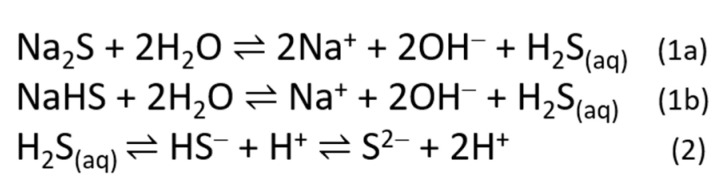Abstract
Hydrogen sulfide (H2S) is an endogenous gasotransmitter recently emerged as an important regulatory mediator of numerous human cell functions in health and in disease. In fact, much evidence has suggested that hydrogen sulfide plays a significant role in many physio-pathological processes, such as inflammation, oxidation, neurophysiology, ion channels regulation, cardiovascular protection, endocrine regulation, and tumor progression. Considering the plethora of physiological effects of this gasotransmitter, the protective role of H2S donors in different disease models has been extensively studied. Based on the growing interest in H2S-releasing compounds and their importance as tools for biological and pharmacological studies, this review is an exploration of currently available H2S donors, classifying them by the H2S-releasing-triggered mechanism and highlighting those potentially useful as promising drugs in the treatment of cardiovascular diseases.
Keywords: hydrogen sulfide, natural H2S donors, synthetic H2S donors, triggered mechanism, H2S release, cardiovascular diseases
1. Introduction
Hydrogen sulfide (H2S), the third endogenous recognized gaseous signaling transmitter [1], among nitric oxide (NO) and carbon monoxide (CO), is a colorless and pungent gas with a boiling point of 60 °C [2].
It is endogenously synthesized by four enzymes: cystathionine γ-lyase (CSE) and cystathionine β-synthetase (CBS), which catalyze the production of H2S by a direct enzymatic desulfhydration of L-cysteine, and 3-mercaptopyruvate sulfurtransferase (3-MST), which produces H2S by an indirect desulfhydration, in concert with cysteine aminotransferase (CAT) and in the presence of reductants [3]. The expression of these enzymes is tissue specific. The amount of CBS is found mostly in the central nervous system, the liver, the kidney, the uterus, and placenta; CSE is primarily concentrated in the cardiovascular system, whereas 3-MST is located predominantly in the mitochondria [4,5,6,7,8].
To study the biological effects of endogenously synthesized H2S, the development of inhibitors selective for the above enzymes is required. To date, some selective inhibitors of CSE and CBS have been identified and widely used in biological systems [9,10,11,12,13]; conversely, no pharmacological inhibitors of the enzyme 3-MST have yet been discovered.
Several studies have shown that H2S plays a significant modulatory role in numerous physio-pathological processes in the human body [14]. In fact, it is involved in the homeostatic regulation of respiratory, cardiovascular, immune, nervous, gastroenteric, and endocrine systems [4,15].
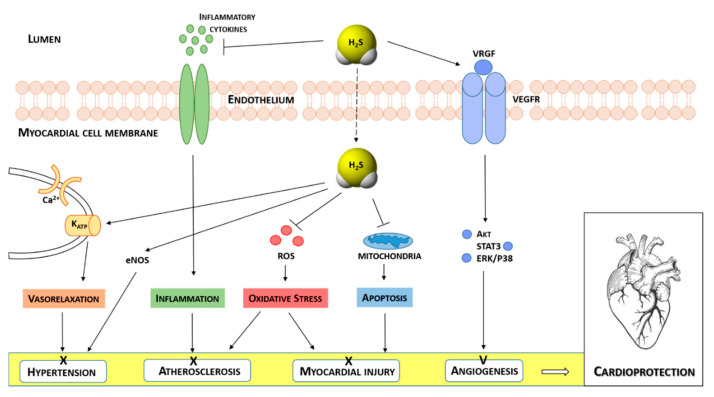
Currently, growing research evidence has confirmed the protective effects of H2S in cardiovascular diseases [16,17,18,19,20,21,22,23], such as cardiac hypertrophy, heart failure, myocardial ischemia/reperfusion (I/R) injury [24], hypertension [18,25] and atherosclerosis [26], acting as an activator of angiogenesis [27], basal vasorelaxant agent [28], blood pressure, and heart rate regulator [29,30]. Moreover, it has been demonstrated that the related mechanisms of action accounting for this cardioprotective activity involve antioxidation, inhibition of cell apoptosis, pro-angiogenesis, anti-inflammatory, and ion channels regulation [31,32] (Figure 1).
Figure 1.
Schematic illustration of the effects of H2S in different heart diseases and the molecular mechanisms underlying H2S-induced cardioprotection.
As depicted in Figure 1, H2S exerts a cardioprotective effect by activating different endothelium-dependent signaling pathways [33]. It reduces blood pressure, by inducing vasorelaxation, a consequence of opening KATP channels and increasing K+ currents resulting in hyperpolarizing membrane of smooth muscle cells [34]. Moreover, H2S shows its anti-hypertensive effect, by activating endothelial nitric oxide synthase (eNOS) and increasing NO bioavailability [35]. It also shows an inhibitory effect on the pathogenesis of atherosclerosis, by preventing an inflammatory response mediated by inflammatory cytokines [26] and exerting antioxidative action through the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent pathway and the protection of tissues by reactive oxygen species (ROS). The cardioprotection of H2S is also associated with the inhibition of cardiomyocyte apoptosis after myocardial injury. In fact, it suppresses the activation of caspase-3 and upregulates the expression of glycogen synthase kinase-3 (GSK-3β). The antioxidant effect of H2S is also embodied in the preservation of mitochondrial functions by inhibiting mitochondrial respiration [36].
Furthermore, H2S stimulates angiogenesis by increasing the expression of VEGF and activating some downstream effectors, such as Akt, STAT3, ERK, and p38 [37].
Recently, considering the interesting involvement of H2S in the cardiovascular system, there has been heightened enthusiasm for the development of compounds able to generate exogenous H2S, which could represent biological tools and promising cardioprotective agents.
2. H2S Donors
For the past several years, inorganic sulfide salts, such as sodium sulfide (Na2S) and hydrosulfide (NaHS), have been commonly used as pharmacological tools. They represent the early H2S donors used in biomedicine field, the results of which make it very useful to elucidate the physio-pathological roles of H2S in mammalian systems.
Although these sulfide salts have been the basic tools used for H2S research for years, some of their significant limitations were demonstrated. In fact, sulfide salts hydrolyze immediately upon reaction with water and the resulting too-rapid release of H2S causes its blood and tissue concentrations to surge to supraphysiological levels followed by a rapid drop [38].
Therefore, this suboptimal pharmacokinetic profile led the researchers to limit the use of these salts as potential therapeutics and to obtain novel organic H2S-releasing compounds, including chemically synthesized molecules and natural plant extracts.
One of the first slow-releasing H2S donors was Lawesson’s reagent, a synthetic compound which is largely used for sulfurization of organic molecules [39]. It has been used in some pharmacological studies as an H2S donor, despite its lack of water solubility.
Therefore, to improve this chemical property, GYY4137, a water-soluble derivative of Lawesson’s reagent, was obtained. Similarly to its parent compound, it also releases H2S upon hydrolysis, but it better mimics the physiological H2S production, due to its slow-releasing nature [40,41]. Moreover, in order to optimize the H2S release properties of GYY4137, structural modifications were made to this compound via substitution of the P-C bond in GYY4137 with a P-O bond, affording a series of O-substituted phosphorodithioate-based H2S donors [42].
Later on, naturally occurring H2S donors derived from some vegetables belonging to Alliaceae and Brassicaceae, such as garlic, onion, broccoli, cabbage, watercress, and garden cress, were also investigated.
So far, among garlic-derived compounds, allicin is one of the best characterized. Due to its instability in aqueous media, it quickly decomposes into four H2S-releasing compounds: diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), and ajoene [43].
Other garlic-derived compounds containing a sulfur atom are S-allylcysteine (SAC) with its structural analogs, S-propyl-L-cysteine (SPC), and S-propargyl-cysteine (SPRC), which are potential sources of H2S [44,45,46].
Similarly, some cruciferous vegetables contain natural isothiocyanates, such as sulforaphane, allyl isothiocyanate, benzyl isothiocyanate, 4-hydroxybenzyl isothiocyanate, and erucin, which exhibit a significant H2S-releasing activity [47].
Moreover, it has been demonstrated that some thioamino acids, such as thioglycine and thiovaline, are also H2S donors, showing a slow release of the gasotransmitter [48].
Recently, the limited clinical application of some naturally occurring H2S donors led to the development of novel synthetic compounds with favorable pharmacological properties, which could be useful both as research tools and as potential therapeutics.
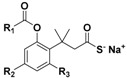
Thus, in order to obtain effective H2S donors with controllable release rates, a series of N-(benzoylthio)benzamides [49], acyl perthiols [50,51], arylthioamides [52], 1,2,4-thiadiazolidin-3,5-diones [53], iminothioethers [54], mercaptopyruvate [55], dithioates [56], isothiocyanate [57,58], and thiocarbamates [59] were developed and evaluated for their H2S-releasing properties.
Each of these donors release H2S with different mechanisms, triggered by hydrolysis, pH modulation, cellular thiols, photo activation, enzymatic reaction, or others.
2.1. Sulfide Salts
Over the years, sulfide salts, such as sodium sulfide (Na2S) and hydrosulfide (NaHS), have been widely used in biological studies to explore the therapeutic prospects of exogenous source of H2S. In particular, these salts showed protective effects against many disease states, such as inflammation [60,61], acute lung injury [62,63], oxidative stress in human neuroblastoma cells [64], Alzheimer’s disease (AD) [65], atherosclerosis [66], and ulcer [67,68].
In addition to these effects, aqueous NaSH solutions delivered in aortic rings led to a 60% greater relaxation over controls, confirming the vasorelaxant properties of H2S [28].
Moreover, several groups demonstrated that the use of sulfide salts was able to induce either pre- and post-conditioning cardioprotection [25,69,70,71,72,73].
Exogenous NaHS has proven to be useful in attenuating ischemia-reperfusion injury [74], protecting against myocardial infarction (MI) [75] and hemorrhagic shock [76].
Furthermore, Calvert et al. reported that long-term Na2S administration attenuates ischemia-induced heart failure, by reducing oxidative stress [24]. The results suggested that the H2S therapy attenuates left ventricular (LV) dilation and cardiac hypertrophy, and leads to an improvement in cardiac function.
Solutions of these sulfide salts are prepared by using hydrates of sodium hydrogen sulfide (NaHS × nH2O), nonahydrate disodium salt (Na2S × 9 H2O), or the anhydrous form.
In water or aqueous solution, these sulfide salts quickly hydrolyze, establishing a dynamic equilibrium among sulfide ions (S2−), bisulfide ions (HS−), and molecular hydrogen sulfide (H2S) [77] (Figure 2); the ratio of these species depends on different parameters, such as temperature, pressure, and pH.
Figure 2.
Mechanism of H2S release from Na2S (1a) and NaHS (1b) in aqueous solution and its dynamic equilibrium among different species (2).
As the pH increases, the H2S dissociates into its ions. At a pH below 6, H2S is the predominant sulfur species; at the physiological pH, H2S and HS− species are both present in solution in the ratio of 1:3; at a pH of ~9 HS− is fully formed, while at a pH above 15, S2− is the predominant species.
2.2. Naturally Occurring Donors
Currently available H2S-releasing compounds can be divided into two groups: naturally occurring donors and synthetic donors. Among the natural source, allium family and cruciferous vegetables are recognized to be rich in organosulfur compounds.
In particular, garlic contains at least thirty-three sulfur compounds, a concentration higher than any other allium species. The organosulfides in the allium family are represented by oil-soluble polysulfides and water-soluble thiosulfinates and appear to be responsible for many garlic’s medicinal effects. It has been demonstrated that the allicin, one of the biologically active components in garlic, and its principal transformation products, which are organic polysulfides, provide a critical role in garlic-induced cardioprotection [78,79,80,81].
In fact, it has been demonstrated that these compounds are responsible for lowering the arterial blood pressure, reducing the serum cholesterol and triglycerides, inhibiting the platelet aggregation, preventing the atherosclerosis, increasing the fibrinolytic activity, and reducing the oxidative stress [82].
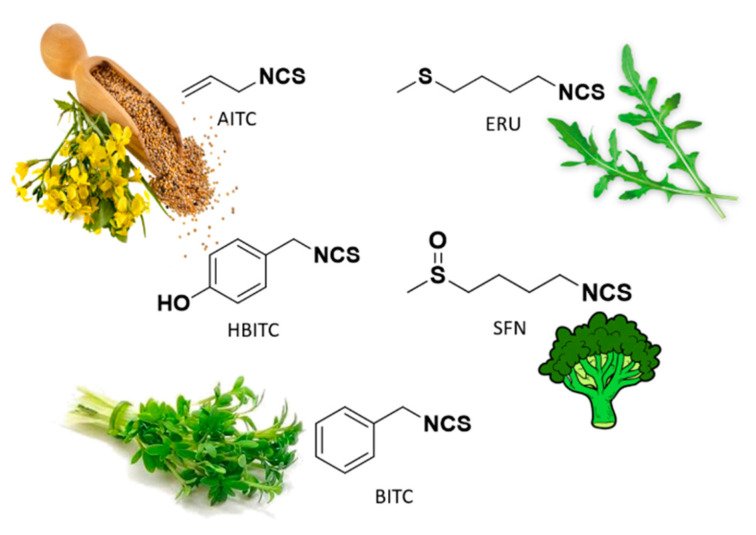
Among Cruciferae, vegetables such as broccoli, watercress, mustard, and garden cress are rich in isothiocyanates, such as sulforaphane (SFN, highly present in broccoli), allyl isothiocyanate (AITC, highly present in black mustard), benzyl isothiocyanate (BITC, highly present in garden cress), 4-hydroxybenzyl isothiocyanate (HBITC, highly present in white mustard), and erucin (ERU, mainly present in broccoli and rocket) (Figure 3).
Figure 3.
Chemical structures of natural isothiocyanates, which are abundant in cruciferous vegetables.
Recently, it has been demonstrated that these secondary metabolites of Brassicaceae, derived from corresponding glucosinolates upon the enzymatic action of myrosinase, show H2S-releasing properties. This finding shed light on the mechanism of action of Brassicaceae and on the role of hydrogen sulfide as relevant player for their nutraceutical and pharmaceutical effects [47].
Based on the mechanism of release, the above compounds belong to thiol-triggered donors; in fact, they react with the thiol groups of glutathione (GSH) or cysteine to release free H2S.
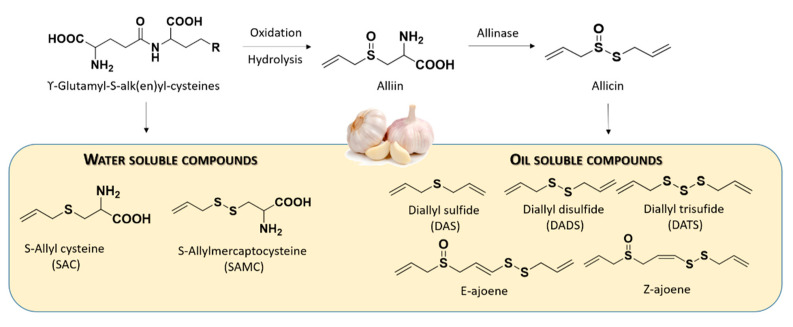
In the intact garlic, the primary sulfur compounds are the γ-glutamyl-S-alk(en)yl-L-cysteines which can be hydrolyzed and oxidized to yield alliin. Alliinase accounts for the conversion of alliin to allicin. The latter is highly instable in aqueous media and instantly decomposes, forming some oil-soluble compounds, such as diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), and ajoene [43]. These allicin-derived compounds led to H2S generation upon reaction with endogenous thiols, such as L-cysteine and GSH.
Furthermore, γ-glutamyl-S-alk(en)yl-L-cysteines can be also converted to water-soluble organosulfur compounds including S-allyl cysteine (SAC) and S-allyl mercaptocysteine (SAMC) (Figure 4).
Figure 4.
Chemical structures of commonly studied organosulfur compounds of garlic.
SAC, a sulfur-containing amino acid, was considered an endogenous H2S-producing agent by providing the substrate for CSE in the H2S synthesis (Figure S1).
It is responsible for many important activities, such as antioxidative [83,84], anticancer [85,86], anti-hepatotoxic [87,88], and cardioprotective activities [89]. In particular, it showed a cardiovascular protective role in MI by decreasing lipid peroxide products and improving the antioxidant status. Indeed, Chuah et al. also demonstrated that SAC could upregulate the expression of CSE, leading to enhanced H2S release. The increased H2S concentration in the myocardial and plasma is responsible for cardioprotection during acute MI [90].
Moreover, it was reported that S-propargyl-cysteine (SPRC) and S-propyl-cysteine (SPC) (Figure S1) also exhibited cardioprotective effects, via CSE/H2S pathway [91,92].
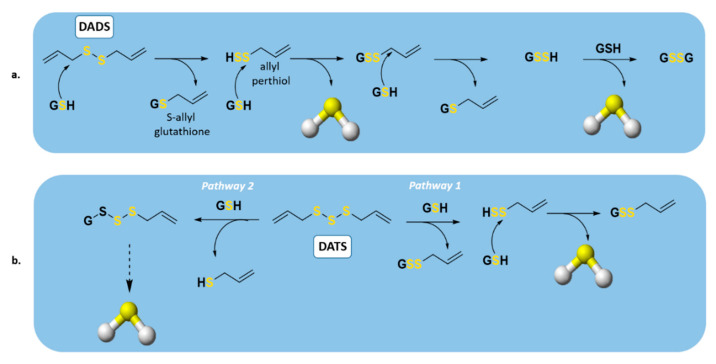
Among the oil-soluble components of garlic, the major organosulfur compounds were reported to be DADS and DATS. In 2007, Benavides et al. demonstrated that these compounds could release H2S rapidly through a thiol-dependent mechanism [93] (Figure 5). In the presence of GSH, DADS can react with it to generate S-allyl glutathione (GSA) and the intermediate allyl perthiol (ASSH), which reacts with another GSH to produce H2S and S-allyl glutathione disulfide (GSSA). Furthermore, the latter compound could also undergo α-carbon nucleophilic substitution, generating H2S rapidly [78] (Figure 5a). This mechanism has been widely confirmed [33,94,95,96,97,98,99], except for the rate of H2S release from DADS. Regarding this topic, in fact, Liang and co-workers demonstrated that DADS releases H2S very slowly through α-carbon nucleophilic substitution. The explanation of the previous misunderstanding of DADS as a fast H2S donor is DATS contamination in commercial samples of DADS; in fact, among these compounds, DATS is responsible for the rapid H2S release.
Figure 5.
H2S production from organic polysulfides by thiol reactions. Proposed mechanism of H2S production from (a) diallyl disulfide (DADS) and (b) diallyl trisulfide (DATS).
Regarding DATS (Figure 5b), two possible thiol−disulfide exchange reaction pathways can occur. Firstly (pathway 1), the nucleophilic attack of GSH on allylic sulfur of DATS can generate GSSA and ASSH, with the latter able to be reduced by GSH and thus releasing H2S; on the other side (pathway 2), the nucleophilic attack of GSH on the central sulfur atom of DATS can lead to the production of ASH and GSSSA. The latter can generate H2S rapidly.
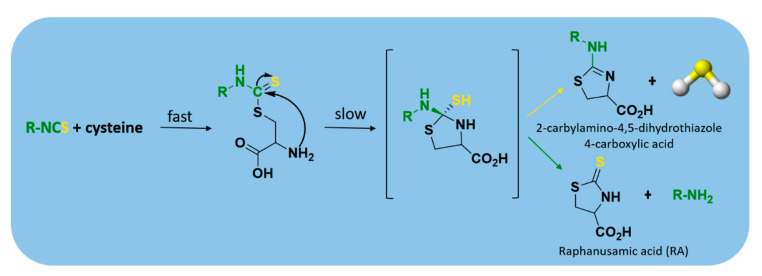
Currently, isothiocyanates are also drawing a lot of attention for their many biological and pharmacological effects in the prevention of important human diseases, such as cancer, neurodegenerative processes, and cardiovascular diseases [100]. In particular, Martelli et al. demonstrated that some selected aryl isothiocyanates were able to release H2S in a biological environment and produce a vasorelaxant effect in rat aortic rings, strongly antagonized by a specific Kv7-blocker, and in the coronary vascular bed, causing an increase of basal coronary flow [57]. Furthermore, the H2S-releasing properties of the secondary metabolites of Brassicaceae, isothiocyanates, are also derived from the rapid reaction with thiols, such as cysteine (Figure 6).
Figure 6.
The mechanism of H2S release from isothiocyanates.
The formed Cys-ITC adduct undergo intramolecular cyclization followed by releasing organic amine R−NH2 and raphanusamic acid (RA), on one hand, and H2S and 2-carbylamino-4,5-dihydrothiazole-4-carboxylic acid, on the other hand [101].
2.3. Synthetic Donors
Although naturally occurring H2S donors represent an attractive tool for in vivo studies, their poor toxicity and the tendency of these compounds to also generate some byproducts, which are not related to H2S production, led researchers to develop novel synthetic H2S donors with more favorable pharmacokinetic and safety profiles.
Based on their mechanism of H2S release, we have classified the synthetic compounds into different groups: hydrolysis-triggered donors, pH-controllable H2S donors, thiol-triggered donors, enzyme-triggered donors, and others (Table 1).
Table 1.
H2S-releasing molecules in cardiovascular system.
| H2S Donors | Chemical Structure | Mechanism of H2S Release |
Drug Development Phases |
|---|---|---|---|
| Lawesson’s reagent |
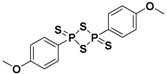
|
Hydrolysis-triggered | Pharmacological studies |
| GYY4137 |
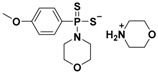
|
Hydrolysis-triggered | Preclinical trials |
| Phosphorodithioates |
|
Hydrolysis-triggered | Pharmacological studies |
| 1,2-dithiole-3-thiones (DTTs) |
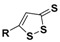
|
Hydrolysis-triggered | Pharmacological studies |
| JK donors |
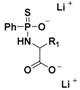
|
pH-controlled | Preclinical trials |
| Ammonium tetrathiomolybdate (ATTM) | (NH4)2MoS4 | pH-controlled | Phase I and II clinical trials for breast cancer |
| N-Benzoylthiobenzamides |
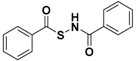
|
Thiol-triggered | Pharmacological studies |
| Acyl perthiols |
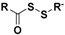
|
Thiol-triggered | Preclinical trials |
| Dithioperoxyanhydrides |
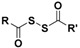
|
Thiol-triggered | Pharmacological studies |
| Arylthioamides |
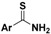
|
Thiol-triggered | Preclinical trials |
| Arylisothiocyanates |
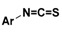
|
Thiol-triggered | Pharmacological studies |
| 1,2,4-Thiadiazolidine-3,5-diones |
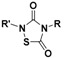
|
Thiol-triggered | Pharmacological studies |
| SG-1002 |
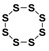
|
Thiol-triggered | Phase I clinical trial complete for heart failure |
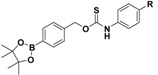
| Trimethyl lock (TML) |
|
Enzyme-triggered | Pharmacological studies |
|
N-Thiocarboxyanhydrides
(NTAs) |
|
Enzyme-triggered | Preclinical trials |
| PeroxyTCM |
|
Enzyme-triggered ROS-triggered |
Preclinical trials |
| Thioamino acids |
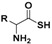
|
Bicarbonate-triggered | Pharmacological studies |
| ZYZ803 |
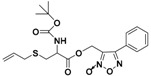
|
CSE/eNOS-dependent | Preclinical trials |
| Zofenopril |
|
ACE inhibitor | Clinical use for CVD |
| ACS 14 |
|
HS–ASA hybrid | Preclinical trials |
| AP-39 |
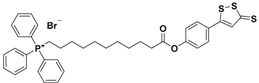
|
Mithocondrial-targeted | Preclinical trials |
2.3.1. Hydrolysis-Triggered Donors
Morpholin-4-ium-4-methoxyphenyl(morpholino)phosphinodithioate (GYY4137), a water-soluble Lawesson’s reagent derivative (Figure S2), is one of the first slow-releasing H2S donors developed [40,41,102] and the most commonly used research tool to investigate the role of H2S in the biological systems.
GYY4137 was synthesized starting from Lawesson’s reagent, previously obtained by heating a mixture of anisole with phosphorus pentasulfide (P4S10) [103,104], which reacts with morpholine in dichloromethane at room temperature (Figure S3) [40].
Much evidence has revealed that GYY4137 exhibits anti-inflammatory, antioxidant, and anticancer properties [52].
It also activates vascular smooth muscle KATP channels, and relaxes rat aortic rings and renal blood vessels, showing its anti-hypertensive activity [40].
Moreover, GYY4137 has been reported to inhibit microvascular thrombus formation and to stabilize atherosclerosis plaque by interfering with platelet activation and adhesion molecule-mediated aggregation [105]. It also protects against diabetic myocardial I/R injury, through activation of the PHLPP-1/Akt/Nrf2 pathway [106].
It has been demonstrated that GYY4137 slowly releases H2S upon hydrolysis [40]. In 2015, Alexander et al. carefully studied the hydrolysis kinetics of GYY4137, monitoring it by a combination of NMR spectroscopy and mass spectrometry [107]. Firstly, a sulfur–oxygen exchange with water occurs, leading to the release of H2S. The formed product, an aryl-phosphonamidothioate, undergoes complete hydrolysis to release a second molecule of H2S (Figure S4).
Despite GYY4137 having been proven to be a useful biological tool, it suffers from some drawbacks, such as its contamination with traces of dichloromethane residual from crystallization and the slow hydrolysis rate. These aspects could make the attribution of biological effects to GYY4137-derived H2S uncertain, because of the possible simultaneous metabolization of dichloromethane to CO, which has biological effects like H2S [108].
Therefore, structural modifications of GYY4137 were designed and the resulting analogs were studied. Park and coworkers developed a series of O-aryl- and alkyl-substituted phosphorodithioates as H2S donors, by replacing the P-C bond in GYY4137 with O-substitution [42] (Figure S2). Their studies evidenced that the gaseous release from these novel H2S donors did not significantly improve. In fact, similarly to the parent compound GYY4137, O-arylated donors showed slow H2S production, whereas O-alkylated donors exhibited very weak H2S generation.
Another class of compounds belonging to the family of hydrolysis-triggered H2S donors are 1,2-dithiole-3-thiones (DTTs) (Figure S2).
DTT compounds are anethole dithiolethione (ADT) and its O-demethylated derivative ADT-OH [5-(p-hydroxyphenyl)-3H-1,2-dithiole-3-thione], which were largely used as H2S donors (Figure S5).
To obtain DTTs, different methods can be applied. The most used synthetic strategy provides dehydrogenation and sulfurization of an allylic methyl group, by treating it with elemental sulfur or phosphorus pentasulfide products [109] (Figure S6). Alternatively, β-ketoesters could react with Lawesson’s reagent to give the desired DTTs [110,111] (Figure S6).
To date, several groups have studied the biological effects of ADT and ADT-OH, observing an important activity related to the H2S-releasing properties of these compounds. ADT is an FDA-approved drug, which can stimulate bile secretion, restoring salivation and relieving dry mouth in patients affected by chemotherapy-induced xerostomia [112]. Additionally, its derivative, ADT-OH, resulted in being useful for reducing cell viability via inhibition of histone deacetylase [113,114] and NF-kB activation [115].
Although their H2S-releasing mechanism is still not completely defined, it is widely accepted that the production of H2S from DTTs occurs via hydrolysis [34,116,117] (Figure S7). Indeed, it has been demonstrated that upon heating to 120 °C in aqueous solution, dithiolethione derivatives gradually release H2S, converting into the corresponding 1,2-dithiole-3-one [39].
Interestingly, H2S donor hybrids were obtained by coupling the OH group of ADT-OH with some commercially available drugs and the resulting compounds were studied for their H2S-releasing properties and therapeutic effects [118].
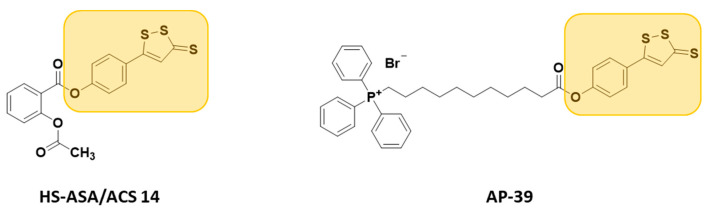
They are usually synthesized by coupling the active compounds with ADT-OH in the presence of N,N’-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) (Figure S8) [119]. These H2S donor hybrids exhibited their efficacy in many pharmacological fields, such as inflammation [120,121] (H2S-donating diclofenac, ACS-15/ATB-337; H2S-donating mesalamine, ATB-429), cancer [122] (H2S-donating sulindac, HS-SUL; H2S-donating aspirin, HS-ASA/ACS-14; H2S-donating ibuprofen, HS-IBU; H2S-donating naproxen, HS-NAP), erectile dysfunction [123] (H2S-donating sildenafil, ACS 6), neurodegeneration (H2S-donating latanoprost, ACS 67, for glaucoma treatment [124], and H2S-donating levodopa, ACS 83, for Parkinson’s disease [125]), and cardioprotection (H2S-donating aspirin, HS-ASA/ACS 14, and AP-39) (Figure 7).
Figure 7.
Chemical structures of H2S donor hybrids involved in cardiovascular disease.
Indeed, Rossoni and co-workers demonstrated that ACS14 exerts strong protective effects against buthionine sulfoximine (BSO)-induced cardiovascular diseases, by increasing systolic blood pressure and lowering heart rates in rats [126]. Additionally, oral administration of ACS 14 reduces BSO-induced hypertensive effects, unlike aspirin which has no effect. Moreover, ACS 14 treatment in BSO rats reduces myocardial I/R injury [126,127,128].
More recently, another hybrid H2S donor, named AP-39, resulting from the reaction of triphenylphosphonium with ADT-OH, was developed [129] and studied for its H2S-releasing properties and potential pharmacological effects.
AP-39 significantly inhibits oxidative stress-induced toxicity and protects against acute cardiac arrest, renal, and myocardial I/R injury, by inhibiting the mitochondrial permeability transition pore [130,131,132,133].
2.3.2. pH-Controllable H2S Donors
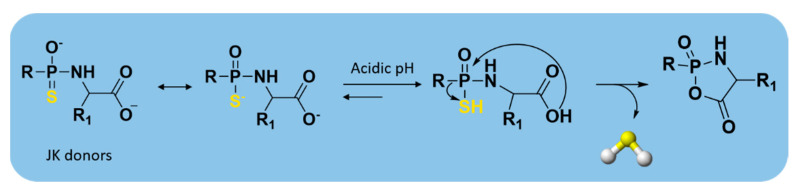
Starting from GYY4137 structure, Kang and coworkers developed a series of phosphonamidothioates, named JK donors [134].
To synthesize these compounds, the phenylphosphonothioic dichloride was treated with 3-hydroxypropionitrile and, consequently, with a selected C-protected amino acid, such as glycine, phenylalanine, valine, alanine, and proline. These steps provided the intermediate, which underwent LiOH-mediated hydrolysis, furnishing the final compounds (Figure S9).
These donors represent the first class of pH-controllable H2S donors. Indeed, in aqueous media under acidic conditions (pH = 2.0–6.0), JK donors had higher H2S release rates, whereas at neutral and mildly basic pH (7.4–8.0), they caused slower and less H2S release.
Kang and co-workers observed that these donors undergo a new hydrolysis mechanism of release. The protonation of phosphonamidothioates leads to the formation of the corresponding phosphorothiols, which cyclize via nucleophilic addition of the carboxylic acid group, resulting in breakage of the P-S bond and in release of H2S (Figure 8).
Figure 8.
H2S-releasing mechanism from JK donors.
Moreover, the two main donors (JK-1 and JK-2) of the series (Figure S10) showed protective effects on cellular and murine models of myocardial I/R injury. They were also successful in reducing infarct size and circulating troponin-I level significantly [134].
Furthermore, ammonium tetrathiomolybdate (ATTM), an excellent copper chelator with the formula (NH4)2MoS4, was identified as a pH-dependent H2S donor [135]. Indeed, it has been demonstrated that H2S can be generated from ATTM under strong acidic conditions (5% H2SO4) (Figure S11).
Later, Xu and co-workers described ATTM as a water-soluble and a pH-sensitive slow-releasing H2S donor [136]. More recently, it has been found that ATTM exerts protective effects on the heart and brain in rat models of myocardial and cerebral I/R injury and attenuates I/R injury [137].
Considering that some preclinical data suggested that H2S is responsible for protective effects in doxorubicin- and adriamycin-induced cardiomyopathy during cancer therapies [138,139,140] and that ATTM is currently in clinical trials for the treatment of breast cancer due to its copper depletion effects [141], the researchers are studying the potential dual role of ATTM as an anticancer and cardioprotective agent in patients with cancer treated with doxorubicin or adriamycin.
2.3.3. Thiol-Triggered Donors
Thiol-activated H2S-releasing compounds were some of the first synthetic donors to be reported. They can release H2S by reacting with endogenous thiol-containing molecules, such as GSH, which are relatively abundant in mammals. Some of these H2S donors were found to exhibit protective effects in cardiovascular diseases (Figure S12).
Among the first nucleophile-triggered H2S donors, a series of N-(benzoylthio)benzamides were synthesized by Zhao et al. [49] with the goal of developing compounds which are stable in aqueous solution. The N-(acylthiol)benzamide derivatives were prepared by treating thiocarboxylic acids with hydroxylamine-O-sulfonic acid under basic conditions; the following reaction between the obtained S-acylthiohydroxylamines and benzoic anhydride led to obtain the final products (Figure S13).
The resulting compounds were proven to be stable in aqueous buffers and to be able to release H2S only following a nucleophilic attack by thiols. The structure–activity relationship studies showed that electron withdrawing groups cause a faster H2S release, unlike electron donating groups. In addition, H2S release from N-(benzoylthio)benzamides was also detected in the plasma, revealing that N-SH compounds have efficacy in complex systems.
These compounds protect human keratinocytes against methylglyoxal (MGO)-induced cell damage and dysfunction, which is prevalent among patients with diabetes mellitus [142]. Moreover, they were evaluated in animal models of myocardial I/R injury. The reduction in infarct size over controls in murine models indicated N-(benzoylthio)benzamides as cardioprotective agents [143].
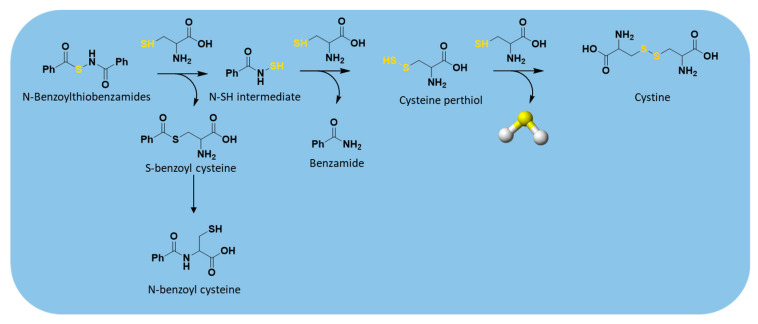
The thiol-triggered mechanism of release from these H2S donors starts with a thioester exchange between the donor and a first molecule of cysteine generating S-benzoyl cysteine and N-mercaptobenzamide, an N-SH intermediate (Figure 9).
Figure 9.
H2S release from N-benzoylthiobenzamides.
The resulting S-benzoyl cysteine, due to its high reactivity, undergoes a fast S to N acyl transfer, leading to the formation of a more stable N-benzoyl cysteine. At the same time, the formed N-SH intermediate reacts with another molecule of cysteine to give benzamide and cysteine perthiol, which finally reacts with cysteine to generate cystine and release H2S.
As cysteine perthiol was found to be the key intermediate for H2S generation from N-mercapto-based donors, in addition to its already-known involvement in H2S biosynthesis catalyzed by CSE [118,144], a library of perthiol-based donors (RC(O)–S–SR’) were designed and developed by Zhao and co-workers [50], aiming to mimic H2S bioproduction.
The authors prepared the above compounds as cysteine and penicillamine derivatives (Figure S14) and protected the unstable –SH residue with acyl groups.
Cys-S-SH-based donors were obtained through the treatment of N-benzoyl cysteine methyl ester with 2,2′-dipyridyl disulfide to attain a reactive intermediate, which then reacted with a thioacid to produce the desired compounds (Figure S15).
To obtain the other derivatives, C- and N-protected penicillamine was treated with 2,2′-dibenzothioazolyldisulfide, furnishing an intermediate, which quickly reacted with different thioacids to produce the desired donors (Figure S16).
Surprisingly, it was found that these donors exhibit different H2S-releasing profiles. Indeed, in the presence of thiols, penicillamine-based donors showed higher efficiency in H2S production than cysteine-based donors, which were able to release very small amounts of H2S. Probably, the two different behaviors could be explained by the presence of the two adjacent methyl groups of penicillamine-based donors, which prevent the cleavage of the disulfide bonds by thiols.
These donors showed tunable release rates by varying the aromatic R substituent. H2S release depends on both steric and electronic factors, with electron-withdrawing substituents accelerating release rates, and bulky substituents on the aromatic ring retarding release rates.
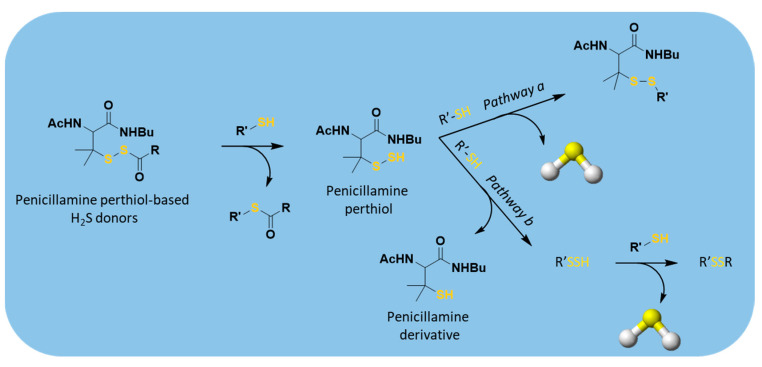
Regarding the H2S-releasing mechanism, the acyl perthiol donors also required disulfide exchange to allow H2S production, similarly to N-mercapto-based donors; therefore, deacylation and subsequent H2S release occurred (Figure 10).
Figure 10.
Proposed mechanism of H2S release from perthiol-based donors; R’-SH = cysteine or GSH.
H2S release from penicillamine-derived perthiol donors was also studied in vivo and in cardiac myocytes (H9c2 cells), confirming their ability to release. Moreover, the selected donors were evaluated into a murine model of myocardial I/R injury. The results proved that they exhibit cardioprotective activity due to their ability to reduce the infarct size.
Analogous perthiol-based donors are the dithioperoxyanhydrides (RC(O)–S–SC(O)R’) [51], which also have the disulfide linkage in their structures (Figure S12).
Both alkyl and aromatic dithioperoxyanhydride donors were obtained by two possible synthetic strategies: iodine oxidation of the thiocarboxylates [145] or reactions between thiocarboxylic acids and methoxycarbonylsulfenyl chloride [146] (Figure S17).
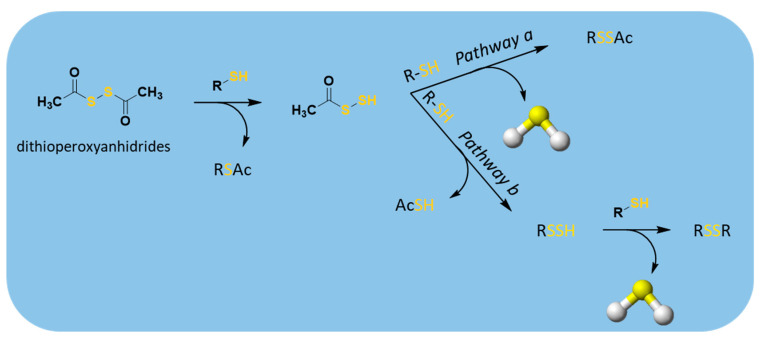
H2S release from these compounds was measured amperometrically and found in both buffers and cellular lysates, confirming H2S-releasing properties of dithioperoxyanhydrides. When treated with thiols, these donors formed a key intermediate for H2S production, the acyl-persulfides, which, reacting with the excess of thiols, could directly release H2S and RSSAc (pathway a, Figure 11); alternatively, it could produce a new perthiol species (RS-SH), which reacting in turn with thiols, leads to disulfide formation and H2S release (pathway b, Figure 11).
Figure 11.
Proposed mechanism for H2S release from dithioperoxyanhydrides; R′-SH = cysteine or GSH.
Furthermore, these donors were found to induce concentration-dependent vasorelaxation of pre-contracted rat aortic rings, presumably owing to H2S release. Moreover, the arylthioamides (Figure S12) were also classified as thiol-activated H2S donors [52]. The non-heterocyclic aromatic compounds were obtained by mixing the corresponding benzonitrile with P4S10; whereas the reaction between amides with Lawesson’s reagent led to heterocyclic aromatic compounds being obtained (Figure S18).
These compounds showed the ability to release H2S in the presence of thiols, similarly to DADS and GYY4137, two slow-releasing donors. To date, the H2S release mechanism and relative intermediates and final products have not yet been clearly described.
After confirming their H2S-releasing profile, Calderone and coworkers tested the effects of these donors on noradrenaline-induced vasoconstriction in isolated rat aortic rings. The results suggested that the lead compound, p-hydroxybenzothioamide (Figure S19), has protective activity in cardiovascular systems, inhibiting norepinephrine-induced vasoconstriction in ex vivo in rat aortic rings, and reducing systolic blood pressure.
Considering the slow and sustained H2S release profile of p-hydroxybenzothioamide and its ease of conjugation to other compounds, this donor was used to develop many hybrid compounds. Among these, p-hydroxybenzothioamide was conjugated with the active compound naproxen, obtaining ATB-346 (Figure S19) [147]. Thus, the efficacy of this hybrid as an anticancer drug was investigated, confirming its apoptotic action in human melanoma cells [148]. Moreover, it has been demonstrated that ATB-346 reduces gastrointestinal tract injury and exhibits chemopreventive action against colorectal cancer when compared to naproxen [149]. Recently, a phase II study was completed and revealed that ATB-346 is useful for the treatment of pain associated with osteoarthritis [150].
These positive preclinical data for ATB-346 suggested that H2S-releasing hybrid drugs could undergo further development for the treatment of cardiovascular disease.
Moreover, Martelli and co-workers reported aryl isothiocyanates as another series of thiol-activated H2S donors (Figure S12) [57]. These compounds showed vasorelaxant effects on conductance and coronary arteries.
Noteworthy, 4-carboxyphenyl-isothiocyanate (4-CPI) and 3-pyridyl-isothiocyanate (Figure S20) exhibited cardioprotective effects against I/R injury, reducing myocardial contractility, ventricular arrhythmias, and oxidative stress [58,151].
The mechanism of H2S release from these compounds has already been described above (Figure 6). The nature of the R groups supporting the isothiocyanate function have a strong impact on H2S-releasing rates. For examples, p-nitrophenyl isothiocyanate produces H2S relatively fast, while benzylisothiocyanate and phenethyl isothiocyanate are considered slow H2S donors [101].
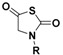
More recently, 1,2,4-thiadiazolidine-3,5-diones (THIA) (Figure S12), which are isothiocyanate derivatives, developed by Severino et al. [53], were demonstrated to be novel thiol-triggered H2S donors.
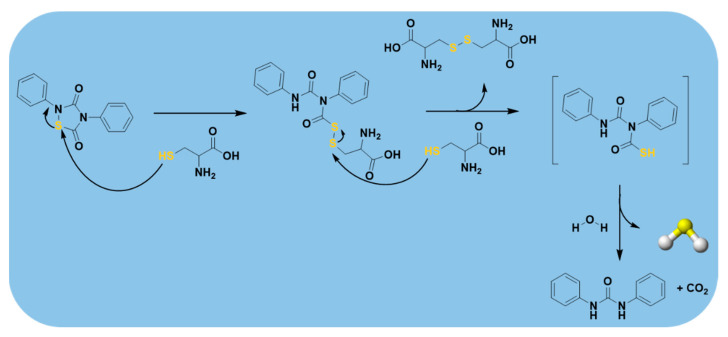
These compounds were obtained through oxidative condensation of isothiocyanates with isocyanates in the presence of SO2Cl2, using both the conventional synthetic strategy, previously described in literature [152], and the microwave-assisted synthesis [53]. The second way led better yields being obtained and the reduction of reaction times (Figure S21). The authors clearly defined the mechanism of H2S release by using a combination of HPLC, MS, and NMR to isolate and characterize the intermediates and the final products.
As depicted in Figure 12, the nucleophilic attack of thiols, for example cysteine, on the electron deficient sulfur atom of the 1,2,4-thiadiazolidine-3,5-dione nucleus opens the ring and produces an intermediate. When this latter reacts with a second molecule of cysteine, it generates cystine and phenyl(phenylcarbamoyl)carbamothioic S-acid, an instable intermediate, which is immediately hydrolyzed, yielding 1,3-diphenylurea, CO2, and H2S.
Figure 12.
H2S-releasing mechanism from 1,2,4-thiadiazolidine-3,5-diones (THIA).
It was also found that these donors exerted vasodilating activity in a concentration-dependent manner on isolated aorta rings.
Furthermore, a sodium polysulthionate, named SG-1002 (Figure S12), was developed. This synthetic H2S prodrug is a water-insoluble microcrystalline material, mostly consisting of α-sulfur (about 99% S8) hydrophilized with traces of ionic substances, such as sodium sulfate, sodium thiosulfate, and sodium polythionates, which enhance its bioavailability [153].
SG-1002 was obtained via comproportionation of sulfur atoms in the -2 and +4 oxidation states in a strongly acidic medium of high ionic strength. This synthetic route, together with properties and some therapeutic applications of SG1002 are described in a patent [154]. H2S-releasing from S8 is activated by thiols and this reaction with GSH is depicted in Figure S22 [155,156,157].
SG-1002, when administered orally, produced a more sustained and consistent increase in H2S and sulfane sulfur levels in models of pressure overload heart failure. Moreover, it improved coronary artery vascular reactivity and attenuated high-fat diet-induced cardiac hypertrophy and dysfunction [153,158,159].
Importantly, SG-1002 has completed the phase I clinical trial where its H2S-donating ability in patients with congestive heart failure was investigated [160]. The results of this trial confirmed that SG-1002 is useful both in healthy patients and in patients with heart failure. Indeed, it effectively enhances sulfide and NO levels in patients with heart failure. Nevertheless, further trials are required to test the drug’s efficacy [161].
To date, it is the only sulfide-based therapy clinically tested in patients with cardiovascular diseases.
2.3.4. Enzyme-Triggered Donors
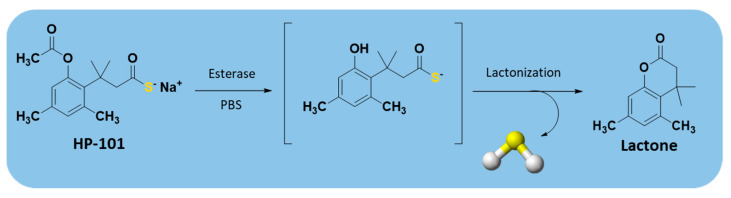
In recent years, enzyme-triggered H2S donors have been received increasing interest due to their many advantages over the other triggers already mentioned above. Among these, a series of esterase-sensitive prodrugs of H2S, based on a lactonization reaction, also known as ‘‘trimethyl lock” (TML) [162], was developed by Zheng et al. [163] (Figure S23).
The first synthesized derivative, HP-101, was obtained starting from compound 1 (Figure S24), which, reacting with Lawesson’s reagent under microwave conditions [164], resulted in an intermediate that was then treated with one equivalent of sodium hydroxide.
The obtained TML-based prodrugs were able to release H2S, through the cleavage of a phenolic ester by an esterase and subsequent lactonization, owing to the steric repulsion of three methyl groups (Figure 13).
Figure 13.
Mechanism of esterase-triggered H2S release.
Interestingly, a TML derivative, HP-102 (Figure S24), was found to attenuate myocardial I/R injury in a dose-dependent manner and exhibited a cardioprotective action [165]. Thus, this compound is currently under investigation to better define its chemical, pharmacological, and safety profile in the hope that it could be tested in clinical trials.
Another class of enzyme-triggered H2S donors is carbonyl sulfide (COS)-releasing compounds. Indeed, COS is a substrate of carbonic anhydrase (CA), which catalyzes its conversion into H2S [166,167].
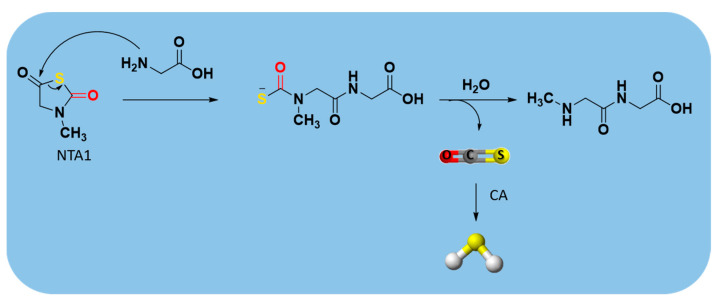
An example of these dual COS/H2S donors are N-thiocarboxyanhydrides (NTAs) (Figure S25) [168,169], which have the advantage of releasing, in addition to COS, only innocuous peptide byproducts [170].
The sarcosine NTA derivative (NTA1) (Figure S25) was synthesized starting from sarcosine (N-methyl glycine) through the route depicted in Figure S26 [171].
The mechanism leading to H2S production from NTA1 consists of ring-opening by a biological nucleophile, such as an amine. The resulting dipeptide byproducts were confirmed by GC-MS and LC-MS, respectively. After COS release, its conversion into H2S occurs through the rapid enzymatic action of CA (Figure 14) [170].
Figure 14.
Mechanism of carbonyl sulfide (COS)/H2S release from NTA1.
Furthermore, Powell et al. showed that the treatment of endothelial cells with NTA1 increased proliferation, which is an important step in the angiogenetic process [170].
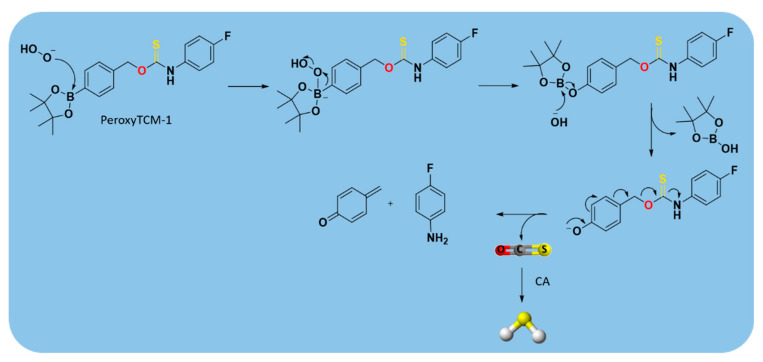
Among the class of COS/H2S donors, some ROS-triggered H2S donors (peroxyTCM-1, 2, 3) were developed [172], using a ROS-cleavable aryl boronate as the protecting group [173,174]. They were obtained as depicted in Figure S27.
The obtained compounds were found to release H2S in the presence of an oxidant, such as H2O2. In this mechanism, the boronate ester was converted to a phenol by ROS, releasing COS, which was quickly converted into H2S by CA [172] (Figure 15).
Figure 15.
Proposed mechanism for H2O2-triggered COS/H2S release.
Moreover, it was demonstrated that the combined role of boronate ester, as ROS scavenger, with the produced H2S, as cytoprotective agent, led the ROS-activated H2S donors to exhibit important activities in many human pathologies driven by high oxidative stress, such as cardiovascular diseases [133].
However, further in vivo studies are required to better characterize the biological nature of these donors and to confirm their efficacy.
2.3.5. Others
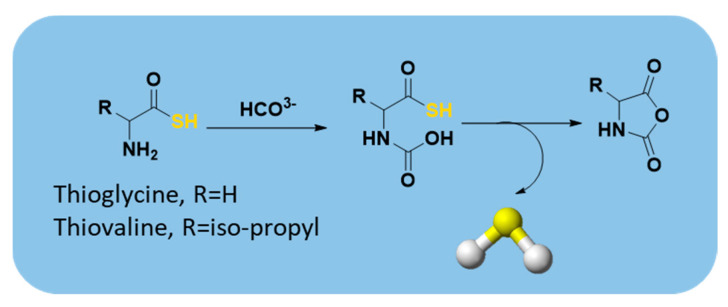
In 2012, Zhou et al. reported that thioamino acids, such as thioglycine and thiovaline, were bicarbonate-triggered H2S donors [48]. They synthesized the selected compounds starting from commercially available Boc-protected amino acids, which were treated with 1,1′-carbonyldiimidazole in dichloromethane and subsequently with gaseous H2S. The following deprotection with trifluoroacetic acid (TFA) in dichloromethane furnished the desired compounds (Figure S28).
The authors proved that, in the presence of bicarbonate, these compounds react through their amine groups and consequently undergo a cyclization, converting to the corresponding amino acid N-carboxyanhydrides with the production of H2S (Figure 16). Considering the proposed mechanism of release and the high concentrations of bicarbonate in blood, thioamino acids could be effective H2S donors.
Figure 16.
Proposed mechanism for H2S release from thioamino acids.
Indeed, H2S-releasing capabilities of thioglycine and thiovaline were evaluated and proved amperometrically. In addition, both thioglycine and thiovaline were found to enhance cGMP formation in a concentration-dependent manner and induce significant relaxation of pre-contracted mouse aortic rings.
However, the high reactivity of these amino acids must be considered. In fact, it was found that under aerobic conditions they could rapidly undergo amidation [175,176,177,178,179,180] and oxidation reactions [51], yielding products which could lead to unwanted side effects.
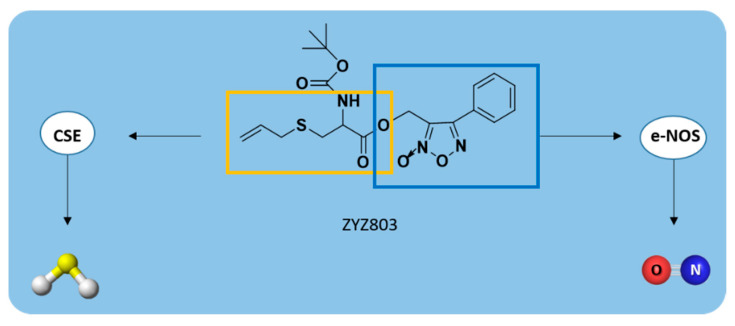
Recently, a novel H2S-NO-releasing molecule, 2-(N-Boc-amino)-3-prop-2-ynylsulfanylpropionic acid, named ZYZ803, was developed [181] by coupling SPRC with furoxan, upon treatment with Boc anhydride (Figure S29) [181,182].
As already described above, SPRC could enhance H2S concentration in plasma through the activation of CSE enzyme [92] while furoxan is a thiol-triggered NO-releasing compound [183].
Therefore, ZYZ803 stimulated both the expression of CSE and the activity of endothelial NO synthase (e-NOS), releasing H2S and NO, respectively (Figure 17). These gasotransmitters promoted angiogenesis through the SIRT1/VEGF/cGMP pathway and via cross-talk between STAT3 and CaMKII [184].
Figure 17.
H2S/NO-releasing mechanism from ZYZ803.
As reported by Hu et al., ZYZ803 significantly promoted endothelial cell angiogenesis both in vitro and in vivo [185].
Indeed, the oral administration of ZYZ803 in a mouse model of hindlimb ischemia stimulated angiogenesis more effectively than individually administered SPRC and furoxan, confirming the additive protective actions of H2S and NO releasing from ZYZ803 [185].
Additionally, ZYZ803 regulated the vascular tone in isolated rat aortic rings [181], attenuated cardiac dysfunction, and improved myocardial injury after heart failure [186], exerting a powerful cardioprotective effect.
Lastly, another H2S donor with activity in the cardiovascular system is zofenopril, an angiotensin-converting enzyme (ACE) inhibitor. Undergoing hydrolysis in the liver, it forms zofenoprilat, its active metabolite containing a thiol group (Figure S30).
It is important to underline that zofenopril is not a new drug; in fact, it was approved for medical use in 2000. Recently, Bucci et al. demonstrated that this compound can release H2S [187], which mediates the beneficial effects of zofenopril, such as anti-inflammatory, pro-angiogenic and anti-apoptotic activity [187,188,189,190,191].
Additionally, zofenopril was reported to improve vascular function in a model of hypertension, combining its ACE inhibitor activity and H2S-releasing properties [187].
Indeed, it was also found that zofenopril administration increases the levels of H2S metabolites in the plasma of mice, protecting against myocardial I/R injury, and pigs, improving endocardial blood flow in myocardial ischemia [192].
3. Conclusions
During last decades, remarkable progress has been made in the understanding of the biological activity of H2S and its mechanism of actions in the cardiovascular system and in the discovering and developing of novel H2S-releasing compounds.
Herein we presented an overview of the currently available H2S donating agents that have been tested in preclinical models of cardiovascular diseases, with a focus on fundamental chemistry and their H2S-releasing properties and mechanisms.
As described above, these H2S donors include inorganic sulfide salts, natural and synthetic organic compounds, characterized by different release triggering mechanisms.
Although many donors have been developed and tested in vitro and in vivo studies, showing their ability to release H2S and the positive effects when treating cardiovascular diseases, to date it is not yet possible to identify an ideal donor, owing to some drawbacks for each of them.
Indeed, the major limitations for many donors are that the release does not mimic the endogenous H2S production and leads to the simultaneous generation of reactive byproducts, whose nature and biological activity are often still unknown.
To overcome these drawbacks, several new classes of donors with a sustained and controlled H2S release were designed, synthesized, and studied for their biological activity, also defining their mechanism of release; moreover, they were successfully tested in various animal models of cardiovascular disease.
Among them, at least two H2S-releasing compounds (ATTM and SG-1002) have completed the phase I clinical trials and registered on clinicaltrials.gov for cardiovascular disease and breast cancer (Table 1).
Nevertheless, there are still too few available donors with oral bioavailability, which is crucial for clinical translation, and being available for testing in in vivo pharmacokinetic studies. Furthermore, the safety and long-term effects of many H2S-releasing compounds have yet to be more tested and fully characterized.
Bearing in mind the above considerations, this work, by reviewing the available H2S donors exhibiting cardioprotective activity and thus providing an insightful knowledge of their chemical nature and mechanisms of release, could enhance the chances to design novel H2S-donating molecules useful to help further reduce the risks of cardiovascular disease in the coming years.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/10/3/429/s1, The following are available online. Figure S1. The chemical structures of SAC, SPC, and SPRC. Figure S2. Chemical structures of hydrolysis-triggered H2S donors. Figure S3. Chemical synthesis of GYY4137. Figure S4. The hydrolytic degradation of GYY4137 and the H2S release. Figure S5. Structures of the most used DTTs as H2S donors. Figure S6. Different chemical strategies to synthesize DTTs. Figure S7. Mechanism of H2S release from DTTs. Figure S8. Chemical synthesis of HS-NSAIDs. Figure S9. Synthetic strategy to obtain JK donors. Figure S10. Chemical structures of two main JK donors. Figure S11. The H2S release from ammonium tetrathiomolybdate (ATTM). Figure S12. Chemical structures of thiol-triggered H2S donors with cardioprotective activity. Figure S13. Chemical synthesis of N-(benzoylthio)benzamides. Figure S14. Chemical structures of perthiol-based donors. Figure S15. Synthetic route to obtain cysteine perthiol-based donors. Figure S16. Chemical synthesis of penicillamine perthiol-based donors. Figure S17. The synthetic strategies to obtain dithioperoxyanhydride-based H2S. Figure S18. Chemical synthesis of (a) non-heterocyclic and (b) heterocyclic p-hydroxy-arylthio-amides. Figure S19. The thioamides: General structure, chemical structures of lead compound (p-hydroxybenzothioamide) and its hybrid with naproxen (ATB-346). Figure S20. Chemical structures of two aryl isothiocyanates exhibiting cardioprotective activity. Figure S21. Synthesis of THIA. Conventional synthetic strategy: (i) SO2Cl2/diethyl ether, 24 h; (ii) H2O, reflux, 30 min; microwave-assisted synthesis: (i) SO2Cl2/diethyl ether/MW 500 W, 50 °C, 1h; (ii) H2O, MW 500 W, 120 °C, 30 min. Figure S22. Mechanism of H2S release from SG1002. Figure S23. Chemical structures of “TML”-based H2S prodrugs HP-101 and HP-102. Figure S24. Synthesis of “TML”-based H2S prodrug HP-101. Figure S25. Chemical structures of N-thiocarboxyanhydrides (NTAs) and sarcosine NTA derivative (NTA1). Figure S26. Chemical synthesis of sarcosine NTA derivative (NTA1). Figure S27. Chemical structures and synthesis of ROS-activated H2S donors. Figure S28. Synthesis of thioamino acids. Figure S29. Chemical structure and synthesis of ZYZ803. Figure S30. Chemical structures of (a) zofenopril and (b) its active metabolite, zofenoprilat.
Author Contributions
Conceptualization, A.C., G.C. and V.S.; resources, F.F. (Francesco Frecentese) and A.S.; writing—original draft preparation, A.C., B.S., E.M. and E.P.; writing—review and editing, B.S. and F.F. (Ferdinando Fiorino); supervision, B.S. and F.F. (Ferdinando Fiorino). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 2.Ungerer P., Wender A., Demoulin G., Bourasseau É., Mougin P. Application of Gibbs Ensemble and NPT Monte Carlo Sim-Ulation to the Development of Improved Processes for H2S-Rich Gases. Mol. Simul. 2004;30:631–648. doi: 10.1080/08927020410001709299. [DOI] [Google Scholar]
- 3.Kabil O., Banerjee R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L., Rose P., Moore P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 5.Gadalla M.M., Snyder S.H. Hydrogen Sulfide as a Gasotransmitter. J. Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miles E.W., Kraus J.P. Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-causing Mutations. J. Biol. Chem. 2004;279:29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 7.Pan L.L., Liu X.H., Gong Q.H., Yang H.B., Zhu Y.Z. Role of Cystathionine C-Lyase/Hydrogen Sulfide Pathway in Cardio-Vascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal. 2011;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxidants Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 9.Marcotte P., Walsh C. Active Site-Directed Inactivation of Cystathionine Gamma-Synthetase and Glutamic Pyruvic Transami-Nase by Propargylglycine. Biochem. Biophys. Res. Commun. 1975;62:677–682. doi: 10.1016/0006-291X(75)90452-0. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q., Collins R., Huang S., Holmberg-Schiavone L., Anand G.S., Tan C.H., van-den-Berg S., Deng L.W., Moore P.K., Karlberg T., et al. Structural Basis for the Inhibition Mechanism of Human Cystathionine Gamma-Lyase, an Enzyme Responsible for the Production of H2S. J. Biol. Chem. 2009;284:3076–3085. doi: 10.1074/jbc.M805459200. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer M., Ressler C. β-Cyanoalanine, An Inhibitor of Rat Liver Cystathionase. Biochem. Pharmacol. 1967;16:2299–2308. doi: 10.1016/0006-2952(67)90217-1. [DOI] [PubMed] [Google Scholar]
- 12.Corvino A., Severino B., Fiorino F., Frecentese F., Magli E., Perissutti E., Santagada V., Bucci M., Cirino G., Kelly G., et al. Fragment-Based de Novo Design of a Cystathionine γ-Lyase Selective Inhibitor Blocking Hydrogen Sulfide Production. Sci. Rep. 2016;6:srep34398. doi: 10.1038/srep34398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asimakopoulou A., Panopoulos P., Chasapis C.T., Coletta C., Zhou Z., Cirino G., Giannis A., Szabo C., Spyroulias G.A., Papapetropoulos A. Selectivity of Commonly Used Pharmacological Inhibitors for Cystathionine β Synthase (CBS) and Cystathi-Onine γ Lyase (CSE) Br. J. Pharm. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore P.K., Bhatia M., Moochhala S. Hydrogen Sulfide: From the Smell of the past to the Mediator of the Future? Trends Pharmacol. Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang R. Hydrogen Sulfide: The Third Gasotransmitter in Biology and Medicine. Antioxid. Redox Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 16.Polhemus D.J., Lefer D.J. Emergence of Hydrogen Sulfide as an Endogenous Gaseous Signaling Molecule in Cardiovascular Disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S., Li H., Ge J. A Cardioprotective Insight of the Cystathionine γ-Lyase/Hydrogen Sulfide Pathway. IJC Hear. Vasc. 2015;7:51–57. doi: 10.1016/j.ijcha.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng G., Ma Y., Xie L., Ferro A., Ji Y. Emerging Role of Hydrogen Sulfide in Hypertension and Related Cardiovascular Diseases. Br. J. Pharmacol. 2015;172:5501–5511. doi: 10.1111/bph.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X.-H., Wang F., You S.-J., Cao Y.-J., Cao L.-D., Han Q., Liu C.-F., Hu L.-F. Dysregulation of Cystathionine γ-Lyase (CSE)/Hydrogen Sulfide Pathway Contributes to Ox-LDL-Induced Inflammation in Macrophage. Cell. Signal. 2013;25:2255–2262. doi: 10.1016/j.cellsig.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Whiteman M., Moore P.K. Hydrogen Sulfide and the Vasculature: A Novel Vasculoprotective Entity and Regulator of Nitric Oxide Bioavailability? J. Cell. Mol. Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y., Du H.-P., Li J., Xu R., Wang Y.-L., You S.-J., Liu H., Wang F., Cao Y.-J., Liu C.-F., et al. Statins Upregulate Cystathionine γ-Lyase Transcription and H2S Generation via Activating Akt Signaling in Macrophage. Pharmacol. Res. 2014;87:18–25. doi: 10.1016/j.phrs.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Yu X.-H., Cui L.-B., Wu K., Zheng X.-L., Cayabyab F.S., Chen Z.-W., Tang C.-K. Hydrogen Sulfide as a Potent Cardiovascular Protective Agent. Clin. Chim. Acta. 2014;437:78–87. doi: 10.1016/j.cca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y., Ji X., Ji K., Wang B. Hydrogen Sulfide Prodrugs-A Review. Acta Pharm. Sin. B. 2015;5:367–377. doi: 10.1016/j.apsb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvert J.W., Elston M., Nicholson C.K., Gundewar S., Jha S., Elrod J.W., Ramachandran A., Lefer D.J. Genetic and Pharmacologic Hydrogen Sulfide Therapy Attenuates Ischemia-Induced Heart Failure in Mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine Gamma-Lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Zhao X., Jin H., Wei H., Li W., Bu D., Tang X., Ren Y., Tang C., Du J. Role of Hydrogen Sulfide in the Devel-Opment of Atherosclerotic Lesions in Apolipoprotein E Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 27.Van Den Born J.C., Mencke R., Conroy S., Zeebregts C.J., Van Goor H., Hillebrands J.L. Cystathionine Gamma-Lyase Is Expressed in Human Atherosclerotic Plaque Microvessels and Is Involved in Micro-Angiogenesis. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep34608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao W., Zhang J., Lu Y., Wang R. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous KATP Channel Opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawe G.S., Han S.P., Bian J.S., Moore P.K. Hydrogen Sulphide in the Hypothalamus Causes an ATP-Sensitive K Chan-Nel-Dependent Decrease in Blood Pressure in Freely Moving Rats. Neuroscience. 2008;152:169–177. doi: 10.1016/j.neuroscience.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Liu W.Q., Chai C., Li X.Y., Yuan W.J., Wang W.Z., Lu Y. The Cardiovascular Effects of Central Hydrogen Sulfide Are Related to K(ATP) Channels Activation. Physiol. Res. 2011;60:729–738. doi: 10.33549/physiolres.932092. [DOI] [PubMed] [Google Scholar]
- 31.Wang R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 32.Xu S., Liu Z., Liu P. Targeting Hydrogen Sulfide as a Promising Therapeutic Strategy for Atherosclerosis. Int. J. Cardiol. 2014;172:313–317. doi: 10.1016/j.ijcard.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 33.Wang R., Szabo C., Ichinose F., Ahmed A., Whiteman M., Papapetropoulos A. The Role of H2S Bioavailability in Endothelial Dysfunction. Trends Pharmacol. Sci. 2015;36:568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan S., Shen X., Kevil C.G. Beyond a Gasotransmitter: Hydrogen Sulfide and Polysulfide in Cardiovascular Health and Im-Mune Response. Antioxid. Redox Signal. 2017;27:634–653. doi: 10.1089/ars.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoki R., Matsuki N., Kimura H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez Aranguren L.C., Ahmad S., Dias I.H.K., Alzahrani F.A., Rezai H., Wang K., Ahmed A. Bioenergetic Effects of Hydrogen Sulfde Suppress Soluble Flt 1 and Soluble Endoglin in Cystathionine Gamma Lyase Compromised Endothelial Cells. Sci. Rep. 2020;10:15810–15820. doi: 10.1038/s41598-020-72371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., et al. Hydrogen Sulfide Is an Endogenous Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes M.N., Centelles M.N., Moore K.P. Making and Working with Hydrogen Sulfide. Free. Radic. Biol. Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Ozturk T., Ertas A.E., Mert O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007;107:5210–5278. doi: 10.1021/cr040650b. [DOI] [PubMed] [Google Scholar]
- 40.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.H., Moore P.K. Characterization of a Novel, Water-Soluble Hydrogen SulfiDe-releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 41.Rose P., Dymock B.W., Moore P.K. GYY4137, a Novel Water-Soluble, H2S-Releasing Molecule. Methods Enzymol. 2015;554:143–167. doi: 10.1016/bs.mie.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Park C., Zhao Y., Zhu Z., Pacheco A., Peng B., Devarie-Baez N.O., Bagdon P., Zhang H., Xian M. Synthesis and Evaluation of Phosphorodithioate-Based Hydrogen Sulfide Donors. Mol. Biosyst. 2013;9:2430–2434. doi: 10.1039/c3mb70145j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brodnitz M.H., Pascale J.V., Van Derslice L. Flavor Components of Garlic Extract. J. Agric. Food Chem. 1971;19:273–275. doi: 10.1021/jf60174a007. [DOI] [Google Scholar]
- 44.Rahman M.S. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007;10:245–268. doi: 10.1080/10942910601113327. [DOI] [Google Scholar]
- 45.Rana S.V., Pal R., Vaiphei K., Sharma S.K., Ola R.P. Garlic in Health and Disease. Nutr. Res. Rev. 2011;24:60–71. doi: 10.1017/S0954422410000338. [DOI] [PubMed] [Google Scholar]
- 46.Butt M.S., Sultan M.T., Butt M.S., Iqbal J. Garlic: Nature’s Protection against Physiological Threats. Crit. Rev. Food Sci. Nutr. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 47.Citi V., Martelli A., Testai L., Marino A., Breschi M.C., Calderone V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effects of Brassicaceae? Planta Medica. 2014;80:610–613. doi: 10.1055/s-0034-1368591. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Z., Rekowski M.V.W., Coletta C., Szabo C., Bucci M., Cirino G., Topouzis S., Papapetropoulos A., Giannis A. Thioglycine and L-Thiovaline: Biologically Active H2S-donors. Bioorganic Med. Chem. 2012;20:2675–2678. doi: 10.1016/j.bmc.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y., Wang H., Xian M. Cysteine-Activated Hydrogen Sulfide (H2S) Donors. J. Am. Chem. Soc. 2010;133:15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y., Bhushan S., Yang C., Otsuka H., Stein J.D., Pacheco A., Peng B., Devarie-Baez N.O., Aguilar H.C., Lefer D.J., et al. Controllable Hydrogen Sulfide Donors and Their Activity against Myocardial Ischemia-Reperfusion Injury. ACS Chem. Biol. 2013;8:1283–1290. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roger T., Raynaud F., Bouillaud F., Ransy C., Simonet S., Crespo C., Bourguignon M.-P., Villeneuve N., Vilaine J.-P., Artaud I., et al. New Biologically Active Hydrogen Sulfide Donors. ChemBioChem. 2013;14:2268–2271. doi: 10.1002/cbic.201300552. [DOI] [PubMed] [Google Scholar]
- 52.Martelli A., Testai L., Citi V., Marino A., Pugliesi I., Barresi E., Nesi G., Rapposelli S., Taliani S., Da Settimo F., et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013;4:904–908. doi: 10.1021/ml400239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Severino B., Corvino A., Fiorino F., Luciano P., Frecentese F., Magli E., Saccone I., Di Vaio P., Citi V., Calderone V., et al. 1,2,4-Thiadiazolidin-3,5-diones as Novel Hydrogen Sulfide Donors. Eur. J. Med. Chem. 2018;143:1677–1686. doi: 10.1016/j.ejmech.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 54.Barresi E., Nesi G., Citi V., Piragine E., Piano I., Taliani S., Da Settimo F., Rapposelli S., Testai L., Breschi M.C., et al. Iminothioethers as Hydrogen Sulfide Donors: From the Gasotransmitter Release to the Vascular Effects. J. Med. Chem. 2017;60:7512–7523. doi: 10.1021/acs.jmedchem.7b00888. [DOI] [PubMed] [Google Scholar]
- 55.Mitidieri E., Tramontano T., Gurgone D., Citi V., Calderone V., Brancaleone V., Katsouda A., Nagahara N., Pa-papetropoulos A., Cirino G., et al. Mercaptopyruvate Acts as Endogenous Vasodi-Lator Independently of 3-Mercaptopyruvate Sulfurtransferase Activity. Nitric Oxide. 2018;75:53–59. doi: 10.1016/j.niox.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Ercolano G., De Cicco P., Frecentese F., Saccone I., Corvino A., Giordano F., Magli E., Fiorino F., Severino B., Calderone V., et al. Anti-metastatic Properties of Naproxen-HBTA in a Murine Model of Cutaneous Melanoma. Front. Pharmacol. 2019;10:66. doi: 10.3389/fphar.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martelli A., Testai L., Citi V., Marino A., Bellagambi F.G., Ghimenti S., Breschi M.C., Calderone V. Pharmacological Characterization of the Vascular Effects of Aryl Isothiocyanates: Is Hydrogen Sulfide the Real Player? Vasc. Pharmacol. 2014;60:32–41. doi: 10.1016/j.vph.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Citi V., Corvino A., Fiorino F., Frecentese F., Magli E., Perissutti E., Santagada V., Brogi S., Flori L., Gorica E., et al. Structure-Activity Relationships Study of Isothiocyanates for H2S Releasing Properties: 3-Pyridyl-Isothiocyanate as a New Promising Cardioprotective Agent. J. Adv. Res. 2021;27:41–53. doi: 10.1016/j.jare.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y., Steiger A.K., Pluth M.D. Cyclic Sulfenyl Thiocarbamates Release Carbonyl Sulfide and Hydrogen Sulfide Inde-pendently in Thiol-Promoted Pathways. J. Am. Chem. Soc. 2019;141:13610–13618. doi: 10.1021/jacs.9b06319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esechie A., Kiss L., Olah G., Horváth E.M., Hawkins H., Szabo C., Traber D.L. Protective Effect of Hydrogen Sulfide in a Murine Model of Acute Lung Injury Induced by Combined Burn and Smoke Inhalation. Clin. Sci. 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- 61.Kloesch B., Liszt M., Steiner G., Bro¨ll J. Inhibitors of p38 and ERK1/2 Mapkinase and Hydrogen Sulphide Block Constitutive and IL-1β-Induced IL-6 and IL-8 Expression in the Human Chondrocyte Cell Line C-28/I2. Rheumatol. Int. 2012;32:729. doi: 10.1007/s00296-010-1682-0. [DOI] [PubMed] [Google Scholar]
- 62.Li T., Zhao B., Wang C., Wang H., Liu Z., Li W., Jin H., Tang C., Du J. Regulatory Effects of Hydrogen Sulfide on IL-6, IL-8 and IL-10 Levels in the Plasma and Pulmonary Tissue of Rats with Acute Lung Injury. Exp. Biol. Med. 2008;233:1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 63.Ali F.F., Abdel-Hamid H.A., Toni N.D. H2S Attenuates Acute Lung Inflammation Induced by Administration of Lipopolysac-Charide in Adult Male Rats. Gen. Physiol. Biophys. 2018;37:421–431. doi: 10.4149/gpb_2018002. [DOI] [PubMed] [Google Scholar]
- 64.Whiteman M., Cheung N.S., Zhu Y.-Z., Chu S.H., Siau J.L., Wong B.S., Armstrong J.S., Moore P.K. Hydrogen Sulphide: A Novel Inhibitor of Hypochlorous Acid-Mediated Oxidative Damage in the Brain? Biochem. Biophys. Res. Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 65.Cao L., Cao X., Zhou Y., Nagpure B.V., Wu Z.-Y., Hu L.F., Yang Y., Sethi G., Moore P.K., Bian J.-S. Hydrogen Sulfide Inhibits ATP-Induced Neuroinflammation and aβ1–42 Synthesis by Suppressing the Activation of STAT3 and Cathepsin S. Brain Behav. Immun. 2018;73:603–614. doi: 10.1016/j.bbi.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Du C., Lin X., Xu W., Zheng F., Cai J., Yang J., Cui Q., Tang C., Cai J., Xu G., et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019;30:184–197. doi: 10.1089/ars.2017.7195. [DOI] [PubMed] [Google Scholar]
- 67.Wallace J.L., Vong L., McKnight W., Dicay M., Martin G.R. Endogenous and Exogenous Hydrogen Sulfide Promotes Resolu-Tion of Colitis in Rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Wallace J.L., Dicay M., McKnight W., Martin G.R. Hydrogen Sulfide Enhances Ulcer Healing in Rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 69.Elrod J.W., Calvert J.W., Morrison J., Doeller J.E., Kraus D.W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., et al. Hydrogen Sulfide Attenuates Myocardial Ischemia-Reperfusion Injury by Preservation of Mitochondrial Function. Proc. Natl. Acad. Sci. USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan T.-T., Chen Y.Q., Bian J.-S. All in the Timing: A Comparison between the Cardioprotection Induced by H2S Preconditioning and Post-infarction Treatment. Eur. J. Pharmacol. 2009;616:160–165. doi: 10.1016/j.ejphar.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The Role of Gasotransmitters NO, H2S and CO in Myocardial Ischaemia/Reperfusion Injury and Cardioprotection by Preconditioning, Postconditioning and Remote Conditioning. Br. J. Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andreadou I., Iliodromitis E.K., Szabo C., Papapetropoulos A. Hydrogen Sulfide and PKG in Ischemia–Reperfusion Injury: Sources, Signaling, Accelerators and Brakes. Basic Res. Cardiol. 2015;110:1–6. doi: 10.1007/s00395-015-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mard S.A., Neisi N., Solgi G., Hassanpour M., Darbor M., Maleki M. Gastroprotective Effect of NaHS against Mucosal Le-Sions Induced by Ischemia-Reperfusion Injury in Rat. Dig. Dis. Sci. 2012;57:1496–1503. doi: 10.1007/s10620-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 75.Li N., Wang M.-J., Jin S., Bai Y.-D., Hou C.-L., Ma F.-F., Li X.-H., Zhu Y.-C. The H2S Donor NaHS Changes the Expression Pattern of H2S-Producing Enzymes after Myocardial Infarction. Oxidative Med. Cell. Longev. 2016;2016:1–11. doi: 10.1155/2016/6492469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao C., Xu D.Q., Gao C.J., Ding Q., Yao L.N., Li Z.C., Chai W. An Exogenous Hydrogen Sulphide Donor, NaHS, Inhibits the Nuclear Factor κB Inhibitor Kinase/Nuclear Factor κB Inhibitor/Nuclear Factor-κB Signaling Pathway and Exerts Cardioprotective Effects in a Rat Hemorrhagic Shock Model. Biol. Pharm. Bull. 2012;35:1029–1034. doi: 10.1248/bpb.b110679. [DOI] [PubMed] [Google Scholar]
- 77.Chen K.Y., Morris J.C. Kinetics of Oxidation of Aqueous Sulfide by Oxygen. Environ. Sci. Technol. 1972;6:529–537. doi: 10.1021/es60065a008. [DOI] [Google Scholar]
- 78.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P., Darley-Usmar V.M., Doeller J.E., Kraus D.W. Hydrogen Sulfide Mediates the Vasoactivity of Garlic. Proc. Natl. Acad. Sci. USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qidwai W., Ashfaq T. Role of Garlic Usage in Cardiovascular Disease Prevention: An Evidence-Based Approach. Evidence-Based Complement. Altern. Med. 2013;2013:1–9. doi: 10.1155/2013/125649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polhemus D.J., Calvert J.W., Butler J., Lefer D.J. The Cardioprotective Actions of Hydrogen Sulfide in Acute Myocardial in-Farction and Heart Failure. Scientifica. 2014;768607:1–8. doi: 10.1155/2014/768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bahadoran Z., Mirmiran P., Momenan A.A., Azizi F. Allium Vegetable Intakes and the Incidence of Cardiovascular Disease, Hypertension, Chronic Kidney Disease, and Type 2 Diabetes in Adults. J. Hypertens. 2017;35:1909–1916. doi: 10.1097/HJH.0000000000001356. [DOI] [PubMed] [Google Scholar]
- 82.Chan J.Y.-Y., Yuen A.C.-Y., Chan R.Y.-K., Chan S.-W. A Review of the Cardiovascular Benefits and Antioxidant Properties of Allicin. Phytother. Res. 2012;27:637–646. doi: 10.1002/ptr.4796. [DOI] [PubMed] [Google Scholar]
- 83.Herrera-Mundo M.N., Silva-Adaya D., Maldonado P.D., Galván-Arzate S., Andrés-Martínez L., La Cruz V.P.-D., Pedraza-Chaverri J., Santamaría A. S-Allylcysteine Prevents the Rat from 3-Nitropropionic Acid-Induced Hyperactivity, Early Markers of Oxidative Stress and Mitochondrial Dysfunction. Neurosci. Res. 2006;56:39–44. doi: 10.1016/j.neures.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 84.Numagami Y., Ohnishi S.T. S-Allylcysteine Inhibits Free Radical Production, Lipid Peroxidation and Neuronal Damage in Rat Brain Ischemia. J. Nutr. 2001;131:1100S–1105S. doi: 10.1093/jn/131.3.1100S. [DOI] [PubMed] [Google Scholar]
- 85.Welch C., Wuarin L., Sidell N. Anti-Proliferative Effect of the Garlic Compound S-Allylcysteine on Human Neuroblastoma Cell in Vitro. Cancer Lett. 1992;63:211–219. doi: 10.1016/0304-3835(92)90263-U. [DOI] [PubMed] [Google Scholar]
- 86.Chu Q., Lee D.T., Tsao S.W., Wang X., Wong Y.C. S-Allylcysteine, a Water-Soluble Garlic Derivative, Suppresses the Growth of a Human Androgen-Independent Prostate Cancer Xenograft, CWR22R, Under in Vivo Conditions. BJU Int. 2007;99:925–932. doi: 10.1111/j.1464-410X.2006.06639.x. [DOI] [PubMed] [Google Scholar]
- 87.Hsu C.C., Lin C.C., Liao T.S., Yin M.C. Protective Effect of S-Allylcysteine and S-Propyl Cysteine on Acetaminophen-Induced Hepatotoxicity in Mice. Food Chem. Toxicol. 2006;44:393–397. doi: 10.1016/j.fct.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 88.Nakagawa S., Kasuga S., Matsura H. Prevention of Liver Damage by Aged Garlic Extract and Its Components in Mice. Phytother. Res. 1989;3:50–53. doi: 10.1002/ptr.2650030203. [DOI] [Google Scholar]
- 89.Padmanabhan M., Prince P.S.M. Preventive Effect of S-Allylcysteine on Lipid Peroxides and Antioxidants in Normal and Isoproterenol-Induced Cardiotoxicity in Rats: A Histopathological Study. Toxicology. 2006;224:128–137. doi: 10.1016/j.tox.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 90.Chuah S.C., Moore P.K., Zhu Y.Z. S-Allylcysteine Mediates Cardioprotection in an Acute Myocardial Infarction Rat Model via a Hydrogen SulfiDe-mediated Pathway. Am. J. Physiol. Circ. Physiol. 2007;293:H2693–H2701. doi: 10.1152/ajpheart.00853.2007. [DOI] [PubMed] [Google Scholar]
- 91.Wang Q., Liu H.-R., Mu Q., Rose P., Zhu Y.Z. S-propargyl-cysteine Protects Both Adult Rat Hearts and Neonatal Cardiomyocytes From Ischemia/Hypoxia Injury: The Contribution of the Hydrogen Sulfide-Mediated Pathway. J. Cardiovasc. Pharmacol. 2009;54:139–146. doi: 10.1097/FJC.0b013e3181ac8e12. [DOI] [PubMed] [Google Scholar]
- 92.Wang Q., Wang X.-L., Liu H.-R., Rose P., Zhu Y.-Z. Protective Effects of Cysteine Analogues on Acute Myocardial Ischemia: Novel Modulators of Endogenous H2S Production. Antioxidants Redox Signal. 2010;12:1155–1165. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 93.Ried K., Frank O., Stocks N.P. Aged Garlic Extract Reduces Blood Pressure in Hypertensives: A Dose–Response Trial. Eur. J. Clin. Nutr. 2012;67:64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacob C., Anwar A., Burkholz T. Perspective on Recent Developments on Sulfur-Containing Agents and Hydrogen Sulfide Signaling. Planta Medica. 2008;74:1580–1592. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- 95.Kashfi K., Olson K.R. Biology and Therapeutic Potential of Hydrogen Sulfide and Hydrogen SulfiDe-releasing Chimeras. Biochem. Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li L., Hsu A., Moore P.K. Actions and Interactions of Nitric Oxide, Carbon Monoxide and Hydrogen Sulphide in the Cardiovascular System and in Inflammation–a Tale of Three Gases! Pharmacol. Ther. 2009;123:386. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Nicholson C.K., Calvert J.W. Hydrogen Sulfide and Ischemia-Reperfusion Injury. Pharmacol. Res. 2010;62:289. doi: 10.1016/j.phrs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olson K.R. The Therapeutic Potential of Hydrogen Sulfide: Separating Hype from Hope. Am. J. Physiol. Integr. Comp. Physiol. 2011;301:R297–R312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 99.Predmore B.L., Lefer D.J., Gojon G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dinkova-Kostova A.T., Kostov R.V. Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Lin Y., Yang X., Lu Y., Liang D., Huang D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019;21:5977–5980. doi: 10.1021/acs.orglett.9b02117. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y., Biggs T.D., Xian M. Hydrogen Sulfide (H2S) Releasing Agents: Chemistry and Biological Applications. Chem. Commun. 2014;50:11788–11805. doi: 10.1039/C4CC00968A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lecher H.Z., Greenwood R.A., Whitehouse K.C., Chao T.H. The Phosphonation of Aromatic Compounds with Phosphorus Pentasulfide. J. Am. Chem. Soc. 1956;78:5018–5022. doi: 10.1021/ja01600a058. [DOI] [Google Scholar]
- 104.Thomsen I., Clausen K., Scheibye S., Lawesson S.-O. Thiation with 2,4-Bis(4-Methoxyphenyl)-1,3,2,4- Dithiadiphosphetane 2,4-Disulfide:N-Methylthiopyrrolidone. Organic Syntheses. 2003;62:158. doi: 10.1002/0471264180.os062.20. [DOI] [Google Scholar]
- 105.Karwi Q.G., Whiteman M., Wood M.E., Torregrossa R., Baxter G.F. Pharmacological Postconditioning against Myocardial Infarction with a Slow-Releasing Hydrogen Sulfide Donor, GYY4137. Pharmacol. Res. 2016;111:442–451. doi: 10.1016/j.phrs.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 106.Qiu Y., Wu Y., Meng M., Luo M., Zhao H., Sun H., Gao S. GYY4137 Protects against Myocardial Ischemia/Reperfusion Injury via Activation of the PHLPP-1/Akt/Nrf2 Signaling Pathway in Diabetic Mice. J. Surg. Res. 2018;225:29–39. doi: 10.1016/j.jss.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 107.Alexander B.E., Coles S.J., Fox B.C., Khan T.F., Maliszewski J., Perry A., Pitak M.B., Whiteman M., Wood M.E. Investigating the Generation of Hydrogen Sulfide from the Phosphonamidodithioate Slow-Release Donor GYY4137. MedChemComm. 2015;6:1649–1655. doi: 10.1039/C5MD00170F. [DOI] [Google Scholar]
- 108.Moore P.K., Whiteman M. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. Springer; Amsterdam, The Netherlands: 2015. pp. 344–354. [Google Scholar]
- 109.Landis P.S. The Chemistry of 1,2-Dithiole-3-thiones. Chem. Rev. 1965;65:237–245. doi: 10.1021/cr60234a004. [DOI] [Google Scholar]
- 110.Curphey T.J., Libby A.A. Dianions of 3-Oxo dithioic Acids: Preparation and Conversion to 3H-1,2-Dithiole-3-thiones. Tetrahedron Lett. 2000;41:6977. doi: 10.1016/S0040-4039(00)01191-6. [DOI] [Google Scholar]
- 111.Pedersen B.S., Lawesson S.O. Syntheses of 3H-1,2-Dithiole-3-Thiones and 4H-1,3,2-Oxazaphosphorine Derivatives from the Dimer of P-Methoxyphenyl-Thionophosphine Sulfide and Der. Tetrahedron. 1979;35:2433–2437. doi: 10.1016/S0040-4020(01)93760-3. [DOI] [Google Scholar]
- 112.Hamada T., Nakane T., Kimura T., Arisawa K., Yoneda K., Yamamoto T., Osaki T. Treatment of Xerostomia with the Bile Secretion-Stimulating Drug Anethole Trithione: A Clinical Trial. Am. J. Med. Sci. 1999;318:146–151. doi: 10.1016/S0002-9629(15)40606-8. [DOI] [PubMed] [Google Scholar]
- 113.Perrino E., Cappelletti G., Tazzari V., Giavini E., Del Soldato P., Sparatore A. New Sulfurated Derivatives of Valproic Acid with Enhanced Histone Deacetylase Inhibitory Activity. Bioorganic Med. Chem. Lett. 2008;18:1893–1897. doi: 10.1016/j.bmcl.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Tazzari V., Cappelletti G., Casagrande M., Perrino E., Renzi L., Del Soldato P., Sparatore A. New Aryldithiolethione Derivatives as Potent Histone Deacetylase Inhibitors. Bioorganic Med. Chem. 2010;18:4187–4194. doi: 10.1016/j.bmc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Sen C.K., Traber K.E., Packer L. Inhibition of NF-jB Activation in Human T-cell Lines by Anetholdithiolthione. Biochem. Biophys. Res. Commun. 1996;218:148–153. doi: 10.1006/bbrc.1996.0026. [DOI] [PubMed] [Google Scholar]
- 116.Zanatta S.D., Jarrott B., Williams S.J. Synthesis and Preliminary Pharmacological Evaluation of Aryl Dithiolethiones with Cyclooxygenase-2-Selective Inhibitory Activity and Hydrogen Sulfide-Releasing Properties. Aust. J. Chem. 2010;63:946–957. doi: 10.1071/CH09517. [DOI] [Google Scholar]
- 117.Qandil A.M. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012;13:17244–17274. doi: 10.3390/ijms131217244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caliendo G., Cirino G., Santagada V., Wallace J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010;53:6275–6286. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 119.Li L., Rossoni G., Sparatore A., Lee L.C., Del Soldato P., Moore P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free. Radic. Biol. Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 120.Wallace J.L., Caliendo G., Santagada V., Cirino G., Fiorucci S. Gastrointestinal Safety and Anti-Inflammatory Effects of a Hydrogen Sulfide–Releasing Diclofenac Derivative in the Rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 121.Fiorucci S., Orlandi S., Mencarelli A., Caliendo G., Santagada V., Distrutti E., Santucci L., Cirino G., Wallace J.L. Enhanced Activity of a Hydrogen SulphiDe-releasing Derivative of Mesalamine (ATB-429) in a Mouse Model of Colitis. Br. J. Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chattopadhyay M., Kodela R., Nath N., Dastagirzada Y.M., Velázquez-Martínez C.A., Boring D., Kashfi K. Hydrogen SulfiDe-releasing NSAIDS Inhibit the Growth of Human Cancer Cells: A General Property and Evidence of a Tissue Type-Independent Effect. Biochem. Pharmacol. 2012;83:715–722. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 123.Shukla N., Rossoni G., Hotston M., Sparatore A., Del Soldato P., Tazzari V., Persad R., Angelini G.D., Jeremy J.Y. Effect of Hydrogen SulfiDe-donating Sildenafil (acs6) on Erectile Function and Oxidative Stress in Rabbit Isolated Corpus Cavernosum and in Hypertensive Rats. BJU Int. 2009;103:1522–1529. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perrino E., Uliva C., Lanzi C., Del Soldato P., Masini E., Sparatore A. New Prostaglandin Derivative for Glaucoma Treatment. Bioorg. Med. Chem. Lett. 2009;19:1639–1642. doi: 10.1016/j.bmcl.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 125.Lee M., Tazzari V., Giustarini D., Rossi R., Sparatore A., Del Soldato P., McGeer E., McGeer P.L. Effects of Hydrogen Sulfide-releasing l-DOPA Derivatives on Glial Activation. J. Biol. Chem. 2010;285:17318–17328. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossoni G., Manfredi B., Tazzari V., Sparatore A., Trivulzio S., Del Soldato P., Berti F. Activity of a New Hydrogen SulfiDe-releasing Aspirin (ACS14) on Pathological Cardiovascular Alterations Induced by Glutathione Depletion in Rats. Eur. J. Pharmacol. 2010;648:139–145. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 127.Kodela R., Chattopadhyay M., Kashfi K. NOSH-Aspirin: A Novel Nitric Oxide–Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med. Chem. Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rossoni G., Berti M., Colonna V.D., Bernareggi M., Del Soldato P., Berti F. Myocardial Protection by the Nitroderivative of Aspirin, NCX 4016: In Vitro and in Vivo Experiments in the Rabbit. Ital. Hear. J. Off. J. Ital. Fed. Cardiol. 2000;1:146–155. [PubMed] [Google Scholar]
- 129.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., Wood M.E., Whiteman M., Szabo C. AP39, A Novel Mitochondria-Targeted Hydrogen Sulfide Donor, Stimulates Cellular Bioenergetics, Exerts Cytoprotective Effects and Protects against the Loss of Mitochondrial DNA Integrity in Oxidatively Stressed Endothelial Cells in Vitro. Nitric Oxide. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ikeda K., Marutani E., Hirai S., Wood M.E., Whiteman M., Ichinose F. Mitochondria-Targeted Hydrogen Sulfide Donor AP39 Improves Neurological Outcomes after Cardiac Arrest in Mice. Nitric Oxide. 2015;49:90–96. doi: 10.1016/j.niox.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ahmad A., Olah G., Szczesny B., Wood M.E., Whiteman M., Szabo C. AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo. Shock. 2016;45:88–97. doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chatzianastasiou A., Bibli S.-I., Andreadou I., Efentakis P., Kaludercic N., Wood M.E., Whiteman M., Di Lisa F., Daiber A., Manolopoulos V.G., et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016;358:431–440. doi: 10.1124/jpet.116.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Karwi Q.G., Bornbaum J., Boengler K., Torregrossa R., Whiteman M., Wood M.E., Schulz R., Baxter G.F. AP39, A Mitochondria-Targeting Hydrogen Sulfide (H2S) Donor, Protects against Myocardial Reperfusion Injury Independently of Salvage Kinase Signalling. Br. J. Pharmacol. 2017;174:287–301. doi: 10.1111/bph.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang J., Li Z., Organ C.L., Park C.M., Yang C.T., Pacheco A., Wang D., Lefer D.J., Xian M. pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2016;138:6336–6339. doi: 10.1021/jacs.6b01373. [DOI] [PubMed] [Google Scholar]
- 135.Wang H., Skeldon P., Thompson G., Wood G. Synthesis and Characterization of Molybdenum Disulphide Formed from Ammonium Tetrathiomolybdate. J. Mater. Sci. 1997;32:497–502. doi: 10.1023/A:1018538424373. [DOI] [Google Scholar]
- 136.Xu S., Yang C.-T., Meng F.-H., Pacheco A., Chen L., Xian M. Ammonium Tetrathiomolybdate as a Water-Soluble and Slow-Release Hydrogen Sulfide Donor. Bioorganic Med. Chem. Lett. 2016;26:1585–1588. doi: 10.1016/j.bmcl.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dyson A., Dal-Pizzol F., Sabbatini G., Lach A.B., Galfo F., Cardoso J.D.S., Mendonça B.P., Hargreaves I., Pinto B.B., Bromage D.I., et al. Ammonium Tetrathiomolybdate Following Ischemia/Reperfusion Injury: Chemistry, Pharmacology, and Impact of a New Class of Sulfide Donor in Preclinical Injury Models. PLoS Med. 2017;14:e1002310. doi: 10.1371/journal.pmed.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H., Zhang A., Guo C., Shi C., Zhang Y., Liu Q., Sparatore A., Wang C. S-diclofenac Protects against Doxorubicin-Induced Cardiomyopathy in Mice via Ameliorating Cardiac Gap Junction Remodeling. PLoS ONE. 2011;6:e26441. doi: 10.1371/journal.pone.0026441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu J., Guo W., Lin S.-Z., Wang Z.-J., Kan J.-T., Chen S.-Y., Zhu Y.-Z. Gp130-Mediated STAT3 Activation by S-Propargyl-cysteine, an Endogenous Hydrogen Sulfide Initiator, Prevents Doxorubicin-Induced Cardiotoxicity. Cell Death Dis. 2016;7:e2339. doi: 10.1038/cddis.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su Y.-W., Liang C., Jin H.-F., Tang X.-Y., Han W., Chai L.-J., Zhang C.-Y., Geng B., Tang C.-S., Du J.-B. Hydrogen Sulfide Regulates Cardiac Function and Structure in Adriamycin-Induced Cardiomyopathy. Circ. J. 2009;73:741–749. doi: 10.1253/circj.CJ-08-0636. [DOI] [PubMed] [Google Scholar]
- 141.Chan N., Willis A., Kornhauser N., Ward M.M., Lee S.B., Nackos E., Seo B.R., Chuang E., Cigler T., Moore A., et al. Influencing the Tumor Microenvironment: A Phase II Study of Copper Depletion Using Tetrathiomolybdate in Patients with Breast Cancer at High Risk for Recurrence and in Preclinical Models of Lung Metastases. Clin. Cancer Res. 2017;23:666–676. doi: 10.1158/1078-0432.CCR-16-1326. [DOI] [PubMed] [Google Scholar]
- 142.Yang C.-T., Zhao Y., Xian M., Li J.-H., Dong Q., Bai H.-B., Xu J.-D., Zhang M.-F. A Novel Controllable Hydrogen Sulfide-Releasing Molecule Protects Human Skin Keratinocytes Against Methylglyoxal-Induced Injury and Dysfunction. Cell. Physiol. Biochem. 2014;34:1304–1317. doi: 10.1159/000366339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao Y., Yang C., Organ C., Li Z., Bhushan S., Otsuka H., Pacheco A., Kang J., Aguilar H.C., Lefer D.J., et al. Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors. J. Med. Chem. 2015;58:7501–7511. doi: 10.1021/acs.jmedchem.5b01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stipanuk M.H., Beck P.W. Characterization of the Enzymic Capacity for Cysteine Desulphhydration in Liver and Kidney of the Rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frank R.L., Blegen J.R. Benzoyl Disulfide. Organic Syntheses. 2003;28:16. doi: 10.1002/0471264180.os028.07. [DOI] [Google Scholar]
- 146.Heimer N.E., Field L., Neal R.A. Biologically Oriented Organic Sulfur Chemistry. 21. Hydrodisulfide of a Penicillamine Derivative and Related Compounds. J. Org. Chem. 1981;46:1374–1377. doi: 10.1021/jo00320a030. [DOI] [Google Scholar]
- 147.Wallace J.L., Caliendo G., Santagada V., Cirino G. Markedly Reduced Toxicity of a Hydrogen SulphiDe-releasing Derivative of Naproxen (ATB-346) Br. J. Pharmacol. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Cicco P., Panza E., Ercolano G., Armogida C., Sessa G., Pirozzi G., Cirino G., Wallace J.L., Ianaro A. ATB-346, A Novel Hydrogen SulfiDe-releasing Anti-inflammatory Drug, Induces Apoptosis of Human Melanoma Cells and Inhibits Melanoma Development in Vivo. Pharmacol. Res. 2016;114:67–73. doi: 10.1016/j.phrs.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 149.Elsheikh W., Blackler R.W., Flannigan K.L., Wallace J.L. Enhanced Chemopreventive Effects of a Hydrogen SulfiDe-releasing Anti-inflammatory Drug (ATB-346) in Experimental Colorectal Cancer. Nitric Oxide. 2014;41:131–137. doi: 10.1016/j.niox.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 150.Wallace J.L., Vaughan D., Dicay M., Macnaughton W.K., De Nucci G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxidants Redox Signal. 2018;28:1533–1540. doi: 10.1089/ars.2017.7068. [DOI] [PubMed] [Google Scholar]
- 151.Testai L., Marino A., Piano I., Brancaleone V., Tomita K., Di Cesare Mannelli L., Martelli A., Citi V., Breschi M.C., Levi R., et al. The Novel H2S-donor 4-Carboxyphenyl Isothiocyanate Promotes Cardioprotective Effects against Ischemia/Reperfusion Injury through Activation of mitoK ATP Channels and Reduction of Oxidative Stress. Pharmacol. Res. 2016;113:290–299. doi: 10.1016/j.phrs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 152.Nasim S., Crooks P.A. N-Chlorosuccinimide Is a Convenient Oxidant for the Synthesis of 2,4-disubstituted 1,2,4-thiadiazolidine-3,5-diones. Tetrahedron Lett. 2009;50:257–259. doi: 10.1016/j.tetlet.2008.10.136. [DOI] [Google Scholar]
- 153.Kondo K., Bhushan S., King A.L., Prabhu S.D., Hamid T., Koenig S., Murohara T., Predmore B.L., Gojon G., Sr., Gojon G., Jr., et al. H2S Protects against Pressure Overload-Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gojon-Romanillos G., Gojon-Zorrilla G. Preparation and Compositions of Highly Bioavailable Zerovalent Sulfur and Uses Thereof. 8771755-B2. U.S. Patent. 2014 Jul 8;
- 155.Rohwerder T., Sand W. The Sulfane Sulfur of Persulfides Is the Actual Substrate of the Sulfur-Oxidizing Enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology. 2003;149:1699–1710. doi: 10.1099/mic.0.26212-0. [DOI] [PubMed] [Google Scholar]
- 156.Suzuki I. Oxidation of Elemental Sulfur by an Enzyme System of Thiobacillus Thiooxidans. Biochim. Biophys. Acta (BBA) Gen. Subj. 1965;104:359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]
- 157.Suzuki I., Werkman C.H. Glutathione and Sulfur Oxidation by Thiobacillus Thiooxidans. Proc. Natl. Acad. Sci. USA. 1959;45:239–244. doi: 10.1073/pnas.45.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barr L.A., Shimizu Y., Lambert J.P., Nicholson C.K., Calvert J.W. Hydrogen Sulfide Attenuates High Fat Diet-Induced Cardiac Dysfunction via the Suppression of Endoplasmic Reticulum Stress. Nitric Oxide. 2015;46:145–156. doi: 10.1016/j.niox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Donnarumma E., Rushing A.M., Boisvert S.F., Scarborough A.L., Polhemus D.J., Trivedi R.K., Lefer D.J., Goodchild T.T. The Novel H2S Pro-drug, SG1002, Preserves Coronary Artery Vascular Reactivity in the Setting of Critical Limb Ischemia in Swine. Circulation. 2016;134:A19028. [Google Scholar]
- 160.ClinicalTrials.gov Assessing the Safety and Ability of SG1002 to Overcome Deficits in Hydrogen Sulfide in Heart Failure Patients. [(accessed on 18 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01989208.
- 161.Polhemus D.J., Li Z., Pattillo C.B., Gojon G., Giordano T., Krum H. A Novel Hydrogen Sulfide Prodrug, SG 1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015;33:216–226. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Borchardt R.T., Cohen L.A. Stereopopulation Control. III. Facilitation of Intramolecular Conjugate Addition of the Carboxyl Group. J. Am. Chem. Soc. 1972;94:9175–9182. doi: 10.1021/ja00781a031. [DOI] [PubMed] [Google Scholar]
- 163.Zheng Y., Yueqin Z., Ji K., Pan Z., Chittavong V., Wang B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2016;55:4514–4518. doi: 10.1002/anie.201511244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rao Y., Li X., Nagorny P., Hayashida J., Danishefsky S.J. A Simple Method for the Conversion of Carboxylic Acids into Thioacids with Lawesson’s Reagent. Tetrahedron Lett. 2009;50:6684–6686. doi: 10.1016/j.tetlet.2009.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Z., Organ C.L., Zheng Y., Wang B., Lefer D.J. Abstract 17903: Novel Esterase Activated Hydrogen Sulfide Donors Attenuate Myocardial Ischemia/ Reperfusion Injury. Circulation. 2016;134:A17903. [Google Scholar]
- 166.Chengelis C.P., Neal R.A. Studies of Carbonyl Sulfide Toxicity: Metabolism by Carbonic Anhydrase. Toxicol. Appl. Pharmacol. 1980;55:198–202. doi: 10.1016/0041-008X(80)90236-7. [DOI] [PubMed] [Google Scholar]
- 167.Chengelis C.P., Neal R.A. Hepatic Carbonyl Sulfide Metabolism. Biochem. Biophys. Res. Commun. 1979;90:993–999. doi: 10.1016/0006-291X(79)91925-9. [DOI] [PubMed] [Google Scholar]
- 168.Steiger A.K., Marcatti M., Szabo C., Szczesny B., Pluth M.D. Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol. 2017;12:2117–2123. doi: 10.1021/acschembio.7b00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Steiger A.K., Pardue S., Kevil C.G., Pluth M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016;138:7256–7259. doi: 10.1021/jacs.6b03780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Powell C.R., Foster J.C., Okyere B., Theus M.H., Matson J.B. Therapeutic Delivery of H2S via COS: Small Molecule and Polymeric Donors with Benign Byproducts. J. Am. Chem. Soc. 2016;138:13477–13480. doi: 10.1021/jacs.6b07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tao X., Deng Y., Shen Z., Ling J. Controlled Polymerization of N-Substituted Glycine N-Thiocarboxyanhydrides Initiated by Rare Earth Borohydrides toward Hydrophilic and Hydrophobic Polypeptoids. Macromolecules. 2014;47:6173–6180. doi: 10.1021/ma501131t. [DOI] [Google Scholar]
- 172.Zhao Y., Pluth M.D. Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Angew. Chem. Int. Ed. 2016;55:14638–14642. doi: 10.1002/anie.201608052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dickinson B.C., Huynh C., Chang C.J. A Palette of Fluorescent Probes with Varying Emission Colors for Imaging Hydrogen Peroxide Signaling in Living Cells. J. Am. Chem. Soc. 2010;132:5906–5915. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miller E.W., Albers A.E., Pralle A., Isacoff E.Y., Chang C.J. Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc. 2005;127:16652–16659. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pan J., Devarie-Baez N.O., Xian M. Facile Amide Formation viaS-Nitrosothioacids. Org. Lett. 2011;13:1092–1094. doi: 10.1021/ol1031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rao Y., Li X., Danishefsky S.J. Thio FCMA Intermediates as Strong Acyl Donors: A General Solution to the Formation of Complex Amide Bonds. J. Am. Chem. Soc. 2009;131:12924–12926. doi: 10.1021/ja906005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang P., Danishefshy S.J. A Promising General Solution to the Problem of Ligating Peptides and Glycopeptides. J. Am. Chem. Soc. 2010;132:17045–17051. doi: 10.1021/ja1084628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu R., Orgel L.E. Oxidative Acylation Using Thioacids. Nat. Cell Biol. 1997;389:52–54. doi: 10.1038/37944. [DOI] [PubMed] [Google Scholar]
- 179.Dawson E.P., Muir T.W., Clark-Lewis I., Kent S.B. Synthesis of Proteins by Native Chemical Ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 180.Crich D., Sharma I. Epimerization-Free Block Synthesis of Peptides from Thioacids and Amines with the Sanger and Mukaiyama Reagents. Angew. Chem. Int. Ed. 2009;48:2355–2358. doi: 10.1002/anie.200805782. [DOI] [PubMed] [Google Scholar]
- 181.Wu D., Hu Q., Ma F., Zhu Y.Z. Vasorelaxant Effect of a New Hydrogen Sulfide-Nitric Oxide Conjugated Donor in Isolated Rat Aortic Rings through cGMP Pathway. Oxidative Med. Cell. Longev. 2016;2016:1–10. doi: 10.1155/2016/7075682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu C., Guo W., Shi X., Kaium M., Gu X., Zhu Y.Z. Leonurine-Cysteine Analog Conjugates as a New Class of Multifunctional Anti-myocardial Ischemia Agent. Eur. J. Med. Chem. 2011;46:3996–4009. doi: 10.1016/j.ejmech.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 183.Ignarro L.J., Napoli C., Loscalzo J. Nitric Oxide Donors and Cardiovascular Agents Modulating the Bioactivity of Nitric Oxide: An Overview. Circ. Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 184.Xiong Y., Chang L.-L., Tran B., Dai T., Zhong R., Mao Y.-C., Zhu Y.-Z. ZYZ-803, a Novel Hydrogen SulfiDe-nitric Oxide Conjugated Donor, Promotes Angiogenesis via Cross-Talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2020;41:218–228. doi: 10.1038/s41401-019-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hu Q., Wu D., Ma F., Yang S., Tan B., Xin H., Gu X., Chen X., Chen S., Mao Y., et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide–Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016;25:498–514. doi: 10.1089/ars.2015.6607. [DOI] [PubMed] [Google Scholar]
- 186.Wu D., Hu Q., Xiong Y., Zhu D., Mao Y., Zhu Y.Z. Novel H2S-NO Hybrid Molecule (ZYZ-803) Promoted Synergistic Effects Against Heart Failure. Redox Biol. 2018;15:243–252. doi: 10.1016/j.redox.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bucci M., Vellecco V., Cantalupo A., Brancaleone V., Zhou Z., Evangelista S., Calderone V., Papapetropoulos A., Cirino G. Hydrogen Sulfide Accounts for the Peripheral Vascular Effects of Zofenopril Independently of ACE Inhibition. Cardiovasc. Res. 2014;102:138–147. doi: 10.1093/cvr/cvu026. [DOI] [PubMed] [Google Scholar]
- 188.Terzuoli E., Monti M., Vellecco V., Bucci M., Cirino G., Ziche M., Morbidelli L. Characterization of Zofenoprilat as an Inducer of Functional Angiogenesis through Increased H2S Availability. Br. J. Pharmacol. 2015;172:2961–2973. doi: 10.1111/bph.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Monti M., Terzuoli E., Ziche M., Morbidelli L. The Sulphydryl Containing ACE Inhibitor Zofenoprilat Protects Coronary Endothelium from Doxorubicin-Induced Apoptosis. Pharmacol. Res. 2013;76:171–181. doi: 10.1016/j.phrs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 190.Monti M., Terzuoli E., Ziche M., Morbidelli L. H2S Dependent and Independent Anti-inflammatory Activity of Zofenoprilat in Cells of the Vascular Wall. Pharmacol. Res. 2016;113:426–437. doi: 10.1016/j.phrs.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 191.Furuta S., Kiyosawa K., Higuchi M., Kasahara H., Saito H., Shioya H., Oguchi H. Pharmacokinetics of Temocapril, an ACE Inhibitor with Preferential Biliary Excretion, in Patients with Impaired Liver Function. Eur. J. Clin. Pharmacol. 1993;44:383–385. doi: 10.1007/BF00316478. [DOI] [PubMed] [Google Scholar]
- 192.Donnarumma E., Ali M.J., Rushing A.M., Scarborough A.L., Bradley J.M., Organ C.L., Islam K.N., Polhemus D.J., Evangelista S., Cirino G., et al. Zofenopril Protects Against Myocardial Ischemia–Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Hear. Assoc. 2016;5:1–17. doi: 10.1161/JAHA.116.003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.