Abstract
Antimicrobial resistance (AMR) has risen as a global threat for human health. One of the leading factors for this emergence has been the massive use of antibiotics growth-promoter (AGPs) in livestock, enhancing the spread of AMR among human pathogenic bacteria. Thus, several alternatives such as probiotics, prebiotics, or phytobiotics have been proposed for using in animal feeding to maintain or improve productive levels while diminishing the negative effects of AGPs. Reducing the use of antibiotics is a key aspect in the pig rearing for production reasons, as well as for the production of high-quality pork, acceptable to consumers. Here we analyze the potential use of Allium extract as an alternative. In this study, weaned piglets were fed with Allium extract supplementation and compared with control and antibiotic (colistin and zinc oxide) treated piglets. The effects of Allium extract were tested by analyzing the gut microbiome and measuring different productive parameters. Alpha diversity indices decreased significantly in Allium extract group in caecum and colon. Regarding beta diversity, significant differences between treatments appeared only in caecum and colon. Allium extract and antibiotic piglets showed better values of body weight (BW), average daily weight gain (ADG), and feed conversion ratio (FCR) than control group. These results indicate that productive parameters can be implemented by modifying the gut microbiota through phytobiotics such as Allium extract, which will drive to drop the use of antibiotics in piglet diet.
Keywords: Allium extract, bacterial community, high-throughput sequencing, phytobiotic, piglet microbiome, productive parameters
1. Introduction
Antibiotics have been used to promote growth and production in livestock (Antibiotic Growth Promoters, AGP [1,2]). However, the inappropriate and indiscriminate use of them contributed to a rising of resistance to antibiotics [3]. This situation drove the World Health Organization (WHO) to call for a global action against Antimicrobial Resistance (AMR; [4]). For this reason, AGPs are banned by the European Union since 2006 (EC Regulation 1831/2003; http://eur-lex.europa.eu/en/index.htm) and by other countries during following years [5,6]. However, this ban has produced an increase in mortality, especially at weaning when many stressors affects piglets’ health, leading to an increase of post-weaning diarrhea caused by Escherichia coli infections [7,8]. This increase in mortality directly affects the pork industry, as pork and its derivatives are product highly consumed daily throughout the world [9]. In this sense, reducing the use of antibiotics is a key aspect in the pig rearing for production reasons, but also for the production of high-quality pork, acceptable to consumers.
The mechanisms through which AGPs act are not very clear but it is believed that growth promotion could be associated with changes in the gut microbiota [10,11]. AGPs may favor the reduction of pathogenic bacteria, the reduction of bacterial competition for nutrients, and reduction of microbial compounds, which can decrease animal growth [12,13]. However, the use of AGPs has undesirable effects such as selection and spread of antibiotic resistance genes [14]. Some studies show evidence of the occurrence of AMR in relation to the use of antibiotics in cattle and specifically in the swine industry [15,16]. Many bacterial strains resistant to a wide variety of antibiotics have been found in the intestinal microbiota of pigs, such as Campylobacter coli, C. jejuni, Salmonella, or the multiresistant Staphylococcus aureus (livestock-associated MRSA) [14,17,18,19]. Given this problem of AMR and the subsequent ban of AGPs in food animal production, there has been a need to look for alternatives that maintain animal health and increase productive levels of pigs while decreasing the use of antibiotics [2,20].
Different compounds have been proposed as substitutes to AGPs in swine industry improving health and performance of pigs. Probiotics, prebiotics, organic acids, enzymes, or phytobiotics have been widely recognized as promising alternatives to antibiotics in feeds [2]. Phytobiotics are plant-derived products used in animal feed to improve performance of livestock. Some studies had demonstrated their antimicrobial, antioxidants, and immunoregulatory effects in poultry and pigs [2,21]. Given these positive properties of phytobiotics, several researchers have tried to demonstrate that their inclusion in diets can improve pig performance. Some studies have shown positive results using different plant extracts including oregano oil [22,23], menthol and cinnamon [22,24], a mixture of different plant extracts [25,26], and garlic [27]. Garlic had also been used due to its antifungal, antimicrobial, and antioxidant properties [28,29]. Currently, several active organosulfur compounds extracted from garlic and other Allium plants, such as PTS (propyl propane thiosulfinate) and PTSO (propyl propane thiosulfonate), have been characterized [30]. An Allium extract, which includes these compounds, has shown high antimicrobial activity against Salmonella, E. coli, Clostridium, methicillin-resistant Staphylococcus aureus (MRSA), Campylobacter jejuni, and Aspergillus pathogens [31,32]. This product had been mainly used in broiler chickens, modulating intestinal microbiota, improving nutrient digestibility, and reducing pathogens and potentially pathogenic bacteria in the intestinal content [31,33]. PTS and PTSO had also been add to pig diet and showed antimicrobial activity against different bacterial groups, decreasing fecal counts of Enterobacteriaceae and coliforms [30].
The aim of the present study was to evaluate the influence of the Allium extract in weaned piglet gut microbiota and how it affects productive parameters such as body weight, daily weight gain, daily feed intake, and feed conversion rate. In this study, we have made a fully randomized experiment using piglets as research animal model supplemented with the Allium extract. We have characterized the microbiota in different gut regions by high-throughput sequencing of 16S rRNA gene at 70 days of life. We suggest that this phytobiotic compound improves piglet productive parameters by means of distal gut microbiota modification.
2. Materials and Methods
2.1. Piglets and Farm Facilities
The experiment was carried out at IMASDE AGROALIMENTARIA S.L. in Granja La Mata (Experimental Authorization Ref No: B-82334855), a swine experimental farm situated in Mata de Cuellar (Segovia, Spain). A total of 240 piglets (50% female, 50% male) were used in the experiment. Piglets were housed in a non-litter housing system consisting of 2 rooms, using a total of 24 blocks (12 of each room). Ten crossbred piglets of the same sex (50% Pietrain × 25% Landrace* 25% Large White) from commercial genetic breeds were kept per block of 6.05 m2 (2.16 × 2.80 m2). Piglets were from stress-free parents. The rooms had natural and artificial lighting, and the temperature was adjusted according to the piglet age. Piglets were weaned at 28 days of life, with an average weight of 7.34 ± 0.89 kg. The farm fulfilled the national regulations and the European directive for the protection of animal welfare in research (Directive 2010/63/EU, European Commission, 2010).
2.2. Experimental Design and Sample Collection
Before starting the experiment, animals were examined and those with signs of illness or injury were removed. Subsequently, groups of 10 piglets of the same sex were assigned randomly to different blocks (8 blocks per treatment, 4 in each room). Piglets were regularly monitored during rearing. No signs of loss of weight, abnormal behaviors or deaths were detected. Control piglets were fed with a basal diet, while experimental piglets received basal diet supplemented with Allium extract (equivalent to 20 mg/kg of thiosulfinates and thiosulfonates). This Allium extract is commercialized under the trademark of Garlicon (DOMCA S.A.U., Spain), and the applied dose is the recommended by the product leaflet. In addition, another group received basal diet supplemented with 120 mg/kg of the antibiotic colistin (Nipoxyme 100) and 3000 ppm of zinc oxide (ZnO) as positive control (colistin was only used for research purpose because it is prohibited for commercial purpose). Basal diet differed in pre-starter (28 to 42 days) and starter (43 to 70 days) (Supplementary Material: Table S1). Both diets and water were supplied ad libitum. Diets were formulated by IMASDE AGROALIMENTARIA S.L. and produced at the factory Gireporc S.A. in Bernuy de Porreros (Segovia, Spain).
Piglets were weighted at weaning (beginning of the experiment—28 days old), at 42 and 70 days old. Other productive parameters were recorded at the end of each experimental stage (42 and 70 days old): Average daily feed intake, ADFI; Average daily weight gain, ADG; and Feed conversion rate, FCR (ADFI divided by ADG). At the end of the experiment (70 days old), one piglet per block (a total of eight piglets of each treatment) was slaughtered by previous electrically stunned and bleed, according to the standardized procedures of slaughterhouse "El cochinillo segoviano" S.L. (Boceguillas, Segovia, Spain). Immediately, pieces of about 10 cm were dissected from different intestinal regions (duodenum and ileum from small intestine; caecum and colon from large intestine) with sterile material. Intestinal pieces were stored in sterile containers and transported to the laboratory, where they were kept at -80 ºC until DNA extraction. Intestinal pieces from different gut regions of piglets were dissected using a sterile scalpel and approximately 100 mg of gut content were collected.
2.3. DNA Extraction
DNA extraction was carried out using FavorPrep Stool DNA Isolation Mini Kit (Favorgen Biotech Corp., Taiwan), according to manufacturer instructions. DNA extraction was checked by 0.7% agarose gel electrophoresis and DNA concentration was measured using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, USA). Samples were standardized at the same DNA concentration (10 ng/µL) and then stored at −20 °C until DNA amplification.
2.4. High-Throughput Sequencing
Amplicon PCR was performed from bacterial total DNA of the V4 region of the 16S rRNA gene using the primer pair U515F (5´ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3´) and E786R (5´-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3´) with overlap partial Illumina primers. This PCR was carried out in a final volume of 25 µL containing 12.5 µL of iProof High-Fidelity DNA Polymerase (Bio-Rad Laboratories, Inc.), 0.3 µM of each primer, and 5 µL of template DNA. The amplification program consisted of an initial denaturing step of 98 °C for 1 min followed by an amplification step of 25 cycles of 10 s at 98 °C, 20 s at 52 °C, and 15 s at 72 °C, and a final extension of 5 min at 72 °C. Then, a second PCR was applied to include specific barcodes by adding a unique combination of a couple of barcodes per sample. This PCR was carried out in a final volume of 25 µL containing 12.5 µL of iProof High-Fidelity DNA Polymerase (Bio-Rad Laboratories, Inc.), 0.4 µM of each primer, and 5 µL of purified PCR product from the previous PCR. The amplification program consisted of an initial denaturing step of 98 °C for 1 min followed by an amplification step of 8 cycles of 10 s at 98 °C, 20 s at 55 °C, and 15 s at 72 °C, and a final extension of 5 min at 72 °C. Purification steps were made using magnetic microparticles with a surface functional group to which DNA can be reversibly linked. Subsequently, the DNA of the magnetic particles were separated by elution [34]. Then, DNA concentration was measured using Qubit® 3.0 Fluorometer (Invitrogen, Carlsbad, CA, USA) and normalized to the same concentration. High-throughput sequencing was carried out on Illumina MiSeq platform in the Scientific Instrumental Center at the University of Granada (CIC-UGR, Spain). Sequences are available in the Genbank-NCBI Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra/), BioProject: PRJNA664026, Accession Nos. SAMN16192455 to SAMN16192544.
2.5. Sequences Processing and Data Analysis
The processing of the sequences obtained from Illumina MiSeq was carried out with QIIME2 v2018.02 (Quantitative Insights In Microbial Ecology [35,36]). First, primers trimming were performed using default parameters using cutadapt plugin [37]. Forward reads were selected for the following analysis due to low quality in reverse reads after 120 bp (Phred score < 20). Quality filtering were performed using default parameters. Afterwards, we used Deblur for sequence clustering into sub-OTUs, a sub-operational-taxonimic-unit (sOTU) approach, in order to remove sequencing errors [38]. Sequences that passed quality filters were truncated to 200 bp, using Phred score of 20 as quality threshold, giving a dataset of 6,548,564 total reads with a mean depth of 70,415 reads per sample. We used fragment insertion script adapted to QIIME2 through the SATé-enabled phylogenetic placement (SEPP) technique, a script that performs the alignment of the sequences and the phylogenetic tree [39]. Taxonomy assignation was made with a classifier pretrained on Greengenes 13.08 with a similarity of 99% [40]. Finally, because the primers used are designed for bacteria, chloroplasts, mitochondria, and non-bacterial DNA were removed from the sOTU table.
2.6. Statistics
To test the effect of treatment on production parameters of pigs, we used Generalized Linear Mixed-Models (GLMM). We used 24 experimental units (2 rooms of 12 experimental units each) with treatment as fixed factor, sex, and room as random factors, and initial body weight as covariate.
For alpha and beta diversity analyses, sOTU table was rarified at 17,000 sequences depth per sample. Samples that did not reach this sequencing depth were excluded for subsequent analyses. Two alpha diversity indices were calculated, i.e., bacterial species richness, as number of observed species; and Faith’s phylogenetic diversity index [41]. We used General Linear Models (GLM) to explore the effect of treatment and gut region in different alpha diversity indices. Piglet was used as experimental unit for alpha and beta diversity analysis.
Productive parameters and alpha diversity analyses were performed in Statistica 10.0 (StatSoft).
Beta diversity distance matrixes were calculated using UniFrac distance [42]. In subsequent analysis, we used both Weighted UniFrac and Unweighted UniFrac distance matrixes as we do not have a priori predictions in the effects of the independent variables (gut region and treatment) in the bacterial community. Weighted UniFrac gives more importance to most abundant bacteria as it takes into account the abundance of sequences per sOTU, while Unweighted UniFrac gives the same importance to all bacterial sOTU presents in the samples, giving more importance to minority bacteria as it takes into account the presence or absence of sOTU [43]. Permutational ANOVA (PERMANOVA) based on Type III sums of squares with 999 permutations was used to test treatment and gut region effects on both UniFrac distance matrixes [44] using PRIMER-7 (PRIMER-e). Principal Coordinates Analysis were calculated and visualizations of the first three axes of the PCoA were plotted using Emperor 2018.2.0 [45].
3. Results
3.1. Effects of Teatment on Piglets’ Gut Bacterial Alpha Diversity
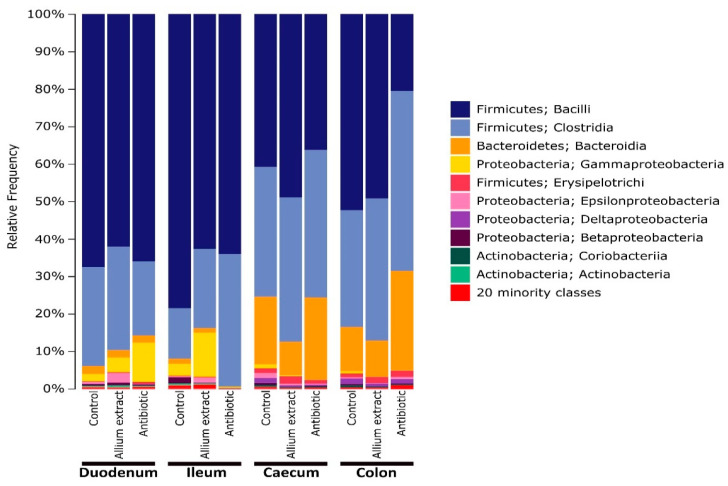
Duodenum and ileum microbiota of 70 days control piglets were mainly dominated at classes Bacilli and Clostridia, representing more than 90% between both groups. This pattern was similar in Allium extract and antibiotic groups, but with a lower proportion of Bacilli and higher proportion of Gammaproteobacteria in duodenum (10.5% and 3.9% in antibiotic and Allium extract group respect to 1.9% in control group) and Clostridia in ileum (35.3% and 21.1% in antibiotic and Allium extract group respect to 13.5% in control group) (Figure 1). At genus level, duodenum and ileum community of piglets was very diverse, dominated by Lactobacillus (more than 65%), followed by an unidentified genus of the family Clostridiaceae (6.7%), Sarcina (5.9%), Streptococcus (3%), and an unidentified genus of the family Peptostreptococcaceae (2.8%). Duodenum microbiota was very similar in three groups, but in the ileum, more differences appeared, with lower proportion of Lactobacillus in both Allium extract and antibiotic group (Supplementary material: Figure S1). However, no statistically significant differences appeared between treatments in duodenum and ileum in neither Species richness (LSD Posthoc test, p > 0.314) nor Faith’s diversity index (LSD Posthoc test, p > 0.253).
Figure 1.
Microbial composition at class level of piglet gut microbiota grouped by gut region and treatment. Classes in the legend are sorted from most abundant to lowest abundant.
Large intestine (caecum and colon) microbiota showed a shift in dominant classes respect to small intestine, with lower proportion of Bacilli and higher proportion of Clostridia and Bacteroidia (Figure 1). Caecum microbiome had a very similar distribution in piglets from different treatments, with a slightly higher proportion in Allium extract fed piglets of Bacilli (48.9% respect to 40.8% in control group) and lower proportion of Bacteroidia (9.0% respect to 18.1% in control group). At the class level, colon microbiome of control and Allium extract groups were very similar, but the Antibiotic group microbiome showed a lower proportion of Bacilli (20.5% compared to 52.3% in control group) and higher proportion of Clostridia and Bacteroidia (Figure 1). At genus level, caecum microbiome of piglets from different treatments was similar, but in colon region, differences appeared in the antibiotic group, with lower proportion of Lactobacillus (14.2%) with respect to control and Allium extract groups (48.9 and 46.5%, respectively) and higher proportion of Prevotella and the rest of minority genera (Additional file 1: Figure S1). Regarding alpha diversity indices, in caecum, Allium extract group showed lower values of Species richness and Faith’s diversity index than Control group (LSD Posthoc test, p = 0.007; LSD Posthoc test, p = 0.034 respectively). In colon, Allium extract group had lower values of Species richness and Faith’s diversity index than antibiotic group (LSD Posthoc test, p = 0.008; LSD Posthoc test, p = 0.019 respectively).
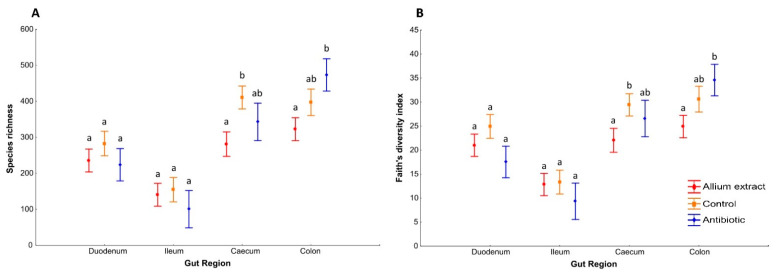
Therefore, none of the small intestine region (duodenum and ileum) showed differences between treatments in Species richness and Faith diversity indices, but significant differences in these alpha diversity indices appeared in large intestine regions (caecum and colon) (Figure 2). Taking into account the whole gut, species richness and Faith’s diversity index differed significantly between treatments and between gut regions (Table 1). However, interactions between treatments and gut region were not significant, indicating that alpha diversity indices along the piglets’ gut of different treatments changed in the same way (see interaction Gut Region and Treatment in Table 1).
Figure 2.
Alpha diversity by gut region. Average ± standard error of the mean of the bacterial species richness (A) and Faith’s diversity index (B) of weaned piglets in different gut regions. Bars with different letter within the same gut region denote significant differences in treatment (LSD Posthoc test; p < 0.05).
Table 1.
General Linear Models exploring the effects of treatment (control, antibiotic and Allium extract) and gut region in the different alpha diversity indices of the bacterial community of weaned piglets. D.f. refers to degree of freedom. The first number is the degree of freedom of the independent variable and the second one for the error term. Significant p-values (p < 0.05) are shown in bold.
| Alpha Diversity Index | Control | Allium Extract | Antibiotic | Explanatory Variables | D.f. | F | p |
|---|---|---|---|---|---|---|---|
| Species richness | 311.75 (27.38) | 243.77 (18.71) | 294.14 (41.60) | Treatment | 2.61 | 4.03 | 0.023 |
| Gut Region | 3.61 | 26.41 | <0.001 | ||||
| Gut Region × Treatment | 6.61 | 1.57 | 0.171 | ||||
| Faith’s diversity index | 24.53 (1.89) | 20.14 (1.31) | 22.59 (2.89) | Treatment | 2.61 | 3.25 | 0.046 |
| Gut Region | 3.61 | 22.11 | <0.001 | ||||
| Gut Region × Treatment | 6.61 | 1.46 | 0.208 |
3.2. Effects of Treatment and Gut Region on Beta Diversity
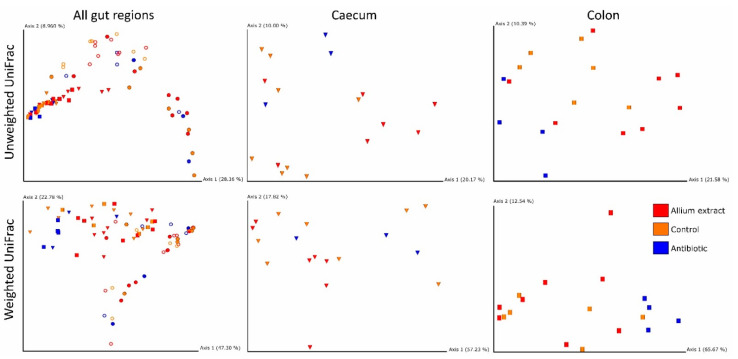
Changes in bacterial communities along different piglets’ gut regions were similar in the three experimental groups (see non-significant interaction terms Gut Region*Treatment of both Unweighted and Weighted UniFrac in Table 2). However, Gut Region and Treatment had a significant effect on the intestinal microbiota of the piglets in both UniFrac indices (Table 2). These differences were observed graphically in the Principal Coordinates Analysis (PCoA) when Gut Region, but not Treatment were taken into account (Figure 3). It can also be observed main clustering between small and large intestine samples.
Table 2.
General Linear Models exploring the effects of treatment, gut region, and their interaction in beta diversity indices of bacterial community of weaned piglets fed with control diet or supplemented with antibiotic or Allium extract. D.f. refers to degree of freedom. The first number is the degree of freedom of the independent variable and the second one for the error term. Significant p-values are shown in bold.
| β-Diversity Distance Matrix |
Explanatory Variables |
D.f. | Pseudo-F | p |
|---|---|---|---|---|
| Unweighted UniFrac | Treatment | 2.61 | 1.84 | 0.001 |
| Gut Region | 3.61 | 7.88 | 0.001 | |
| Gut Region × Treatment | 6.61 | 1.06 | 0.303 | |
| Weighted UniFrac | Treatment | 2.61 | 2.35 | 0.044 |
| Gut Region | 3.61 | 9.14 | 0.001 | |
| Gut Region × Treatment | 6.61 | 1.02 | 0.412 |
Figure 3.
Dimensional figures showing the first two axes of Principal Coordinate Analysis and representing bacterial communities of weaned piglets in all gut regions and taking into account only caecum and colon using Unweighted and Weighted UniFrac distance matrixes. Samples are colored by treatment (Control—yellow; Antibiotic—blue; Allium extract —red) and samples from each intestinal region are represented by different shapes (Duodenum—ring; Ileum—sphere; Caecum—cone; Colon—square). Proportion of explained variance by each PCo axes is shown.
When we studied the effect of Treatment within each gut region, significant differences appeared at the. caecum level with Unweighted UniFrac (Figure 3) and at the colon level with both Unweighted and Weighted UniFrac (Figure 3). Antibiotic samples grouped in a cluster separated from control and Allium extract samples. Therefore, our treatment affected mainly to large intestine regions (Table 3).
Table 3.
General Linear Models exploring the effects of treatment in beta diversity indices of bacterial community of weaned piglets fed with control diet or supplemented with antibiotic or Allium extract. D.f. refers to degree of freedom. The first number is the degree of freedom of the independent variable and the second one for the error term. Significant p-values are shown in bold.
| β-Diversity Distance Matrix | D.f. | Pseudo-F | p | |
|---|---|---|---|---|
| Duodenum | Unweighted UniFrac | 2.16 | 1.23 | 0.099 |
| Weighted UniFrac | 2.16 | 0.25 | 0.977 | |
| Ileum | Unweighted UniFrac | 2.15 | 0.93 | 0.502 |
| Weighted UniFrac | 2.15 | 1.08 | 0.377 | |
| Caecum | Unweighted UniFrac | 2.15 | 1.56 | 0.007 |
| Weighted UniFrac | 2.15 | 1.48 | 0.191 | |
| Colon | Unweighted UniFrac | 2.15 | 1.55 | 0.017 |
| Weighted UniFrac | 2.15 | 4.18 | 0.009 |
3.3. Effects of Treatment on Piglets’ Productive Parameters
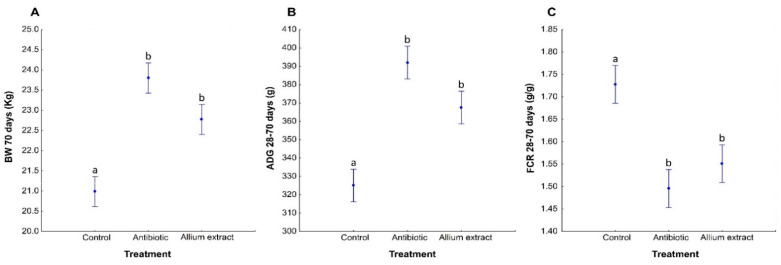
Body weight significantly differed between treatments at day 70 (Table 4; Supplementary material: Table S2). Antibiotic and Allium extract fed piglets showed higher values of body weight than Control piglets (Table 4; Supplementary material: Table S2; Figure 4A). Allium extract group showed lower values of body weight than Antibiotic one, although this difference was marginally significant (LSD Posthoc test; p = 0.080).
Table 4.
General Linear Models exploring the effects of treatment as factor, sex, and block as random factors and initial body weight as covariate, in weaned piglets fed with control diet or supplemented with antibiotic or Allium extract. BW refers to body weight, ADG to average daily gain, FCR to feed conversion rate, and ADFI to average daily feed intake. D.f. refers to degree of freedom. The first number is the degree of freedom of the independent variable and the second one for the error term. Significant p-values are shown in bold.
| Dependent Variable | Control | Allium Extract | Antibiotic | Independent Variables | F | D.f. | p |
|---|---|---|---|---|---|---|---|
| Initial BW (28 days), kg | 7.34 (0.35) | 7.34 (0.33) | 7.32 (0.37) | Treatment | <0.01 | 2.19 | 0.998 |
| Sex | 0.21 | 1.19 | 0.653 | ||||
| Room | 0.93 | 1.19 | 0.346 | ||||
| BW 42 days, kg | 10.50 (0.49) | 10.87 (0.57) | 11.40 (0.55) | Treatment | 5.69 | 2.18 | 0.012 |
| Sex | 4.98 | 1.18 | 0.039 | ||||
| Room | 29.67 | 1.18 | <0.001 | ||||
| Initial BW | 116.14 | 1.18 | <0.001 | ||||
| BW 70 days, kg | 21.01 (0.76) | 22.79 (0.98) | 23.76 (0.92) | Treatment | 14.59 | 2.18 | <0.001 |
| Sex | 8.96 | 1.18 | 0.008 | ||||
| Room | 2.46 | 1.18 | 0.134 | ||||
| Initial BW | 86.30 | 1.18 | <0.001 | ||||
| ADG 28–70 days, g/d | 325.25 (11.01) | 367.71 (16.84) | 391.53 (15.37) | Treatment | 14.59 | 2.18 | <0.001 |
| Sex | 8.96 | 1.18 | 0.008 | ||||
| Room | 2.46 | 1.18 | 0.134 | ||||
| Initial BW | 26.13 | 1.18 | <0.001 | ||||
| ADFI 28–70 days, g/d | 562.73 (30.24) | 566.26 (23.25) | 583.79 (19.97) | Treatment | 0.49 | 2.18 | 0.620 |
| Sex | 2.14 | 1.18 | 0.161 | ||||
| Room | 18.45 | 1.18 | <0.001 | ||||
| Initial BW | 11.22 | 1.18 | 0.004 | ||||
| FCR 28–70 days, g/g | 1.73 (0.06) | 1.55 (0.06) | 1.50 (0.04) | Treatment | 8.27 | 2.18 | 0.003 |
| Sex | 1.64 | 1.18 | 0.216 | ||||
| Room | 11.88 | 1.18 | 0.003 | ||||
| Initial BW | 1.26 | 1.18 | 0.277 | ||||
| Mortality 28–70 days, % | 5.00 (2.67) | 2.50 (1.64) | 1.25 (1.25) | Treatment | 0.90 | 2.18 | 0.423 |
| Sex | 0.08 | 1.18 | 0.787 | ||||
| Room | 1.52 | 1.18 | 0.233 | ||||
| Initial BW | 0.66 | 1.18 | 0.428 |
Figure 4.
Average ± standard error of the mean of the Body Weight (BW) at 70 days of life (A), Average Daily Gain (ADG) (B), and Feed Conversion Ratio (FCR) (C) from 28 to 70 days of life of weaned piglets fed with control diet or antibiotic or Allium extract supplemented diets. Bars with different letter denote significant differences in treatment (LSD Posthoc test; p < 0.05).
During pre-starter stage (from 28 to 42 days), Antibiotic piglets had significantly more ADG and showed a better FCR than Control piglets, while Allium extract fed piglets showed intermediate values in both parameters. During starter stage (from days 43 to 70) Antibiotic and Allium extract showed higher values of ADG than Control piglets (Supplementary material: Table S2). Analyzing global stage (from 28 to 70 days), results showed that Antibiotic and Allium extract fed piglets significantly had higher ADG and lower FCR than Control piglets (Table 4; Supplementary material: Table S2; Figure 4B,C). No differences were observed between treatments in average daily feed intake (ADFI) or mortality (Table 4; Supplementary material: Table S2).
4. Discussion
The addition of Allium extract in the diet of weaned piglets had a significant increase of body weight (BW) and average daily gain (ADG), and decrease of feed conversion ratio (FCR) respect to control diet. Allium extract fed piglets reached similar productive levels to those of antibiotic group (colistin + ZnO), but marginally significant differences appeared in BW and ADG. These beneficial productive changes were accompanied by significant changes in bacterial community as diminution of alpha diversity indices and significant changes in beta diversity in large intestine regions (caecum and colon). These changes in beta diversity only appeared in the caecum and colon but general behavior of gut microbiota was not affected by the treatment (no differences in interaction between Gut and Treatment; Table 2).
Alternatives to antibiotics that maintain productive parameters in pig breeding is essential to fight AMR spreading and improve animal welfare. Several alternatives to antibiotic growth promoters such as probiotics, prebiotics, enzymes, and plant extracts had been proposed to achieve it and also to reduce the probability of AMR appearing [2,21]. From this point of view, plant extracts or phytobiotic, which can modulate microbiota and increase productive parameters, appear to be good and safe alternative to antibiotics [46]. Different plant extracts improve animal performance, productive parameters, and induce changes in gut microbiome of animals. For instance, oregano oil in growing-finishing pigs improved growth performance and nutrient digestibility by modulating gut microbiota [47], and oregano oil had been also used together with carbohydrases in piglets, improving feed conversion ratio with respect to control and antibiotic growth promoter diets [23]. Other essential oils obtained from thyme and cinnamon improved body weight of weaning pigs and decreased the number of pathogens as E. coli in different gut regions [22]; and a mixture of essential oil from mint and cinnamon improved feed efficiency in piglets [24]. Allium extract, mainly garlic extract, had also been used in piglets’ diet in different studies, reducing diarrhea and inflammation caused by E. coli [27] and improving piglet performance and body weight [29]. In our study, piglet diet was supplemented with Allium extract, an extract of onion and garlic, of which the principal active components are propyl propane thiosulfinate (PTS) and propyl propane thiosulfonate (PTSO). Our results support the use of this phytobiotic compound in piglet diet given that animals showed a performance improvement characterized by an increase of body weight (BW) and average daily gain (ADG), and a decrease of feed conversion ratio (FCR) with respect to control group. Furthermore, in our study, piglets fed with Allium extract reached productive levels similar to those obtained using an antibiotic growth promoter (colistin) and ZnO. These results are promising as pork is one of the most consumed meat all over the world [9,48], thus Allium extract could be a good alternative to antibiotic growth promoters in pig diet given that improve productive parameters. Results obtained in other studies carried out with piglets suffering from diarrhea fed with plant extracts suggest that the growth promoting effects may be due to their antimicrobial activity [49,50]. This conclusion was also obtained in studies of [30] using both PTS and PTSO in swine, which had antimicrobial activity against different bacterial group in pig feces, especially against Enterobacteriaceae and other coliforms. Other studies pointed out that plant extracts increase productive parameters stimulating feed consumption [51], but other authors found that plant extracts decrease feed consumption [52]. Nevertheless, our results shown that piglets fed with Allium extract had similar levels of average daily feed intake (ADFI) compared to control and antibiotic groups.
Microbiome of intestine of pigs is dominated by Firmicutes, followed by Proteobacteria in the small intestine and Bacteroidetes in the large intestine [53,54,55,56]. At the class level, dominant classes of each phylum are Bacilli and Clostridia (Firmicutes), Bacteroidia (Bacteroidetes), and Gammaproteobacteria (Proteobacteria). Our results are consistent with these previous findings, especially at the phylum level. Other studies had shown that some Lactobacillus species play an important role in intestinal health of piglets by influencing intestinal physiology, regulating the immune system, and balancing the intestinal ecology of the host [57,58]. In our experiment, in caecum and colon, piglets supplemented with Allium extract showed similar levels of Bacilli versus control group, mainly due to the genus Lactobacillus. However, antibiotic group showed lower proportion of Lactobacillus, especially in the colon, showing that colistin and ZnO would have an effect on Lactobacillus depletion, whereas the genus Prevotella had an increase occupying its niche. This decrease in Lactobacillus abundance in colistin and ZnO piglets may be related to a depletion in carbohydrate levels in distal parts of the gut. In vitro studies have demonstrated that shifts in pig gut microbiome composition can be produced by changes in substrate structure [59]. Different Allium extracts produce changes in the physiology and histology of the gut of animals. In broilers, onion powder increased length, width, and surface area of intestinal villus [60]. In piglets, aged garlic extract improved body weight, the morphology of intestinal villi, and non-specific immune response [29]. Other studies using Allium extract in growing-finishing pigs showed an increase in productive parameters and an increase of short-chain fatty acids (SCFA) in feces, which is related to high Lactobacillus abundance in distal gut [61]. These changes may suggest that Allium extracts produce changes in the availability of some substrates necessary for the growth of beneficial bacteria. However, an in vitro study showed that PTSO extracted from Allium plants have antimicrobial activity against lactobacilli, bifidobacteria, Bacteroides, and Clostridia, and strongly reduce enterobacteria and coliforms in swine microbiota [30]. Whether PTSO and Allium extracts affect bacterial community directly or indirectly by change the substrate availability deserve future research.
Changes due to the supplementation of antimicrobials showed that main changes in bacterial community were produced in caecum and colon [62]. Our results are consistent with these previous findings, showing differences between treatments in large intestine regions (caecum and colon) in both alpha and beta diversity indices. These changes in bacterial community indices may be due to differential bioavailability of Garlicon in these intestinal regions. In vitro digestion studies of [63] showed that Garlicon bioavailability increases as it progresses in the gastrointestinal tract of pigs. Alpha diversity indices in the colon in the Antibiotic group were higher than in the Allium extract group. This reduction in alpha diversity levels could be related to the increase of body weight since reduction of alpha diversity has been associated with obesity in several human studies [64,65,66]. Different studies have found evidences that differences in microbial composition could be due to body weight [67] while other studies showed that changes induced by feed additives in gut microbiota can produce changes in body weight [68].
5. Conclusions
Our experiment supports the use of Allium extract supplemented in the diet of weaning piglets for successfully improving productive parameters such as body weight, average daily gain, or feed conversion ratio levels with respect to control diet. These beneficial effects in productivity correlates with significant changes in the bacterial community of the distal gut. These results are preliminary as further experiments are necessary to untangle whether Allium extracts directly affect the gut microbiota and hence the productivity parameters or whether the effects are directly on the bacterial community or on specific bacterial groups related to immune system or piglet’s health.
Acknowledgments
The authors thank their colleague Ana Llopis Valdivia for revising their English text.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/10/3/269/s1: Table S1. Calculated composition and analysis (% per Kg of feed) of the diet used for piglets; Table S2. Average ± standard error of the mean of the Body Weight (BW) at 28, 42 and 70 days of life; and Average Daily Gain (ADG), Average Daily Feed Intake (ADFI), Feed Conversion Ratio (FCR) and mortality in different experimental stages and global stage of weaned piglets fed with control diet or Allium extract or antibiotic supplemented diets. Rows with different letter denote significant differences in treatment (LSD Posthoc test; p < 0.05); Figure S1. Microbial composition at genus level of piglets gut microbiota grouped by gut region and treatment. Genera in the legend are sorted from most abundant to lowest abundant.
Author Contributions
Conceptualization, M.R.-R., C.T.-P., E.G., M.A.A.-C., A.B., and M.M.-B.; methodology, M.R.-R., C.T.-P., J.M.P.-S., A.M.M.-P., J.J.A., Ó.C.-R., P.V.-C., A.B., and M.M.-B.; validation, J.M.P.-S., A.M.M.-P., A.B., and M.M.-B.; formal analysis, M.R.-R., C.T.-P., and J.M.P.-S; investigation, M.R.-R., C.T.-P., and J.M.P.-S.; resources, E.G., A.B., and M.M.-B.; data curation, M.R.-R., C.T.-P., and J.M.P.-S.; writing—original draft preparation, M.R.-R.; writing—review and editing, M.R.-R., C.T.-P., J.M.P.-S., A.M.M.-P., M.M., E.V., A.B., and M.M.-B.; supervision, J.M.P.-S., A.M.M.-P., and M.M.-B.; project administration, E.G., A.B., and M.M.-B.; funding acquisition, E.G., A.B., and M.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FEDER-INNTERCONECTA-CDTI program (CDTI, Spanish Ministry of Economy and Competitiveness; MBIOPOR Project). M.R.-R was funded by Programa Operativo de Empleo Juvenil (Fondo Social Europeo, Junta de Andalucia, Ref 6127). J.M.P.-S was economically financed by Proyectos de Excelencia 2011-Junta de Andalucía (Project No: RNM-8147).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and ethical review and approval were waived for this study. This study was carried out in accordance with the national regulations and the European directive for the protection of animal welfare in research (Directive 2010/63/EU, European Commission, 2010). The experiment was performed at IMASDE AGROALIMENTARIA S.L. in Granja La Mata (Mata de Cuellar, Segovia, Spain). This farm has experimental authorization (Ref No: B-82334855). Gut samples were collected in the course of the regular farm and slaughtering procedures in "El cochinillo segoviano" S.L. (Boceguillas, Segovia, Spain).
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences are available in the Genbank-NCBI Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra/), BioProject: PRJNA664026, Accession Nos. SAMN16192455 to SAMN16192544.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillips I., Casewell M., Cox T., De Groot B., Friis C., Jones R., Nightingale C., Preston R., Waddell J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2003;53:28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- 2.A Thacker P. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013;4:35. doi: 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capita R., Alonso-Calleja C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013;53:11–48. doi: 10.1080/10408398.2010.519837. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Antimicrobial Resistance: Global Report on Surveillance. WHO; Geneva, Switzerland: 2014. pp. 1–257. [Google Scholar]
- 5.U.S. FDA . FDA Reminds Retail Establishments of Upcoming Changes to the Use of Antibiotics in Food Animals. U.S. FDA; Silver Spring, MA, USA: 2016. [Google Scholar]
- 6.Maron D.F., Smith T.J.S., E Nachman K. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health. 2013;9:48. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casewell M., Friis C., Marco E., McMullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- 8.Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013;4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietze K. Pigs for Prosperity (FAO Diversification Booklet 15) U.S. FAO; Hot Springs, VA, USA: 2011. [Google Scholar]
- 10.Kim H.B., Borewicz K., White B.A., Singer R.S., Sreevatsan S., Tu Z.J., Isaacson R.E. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc. Natl. Acad. Sci. USA. 2012;109:15485–15490. doi: 10.1073/pnas.1205147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 12.Gaskins H.R., Collier C.T., Anderson D.B. Antibiotics as growth promotants: Mode of action. Anim. Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 13.Niewold T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- 14.Barton M.D. Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 2014;19:9–15. doi: 10.1016/j.mib.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Faldynova M., Videnska P., Havlickova H., Sisak F., Juricova H., Babak V., Steinhauser L., Rychlik I. Prevalence of antibiotic resistance genes in faecal samples from cattle, pigs and poultry. Vet. Med. 2013;58:298–304. doi: 10.17221/6865-VETMED. [DOI] [Google Scholar]
- 16.Gerzova L., Babak V., Sedlar K., Faldynova M., Videnska P., Cejkova D., Jensen A.N., Denis M., Kerouanton A., Ricci A., et al. Characterization of antibiotic resistance gene abundance and microbiota composition in feces of organic and conventional pigs from four EU countries. PLoS ONE. 2015;10:e0132892. doi: 10.1371/journal.pone.0132892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes-Neves E., Antunes P., Manageiro V., Gartner F., Caniça M., Da Costa J.M.C., Peixe L. Clinically relevant multidrug resistant Salmonella enterica in swine and meat handlers at the abattoir. Vet. Microbiol. 2014;168:229–233. doi: 10.1016/j.vetmic.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Peeters L.E., Argudín M.A., Azadikhah S., Butaye P., Regueiro M. Ángeles A. Antimicrobial resistance and population structure of Staphylococcus aureus recovered from pigs farms. Vet. Microbiol. 2015;180:151–156. doi: 10.1016/j.vetmic.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Thakur S., Gebreyes W.A. Prevalence and antimicrobial resistance of Campylobacter in antimicrobial-free and conventional pig production systems. J. Food Prot. 2005;68:2402–2410. doi: 10.4315/0362-028X-68.11.2402. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Espinosa C.D., Abelilla J.J., Casas G.A., Lagos L.V., Lee S.A., Kwon W.B., Mathai J.K., Navarro D.M., Jaworski N.W., et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- 22.Li P., Piao X., Ru Y., Han X., Xue L., Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian Australas. J. Anim. Sci. 2012;25:1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragland D., Stevenson D., Hill M.A. Oregano oil and multi-component carbohydrases as alternatives to antimicrobials in nursery diets. J. Swine Health Prod. 2008;16:238–243. [Google Scholar]
- 24.Maenner K., Vahjen W., Simon O. Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. J. Anim. Sci. 2011;89:2106–2112. doi: 10.2527/jas.2010-2950. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed S.T., Hossain M.E., Kim G.M., Hwang J.A., Ji H., Yang C.J. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian Australas. J. Anim. Sci. 2013;26:683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Yoo J.S., Kim H.J., Wang Y., Chen Y.J., Cho J.H., Kim I.H. Effects of dietary supplementation with blended essential oils on growth performance, nutrient digestibility, blood profiles and fecal characteristics in weanling pigs. Asian Australas. J. Anim. Sci. 2010;23:607–613. doi: 10.5713/ajas.2010.80120. [DOI] [Google Scholar]
- 27.Liu Y., Song M., Che T.M., Almeida J.A.S., Lee J.J., Bravo D., Maddox C.W., Pettigrew J.E. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 2013;91:5294–5306. doi: 10.2527/jas.2012-6194. [DOI] [PubMed] [Google Scholar]
- 28.Harris J.C., Cottrell S.L., Plummer S., Lloyd D. Antimicrobial properties of Allium sativum (garlic) Appl. Microbiol. Biotechnol. 2001;57:282–286. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- 29.Tatara M.R., Sliwa E., Dudek K., Gawron A., Piersiak T., Dobrowolski P., Mosiewicz J., Siwicki A., Studzinski T. Aged garlic extract and allicin improve performance and gastrointestinal tract development of piglets reared in artificial sow. Ann. Agric. Environ. Med. 2008;15:63–69. [PubMed] [Google Scholar]
- 30.Ruiz R., García M.P., Lara A., Rubio L.A. Garlic derivatives (PTS and PTS-O) differently affect the ecology of swine faecal microbiota in vitro. Vet. Microbiol. 2010;144:110–117. doi: 10.1016/j.vetmic.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Peinado M.J., Ruiz R., Echávarri A., Rubio L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012;91:2148–2157. doi: 10.3382/ps.2012-02280. [DOI] [PubMed] [Google Scholar]
- 32.Sorlozano-Puerto A., Albertuz-Crespo M., Lopez-Machado I., Ariza-Romero J.J., Baños-Arjona A., Exposito-Ruiz M., Gutierrez-Fernandez J. In vitro antibacterial activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate derived from allium spp. against gram-negative and gram-positive multidrug-resistant bacteria isolated from human samples. BioMed Res. Int. 2018;2018:7861207. doi: 10.1155/2018/7861207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peinado M., Ruiz R., Echávarri A., Aranda-Olmedo I., Rubio L. Garlic derivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed. Sci. Technol. 2013;181:87–92. doi: 10.1016/j.anifeedsci.2013.03.001. [DOI] [Google Scholar]
- 34.Hawkins T. DNA Purification and Isolation Using Magnetic Particles. 5,705,628. U.S. Patent. 1998 Jan 6;
- 35.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 38.Amir A., McDonald D., Navas-Molina J.A., Kopylova E., Morton J.T., Zech Xu Z., Kightley E.P., Thompson L.R., Hyde E.R., Gonzalez A., et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems. 2017;2:e00191-16. doi: 10.1128/mSystems.00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssen S., McDonald D., Gonzalez A., Navas-Molina J.A., Jiang L., Xu Z.Z., Winker K., Kado D.M., Orwoll E., Manary M., et al. Phylogenetic placement of exact amplicon sequences improves associations with clinical information. mSystems. 2018;3:e00021-18. doi: 10.1128/mSystems.00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faith D.P., Baker A.M. Phylogenetic diversity (PD) and biodiversity conservation: Some bioinformatics challenges. Evol. Bioinform. 2006;2:121–128. doi: 10.1177/117693430600200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lozupone C., Knight R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lozupone C.A., Hamady M., Kelley S.T., Knight R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collyer M.L., Sekora D.J., Adams D.C. A method for analysis of phenotypic change for phenotypes described by high-dimensional data. Hered. 2015;115:357–365. doi: 10.1038/hdy.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vázquez-Baeza Y., Pirrung M., Gonzalez A., Knight R. EMPeror: A tool for visualizing high-throughput microbial community data. GigaScience. 2013;2:16. doi: 10.1186/2047-217X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soler C., Goossens T., Bermejo A., Migura-García L., Cusco A., Francino O., Fraile L. Digestive microbiota is different in pigs receiving antimicrobials or a feed additive during the nursery period. PLoS ONE. 2018;13:e0197353. doi: 10.1371/journal.pone.0197353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng C., Xia M., Zhang X., Wang C., Jiang S., Peng J. Supplementing oregano essential oil in a reduced-protein diet improves growth performance and nutrient digestibility by modulating intestinal bacteria, intestinal morphology, and antioxidative capacity of growing-finishing pigs. Animals. 2018;8:159. doi: 10.3390/ani8090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resano H., Perez-Cueto F.J., De Barcellos M.D., Veflen-Olsen N., Grunert K.G., Verbeke W. Consumer satisfaction with pork meat and derived products in five European countries. Appetite. 2011;56:167–170. doi: 10.1016/j.appet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Hanczakowska E., Świątkiewicz M., Grela E.R. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. 2015;108:61–66. doi: 10.1016/j.meatsci.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Hernández F., Madrid J., García V., Orengo J., Megías M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- 51.Hanczakowska E., Swiatkiewicz M. Effect of herbal extracts on piglet performance and small intestinal epithelial villi. Czech J. Anim. Sci. 2012;57:420–429. doi: 10.17221/6316-CJAS. [DOI] [Google Scholar]
- 52.Nowak P., Kasprowicz-Potocka M., Zaworska A., Nowak W., Stefańska B., Sip A., Grajek W., Juzwa W., Taciak M., Barszcz M., et al. The effect of eubiotic feed additives on the performance of growing pigs and the activity of intestinal microflora. Arch. Anim. Nutr. 2017;71:455–469. doi: 10.1080/1745039X.2017.1390181. [DOI] [PubMed] [Google Scholar]
- 53.De Rodas B., Youmans B.P., Danzeisen J.L., Tran H., Johnson T.J. Microbiome profiling of commercial pigs from farrow to finish. J. Anim. Sci. 2018;96:1778–1794. doi: 10.1093/jas/sky109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Y., Kong F., Xiang Y., Zhou W., Wang J., Yang H., Zhang G., Zhao J. Comparative biogeography of the gut microbiome between Jinhua and Landrace pigs. Sci. Rep. 2018;8:5985. doi: 10.1038/s41598-018-24289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H., Huang X., Fang S., Xin W., Huang L., Chen C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci. Rep. 2016;6:27427. doi: 10.1038/srep27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L., Wu W., Lee Y.-K., Xie J., Zhang H. Spatial Heterogeneity and Co-occurrence of Mucosal and Luminal Microbiome across Swine Intestinal Tract. Front. Microbiol. 2018;9:48. doi: 10.3389/fmicb.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park S.-J., Kim J., Lee J.-S., Rhee S.-K., Kim H. Characterization of the fecal microbiome in different swine groups by high-throughput sequencing. Anaerobe. 2014;28:157–162. doi: 10.1016/j.anaerobe.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Gong L., Wu Y.-P., Cui Z.-W., Wang Y.-Q., Huang Y., Zhang X.-P., Li W.-F. Oral administration of Lactobacillus rhamnosus GG to newborn piglets augments gut barrier function in pre-weaning piglets. J. Zhejiang Univ. Sci. B. 2019;20:180–192. doi: 10.1631/jzus.B1800022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warren F.J., Fukuma N.M., Mikkelsen D., Flanagan B.M., Williams B.A., Lisle A.T., Cuív P.Ó., Morrison M., Gidley M.J. Food starch structure impacts gut microbiome composition. mSphere. 2018;3:e00086-18. doi: 10.1128/mSphere.00086-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman S.U., Khan S., Chand N., Sadique U., Khan R.U. In vivo effects of Allium cepa L. on the selected gut microflora and intestinal histomorphology in broiler. Acta Histochem. 2017;119:446–450. doi: 10.1016/j.acthis.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Sánchez C.J., Martínez-Miró S., Ariza J.J., Madrid J., Orengo J., Aguinaga M.A., Baños A., Hernández F. Effect of Alliaceae extract supplementation on performance and intestinal microbiota of growing-finishing pig. Animals. 2020;10:1557. doi: 10.3390/ani10091557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Looft T., Allen H.K., Cantarel B.L., Levine U.Y., O Bayles D., Alt D.P., Henrissat B., Stanton T.B. Bacteria, phages and pigs: The effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 2014;8:1566–1576. doi: 10.1038/ismej.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abad P., Arroyo-Manzanares N., Rivas-Montoya E., Ochando-Pulido J.M., Guillamon E., Garcia-Campaña A.M., Martinez-Ferez A. Effects of different vehiculization strategies for the allium derivative propyl propane thiosulfonate during dynamic simulation of the pig gastrointestinal tract. Can. J. Anim. Sci. 2019;99:244–253. doi: 10.1139/cjas-2018-0063. [DOI] [Google Scholar]
- 64.Menni C., A Jackson M., Pallister T., Steves C.J., Spector T.D., Valdes A.M. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 2017;41:1099–1105. doi: 10.1038/ijo.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A core gut microbiome in obese and lean twins. Nature. 2008;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters B.A., Shapiro J.A., Church T.R., Miller G., Trinh-Shevrin C., Yuen E., Friedlander C., Hayes R.B., Ahn J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-28126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han G.G., Lee J.-Y., Jin G.-D., Park J., Choi Y.H., Chae B.J., Kim E.B., Choi Y.-J. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl. Microbiol. Biotechnol. 2017;101:5903–5911. doi: 10.1007/s00253-017-8304-7. [DOI] [PubMed] [Google Scholar]
- 68.Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences are available in the Genbank-NCBI Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra/), BioProject: PRJNA664026, Accession Nos. SAMN16192455 to SAMN16192544.