Abstract
Background
Long noncoding RNAs (lncRNAs) have been reported as important molecules in cholangiocarcinoma (CCA) occurrence and development. A previous study showed that lncRNA GAS5 (GAS5) was an oncogene in some tumors. But the role of GAS5 in CCA progression reminds unclear. This research was designed to study the expression and potential effects of GAS5 in the progression of CCA.
Methods
The expression of GAS5 in CCA tissues was evaluated through mining of the TCGA and GEPIA databases. qRT-PCR was applied to validate the results in our clinical samples. χ2 test was used to analyze the association between the expression level of tissue GAS5 and different clinicopathological parameters of CCA patients. The target gene of GAS5 was predicted by bioinformatic databases, and further verified by luciferase reporter assays. Finally, the role of GAS5 in CCA cells invasion and proliferation was detected by Transwell assay and CCK-8 assay.
Results
Compared to the adjacent nontumor tissues and the normal human intrahepatic biliary epithelial cell, the expression of GAS5 was markedly increased in CCA tissues (p<0.001) and cell lines (p<0.01), respectively. CCA patients with high GAS5 expression tended to present lymph node metastasis (p<0.001) and had advanced clinical stage (p=0.006). The bioinformatics analysis predicted that hsa-miR-1297 was the potential target gene of GAS5, which was validated by luciferase reporter assays. In addition, the function study showed that GAS5 acted as a “sponge” to downregulate hsa-miR-1297, thus modulating CCA cell proliferation and invasion.
Conclusion
GAS5 acts as an endogenous sponge of hsa-miR-1297 to promote CCA cell proliferation and invasion, which might be a potential biomarker and therapeutic target for CCA.
Keywords: metastasis, GAS5, miR-1297, invasion, proliferation
Introduction
Cholangiocarcinoma (CCA) is a highly metastatic and invasive cancer with diagnostic difficulties and high mortality, and mainly originates from cholangiocytes of small intrahepatic bile ducts.1,2 The incidence of CCA is relatively higher in Asian countries compared to western countries. The highest annual morbidity of CCA is in Southeast Asia. Recently, the incidence of CCA tends to show a worldwide rise.3,4 Despite the improvements in early diagnosis, the postoperative recurrence rate is still high and the 5-year survival rate is only about 5%.5,6 Studies showed that cholestatic liver diseases, certain bacterial, viral or parasitic infections such as hepatitis B and C, metabolic conditions and inflammatory disorders may be the risk factors for CCA, but the molecular mechanism underlying the initiation and progression of CCA remains unclear.7,8
LncRNAs are longer than 200nt in length and contribute to a major part of human RNA, according to transcriptome analysis.9,10 Early studies have shown that lncRNAs exert function by affecting gene transcription, regulating splicing, interacting with RNA molecules, and taking participate in cellular biological function, such as cell proliferation, apoptosis, invasion and differentiation.11 Increasing evidence demonstrated that lncRNAs expressed abnormally in various human cancer types, including CCA. For example, CCA patients with upregulated lncRNA CPS1-IT1 tended to have a larger tumor size, advanced tumor stage and present lymph node metastasis. In addition, the aberrant expression of lncRNAs may regulate CCA cell proliferation and invasion by activating oncogenic signaling pathways.12,13 LncRNA GAS5 was initially described as potential tumor-suppressors. GAS5 is located on chromosome 1q25, with ~630 nucleotides. It is a 5′-terminal oligopyrimidine RNA that is comprised of 12 non-conserved exons. GAS5 introns are transcribed into 10 box C/D small nucleolar RNA (snoRNA) molecules and 2 mature lncRNAs (GAS5a and GAS5b).14,15 Recently, lncRNA GAS5 has been reported to be downregulated in ovarian cancer and low expression of lncRNA GAS5 promotes the progression of ovarian cancer.16 Notably, GAS5 is overexpressed in liver cancer, potentially functioning as an oncogene, Tao et al reported that GAS5 may act as a pro-oncogene in hepatocellular carcinoma.17 However, the role of GAS5 in the occurrence and progression of CCA has not been investigated previously.
In this study, firstly, the expression of GAS5 in CCA tissues and cell lines was detected. Then, the role of GAS5 in CCA cell proliferation and invasion was observed. In addition, the target miRNAs of GAS5 were predicted and verified. Finally, the role of the target miRNA on GAS5 inducing CCA cell invasion and proliferation was discussed. This study aimed to explore the role and potential mechanism of GAS5 in CCA progression.
Materials and Methods
Gene Expression Profiling Interactive Analysis (GEPIA)
GEPIA (http://gepia.cancer-pku.cn/) is an interactive and open website based on The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEX) project data. We extracted the expression profiles of GAS5 in various types of human cancer and adjacent normal tissues from GEPIA data. Then, the expression of GAS5 in CCA and adjacent normal tissues were analyzed. In addition, GEPIA data were used to explore the expression of GAS5 in different stages of CCA patients.
Patient Samples
Fifty cases of CCA patients were recruited from the Department of Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital. The tumor tissues and the adjacent non-tumor tissues were collected and immediately frozen in liquid nitrogen and then stored at −80°C once they were excised. The matched normal adjacent tissues were collected from 30 cases of the 50 malignant samples mentioned above. All the patients did not have any treatment and signed a written informed consent form before enrollment. The Ethical Committee of The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital for Clinical Research approved the protocol for this study. Informed consent was obtained from all study participants according to the Helsinki Declaration.
siRNA Transfection
CCA cell lines, including RBE, CCLP1, HuCCT1 and HCCC-9810 cholangiocarcinoma cell lines and a normal human intrahepatic biliary epithelial cell (HIBEC) line were purchased from ATCC. Cells were cultured in the atmosphere with 5% CO2 at 37 °C. The medium of cell culture is Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. si-GAS5 and hsa-miR-1297 inhibitor were purchased from GenePharma (Shanghai China). Lipofectamine 3000 reagent (Invitrogen, USA) was used for cell transfection. The transfection of cell with si-GAS5 and hsa-miR-1297 inhibitor were performed according to the manufacturer’s instructions. The PCR primers were miR-1297-forward: 5′-TCGGCAGGTTCAAGTAATT-3′; miR-1297-reverse: 5′-CTCAACTGGTGTCGTGGA-3′. The sequence of siRNA targeting GAS5 was 5′-UCUUCAAUCAUGAAUUCUGAG-3′. The negative control nontargeting sequence was 5′-ACGUGACACGUUCGGAGAATT-3′.
RNA Isolation and qRT-PCR
Total RNA from CCA tissues and cell lines was isolated with TRIzol reagent (Invitrogen). To examine the levels of GAS5, reverse transcription of 1 µg of total RNA into synthesized cDNA was performed using a cDNA synthesis kits (Invitrogen, Carlsbad, CA, USA) according to the protocols in the kits. SYBR Master Mixture (TAKARA, Tokyo, Japan) was applied to detect the expression of GAS5 by quantitative real-time PCR (qRT-PCR). The 2ˆ-ΔΔCT method was used to determine the relative mRNA expression. The primers were as follows: GAS5-F: ACTCCTGTGAGGTATGGTGCTGG, GAS5-R: GCTCTTTAGGACTTCAGTAGCTATTC; U6-F: 5′‐ATTGGAACGATACAGAGAAGATT‐3′, and U6-R: 5′‐GGAACGCTTCACGAATTTG‐3′. The thermocycling profile was as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 30 s.
CCK-8 Assay
RBE cells and si-GAS5 transfected RBE cells were seeded in 96-well plates at a density of 4.0 × 103 cells/well for 48h. Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan) was used to assess cell proliferation according to the manufacturer’s protocol. After incubation at 37°C for 0, 24, 48 or 72 h, 10 µL CCK-8 solution was added to each well. The absorbance was measured at 450 nm on a microplate reader (Thermo Scientific, Waltham, MA, USA).
Cell Invasion Assay
The role of GAS5 in CCA cell invasion was evaluated by Transwell assay (Corning, Corning, NY, USA). The lower chamber of Transwell was filled with 700 μL of DMEM supplemented with 10% fetal bovine serum. Then, 1×105 RBE cells and si-GAS5 transfected RBE cells suspended in 200 µL DMEM were seeded into the upper chambers. After incubation for 2 days in the atmosphere with 5% CO2 at 37 °C, cotton swabs were applied to remove the non-invading cells on the surface of Transwell. The invasive RBE cells on the lower surface of the membrane were stained with 1% crystal violet solution at room temperature for 15 min. Images of invading cells were captured under an inverted microscope.
Bioinformatics Analysis and Luciferase Reporter Assay
miRDB14 (http://mirdb.org/), DIANA IncBase v212 (http://diana.imis.athena-innovation.gr/) and RegRNA2.013 (http://regrna2.mbc.nctu.edu.tw/detection.html) were used to predict target miRNAs of GAS5. After screening and analysis, hsa-miR-1297 was selected as the target miRNA of GAS5. The wild-type (WT) and mutant-type (MT) GAS5 luciferase reporter plasmids (pmirGLO-GAS mild and pmirGLO-GAS5 mutant) were obtained from Gene Pharma (Shanghai, China), which contain the miR-1297 binding site of GAS5. To explore the target association between GAS5 and miR-1297, Lipofectamine 3000 was used to co-transfect RBE cells with WT or MT GAS5 luciferase reporter plasmid and miR-1297 or NC mimics. Following 48 h, the luciferase activity was measured by the Dual Luciferase Reporter Assay System (Promega Corp., Madison, WI, USA) according to the manufacturer’s protocol.
Statistical Analysis
The analyses are performed using SPSS 20.0 statistical software (IBM Corporation, USA). The differences between two groups were analysed using two-tailed Student’s t-tests. χ2 tests were used to analyze the correlation between clinicopathological features and GAS5 expression in CCA patients. *p < 0.05 was considered significant. All experiments were performed at least three independent experiments.
Results
The Level of GAS5 Was Increased in CCA Patients
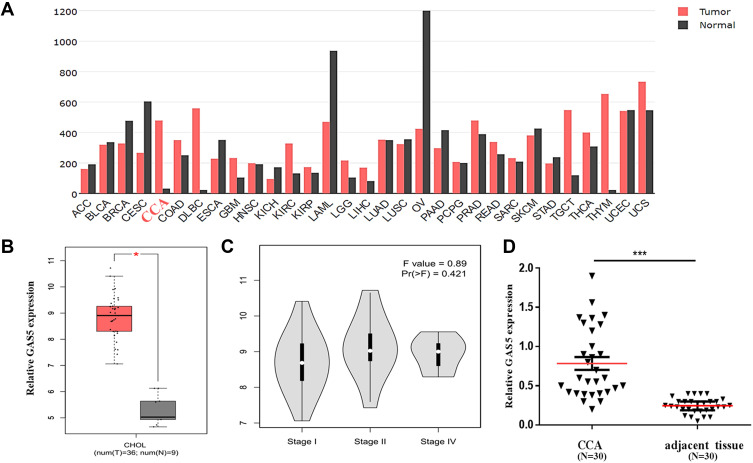
To explore the expression of GAS5 in human CCA, an open and online dataset (GEPIA) was analyzed. The result showed that GAS5 was differentially expressed in a variety of tumors and healthy tissues, and the expression of GAS5 was significantly increased in human CCA tissues, and GAS5 expression in human CCA tissues was higher than that in adjacent tissues (Figure 1A). A total of 9 healthy tissues with 36 CCA tissues were included in the study, and the results showed that the expression of GAS5 in CCA was markedly upregulated (p<0.05, Figure 1B). CCA can be classified by tumor stages I, II, and IV, and the expression level of GAS5 was elevated significantly with the progression of the disease stage on the GEPIA dataset (p<0.05, Figure 1C). In order to further validate the differential expression of GAS5, we collected 30 CCA tissues and paired adjacent non-tumor tissues. qRT-PCR was used to explore the expression of GAS5 in CCA tissues and adjacent tissues. The results showed that the expression of GAS5 in CCA tissues was dramatically upregulated, compared with the adjacent tissue (p<0.001, Figure 1D).
Figure 1.
Clinical expression of GAS5 in CCA patients. (A) The expression profile of GAS5 by bioinformatics analysis according to the GEPIA database. (B) Differential expression of GAS5 between CCA and healthy tissues based on the GEPIA database. *p<0.05. (C) Differential expression of GAS5 in stage I, stage II and stage IV of CCA. (D) qRT-PCR was applied to detect the differential expression of GAS5 in CCA tissues and adjacent tissues, ***p<0.001.
The Elevated Expression of GAS5 in CCA Patients Was Associated with Clinicopathological Characteristics
To analyze the role of GAS5 in CCA, according to the median value of GAS5 expression in CCA patients, CCA patients were divided into high GAS5 and low GAS5 expression groups. There was no significant relationship between GAS5 expression and clinical features, such as age, gender and tumor size. However, the results showed that increased GAS5 expression in CCA patients was associated with lymph node metastasis (p<0.001) and a more advanced clinical stage (p=0.006), which suggested that GAS5 might exert a significant role in CCA metastasis (Table 1).
Table 1.
Relationship Between GAS5 Expression and Clinical Features of CCA Patients
| Variables | Number of Cases | GAS5 Expression | χ2 | P | |
|---|---|---|---|---|---|
| High (n=28) | Low (n=22) | ||||
| Gender | |||||
| Male | 25 | 13 | 12 | 0.321 | 0.568 |
| Female | 25 | 15 | 10 | ||
| Age (years) | |||||
| <60 | 19 | 11 | 8 | 0.044 | 0.832 |
| ≥60 | 31 | 17 | 14 | ||
| Tumor size (cm) | |||||
| <5 | 20 | 11 | 9 | 0.013 | 0.907 |
| ≥5 | 30 | 17 | 13 | ||
| Lymph node metastasis | |||||
| Presence | 33 | 26 | 7 | 20.461 | <0.001 |
| Absence | 17 | 2 | 15 | ||
| Clinical stage | |||||
| I/II | 19 | 6 | 13 | 7.417 | 0.006 |
| III/IV | 31 | 22 | 9 | ||
Knockdown of GAS5 Reduced CCA Cell Proliferation and Invasion
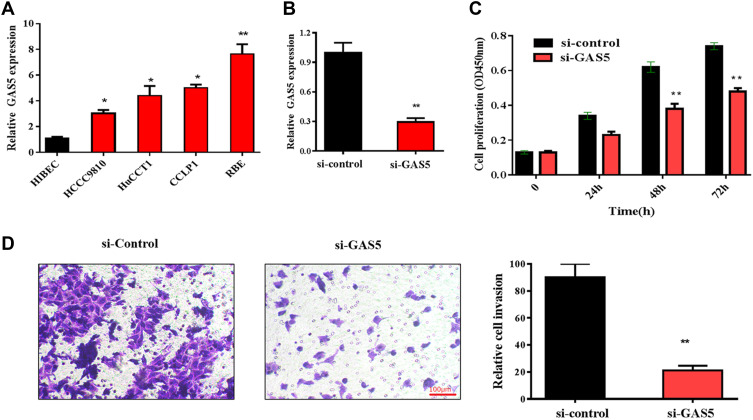
To further explore the role of GAS5 in CCA progression, the expression of GAS5 was firstly analyzed in CCA cell lines. Consistent with the data of clinical tissues, the expression of GAS5 was significantly elevated in CCA cell lines (RBE, CCLP1, HuCCT1 and HCCC-9810), compared with that in the control cell line (HIBEC). As showed in Figure 2A, the expression of GAS5 was most increased in REB cell line; thus, REB cell line was selected for the subsequent experiments. RNA interference method was used to knock down endogenous GAS5 expression. The expression of GAS5 in RBE cells transfected with si-GAS5 was significantly decreased, compared with that transfected with si-control (Figure 2B). CCK-8 assays and transwell invasion assays were applied to evaluate the role of GAS5 in CCA cell proliferation and invasion. The result of CCK-8 assays showed that the proliferation of the RBE cell transfected with si-GAS5 was markedly inhibited, compared with the cell transfected with si-control (p<0.01) (Figure 2C). Similarly, transwell assays showed that the transfection of si-GAS5 significantly inhibited RBE cells invasion, compared with the si-control group (p<0.01) (Figure 2D).
Figure 2.
Knockdown of GAS5 suppressed RBE cell proliferation and invasion. (A) GAS5 is upregulated in CCA cell lines compared with that in the HIBEC cell, *p<0.05, **p<0.01. (B) qRT-PCR was used to detect the expression of GAS5 in RBE cells transfected with si-GAS5, **p<0.01. (C) CCK-8 assay was applied to observe the effect of GAS5 on RBE cell proliferation, **p<0.01. (D) Transwell assay was used to explore the role of GAS5 on RBE cell invasion. **p < 0.01 compared to the control group.
Bioinformatics Analysis Predicted That miR-1297 Was the Potential Target of GAS5
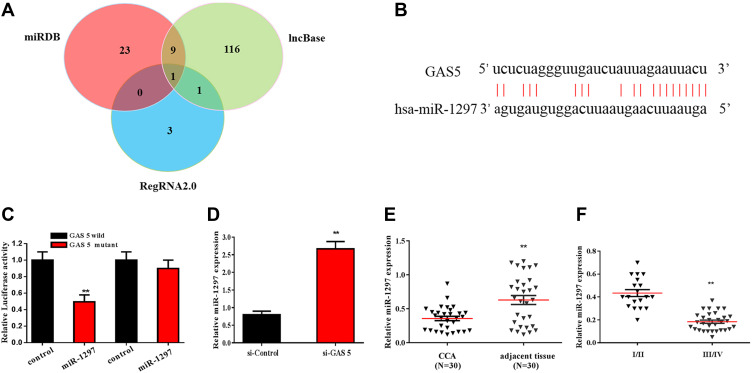
Studies showed that lncRNAs exert regulatory functions through targeting miRNAs that bind to the 3-UTR, function as miRNA “sponge” or ceRNA. Bioinformatics analysis was applied to predict the potential target miRNAs of GAS5 in CCA. DIANA IncBase v2, miRDB and RegRNA2.0 identified 127, 33 and 5 miRNAs, which may be the target gene of GAS5, respectively. As showed in Figure 3A, the intersection of miRNA was jointly predicted by three bioinformatics analyses. After screening and analysis, miR-1297 was found to be as the potential target miRNA of GAS5. The complementary binding sites of miR-1297 and GAS5 are shown in Figure 3B. The luciferase reporter assay was used to confirm the predicted binding site (Figure 3C). The qRT-PCR results showed that the expression of miR-1297 was increased after transfected with si-GAS5 in RBE cell (Figure 3D). The expression of miR-1297 was decreased in cholangiocarcinoma, compared with the adjacent normal tissues (Figure 3E). The expression of miR-1297 in CCA patients with clinical stage I–II and III–IV is shown in Figure 3F. We found that miR-1297 was markedly increased in clinical stage I–II, compared with clinical stage III–IV.
Figure 3.
miR-1297 was the downstream target of GAS5, which was predicted and validated by bioinformatics analysis and luciferase reporter assay, respectively. (A) A chart screen the downstream target miRNAs of GAS5. (B) The complementary binding site of GAS5 and miR-1297. (C) Luciferase reporter assay was applied to verified the predicted binding of GAS5 and miR-1297. (D) qRT-PCR was applied to observe the expression of miR-1297 in RBE cell transfected with si-GAS5. (E) qRT-PCR was applied to detect the expression of miR-1297 of CCA tissues and adjacent normal tissues. (F) qRT-PCR was used to assess the expression of miR-1297 of CCA tissues with different stages. **p < 0.01, compared to the control group.
GAS5 Promoted CCA Cell Proliferation and Invasion Through miR-1297
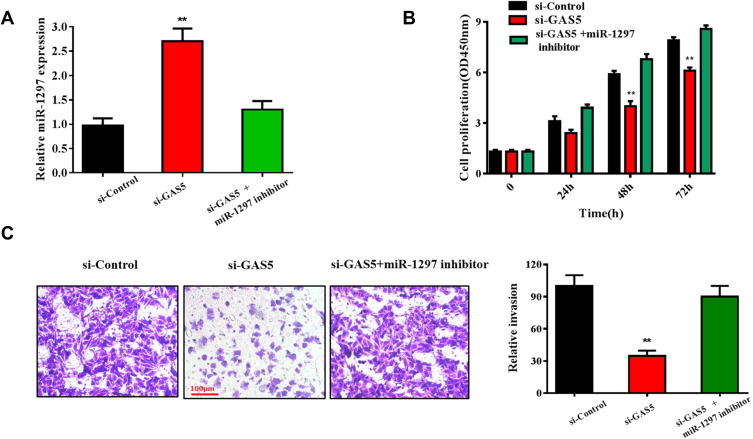
Functional studies were conducted to confirm whether GAS5 could regulate CCA progression by targeting the miR-1297. As shown in Figure 4A, the expression of miR-1297 was dramatically upregulated in the si-GAS5 transfected group (p<0.01), but decreased in the si-GAS5 and miR-1297 inhibitor co-transfected group. The result of CCK-8 assays showed that the miR-1297 inhibitor could prominently reverse the inhibitory effect of si-GAS5 on RBE cell proliferation (p<0.01) (Figure 4B). Similarly, RBE cell co-transfected with miR-1297 inhibitor and si-GAS5 possessed an increased invasion rate compared with those only transfected with si-GAS5 (p<0.01) (Figure 4C). These results suggested that GAS5 played an important role in CCA progression by targeting miR-1297.
Figure 4.
GAS5 promoted RBE cell proliferation and invasion through targeting miR-1297. (A) qRT-PCR was conducted to evaluate the expression of miR-1297 in RBE cell lines transfected with si-GAS5 and si-GAS5+miR-1297 inhibitor, respectively. (B) CCK-8 assay was used to observe the effect of si-GAS5+miR-1297 inhibitor on RBE cells proliferation ability. (C) Transwell assay was applied to detect the invasive capabilities of RBE cells transfected with si-GAS5 and miR-1297 inhibitor. **p < 0.01 compared to the control group.
Discussion
In the current study, we firstly analyzed the GEPIA databases to evaluate the expression of GAS5 in CCA. The results showed that GAS5 expression was significantly increased in CCA tissues compared with normal tissues. This argued with the previously reported tumor-suppressive role of GAS5 in several cancer types, such as pancreatic cancer, bladder cancer and cervical cancer.18–20 The explanation for this difference might be that lncRNA exhibits its distinctive function in different tissues. GAS5 may exhibit tumor suppressor and oncogenic roles in different cancer types.21 Subsequently, we confirmed the above-mentioned result by qRT-PCR in our clinical samples. The clinical results showed that CCA patients with high GAS5 expression were positively associated with lymph node metastasis and advanced clinical stage, indicating that GAS5 may play a promotive role in CCA metastasis. Previous evidence showed that GAS5 influences cell growth, cell cycle progression and cell division.22 In this study, the results of CCK-8 assay and invasion assay demonstrated that down-regulation of GAS5 markedly inhibited CCA cell proliferation and invasion. For the first time, the results of our experiment offered evidence that upregulation of GAS5 may induce CCA progression. Different from our results, a previous study showed that downregulation of GAS5 promoted bladder cancer cell proliferation.23 Thus, our data, together with previous findings, suggested that the abnormal expression of GAS5 might involve cell growth and invasion.
Generally, lncRNAs could function as a miRNA sponge to modulate their downstream targets. In breast cancer, it was shown that GAS5 could be used as a miR-23a sponge, which promotes autophagy and enhances the formation of autophagosomes after GAS5 overexpression.24 Another study showed that GAS5 serves as a competing endogenous RNA for miR-223; and the inhibition of GAS5 reduces the activity of miR-223 inhibitor on cell growth, apoptosis and invasion.25 In the present study, the mechanism underlying GAS5 accelerated CCA progression was revealed. Based on the interaction between miRNAs and lncRNAs, DIANA IncBase v2, miRDB and RegRNA2.0 were applied to predict the target miRNAs of GAS5.26 We found that miR-1297 might interact with GAS5. The luciferase reporter assays confirmed the interaction between miR-1297 and GAS5. Studies have suggested that miR-1297 is related to the proliferation, apoptosis and metastasis of cancer cells. However, opinions about the role of miR-1297 in tumor progression are controversial. A study showed that the expression of miR-1297 was significantly decreased in gastric cancer tissue, compared to adjacent normal tissue. Upregulation of miR-1297 significantly inhibited cell proliferation in vitro and tumor growth in vivo.27 While others found that miR-1297 was an oncogene in oral squamous cell carcinoma and promoted tumor progression through PTEN.28 This difference could be explained by the diversity of miRNA’s function. Our study showed that miR-1297 was significantly downregulated in clinical stage III–IV of CCA, compared with clinical stage I–II. MiR-1297 inhibitor could reverse the inhibition of si-GAS5 on CCA cell proliferation and invasion. Our data indicated that GAS5 accelerated CCA cell proliferation and invasion by regulating miR-1297.
Conclusions
In conclusion, our research is the first time to reveal the increased level and biofunction of GAS5 in CCA, and verified that miR-1297 was the target miRNA of GAS5 in CCA. GAS5 promoted CCA proliferation and invasion through targeting miR-1297. Given the clinical and functional significance of GAS5 in CCA, we summarized that GAS5 may be a pivotal biomarker and target for the diagnosis and treatment of CCA.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Sempoux C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;4(31):49–60. doi: 10.1055/s-0031-1272839 [DOI] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(2):2168–2179. doi: 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragazzi MC, Ridola L, Safarikia S, et al. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol. 2018;31(5):42–55. doi: 10.20524/aog.2017.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel T. New insights into the molecular pathogenesis of intrahepatic cholangiocarcinoma. J Gastroenterol. 2014;49(2):165–172. doi: 10.1007/s00535-013-0894-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith-Cohn MA, Gill D, Voorhies BN, et al. Case report: pembrolizumab-induced type 1 diabetes in a patient with metastatic cholangiocarcinoma. Immunotherapy. 2017;9(2):797–804. doi: 10.2217/imt-2017-0042 [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Liu Y, Wang B, et al. Sumoylation in p27kip1 via RanBP2 promotes cancer cell growth in cholangiocarcinoma cell line QBC939. BMC Mol Biol. 2017;18(7):23–33. doi: 10.1186/s12867-017-0100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labib PL, Goodchild G, Pereira SP. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer. 2019;19(1):185. doi: 10.1186/s12885-019-5391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagante F, Gamblin TC, Pawlik TM. Cholangiocarcinoma risk factors and the potential role of aspirin. Hepatology. 2016;6(3):708–710. doi: 10.1002/hep.28613 [DOI] [PubMed] [Google Scholar]
- 9.Bertone P, Stolc V, Royce TE. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004;306(11):2242–2246. doi: 10.1126/science.1103388 [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Dong S, Duan B. HOTAIR is a predictive and prognostic biomarker for patients with advanced gastric adenocarcinoma receiving fluorouracil and platinum combination chemotherapy. Am J Transl Res. 2015;1295‐1302. [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Sun Q, Zhao L, et al. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol. 2016;33(8):88–97. doi: 10.1007/s12032-016-0804-2 [DOI] [PubMed] [Google Scholar]
- 12.Sand M, Bechara FG, Sand D, et al. Expression profiles of long noncoding RNAs in cutaneous squamous cell carcinoma. Epigenomics. 2016;8(4):501–518. doi: 10.2217/epi-2015-0012 [DOI] [PubMed] [Google Scholar]
- 13.Ma SL, Li AJ, Hu ZY, et al. Coexpression of the carbamoylphosphate synthase 1 gene and its long noncoding RNA correlates with poor prognosis of patients with intrahepatic cholangiocarcinoma. Mol Med Rep. 2015;12(9):7915–7926. doi: 10.3892/mmr.2015.4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Huang H, Li Y, et al. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol Rep. 2016;36(5):3241. doi: 10.3892/or.2016.5200 [DOI] [PubMed] [Google Scholar]
- 15.Fleming JV, Fontanier N, Harries DN, et al. The growth arrest genes gas5, gas6, and CHOP-10 (gadd153) are expressed in the mouse preimplantation embryo. Mol Reprod Dev. 1997;48(3):310–316. doi: [DOI] [PubMed] [Google Scholar]
- 16.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54(6):787–793. doi: 10.1016/S0092-8674(88)91065-3 [DOI] [PubMed] [Google Scholar]
- 17.Tao R, Hu S, Wang S, et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis. 2015;36(10):1136–1143. doi: 10.1093/carcin/bgv099 [DOI] [PubMed] [Google Scholar]
- 18.Cao S, Liu W, Li F, et al. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7(10):6776–6783. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Wu S, Ma J, et al. lncRNA GAS5 reverses EMT and tumor stem cell-mediated gemcitabine resistance and metastasis by targeting miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:472–482. doi: 10.1016/j.omtn.2018.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Li J, Xiong G, et al. Negative regulation of lncRNA GAS5 by miR-196a inhibits esophageal squamous cell carcinoma growth. Biochem Biophys Res Commun. 2018;495(1):1151–1157. doi: 10.1016/j.bbrc.2017.11.119 [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Xie Z, Lei X, et al. Long non-coding RNA GAS5 in human cancer. Oncol Lett. 2020;20(3):2587–2594. doi: 10.3892/ol.2020.11809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin D, He X, Zhang E, et al. Long non-coding RNA GAS5 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Med Oncol. 2014;31(11):253. doi: 10.1007/s12032-014-0253-8 [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Wang W, Jiang J, et al. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One. 2013;8(9):e73991. doi: 10.1371/journal.pone.0073991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu J, Wang Y, Wang X, et al. Effect of the lncRNA GAS5-miR-23a-ATG3 axis in regulating autophagy in patients with breast cancer. Cell Physiol Biochem. 2018;48(1):194–207. doi: 10.1159/000491718 [DOI] [PubMed] [Google Scholar]
- 25.Dong X, Kong C, Liu X, et al. GAS5 functions as a ceRNA to regulate hZIP1 expression by sponging miR-223 in clear cell renal cell carcinoma. Am J Cancer Res. 2018;8(8):1414–1426. [PMC free article] [PubMed] [Google Scholar]
- 26.Guo LL, Wang SF. Downregulated long noncoding RNA GAS5 fails to function as decoy of CEBPB, resulting in increased GDF15 expression and rapid ovarian cancer cell proliferation. Cancer Biother Radiopharm. 2019;1(2):1–10. [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Cao Y, Guo P, et al. Downregulation of MiR-1297 predicts poor prognosis and enhances gastric cancer cell growth by targeting CREB1. Biomed Pharmacother. 2019;9:413–419. [DOI] [PubMed] [Google Scholar]
- 28.Liang L, Feng L, Wei B. microRNA-1297 involves in the progression of oral squamous cell carcinoma through PTEN. Saudi J Biol Sci. 2018;5(5):923–927. doi: 10.1016/j.sjbs.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]