Abstract
Purpose
Disorders with systematic inflammation were prognostic for secondary frozen shoulder (sFS) following rotator cuff repair (RCR); however, how systematic inflammation affects sFS remains unclear. The aim of this study was to observe the effect of pre-operative serum from patients with sFS and the serum from those without on shoulder capsule in mice, and on macrophages and fibroblasts in vitro.
Methods
Serum samples of a consecutive cohort of patients for RCR were collected pre-operatively. Three months after RCR, patients who developed sFS (Group S) were identified. Serum samples from gender- and age-matched controls without sFS (group NS) were also picked out. Firstly, the effect of serum on shoulder capsule fibrosis was observed histologically and biomechanically in a mouse model of RCR. Secondly, the roles of the serum on macrophage polarization and fibroblast activation were investigated, and the potentially involved signaling pathways were identified. Finally, inflammation and fibrosis-related cytokines in serum were quantified.
Results
In our cohort, all patients had free pre-operative shoulder range of motion. Seven patients developed sFS at 3 months after surgery. Seven matched patients without sFS were selected as control. The inter-group difference of basic characteristics was not significant. Compared to the serum of group NS, the serum of group S significantly induced hypercellularity, capsular thickening, and range of motion deficiency in mice shoulders after RCR. Compared to the serum of group NS, samples of group S significantly promoted M2 polarization of THP-1 human macrophages and the activation of human capsule-derived fibroblasts. Meanwhile, Smad3 and p-Smad3 in macrophages and fibroblasts were significantly up-regulated. On the other hand, levels of inflammation and fibrosis-related cytokines were not significantly different between serum in group S and group NS.
Conclusion
Although all patients in this cohort had free range of motion pre-operatively, the pre-operative serum from patients with sFS at 3 months after RCR could act as a trigger of shoulder capsule fibrosis post-operatively. This effect may be related to its promotion on macrophage polarization to M2 phenotype and fibroblast activation.
Keywords: rotator cuff, secondary frozen shoulder, serum, macrophage, fibroblast
Introduction
Rotator cuff repair (RCR) has been widely used for rotator cuff tear (RCT) with overall satisfactory results;1 however, 4.9% to 32.7% cases were reported to develop shoulder stiffness, or secondary frozen shoulder (sFS),2 which may last more than 2 years.3 Many systematic conditions, such as diabetes,4 hypothyroidism5 and low serum vitamin D level,6 are found to be related to sFS, and cases with these disorders usually have high systematic inflammatory level.7–9 Considering that systematic inflammation can be evaluated by blood routine,10,11 recent studies confirmed that white cell counts and ratios correlate with compromised RCR outcomes and sFS.3,12
In the serum of the patients with systematic inflammation, levels of pro-inflammatory and pro-fibrotic cytokines, metabolites and other pathological factors are usually elevated.13,14 These factors promote macrophagespolarization and fibroblastsactivation via NF-κB, Smads and other pathways,15–17 and are potential biomarkers and drivers of liver,18 renal19 or lung fibrosis.20 Similarly, for FS, it is also characterized by capsular fibrosis, during which macrophages and fibroblasts are the two pivotal cellular players.21,22
In light of this, we hypothesized that the patients with sFS after RCR had high systematic inflammatory level pre-operatively, and their serum would induce shoulder capsular fibrosis and influence macrophages and fibroblasts phenotypes. In detail, the effect of serum of from patients on shoulder capsule was investigated in a mouse model of RCR23,24 in vivo and on human macrophage cell line and primary capsule-derived fibroblasts (CDFs) in vitro. Potentially involved signaling pathways were investigated.
Methods
The current research was approved by local ethical committee of Huashan Hospital, Fudan University (KY-2018-0390). All patients provided informed consent, and that this study was conducted in accordance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
The inclusion and exclusion process consists of two independent steps: pre-operative screening and intra-operative screening. Pre-operative inclusion criteria were: (1) small to large-sized supraspinatus tendon tear; (2) acromiohumeral distance >6 mm;25 (3) muscle fatty infiltration less than grade II according to Goutallier classification; (4) no muscle atrophy according to tangent sign on MRI. Pre-operative exclusion criteria were: (1) cuff tear arthropathy; (2) glenohumeral joint osteoarthritis; (3) concomitant ipsilateral shoulder stiffness; (4) concomitant shoulder instability; (5) previous surgery on ipsilateral shoulder; (6) injury or operation on the ipsilateral shoulder after RCR; (7) local steroid injection within 3 months before surgery; and (8) autoimmune diseases or systematic inflammatory diseases, such as rheumatic arthritis of the ipsilateral shoulder. The intra-operative exclusion criteria were: (1) concomitant infraspinatus or subscapularis tendon tear, (2) biceps surgery, and (3) combined labrum repair.
Serum Preparation
From February 1st 2019 to October 15th 2019, for patients with RCR in our department who met the pre-operative inclusion and exclusion criteria, serum samples were collected. In detail, ~20mL of whole blood was collected in tubes and centrifuged at 4°C for 10 min at 3000 rpm. The serum was then collected and divided into EP tubes in sterile vertical flow clean bench. The serum samples were stored at −80°C for further experiments.
Data Collection
According to Chung et al, sFS was diagnosed if the passive range of motion (ROM) met any of the following: forward elevation ≤120°, external rotation at the side ≤30°, or internal rotation at the back ≤ L-3.26 In this cohort, seven cases had developed sFS at 3 months after arthroscopic RCR, and were labeled as group S.
Then, seven age- and gender-matched patients with no sFS in the same cohort were labeled as group NS, and serum samples were pinpointed. Considering the limited number of patients included, a strict age-match procedure was unable to be conducted. Instead, on the basis of the same gender, serum samples of patients with an age difference smaller than 5 years were selected. In case of more than one eligible samples, the one with the closest age was selected.
The following data were collected: (1) general demographic data, including age, height, weight, and gender; history of trauma, duration, side, diabetes, and hypertension; laboratory examinations including blood glucose and neutrophil/lymphocyte ratio (NLR), (2) pre- and post-operative (3 months after surgery) data of clinical outcome, such as pain visual analog scale (VAS), passive ROM including forward flexion (FF), abduction (AB), external rotation at side (ER0), and internal rotation at the back (IR0),27 shoulder functional scores including American Shoulder and Elbow Surgeon (ASES) score,28 Constant-Murley (Constant) score,29 and Fudan University Shoulder Score (FUSS),30 (3) surgical data, such as tear size, single-row or double-row technique, and anchors. High inflammation was defined as NLR > 3.31 All surgeries were performed by one senior surgeon with the identical rehabilitation protocol.
Establishment of Mice RCR Model and Serum Injection
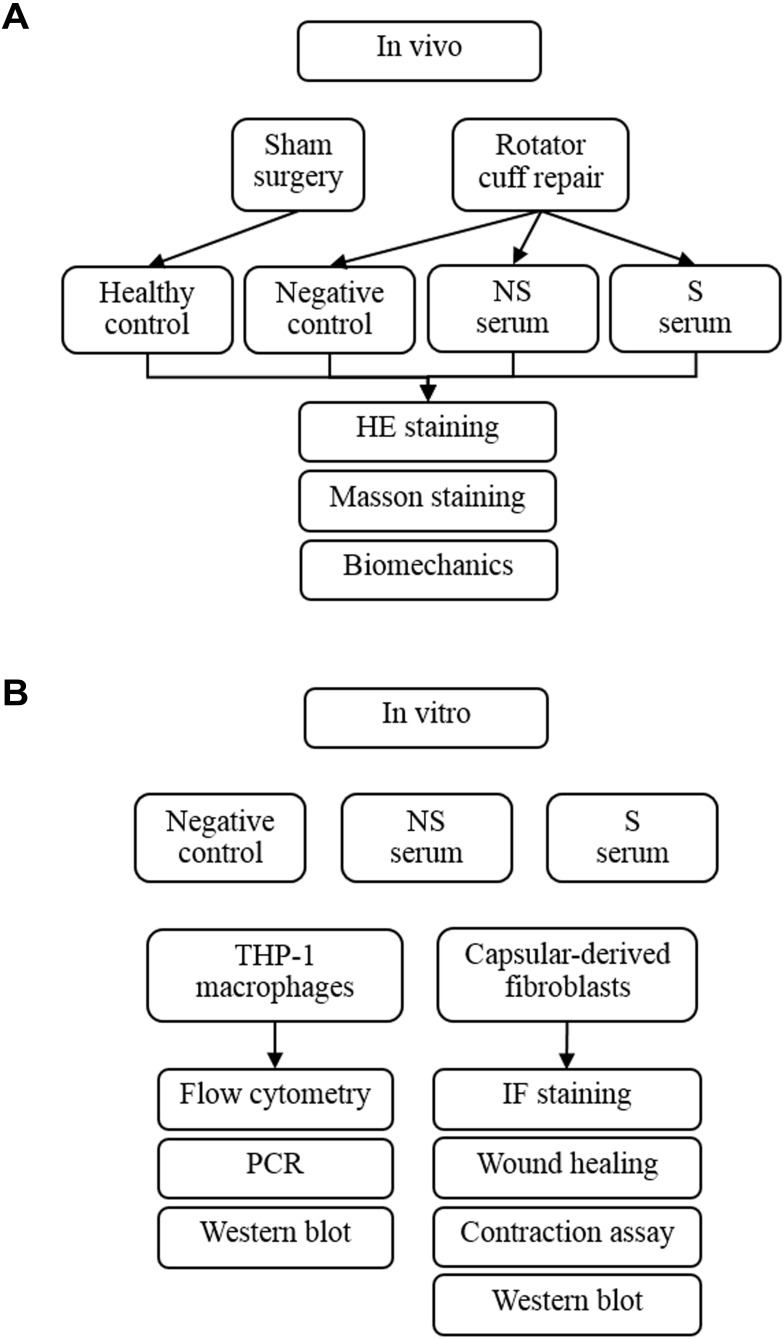
Animal experiments were approved by the Institutional Animal Care and Use Committee (202004003S) of Shanghai Medical School, Fudan University, and were conducted according to the Guide for the Care and Use of Laboratory Animals. Twenty-eight male C57/6J mice of ~12 weeks were randomly and equally divided into four groups, ie, mice with sham left RCR (healthy control, group HC), mice with left RCR (negative control, NC group), mice with left RCR and intra-articular injection of serum of NS patients (group NS), and mice with left RCR and an intra-articular injection of serum of S patients (group S). The RCR procedure was performed with the reference to previous studies.23,24,32 Intra-articular injection of 20μL serum was conducted using micro-syringe right before the suture of RCR was knotted. All animals were allowed to move freely in the cage after surgery in 12h light-dark circle. According to our preliminary experiments, at least 6 mice were required to reach a statistical significance between group HC and group S regarding the difference of ROM. Considering that 7 samples from group S and from group NS were used, and each sample was intra-articularly injected into one mouse, a total of 28 mice (7 in each group) were used. Group allocation and analysis methods are briefly drawn in Figure 1A.
Figure 1.
The flowchart of experiments in vivo (A) and in vitro (B).
Shoulder Sample Harvest and Analysis
Ten days after the procedure, mice were euthanized by CO2 overdose to harvest their left shoulders. Immediately following sample collection, passive ROM was measured using the manner reported by Oki et al.33 Then, the samples were fixed in 10% neutral buffered formalin for one day, and were decalcified, dehydrated, and embedded in paraffin wax for histological examination. HE and Masson staining were conducted based on the established method.34,35 The thickness of joint capsule was obtained by five random measurements to obtain a mean value using ImageJ software. The relative thickness of joint capsule in different groups compared to group NC was calculated. These aforementioned measurements were conducted by the third author unaware of animal interventions.
Cell Harvest, Culture, and Intervention
Experiments in vitro were conducted in two types of cells (Figure 1B). For human macrophages, THP-1 cells were purchased from Shanghai Cell Bank and cultured in RPMI 1640 medium (Thermo Fisher Scientific, USA) + 10% fetal bovine serum (FBS) (Gibco, USA). Prior to stimulations, 2×106 cells were seeded in a 6-well plate and differentiated into macrophages with 500 ng/mL phorbol-12-myristate-13-acetate (PMA, Sigma Aldrich, USA) for 24h.
In order to collect the human capsule-derived fibroblasts (CDFs), fresh capsular samples were harvested in three patients (female: 2) with anterior shoulder instability during the arthroscopic surgery of Bankart repair. The CDFs were then collected following the published protocol.36 CDFs were maintained in high-glucose DMEM medium (Thermo Fisher Scientific) + 10% FBS (Gibco). The harvested CDFs were entitled as passage 0. When reading ~90% confluence, CDFs were subcultured, and the cells at passage 3 were used in experiments.
In order to assess the impact of serum on macrophages and fibroblasts, three groups, ie, group negative control medium (NC), group NS, and group S, were set up. For group NC, cells were cultured in the original medium with 10% FBS. For group NS and S, cells were cultured in medium with 10% mixed serum (5% FBS + 5% serum from patients of group NS or group S, respectively). The data of different experiments were collected at specific time points.
Flow Cytometry
Macrophages for flow cytometry were first harvested and blocked with 3% BSA in PBS for 30 min, followed by incubation with PE-anti-CD86 or PE-anti-CD206 antibodies (BioLegend; San Diego, CA) in the dark. The cells incubated with PE-isotype (BioLegend) were used to exclude nonspecific binding by the fluorescent antibodies. Data files were analyzed using FlowJo software (Tree Star, Ashland, OR). M1 macrophages were defined as CD86+ and M2 macrophages as CD206+.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
To quantify the expression of certain mRNAs, total RNA was isolated by TRIzol Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Complementary DNA (cDNA) synthesis for mRNA detection was carried out using the cDNA Synthesis kit (Takara, Otsu, Japan). Real-time PCR was performed using SYBR Green PCR Master Mix (Takara).37 The relative fold changes of target genes were normalized to the endogenous reference gene GAPDH by using an optimized comparative Ct (2ΔΔCt) value method. The expression of genes was normalized to that expressed in NC group. Primer sequences are listed in Table 1.
Table 1.
Sequences of Target Genes
| Gene Name | Sequence | |
|---|---|---|
| Arg-1 | F | TCACCTGAGCTTTGATGTCG |
| R | CTGAAAGGAGCCCTGTCTTG | |
| TGF-β | F | ACCTGCAAGACCATCGACAT |
| R | GGTTTTCTCATAGATGGCGT | |
| IL-10 | F | CCAGGGAGATCCTTTGATGA |
| R | AACTGGCCACAGTTTTCAGG | |
| iNOS | F | AAGGCCACATCGGATTTCAC |
| R | GATGGACCCCAAGCAATACTT | |
| TNF-α | F | GGATCTCAAAGACAACCAAC |
| R | ACAGAGCAATGACTCCAAAG | |
| IL-1β | F | TTCAGGCAGGCAGTATCACTC |
| R | CCACGGGAAAGACACAGGTAG | |
| GAPDH | F | GGCATGGACTGTGGTCATGAG |
| R | TGCACCACCAACTGCTTAGC |
Abbreviations: F, forward; R, reverse; Arg-1, arginase-1; TGF-β, transforming growth factor-β; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Immunofluorescent (IF) Staining
CDFs were cultured on glass slides in 6-well plates and cultured until 80–90% confluent. After medium is removed, cells were fixed in 4% paraformaldehyde for 10 min at room temperature, followed by blocking and permeabilization with 0.5% Trion-X-100/3% BSA in PBS for 30 min at room temperature. Then, slides were incubated with the primary antibody α-SMA overnight at 4°C, and then with the secondary antibody conjugated with FITC for 45 min at 37°C. Immediately after DAPI was added, images were taken using the microscope (Olympus).
Wound Healing Assay
CDFs were cultured in 6-well-plate to confluency and scratched using a 100 μL pipette tip under suction. Medium was changed after wounding and digital photos were obtained by screening at 0, 12, and 24 h. Wound closure was quantified at the indicated time points using ImageJ software (NIH, Bethesda, MD, USA) and calculated as percent reduction of cell-free surface area compared with immediately after wounding (t = 0).38
Collagen Gel Contraction Assay
CDFs (2.0×105 cells/mL) were suspended in 0.5 mL of 2 mg/mL collagen solution (Solarbio, Beijing, China) and added into each well of a 24-well plate and allowed to polymerize for 1 h at 37°C. After gelatinization, 0.5 mL of DMEM was poured onto the surface of the gels, and sterile pipette tips were applied to detach the collagen disk from the culture dish. Then, the plates were placed in a 37°C incubator with 5% CO2, and digital photos were obtained by screening at 0, 12, and 24 h in group NC, group NS, and group S. Contraction of the gels were measured using ImageJ software (NIH, Bethesda, MD, USA) to calculate the relative-contraction ratio.
Western Blot (WB)
The measurement of signaling pathways involved in macrophage polarization and fibroblast activation was achieved by WB. Cells were lysed using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Haimen, China) supplemented with protease and phosphatase inhibitor cocktail (Pierce, Thermo Fisher Scientific) for 30min on ice, and then centrifuged at 15,000×g for 30 min at 4°C. Cell extracts were heated for 5 min at 100°C. The proteins were analyzed by Western blot as previously described.17 p-Smad3 (1:1000; Affinity, AF3362), Smad3 (1:1000; Affinity, AF6362), GAPDH antibody (1:1000; Affinity, AF7021), and Goat anti-rabbit IgG (1:5000; Affinity, S0001) were used according to the manufacturer’s protocol. Immunoreactive bands were detected with electrochemiluminescence (ECL) reagent (Beyotime Biotechnology) and imaged with a Tanon-5200 Multi Fluorescence imager.
Enzyme-Linked Immune-Sorbent Assay (ELISA) Analysis
To elucidate the constituents of serum samples, the samples were subjected to. The concentrations of TGF-β, inter-cellular adhesion molecule 1 (ICAM-1), tumor necrosis factor-α (TNF-α), IL-1β, hsCRP, matrix metalloproteinase-1 (MMP-1), MMP-2, tissue inhibitor metalloproteinase-1 (TIMP-1), and TIMP-2 were examined utilizing ELISA kits (Abcam) and following the instructions of the manufacturer. MMPs/TIMPs were calculated based on an established method.39 Considering the limited volume of serum harvested, the samples were mixed with normal saline at a ratio of 1:9 prior to measurements.
Statistical Analysis
Continuous data were presented as mean ± SD. Based on the normal distribution of data or not, differences between groups were determined using non-parametric Mann–Whitney U-test, Student t-test, or analysis of variance, followed by post hoc Bonferroni test. Difference in categorical data between groups was examined by Fisher exact test. Two-side P<0.05 was considered significant. Specifically, for the detection of systematic inflammation level between patients with and without sFS, one-side P < 0.05 was considered significant, since it is proved that patients with sFS had higher systematic inflammation level than those without.3
Results
Basic Characteristics of Included Patients
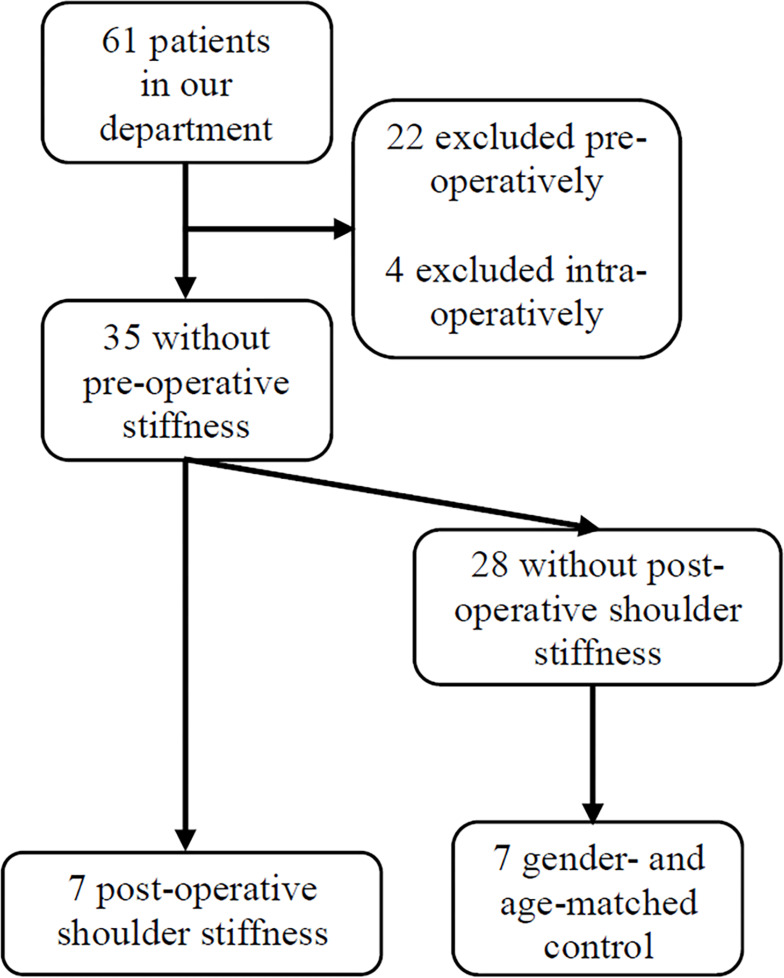
From February 1st 2019 to October 15th 2019, 39 patients for RCR met the pre-operative inclusion and exclusion criteria, and their serum were collected before the surgery. According to the exclusion criteria, 18 cases were excluded pre-operatively, while eight were excluded due to combined procedure during surgery, including one subscapularis tendon repair, six biceps surgery, and one labrum repair. Among the remaining 35 patients, 7 developed sFS at 3 months after surgery (group S), and 7 gender- and age-matched patients with no sFS were pinpointed (group NS) (Figure 2).
Figure 2.
The flowchart of patient inclusion and exclusion.
The basic characteristics of the 14 included patients are shown in Table 2. There was no significant difference between group S and group NS regarding age, height, weight, gender, history of trauma, duration of symptom, right side affected, diabetes, hypertension, or blood glucose. Four patients in group S had NLR >3, while no patient in group NS exceeded this level (p = 0.035), confirming a high systematic inflammation level in group S.
Table 2.
General Characteristics and Baseline Conditions
| Items | Group NS (7) | Group S (7) | P value |
|---|---|---|---|
| Age (y) | 48.86±7.34 | 52.14±15.51 | 0.622 |
| Height (cm) | 162.71±7.16 | 165.00±6.95 | 0.555 |
| Weight (kg) | 61.86±9.92 | 68.14±8.44 | 0.226 |
| Gender (male) | 3 | 3 | 1.000 |
| Trauma | 2 | 3 | 0.577 |
| Duration (m) | 9.94±7.13 | 12.00±8.19 | 0.625 |
| Side (right)* | 3 | 2 | 0.577 |
| Diabetes* | 0 | 1 | 0.299 |
| Hypertension | 0 | 2 | 0.127 |
| Blood Glucose (mmol/L) | 5.41±0.46 | 5.80±1.12 | 0.419 |
| Neutrophil/lymphocyte ratio > 3 (n) | 0 | 4 | 0.035 |
Note: *Fisher exact test.
The comparison of pre-, intra-, and post-operative data between the two groups is shown in Table 3. The differences in pre-operative VAS, ROM, and functional scores were not significant. The tear sizes and surgical techniques adopted in two groups were similar. However, passive FL, AB, ER0, and IR0 at 3 months post-operative were significantly lower in group S than those in group NS.
Table 3.
Pre-, Intra- and Post-Operative Data
| Items | Group NS (7) | Group S (7) | P value |
|---|---|---|---|
| Pre-operative VAS† | 4.71±1.67 | 4.13±1.83 | 0.511 |
| Pre-operative flexion† | 167.14±4.52 | 160.00±13.09 | 0.511 |
| Pre-operative abduction | 161.42±6.39 | 158.57±14.57 | 0.644 |
| Pre-operative external rotation | 47.86±7.49 | 54.29±18.60 | 0.421 |
| Pre-operative internal rotation# | 13.86±2.23 | 11.63±2.05 | 0.075 |
| Pre-operative Constant score | 72.43±8.94 | 67.29±14.12 | 0.432 |
| Pre-operative ASES score | 68.33±8.91 | 68.81±13.91 | 0.940 |
| Pre-operative FUSS | 73.71±5.57 | 68.86±13.42 | 0.403 |
| Tear size (cm) | 2.29±0.88 | 2.71±1.36 | 0.550 |
| Single-row* | 1 | 3 | 0.236 |
| Anchors† | 2.57±1.05 | 2±0.53 | 0.432 |
| Post-operative pain VAS** | 1.71±0.70 | 2.00±1.07 | 0.560 |
| Post-operative flexion** | 162.86±12.78 | 107.14±12.78 | <0.001 |
| Post-operative abduction†, ** | 161.43±13.55 | 95.00±9.64 | 0.002 |
| Post-operative external rotation** | 59.29±14.00 | 34.29±15.68 | 0.037 |
| Post-operative internal rotation** | 11.34±2.35 | 15.00±1.31 | 0.004 |
Notes: †The difference was calculated by Mann–Whitney U-test; *Fisher exact test; **the data was collected at 3 months post-operatively; #the highest vertebral level that a patient can reach with the thumb was recorded and numbered serially as 1 to 12 for the 1st to 12th thoracic vertebrae, 13 to 17 for the 1st to 5th lumbar vertebrae, and 18 for the level below the sacral vertebrae.
Abbreviation: VAS, visual analog scale.
The Effect of Serum on Mice Shoulders
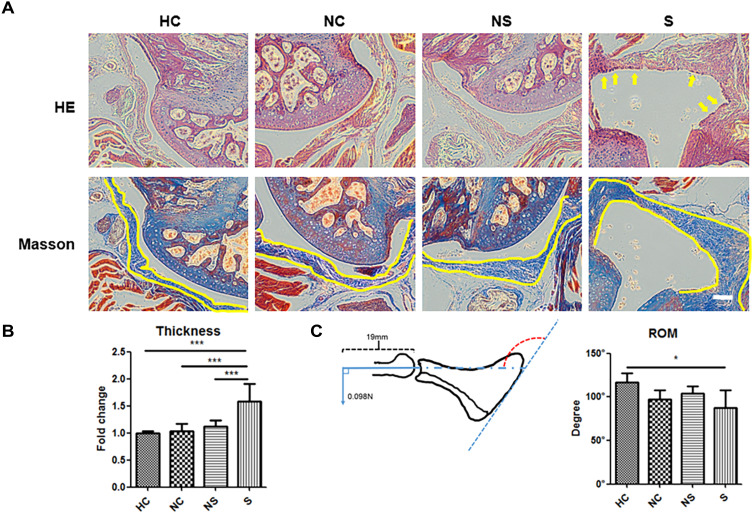
Experiments in vivo were organized to testify the pro-fibrotic role of the serum. We found that, compared to the mice in group HC, group NC or group NS showed no obvious hypercellularity of the shoulder capsule, but the serum of group S remarkably induced hypercellularity of the index shoulder capsule (indexed by yellow arrow in Figure 3A by HE staining). In line with this finding was the thickness of joint capsule illustrated by Masson staining (indexed by yellow line, and quantified in Figure 3B), which was significantly greater in group S than the other three groups. The data of passive ROM obtained were consistent with the histological observations. Multi-group comparison showed that the ROM of group NC (96.52° ± 10.40°), group NS (104.30° ± 7.10°), and group HC (117.12° ± 9.65°) were not significantly different, while that of group S was significantly lower (87.39° ± 19.04°, p<0.05) than that of group HC (Figure 3C).
Figure 3.
The impact of different injection materials on the histological and biomechanical appearance of mice shoulders with immobilization. (A) The representative image of joint capsule of the shoulder on HE staining and Masson staining. (B) The quantification of capsule thickness and inter-group comparison. (C) The schematic image of the method to measure passive range of motion and the quantification. The yellow arrow in A indicated the hypercellular status of joint capsule. The yellow line in A indicated the inner and outer border of joint capsule. The red dotted arch indicated the angle of the glenohumeral joint under a certain torque, which represented the range of motion to be measured. N=7. *p<0.05, ***p<0.001. Bar: 200μm.
The Effect of Serum on Human Macrophage Polarization
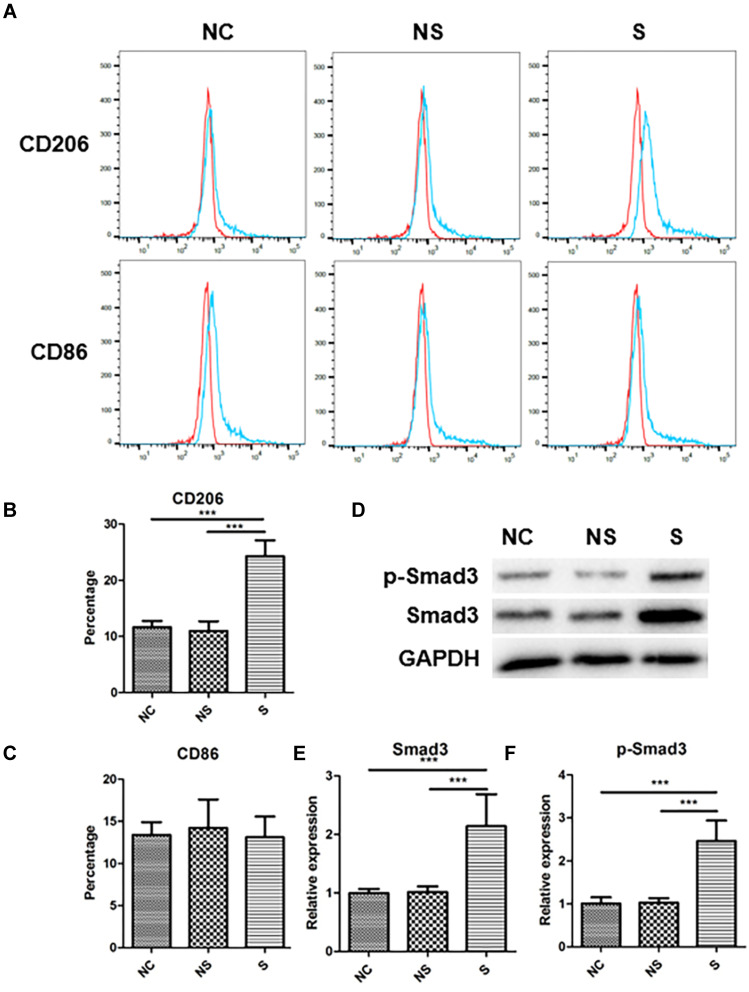
Compared to NC, serum from group NS showed no significant impact on macrophage polarization, while those of group S could stimulate M2 polarization (representative flow cytometry image in Figure 4A). Quantification analysis (Figure 4B) indicated that the rate of M2 macrophages was 24.30% ± 2.62% in group S, as indicated by the rate of CD206+ cells, which was significantly higher than those in group NC (11.64% ± 1.04%, p<0.001) and group NS (11.00% ± 1.59%, p<0.001). Nevertheless, the serum from neither group S nor group NS had remarkable influence on M1 polarization, as indicated by the rate of CD86+ cells (Figure 4C). The expression of M2 polarization-related protein, Smad3, together with the activated type, p-Smad3, were also significantly up-regulated in group S than group NC or group NS (representative image in Figure 4D, and quantified in 4E and 4F).
Figure 4.
The phenotype in THP-1 macrophages under stimulations of serum with different origins. (A) The representative image of flow-cytometry of CD206 expression and CD86 expression in THP-1 macrophages with different stimulations. (B) The quantification of CD206 expression in THP-1 macrophages with different stimulations. (C) The quantification of CD86 expression in THP-1 macrophages with different stimulations. (D) The expression of Smad3 and p-Smad3 in THP-1 macrophages with different stimulations, and related quantification (E and F). N=7. ***p<0.001.
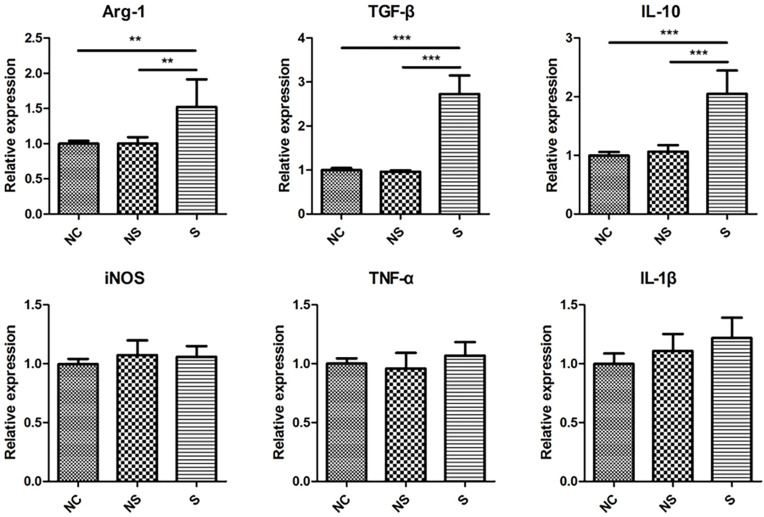
To further assure the phenotype of macrophages in different groups, M2- and M1-related genes were measured. As illustrated in Figure 5, the level of M2 markers, Arg-1, TGF-β, and IL-10, were significantly up-regulated in macrophages in group S compared to group NC or group NS, while the level of M1 markers, iNOS, TNF-α, and IL-1β, were not influenced by different stimulations.
Figure 5.
The expression of M2 markers and M1 markers in THP-1 macrophages under stimulations of serum with different origins. M2 markers were shown in upper row while M1 markers in lower row. N=7. **p<0.01, ***p<0.001.
The Effect of Serum on Human Fibroblast Activation
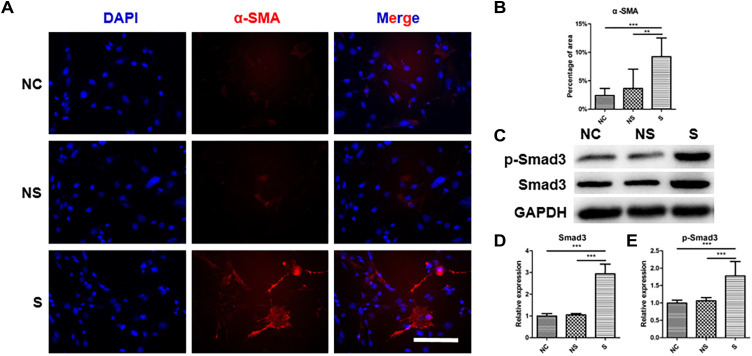
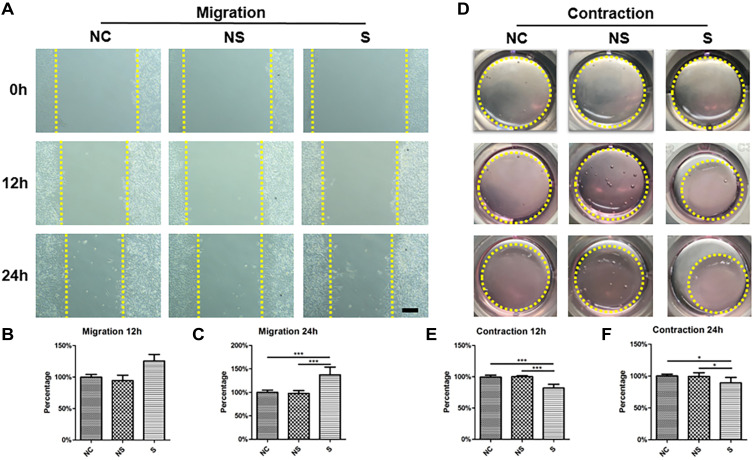
As shown in Figure 6A, α-SMA (stained in red) was highly expressed in CDFs in group S, which was verified by the quantification of positive area (Figure 6B). Similarly, the expression of total Smad3 and p-Smad3, which were also involved in fibroblast activation,40 were significantly higher in CDFs stimulated by the serum of group S (representative WB in Figure 6C and quantified in 6D and 6E). Moreover, migration assay (Figure 7A and quantified in 7B and 7C) and contraction assay (Figure 7D and quantified in 7E and F) showed that CDFs had the character of migration and contraction, which was significantly exacerbated by the serum of group S.
Figure 6.
The level of fibroblast activation under stimulations with serum from different origins. (A) The representative image of immunofluorescent staining of α-SMA in fibroblasts in different groups. (B) The quantification of the positive area of α-SMA relative to the total image in different groups. (C) The expression of Smad3 and p-Smad3 in fibroblasts with different stimulations, and related quantification (D and E). N=7. **p<0.01, ***p<0.001. Bar: 50μm.
Figure 7.
The migration and contraction ability of fibroblasts under stimulations of serum with different origins. (A) The representative image of fibroblasts migration under different stimulations (yellow dotted line indicated the border of cells that reached). (B) The relative quantification of migration distance of fibroblasts in different groups compared to that of group NC at 12h. (C) The relative quantification of migration distance of fibroblasts in different groups compared to that of group NC at 24h. (D) The representative image of the ability of collagen contraction of fibroblasts in different groups (yellow dotted line indicated the collagen gel). (E) The relative quantification of the area of collagen gel in different groups compared to that of group NC at 12h. (F) The relative quantification of the area of collagen gel in different groups compared to that of group NC at 24h. The yellow dotted line in (A) indicated the distance between fibroblasts at two sides of the gap. The yellow dotted cycle in (D) indicated the area of collagen gel. N=7. *p<0.05, ***p<0.001. Bar: 100μm.
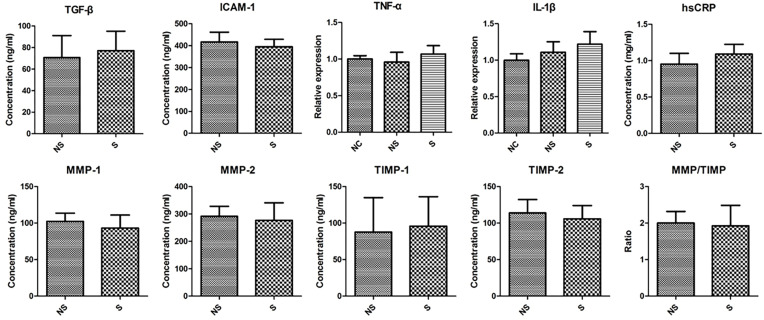
The Level of Cytokines in Serum
The representative cytokines that were reported to be related to shoulder stiffness were measured (Figure 8). Contrary to our anticipation, we did not find any significant difference between the serum from group S and group NS regarding TGF-β, ICAM-1, TNF-α, IL-1β, hsCRP, MMP-1, MMP-2, TIMP-1, TIMP-2, or MMPs/TIMPs.
Figure 8.
The concentration of various cytokines in the serum of patients in two groups.
Discussion
Recently, the influence of systematic disorders, such as diabetes and vitamin D deficiency, on the structural and functional recovery following RCR has attracted much attention.5,41 These systematic comorbidities are usually accompanied by high systematic inflammation levels,7–9 and their serum contains various pathogenic factors that can induce macrophage polarization and fibroblast activation to promote the progress of the disease.42,43 In this study, we found that group S had higher NLR than group NS, indicating a high systematic inflammation level. Serum of group S could significantly induce shoulder stiffness and capsular fibrosis in a mouse model of RCR, polarize macrophages towards M2 phenotype and activate CDFs in vitro. These results indicated that the pre-operative serum of the patients who developed sFS after RCR was sufficient to induce shoulder capsule fibrosis, which was related to its effect on macrophage polarization and fibroblast activation.
NLR can be influenced by various factors, such as smoking, gender, aging et al,44,45 therefore is hard to figure out the exact reason behind its variation among population. Instead, we directly investigated the effect of serum on shoulder capsule in a mouse model. Rodent shoulders are suitable models due to the high level of similarity in shoulder stiffness between mice and human, such as thickening of joint capsule, infiltration of inflammatory cells, and loss of ROM.46,47 In this study, serum of group S significantly thickened mice shoulder capsule, increased cellularity, and decreased ROM, demonstrating a pro-fibrotic role.
To examine the pro-fibrotic effect of serum of group S in vitro, human THP-1 macrophages and CDFs were used. In response to different stimulations, macrophages are polarized to either M1 type or M2 type.48 The former mediates the inflammatory environment while the latter can antagonize inflammation but may also induce fibrosis.49 As surface marker of M1 and M2 macrophages, the levels of CD86 and CD206 have been widely used to evaluate M1 and M2 polarization, respectively.50 iNOS, TNF-α and IL-1β are the representative cytokines secreted by M1 macrophages, while Arg1, TGF-β, and IL-10 are the ones by M2 macrophages.48,51 By measuring the level of these markers, we confirmed that the serum of group S could induce M2 polarization. As for fibroblast activation, its hallmark is α-SMA expression, with enhanced ability of migration and contraction,52 which was also validated by our experiments. In sum, serum of group S was capable of polarizing macrophages to M2 type and activating CDFs, testifying the pro-fibrotic potential.
Then, signaling pathways related to macrophage polarization and fibroblast activation were investigated. TGF-β/Smad3 pathway was reported to be essential in macrophage polarization53 and fibroblast activation.40 Wang et al reported that Smad3 mediated the inhibitory role of growth differentiation factor 3 on M1 polarization of macrophages,54 and others reported that Smad3 was indispensable for mediating the pro-fibrotic effect of TGF-β in fibroblasts from various origins.40 Similarly, our study noted that, after being educated by serum of group S, both the total level and phosphorylated level of Smad3 were up-regulated in polarized macrophages and activated fibroblasts. Whether this was a concomitant phenomenon or was necessary for the regulatory effect of serum shoulder be further studied.
Paradoxically, even though the patients with high systematic inflammation level had serum that was capable of inducing capsular fibrosis and ROM deficiency in vivo, and polarizing macrophages and activating fibroblasts in vitro, their pre-operative ROM was normal. One possible explanation is that the migration and accumulation of macrophages and fibroblasts into local tissue requires a certain stimuli, which could be the surgical intervention in our cohort.
It is well acknowledged that various cytokines and ratios, including TGF-β, TNF-α, IL-1β, ICAM-1, hsCRP, MMP and the MMPs/TIMPs ratio, are essential in the pathology of capsular fibrosis.55–59 Surprisingly, we did not detect any difference in these cytokines between the serum of group S and group NS, which means other components of the serum must be at work here. It could be cytokines other than those we measured. For example, IL-10 can induce idiopathic pulmonary fibrosis,60 and IL-33 promotes scar formation in skin wound.61 It could also be that compounds other than cytokines mediated the effect. In recent years, much advancement has been achieved on the contents of serum. Exosomes and other micro-vehicles have been found to shuttle DNA, RNA, and even protein to target cells62 and influence cell phenotype.63 Considering the complexity of pinpointing the specific pro-fibrotic component, the current study mainly focused on the general effect of serum. Further researches are necessary to figure out which factors in the serum play a role in this regulation.
The current findings were guaranteed by the following advantages. First, variables were controlled as much as possible such that the influence of basic characteristics, surgical techniques and other confounders were minimized. Second, the experiments were first conducted on animal model of RCR and then on cells of homo sapiens species in vitro. Observing the biological activities of fibroblasts derived from shoulder capsule in the condition of patient serum mimicked the environment fibroblasts grow in. On the other hand, the study also had limitations. The sample size was relatively small, so a strict matching procedure of two groups could not be conducted, and statistical bias might be introduced. Reliability and repeatability need further verification in larger cohorts. In addition, although Smad3 signaling pathway was activated in macrophages and fibroblasts, more tests are needed to verify whether it played a pivotal role in regulating cellular phenotypes. Lastly, the component responsible for phenotype regulations requires confirmation.
Conclusion
In conclusion, for patients who had normal pre-operative ROM but developed sFS at 3 months after RCR, their pre-operative serum could trigger shoulder capsule fibrosis by inducing M2 polarization of macrophages and fibroblasts activation. Deeper investigations are needed to figure out the pro-fibrotic content.
Acknowledgment
The current work was supported by National Natural Foundation of China (Nos. 81972062 and 81772419).
Abbreviations
RCR, rotator cuff repair; RCT, rotator cuff tear; sFS, secondary frozen shoulder; TGF-β, transforming growth factor-β; ICAM-1, inter-cellular adhesion molecule-1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; hsCRP, high-sensitive C-reactive protein; MMP-1, matrix metalloproteinase-1; MMP-2, matrix metalloproteinase-2; TIMP-1, tissue inhibitor of metalloproteinase-1; TIMP-2, tissue inhibitor of metalloproteinase-2; NLR, neutrophil/lymphocyte ratio; VAS, pain visual analog scale; FF, forward flexion; AB, abduction; ER0, external rotation at side; IR0, internal rotation at the back; ASES, American Shoulder and Elbow Surgeon score; Constant, Constant-Murley score; FUSS, Fudan University Shoulder Score; ROM, range of motion; CDFs, capsule-derived fibroblasts; NC, negative control; cDNA, complementary DNA.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fermont AJ, Wolterbeek N, Wessel RN, Baeyens JP, de Bie RA. Prognostic factors for successful recovery after arthroscopic rotator cuff repair: a systematic literature review. J Orthop Sports Phys Ther. 2014;44(3):153–163. doi: 10.2519/jospt.2014.4832 [DOI] [PubMed] [Google Scholar]
- 2.Papalia R, Franceschi F, Vasta S, Gallo A, Maffulli N, Denaro V. Shoulder stiffness and rotator cuff repair. Br Med Bull. 2012;104:163–174. doi: 10.1093/bmb/lds006 [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Lin J, Luo Z, Chen J. Preoperative lymphocyte to monocyte ratio can be a prognostic factor in arthroscopic repair of small to large rotator cuff tears. Am J Sports Med. 2020;48(12):3042–3050. doi: 10.1177/0363546520953427 [DOI] [PubMed] [Google Scholar]
- 4.Cho CH, Bae KC, Kim DH. Patients who have undergone rotator cuff repair experience around 75% functional recovery at 6 months after surgery. Knee Surg Sports Traumatol Arthrosc. 2020. doi: 10.1007/s00167-020-06019-z [DOI] [PubMed] [Google Scholar]
- 5.Blonna D, Fissore F, Bellato E, et al. Subclinical hypothyroidism and diabetes as risk factors for postoperative stiff shoulder. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2208–2216. doi: 10.1007/s00167-015-3906-z [DOI] [PubMed] [Google Scholar]
- 6.Harada GK, Arshi A, Fretes N, et al. Preoperative vitamin D deficiency is associated with higher postoperative complications in arthroscopic rotator cuff repair. J Am Acad Orthop Surg Glob Res Rev. 2019;3(7):e075. doi: 10.5435/JAAOSGlobal-D-19-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erkus E, Aktas G, Atak BM, Kocak MZ, Duman TT, Savli H. Haemogram parameters in vitamin D deficiency. J Coll Physicians Surg Pak JCPSP. 2018;28(10):779–782. [PubMed] [Google Scholar]
- 8.Akbas EM, Gungor A, Ozcicek A, Akbas N, Askin S, Polat M. Vitamin D and inflammation: evaluation with neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Arch Med Sci. 2016;12(4):721–727. doi: 10.5114/aoms.2015.50625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S, Hou G, Liu Z, et al. Risk prediction of in-hospital mortality among patients with type 2 diabetes mellitus and concomitant community-acquired pneumonia. Ann Palliat Med. 2020;9(5):3313–3325. doi: 10.21037/apm-20-1489 [DOI] [PubMed] [Google Scholar]
- 10.Khaled SAA, NasrEldin E, Makarem YS, Mahmoud HFF. Value of platelet distribution width and mean platelet volume in disease activity score of rheumatoid arthritis. J Inflamm Res. 2020;13:595–606. doi: 10.2147/JIR.S265811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He S, Mao X, Lei H, et al. Peripheral blood inflammatory-immune cells as a predictor of infertility in women with polycystic ovary syndrome. J Inflamm Res. 2020;13:441–450. doi: 10.2147/JIR.S260770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oner K, Okutan AE, Ayas MS, Paksoy AE, Polat F. Predicting postoperative pain with neutrophil/lymphocyte ratio after arthroscopic rotator cuff repair. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2020;20:24–27. doi: 10.1016/j.asmart.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Jiang M, Meng J, Tao L. Exosomes: carriers of pro-fibrotic signals and therapeutic targets in fibrosis. Curr Pharm Des. 2019;25(42):4496–4509. doi: 10.2174/1381612825666191209161443 [DOI] [PubMed] [Google Scholar]
- 14.Kartika R, Purnamasari D, Pradipta S, Larasati RA, Wibowo H. Impact of low interferon-gamma and IL-10 levels on TNF-alpha and IL-6 production by PHA-induced PBMCs in type 2 diabetes mellitus. J Inflamm Res. 2020;13:187–193. doi: 10.2147/JIR.S245064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Cao N, Yang Z, et al. Bilobalide alleviated dextran sulfate sodium-induced experimental colitis by inhibiting M1 macrophage polarization through the NF-kappaB signaling pathway. Front Pharmacol. 2020;11:718. doi: 10.3389/fphar.2020.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie MF, Xie Q, Wu YH, et al. Serum and ectopic endometrium from women with endometriosis modulate macrophage M1/M2 polarization via the Smad2/Smad3 pathway. J Immunol Res. 2018;2018:6285813. doi: 10.1155/2018/6285813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Wang H, Li Y, Liu S, Chen J, Ying H. miR-24 and miR-122 negatively regulate the transforming growth factor-beta/smad signaling pathway in skeletal muscle fibrosis. Mol Ther Nucleic Acids. 2018;11:528–537. doi: 10.1016/j.omtn.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darmadi D, Ruslie RH. Association between serum interleukin (IL)-12 level and severity of non-alcoholic fatty liver disease (NAFLD). Rom J Intern Med. 2021;59:66–72. [DOI] [PubMed] [Google Scholar]
- 19.Suthanthiran M, Gerber LM, Schwartz JE, et al. Circulating transforming growth factor-beta1 levels and the risk for kidney disease in African Americans. Kidney Int. 2009;76(1):72–80. doi: 10.1038/ki.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Yang D, Zhu S, et al. Bleomycin-induced pulmonary fibrosis is attenuated by an antibody against KL-6. Exp Lung Res. 2013;39(6):241–248. doi: 10.3109/01902148.2013.798056 [DOI] [PubMed] [Google Scholar]
- 21.Tamai K, Akutsu M, Yano Y. Primary frozen shoulder: brief review of pathology and imaging abnormalities. J Orthop Res. 2014;19(1):1–5. doi: 10.1007/s00776-013-0495-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbar M, McLean M, Garcia-Melchor E, et al. Fibroblast activation and inflammation in frozen shoulder. PLoS One. 2019;14(4):e0215301. doi: 10.1371/journal.pone.0215301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Tan H, Lebaschi AH, et al. Kartogenin enhances collagen organization and mechanical strength of the repaired enthesis in a murine model of rotator cuff repair. Arthroscopy. 2018;34(9):2579–2587. doi: 10.1016/j.arthro.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyrna F, Zakko P, Pauzenberger L, McCarthy MB, Mazzocca AD, Dyment NA. Human subacromial bursal cells display superior engraftment versus bone marrow stromal cells in murine tendon repair. Am J Sports Med. 2018;46(14):3511–3520. doi: 10.1177/0363546518802842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92–96. [PubMed] [Google Scholar]
- 26.Chung SW, Huong CB, Kim SH, Oh JH. Shoulder stiffness after rotator cuff repair: risk factors and influence on outcome. Arthroscopy. 2013;29(2):290–300. doi: 10.1016/j.arthro.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Liu S, Chen S, Chen J. The effect of corticosteroid injection into rotator interval for early frozen shoulder: a randomized controlled trial. Am J Sports Med. 2018;46(3):663–670. doi: 10.1177/0363546517744171 [DOI] [PubMed] [Google Scholar]
- 28.Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347–352. doi: 10.1016/S1058-2746(09)80019-0 [DOI] [PubMed] [Google Scholar]
- 29.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160–164. [PubMed] [Google Scholar]
- 30.Ge Y, Chen S, Chen J, Hua Y, Li Y. The development and evaluation of a new shoulder scoring system based on the view of patients and physicians: the Fudan University shoulder score. Arthroscopy. 2013;29(4):613–622. doi: 10.1016/j.arthro.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 31.Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol. 2016;37(3):4135–4142. doi: 10.1007/s13277-015-4233-1 [DOI] [PubMed] [Google Scholar]
- 32.Moser HL, Doe AP, Meier K, et al. Genetic lineage tracing of targeted cell populations during enthesis healing. J Orthop Res. 2018;36(12):3275–3284. doi: 10.1002/jor.24122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oki S, Shirasawa H, Yoda M, et al. Generation and characterization of a novel shoulder contracture mouse model. J Orthop Res. 2015;33(11):1732–1738. doi: 10.1002/jor.22943 [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Chen W, Hao Y, et al. Stem cell-conditioned medium promotes graft remodeling of midsubstance and intratunnel incorporation after anterior cruciate ligament reconstruction in a rat model. Am J Sports Med. 2019;47(10):2327–2337. doi: 10.1177/0363546519859324 [DOI] [PubMed] [Google Scholar]
- 35.Feeley BT, Liu M, Ma CB, et al. Human rotator cuff tears have an endogenous, inducible stem cell source capable of improving muscle quality and function after rotator cuff repair. Am J Sports Med. 2020;48(11):2660–2668. doi: 10.1177/0363546520935855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang R, Deng H, Hou J, et al. Investigation of salmon calcitonin in regulating fibrosis-related molecule production and cell-substrate adhesion in frozen shoulder synovial/capsular fibroblasts. J Orthop Res. 2020;38(6):1375–1385. doi: 10.1002/jor.24571 [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Sun Y, Gu X, et al. Conditioned medium of mesenchymal stem cells delays osteoarthritis progression in a rat model by protecting subchondral bone, maintaining matrix homeostasis, and enhancing autophagy. J Tissue Eng Regen Med. 2019;13(9):1618–1628. doi: 10.1002/term.2916 [DOI] [PubMed] [Google Scholar]
- 38.Reed M, Luissint AC, Azcutia V, et al. Epithelial CD47 is critical for mucosal repair in the murine intestine in vivo. Nat Commun. 2019;10(1):5004. doi: 10.1038/s41467-019-12968-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117(3):524–529. doi: 10.1172/JCI31487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 41.Entezari V, Lazarus M. Surgical considerations in managing osteoporosis, osteopenia, and vitamin D deficiency during arthroscopic rotator cuff repair. Orthop Clin North Am. 2019;50(2):233–243. doi: 10.1016/j.ocl.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 42.Feng J, Dong C, Long Y, et al. Elevated Kallikrein-binding protein in diabetes impairs wound healing through inducing macrophage M1 polarization. Cell Commun Signal. 2019;17(1):60. doi: 10.1186/s12964-019-0376-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin K, You Y, Swier V, et al. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015;35(11):2432–2442. doi: 10.1161/ATVBAHA.115.306132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine. 2018;97(26):e11138. doi: 10.1097/MD.0000000000011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pujani M, Chauhan V, Singh K, Rastogi S, Agarwal C, Gera K. The effect and correlation of smoking with platelet indices, neutrophil lymphocyte ratio and platelet lymphocyte ratio. Hematol Transfus Cell Ther. 2020. doi: 10.1016/j.htct.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villa-Camacho JC, Okajima S, Perez-Viloria ME, et al. In vivo kinetic evaluation of an adhesive capsulitis model in rats. J Shoulder Elbow Surg. 2015;24(11):1809–1816. doi: 10.1016/j.jse.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 47.Kanno A, Sano H, Itoi E. Development of a shoulder contracture model in rats. J Shoulder Elbow Surg. 2010;19(5):700–708. doi: 10.1016/j.jse.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 48.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090 [DOI] [PubMed] [Google Scholar]
- 49.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144–158. [DOI] [PubMed] [Google Scholar]
- 50.Xu HT, Lee CW, Li MY, Wang YF, Yung PS, Lee OK. The shift in macrophages polarisation after tendon injury: a systematic review. J Orthop Translat. 2020;21:24–34. doi: 10.1016/j.jot.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429 [DOI] [PubMed] [Google Scholar]
- 52.Hettrich CM, DiCarlo EF, Faryniarz D, Vadasdi KB, Williams R, Hannafin JA. The effect of myofibroblasts and corticosteroid injections in adhesive capsulitis. J Shoulder Elbow Surg. 2016;25(8):1274–1279. doi: 10.1016/j.jse.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 53.Liu F, Qiu H, Xue M, et al. MSC-secreted TGF-beta regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10(1):345. doi: 10.1186/s13287-019-1447-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Li Y, Wang X, et al. GDF3 protects mice against sepsis-induced cardiac dysfunction and mortality by suppression of macrophage pro-inflammatory phenotype. Cells. 2020;9(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg Br. 2000;82(5):768–773. doi: 10.1302/0301-620X.82B5.0820768 [DOI] [PubMed] [Google Scholar]
- 56.Andronic O, Ernstbrunner L, Jungel A, Wieser K, Bouaicha S. Biomarkers associated with idiopathic frozen shoulder: a systematic review. Connect Tissue Res. 2020;61(6):509–516. doi: 10.1080/03008207.2019.1648445 [DOI] [PubMed] [Google Scholar]
- 57.Kim YS, Kim JM, Lee YG, Hong OK, Kwon HS, Ji JH. Intercellular adhesion molecule-1 (ICAM-1, CD54) is increased in adhesive capsulitis. J Bone Joint Surg Am. 2013;95(4):e181–188. doi: 10.2106/JBJS.K.00525 [DOI] [PubMed] [Google Scholar]
- 58.Park HB, Gwark JY, Kam M, Jung J. Association between fasting glucose levels and adhesive capsulitis in a normoglycemic population: a case-control study. J Shoulder Elbow Surg. 2020;29(11):2240–2247. doi: 10.1016/j.jse.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 59.Park HB, Gwark JY, Jung J, Jeong ST. Association between high-sensitivity C-reactive protein and idiopathic adhesive capsulitis. J Bone Joint Surg Am. 2020;102(9):761–768. doi: 10.2106/JBJS.19.00759 [DOI] [PubMed] [Google Scholar]
- 60.Wijsenbeek MS, Kool M, Cottin V. Targeting interleukin-13 in idiopathic pulmonary fibrosis: from promising path to dead end. Eur Respir J. 2018;52(6):1802111. doi: 10.1183/13993003.02111-2018 [DOI] [PubMed] [Google Scholar]
- 61.Wulff BC, Pappa NK, Wilgus TA. Interleukin-33 encourages scar formation in murine fetal skin wounds. Wound Repair Regen. 2019;27(1):19–28. doi: 10.1111/wrr.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen HP, Wang XY, Pan XY, et al. Circulating neutrophil-derived microparticles associated with the prognosis of patients with sepsis. J Inflamm Res. 2020;13:1113–1124. doi: 10.2147/JIR.S287256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martellucci S, Orefice NS, Angelucci A, Luce A, Caraglia M, Zappavigna S. Extracellular vesicles: new endogenous shuttles for miRNAs in cancer diagnosis and therapy? Int J Mol Sci. 2020;21:18. [DOI] [PMC free article] [PubMed] [Google Scholar]