Abstract
Neuroscience research links alexithymia, the difficulty in identifying and describing feelings and emotions, with left hemisphere dominance and/or right hemisphere deficit. To provide behavioral evidence for this neuroscientific hypothesis, we explored the relationship between alexithymia and performance in a line bisection task, a standard method for evaluating visuospatial processing in relation to right hemisphere functioning. We enrolled 222 healthy participants who completed a version of the 20-item Toronto Alexithymia Scale (TAS-20), which measures alexithymia, and were asked to mark (bisect) the center of a 10-cm horizontal segment. The results document a significant rightward shift in the center of the line in participants with borderline and manifest alexithymia compared with non-alexithymic individuals. The higher the TAS-20 score, the greater the rightward shift in the line bisection task. This finding supports the right hemisphere deficit hypothesis in alexithymia and suggests that visuospatial abnormalities may be an important component of this mental condition.
Keywords: alexithymia, right hemisphere, line bisection, pseudoneglect
1. Introduction
Alexithymia is a stable personality characteristic [1,2] characterized by a disturbance of affective-emotional processing, which causes difficulties in verbally identifying and describing feelings and emotions [3]. Research in the field reports an alexithymia prevalence rate of 13% in the non-clinical population, where the alexithymia rate appears to be nearly double in men (17%) compared with women (10%) [4]. In terms of impact, alexithymia may be associated with somatic sensations that accompany emotional arousal and may be related to somatic diseases such as inflammatory bowel disease and type 2 diabetes mellitus [5,6]. It is frequently associated with various psychopathological conditions such as depression [7], anxiety [8], and addiction [9], where the prevalence rate is higher than 30% [10].
Research in cognitive neuroscience has provided a brain basis for this affective deficit, showing that alexithymia can be characterized by left hemisphere dominance and/or right hemisphere deficit [11]. Neuroimaging investigations have shown a broad network of cortical and subcortical regions, including regions that are outside the canonical circuit of emotion processing.
This is the case for the parietal cortex, a cortical region involved in several cognitive abilities including spatial attention [12] and magnitude processing [13,14,15]. For example, Kano et al. [16] reported that alexithymics show less activation in the right inferior parietal cortex (and right prefrontal cortex) and greater activation in the left inferior parietal cortex compared with non-alexithymic individuals. A negative correlation was found between the severity of alexithymia and the activation of the right inferior parietal lobe [17]. More recently, Imperatori et al. [18] found a decrease in alpha connectivity between the right parietal lobe and the right temporal lobe.
In the literature, a lesion in the right inferior-parietal cortex was reported to be frequently associated with hemispatial neglect [19], a syndrome characterized by reduced or absent awareness for the contralesional space (i.e., the left spatial side) and an attentional bias to the right side. Similar deficits in spatial processing were found for lesions in the right frontal lobe (e.g., [20]).
An ideal test for assessing the visuospatial and attentional deficits described above [21] is the line bisection task. Over the years, this task has become increasingly popular as a diagnostic tool due to its ease of use and the relative ease with which results are interpreted [22]. The line bisection task requires participants to identify and mark the center of a straight line printed on a piece of paper. Patients with a lesion in the right hemisphere, such as patients with hemispatial neglect, identify and mark the center of the segment in the direction of the damaged hemisphere (i.e., the right hemisphere) [23].
In contrast to this spatial attention bias to the right side, neurologically normal individuals generally shift to the left of the true center when dividing horizontal lines [24]. This behavioral phenomenon, called pseudoneglect [25], has been associated with a dominant right hemisphere, which includes regions of the parietal and frontal lobes such as the intraparietal sulcus [26] and the inferior frontal gyrus [27].
Given the close relationship between the activation level of the right fronto-parietal network, the performance in the line bisection task, and evidence of the hypoactivation of the right fronto-parietal cortex in alexithymia, we aimed to test whether this personality trait [1] predicts spatial representation in healthy individuals. In agreement with the right hemisphere deficit hypothesis in alexithymia [11], we predicted a reduced or even absent pseudoneglect in individuals with this mental condition.
2. Participants
In total, 225 healthy participants (college students) participated in this study (113 women, age range between 18 and 30 years). The average age was 23.58 years ± 2.98 standard deviation (SD). All participants provided their written informed consent prior to inclusion in the study and were naïve about its purpose. The data were anonymously collected. The study was approved by the local ethics board (COSPECS Department, approval code: COSPECS_10_2020) and was conducted in agreement with the principles of the Declaration of Helsinki.
3. Materials and Measures
3.1. The 20-Item Toronto Alexithymia Scale (TAS-20)
The TAS-20 is a self-report scale used to measure alexithymia. It is composed of three subscales: (a) difficulty with identifying feelings, (b) difficulty with communicating feelings to others, and (c) external-oriented thought. Bagby et al. (1994) [28] proposed three cut-off scores for the classification of individuals: alexithymic subjects (≥61), borderline (score range between 51 to 60), and non-alexithymic subjects (≤50). In this study, the Italian version of the Toronto Alexithymia Scale was used, which was validated by Bressi et al. [29] (1996, Cronbach’s alpha: 0.75).
3.2. Line Bisection Task
Participants were asked to sit in a chair and use their preferred hand to mark the center of a single 10-cm horizontal line printed on a A4 sheet with a pen. The sheet was placed on a desk in front of the participant. They were asked to bisect the line within a few seconds, and were not allowed to move the sheet at will. The line bisection task was always performed after the completion of the TAS-20. Participants were tested in a quiet room located in the Faculty of Human and Social Sciences, UKE-Kore University of Enna, and in the department of Cognitive Sciences, University of Messina. Scores were measured manually. Pseudoneglect was indicated by line bisection scores less than 5 cm.
3.3. Data Analysis
Statistical analysis was performed using STATISTICA (StatSoft. Inc., Tulsa, OK, USA) version 8.0. Data were entered into a one-way ANOVA to identify differences between the three groups (alexithymic, borderline, and non-alexithymic) in bisection line performance and demographic variables. Student’s t-tests (Bonferroni corrected) were conducted in the case of significant ANOVA results. Additional Student’s t-tests were performed to verify significant shifts from the real center of the presented segment. Finally, Pearson’s correlation analysis was performed to investigate the association between TAS-20 scores and bisection line performance. For all analyses, the statistical significance level was set to p < 0.05.
4. Results
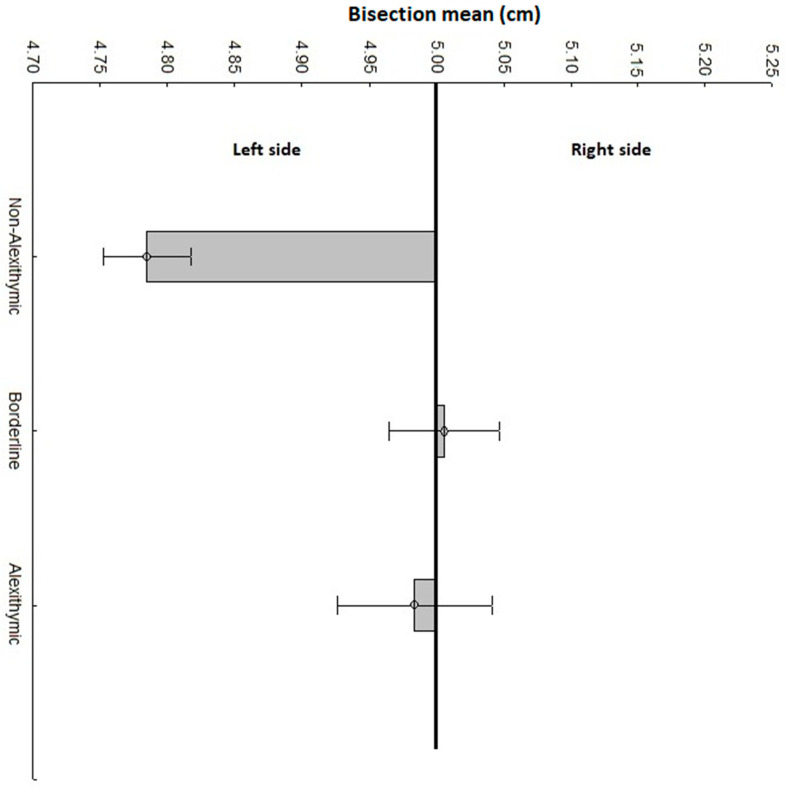
Three participants were removed from the final analysis as their bisection line performance was ±3 SD from the average. Our final sample was composed of N = 37 alexithymic (TAS-20 score: M = 66.48 ± 0.927), N = 72 borderline (TAS-20 score: M = 55.44 ± 0.664), and N = 113 non-alexithymic (TAS-20 score: M = 39.29 ± 0.927) participants. The TAS-20 score was statistically different between the three groups (F(2, 219) = 392, p < 0.001, ηp2 = 0.781). No significant difference was reported for the sex (F(2, 219) = 1.99, p = 0.138) or age (F(2, 219) = 0.403, p = 0.668) variables between the three groups. The ANOVA documented a significant group difference in bisection line performance (F(2, 219) = 10.46, p < 0.001, ηp2 = 0.087). Post-hoc comparison documented a lower line bisection score in non-alexithymic participants (M = 4.784 ± 0.032 cm) compared with borderline (M = 5.005 ± 0.041 cm, p < 0.001) and alexithymic (M = 4.983 ± 0.057 cm, p = 0.008) participants. No difference was identified between borderline and alexithymic participants (p = 1.000). See Figure 1 for details. One sample t-test against the real center of the segment (i.e., 5 cm) documented a significant difference for non-alexithymic participants (t(112) = −6.060, p < 0.001). This result confirms pseudoneglect in non-alexithymic participants. On the other hand, no difference was reported for borderline (t(71) = 0.146, p = 0.883) or alexithymic (t(36) = −0.325, p = 0.756) participants.
Figure 1.
Performance in the line bisection task of alexithymic, borderline, and non-alexithymic participants. Deviations from the central vertical line indicate the mean bisection shift of the three groups of participants for the 10-cm segment. Scores less than 5 cm indicate a deviation to the left (i.e., pseudoneglect). Scores greater than 5 cm indicate a deviation to the right. Vertical bars indicate standard error.
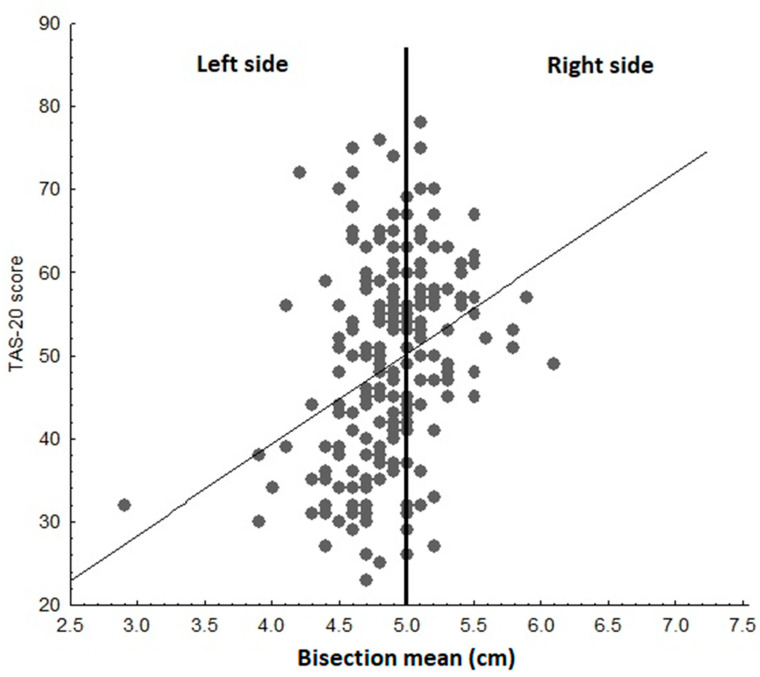
Correlation analysis provided further support to the ANOVA results. We found a positive correlation between TAS-20 and line bisection scores (r = 0.329, p < 0.001). Therefore, the higher the TAS-20 score, the larger the rightward shift in the line bisection task (Figure 2).
Figure 2.
A plot of the 20-item Toronto Alexithymia Scale (TAS-20) scores and line bisection performance of all participants. The figure shows a positive correlation between TAS-20 scores and rightward bias.
5. Discussion
Motivated by neuroimaging evidence [16,17] of reduced activation in the right fronto-parietal network in alexithymia, here, we used the line bisection task to provide behavioral evidence to this functional asymmetry. As expected, the results of our sub-group of non-alexithymic participants replicate the pseudoneglect bias (i.e., leftward shift in line bisection) reported in previous studies on neurologically normal individuals [24]. We also confirmed our research hypothesis of no pseudoneglect in participants classified as overtly alexithymic compared with non-alexithymic participants. Interestingly, the absence of pseudoneglect was also confirmed for participants with borderline TAS-20 scores. This result is also corroborated by the overall positive correlation between TAS-20 and rightward bias. Therefore, the greater the severity of the alexithymia trait, the greater the tendency to mark the center on the right side of the presented segment.
Overall, our results are in line with neuroimaging evidence of reduced right fronto-parietal activation in this mental condition and, more generally, with models proposing a left hemisphere dominance and/or a right hemisphere deficit [11] in alexithymia.
Evidence of reduced or even absent pseudoneglect in alexithymia provides new insights into understanding this mental condition, as it suggests that this personality trait is also characterized by non-emotional features. It adds new evidence to the results of altered cognitive processing in alexithymia [30] by documenting an abnormal representation of space in this mental condition.
Although we investigated the relationship between alexithymia and line bisection in healthy participants, the results of our study may be useful for the interpretation of altered pseudoneglect (i.e., rightward bias) in psychopathology and mental illnesses such as schizophrenia and attention deficit hyperactivity disorder (ADHD) [31]. Schizophrenia is a severe psychiatric illness frequently associated with alexithymia [32] and there is evidence of a link between alexithymia and ADHD [33]. Evidence of a rightward bias in healthy individuals with borderline/manifest alexithymia in the absence of psychiatric conditions suggests that the origin of the rightward shift in the clinical populations mentioned above (i.e., schizophrenia and ADHD) may be linked, at least in part, with this personality trait. This could question the suggestion that an alteration in pseudoneglect should be considered an endophenotype of schizophrenia [34]. However, this question remains to be investigated, as no research has explored the mediating role of the alexithymia trait in the line bisection performance of the aforementioned clinical populations. Further investigation in the field is needed to explore this hypothesis in dedicated studies.
In conclusion, this is the first evidence of abnormal spatial representation in individuals with borderline and manifest alexithymia. In future investigations, it would be interesting to address the role of handedness, which was not considered in the present study. It would also be important to explore the existence of any dissociation between near and far space in the line bisection task, given the evidence that distinct neural networks are involved in the bisection of lines placed in the near and far space (i.e., the dorsal stream for the bisection of lines placed in the near space and the ventral stream for the bisection of lines placed in the far space [35].
Finally, given the close relationship between space and numbers [36,37,38,39], as well as between space and time [40,41,42,43], it would be intriguing to explore the bisection of time and numbers in alexithymia, as a pseudoneglect-like effect for this information has been documented in healthy individuals [44,45].
Author Contributions
Conceptualization, C.M.V., G.M., G.C.; methodology, C.M.V.; software, C.M.V.; formal analysis, C.M.V.; investigation, G.C., A.M.; writing—original draft preparation, C.M.V.; writing—review and editing, C.M.V., G.M., G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of COSPECS Department (protocol code COSPECS_10_2020. Approval date 2 September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data access is granted by forwarding a request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor G.J., Bagby R.M. The Alexithymia Personality Dimension. In: Widiger T.A., editor. Oxford Library of Psychology. The Oxford Handbook of Personality Disorders. Oxford University Press; Oxford, UK: 2012. pp. 648–673. [DOI] [Google Scholar]
- 2.Martínez-Sánchez F., Ato-García M., Ortiz-Soria B. Alexithymia--state or trait? Span. J. Psychol. 2003;6:51–59. doi: 10.1017/S1138741600005205. [DOI] [PubMed] [Google Scholar]
- 3.Craparo G., Gori A., Dell’Aera S., Costanzo G., Fasciano S., Tomasello A., Vicario C.M. Impaired emotion recognition is linked to alexithymia in heroin addicts. PeerJ. 2016;4:e1864. doi: 10.7717/peerj.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salminen J.K., Saarijärvi S., Äärelä E., Toikka T., Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of finland. J. Psychosom. Res. 1999;46:75–82. doi: 10.1016/S0022-3999(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 5.Martino G., Caputo A., Schwarz P., Bellone F., Fries W., Quattropani M.C., Vicario C.M. Alexithymia and Inflammatory Bowel Disease: A Systematic Review. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martino G., Caputo A., Vicario C.M., Catalano A., Schwarz P., Quattropani M.C. The Relationship Between Alexithymia and Type 2 Diabetes: A Systematic Review. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honkalampi K., Hintikka J., Laukkanen E., Viinamäki J.L.H. Alexithymia and Depression: A Prospective Study of Patients with Major Depressive Disorder. J. Psychosom. Res. 2001;42:229–234. doi: 10.1176/appi.psy.42.3.229. [DOI] [PubMed] [Google Scholar]
- 8.Palser E.R., Palmer C.E., Galvez-Pol A., Hannah R., Fotopoulou A., Kilner J.M. Alexithymia mediates the relationship between intero-ceptive sensibility and anxiety. PLoS ONE. 2018;13:e0203212. doi: 10.1371/journal.pone.0203212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coriale G., Bilotta E., Leone L., Cosimi F., Porrari R., De Rosa F., Ceccanti M. Avoidance coping strategies, alexithymia and alcohol abuse: A mediation analysis. Addict. Behav. 2012;37:1224–1229. doi: 10.1016/j.addbeh.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Çelikel F.Ç., Kose S., Erkorkmaz U., Sayar K., Cumurcu B.E., Cloninger C.R. Alexithymia and temperament and character model of personality in patients with major depressive disorder. Compr. Psychiatry. 2010;51:64–70. doi: 10.1016/j.comppsych.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Bermond B., Vorst H.C.M., Moormann P.P. Cognitive neuropsychology of alexithymia: Implications for personality typology. Cogn. Neuropsychiatry. 2006;11:332–360. doi: 10.1080/13546800500368607. [DOI] [PubMed] [Google Scholar]
- 12.Shomstein S. Cognitive functions of the posterior parietal cortex: Top-down and bottom-up attentional control. Front. Integr. Neurosci. 2012;6:38. doi: 10.3389/fnint.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh V. A theory of magnitude: Common cortical metrics of time, space and quantity. Trends Cogn. Sci. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Vicario C.M., Rappo G., Pepi A., Pavan A., Martino D. Temporal Abnormalities in Children with Developmental Dyscalculia. Dev. Neuropsychol. 2012;37:636–652. doi: 10.1080/87565641.2012.702827. [DOI] [PubMed] [Google Scholar]
- 15.Vicario C., Martino D., Koch G. Temporal accuracy and variability in the left and right posterior parietal cortex. Neuroscience. 2013;245:121–128. doi: 10.1016/j.neuroscience.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Kano M., Fukudo S., Gyoba J., Kamachi M., Tagawa M., Mochizuki H., Itoh M., Hongo M., Yanai K. Specific brain processing of facial expressions in people with alexithymia: An H215O-PET study. Brain. 2003;126:1474–1484. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- 17.Sturm V.E., Levenson R.W. Alexithymia in neurodegenerative disease. Neurocase. 2011;17:242–250. doi: 10.1080/13554794.2010.532503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imperatori C., Della Marca G., Brunetti R., Carbone G.A., Massullo C., Valenti E.M., Amoroso N., Maestoso G., Contardi A., Farina B. Default Mode Network alterations in alexithymia: An EEG power spectra and connectivity study. Sci. Rep. 2016;15:36653. doi: 10.1038/srep36653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain M., Nachev P. Space and the parietal cortex. Trends Cogn. Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeshima S., Funahashi K., Ogura M., Itakura T., Komai N. Unilateral spatial neglect due to right frontal lobe haematoma. J. Neurol. Neurosurg. Psychiatry. 1994;57:89–93. doi: 10.1136/jnnp.57.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer M.H. Cognition in the bisection task. Trends Cogn. Sci. 2001;5:460–462. doi: 10.1016/S1364-6613(00)01790-3. [DOI] [PubMed] [Google Scholar]
- 22.Wilson B., Cockburn J., Halligan P. Behavioural Inattention Test. Thames Valley Test Company; Titchfield, UK: 1987. [Google Scholar]
- 23.Albert M.L. A simple test of visual neglect. Neurology. 1971;23:658–664. doi: 10.1212/WNL.23.6.658. [DOI] [PubMed] [Google Scholar]
- 24.Jewell G., McCourt M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/S0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 25.Bowers D., Heilman K.M. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychol. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Lee A.C., O’Neil E.B., Abdul-Nabi M., Niemeier M. Mapping the anatomy of perceptual pseudoneglect. A multivariate approach. NeuroImage. 2020;207:116402. doi: 10.1016/j.neuroimage.2019.116402. [DOI] [PubMed] [Google Scholar]
- 27.Zago L., Petit L., Jobard G., Hay J., Mazoyer B., Tzourio-Mazoyer N., Karnath H.-O., Mellet E. Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial attention dominance in right-handers. Neuropsychol. 2017;94:75–83. doi: 10.1016/j.neuropsychologia.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Bagby R.M., Taylor G.J., Parker J.D.A. The twenty-item Toronto Alexithymia Scale. II. Convergent, discriminant, and concurrent validity. J. Psychiatr. Res. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 29.Bressi C., Taylor G.J., Parker J.D.A., Bressi S., Brambilla V., Aguglia E., Allegranti S., Bongiorno A., Giberti F., Bucca M., et al. Cross validation of the factor structure of the 20-Item Toronto Alexithymia Scale: An Italian multicenter study. J. Psychosom. Res. 1996;41:551–559. doi: 10.1016/S0022-3999(96)00228-0. [DOI] [PubMed] [Google Scholar]
- 30.Koven N.S., Thomas W. Mapping facets of alexithymia to executive dysfunction in daily life. Pers. Individ. Differ. 2010;49:24–28. doi: 10.1016/j.paid.2010.02.034. [DOI] [Google Scholar]
- 31.Ribolsi M., Di Lorenzo G., Lisi G., Niolu C., Siracusano A. A critical review and meta-analysis of the perceptual pseudoneglect across psychiatric disorders: Is there a continuum? Cogn. Process. 2015;16:17–25. doi: 10.1007/s10339-014-0640-2. [DOI] [PubMed] [Google Scholar]
- 32.O’Driscoll C., Laing J., Mason O. Cognitive emotion regulation strategies, alexithymia and dissociation in schizophrenia, a review and meta-analysis. Clin. Psychol. Rev. 2014;34:482–495. doi: 10.1016/j.cpr.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Donfrancesco R., Di Trani M., Gregori P., Auguanno G., Melegari M.G., Zaninotto S., Luby J. Attention-deficit/hyperactivity disorder and alexithymia: A pilot study. Atten. Deficit Hyperact. Disord. 2013;5:361–367. doi: 10.1007/s12402-013-0115-9. [DOI] [PubMed] [Google Scholar]
- 34.Ribolsi M., Lisi G., Di Lorenzo G., Koch G., Oliveri M., Magni V., Pezzarossa B., Saya A., Rociola G., Rubino I.A., et al. Perceptual Pseudoneglect in Schizophrenia: Candidate Endophenotype and the Role of the Right Parietal Cortex. Schizophr. Bull. 2012;39:601–607. doi: 10.1093/schbul/sbs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss P.H., Marshall J.C., Wunderlich G., Tellmann L., Halligan P.W., Freund H.-J., Zilles K., Fink G.R. Neural consequences of acting in near versus far space: A physiological basis for clinical dissociations. Brain. 2000;123:2531–2541. doi: 10.1093/brain/123.12.2531. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard E.M., Piazza M., Pinel P., Dehaene S. Interactions between number and space in parietal cortex. Nat. Rev. Neurosci. 2005;6:435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- 37.Dehaene S., Brannon E.M. Space, time, and number: A Kantian research program. Trends Cogn. Sci. 2010;14:517–519. doi: 10.1016/j.tics.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Vicario C.M., Martino D. The neurophysiology of magnitude: One example of extraction analogies. Cogn. Neurosci. 2010;1:144–145. doi: 10.1080/17588921003763969. [DOI] [PubMed] [Google Scholar]
- 39.Vicario C.M. Perceiving Numbers Affects the Internal Random Movements Generator. Sci. World J. 2012;2012:1–6. doi: 10.1100/2012/347068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicario C.M., Pavone E.F., Martino D., Fuggetta G. Lateral Head Turning Affects Temporal Memory. Percept. Mot. Ski. 2011;113:3–10. doi: 10.2466/04.22.PMS.113.4.3-10. [DOI] [PubMed] [Google Scholar]
- 41.Vicario C.M., Rappo G., Pepi A.M., Oliveri M. Timing Flickers across Sensory Modalities. Perception. 2009;38:1144–1151. doi: 10.1068/p6362. [DOI] [PubMed] [Google Scholar]
- 42.Vicario C.M., Bonní S., Koch G. Left hand dominance affects supra-second time processing. Front. Integr. Neurosci. 2011;5:65. doi: 10.3389/fnint.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicario C.M., Yates M.J., Nicholls M.E.R. Shared deficits in space, time, and quantity processing in childhood genetic disorders. Front. Psychol. 2013;4:43. doi: 10.3389/fpsyg.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loftus A.M., Nicholls M.E.R., Mattingley J.B., Chapman H.L., Bradshaw J.L. Pseudoneglect for the Bisection of Mental Number Lines. Q. J. Exp. Psychol. 2009;62:925–945. doi: 10.1080/17470210802305318. [DOI] [PubMed] [Google Scholar]
- 45.Schwan N., Brugger P., Huberle E. Spatial Representation of Time in Backspace. Timing Time Percept. 2018;6:154–168. doi: 10.1163/22134468-20181120. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data access is granted by forwarding a request to the corresponding author.