Abstract
Diagnosis of HIV-associated neurocognitive impairment (NCI) continues to be a clinical challenge. The purpose of this study was to develop a prediction model for NCI among people with HIV using clinical- and magnetic resonance imaging (MRI)-derived features. The sample included 101 adults with chronic HIV disease. NCI was determined using a standardized neuropsychological testing battery comprised of seven domains. MRI features included gray matter volume from high-resolution anatomical scans and white matter integrity from diffusion-weighted imaging. Clinical features included demographics, substance use, and routine laboratory tests. Least Absolute Shrinkage and Selection Operator Logistic regression was used to perform variable selection on MRI features. These features were subsequently used to train a support vector machine (SVM) to predict NCI. Three different classification tasks were performed: one used only clinical features; a second used only selected MRI features; a third used both clinical and selected MRI features. Model performance was evaluated by area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, and specificity with a tenfold cross-validation. The SVM classifier that combined selected MRI with clinical features outperformed the model using clinical features or MRI features alone (AUC: 0.83 vs. 0.62 vs. 0.79; accuracy: 0.80 vs. 0.65 vs. 0.72; sensitivity: 0.86 vs. 0.85 vs. 0.86; specificity: 0.71 vs. 0.37 vs. 0.52). Our results provide preliminary evidence that combining clinical and MRI features can increase accuracy in predicting NCI and could be developed as a potential tool for NCI diagnosis in HIV clinical practice.
Keywords: Neurocognitive impairment, People with HIV, MRI, Machine learning, Prediction
Introduction
Although widespread use of combination antiretroviral therapy (cART) has prolonged the survival of people with HIV, neurocognitive impairment (NCI) continues to be a prevalent complication. It is estimated that about 20 to 50% of people with HIV experience NCI, and the prevalence of Asymptomatic Neurocognitive Impairment (ANI) and Mild Neurocognitive Disorder (MND) have increased in the cART era (Heaton et al. 2010; Kumar et al. 2019; Santos et al. 2019). The high risk of NCI among people with HIV may be due to irreversible central nervous system (CNS) injury before treatment, a so-called legacy effect, or a persistent reservoir of HIV infection and inflammation even when HIV RNA is undetectable in blood (Nightingale et al. 2014). NCI can interfere with the ability to perform important everyday activities and has been linked to HIV disease progression and increased risk of mortality (Banerjee et al. 2019; Belete et al. 2017; Grabyan et al. 2018; Heaton et al. 2004; Wagner et al. 2016).
While the diagnosis of HIV-associated neurocognitive disorder (HAND) is essential in the long-term clinical management of HIV, the identification of NCI continues to be a clinical challenge. HAND is diagnosed based on comprehensive neuropsychological testing that assesses performance in different cognitive domains and is defined by NCI in at least two domains (Antinori et al. 2007). In addition to requiring specialized training, neuropsychological testing batteries are relatively time-consuming to implement, language- and education-dependent, and susceptible to contextual influences (e.g., sleepiness, effort level, time of day) (Benloucif et al. 2004; Seelye et al. 2015).
Metrics of brain structure derived from magnetic resonance imaging (MRI) have great potential for predicting the diagnosis and prognosis of NCI (Zandvakili et al. 2019). Previous studies identified a wide distribution of gray matter (GM) atrophy in people with HIV (Israel et al. 2019; Kuper et al. 2011), with volume reduction in specific regions such as the frontal cortex and striatum linked to NCI (Israel et al. 2019). Diffusion-weighted imaging (DWI) can detect microstructure differences within white matter (WM) tracts, as captured by fractional anisotropy (FA). While NCI is associated with decreased FA values, the specific regions identified differ across studies (Cysique et al. 2017; Oh et al. 2018; Sexton et al. 2011). However, most of the studies were based on univariate analyses (e.g., t test and ANOVA) comparing unimodal imaging metrics between people with and without NCI. These approaches fail to make use of correlations among imaging metrics or to leverage across multimodal imaging metrics to improve predictive accuracy.
Machine learning methods that incorporate multiple imaging metrics and data sources may exhibit much greater accuracy in discriminating individuals with and without NCI. Machine learning algorithms have been used to predict brain disorders and recognize patterns of brain disease (Dyrba et al. 2015; Haller et al. 2010; Mansson et al. 2015; Zandvakili et al. 2019). There are two main approaches for implementing machine learning: unsupervised and supervised learning (Libbrecht et al. 2015). In unsupervised machine learning, characterized by clustering, algorithms seek to learn structure in the data without the benefit of labels (e.g., diagnoses). These approaches are commonly used to model the distribution or pattern of MRI data within the input space without prior knowledge (Chockanathan et al. 2018, 2019; DSouza et al. 2018; Tang et al. 2017a, 2017b; Zeng et al. 2014). In contrast, supervised learning attempts to predict a set of labels from other input variables. The support vector machine (SVM) is a type of supervised learning method that aims to optimally distinguish data points into separate groups in a high-dimensional space (Cortes and Vapnik 1995). The method has been successfully used to distinguish persons with and without neurocognitive disorder based on MRI data involving GM volume (Abdulkadir et al. 2011; Xiao et al. 2017) and DWI metrics (Haller et al. 2012; Zhou et al. 2018) across diseases, such as Alzheimer’s disease, schizophrenia, obsessive-compulsive disorder, and Parkinson’s disease. SVM also has been applied to the analysis of neuroimaging data in NeuroHIV research to identify anatomical and functional changes associated with NCI (Chockanathan et al. 2018, 2019; DSouza et al. 2018; Mansson et al. 2015; Tang et al. 2017a, 2017b). However, whether the addition of MRI features improves the prediction of NCI above machine learning models including only clinical features is still unknown.
In this study, we developed SVM models using multimodal MRI data to predict NCI among people with HIV. Specifically, the purpose of this study was to determine whether the addition of MRI data would improve the classification of NCI above a model using only clinical features based on interview and medical record data. The MRI features included GM volume obtained from an anatomical MRI and FA from DWI. The clinical features included demographics, substance use, and routine laboratory tests. We expected that the model combining clinical and MRI features would outperform the model including only clinical features.
Methods
Participants and study procedure
This is a secondary analysis of several projects that examined how substance use and HIV infection together impact brain structure and associated NCI (Meade et al. 2018, 2019, 2017). Participants were recruited from the Raleigh-Durham area in North Carolina, USA, via advertisements in local newspapers and websites, flyers and brochures at community-based organizations, and infectious diseases clinics. After completing a brief pre-screening, eligible participants had an in-depth screening to determine eligibility. This analysis included people who were 18–55 years old. Exclusion criteria were less than an 8th-grade education, English non-fluency or illiteracy, serious neurological conditions, severe traumatic brain injury, or serious mental illness. As part of the original protocols, participants were excluded for 2 or more days of use of any illicit drug other than cocaine and marijuana. The screening visit included clinical interviews and questionnaires, urine drug testing, and MRI safety evaluation. HIV diagnosis was confirmed via medical record review. Eligible participants returned on another day to complete neuropsychological testing and an MRI scan. This study was approved by the institutional review board at Duke University Health System.
Neuropsychological testing
Trained psychometrists administered a 45-min battery to assess neurocognitive function in seven domains: (1) executive function (Trail Making Test Part B (Reitan and Wolfson 1993) and Stroop Interference (Golden 1978)), (2) processing speed (Trail Making Test Part A (Reitan and Wolfson 1993) and Stroop Color Naming (Golden 1978)), (3) attention/working memory (Paced Auditory Serial Addition Task-50 (Diehr et al. 2003) and NAB Digit Span: forward and backward (Stern and White 2009)), (4) verbal fluency (FAS letter fluency and category fluency–animals (Benton et al. 1983)), (5) learning (Hopkins Verbal Learning Test—Revised (HVLT-R), immediate trials (Brandt and Benedict 2001)), (6) motor functioning (Grooved Pegboard Test, dominant and non-dominant hands (Klove 1963)), and (7) Memory (HVLT-R, delayed trial (Brandt and Benedict 2001)).
Raw scores of each neuropsychological measure were converted to demographically-corrected T-scores with mean = 50, and standard deviation = 10 (Diehr et al. 2003; Golden and Freshwate 2002; Heaton et al. 2004b; Stern and White 2009). T-scores for each test were converted to a deficit score ranging from 0 to 5 with 0 representing no impairment and 5 representing severe impairment (Carey et al. 2004). Overall domain deficit scores (DDSs) were computed by averaging the deficit scores of the tests within a domain, and the DDSs were then averaged across the seven domains to create the global deficit score (GDS). Previous studies have found that a GDS cutoff score of 0.5 yields the most optimal balance between sensitivity and specificity and the best discriminatory power in predicting NCI among people with HIV (Carey et al. 2004; Heaton et al. 2004). This cutoff was utilized in the current study, with GDS ≥ 0.5 categorized as impaired (NCI+) and all others categorized as not impaired (NCI−). A dichotomized GDS was used to establish the SVM model because the neuropsychological physicians usually diagnose a patient as “impaired” or “not impaired.” Results based on dichotomized GDS are meant especially for clinical diagnosis.
Clinical features
Demographic characteristics (age, gender, and education) were assessed using self-report. Substance use in the past 30 days (cigarettes, alcohol, marijuana, and cocaine) was assessed using the Addiction Severity Index (McLellan et al. 1992). HIV clinical characteristics (years since HIV diagnosis, recent viral load and CD4+ T cell counts, and nadir CD4+ T cell counts) and general health information (e.g., body mass index [BMI], complete blood count, comprehensive metabolic panel, hepatitis C co-infection) were abstracted from medical records. Using this information, the Veterans Aging Cohort Study (VACS) Index 2.0 was computed to provide a summary of the overall HIV disease burden (Marquine et al. 2014). The VACS Index was first developed and found to be predictive of multiple clinical outcomes and health conditions in people with HIV, including all-cause mortality (Chichetto et al. 2019; Tate et al. 2013), hospitalization (Akgun et al. 2013), cardiovascular disease (Salinas et al. 2016), and NCI (Marquine et al. 2016). The recently developed VACS Index 2.0 further improved discrimination and external validation across a variety of subgroups (Tate et al. 2019).
MRI imaging acquisition
All MRI data were acquired using the same 3T GE Discovery MR750 scanner equipped with an 8-channel volume head coil at the Brain Imaging and Analysis Center at Duke University. High-resolution T1-weighted structural images were acquired using the following parameters: TR = 8.10 ms, TE = 3.18 ms, FOV = 25.6 cm, flip angle = 12°, in-plane matrix = 256 * 256; slice thickness = 1 mm, number of slices = 166. DWI were acquired in the axial plane using a single-shot spin-echo diffusion sensitized EPI sequence (FOV = 25.6 cm, in-plane matrix size = 256 × 256, slice thickness = 2 mm; parallel acceleration factor = 2). Additional parameters differed between the three protocols. Protocol 1 used parameters: b-factor = 900 s/mm2, TR/TE = 10,000/83.2 ms, 73 slices, 30 directions; Protocol 2 used parameters: b-factor = 800 s/mm2, TR/TE = 8,000/77.9 ms and 78.2 ms, 67 slices, 30 directions; Protocol 3 used parameters b-factor = 800 s/mm2, TR/TE = 8,000/78.6 ms, 67 slices, 80 directions. To harmonize the number of diffusion directions across protocols, the 80-direction protocol was downsampled using a MATLAB dot product function that identified the diffusion-encoding direction that was most similar to those in the 30-direction protocol.
MRI preprocessing
Case-specific pre-processing steps were implemented to improve data quality. The structural images were visually inspected to ensure full coverage and identify significant motion-related artifacts that could affect processing. Anatomical data were analyzed with FSL-VBM (Douaud et al. 2007, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol (Good et al. 2001) carried out with FSL tools (Smith et al. 2004). First, anatomical images were brain-extracted and segmented for maximum GM inclusion before being registered to the MNI 152 standard space using non-linear registration (Andersson et al. 2007). The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific GM template that contained equal representation from both groups. Second, all native GM images were non-linearly registered to this study-specific template and “modulated” to correct for local expansion/contraction due to the non-linear component of the spatial transformation. The modulated GM images were then smoothed with an isotropic Gaussian kernel with a sigma of 2 mm. GM images were then segmented into regions using the Havard-Oxford cortical and subcortical atlases within FSL. The averaged GM volume within each segmented cortical and subcortical region was then extracted using fslmeants (Jenkinson et al. 2012).
For the DWI data, all cases were run through a denoising procedure that substantially improves signal quality by reducing non-Gaussian noise-induced signal bias while maintaining anatomic resolvability (Chen et al. 2018). The DWI data were then preprocessed using DTIPrep, a quality control pipeline that includes image dimension, slice-wise, interlace-wise, and gradient-wise checking along with baseline averaging, head motion correction, and eddy current correction (Oguz et al. 2014). After DTIPrep, all included participants had a minimum of 24 diffusion directions. A final visual check was performed on all cases to ensure whole-brain coverage and the absence of uncorrectable motion or scanner artifacts.
The DWI data went through the following processing steps using tools from FSL 5.0.9 and MRtrix3 (https://www.mrtrix.org/) (Tournier et al. 2019). First, B1 bias-correction was performed to correct for field inhomogeneity using dwibiascorrect in MRtrix3 and the ANTS N4 method for estimating the bias field (Tustison et al. 2010). Global DWI intensity normalization was done using dwiintensitynorm in MRtrix3. A mask was then created from both B0 and DWI using dwi2mask. We next calculated rigid-body (6 DOF) registration between subjects’ mean B0 image and the T1-weighted image, and non-linear registration between the T1-weighted image and the standard MNI brain using FSL’s mainfeatreg.
Probabilistic tractography was conducted using tckgen, using an anatomically-constrained procedure (Smith et al. 2012) with a five-tissue-type segmentation of the T1-weighted image using the FSL-based algorithm 5ttgen (Smith et al. 2004), generating 1 million tracks seeded from the mask image at a step size of 0.2 mm (Jones 2008). For quality assurance, 10% of the tracks generated by the probabilistic tractography were overlaid on each participant’s MNI-registered T1-weighted image and visually inspected. FA was calculated using tensor2metric in MRtrix3. A summary “track mean” of the FA measure sampled over each track was computed using tcksample. White matter (WM) images were then segmented into regions using the ICBM-DTI-81 WM label atlas within FSL. The averaged WM volume within each segmented tract was then extracted using fslmeants (Jenkinson et al. 2012).
Feature selection
Before predictive model fitting, we used the least absolute shrinkage and selection operator (LASSO) logistic regression to perform variable selection among MRI features (Tibshirani 1996). This approach is similar to linear regression in that it predicts outcomes using a weighted combination of input features, but unlike linear regression, regression coefficients are penalized with the sparsity-inducing L1 norm, favoring solutions with most coefficients shrunk to 0. We estimated the mean area under receiver operating curve (AUC) for each shrinkage parameter (lambda) using tenfold cross-validation (Fig. 1). The lambda with the maximum AUC was selected as the optimal shrinkage parameter, and all features with nonzero coefficients in the model with the optimal shrinkage parameter were used for training the subsequent predictive models.
Fig. 1.
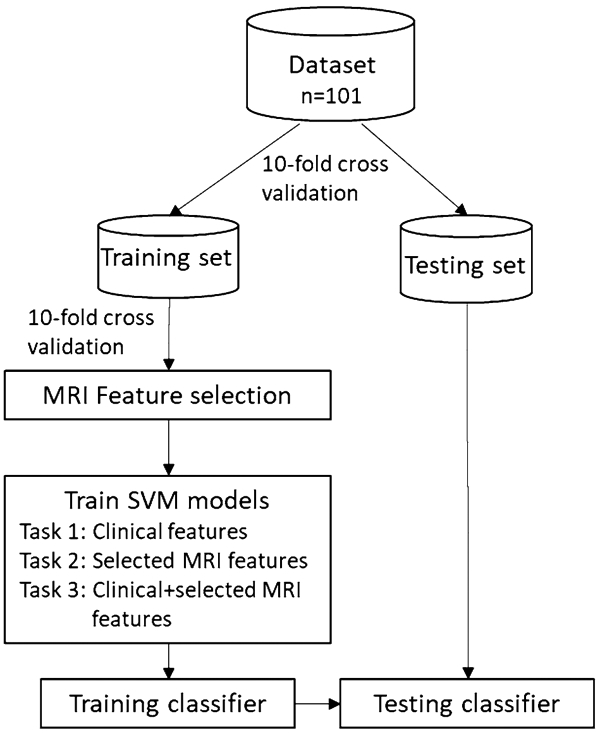
Diagram of the classification approach employed in this study
Support vector machine classification
We trained an SVM with a linear kernel to perform a two-class classification of NCI. Modeling was also performed using other kernels with no improvement of classification (Appendix Table 1). A tenfold cross-validation scheme was employed to train and test the model (Fig. 1). Three different classification tasks were performed, with one involving clinical variables only (i.e., VACS index 2.0, gender, race, nadir CD4+ T cell counts, years of HIV diagnosis, and the use of cigarettes, alcohol, marijuana, and cocaine), one involving the selected MRI features only, and one involving both clinical and selected MRI features. The classification quality was assessed by AUC, accuracy, sensitivity, and specificity.
Statistical analysis
Clinical characteristics between people with and without NCI were compared using t tests for continuous variables with normal distribution, Wilcoxon’s rank sum test for continuous variables with non-normal distribution, chi-square tests for categorical variables, and Fisher’s exact test for categorical variables with cell sizes < 5. Differences in MRI features between NCI+ and NCI− groups were tested using generalized linear models, controlling for age and protocol. Statistical significance was set at p < 0.05 (two-tail). All analyses were performed using R 6.7 and SAS 9.7.Results
Results
Sample characteristics
The characteristics of the 101 participants are summarized in Table 1. The mean age was 41.5 (standard deviation [SD] = 9.6); the majority of the participants were male (73.3%) and African American (81.2%). More than half of the sample (58.4%) had NCI based on the GDS. The median years since HIV diagnosis was 8.2 (interquartile range [IQR] = 4.3, 16.6), and 75.8% were virologically suppressed at < 50 copies/ml. NCI+ status was associated with African American race, eGRF > 60 ml/min, lack of alcohol use, higher hemoglobin level, and lower albumin levels.
Table 1.
Participant characteristics (N = 101)
| Variables | NCI− N = 42 |
NCI+ n = 59 |
Statistic |
|---|---|---|---|
| Demographic characteristics | |||
| Age, mean (SD) | 42.1 (9.7) | 40.9 (9.5) | t(99) = 0.52 |
| Male, n (%) | 28 (66.7) | 46 (78.0) | X2(1) = 1.60 |
| African-American race, n (%) | 29 (69.1) | 53 (89.8) | X2(1) = 6.94** |
| Current substance use | |||
| Daily cigarette smoking, n (%) | 20 (47.6) | 34 (57.6) | X2(1) = 0.99 |
| Alcohol, n (%) | 38 (90.5) | 43 (72.9) | p = 0.04* |
| Cocaine, n (%) | 14 (33.3) | 17 (28.8) | X2(1) = 0.24 |
| Marijuana, n (%) | 17 (40.5) | 24 (40.7) | X2(1) = 0.00 |
| HIV characteristics | |||
| Years since HIV diagnosis, median (IQR) | 8.4 (5.2, 15.5) | 8.0 (3.7, 17.6) | S = 2239 |
| Recent CD4+ T cell counts, median (IQR) | 711 (480, 857) | 513 (300, 860) | S = 2329 |
| Nadir CD4+ T cell counts, median (IQR) | 305 (105, 500) | 228 (100, 386) | S = 2274 |
| Recent viral load < 50 copies/ml, n (%) | 31 (75.6) | 44 (75.9) | X2(1) = 0.01 |
| Other clinical characteristics | |||
| Hemoglobin (g/dl), median (IQR) | 14.4 (13.4–5.3) | 13.6 (12.6, 14.8) | S = 2142* |
| FIB-4, median (IQR) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.3) | S = 1859 |
| eGRF > 60 ml/min, n (%) | 35 (89.7) | 51 (94.4) | p = 0.45* |
| Chronic HCV infection, n (%) | 3 (7.1) | 9 (15.5) | p = 0.23 |
| Albumin (g/dl), median (IQR) | 4.1 (3.8, 4.4) | 3.9 (3.6, 4.2) | S = 1741* |
| WBC (103 cells/ml), median (IQR) | 6.0 (4.5, 7.0) | 5.3 (4.6, 6.8) | S = 1654 |
| BMI, mean (SD) | 25.8 (22.5, 30.7) | 25.4 (21.9, 28.1) | S = 2262 |
| VACS index, median (IQR) | 39.9 (32.2, 47.0) | 46.7 (29.8, 57.5) | S = 1966 |
BMI body mass index, IQR interquartile range, NCI neurocognitive impairment, SD standard deviation, VACS veterans aging cohort study, WBC white blood count
P < 0.05
P < 0.01
MRI features selected in LASSO regression
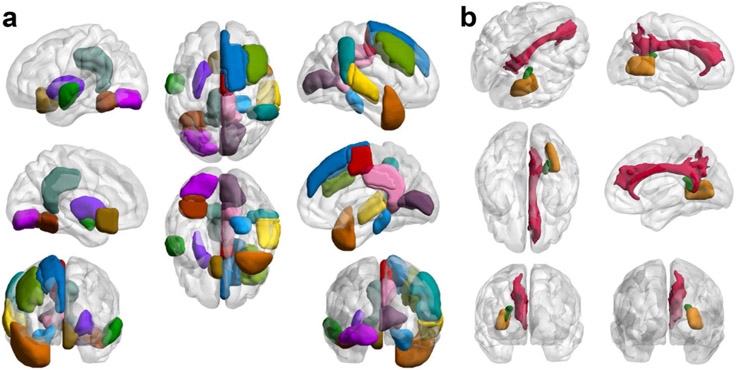
Three WM regions in the right hemisphere were selected by the LASSO regression analysis. The FA of the right tapetum was significantly lower in the NCI+ group compared with the NCI− group. Six GM regions in the left hemisphere and nine in the right hemisphere were selected. The GM volumes of the left occipital fusiform gyrus, left subcallosal cortex, posterior division of right superior temporal gyrus, right temporal pole, right supplementary motor area, and right superior frontal gyrus were significantly different between NCI+ and NCI− group (Fig. 2; Table 2).
Fig. 2.
Gray matter (a) and white matter (b) regions identified in LASSO regression analysis
Table 2.
Comparison of the selected gray matter and white matter regions between NCI+ and NCI− groups
| Regions | Mean (95% CI) |
Diff. (95% CI) | p value | |
|---|---|---|---|---|
| NCI− (n = 42) |
NCI+ (n = 59) |
|||
| White matter | ||||
| R. posterior thalamic radiation | 0.49 (0.48, 0.51) | 0.49 (0.48, 0.50) | − 0.001 (− 0.015, 0.012) | 0.866 |
| R. cingulum | 0.31 (0.30, 0.32) | 0.32 (0.31, 0.33) | − 0.006 (− 0.020, 0.008) | 0.370 |
| R. tapetum | 0.44 (0.42, 0.46) | 0.41 (0.39, 0.43) | 0.032 (0.011, 0.052) | 0.003 |
| Gray matter | ||||
| L. posterior cingulate gyrus | 0.56 (0.54, 0.58) | 0.56 (0.54, 0.58) | − 0.003 (− 0.024, 0.018) | 0.773 |
| L. occipital fusiform gyrus | 0.62 (0.59, 0.65) | 0.58 (0.56, 0.61) | 0.038 (0.007, 0.068) | 0.016 |
| L. subcallosal cortex | 0.57 (0.55, 0.60) | 0.55 (0.53, 0.57) | 0.028 (0.002, 0.054) | 0.037 |
| L. superior temporal gyrus (anterior division) | 0.53 (0.50, 0.55) | 0.51 (0.48, 0.53) | 0.020 (− 0.010, 0.051) | 0.180 |
| L. temporal occipital fusiform cortex | 0.70 (0.67, 0.72) | 0.68 (0.65, 0.70) | 0.017 (− 0.014, 0.048) | 0.268 |
| L. putamen | 0.32 (0.30, 0.33) | 0.33 (0.31, 0.35) | − 0.013 (− 0.035, 0.009) | 0.251 |
| R. posterior cingulate gyrus | 0.52 (0.50, 0.54) | 0.52 (0.51, 0.54) | − 0.004 (− 0.024, 0.016) | 0.710 |
| R. intracalcarine cortex | 0.49 (0.47, 0.52) | 0.52 (0.50, 0.54) | − 0.024 (− 0.051, 0.003) | 0.080 |
| R. supplementary motor area | 0.49 (0.47, 0.52) | 0.52 (0.50, 0.55) | − 0.032 (− 0.061, − 0.002) | 0.032 |
| R. DLPFC | 0.45 (0.44, 0.47) | 0.47 (0.46, 0.48) | − 0.016 (− 0.038, 0.005) | 0.131 |
| R. parahippocampal gyrus (posterior division) | 0.55 (0.53, 0.57) | 0.53 (0.52, 0.55) | 0.019 (− 0.005, 0.042) | 0.114 |
| R. superior frontal gyrus | 0.45 (0.43, 0.47) | 0.47 (0.45, 0.48) | − 0.020 (− 0.039, − 0.000) | 0.050 |
| R. superior temporal gyrus (posterior division) | 0.49 (0.47, 0.51) | 0.46 (0.45, 0.48) | 0.027 (0.006, 0.047) | 0.012 |
| R. posterior parietal cortex | 0.48 (0.47, 0.50) | 0.47 (0.45, 0.48) | 0.019 (− 0.001, 0.039) | 0.066 |
| R. temporal pole | 0.42 (0.41, 0.44) | 0.40 (0.39, 0.41) | 0.022 (0.004, 0.040) | 0.016 |
NCI neurocognitive impairment, SD standard deviation, SE standard error
Support vector machine classification
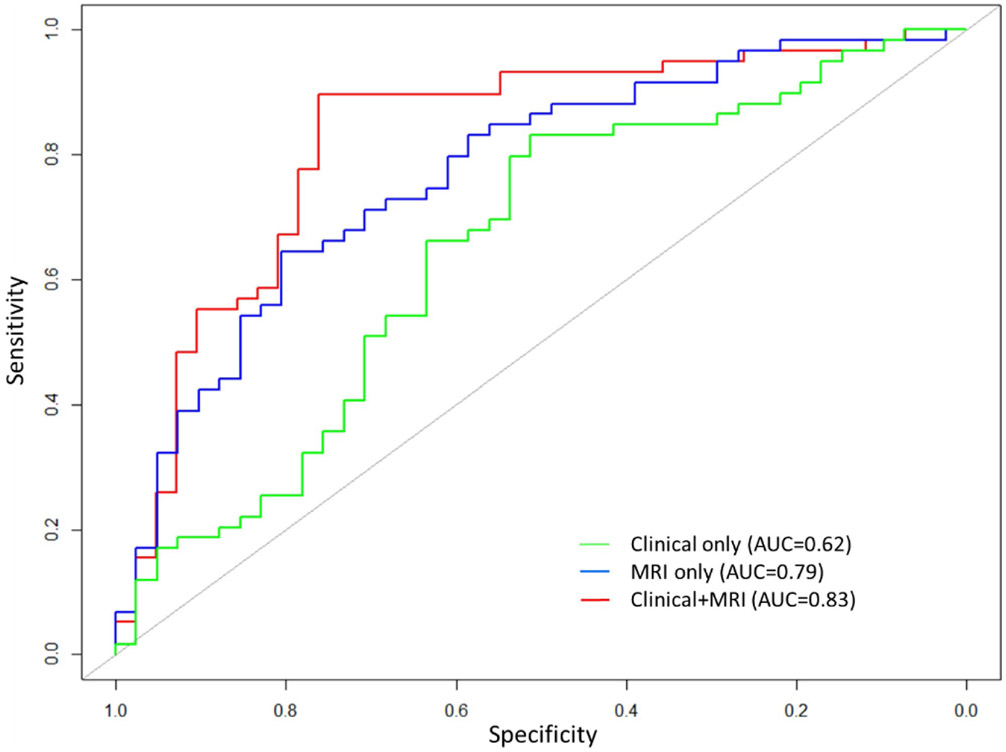
Table 3 and Fig. 3 show AUC, accuracy, sensitivity, and specificity of the SVM classification for NCI. Using only clinical features, the SVM model correctly classified participants with and without NCI with AUC of 0.62, accuracy of 0.65, sensitivity of 0.85, and specificity of 0.37. The model including only MRI features had AUC of 0.79, accuracy of 0.72, sensitivity of 0.86, and specificity of 0.52. SVM model involving both clinical and MRI features had the best performance with AUC of 0.83, accuracy of 0.80, sensitivity of 0.86, and specificity of 0.71.
Table 3.
Accuracy, sensitivity, and specificity of support vector machine classification of neurocognitive impairment
| Model | Accuracy | Sensitivity | Specificity |
|---|---|---|---|
| Clinical | 0.65 | 0.85 | 0.37 |
| MRI | 0.72 | 0.86 | 0.52 |
| Clinical+ MRI | 0.80 | 0.86 | 0.71 |
Fig. 3.
Receiver operating characteristic curve for support vector machine classification of neurocognitive impairment
Discussion
In this study, we developed SVM models to predict NCI among people with HIV using clinical and MRI features. Models using MRI features alone outperformed those using clinical features, but the combination of clinical and MRI features led to the best classification of NCI. The sensitivity of the model was high and very similar across the three models, while the specificity was substantially improved by the addition of MRI features. The results of this study suggest the potential value of the SVM model in diagnosis of NCI and support the benefit of including MRI data to improve prediction among people with HIV. While the collection of MRI data is expensive and requires specialized equipment, the addition of neural biomarkers could be especially useful for patients who are at risk of NCI.
Given that neuropsychological testing is time-consuming and costly, several screening tools have been developed. However, existing screening tools poorly distinguish NCI+ from NCI− in adults with HIV due to low sensitivity and/or specificity. Screening tools with low sensitivity miss a high proportion of people with NCI, which leads to a diagnostic delay and lack of appropriate intervention. In contrast, low specificity means that a high proportion of people without NCI are incorrectly identified as screening positive which might lead to inappropriate medical interventions and increase the already high cost of HIV treatment (Nunn et al. 2009). A meta-analysis of 31 studies showed poor to moderate pooled sensitivities in classifying HAND in people with HIV for HIV Dementia Scale (HDS) (0.48) and International HIV Dementia Scale (IHDS) (0.62) (Zipursky et al. 2013). In studies that evaluated the ability of IHDS to identify HIV dementia, the specificity of the scale significantly decreased as sensitivity increased (Diehr et al. 2003; Joska et al. 2016). A recent study compared the ability of five scales in discriminating people with HIV who had or not had HAND (Joska et al. 2016). Both sensitivity and specificity are lower than our machine learning model (sensitivity: 0.86; specificity: 0.71) for the Cognitive Assessment Tool-rapid version (CAT-Rapid) (sensitivity: 0.64; specificity: 0.52) and Simioni symptom questions (SSQ) (sensitivity: 0.77; specificity: 0.32). The MoCA with a slightly higher sensitivity (0.89) than the proposed machine learning model has an unacceptably low specificity (0.22). In contrast, for IHDS and Mini Mental Status Exam (MMSE), the sensitivity (IHDS: 0.40; MMSE: 0.23) is very low despite of a high specificity (IHDS: 0.86; MMSE: 0.97).
Compared with the current NCI screening tools for patients with HIV, the proposed model combining clinical and MRI features is more promising with both high sensitivity and specificity (sensitivity: 0.86; specificity: 0.71). Findings of this study show that involving MRI features in the machine learning model substantially elevated specificity without any attenuation of sensitivity. The model including only clinical features had a high sensitivity of 0.85 but a low specificity of 0.37. There was a 15% increase in accuracy by further adding MRI features in the clinical model, with 1% increase in sensitivity but a large increase in specificity (34%). Thus, the addition of MRI data could be a potential means to reduce the false-positive rate among people who are screened positive.
In the current study, the most predictive MRI features were selected based on LASSO algorithm, which reveals the brain regions that may be associated with NCI among people with HIV. Our findings are consistent with previous machine learning studies on neural structure and HIV infection. Tang et al.’s study revealed WM impairment in the right tapetum was able to effectively distinguish HIV patients from controls without HIV (Tang et al. 2017a, 2017b). When examining GM, Adeli et al. found HIV-specific impairment of brain regions including the parahippocampal gyrus, dorsolateral prefrontal cortex, and superior frontal gyrus using Chained-Regularization within a multiple kernel learning framework (Adeli et al. 2018). In another study, the same group found that volumetric changes in parahippocampal gyrus, posterior cingulate gyrus, and posterior parietal cortex could successfully discriminate people with and without HIV (Adeli et al. 2019). Similar to the findings from these previous studies, a large number of anatomic regions in the brain were identified in this study. Thus, it could be speculated that NCI in people with HIV may be predicted by spatially diffuse abnormalities across the brain, a broad number of brain regions impacted, or a specific set of these identified regions.
This study has several limitations. First, we did not verify the classification results by independent validation. Although we employed cross-validation in this study, the true test of generalization is the model’s performance on new data. Second, the ANI may be a long-term course and the activities of daily living may be obviously affected when people develop to more severe forms of NCI. Our study was only based on neuropsychological assessments and MRI, which did not assess daily functional impairment and long-term outcomes. Third, although commonly used in research and clinical settings, the GDS has been shown to over-diagnose NCI in people with HIV (Carey et al. 2004a; De Francesco et al. 2016; Gisslen et al. 2011; Heaton et al. 1995; Heaton et al. 1994; Underwood et al. 2018). In addition, comorbidities and coinfections, such as major depression and HCV, may have contributed to NCI in our sample. Fifth, since we selected MRI features using LASSO, the identified brain regions may not have biological relevance. Finally, we used mean volume across relatively large neural regions, which may mask more subtle changes that are confined to smaller sub-regions in the brain.
In conclusion, the findings of this study show that SVM learning models incorporating both clinical and MRI features may provide an effective means of classifying persons with HIV as having NCI or not with high sensitivity and specificity. The addition of neural biomarkers in the NCI diagnosis model largely increases specificity while maintaining high sensitivity. This study only included anatomical data measured by structural MRI and DWI. Further studies are needed to test the performance of machine learning models that also include functional neuroimaging data. External validation is also needed to test the generalizability of the proposed models. In addition, future machine learning studies should utilize formal HAND diagnoses to determine accuracy of the models.
Supplementary Material
Acknowledgements
This study was funded by grant R01-DA045565 and R25-AI140495 from the US National Institutes of Health.
Funding National Institute on Drug Abuse (R01-DA045565) Dr. Christina S. Meade, Division of Intramural Research, National Institute of Allergy and Infectious Diseases (R25-AI140495) Dr. Cliburn Chan.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s13365-020-00930-4.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulkadir A, Mortamet B, Vemuri P, Jack CR Jr, Krueger G, Kloppel S , Alzheimer’s Disease Neuroimaging Initiative (2011) Effects of hardware heterogeneity on the performance of SVM Alzheimer’s disease classifier. Neuroimage 58(3):785–792. 10.1016/j.neuroimage.2011.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli E, Kwon D, Zhao Q, Pfefferbaum A, Zahr NM, Sullivan EV, Pohl KM (2018) Chained regularization for identifying brain patterns specific to HIV infection. Neuroimage 183:425–437. 10.1016/j.neuroimage.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli E, Zahr NM, Pfefferbaum A, Sullivan EV, Pohl KM (2019) Novel machine learning identifies brain patterns distinguishing diagnostic membership of human immunodeficiency virus, alcoholism, and their comorbidity of individuals. Biol Psychiatry Cogn Neurosci Neuroimaging 4(6):589–599. 10.1016/j.bpsc.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun KM, Gordon K, Pisani M, Fried T, McGinnis KA, Tate JP, Butt AA, Gibert CL, Huang L, Rodriguez-Barradas MC, Rimland D, Justice AC, Crothers K (2013) Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected Veterans. J Acquir Immune Defic Syndr 62(1):52–59. 10.1097/QAI.0b013e318278f3fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S (2007) Non-linear Registration, aka Spatial Normalisation, in FMIRB Analysis Group Technical Reports. Oxford Centre for Functional MRI of the Brain: Oxford, United Kingdom. [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69(18):1789–1799. 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N, McIntosh RC, Ironson G (2019) Impaired neurocognitive performance and mortality in HIV: assessing the prognostic value of the hiv-dementia scale. AIDS Behav 23(12):3482–3492. 10.1007/s10461-019-02423-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belete T, Medfu G, Yemiyamrew E (2017) Prevalence of HIV associated neurocognitive deficit among HIV positive people in Ethiopia: a cross sectional study at Ayder Referral Hospital. Ethiop J Health Sci 27(1):67–76. 10.4314/ejhs.v27i1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J, Zee PC (2004) Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep 27(8):1542–1551. 10.1093/sleep/27.8.1542 [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A (1983) Multilingual Aphasia Examination, 3rd edn. AJA Associates, Iowa City, IA [Google Scholar]
- Brandt J, Benedict RHB (2001) Hopkins Verbal Learning Test—revised professional manual. Psychological Assessment Resources Inc, Lutz, FL [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK (2004) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26(3):307–319. 10.1080/13803390490510031 [DOI] [PubMed] [Google Scholar]
- Chen NK, Chang HC, Bilgin A, Bernstein A, Trouard TP (2018) A diffusion-matched principal component analysis (DM-PCA) based two-channel denoising procedure for high-resolution diffusion-weighted MRI. PLoS ONE 13(4):e0195952. 10.1371/journal.pone.0195952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichetto NE, Kundu S, Freiberg MS, Butt AA, Crystal S, So-Armah KA, Cook RL, Braithwaite RS, Fiellin DA, Khan MR, Bryant KJ, Gaither JR, Barve SS, Crothers K, Bedimo RJ, Warner AL, Tindle HA, Veterans Aging Cohort S (2019) Association of Syndemic Unhealthy Alcohol Use, Cigarette Use, and Depression With All-Cause Mortality Among Adults Living With and Without HIV Infection: Veterans Aging Cohort Study. Open Forum Infect Dis, 6(6), ofz188. 10.1093/ofid/ofz188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockanathan U, AM DS, Abidin AZ, Schifitto G, Wismuller A (2018) Identification and functional characterization of HIV-associated neurocognitive disorders with large-scale Granger causality analysis on resting-state functional MRI. Proc SPIE Int Soc Opt Eng, 10575. 10.1117/12.2293888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockanathan U, AM DS, Abidin AZ, Schifitto G, Wismuller A (2019) Automated diagnosis of HIV-associated neurocognitive disorders using large-scale Granger causality analysis of resting-state functional MRI. Comput Biol Med 106:24–30. 10.1016/j.compbiomed.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20(3):273–297. 10.1007/BF00994018 [DOI] [Google Scholar]
- Cysique LA, Soares JR, Geng G, Scarpetta M, Moffat K, Green M, Brew BJ, Henry RG, Rae C (2017) White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. J Neurovirol 23(4):539–547. 10.1007/s13365-017-0524-1 [DOI] [PubMed] [Google Scholar]
- De Francesco D, Underwood J, Post FA, Vera JH, Williams I, Boffito M, Sachikonye M, Anderson J, Mallon PW, Winston A, Sabin CA, Group PS (2016) Defining cognitive impairment in people-living-with-HIV: the POPPY study. BMC Infect Dis 16(1):617. 10.1186/s12879-016-1970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK (2003) The 50 and 100-item short forms of the Paced Auditory Serial Addition Task (PASAT): demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. J Clin Exp Neuropsychol 25(4):571–585. 10.1076/jcen.25.4.571.13876 [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A (2007) Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130(9):2375–2386 [DOI] [PubMed] [Google Scholar]
- DSouza AM, Abidin AZ, Chockanathan U, Wismüller A (2018) Regional autonomy changes in resting-state functional MRI in patients with HIV associated neurocognitive disorder. Paper presented at the Medical Imaging 2018: Image Processing. [Google Scholar]
- Dyrba M, Barkhof F, Fellgiebel A, Filippi M, Hausner L, Hauenstein K, Kirste T, Teipel SJ, Group ES (2015) Predicting prodromal Alzheimer’s disease in subjects with mild cognitive impairment using machine learning classification of multimodal multicenter diffusion-tensor and magnetic resonance imaging data. J Neuroimaging 25(5):738–747. 10.1111/jon.12214 [DOI] [PubMed] [Google Scholar]
- Gisslen M, Price RW, Nilsson S (2011) The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 11:356. 10.1186/1471-2334-11-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ (1978) Stroop Color and Word Test. Stoelting, Chicago, IL [Google Scholar]
- Golden CJ, Freshwater SM (2002) The Stroop color and word test: a manual for clinical and experimental uses. Stoelting, Chicago, IL [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J Richard, Henson NA, Friston KJ, Frackowiak RSJ (2001) A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 14(1):21–36 [DOI] [PubMed] [Google Scholar]
- Grabyan JM, Morgan EE, Cameron MV, Villalobos J, Grant I, Woods PS, Group HIVNRP (2018) Deficient emotion processing is associated with everyday functioning capacity in HIV-associated neurocognitive disorder. Arch Clin Neuropsychol 33(2):184–193. 10.1093/arclin/acx058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Badoud S, Nguyen D, Garibotto V, Lovblad KO, Burkhard PR (2012) Individual detection of patients with Parkinson disease using support vector machine analysis of diffusion tensor imaging data: initial results. AJNR Am J Neuroradiol 33(11):2123–2128. 10.3174/ajnr.A3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Nguyen D, Rodriguez C, Emch J, Gold G, Bartsch A, Lovblad KO, Giannakopoulos P (2010) Individual prediction of cognitive decline in mild cognitive impairment using support vector machine-based analysis of diffusion tensor imaging data. J Alzheimers Dis 22(1):315–327. 10.3233/JAD-2010-100840 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C (2010) HIV associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER. Study Neurology 75(23):2087–2096. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ et al. (1995) The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc, 1(3):231–251. 10.1017/s1355617700000230 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Kirson D, Velin RA, IGRO Grant, Group H (1994) The utility of clinical ratings for detecting cognitive change in HIV infection. Neuropsychology of HIV infection (pp. 188–206). New York: Oxford University. [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, Group H (2004) The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10(3):317–331. 10.1017/S1355617704102130 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I (2004) Revised comprehensive norms for an expanded halstead-reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources Inc, Lutz, FL [Google Scholar]
- Heaton RK, Psychological Assessment Resources I (2004) Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources, Professional Manual [Google Scholar]
- Israel SM, Hassanzadeh-Behbahani S, Turkeltaub PE, Moore DJ, Ellis RJ, Jiang X (2019) Different roles of frontal versus striatal atrophy in HIV-associated neurocognitive disorders. Hum Brain Mapp 40(10):3010–3026. 10.1002/hbm.24577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL NeuroImage 62(2):782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jones DK (2008) Tractography gone wild: probabilistic fibre tracking using the wild bootstrap with diffusion tensor MRI. IEEE Trans Med Imaging 27(9):1268–1274 [DOI] [PubMed] [Google Scholar]
- Joska JA, Witten J, Thomas KG, Robertson C, Casson-Crook M, Roosa H, Creighton J, Lyons J, McArthur J, Sacktor NC (2016) A comparison of five brief screening tools for HIV-associated neurocognitive disorders in the USA and South Africa. AIDS Behav 20(8):1621–1631. 10.1007/s10461-016-1316-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H (1963) Grooved Pegboard. Lafayette Instruments, Lafayette, IN [Google Scholar]
- Kumar S, Himanshu D, Tandon R, Atam V, Sawlani KK, Verma SK (2019) Prevalence of HIV Associated neurocognitive disorder using modified Mini Mental State Examination and its correlation with CD4 counts and anti-retroviral therapy. J Assoc Physicians India 67(4):47–51 [PubMed] [Google Scholar]
- Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M (2011) Structural gray and white matter changes in patients with HIV. J Neurol 258(6):1066–1075. 10.1007/s00415-010-5883-y [DOI] [PubMed] [Google Scholar]
- Libbrecht MW, Noble WS (2015) Machine learning applications in genetics and genomics. Nat Rev Genet 16(6):321–332. 10.1038/nrg3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson KN, Frick A, Boraxbekk CJ, Marquand AF, Williams SC, Carlbring P, Andersson G, Furmark T (2015) Predicting long-term outcome of Internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Transl Psychiatry 5:e530. 10.1038/tp.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Umlauf A, Rooney AS, Fazeli PL, Gouaux BD, Paul Woods S, Letendre SL, Ellis RJ, Grant I, Moore DJ, Group HIVNRP (2016) The Veterans Aging Cohort Study (VACS) index and neurocognitive change: a longitudinal study. Clin Infect Dis 63(5):694–702. 10.1093/cid/ciw328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Umlauf A, Rooney AS, Fazeli PL, Gouaux BD, Paul Woods S, Letendre SL, Ellis RJ, Grant I, Moore DJ, Group HIVNRP (2014) The veterans aging cohort study index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr 65(2):190–197. 10.1097/QAI.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992) The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat, 9(3):199–213. 10.1016/0740-5472(92)90062-s [DOI] [PubMed] [Google Scholar]
- Meade CS, Addicott M, Hobkirk AL, Towe SL, Chen NK, Sridharan S, Huettel SA (2018) Cocaine and HIV are independently associated with neural activation in response to gain and loss valuation during economic risky choice. Addict Biol 23(2):796–809. 10.1111/adb.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Bell RP, Towe SL, Chen NK, Hobkirk AL, Huettel SA (2019) Synergistic effects of marijuana abuse and HIV infection on neural activation during a cognitive interference task. Addict Biol 24(6):1235–1244. 10.1111/adb.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Hobkirk AL, Towe SL, Chen NK, Bell RP, Huettel SA (2017) Cocaine dependence modulates the effect of HIV infection on brain activation during intertemporal decision making. Drug Alcohol Depend 178:443–451. 10.1016//j.drugalcdep.2017.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, Solomon T (2014) Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 13(11):1139–1151. 10.1016/S1474-4422(14)70137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn AS, da Fonseca EM, Bastos FI, Gruskin S (2009) AIDS treatment in Brazil: impacts and challenges. Health Aff (Millwood) 28(4):1103–1113. 10.1377/hlthaff.28.4.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, Johnson HJ, Styner M (2014) DTIPrep: quality control of diffusion-weighted images. Frontiers in Neuroinformatics 8:4. 10.3389/fninf.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Shin NY, Choi JY, Lee SK, Bang MR (2018) Altered white matter integrity in human immunodeficiency virus-associated neurocognitive disorder: a tract-based spatial statistics study. Korean J Radiol 19(3):431–442. 10.3348/kjr.2018.19.3.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1993) The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation, 2nd edn. Neuropsycholgy Press, Tucson, AZ [Google Scholar]
- Salinas JL, Rentsch C, Marconi VC, Tate J, Budoff M, Butt AA, Freiberg MS, Gibert CL, Goetz MB, Leaf D, Rodriguez-Barradas MC, Justice AC, Rimland D (2016) Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis 63(11):1423–1430. 10.1093/cid/ciw564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GMA, Locatelli I, Metral M, Calmy A, Lecompte TD, Nadin I, Hauser C, Cusini A, Hasse B, Kovari H, Tarr P, Stoeckle M, Fux C, Di Benedetto C, Schmid P, Darling KEA, Du Pasquier R, Cavassini M, Neurocognitive Assessment in the, M., & Aging Cohort Study, G (2019) Cross-Sectional and Cumulative Longitudinal Central Nervous System Penetration Effectiveness Scores Are Not Associated With Neurocognitive Impairment in a Well Treated Aging Human Immunodeficiency Virus-Positive Population in Switzerland. Open Forum Infect Dis, 6(7), ofz277. 10.1093/ofid/ofz277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelye A, Mattek N, Howieson D, Riley T, Wild K, Kaye J (2015) The impact of sleep on neuropsychological performance in cognitively intact older adults using a novel in-home sensor-based sleep assessment approach. Clin Neuropsychol, 29(1):53–66. 10.1080/13854046.2015.1005139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP (2011) A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging, 32(12), 2322 e2325–2318. 10.1016/j.neurobiolaging.2010.05.019 [DOI] [PubMed] [Google Scholar]
- Smith RE, Tournier J-D, Calamante F, Connelly A (2012) Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage 62(3):1924–1938 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Stern RA, White T (2009) NAB Digits Forward/Digits Backward Test: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc. (PAR) [Google Scholar]
- Tang Z, Liu Z, Li R, Yang X, Cui X, Wang S, Yu D, Li H, Dong E, Tian J (2017a) Identifying the white matter impairments among ART-naive HIV patients: a multivariate pattern analysis of DTI data. Eur Radiol 27(10):4153–4162. 10.1007/s00330-017-4820-1 [DOI] [PubMed] [Google Scholar]
- Tang Z, Liu Z, Li R, Yang X, Cui X, Wang S, Yu D, Li H, Dong E, Tian J (2017b) Identifying the white matter impairments among ART-naïve HIV patients: a multivariate pattern analysis of DTI data. Eur Radiol 27(10):4153–4162 [DOI] [PubMed] [Google Scholar]
- Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Nattermann J, Lampe FC, Bucher HC, Sterling TR, Crane HM, Kitahata MM, May M, Sterne JAC (2013) An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 27(4):563–572. 10.1097/QAD.0b013e32835b8c7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JP, Sterne JAC, Justice AC, Veterans Cohort Collaboration Study and the Antiretroviral Therapy Cohort Collaboration (2019) Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS 33(5):903–912. 10.1097/QAD.0000000000002140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R (1996) Regression shrinkage and selection via the Lasso. J Roy Stat Soc: Ser B (Methodol) 58(1):267–288. 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, Connelly A (2019) MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29(6):1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J, De Francesco D, Leech R, Sabin CA, Winston A, Pharmacokinetic and Clinical Observations in PeoPle Over fifty (2018) Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS One, 13(4), e0194760. 10.1371/journal.pone.0194760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GA, Chaillon A, Liu S, Franklin DR Jr, Caballero G, Kosakovsky Pond SL, Vaida F, Heaton RK, Letendre SL, Grant I, Richman DD, Smith DM (2016) HIV-associated neurocognitive disorder is associated with HIV-1 dual infection. AIDS 30(17):2591–2597. 10.1097/QAD.0000000000001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Yan Z, Zhao Y, Tao B, Sun H, Li F, Yao L, Zhang W, Chandan S, Liu J, Gong Q, Sweeney JA, Lui S (2017) Support vector machine-based classification of first episode drug-naive schizophrenia patients and healthy controls using structural MRI. Schizophr Res. 10.1016/j.schres.2017.11.037 [DOI] [PubMed] [Google Scholar]
- Zandvakili A, Philip NS, Jones SR, Tyrka AR, Greenberg BD, Carpenter LL (2019) Use of machine learning in predicting clinical response to transcranial magnetic stimulation in comorbid posttraumatic stress disorder and major depression: a resting state electroencephalography study. J Affect Disord 252:47–54. 10.1016/j.jad.2019.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Hu D (2014) Unsupervised classification of major depression using functional connectivity MRI. Hum Brain Mapp 35(4):1630–1641. 10.1002/hbm.22278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Cheng Y, Ping L, Xu J, Shen Z, Jiang L, Shi L, Yang S, Lu Y, Xu X (2018) Support vector machine classification of obsessive-compulsive disorder based on whole-brain volumetry and diffusion tensor imaging. Front Psychiatry 9:524. 10.3389/fpsyt.2018.00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, Gill MJ, Rachlis A, Rosenes R, Arbess G, Marcotte T, Rourke SB (2013) Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS 27(15):2385–2401. 10.1097/QAD.0b013e328363bf56 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.