in cyathostomin parasites: Investigating the benefits of refugia-based strategies. Int. J. Parasitol. Drugs Drug Resist. 2019;10:118–124. doi: 10.1016/j.ijpddr.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James C.E., Hudson A.L., Davey M.W. Drug resistance mechanisms in helminths: Is it survival of the fittest? Trends Parasitol. 2009;25:328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Gibson T.E. Some experiences with small daily doses of phenothiazine as a means of control of strongylid worms in the horse. Vet. Rec. 1960;72:37–41. [Google Scholar]
- 9.Pawar P., Das Singla L., Kaur P., Bal M.S., Javed M. Evaluation and correlation of multiple anthelmintic resistances to gastrointestinal nematodes using different fecal egg count reduction methods in small ruminants of Punjab, India. Acta Parasitol. 2019;64:456–463. doi: 10.2478/s11686-019-00083-3. [DOI] [PubMed] [Google Scholar]
- 10.Castro P.J., Howell S.B., Schaefer J.J., Avramenko R.W., Gilleard J.S., Kaplan R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: An emerging threat? Parasites Vectors. 2019;12:1–15. doi: 10.1186/s13071-019-3828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Ihler C.E. A field survey on anthelmintic resistance in equine small strongyles in Norway. Acta Vet. Scand. 1995;36:135–143. doi: 10.1186/BF03547710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews J.B. An update on cyathostomins: Anthelmintic resistance and worm control. Equine Vet. Educ. 2008;20:552–560. doi: 10.2746/095777308X363912. [DOI] [Google Scholar]
- 14.Von Samson-Himmelstjerna G., Fritzen B., Demeler J., Schürmann S., Rohn K., Schnieder T., Epe C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet. Parasitol. 2007;144:74–80. doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Campbell C., Cringoli G., Coles G. Ivermectine Resistance in Cyathostomins in UK horses; Proceedings of the 21st Interna-tional Conference of the World Association for the Advancement of Veterinary Parasitology (WAAVP); Ghent, Belgium. 19–23 August 2007; p. 389. [Google Scholar]
- 16.Traversa D., Von Samson-Himmelstjerna G., Demeler J., Milillo P., Schürmann S., Barnes H., Otranto D., Perrucci S., Di Regalbono A.F., Beraldo P., et al. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasites Vectors. 2009;2:S2. doi: 10.1186/1756-3305-2-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molento M.B., Nielsen M.K., Kaplan R.M. Resistance to avermectin/milbemycin anthelmintics in equine cyathostomins—Current situation. Vet. Parasitol. 2012;185:16–24. doi: 10.1016/j.vetpar.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Corning S. Equine cyathostomins: A review of biology, clinical significance and therapy. Parasites Vectors. 2009;2:S1. doi: 10.1186/1756-3305-2-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind E.O., Höglund J., Ljungström B.-L., Nilsson O., Uggla A. A field survey on the distribution of strongyle infections of horses in Sweden and factors affecting faecal egg counts. Equine Vet. J. 1999;31:68–72. doi: 10.1111/j.2042-3306.1999.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 20.Kornaś S., Cabaret J., Skalska M., Nowosad B. Horse infection with intestinal helminths in relation to age, sex, access to grass and farm system. Vet. Parasitol. 2010;174:285–291. doi: 10.1016/j.vetpar.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Chapman M., Klei T., French D. Identification of thiabendazole-resistant cyathostome species in Louisiana. Vet. Parasitol. 1991;39:293–299. doi: 10.1016/0304-4017(91)90046-X. [DOI] [PubMed] [Google Scholar]
- 22.Young K.E., Garza V., Snowden K., Dobson R., Powell D., Craig T. Parasite diversity and anthelmintic resistance in two herds of horses. Vet. Parasitol. 1999;85:205–214. doi: 10.1016/S0304-4017(99)00100-4. [DOI] [PubMed] [Google Scholar]
- 23.De Castro L.L.D., Abrahão C.L., Buzatti A., Molento M.B., Bastianetto E., Rodrigues D.S., Lopes L.B., Silva M.X., De Freitas M.G., Conde M.H., et al. Comparison of McMaster and Mini-FLOTAC fecal egg counting techniques in cattle and horses. Vet. Parasitol. Reg. Stud. Rep. 2017;10:132–135. doi: 10.1016/j.vprsr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Bevilaqua C.M.L., Rodrigues M.L., Concordet D. Identification of infective larvae of some common nematode strongylids of horses Strongylus vulgaris, S. equinus, S. edentatus, Triodontophorus spp., Poteriostomum spp., Gyalocephalus capitatus, Cylicocyclus radiatus, C. nassatus, C. minutus, C. poculatu. Rev. Med. Vet. 1993;144:989–995. [Google Scholar]
- 25.Kornaś S., Gawor J., Cabaret J., Molenda K., Skalska M., Nowosad B. Morphometric identification of equid cyathostome (Nematoda: Cyathostominae) infective larvae. Vet. Parasitol. 2009;162:290–294. doi: 10.1016/j.vetpar.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Santos D.W., De Castro L.L.D., Giese E.G., Molento M.B. Morphometric study of infective larvae of cyathostomins of horses and their distribution. J. Equine Vet. Sci. 2016;44:49–53. doi: 10.1016/j.jevs.2016.02.237. [DOI] [Google Scholar]
- 27.Santos D.W., De Carvalho L.M.M., Molento M.B. Identification of third stage larval types of cyathostomins of equids: An improved perspective. Vet. Parasitol. 2018;260:49–52. doi: 10.1016/j.vetpar.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Von Samson-Himmelstjerna G. Molecular diagnosis of anthelmintic resistance. Vet. Parasitol. 2006;136:99–107. doi: 10.1016/j.vetpar.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Development Core Team . R: A Language and Environment for Statistical Computing. Foundation for Statistical Computing; Vienna, Austria: 2018. [Google Scholar]
- 30.European Scientific Counsel on Companion Animal Parasites [(accessed on 14 December 2019)]; Available online: https://www.esccap.org/guidelines/
- 31.American Association of Equine Practitioners [(accessed on 14 December 2019)]; Available online: https://aaep.org/guidelines.
- 32.Scott I., Bishop R., Pomroy W., Pomroy W. Anthelmintic resistance in equine helminth parasites—A growing issue for horse owners and veterinarians in New Zealand? N. Z. Vet. J. 2015;63:188–198. doi: 10.1080/00480169.2014.987840. [DOI] [PubMed] [Google Scholar]
- 33.Easton S., Bartley D.J., Hotchkiss E., Hodgkinson J.E., Pinchbeck G.L., Matthews J.B. Use of a multiple choice questionnaire to assess UK prescribing channels’ knowledge of helminthology and best practice surrounding anthelmintic use in livestock and horses. Prev. Vet. Med. 2016;128:70–77. doi: 10.1016/j.prevetmed.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Wilkes E.J.A., Woodgate R.G., Raidal S.L., Hughes K.J. The application of faecal egg count results and statistical inference for clinical decision making in foals. Vet Parasitol. 2019;270:7–12. doi: 10.1016/j.vetpar.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Geurden T., Van Doorn D., Claerebout E., Kooyman F., De Keersmaecker S., Vercruysse J., Besognet B., Vanimisetti B., Di Regalbono A.F., Beraldo P., et al. Decreased strongyle egg re-appearance period after treatment with ivermectin and moxidectin in horses in Belgium, Italy and The Netherlands. Vet. Parasitol. 2014;204:291–296. doi: 10.1016/j.vetpar.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Näreaho A., Vainio K., Oksanen A. Impaired efficacy of ivermectin against Parascaris equorum, and both ivermectin and pyrantel against strongyle infections in trotter foals in Finland. Vet. Parasitol. 2011;182:372–377. doi: 10.1016/j.vetpar.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 37.Traversa N., Klei T.R., Iorio R., Paoletti B., Lia R.P., Otranto D., Sparagano O.A., Giangaspero A. Occurrence of anthelmintic resistant equine cyathostome populations in central and southern Italy. Prev. Vet. Med. 2007;82:314–320. doi: 10.1016/j.prevetmed.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Kuzmina T.A., Zvegintsova N.S., Yasynetska N.I., Kharchenko V.A. Anthelmintic resistance in strongylids (Nematoda: Strongylidae) parasitizing wild and domestic equids in the Askania Nova Biosphere Reserve, Ukraine. Ann. Parasitol. 2020;66:49–60. [PubMed] [Google Scholar]
- 39.Relf V.E., Lester H.E., Morgan E.R., Hodgkinson J.E., Matthews J.B. Anthelmintic efficacy on UK Thoroughbred stud farms. Int. J. Parasitol. 2014;44:507–514. doi: 10.1016/j.ijpara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Meier A., Hertzberg H. Strongyliden beim Pferd. II. vorkommen von anthelminthika-resistenzen in der Schweiz. Schweiz Arch. Tierheilkd. 2005;147:389–396. doi: 10.1024/0036-7281.147.9.389. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson O., Lindholm A., Christensson D. A field evaluation of anthelmintics in horses in Sweden. Vet. Parasitol. 1989;32:163–171. doi: 10.1016/0304-4017(89)90117-9. [DOI] [PubMed] [Google Scholar]
- 42.Bjørn H., Sommer C., Schougård H., Henriksen S.A., Nansen P. Resistance to benzimidazole anthelmintics in small stron-gyles (Cyathostominae) of horses in Denmark. Acta Vet. Scand. 1991;32:253–260. doi: 10.1186/BF03546987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Čerňanská D., Paoletti B., Králová-Hromadová I., Iorio R., Čudeková P., Milillo P., Traversa D. Application of a Reverse Line Blot hybridisation assay for the species-specific identification of cyathostomins (Nematoda, Strongylida) from benzimidazole-treated horses in the Slovak Republic. Vet. Parasitol. 2009;160:171–174. doi: 10.1016/j.vetpar.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 44.Lind E.O., Kuzmina T., Uggla A., Waller P.J., Höglund J. A field study on the effect of some anthelmintics on cyathostomins of horses in Sweden. Vet. Res. Commun. 2006;31:53–65. doi: 10.1007/s11259-006-3402-5. [DOI] [PubMed] [Google Scholar]
- 45.Craven J., Bjørn H., Henriksen S.A., Nansen P., Larsen M., Lendal S. Survey of anthelmintic resistance on Danish horse farms, using 5 different methods of calculating faecal egg count reduction. Equine Vet. J. 1998;30:289–293. doi: 10.1111/j.2042-3306.1998.tb04099.x. [DOI] [PubMed] [Google Scholar]
- 46.Lyons E.T., Tolliver S.C., Collins S.S. Study (1991 to 2001) of drug-resistant Population B small strongyles in critical tests in horses in Kentucky at the termination of a 40-year investigation. Parasitol. Res. 2007;101:689–701. doi: 10.1007/s00436-007-0535-6. [DOI] [PubMed] [Google Scholar]
- 47.Matthews J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014;4:310–315. doi: 10.1016/j.ijpddr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scare J., Steuer A., Gravatte H., Kálmán C., Ramires L., De Castro L.D., Norris J., Miller F., Camargo F., Lawyer A., et al. Management practices associated with strongylid parasite prevalence on horse farms in rural counties of Kentucky. Vet. Parasitol. Reg. Stud. Rep. 2018;14:25–31. doi: 10.1016/j.vprsr.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Fritzen B., Rohn K., Schnieder T., Von Samson-Himmelstjerna G. Endoparasite control management on horse farms—Lessons from worm prevalence and questionnaire data. Equine Vet. J. 2009;42:79–83. doi: 10.2746/042516409X471485. [DOI] [PubMed] [Google Scholar]
- 50.Tzelos T., Barbeito J.S., Nielsen M.K., Morgan E.R., Hodgkinson J.E., Matthews J.B. Strongyle egg reappearance period after moxidectin treatment and its relationship with management factors in UK equine populations. Vet. Parasitol. 2017;237:70–76. doi: 10.1016/j.vetpar.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Love S., Burden F.A., McGirr E.C., Gordon L., Denwood M.J. Equine Cyathostominae can develop to infective third-stage larvae on straw bedding. Parasites Vectors. 2016;9:478. doi: 10.1186/s13071-016-1757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the text.