Abstract
Cannabidiol (CBD), a lipophilic cannabinoid compound without psychoactive effects, has emerged as adjuvant of anti-epileptic drugs (AEDs) in the treatment of refractory epilepsy (RE), decreasing the severity and/or frequency of seizures. CBD is considered a multitarget drug that could act throughout the canonical endocannabinoid receptors (CB1-CB2) or multiple non-canonical pathways. Despite the fact that the CBD mechanism in RE is still unknown, experiments carried out in our laboratory showed that CBD has an inhibitory role on P-glycoprotein excretory function, highly related to RE. Since CB2 is expressed mainly in the immune cells, we hypothesized that CBD treatment could alter the activity of polymorphonuclear neutrophils (PMNs) in a similar way that it does with microglia/macrophages and others circulating leukocytes. In vitro, CBD induced PMN cytoplasmatic vacuolization and proapoptotic nuclear condensation, associated with a significantly decreased viability in a concentration-dependent manner, while low CBD concentration decreased PMN viability in a time-dependent manner. At a functional level, CBD reduced the chemotaxis and oxygen consumption of PMNs related with superoxide anion production, while the singlet oxygen level was increased suggesting oxidative stress damage. These results are in line with the well-known CBD anti-inflammatory effect and support a potential immunosuppressor role on PMNs that could promote an eventual defenseless state during chronic treatment with CBD in RE.
Keywords: cannabidiol, refractory epilepsy, polymorphonuclear neutrophils (PMNs), chemotaxis, oxygen consumption, oxidative stress
1. Introduction
Epilepsy is a neurological disease affecting about 50 million people worldwide, and nearly 30–40% of patients will develop a multidrug-resistant (MDR) phenotype defined as refractory epilepsy (RE), characterized by high recurrence of seizures that cannot be controlled by at least two well-tolerated antiepileptic drugs (AEDs) appropriate for the particular epilepsy type [1,2,3]. Despite the constant develop of the AEDs with several improves in absorption, tolerance, molecular target and diminished side effects, the rate of RE remains unchanged through the decades. Several mechanisms have been described that explain this phenotype, including the role of ATP binding cassette (ABC)-transporters, particularly P-glycoprotein (P-gp) [4,5].
Several alternative therapies such as ketogenic diet and vagal stimulation have been applied with variable results. In this context, the treatment with cannabinoids compounds such as cannabidiol (CBD) have emerged as a promising adjuvant therapy for seizure control [6]. Several reports showed a decrease in the seizures frequency and intensity [7,8,9] and that correlates with an increased plasmatic AEDs concentration [10,11,12]. Despite the beneficial effect of CBD, only Epidiolex® was approved by the Food and Drug Administration, and their action mechanism is still unknown [13]. Several lines of evidence highlight CBD as a multitarget drug that could act throughout the endocannabinoid receptors (CB1 in the central nervous system and CB2 in immune cells) as an inverse agonist [14,15] as well as throughout of others metabotropic receptors such as serotonin receptors (5-HT1A and 5-HT2A) [16], adenosine receptors (A1 and A2) [17] and G protein-coupled receptor 55 (GPR55) [16]. CBD has also been demonstrated to regulate current of Ca interacting TRPV1 [18,19] or voltage-gated T-type calcium channels (VGCC) [20]. Furthermore, CBD is able to inhibit the activity of several cytochromes responsible for AEDs metabolization. In this way, CBD inhibits the activity of CYP2C19, CYP2D6 and CYP2C9 that metabolize Phenytoin and Carbamazepine between others [21,22]. Recently, it was demonstrated that CBD has an inhibitory action on the excretory function of P-glycoprotein, highly related to drug-resistant epilepsies [5,23]. So, the chronic use of CBD in addition to common anti-epileptic drugs (AEDs) could help in the control of severity and/or frequency of seizures in patients with RE supporting the CBD adjuvant role.
The endocannabinoid system can regulate the immune response through the stimulation of CB2 receptors, which is mainly expressed in most of the immune cells including the neutrophils polymorphonuclear cells (PMNs) [24]. PMNs are produced in large numbers in the bone marrow (~1011 cell per day), and they represent the more abundant leukocytes in peripheral blood and are renewed from circulation three times a day [25]. The stimulation of PMN´s CB receptors with the endogenous agonist 2-arachidonoylglycerol usually leads to an anti-inflammatory effect [26]. In a rat uveitis model, the stimulation of CB2 decreased leukocyte adhesion in the iris microcirculation, inhibited the release of pro-inflammatory cytokines and chemokines and increased iridial microcirculation blood flow [27,28]. Furthermore, the in vivo and in vitro stimulations of CB2 have been related with a decreased release of metalloproteases from PMNs [29]. In the same way, the lack of CB2 increased the PMN recruitment in the CB2 knockout (CB2-KO) mice endotoxemia model [30]. Additionally, the level of the intestinal lumen in these CB2-KO mice, a decreased N-acyl ethanolamine-type endocannabinoids release allowed an increased PMN transmigration that correlated with the ablation of CB2 [31].
Cellular immunity activity of PMNs needs initial processes for neutrophils activation, triggering an ordered sequence of events as rolling and adhesion to activated endothelium; migration into the affected tissues by chemotaxis; recognition and binding to microorganisms; engulfment and phagocytosis of them and occasionally neutrophil extracellular tramps (NETs) production. All these processes are orchestrated by a complex tangle of cytokines that control and drive the PMNs activity [32]. The PMNs transmigration occurring prominently at the borders of endothelial cells, secondary to signals delivered by “end target-derived” chemoattractants, such as formyl peptides, released by bacteria or by mitochondria from dying cells. These are dominant signals that override “regulatory cell-derived”, such as bioactive peptides (LTB4) or chemokines (IL-8) [33]. When the PMNs arrive at the site of infection and recognize the pathogens, they activate the NADPH (nicotinamide adenine dinucleotide phosphate-oxidase/Nox2) oxidase complex to produce reactive oxygen species (ROS) with which to attack the microorganisms at the extracellular level or in phagosomes. After assembly, the NADPH oxidase complex transfer electrons from NADPH to molecular oxygen generating superoxide anions (O2−) as the primary product. To minimize damage, cells are equipped with antioxidant scavenging enzymes, such as superoxide dismutase (SOD) and catalase. SOD, which dismutates O2− to hydrogen peroxide (H2O2) and glutathione peroxidase, can further convert these species into water, which limit damages to the host. Granule-localized myeloperoxidase (MPO) can convert H2O2 to hypochlorous acid (HOCl), which can enhance clearance of invading pathogens. MPO can also directly convert O2− into singlet oxygen (1O2*). In addition, ferric iron can convert O2− and H2O2 into hydroxyl radical (HO•) [34,35].
Cannabinoids compounds can also facilitate different infections supporting an immunosuppressive role [36]. Additionally, it was reported that activation of CB2 blocks monocyte migration, dampens LPS induced secretion of the pro-inflammatory cytokine tumor necrosis-alpha (TNF-α) and can to inhibits the differentiation of human monocytes into antigen-presenting dendritic cells [37], altering the inflammatory responses to infection [38]. Furthermore, it was described that CBD can induce apoptosis in human monocytes by mitochondrial oxidative stress [39]. In the same way, similar effects on microglia proliferation, migration and inflammatory response were elicited by CBD [40,41,42]. In light of these evidences, we hypothesized that CBD treatment could alter the activity of PMNs in a similar way that it does with microglia/macrophages and others circulating leukocytes leading to reduce the immunocompetence of PMNs, by producing functional alterations and/or affecting their viability.
2. Results
2.1. Effect of CBD on PMN Viability
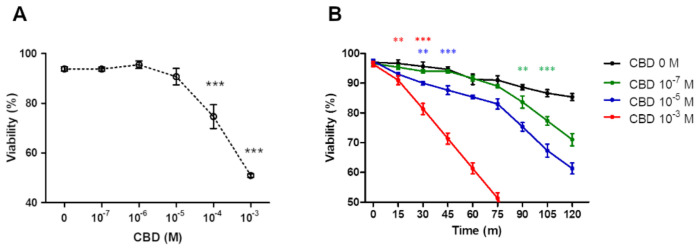
Under the hypothesis of the anti-inflammatory effect of CBD, argued about an increased PMN mortality, we tested the cell viability incubating 106 PMN/mL with increased concentrations of CBD (10−7 M to 10−3 M). After 1 h of incubation, the micromolar CBD range did not produced any change in PMN viability, while a substantially decreased viability (77 ± 3.1%) at submilimolar CBD concentration was observed, while approximately 50% of viability was reached with millimolar CBD concentration (Figure 1A). Next, the time course of PMNs viability at low, middle and high CBD concentrations was analyzed. When the PMNs were incubated with DMSO as vehicle (DMSO was added at same concentration as CBD a 10−3 M) a PMN’s viability of 10% at 60 min (Figure 1B, black line) was observed. Similar results were observed with CBD 10−7 M, but that fall accelerated at 90–120 min. In contrast, intermediate and very pronounced loss of viability were observed with CBD at 10−5 M and 10−3 M, respectively (Figure 1B).
Figure 1.
CBD affect the viability of PMNs. PMNs viability is significantly affected with CBD concentrations >10−5 M (A) and correlated with shorter survival time courses at the same CBD concentrations (B). Results are expressed as mean ± SD (p < 0.05). ** and *** indicate the significance differences relative to 0 M of CBD. In (B) the asterisks indicate the point from which the curves for each CBD concentration are different from the control, while the color of the asterisks indicates which CBD concentration is compared.
2.2. Morphological Analysis
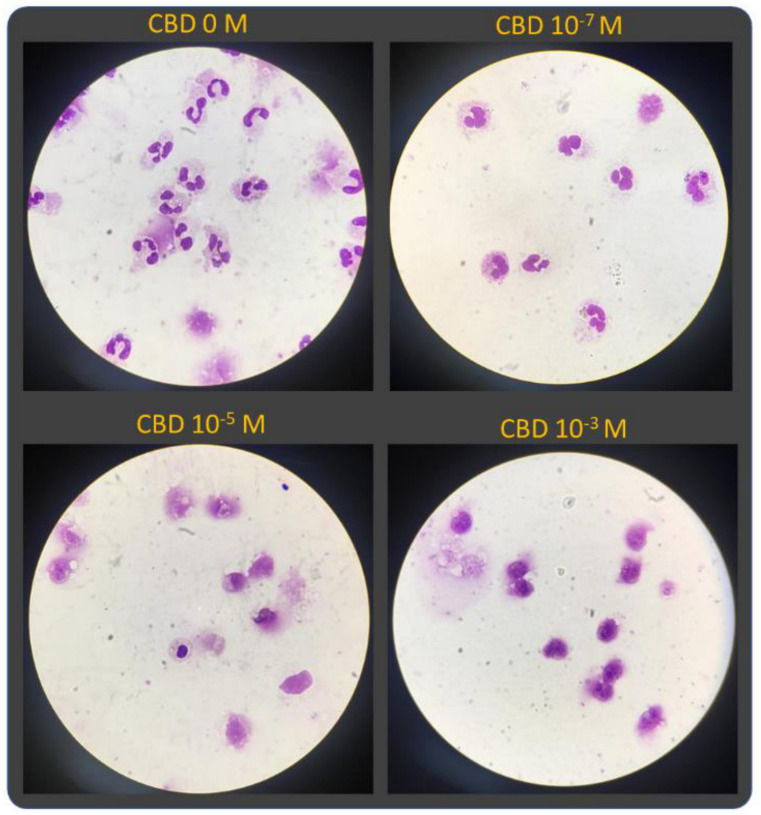
The morphological analysis revealed that PMN exposed at lower CBD concentrations did not show any significant morphological alteration compared to normal neutrophils. However, the higher concentration of CBD gradually increased the morphological alterations characterized by cytoplasmic vacuolization, nuclei with pseudo-maturation aspects, degranulation and rupture of the cytoplasmic membrane (Figure 2).
Figure 2.
The morphology of PMNs is modified by CBD in a concentration-dependent manner. All images were captured with a magnification of 40×.
In normal conditions, a spontaneous nuclear modification can be observed across the time, where normal polymorphonuclear (PMN) neutrophils’ population decreases proportionally to the increasing of abnormal mononuclear (MN) forms’ population (Figure 3A). The PMN/MN rate indicated that Figure 3B showed that the PMN/MN rate decreases significantly after one hour incubation only with higher CBD concentrations (10−3 M) (Figure 3).
Figure 3.
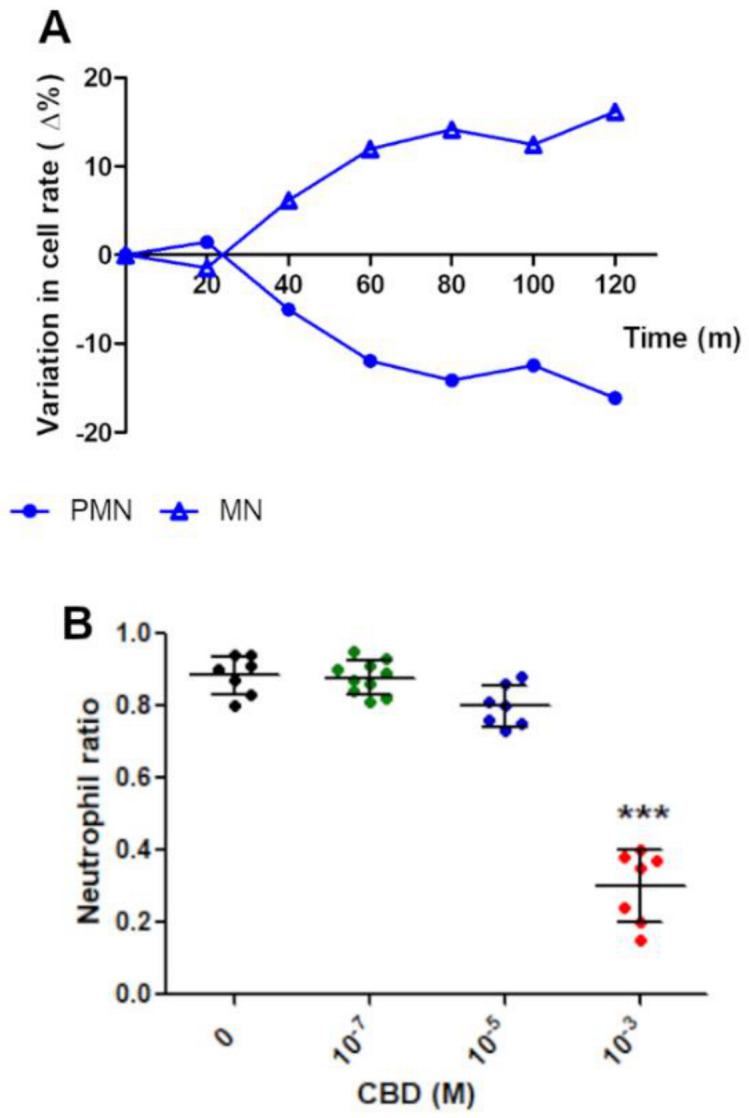
CBD accelerates the decrease in PMN/MN rate. (A) Spontaneous nuclear transformation from PMN to MN forms affects no more than 20% of normal PMN population in a symmetric mode. (B) A significant drop in the PMN/MN ratio is observed when PMNs are exposed to higher CBD concentrations. Results are expressed as mean ± SD (p < 0.0001). *** indicate the significance differences relative to 0 M of CBD.
2.3. Effect of CBD on PMN Functionality
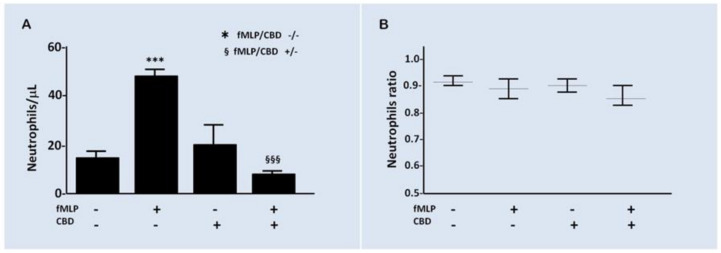
As mentioned above, 10−7 M CBD did not produce a significant effect on the viability to short times, and then, we use this concentration to test the effect of CBD on the PMN chemotaxis elicited by fMLP 10−7 M using a Boyden chamber. After an incubation of 45 min, the chemotaxis induced by fMLP was increased by three times over the spontaneous basal level. On the other hand, CBD reduced the induced chemotaxis in comparison to vehicle (Figure 4A). Additionally, the PMN/MN ratio in the upper well of Boyden chamber was measured (Figure 4B) to discard if any differences of PMN/MN ratio could be related with any altered chemotaxis results. No statistical differences were observed in the PMN/MN ratio between CBD and vehicle.
Figure 4.
CBD modifies the PMN chemotaxis. (A) The activation of chemotaxis by fMLP is significantly inhibited by CBD (p < 0.01). *** compared to fMLP/CBD -/- while §§§ compared to fMLP/CBD +/-. (B) During the time of the experiment, no differences were observed in the ratio PMN/MN, indicating that chemotaxis results were independent of morphologic modifications. Results are expressed as mean ± SD (p = 0.1865).
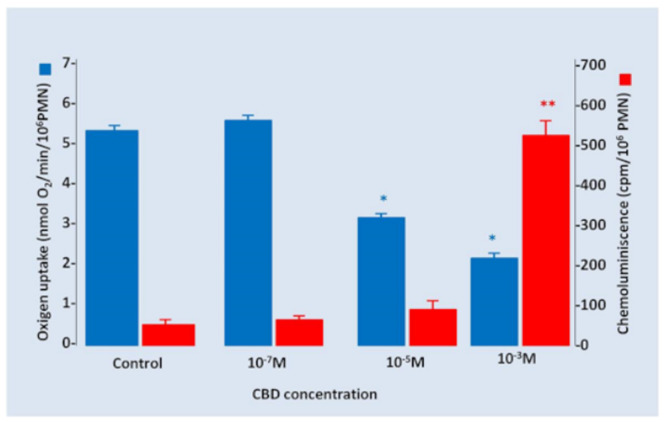
Additionally, the PMN oxygen consumption and chemiluminescence under CBD gradually increased concentrations were measured. We found that CBD 10−7 M had no effects on oxygen uptake by PMNs; however, a significant decrease in oxygen consumption was observed, in an apparent inverse relationship with the increase in CBD concentrations. Inversely, chemiluminescence was significantly increased only when PMNs were incubated with high concentrations of CBD 10−3 M (Figure 5).
Figure 5.
Oxygen uptake and chemiluminescence under several CBD concentrations. An inverse effect between oxygen uptake and chemiluminescence was observed secondary to the incubations of PMNs with increased concentrations of CBD. Results are expressed as mean ± SD (p < 0.05). * and ** indicate the significance differences relative to 0 M of CBD.
3. Discussion
It was described that cannabidiol (CBD), a non-psychoactive metabolite of Cannabis sativa, is popularized as a potential medicinal product with anti-inflammatory, antioxidant and analgesic effects. Because PMN cells are clear protagonists of these mentioned conditions, we hypothesized that CBD could play an inhibitory effect on these properties, irrespective to the CBD actions on other tissues and systems. Some endocannabinoids or phytocannabinoids have been reported to inhibits the human neutrophils migration, but without effects on their viability [43].
In our study, the viability as well as chemotaxis were significantly reduced by CBD 10−3 M and 10−7 M (50 and 80%, respectively). Additionally, a loss in nuclear lobulation and morphology changes were observed in PMN exposed to CBD. Additionally, we report that the oxygen uptake of PMNs also was diminished, while chemiluminescence was significantly induced by CBD at 10−3 M. This dissociating effect on the two parameters of oxidative stress (O2 consumption and chemiluminescence) could be secondary to the poly-target CBD properties, acting on different receptors presents on PMNs and activating as well as inhibiting a wide spectrum of cell’s functionalities.
Structural and functional changes in PMNs membrane trigger the respiratory burst either by the uptake of microorganisms or by active agents at membrane level. This process includes activation of both multi-membrane-bound NADPH oxidase complex [44,45] and constitutive nitric oxide synthase (cNOS) in the external membrane of PMNs and myeloperoxidase enzyme in the internal granules. In response to a stimulus, PMNs consume oxygen (O2) to generate O2− and H2O2. Experimentally, it was described that the activity of NOX2 is inhibited by CBD in PMNs and, consequently, the generation of O2− and H2O2, preventing the oxidative burst in a direct form, interfering with translocation of the NOX2 subunits to the membrane, or indirectly, on signaling pathways needed for its activation [46].
In our experiments, CBD produced an inhibitory effect on O2− and H2O2 generation; this effect was increased when PMNs were exposed to increasing CBD concentrations (40% for 1 × 10−5 M, p < 0.05 and 60% for 1 × 10−3 M, p < 0.01) (Figure 5). These results suggest that the inhibitory effect of CBD on NOX2 activity could be associated to a direct inhibitory effect on this protein or inducing structural changes in the membrane that prevent the binding of NOX2 subunits (p47phox and p40phox) necessary for its normal function, as was described for chronic granulomatous disease (CGD) [47]. These results are in the same line with a recent report showing the decreased activity in microglial NADPH oxidase complex [48]. Potential mechanisms of NOX2 deactivation by CBD can include the dephosphorylation of phox subunits, decreased activity of guanine activating protein (GAPs) on Rac (small GTPase) proteins and dissemble of the complex or diminution of amount of FAD [35].
Nitric oxide (NO) is generated together with O2− and H2O2 in the respiratory burst in PMNs exposed to chemoattractant agents as phorbol esters (PMA), by the activation of cNOS [49]. Carreras et al. showed that NO production is about 30% of the total oxygen consumption in PMN stimulated with PMA, and 70% of oxygen is used, simultaneously, for O2− generated by NOX2 activity, involving protein kinase C as a common mechanism [50]. NO inhibits O2− production by NOX2 and other potent oxidants in PMNs activated, the peroxynitrite (ONOO−) when NO reacts with O2− [51]. However, the increase in spontaneous chemiluminescence by adding CBD in the highest concentration evaluated in this study would be indicating the generation of toxic for PMN and reactive end products of lipid peroxidation and proteins. This indicates the generation of the electronically excited product, 1O2*, in the neutrophils. The chemical reactivity of 1O2* in solution is mainly due to electron configuration, higher energy and short life of this reactive oxygen species; 1O2* emits light, as excited electrons fall to lower energy levels, and is the final product of lipid and protein oxidation process [52,53].
MPO needs H2O2 to induce toxic effects on microorganisms by mechanisms of halogenation or oxidation mediated mechanisms in neutrophils and is inhibited by hypoxia condition. MPO released from azurophilic granules catalyzes the synthesis of HO• and 1O2* from of H2O2 and O2− reaction or generates hypochlorite anion (ClO-) from H2O2 and chloride anion (Cl-). The ClO− anion may react with H2O2 in excess to generate 1O2* [54]. Nevertheless, the finding that phagocytosis is associated to light emission, that NADPH oxidase system is inhibited with CBD and that myeloperoxidase is present in very high concentrations in the azurophil granules of PMN indicate that products of oxidation may be formed by PMN and lipid peroxidation may be initiated for oxidants other than HO•. The molecular mechanism for H2O2 production in CBD exposed PMNs are unknown, but it seems be similar to the CGD characteristics, in which the assembly of O2− and H2O2-generating system, NOX2, is defective [55]. In neutrophils, the ROS production by activation of NADPH oxidase occurs not only at the plasma membrane but also at an intracellular location. Regardless of the ROS released out of the cell, and in order to give rise to an intracellular chemiluminescence reaction, ROS also needs access to MPO that is stored in the azurophil granules [56].
PMN-NADPH oxidase consists of at least of three cytosolic components and two subunits (cytochrome b), bound to both the plasma membrane and specific granule membranes, and activation of this enzyme could be related to the vesicular location of P-gp in neutrophils [57]. Furthermore, the neutrophil not only requires the presence of P-gp, but also the cystic fibrosis transmembrane conductance regulator (CFTR) into the phagosomal membrane to allow an increase in Cl- transport into the phagosome for the support of HOCl generation [58]. Because both P-gp and CFTR are members of the ABC-transporters superfamily, the inhibitory effects of CBD on P-gp, as previously demonstrated [23] could also inhibit the CFTR activity and, in consequence, increase the risk of infection, resulting in inadequate microbial killing, as described in the cystic fibrosis disease [59,60].
Increasing clinical evidence supports the role of CBD as an adjuvant therapy for the control of refractory epileptic seizures. In this context, CBD has been shown to decrease the frequency and intensity of epileptic seizures, even keeping a variable proportion of people seizure-free [7,8,9]. Additionally, treatment with CBD showed an increase in the AEDs plasma concentration [10,11,12]. Although the mechanism of this increase is not clear, it is known that CBD blocks CYP3A and CYP2C cytochromes that are responsible for the AEDs metabolization [9]. Recently, our group has shown that CBD can block the excretory activity of P-gp in a similar way to the specific blocker Tariquidar [23]. This would possibly contribute to reducing the clearance of AEDs and also make their access to the brain parenchyma more effective, bypassing the excretory activity of P-gp in the blood–brain barrier. Several reports show that CBD has an anti-inflammatory effect by reducing the secretion of pro-inflammatory mediators and that it can produce an immunoparalysis acting on lymphocytes, monocytes, macrophages and even microglia [37,38,39,40,41,42]. However, the role of CBD on the activity of PMNs has been little investigated. In this context, we show that CBD decreases the viability and functionality of PMNs consequently with its anti-inflammatory effect. From a clinical perspective, the anti-inflammatory effect of CBD is appropriate for reducing brain inflammation that accompanies epileptic crises. However, the chronic use of CBD can cause a state of immune paralysis, responsible for a greater susceptibility to infections. Although CBD facilitates better control of seizures in patients with RE, its harmful effect on the functionality of the neutrophils shown here, and as well as on other cells of the immune system [61], may require a clinical-immunological control in patients with RE who are chronically medicated CBD.
4. Materials and Methods
4.1. Ethic Statements and Samples
Samples were collected from the Hematology Laboratory of the Clinical Biochemistry Department of Clinical Hospital “José de San Martín”. The blood samples were collected with EDTA and were only used if they presented normal results in the cell blood counter analysis. This procedure was approved by CEIC (Comité de Ética en Investigación Clínica) from Facultad de Farmacia y Bioquímica—Universidad de Buenos Aires (Exp-UBA 77388/2017).
4.2. Materials
Cell culture reagents were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). Giemsa solution was provided by Biopack (cod 1100.08- Biopack-Buenos Aires, Argentina). Other reagents such as Ficoll–Paque plus (GE17-1440-03), fMLP (F3506) and Trypam Blue (T6146) were obtained from Merck (Darmstadt, Germany).
4.3. CBD Preparation
CBD was diluted in dimethyl sulfoxide (DMSO) at a final concentration of 10−1 M. This stock solution was aliquoted and stored at −20 °C. For each experiment, intermedial solutions were prepared from stock solution with appropriated volume of buffer phosphate saline (PBS). A final CBD experimental concentration was reached using an appropriated volume of PMN suspension. Control experiments using DMSO as a vehicle were performed, adding an equal volume of DMSO that needed of the stock solution to reach a CBD 10° M concentration.
4.4. PMNs Isolation
PMNs were isolated, as described previously [62]. Briefly, PMNs were isolated from blood samples using the Ficoll–Paque plus method and exposed to hypotonic shock with sterile NaCl solution (0.2% p/v) for 20 s and rebalanced with an equal volume of sterile NaCl solution (1.6% p/v). This suspension of PMNs was centrifuged 10 min at 450 g for 10 min at 20 °C, the supernatant fluid was discarded, and the pellet was resuspended in 10 mL of sterile RPMI 1640 medium at 37 °C.
In all assay, aliquots of a final PMN 106/mL suspension were incubated with increased concentrations of CBD (10−7 M, 10−5 M and 10−3 M) at 37 °C and compared with aliquots of PMN 106/mL suspension without CBD that were used as normal control.
4.5. PMNs Count and Viability
Total and differential cells account of viable PMNs in suspension were developed using the Sysmex XN-1000 AS-01 Automated Hematology Analyzer (XN-Series, Sysmex® Chuo-ku, Kobe, Hyogo 651-0073, Japan), in a mode that allowed for distinguishing the mono-nuclear (MN) cells from neutrophils. These counts were confirmed manually with Neubauer´s chamber, using a 1/400 dilution of the PMNs suspension in Turk’s solution (1–2% acetic acid with aqueous methylene blue).
The PMNs’ viability was measured with equal volume of Trypan Blue solution 0.4% using a 1/100 dilution of the abovementioned neutrophils suspension. PMN/MN ratio as well as the cell´s viability was determined before and after each experiment, and only cells suspensions with viability >95% were used.
4.6. Morphological Analysis
Sample film preparations was developed using a volume of 100 µL of 105 PMN/mL suspension, spread-out on glasses by cito-spinning centrifugation, fixed with methanol 10% for 10 min and stained with Giemsa. After washes with water, glasses were dried at room temperature overnight. Glasses were observed at 40× magnification and photographed using an Olympus IX-81 microscope equipped with a DP71 camera (Olympus, Tokyo, Japan).
Additionally, the transformation of poly-lobed morphology of normal PMN to mono-nuclear (MN) cells can indicate the kinetics of viability loss secondary to the incubation of PMN with CBD detected as the PMN/MN cells ratio, by Sysmex XN-1000 AS-01 Automated Hematology Analyzer. So, the PMN/MN rate can reflex the nuclear condensation index related to unviable PMN amounts.
4.7. Chemotaxis Assay
PMN migration assays were performed in 12 multi-wells Boyden chambers, and polyvinylpyrrolidone-free polycarbonate filters with pores 5 µm in diameter were used. The chemo-attracter N-formyl-Met-Leu-Phe peptide (fMLP) (10−7 M) and/or CBD (10−3 M) were carefully loaded in the lower well, avoiding the formation of air bubbles. After the chambers were assembled, upper wells were filled with the suspension of 106 PMNs/mL. In the settings of the experiment, the chambers were incubated at 5% CO2 and 37 °C for 0, 30, 60 and 90 min, establishing that the optimal incubation time was 45 min. Finally, transmigrated cells were counted using a Sysmex XN-1000 AS-01, as described above. Additionally, the ration of PMN/MN was performed in both the upper and lower cells’ suspensions. All experiments were assayed by triplicate.
4.8. Stimuli and Activation of PMN
Human PMN cells (1 × 106 cells) suspended in buffer phosphate solution (PBS: 138 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4 and 1.47 mM KH2PO4 buffer, pH 7.4) were preincubated for 15 min at 37 °C with increasing concentrations of CBD (10−7 M; 10−5 M and 10−3 M).
4.9. PMN Oxygen Consumption
A liquid-phase respiration measurement system, the oxygraph plus model, was used for the determination of oxygen consumption by PMN, consisting of an oxygen electrode chamber (DW1/AD model) with integral Clark type polarographic oxygen electrode (S1) (Hansatech Instruments Ltd., Norfolk, British), thermostated at 37 °C, with human PMN (106/mL) in PBS supplemented with 0.9 mM CaCl2, 0.5 mM MgCl2 and 7.5 mM glucose (PBSG). For the assay, respiratory buffer (for 1 mL final volume) was placed in the electrode chamber. The chamber was covered. Substrates were added with a Hamilton syringe. The rate of oxygen uptake was calculated from the initial time course and expressed as nmol of oxygen/min/106 cells, as previously described [49].
4.10. PMN Chemiluminescence
Chemiluminescence is a useful approach to determine the occurrence of oxidative stress in cells or tissues. Spontaneous chemiluminescence in fresh PMNs was assayed, applying a photon counter and the procedure for the light emission reading use, as previously described [63,64]. Results are expressed in cps/mL PMN (cps: counts per second, 1 cps corresponds to about 103 photons per second).
4.11. Statistics
All samples for PMN viability and chemotaxis experiments were run three times, while samples for PMN chemiluminescence and oxygen consumption measurements were tested once. All experiments were performed by triplicate. After checking the normal distribution, differences were analyzed by Student t-test or one-way ANOVA with a Dunnett’s post-test comparison with a significance level alpha = 0.05.
5. Conclusions
Cannabidiol, a lipophilic cannabinoid compound without psychoactive effects, has emerged as adjuvant of AEDs in the treatment of RE. The treatment with CBD as adjuvant has been reported for decreasing the severity and/or frequency of seizures. Additionally, CBD has a well-documented anti-inflammatory role, decreasing the microglial reactivity between others. In this sense, microglia and PMN share several immune activities. The results of this work show that CBD decreases PMN survival, chemotaxis and oxygen uptake, while increasing the singlet oxygen formation, suggesting that CBD could affect the PMN functionality. In a clinical perspective, a prolonged CBD treatment could lead a defenseless state, increasing the infections risk. This side effect could be bypassed with an antioxidants treatment, since CBD enhances the singlet oxygen as a result of the protein and lipid peroxidation. In sum, research on the CBD effects must continue in order to establish how it influences the physiological roles of different cell types with clinical relevance.
Acknowledgments
Special thanks to Enecta for the CBD provision.
Author Contributions
Conceptualization, A.L., A.M. and J.A.; methodology, C.T.G., F.L. and J.A.; software, C.T.G., F.L. and M.R.; validation, M.R. and J.A.; formal analysis, C.T.G., F.L., M.R., A.M. and J.A.; investigation, A.L., A.M. and J.A.; resources, M.R., A.L. and A.M.; data curation, M.R. and J.A.; writing—original draft preparation, M.R., M.E., A.L. and J.A.; writing—review and editing, M.R., A.L. and J.A.; visualization, A.L. and J.A.; supervision, M.R., A.L. and J.A.; project administration A.L., A.M. and J.A.; funding acquisition A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Buenos Aires, UBACyT 20720170200024BA.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the CEIC (Comité de Ética en Investigacion Clínica) from Facultad de Farmacia y Bioquímica—Universidad de Buenos Aires (Exp-UBA 77388/2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Kwan P., Brodie M.J. Definition of refractory epilepsy: Defining the indefinable? Lancet Neurol. 2010;9:27–29. doi: 10.1016/S1474-4422(09)70304-7. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Hauser W.A., Mathern G., Moshé S.L., Perucca E., Wiebe S., French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Pérez D., Frías-Soria C.L., Rocha L. Drug-resistant epilepsy: From multiple hypotheses to an integral explanation using preclinical resources. Epilepsy Behav. 2019:106430. doi: 10.1016/j.yebeh.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Lazarowski A., Czornyj L., Lubienieki F., Girardi E., Vazquez S., D’Giano C. ABC Transporters during Epilepsy and Mechanisms Underlying Multidrug Resistance in Refractory Epilepsy. Epilepsia. 2007;48:140–149. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 6.Rocha L., Frías-Soria C.L., Ortiz J.G., Auzmendi J., Lazarowski A. Is Cannabidiol a drug acting on unconventional targets to control drug-resistant epilepsy? Epilepsia Open. 2020;5:36–49. doi: 10.1002/epi4.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huntsman R.J., Tang-Wai R., Alcorn J., Vuong S., Acton B., Corley S., Laprairie R., Lyon A.W., Meier S., Mousseau D.D., et al. Dosage Related Efficacy and Tolerability of Cannabidiol in Children With Treatment-Resistant Epileptic Encephalopathy: Preliminary Results of the CARE-E Study. Front. Neurol. 2019;10:716. doi: 10.3389/fneur.2019.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco V., Perucca E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs. 2019;79:1435–1454. doi: 10.1007/s40265-019-01171-4. [DOI] [PubMed] [Google Scholar]
- 9.Golub V., Reddy D.S. Advances in Experimental Medicine and Biology. Volume 1264. Springer; Berlin/Heidelberg, Germany: 2021. Cannabidiol Therapy for Refractory Epilepsy and Seizure Disorders; pp. 93–110. [DOI] [PubMed] [Google Scholar]
- 10.Chang B.S. Cannabidiol and serum antiepileptic drug levels: The ABCs of CBD with AEDs. Epilepsy Curr. 2018;18:33–34. doi: 10.5698/1535-7597.18.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geffrey A.L., Pollack S.F., Bruno P.L., Thiele E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–1251. doi: 10.1111/epi.13060. [DOI] [PubMed] [Google Scholar]
- 12.Franco V., Bialer M., Perucca E. Cannabidiol in the treatment of epilepsy: Current evidence and perspectives for further research. Neuropharmacology. 2021;185:108442. doi: 10.1016/j.neuropharm.2020.108442. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration . FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. US Food and Drug Administration; Rockville, MD, USA: Jun 25, 2018. [Google Scholar]
- 14.Thomas A., Baillie G.L., Phillips A.M., Razdan R.K., Ross R.A., Pertwee R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB 1 and CB 2 receptor agonists in vitro. Br. J. Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertwee R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelz M.C., Schoolcraft K.D., Larson C., Spring M.G., López H.H. Assessing the role of serotonergic receptors in cannabidiol’s anticonvulsant efficacy. Epilepsy Behav. 2017;73:111–118. doi: 10.1016/j.yebeh.2017.04.045. [DOI] [PubMed] [Google Scholar]
- 17.Castillo A., Tolón M.R., Fernández-Ruiz J., Romero J., Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010;37:434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Bisogno T., Hanuš L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace M.J., Wiley J.L., Martin B.R., DeLorenzo R.J. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 2001;428:51–57. doi: 10.1016/S0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- 20.Vezzani A., Aronica E., Mazarati A., Pittman Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Van der Weide J., Steijns L.S.W., Van Weelden M.J.M., De Haan K. The effect of genetic polymorphism of cytochrome P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics. 2001;11:287–291. doi: 10.1097/00008571-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tate S.K., Depondt C., Sisodiya S.M., Cavalleri G.L., Schorge S., Soranzo N., Thom M., Sen A., Shorvon S.D., Sander J.W., et al. Genetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoin. Proc. Natl. Acad. Sci. USA. 2005;102:5507–5512. doi: 10.1073/pnas.0407346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auzmendi J., Palestro P., Blachman A., Gavernet L., Merelli A., Talevi A., Calabrese G.C., Ramos A.J., Lazarowski A. Cannabidiol (CBD) Inhibited Rhodamine-123 Efflux in Cultured Vascular Endothelial Cells and Astrocytes Under Hypoxic Conditions. Front. Behav. Neurosci. 2020;14:32. doi: 10.3389/fnbeh.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Cervantes R., Méndez-DÍaz M., Prospéro-García Ó., Morales-Montor J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation. 2018;24:183–199. doi: 10.1159/000481824. [DOI] [PubMed] [Google Scholar]
- 25.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000;80:617–654. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 26.Turcotte C., Archambault A.S., Dumais É., Martin C., Blanchet M.R., Bissonnette E., Ohashi N., Yamamoto K., Itoh T., Laviolette M., et al. Endocannabinoid hydrolysis inhibition unmasks that unsaturated fatty acids induce a robust biosynthesis of 2-arachidonoyl-glycerol and its congeners in human myeloid leukocytes. FASEB J. 2020;34:4253–4265. doi: 10.1096/fj.201902916R. [DOI] [PubMed] [Google Scholar]
- 27.Toguri J.T., Moxsom R., Szczesniak A.M., Zhou J., Kelly M.E.M., Lehmann C. Cannabinoid 2 receptor activation reduces leukocyte adhesion and improves capillary perfusion in the iridial microvasculature during systemic inflammation. Clin. Hemorheol. Microcirc. 2015;61:237–249. doi: 10.3233/CH-151996. [DOI] [PubMed] [Google Scholar]
- 28.Toguri J.T., Lehmann C., Laprairie R.B., Szczesniak A.M., Zhou J., Denovan-Wright E.M., Kelly M.E.M. Anti-inflammatory effects of cannabinoid CB2 receptor activation in endotoxin-induced uveitis. Br. J. Pharmacol. 2014;171:1448–1461. doi: 10.1111/bph.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montecucco F., Di Marzo V., Da Silva R.F., Vuilleumier N., Capettini L., Lenglet S., Pagano S., Piscitelli F., Quintao S., Bertolotto M., et al. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur. Heart J. 2012;33:846–856. doi: 10.1093/eurheartj/ehr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapellos T.S., Recio C., Greaves D.R., Iqbal A.J. Cannabinoid Receptor 2 Modulates Neutrophil Recruitment in a Murine Model of Endotoxemia. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/4315412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabady R.L., Louissaint C., Lubben A., Xie B., Reeksting S., Tuohy C., Demma Z., Foley S.E., Faherty C.S., Llanos-Chea A., et al. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Investig. 2018;128:4044–4056. doi: 10.1172/JCI96817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham E., Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit. Care Med. 2007;35:2408–2416. doi: 10.1097/01.CCM.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 33.Foxman E.F., Kunkel E.J., Butcher E.C. Integrating conflicting chemotactic signals: The role of memory in leukocyte navigation. J. Cell Biol. 1999;147:577–587. doi: 10.1083/jcb.147.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Benna J., Hurtado-Nedelec M., Marzaioli V., Marie J.C., Gougerot-Pocidalo M.A., Dang P.M.C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016;273:180–193. doi: 10.1111/imr.12447. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen G.T., Green E.R., Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell. Infect. Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenstein T.K., Meissler J.J. Effects of Cannabinoids on T-cell Function and Resistance to Infection. J. Neuroimmune Pharmacol. 2015;10:204–216. doi: 10.1007/s11481-015-9603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth M.D., Castaneda J.T., Kiertscher S.M. Exposure to Δ9-Tetrahydrocannabinol Impairs the Differentiation of Human Monocyte-derived Dendritic Cells and their Capacity for T cell Activation. J. Neuroimmune Pharmacol. 2015;10:333–343. doi: 10.1007/s11481-015-9587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidd P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 39.Wu H.Y., Huang C.H., Lin Y.H., Wang C.C., Jan T.R. Cannabidiol induced apoptosis in human monocytes through mitochondrial permeability transition pore-mediated ROS production. Free Radic. Biol. Med. 2018;124:311–318. doi: 10.1016/j.freeradbiomed.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Walter L., Franklin A., Witting A., Wade C., Xie Y., Kunos G., Mackie K., Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mechoulam R., Peters M., Murillo-Rodriguez E., Hanuš L.O. Cannabidiol—Recent advances. Chem. Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- 42.Croxford J.L., Yamamura T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 43.McHugh D., Tanner C., Mechoulam R., Pertwee R.G., Ross R.A. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: Evidence for a site distinct from CB1 and CB 2. Mol. Pharmacol. 2008;73:441–450. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- 44.Kakinuma K., Boveris A., Chance B. H2O2 Generation in subcellular fractions of leukocytes assayed by cytochrome c peroxidase method. FEBS Lett. 1977;74:295–299. doi: 10.1016/0014-5793(77)80868-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaminski K.A., Bonda T.A., Korecki J., Musial W.J. Oxidative stress and neutrophil activation—The two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002;86:41–59. doi: 10.1016/S0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Mukhopadhyay P., Cao Z., Wang H., Feng D., Haskó G., Mechoulam R., Gao B., Pacher P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-10924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon S. Phagocytosis: The Legacy of Metchnikoff. Cell. 2016;166:1065–1068. doi: 10.1016/j.cell.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Dos-Santos-Pereira M., Guimarães F.S., Del-Bel E., Raisman-Vozari R., Michel P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia. 2020;68:561–573. doi: 10.1002/glia.23738. [DOI] [PubMed] [Google Scholar]
- 49.Valdez L.B., Boveris A. Nitric oxide and superoxide radical production by human mononuclear leukocytes. Antioxid. Redox Signal. 2001;3:505–513. doi: 10.1089/15230860152409130. [DOI] [PubMed] [Google Scholar]
- 50.Carreras M.C., Pargament G.A., Catz S.D., Poderoso J.J., Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 1994;341:65–68. doi: 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 51.Antunes F., Boveris A., Cadenas E. On the biologic role of the reaction of NO with oxidized cytochrome C oxidase. Antioxid. Redox Signal. 2007;9:1569–1579. doi: 10.1089/ars.2007.1677. [DOI] [PubMed] [Google Scholar]
- 52.Repetto M., Semprine J., Boveris A. Lipid peroxidation: Chemical mechanisms, biological implications and analytical determinations. In: Catala A., editor. Lipid Peroxidation. Intech; London, UK: Rijeka, Croatia: 2012. pp. 3–30. [Google Scholar]
- 53.Repetto M. Clinical use of chemiluminescence assays for the determination of systemic oxidative stress. In: Popov I., Lewin G., editors. Handbook of Chemiluminescent Methods in Oxidative Stress Assessment. Transword Research Network; Kerala, India: 2008. pp. 163–194. [Google Scholar]
- 54.Levine A.P., Segal A.W. What Is wrong with granulocytes in inflammatory bowel diseases. Dig. Dis. 2013;31:321–327. doi: 10.1159/000354686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roos D. Chronic granulomatous disease. Br. Med. Bull. 2016;118:50–63. doi: 10.1093/bmb/ldw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Björkman L., Dahlgren C., Karlsson A., Brown K.L., Bylund J. Phagocyte-derived reactive oxygen species as suppressors of inflammatory disease. Arthritis Rheum. 2008;58:2931–2935. doi: 10.1002/art.23941. [DOI] [PubMed] [Google Scholar]
- 57.Lazarowski A. ABC-transporters and drug efflux in hematologic cancers. In: Sosnik A., Bendayan R., editors. Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy. Elsevier Inc.; Amsterdam, The Netherlands: 2020. pp. 149–195. [Google Scholar]
- 58.Bréchard S., Plançon S., Tschirhart E.J. New insights into the regulation of neutrophil NADPH oxidase activity in the phagosome: A focus on the role of lipid and Ca2+ signaling. Antioxid. Redox Signal. 2013;18:661–676. doi: 10.1089/ars.2012.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Painter R.G., Bonvillain R.W., Valentine V.G., Lombard G.A., LaPlace S.G., Nauseef W.M., Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 2008;83:1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Painter R.G., Marrero L., Lombard G.A., Valentine V.G., Nauseef W.M., Wang G. CFTR-mediated halide transport in phagosomes of human neutrophils. J. Leukoc. Biol. 2010;87:933–942. doi: 10.1189/jlb.1009655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nichols J.M., Kaplan B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020;5:12–31. doi: 10.1089/can.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhns D.B., Priel D.A.L., Chu J., Zarember K.A. Isolation and Functional Analysis of Human Neutrophils. Curr. Protoc. Immunol. 2015;111:7–23. doi: 10.1002/0471142735.im0723s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 64.Musacco Sebio R., Ferrarotti N., Saporito Magriñá C., Fuda J., Torti H., Lairión F., Boveris A., Repetto M.G. Redox dyshomeostasis in the experimental chronic hepatic overloads with iron or copper. J. Inorg. Biochem. 2019;191:119–125. doi: 10.1016/j.jinorgbio.2018.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.