Abstract
Bats are often unfairly depicted as the direct culprit in the current COVID-19 pandemic, yet the real causes of this and other zoonotic spillover events should be sought in the human impact on the environment, including the spread of domestic animals. Here, we discuss bat predation by cats as a phenomenon bringing about zoonotic risks and illustrate cases of observed, suspected or hypothesized pathogen transmission from bats to cats, certainly or likely following predation episodes. In addition to well-known cases of bat rabies, we review other diseases that affect humans and might eventually reach them through cats that prey on bats. We also examine the potential transmission of SARS-CoV-2, the causal agent of COVID-19, from domestic cats to bats, which, although unlikely, might generate a novel wildlife reservoir in these mammals, and identify research and management directions to achieve more effective risk assessment, mitigation or prevention. Overall, not only does bat killing by cats represent a potentially serious threat to biodiversity conservation, but it also bears zoonotic implications that can no longer be neglected.
Keywords: bat, cat, COVID-19, SARS-CoV-2, spillback, spillover, zoonotic risk
1. Introduction
The ongoing COVID-19 (COronaVIrus Disease 2019) pandemic has highlighted the primary role of wildlife in zoonotic events (e.g., [1]) that due to the high density and mobility of the human population can spread rapidly over large regions of the globe.
While much of the public attention has focused on the epidemiology of SARS-CoV-2 as a human pathogen, there is little doubt that the causal factors that originated the spillover lie directly or indirectly in the consumption of bats by humans, frequent in Asia [2]. More generally, there is consensus on the fact that zoonotic diseases are strongly favored by the ever-growing deforestation and expansion of farmland and urban areas at the expense of natural habitats, increasing the opportunities for wildlife–human interactions, as well as for wildlife traffic and consumption [3]. It is therefore clear that a holistic approach to the prevention of zoonotic diseases taking into account humans, wildlife and environmental socio-ecological dynamics is necessary to prevent future spillover processes and pandemic events.
The zoonotic risk associated with wildlife is often multifaceted and may involve several actors. Therefore, only accurate surveillance of all the species that take part in the human–wildlife interaction network may indeed lead to effective prevention and mitigation. Domestic animals may play a crucial zoonotic role in bridging wildlife and humans in situations where direct human–wild animal contact would otherwise be rare, amplifying the pathogen or acting as a vessel for genetic variation [4]. To mention one example, in the case of the Nipah virus in Malaysia, domestic pigs acted as amplifiers of the virus carried by fruit bats and passed it on to humans [5].
Bats are often mentioned as species characterized by a high zoonotic potential ([6]; but see [7]) and natural reservoirs of pathogens (especially viruses) that may affect humans directly or after a more complex pathway involving other animal species. While the risk posed by bats is overwhelmed by the vital ecosystem services these mammals provide, and misrepresentation of such zoonotic risks may harm bat conservation [8], an often-overlooked argument is that direct contacts between bats and people are relatively unlikely if bushmeat or human encroachment on bat habitat does not occur. Bats being nocturnal, elusive mammals that typically avoid contact with humans and are highly sensitive to anthropogenic disturbance, such contacts are in fact very rare. Nonetheless, predation on bats by domestic animals which have frequent contact with humans might represent an important epidemiological link so far only partly explored.
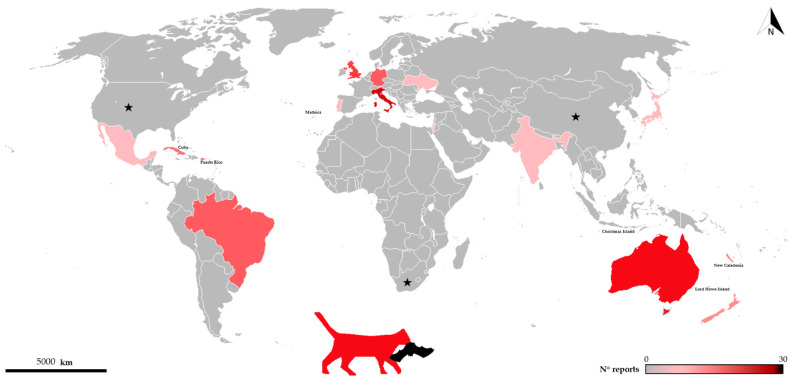
As documented worldwide, domestic cats are well-known predators of many wildlife species [9], sometimes in ecologically vulnerable systems such as islands [10] where their impact may be especially pronounced. As for bats, cases of predation by domestic cats cover 48 bat species and ca. 20 countries (see Figure 1 and Table 1), and due to the difficulties in recording such events, there is no doubt that this phenomenon is much more widespread than previously imagined.
Figure 1.
Distribution of records of bats killed by cats throughout the world. Color intensity increases with number of records. Black stars show countries where predation on bats by domestic cats is reported but number of records/species is not available [11,12].
Table 1.
Cases of bat predation by cats documented in the scientific literature. Cat classified as: F = feral; SF = semi-feral; O = owned; U = unknown. Habitat: H = human habitat such as agricultural or urban; N = natural habitat; U = unknown. International Union for Conservation of Nature (IUCN) status as follows: DD = Data Deficient; LC = Least Concern; NT = Near Threatened; VU = Vulnerable.
| Family | Species | Cat Type | Sample | Country | Habitat | IUCN Status | Reference |
|---|---|---|---|---|---|---|---|
| Miniopteridae | Miniopterus schreibersii | U | Records of rescue | Italy | H | NT | [13] |
| O | Survey | Italy | H | [14] | |||
| Molossidae | Austronomus australis | F | Stomach | Australia | N | LC | [15] |
| Mormopterus planiceps | F | Stomach | Australia | H | LC | [16] | |
| Tadarida brasiliensis | U | Camera | Argentina | H | LC | [17] | |
| Tadarida teniotis | U | Records of rescue | Italy | H | LC | [13] | |
| Mormoopidae | Mormoops blainvillei | F | Camera, scats and body remains | Puerto Rico | N | LC | [18] |
| Pteronotus quadridens | F | Camera, scats and body remains | Puerto Rico | N | LC | [18] | |
| Mystacinidae | Mystacina tuberculata | F | DNA and body remains | New Zealand | N | VU | [19] |
| U | Survey | U | [20] | ||||
| Natalidae | Chilonatalus macer | F | Scats | Cuba | N | DD | [21] |
| Natalus primus | F | Scats | Cuba | N | VU | [21] | |
| Phyllostomidae | Artibeus lituratus | O | Observations and body remains and rescue | Brazil | H | LC | [22] |
| U | Observations | Brazil | U | [23] | |||
|
Brachyphylla
cavernarum |
F | Camera, scats and body remains | Puerto Rico | N | LC | [18] | |
| Carollia perspicillata | U | Observations | Brazil | U | LC | [23] | |
| Desmodus rotundus | O | Observations and body remains | Brazil | H | LC | [24] | |
| Erophylla bombifrons | F | Camera, scats and body remains | Puerto Rico | N | LC | [18] | |
| Monophyllus redmani | F | Camera, scats and body remains | Puerto Rico | N | LC | [18] | |
| Phyllostomus discolor | O | Observations, body remains and rescue | Brazil | H | LC | [22] | |
| Phyllonycteris poeyi | U | Observations of predator in the cave | Cuba | U | LC | [25] | |
| U | Scats | Cuba | N | [21] | |||
| Sturnira lilium | U | Observations | Brazil | U | LC | [23] | |
| Pteropus dasymallus | F/SF | Survey | Japan | H | VU | [26] | |
| Pteropus natalis | F | Scats and stomach | Christmas Island | H | VU | [27] | |
| Pteropus ornatus | F | Scats | New Caledonian | N | VU | [28] | |
| Pteropodidae | Pteropus tonganus | F | Scats | New Caledonian | N | LC | [28] |
| Pteropus vetulus | F | Scats | New Caledonian | N | VU | [28] | |
| Rousettus aegyptiacus | O | Brought home | Israel | H | LC | [29] | |
| Syconycteris australis | O | Corpses brought | Australia | N | LC | [30] | |
| Rhinolophidae |
Rhinolophus
ferrumequinum |
U | Camera trap | Italy | H | LC | [13] |
| O | Survey | Italy | H | [14] | |||
|
Rhinolophus
hipposideros |
U | Records of rescue | Italy | H | LC | [13] | |
| O | Survey | Italy | H | [14] | |||
| Vespertilionidae | Chalinolobus gouldii | F | Stomach | Australia | N | LC | [15] |
| F | Stomach | Australia | H | [16] | |||
| F | Stomach | Australia | H | [31] | |||
|
Chalinolobus
turbeculatus |
U | Survey | New Zealand | N and H | VU | [20] | |
| Eptesicus serotinus | U | Records of rescue | Italy | H | LC | [13] | |
| O | Survey | Italy | H | [14] | |||
| U | Molecular analysis | United Kingdom | U | [32] | |||
| Hypsugo savii | O | Records of rescue | Italy | H | LC | [13] | |
| Myotis bechsteinii | U | Records of rescue | H | NT | [13] | ||
| Myotis mystacinus | O | Survey | Italy | H | LC | [14] | |
| U | Necropsy | Germany | U | [33] | |||
| U | Molecular analysis | United Kingdom | U | [32] | |||
| Myotis crypticus | U | Records of rescue | Italy | H | LC | [13] | |
| O | Survey | Italy | H | [14] | |||
| U | Molecular analysis | United Kingdom | U | [32] | |||
| Myotis vivesi | U | Scats | Mexico | N | VU | [34] | |
| Nyctalus leisleri | U | Records of rescue | Italy | H | LC | [13] | |
| O | Survey | Italy | [14] | ||||
| Nyctalus noctula | U | Body remains | Ukraine | H | LC | [35] | |
| Nyctophilus geoffroyi | F | Stomach | Australia | N | LC | [15] | |
| O | Corpses brought | Australia | H | [36] | |||
| O | Corpses brought/Scats | Australia | H | [37] | |||
| F | Scats | Australia | N | [38] | |||
| F | Stomach | Australia | H | [39] | |||
| F | Stomach | Australia | N | [40] | |||
| F | Scats | Australia | N | [41] | |||
|
Pipistrellus
coromandra |
SF | Rescue and free | India | H | LC | [42] | |
| Pipistrellus kuhlii | O | Records of rescue | Italy | H | LC | [13] | |
| Pipistrellus maderensis | O | Corpses brought | Portugal | H | VU | [43] | |
| Pipistrellus nathusii | U | Records of rescue | Italy | H | LC | [13] | |
| O | Survey | Italy | H | [14] | |||
| U | Necropsy | Germany | U | [33] | |||
| Pipistrellus pipistrellus | O | Records of rescue | Italy | H | LC | [13] | |
| U | Wing damage and molecular analyses | United Kingdom | U | [32,44] | |||
| U | Necropsy | Germany | H | [33] | |||
| Pipistrellus pygmaeus | O | Records of rescue | Italy | H | LC | [13] | |
| U | Wing damage and molecular analysis | United Kingdom | U | [32,44] | |||
| Plecotus auritus | U | Records of rescue | Italy | H | LC | [13] | |
| O | Survey | Italy | H | [14] | |||
| U | Necropsy | Germany | H | [33] | |||
| U | Molecular analysis | United Kingdom | U | [32] | |||
| Vespadelus darlingtoni | O | Corpses brought | Lord Howe Island | N/U | LC | [45] | |
| Vespertilio murinus | U | Necropsy | Germany | U | LC | [33] |
For instance, cat predation was found to be the first cause of admittance to Italian wildlife rescue centers for adult bats [13].
While the actual population effects on wildlife of cat predation are unknown, yet suspected to be important, there is overall little doubt that where bats and domestic cats co-occur, this typically results in predation episodes (see Table 1 and references therein). The topic is often discussed in terms of conservation consequences since many bat species are at risk, and cat predation may at least act synergically with other threats contributing to jeopardize bat conservation status, especially when already dwindling small bat populations are involved.
In general, obligate zoonotic pathogens are usually transmitted from an animal to people (in which case, the latter normally receive the pathogen from animals). Human pathogens, on the other hand, are typically passed on from human to human, but when they have an animal reservoir, they may also be transmitted from an animal to a person. Finally, an animal pathogen may evolve and adapt to our species, becoming a new human pathogen which is then passed on predominantly from human to human. In the last case, the novel human pathogen might still be transferred from people to an animal reservoir, which represents a further way of transmission to humans when it comes into contact with the latter.
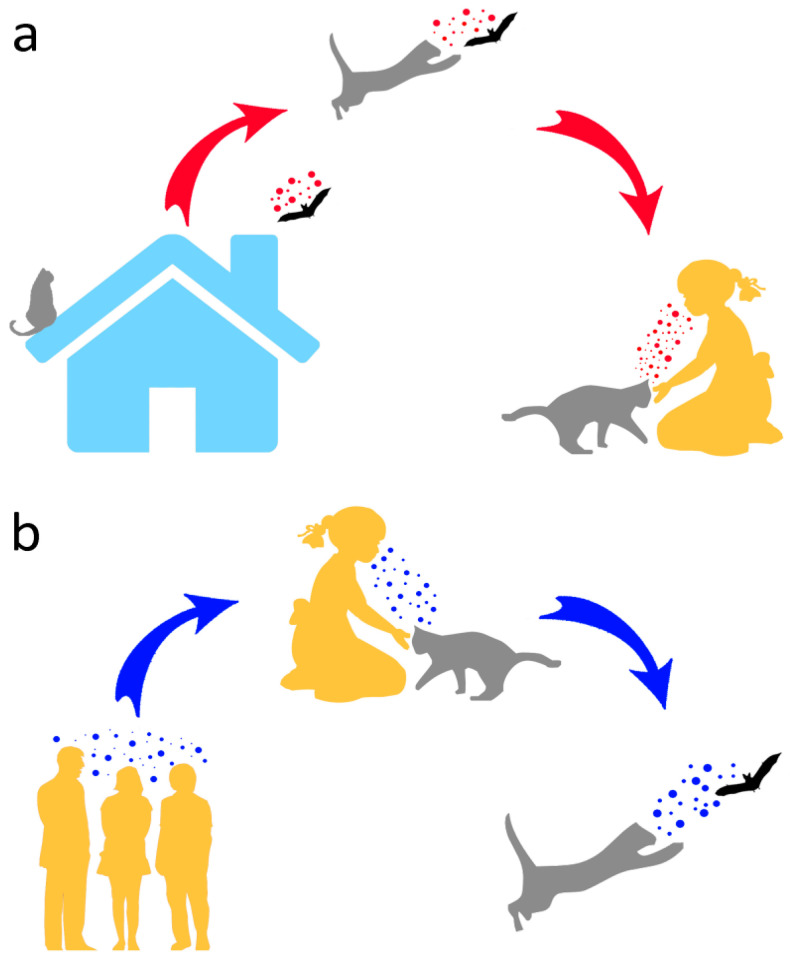
Therefore, in addition to posing conservation problems, as opportunistic predators of wild animals, domestic cats may help to open the Pandora’s box of wildlife (including bats) pathogens by (a) contracting such pathogens from their wild prey and transmitting them to humans and (b) providing a further evolutionary environment that might ultimately lead to diseases affecting humans or other animals (Figure 2). Moreover, due to their frequent interactions with people, typically involving physical contact, cats might host human pathogens and pass these on to bats (Figure 2).
Figure 2.
Potential human–cat–bat interactions and associated pathogen transmission. In (a), pathogens are transmitted by a bat caught by a cat, and eventually to humans from the latter. In (b), a reverse pathogen transmission from humans to bats via cat predation is shown.
Because of their peculiar physiology and natural history, bats are highly resistant to viruses [46], so in the worst-case scenarios, the above-described interactions might make bats viral reservoirs of pathogens that in the absence of predation-mediated transmission would never reach them. In addition to being species of conservation concern strictly protected in most countries (in the EU, all bat species and their habitat are protected under the 92/43 Habitats Directive), bats provide vital ecosystem services such as insectivory, pollination and seed dispersal [47,48], and insect suppression by bats is in fact an important natural defense from arthropod-borne diseases. Therefore, should bats become a novel pathogen reservoir, culling bats even under hypothetical emergency scenarios must be firmly ruled out as a solution as it would surely have detrimental consequences for ecosystem functioning, human economy and health.
2. State of Art of Bat–Cat Exchange of Pathogens
2.1. Rabies
The best-known disease that may pass on from bats to cats and eventually humans is rabies, a zoonosis that causes 590,000 human deaths per year and affects >100 countries worldwide [49]. Human contagion is caused by contact with saliva of an infected animal. Cases of Rabies Lyssavirus (RABV) in the Americas are often associated with bats [50,51], and the virus was also recurrently isolated from domestic cats. For example, in 2007 alone, 3.8% of RABV cases reported for the US and Puerto Rico were cats (n = 274; [52]). In the same geographic area, in 2018, cats were 4.9% of 4951 cases of animal rabies [53]. Lyssaviruses other than RABV such as European Bat Lyssaviruses (EBLV) types 2 and 3, also potentially fatal to people yet with extremely low human incidence across Europe, have bats as the main host and have been also rarely isolated from domestic cats in the European territory [54]. Contacts between domestic cats and rabid Eptesicus serotinus bats were reported for the Netherlands [55]. Most recently, a cat in Central Italy died after biting humans and tested positive for another lyssavirus, the West Caucasian bat lyssavirus [49], previously isolated in the Miniopterus schreibersii from South-Eastern Europe [56]—a partly migratory species also occurring in Italy [57]. Isolates of the African Mokola virus (genus Lyssavirus) are also retrieved in domestic cats and might result from predation over a bat or rodent natural reservoir [58].
Although in many cases, cats are likely to be infected from other wildlife such as carnivores, this picture still points at a non-negligible risk of spillover events from bats to domestic cats [59], which has in fact been observed [60]. Although cats seem to be resistant to acquiring rabies through the ingestion of contaminated tissue [61], they may still be exposed to (and infected through) bat bites when capturing bats on dusk emergence from the roost. Due to its altered movement and reduced reactivity, a rabid bat fallen to the ground in the surroundings of its roost [59] may be especially attractive to the predator [62,63]. While the risk run by humans of being bitten by a rabid wildlife species is small [64], this is intuitively substantially higher when it comes to domestic animals such as cats. Through this way, even historically rabies-free areas—particularly when involving migratory bat species [65]—and urban or rural environments [66,67,68] may be affected. For instance, in Brazil, in 2008–2016, only 1.4% cases of people bitten by animals concerned wildlife vs. 94% of bites by dogs and cats [64]. In the same country, variants of rabies viruses circulating in populations of vampire bats Desmodus rotundus were also isolated in domestic cats and transmitted to humans from both animal species [69]. Moreover, rabies may potentially be transmitted from cats to humans through scratches, which are common injuries associated with people–cat interactions [70]. Domestic cats may therefore represent a new spillover-spreading route [71] through which an otherwise negligible incidence of bat rabies transmission to humans is magnified.
2.2. Other Diseases
In addition to rabies, a range of other diseases may directly or indirectly involve cats, bats and humans. For instance, domestic cats have been found to be susceptible to henipaviruses and filoviruses isolated from bats and might therefore act as intermediate hosts in outbreaks of these pathogens, but important knowledge gaps exist on this issue ([72], for a review). Other viruses such as the EfHV (Eptesicus fuscus Herpesvirus, a Gammaherpesuvirus) may replicate in bat, cat and human cells [73]. Further, interspecies transmission involving bats, cats and humans may foster viral genetic reassortments, which may also take place in viruses that are currently pathogenic to humans [74].
Gram-negative bacteria from the genus Pasteurella are commensal of dogs’ and cats’ oral cavities and may occasionally infect humans through bites or scratches [75]. Among these, P. multocida was reported to infect and kill free-ranging vespertilionid bats, which typically die by septicemia following pasteurellosis [33]. There is substantial evidence that P. multocida strains of cat origin were transmitted to bats following cat predation attempts [33], so even bats that survive the attack may develop wound infections and die afterwards. Other bacteria transmitted to bats in the same way that may also harm these mammals are those in the family Enterobacteriaceae [33]. Among protozoans, Toxoplasma gondii, a feline–zoonotic pathogen causing toxoplasmosis in humans to whom it is often transmitted by domestic cats, was also (albeit rarely) observed in bats, comprising genotypes previously isolated from cats [76,77]. Other protozoans harmful to humans such as Trypanosoma cruzi have a range of wild and domestic reservoirs, including bats and cats [78], yet no direct transmission between such species is documented. Yeasts that affect animals and humans such as the Malassezia spp., some of which cause skin disorders in people, are frequent on the skin and in the auricular canal of domestic cats and dogs and have also been detected in bats, suggestive of the hypothetic emerging zoonotic pathways involving, this time, fungi [79].
Arthropod ectoparasites are well-known vectors that transmit diseases to their hosts and can be directly involved in spillover events [80]. Although such parasites are often host-specific, some are opportunistic, such as the badger flea Paraceras melis, observed in several wild mammals, but also in dogs, cats, bats (Rhinolophus hipposideros) and humans [81]. The flea in question is the vector of Trypanosoma pestanai, affecting European badgers [82]. Likewise, mites such as Demodex canis may parasitize cats and dogs as well as bats [83] and rarely cause demodicosis in humans [84]. In such cases, arthropod ectoparasites might provide an epidemiological connection among different hosts and link humans to bats via cats, but this potential route is unstudied and warrants investigation.
3. SARS-CoV-2 and the Risk of Human-to-Bat Transmission. A Role for Cats?
Over the last few months, in the course of the (at the time of writing) still ongoing tragic pandemic, the media have too often presented bats as the epidemiological source of SARS-CoV-2, the virus causing COVID-19, framing these mammals in a negative light and exposing them to potentially adverse conservation consequences such as deliberate killing and roost disturbance [85]. In fact, rhinolophid bats from Southern China were found to host other coronaviruses (Bat CoV RaTG13 and RmYN02) that are phylogenetically strictly related to SARS-CoV-2, pointing at a common origin of this virus [86]. Although bats are therefore unfairly indicated as the culprit of the pandemic, the real causes are to be found in bat hunting and trading for human consumption [87]. Whether the SARS-CoV-2 evolution may have involved other hosts such as pangolins [88] is unclear and was recently questioned [89], but there is no doubt that live animal markets where bats, pangolins and other wildlife are stored together and in strict contact with people, slaughtered and sold in situ provide ideal conditions for the emergence of novel viral diseases [87]. Although COVID-19 is a human disease that had a zoonotic origin, research showed that SARS-CoV-2 spike proteins may bind to the ACE2 proteins found on the cell surface of several other mammals in addition to humans [90]. There have been, in fact, cases of non-human mammals infected with SARS-CoV-2 comprising captive tigers and lions, American minks in breeding facilities as well as in the wild, and pets (see [91] for a review). As for bats, the picture is unclear and far from being comprehensively explored. Rousettus aegyptiacus may be experimentally infected but show no symptoms [92], whereas other species, including Tadarida brasiliensis and rhinolophids, are deemed possibly susceptible to human-to-bat transmission [91]. On the other hand, big brown bats Eptesicus fuscus experimentally inoculated with SARS-CoV-2 proved resistant to infection [93]. Overall, reactions to the virus can be highly species-specific, which is of little relief considering the high global species richness of bats (>1400 species) and the widespread occurrence of SARS-CoV-2 among humans.
Based on the high diversity of coronaviruses found in bats and the tendency shown by such pathogens to change hosts [94], conservationists’ main concern is that transmission of SARS-CoV-2 from humans to bats (or other wildlife) would establish a novel reservoir in these mammals from which further waves of infection might be sparked (Figure 2). Should this happen, it would clearly constitute a very challenging managing issue with adverse consequences for bat conservation, especially in urban areas [91]. Moreover, by spreading in wildlife, including bats, SARS-CoV-2 might have the chance to evolve and adapt to a range of new hosts. On such a basis, in 2020, the EUROBATS Advisory Committee promptly published recommendations on potential risks of SARS-CoV-2 transmission from humans to bats (EUROBATS is the UNEP Agreement on the Conservation of Populations of European Bats: www.eurobats.org, accessed on 25 January 2021). In the same year, to prevent such risks, the International Union for Conservation of Nature Bat Specialist Group issued guidelines aimed at a diverse range of stakeholders including bat researchers, rehabilitators, cavers and cave visitors and guano collectors (https://www.iucnbsg.org/bsg-publications.html, accessed on 25 January 2021).
The presence of house-dwelling bats in their roosts is unlikely to pose a realistic risk of direct human-to-bat transmission of SARS-CoV-2; however, especially in rural or urban contexts, domestic cats may hypothetically bridge the physical gap separating people from bats and contribute to spread the virus to the latter through predation. People may in fact transmit SARS-CoV-2 to cats, which are susceptible to the virus and are subject to efficient (also airborne) transmission of the virus, as known from both recorded cases and experimental work [95,96,97,98,99,100]. While it is difficult to predict the likelihood of cat-to-bat transmission of SARS-CoV-2, which based on the scarce available knowledge seems negligible, there is no doubt that the issue merits full attention due to its considerable implications in terms of zoonotic risk and conservation consequences.
4. Preventing Zoonotic Risks Associated with Bat Predation by Cats: Research and Management Directions
From what has been illustrated so far, it should be clear that not only do domestic cats left outdoor pose threats to bat conservation, they also represent an epidemiologic link between humans and bats across which pathogens may spread in either direction. On such a basis, we urge that appropriate measures be taken to prevent the zoonotic risk associated with cats, especially in the proximity of known bat roosts, as follows:
Domestic cat populations should be part of active surveillance programs aiming at detecting the possible presence of pathogens that may be involved in the bat–cat–human transmission chain. The program should target cats left outdoor, which not only includes stray cats but also free-roaming owned cats;
In countries such as Italy, urban free-roaming cats living in colonies are protected by the law; cats are recognized the right to live free, neither killed nor moved, are neutered by the veterinary service of the local health authorities and are looked after by institutionalized cat carers [101]. While this degree of protection has certainly improved conditions of stray cats and pursued important animal welfare objectives, its consequences in terms of the impact on urban animal biodiversity are unknown and neglected. We propose these colonies be systematically monitored for their effects on wildlife, and more specifically bats, and the possible zoonotic consequences assessed to develop appropriate management strategies. Special measures should also be taken for cat colonies that occur in sites where a bat roost is known to be present;
Outdoor cats should be included as a threat factor in all conservation plans aiming at the management of known bat colonies, and action should be taken, including legal measures, to prevent contacts between cats and bats;
Where applicable, vaccination campaigns of cats living outdoor should be promoted to reduce major zoonotic risks such as that associated with rabies, and more investigation is warranted to see whether commercially available rabies–inactivated veterinary vaccines, prepared from RABV strains, can cross-protect cats against the different lyssaviruses circulating in Europe [102]. The risk of stopping vaccination campaigns in areas that have been recently declared rabies-free should also be evaluated [103];
Cat predation on wildlife is a complex process that is deeply rooted in cats’ evolutionary history but also depends on a range of variables including dietary requirements and physiological traits, early life history, individual personality and environmental factors [104]. A better understanding of the relative weights of such variables is key to develop appropriate strategies to prevent cat predation on wildlife and bats more specifically, and ad hoc work should be done on bat–cat interactions, including those involving sick bats. For instance, in a study on spatiotemporal trends in bat rabies in Washington State which assessed the associated zoonotic risk, cats caught almost 90% of bats captured by pets, but dogs tended to catch rabid bats more often [105]. Patterns such as this are clearly linked with the behavioral characteristics of cat predation and their comprehension would help to prevent or mitigate zoonotic risks. Systematic studies of cat predation on bats should include, in addition to behavioral observations, molecular assessments of the diet of cats and strict cooperation with wildlife rehabilitation centers, where bats injured by cats are often admitted [13]. Confirming that a given bat was killed or injured by a cat is today possible thanks to forensic DNA analysis techniques, which can even be used to link predation events to individual cats [32] and allow inspection of both predator and prey for the potential presence, or transmission, of pathogens. Estimating survival rates among bats following predation attempts from cats is also key to better evaluate the potential zoonotic risk associated with these events;
Since tools to explore epidemiological scenarios are available [106], including the use of GIS and spatial modeling, applications to situations involving people, cats and bats in rural and urban areas are needed;
Domestic cats are highly popular and many people, including animal lovers in good faith, keep cats outdoor to meet their pet’s supposed need for “freedom”. Cat impact on wildlife is often deliberately glossed over, generates animosity among cat lovers and is sometimes even exacerbated by a real “code of silence”. There is ever growing evidence, however, that cat predation on wildlife (e.g., [9,14,28]), including bats ([107]; this study), is largely underestimated. Awareness-raising campaigns should be carried out to encourage people to keep cats indoor, which would in fact protect not only wildlife from predation, but also cats from the many risks posed by living outdoors, and people from the zoonotic risks we discussed;
Finally, we urge that a stronger cooperation among potential stakeholders (conservationists, animal welfare organizations, human health authorities, virologists, etc.) is developed to implement appropriate one-health strategies and prevent or at least mitigate the risks of bat–cat–human transmission of pathogens.
Acknowledgments
We thank two anonymous reviewers for their valuable comments on a first draft of this article.
Author Contributions
Conceptualization, V.B.S.-R. and D.R.; investigation, V.B.S.-R. and D.R.; writing—original draft preparation, V.B.S.-R., L.B., E.M., L.A. and D.R.; writing—review and editing, V.B.S.-R., L.B., E.M., L.A. and D.R.; supervision, D.R.; funding acquisition, V.B.S.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT, grant number [294178], recipient V.B.S.-R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bengis R.G., Leighton F.A., Fischer J.R., Artois M., Morner T., Tate C.M. The role of wildlife in emerging and re-emerging zoonoses. Rev. Sci. Tech. 2004;23:497–512. [PubMed] [Google Scholar]
- 2.Huong N.Q., Nga N.T., Long N.V., Luu B.D., Latinne A., Pruvot M., Phuong N.T., Quang L.T., Hung V.V., Lan N.T., et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Vietnam, 2013–2014. PLoS ONE. 2004;15:e0237129. doi: 10.1371/journal.pone.0237129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora N.K., Mishra J. COVID-19 and importance of environmental sustainability. Environ. Sustain. 2020;3:117–119. doi: 10.1007/s42398-020-00107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J.A., Chen F., Fan S. Effect of intermediate hosts on emerging zoonoses. Vector Borne Zoonotic Dis. 2017;17:599–609. doi: 10.1089/vbz.2016.2059. [DOI] [PubMed] [Google Scholar]
- 5.Nor M.M., Gan C.H., Ong B.L. Nipah virus infection of pigs in peninsular Malaysia. Rev. Sci. Tech. 2000;19:160–165. doi: 10.20506/rst.19.1.1202. [DOI] [PubMed] [Google Scholar]
- 6.Hayman D.T.S., Bowen R.A., Cryan P.M., McCracken G.F., O’shea T.J., Peel A.J., Gilbert A., Webb C.T., Wood J.L.N. Ecology of zoonotic infectious diseases in bats: Current knowledge and future directions. Zoonoses Public Health. 2013;60:2–21. doi: 10.1111/zph.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollentze N., Streicker D.G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl. Acad. Sci. USA. 2020;117:9423–9430. doi: 10.1073/pnas.1919176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Baucells A., Rocha R., Fernández-Llamazares Á. When bats go viral: Negative framings in virological research imperil bat conservation. Mamm. Rev. 2018;48:62–66. doi: 10.1111/mam.12110. [DOI] [Google Scholar]
- 9.Woolley L.A., Geyle H.M., Murphy B.P., Legge S.M., Palmer R., Dickman C.R., Augusteyn J., Comer S., Doherty T.S., Eager C., et al. Introduced cats Felis catus eating a continental fauna: Inventory and traits of Australian mammal species killed. Mamm. Rev. 2019;49:354–368. doi: 10.1111/mam.12167. [DOI] [Google Scholar]
- 10.Capizzi D. A review of mammal eradications on Mediterranean islands. Mamm. Rev. 2020;50:124–135. doi: 10.1111/mam.12190. [DOI] [Google Scholar]
- 11.Marra P., Santella C. Cat Wars: The Devastating Consequences of a Cuddly Killer. Princeton University Press; Princeton, NJ, USA: Oxford, UK: 2016. p. 223. [Google Scholar]
- 12.Li Y., Wan Y., Shen H., Loss S.R., Marra P.P., Li Z. Estimates of wildlife killed by free-ranging cats in China. Biol. Conserv. 2021;253:108929. doi: 10.1016/j.biocon.2020.108929. [DOI] [Google Scholar]
- 13.Ancillotto L., Serangeli M.T., Russo D. Curiosity killed the bat: Domestic cats as bat predators. Mamm. Biol. 2013;78:369–373. doi: 10.1016/j.mambio.2013.01.003. [DOI] [Google Scholar]
- 14.Mori E., Menchetti M., Camporesi A., Cavigioli L., Tabarelli de Fatis K., Girardello M. Licence to kill? Domestic cats affect a wide range of native fauna in a highly biodiverse Mediterranean country. Front. Ecol. Evol. 2019;7:477. doi: 10.3389/fevo.2019.00477. [DOI] [Google Scholar]
- 15.Woinarski J.C., South S.L., Drummond P., Johnston G.R., Nankivell A. The diet of the feral cat (Felis catus), red fox (Vulpes vulpes) and dog (Canis familiaris) over a three-year period at Witchelina Reserve, in arid South Australia. Aust. Mammal. 2018;40:204–213. doi: 10.1071/AM17033. [DOI] [Google Scholar]
- 16.Holden C., Mutze G. Impact of rabbit haemorrhagic disease on introduced predators in the Flinders Ranges, South Australia. Wildl. Res. 2002;29:615–626. doi: 10.1071/WR00101. [DOI] [Google Scholar]
- 17.Romano M.C., Maidagan J.I., Pire F.E. Behavior and demography in an urban colony of Tadarida brasiliensis (Chiroptera: Molossidae) in Rosario, Argentina. Rev. Biol. Trop. 1999;47:1121–1127. doi: 10.15517/rbt.v47i4.19344. [DOI] [Google Scholar]
- 18.Rodríguez-Durán A., Pérez J., Montalbán M.A., Sandoval J.M. Predation by free-roaming cats on an insular population of bats. Acta Chiropt. 2010;12:359–362. doi: 10.3161/150811010X537945. [DOI] [Google Scholar]
- 19.Scrimgeour J., Beath A., Swanney M. Cat predation of short-tailed bats (Mystacina tuberculata rhyocobia) in rangataua forest, Mount Ruapehu, central North Island, New Zealand. N. Z. J. Zool. 2012;39:257–260. doi: 10.1080/03014223.2011.649770. [DOI] [Google Scholar]
- 20.Daniel M.J., Williams G.R. A survey of the distribution, seasonal activity and roost sites of New Zealand bats. N. Z. J. Ecol. 1984;7:9–25. [Google Scholar]
- 21.Borroto-Páez R., Mancina C.A. Biodiversity and conservation of Cuban mammals: Past, present, and invasive species. J. Mammal. 2017;98:964–985. doi: 10.1093/jmammal/gyx017. [DOI] [Google Scholar]
- 22.da Costa-Pinto A.L. First record of natural predation on bats by domestic cat in Brazil, with distribution extension for Phyllostomus discolor. Oecol. Aust. 2020;24:242–248. doi: 10.4257/oeco.2020.2401.24. [DOI] [Google Scholar]
- 23.Bigai L.R., Faria M.B. Eventos predatórios em morcegos no Brasil. Rev. Biol. Neotrop. 2018;15:96–108. doi: 10.5216/rbn.v15i2.53478. [DOI] [Google Scholar]
- 24.Delpietro H., Konolsaisen F., Marchevsky N., Russo G. Domestic cat predation on vampire bats (Desmodus rotundus) while foraging on goats, pigs, cows and human beings. Appl. Anim. Behav. Sci. 1994;39:141–150. doi: 10.1016/0168-1591(94)90134-1. [DOI] [Google Scholar]
- 25.Mancina C.A. Phyllonycteris poeyi (Chiroptera: Phyllostomidae) Mamm. Species. 2010;42:41–48. doi: 10.1644/852.1. [DOI] [Google Scholar]
- 26.Vincenot C.E., Koyama L., Russo D. Near threatened? First report of unsuspected human-driven decline factors in the Ryukyu flying fox (Pteropus dasymallus) in Japan. Mamm. Biol. 2015;80:273–277. doi: 10.1016/j.mambio.2015.03.003. [DOI] [Google Scholar]
- 27.Tidemann C.R., Yorkston H.D., Russack A.J. The diet of cats, Felis catus, on Christmas Island, Indian ocean. Wildl. Res. 1994;21:279–285. doi: 10.1071/WR9940279. [DOI] [Google Scholar]
- 28.Palmas P., Jourdan H., Rigault F., Debar L., De Meringo H., Bourguet E., Mathiveta M., Leea M., Adjouhgniopea R., Papillona Y., et al. Feral cats threaten the outstanding endemic fauna of the New Caledonia biodiversity hotspot. Biol. Conserv. 2017;214:250–259. doi: 10.1016/j.biocon.2017.08.003. [DOI] [Google Scholar]
- 29.Brickner-Braun I., Geffen E., Yom-Tov Y. The domestic cat as a predator of Israeli wildlife. Isr. J. Ecol. Evol. 2007;53:129–142. doi: 10.1560/IJEE.53.2.129. [DOI] [Google Scholar]
- 30.Phillips S., Coburn D., James R. An observation of cat predation upon an eastern blossom bat Syconycteris australis. Aust. Mammal. 2001;23:57–58. doi: 10.1071/AM01057. [DOI] [Google Scholar]
- 31.Kutt A.S. The diet of the feral cat (Felis catus) in north-eastern Australia. Acta Theriol. 2011;56:157–169. doi: 10.1007/s13364-010-0016-7. [DOI] [Google Scholar]
- 32.Khayat R.O.S., Grant R.A., Ryan H., Melling L.M., Dougill G., Killick D.R., Shaw K.J. Investigating cat predation as the cause of bat wing tears using forensic DNA analysis. Ecol. Evol. 2020;20:8368–8378. doi: 10.1002/ece3.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mühldorfer K., Speck S., Wibbelt G. Diseases in free-ranging bats from Germany. BMC Vet. Res. 2011;7:61. doi: 10.1186/1746-6148-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vázquez-Domínguez E., Ceballos G., Cruzado J. Extirpation of an insular subspecies by a single introduced cat: The case of the endemic deer mouse Peromyscus guardia on Estanque Island, Mexico. Oryx. 2004;38:347–350. doi: 10.1017/S0030605304000602. [DOI] [Google Scholar]
- 35.Vlaschenko A., Kovalov V., Hukov V., Kravchenko K., Rodenko O. An example of ecological traps for bats in the urban environment. Eur. J. Wildl. Res. 2019;65:20. doi: 10.1007/s10344-019-1252-z. [DOI] [Google Scholar]
- 36.Calver M., Thomas S., Bradley S., McCutcheon H. Reducing the rate of predation on wildlife by pet cats: The efficacy and practicability of collar-mounted pounce protectors. Biol. Conserv. 2007;137:341–348. doi: 10.1016/j.biocon.2007.02.015. [DOI] [Google Scholar]
- 37.Hall C.M., Fontaine J.B., Bryant K.A., Calver M.C. Assessing the effectiveness of the Birdsbesafe® anti-predation collar cover in reducing predation on wildlife by pet cats in Western Australia. Appl. Anim. Behav. Sci. 2015;173:40–51. doi: 10.1016/j.applanim.2015.01.004. [DOI] [Google Scholar]
- 38.Triggs B., Brunner H., Cullen J.M. The food of fox, dog and cat in Croajingalong National Park, south-eastern Victoria. Wildl. Res. 1984;11:491–499. doi: 10.1071/WR9840491. [DOI] [Google Scholar]
- 39.Jones E., Coman B.J. Ecology of the feral cat, Felis catus (L.), in south-eastern Australia I. Diet. Wildl. Res. 1981;8:537–547. doi: 10.1071/WR9810537. [DOI] [Google Scholar]
- 40.Read J.L., Dagg E., Moseby K.E. Prey selectivity by feral cats at central Australian rock-wallaby colonies. Aust. Mammal. 2019;41:132–141. doi: 10.1071/AM17055. [DOI] [Google Scholar]
- 41.Rismiller P.D., McKelvey M.W. Feral Cat Control, Some New Ideas for 2003, Kangaroo Island/Roxby Downs. Kangaroo Island Rotary Club; Kingscote, Australia: 2003. Twenty-seven years of wildcats and kittens, case history of a feral predator on the Pelican Lagoon Peninsula, Kangaroo Island; pp. 9–16. [Google Scholar]
- 42.Virkar P.S., Shrotriya S. Threat to wildlife from carnivorous pets: A case of cat attacking Indian Pipistrelle Pipistrellus coromandra (Gray, 1838) Zoo’s Print. 2013;28:25–27. [Google Scholar]
- 43.Rocha R. Look what the cat dragged in: Felis silvestris catus as predators of insular bats and instance of predation on the endangered Pipistrellus maderensis. Barb. 2015;8:18–21. [Google Scholar]
- 44.Khayat R.O.S., Shaw K.J., Dougill G., Melling L.M., Ferris G.R., Cooper G., Grant R.A. Characterizing wing tears in common pipistrelles (Pipistrellus pipistrellus): Investigating tear distribution, wing strength, and possible causes. J. Mammal. 2019;100:1282–1294. doi: 10.1093/jmammal/gyz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller B., Mullette K.J. Rehabilitation of an endangered Australian bird: The Lord Howe Island woodhen Tricholimnas sylvestris (Sclater) Biol. Conserv. 1985;34:55–95. doi: 10.1016/0006-3207(85)90057-6. [DOI] [Google Scholar]
- 46.Hayman D.T. Bat tolerance to viral infections. Nat. Microbiol. 2019;4:728–729. doi: 10.1038/s41564-019-0430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunz T.H., Braun de Torrez E., Bauer D., Lobova T., Fleming T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011;1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- 48.Russo D., Bosso L., Ancillotto L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Environ. 2018;266:31–38. doi: 10.1016/j.agee.2018.07.024. [DOI] [Google Scholar]
- 49.Gossner C.M., Mailles A., Aznar I., Dimina E., Echevarría J.E., Feruglio S.L., Lange H., Maraglino F.P., Parodi P., Perevoscikovs J., et al. Prevention of human rabies: A challenge for the European Union and the European Economic Area. Eurosurveillance. 2020;25:2000158. doi: 10.2807/1560-7917.ES.2020.25.38.2000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization (WHO) WHO Expert Consultation on Rabies: Third Report. WHO; Geneva, Switzerland: 2018. [(accessed on 25 January 2021)]. Available online: https://apps.who.int/iris/handle/10665/272364. [Google Scholar]
- 51.Velasco-Villa A., Orciari L.A., Juárez-Islas V., Gómez-Sierra M., Padilla-Medina I., Flisser A., Souza V., Castillo A., Franka R., Escalante-Mañe M., et al. Molecular diversity of rabies viruses associated with bats in Mexico and other countries of the Americas. J. Clin. Microbiol. 2006;44:1697–1710. doi: 10.1128/JCM.44.5.1697-1710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanton J.D., Palmer D., Christian K.A., Rupprecht C.E. Rabies surveillance in the United States during 2008. J. Am. Vet. Med. Assoc. 2009;235:676–689. doi: 10.2460/javma.235.6.676. [DOI] [PubMed] [Google Scholar]
- 53.Ma X., Monroe B.P., Cleaton J.M., Orciari L.A., Gigante C.M., Kirby J.D., Chipman R.B., Fehlner-Gardiner C., Gutiérrez Cedillo V., Petersen B.W., et al. Public Veterinary Medicine: Public Health: Rabies surveillance in the United States during 2018. J. Am. Vet. Med. Assoc. 2020;256:195–208. doi: 10.2460/javma.256.2.195. [DOI] [PubMed] [Google Scholar]
- 54.Dacheux L., Larrous F., Mailles A., Boisseleau D., Delmas O., Biron C., Bouchier C., Capek I., Muller M., Ilari F., et al. European bat lyssavirus transmission among cats, Europe. Emerg. Infect. Dis. 2009;15:280. doi: 10.3201/eid1502.080637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takumi K., Lina P.H.C., Van der Poel W.H.M., Kramps J.A., Van Der Giessen J.W.B. Public health risk analysis of European bat lyssavirus infection in The Netherlands. Epidemiol. Infect. 2009;137:803–809. doi: 10.1017/S0950268807000167. [DOI] [PubMed] [Google Scholar]
- 56.Botvinkin A.D., Poleschuk E.M., Kuzmin I.V., Borisova T.I., Gazaryan S.V., Yager P., Rupprecht C.E. Novel lyssaviruses isolated from bats in Russia. Emerg. Infect. Dis. 2003;9:1623–1625. doi: 10.3201/eid0912.030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright P.G., Newton J., Agnelli P., Budinski I., Di Salvo I., Flaquer C., Fulco A., Georgiakakis P., Martinoli A., Mas M., et al. Hydrogen isotopes reveal evidence of migration of Miniopterus schreibersii in Europe. BMC Ecol. 2020;20:1–7. doi: 10.1186/s12898-020-00321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabeta C., Blumberg L., Miyen J., Mohale D., Shumba W., Wandeler A. Mokola virus involved in a human contact (South Africa) FEMS Immunol. Med. Microbiol. 2010;58:85–90. doi: 10.1111/j.1574-695X.2009.00609.x. [DOI] [PubMed] [Google Scholar]
- 59.Shankar V., Orciari L.A., Mattos C.D., Kuzmin I.V., Pape W.J., O’Shea T.J., Rupprecht C.E. Genetic divergence of rabies viruses from bat species of Colorado, USA. Vector Borne Zoonotic Dis. 2005;5:330–341. doi: 10.1089/vbz.2005.5.330. [DOI] [PubMed] [Google Scholar]
- 60.Jaramillo-Reyna E., Almazán-Marín C., de la O-Cavazos M.E., Valdéz-Leal R., Bañuelos-Álvarez A.H., Zúñiga-Ramos M.A., Melo-Munguía M., Gómez-Sierra M., Sandoval-Borja A., Chávez-López S., et al. Public Veterinary Medicine: Public Health Rabies virus variants identified in Nuevo Leon State, Mexico, from 2008 to 2015. J. Am. Vet. Med. Assoc. 2020;256:438–443. doi: 10.2460/javma.256.4.438. [DOI] [PubMed] [Google Scholar]
- 61.Shirakawa R.K., Cortez A., Richtzenhain L.J., Itoou T., Sakai T., Ito F.H. Ensaios sobre inoculação intramuscular e alimentação de gatos domésticos (Felis catus) com cérebros de camundongos préviamente inoculados com vírus da raiva. Braz. J. Vet. Res. Anim. Sci. 2007;44:125–133. doi: 10.11606/issn.1678-4456.bjvras.2007.26601. [DOI] [Google Scholar]
- 62.Genaro G. Gato doméstico: Futuro desafio para controle da raiva em áreas urbanas? Pesqui. Vet. Bras. 2010;30:186–189. doi: 10.1590/S0100-736X2010000200015. [DOI] [Google Scholar]
- 63.Ribeiro J., Staudacher C., Martins C.M., Ullmann L.S., Ferreira F., Araujo J.P., Biondo A.W. Bat rabies surveillance and risk factors for rabies spillover in an urban area of Southern Brazil. BMC Vet. Res. 2018;14:1–8. doi: 10.1186/s12917-018-1485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benavides J.A., Megid J., Campos A., Hampson K. Using surveillance of animal bite patients to decipher potential risks of rabies exposure from domestic animals and wildlife in Brazil. Front. Public Health. 2020;8:318. doi: 10.3389/fpubh.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morikawa V.M., Ribeiro J., Biondo A.W., Fellini A., Bier D., Molento M.B. Cat infected by a variant of bat rabies virus in a 29-year disease-free urban area of southern Brazil. Rev. Soc. Bras. Med. Trop. 2012;45:255–256. doi: 10.1590/S0037-86822012000200022. [DOI] [PubMed] [Google Scholar]
- 66.Miranda A.O., Núñez S.E., Martinez L., Gury–Dohmen D.M. Molecular analysis of urban rabies case from vampire bat in Corrientes, Argentina. Rev. Vet. 2009;20:77–80. doi: 10.30972/vet.2021853. [DOI] [Google Scholar]
- 67.Castilho J.G., De Souza D.N., Oliveira R.N., Carnieli P., Jr., Batista H.B.C.R., Pereira P.M.C., Achkar S.M., Macedo C.I. The epidemiological importance of bats in the transmission of rabies to dogs and cats in the state of São Paulo, Brazil, between 2005 and 2014. Zoonoses Public Health. 2017;64:423–430. doi: 10.1111/zph.12320. [DOI] [PubMed] [Google Scholar]
- 68.Bannazadeh Baghi H., Alinezhad F., Kuzmin I., Rupprecht C.E. A Perspective on Rabies in the Middle East—Beyond Neglect. Vet. Sci. 2018;5:67. doi: 10.3390/vetsci5030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araújo I.L., Oliveira T.M., Diniz S.A., Silva M.X. Análise epidemiológica dos atendimentos da profilaxia antirrábica humana associados a acidentes com gatos. Arq. Bras. Med. Vet. Zootec. 2020;72:814–822. doi: 10.1590/1678-4162-10413. [DOI] [Google Scholar]
- 70.Wang X., Werner B.G., Konomi R., Hennigan D., Fadden D., Caten E., Soliva S., DeMaria A. Animal rabies in Massachusetts, 1985–2006. J. Wildl. Dis. 2009;45:375–387. doi: 10.7589/0090-3558-45.2.375. [DOI] [PubMed] [Google Scholar]
- 71.Teng C.H.E.N., Miao F.M., Ye L.I.U., Zhang S.F., Zhang F., Nan L.I., Hu R.L. Possible transmission of irkut virus from dogs to humans. Biomed. Environ. Sci. 2018;31:146–148. doi: 10.3967/bes2018.017. [DOI] [PubMed] [Google Scholar]
- 72.Glennon E.E., Restif O., Sbarbaro S.R., Garnier R., Cunningham A.A., Suu-Ire R.D., Osei-Amponsah R., Wood J.L.N., Peel A.J. Domesticated animals as hosts of henipaviruses and filoviruses: A systematic review. Vet. J. 2018;233:25–34. doi: 10.1016/j.tvjl.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 73.Subudhi S., Rapin N., Dorville N., Hill J.E., Town J., Willis C.K., Bollinger T.K., Misra V. Isolation, characterization and prevalence of a novel Gammaherpesvirus in Eptesicus fuscus, the North American big brown bat. Virology. 2018;516:227–238. doi: 10.1016/j.virol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 74.Okitsu S., Hikita T., Thongprachum A., Khamrin P., Takanashi S., Hayakawa S., Maneekarn N., Ushijima H. Detection and molecular characterization of two rare G8P[14] and G3P[3] rotavirus strains collected from children with acute gastroenteritis in Japan. Infect. Genet. Evol. 2018;62:95–108. doi: 10.1016/j.meegid.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Westling K., Farra A., Cars B., Ekblom A.G., Sandstedt K., Settergren B., Wretlind B., Jorup C. Cat bite wound infections: A prospective clinical and microbiological study at three emergency wards in Stockholm, Sweden. J. Infect. 2006;53:403–407. doi: 10.1016/j.jinf.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Fournier G.F.D.S.R., Lopes M.G., Marcili A., Ramirez D.G., Acosta I.C.L., Ferreira J.I.G.D.S., Cabral A., Lima J.T.R.D., Pena H., Dias R., et al. Toxoplasma gondii in domestic and wild animals from forest fragments of the municipality of Natal, northeastern Brazil. Rev. Bras. Parasitol. Vet. 2014;23:501–508. doi: 10.1590/s1984-29612014092. [DOI] [PubMed] [Google Scholar]
- 77.Cabral A.D., Gama A.R., Sodré M.M., Savani E.S.M.M., Galvão-Dias M.A., Jordão L.R., Maeda M.M., Yai L.E.O., Gennari S.M., Pena H.F.J. First isolation and genotyping of Toxoplasma gondii from bats (Mammalia: Chiroptera) Vet. Parasitol. 2013;193:100–104. doi: 10.1016/j.vetpar.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Wehrendt D.P., Gómez-Bravo A., Ramirez J.C., Cura C., Pech-May A., Ramsey J.M., Abril M., Guhl F., Schijman A.G. Development and evaluation of a duplex TaqMan qPCR assay for detection and quantification of Trypanosoma cruzi infection in domestic and sylvatic reservoir hosts. Parasit. Vectors. 2019;12:1–9. doi: 10.1186/s13071-019-3817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gandra R.F., Gambale W., Simao R.D.C.G., da Silva Ruiz L., Durigon E.L., de Camargo L.M.A., Giudice M.C., Sanfilippo L.F., de Araújo J., Paula C.R. Malassezia spp. in acoustic meatus of bats (Molossus molossus) of the Amazon Region, Brazil. Mycopathologia. 2008;165:21–26. doi: 10.1007/s11046-007-9079-7. [DOI] [PubMed] [Google Scholar]
- 80.Marshall A.G. The Ecology of Ectoparasitic Insects. Academic Press Inc.; London, UK: 1981. p. 446. [Google Scholar]
- 81.Ancillotto L., Mazza G., Menchetti M., Mori E. Host specificity of the badger’s flea (Paraceras melis) and first detection on a bat host. Parasitol. Res. 2014;113:3909–3912. doi: 10.1007/s00436-014-4136-x. [DOI] [PubMed] [Google Scholar]
- 82.Lizundia R., Newman C., Buesching C.D., Ngugi D., Blake D., Sin Y.W., Macdonald D.W., Wilson A., McKeever D. Evidence for a role of the host-specific flea (Paraceras melis) in the transmission of Trypanosoma (Megatrypanum) pestanai to the European badger. PLoS ONE. 2011;6:e16977. doi: 10.1371/journal.pone.0016977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sastre N., Francino O., Curti J.N., Armenta T.C., Fraser D.L., Kelly R.M., Hunt E., Silbermayr K., Zewe C., Sánchez A., et al. Detection, prevalence and phylogenetic relationships of Demodex spp and further skin prostigmata mites (Acari, Arachnida) in wild and domestic mammals. PLoS ONE. 2016;11:e0165765. doi: 10.1371/journal.pone.0165765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dik B. A dog related Demodex spp. infestation in a student: A rare Demodex case. Mikrobiyol Bul. 2018;52:214–220. doi: 10.5578/mb.66410. [DOI] [PubMed] [Google Scholar]
- 85.Rocha R., Aziz S.A., Brook C.E., Carvalho W.D., Cooper-Bohannon R. Bat conservation and zoonotic disease risk: A research agenda to prevent misguided persecution in the aftermath of COVID-19. Anim. Conserv. 2020 doi: 10.1111/acv.12636. [DOI] [Google Scholar]
- 86.Zhou H., Chen X., Hu T., Li J., Song H., Liu Y., Wang P., Liu D., Yang J., Holmes E.C., et al. A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr. Biol. 2020;30:2196–2203. doi: 10.1016/j.cub.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aguirre A.A., Catherina R., Frye H., Shelley L. Illicit wildlife trade, wet markets, and COVID-19: Preventing future pandemics. World Med. Health Policy. 2020;12:256–265. doi: 10.1002/wmh3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang T., Qunfu W., Zhigang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frutos R., Serra-Cobo J., Chen T., Devaux C.A. COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans. Infect. Genet. Evol. 2020;84:104493. doi: 10.1016/j.meegid.2020.104493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94:e00127-20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gryseels S., De Bruyn L., Gyselings R., Calvignac-Spencer S., Leendertz F.H., Leirs H. Risk of human-to-wildlife transmission of SARS-CoV-2. Mamm. Rev. 2020 doi: 10.1111/mam.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., Höper D., Mettenleiter D.C., Balkema-Buschmann A., Harder T., et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe. 2020;1:218–225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hall J.S., Knowles S., Nashold S.W., Ip H.S., Leon A.E., Rocke T., Keller S., Carossino M., Balasuriya D., Hofmeister E. Experimental challenge of a North American bat species, big brown bat (Eptesicus fuscus), with SARS-CoV-2. Transbound Emerg. Dis. 2020 doi: 10.1111/tbed.13949. [DOI] [PubMed] [Google Scholar]
- 94.Colunga-Salas P., Hernández-Canchola G. Bats and humans during the SARS-CoV-2 outbreak: The case of bat-coronaviruses from Mexico. Transbound Emerg. Dis. 2020 doi: 10.1111/tbed.13751. [DOI] [PubMed] [Google Scholar]
- 95.Sailleau C., Dumarest M., Vanhomwegen J., Delaplace M., Caro V., Kwasiborski A., Hourdel V., Chevaillier P., Barbarino A., Comtet L., et al. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound Emerg. Dis. 2020:1–5. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MANFQ Update Corona Bij Dieren. Ministry of Agriculture, Nature and Food Quality of the Netherlands. [(accessed on 25 January 2021)];2020 Available online: https://www.rijksoverheid.nl/documenten/kamerstukken/2020/05/15/kamerbrief-over-corona-bij-dieren.
- 97.USDA Confirmed Cases of SARS-CoV-2 in Animals in the United States. Animal and Plant Health Inspection Service of the United States Department of Agriculture. [(accessed on 25 January 2021)];2020 Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/sa_one_health/sars-cov-2-animals-us.
- 98.AFCD Pet Cat Tests Positive for COVID-19. Agriculture Fisheries and Conservation Department of the Hong Kong Special Administrative Region. [(accessed on 25 January 2021)];2020 Available online: https://www.news.gov.hk/eng/2020/03/20200331.
- 99.FASFC . Zoönotisch risico van het SARS-CoV2 Virus (Covid-19) bij Gezelschapsdieren: Infectie van dier naar mens en van mens naar dier. Federal Agency for the Safety of the Food Chain; Brussels, Belgium: 2020. [(accessed on 25 January 2021)]. Available online: http://www.afsca.be/wetenschappelijkcomite/adviezen/2020/_documents/Spoedraadgeving04-2020_SciCom2020-07_Covid-19gezelschapdieren_27-03-20.pdf. [Google Scholar]
- 100.Newman A., Smith D., Ghai R.R., Wallace R.M., Torchetti M.K., Loiacono C., Murrell L.S., Carpenter A., Moroff S., Rooney J.A., et al. First reported cases of SARS-CoV-2 infection in companion animals—New York, March–April 2020. MMWR Morb. Mortal. Wkl. Rep. 2020;69:710–713. doi: 10.15585/mmwr.mm6923e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Natoli E., Malandrucco L., Minati L., Verzichi S., Perino R., Longo L., Pontecorvo F., Faini A. Evaluation of Unowned Domestic Cat Management in the Urban Environment of Rome After 30 Years of Implementation of the No-Kill Policy (National and Regional Laws) Front. Vet. Sci. 2019;6:31. doi: 10.3389/fvets.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Servat A., Wasniewski M., Cliquet F. Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe. Viruses. 2019;11:936. doi: 10.3390/v11100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dias R.A., Rocha F., Ulloa-Stanojlovic F.M., Nitsche A., Castagna C., de Lucca T., Rodrigues R.C.A. Spatiotemporal distribution of a non-haematophagous bat community and rabies virus circulation: A proposal for urban rabies surveillance in Brazil. Epidemiol. Infect. 2019;147:e130. doi: 10.1017/S0950268818003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cecchetti M., Crowley S.L., McDonald R.A. Drivers and facilitators of hunting behaviour in domestic cats and options for management. Mamm. Rev. 2020 doi: 10.1111/mam.12230. [DOI] [Google Scholar]
- 105.Bonwitt J., Oltean H., Lang M., Kelly R.M., Goldoft M. Bat rabies in Washington State: Temporal-spatial trends and risk factors for zoonotic transmission (2000–2017) PLoS ONE. 2018;13:e0205069. doi: 10.1371/journal.pone.0205069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arias Caicedo M.R., Xavier D.D.A., Arias Caicedo C.A., Andrade E., Abel I. Epidemiological scenarios for human rabies exposure notified in Colombia during ten years: A challenge to implement surveillance actions with a differential approach on vulnerable populations. PLoS ONE. 2019;14:e0213120. doi: 10.1371/journal.pone.0213120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oedin M., Brescia F., Millon A., Murphy B.P., Palmas P., Woinarski J.C.Z., Vidal E. Cats Felis catus as a threat to bats worldwide: A review of the evidence. Mamm. Rev. 2021 doi: 10.1111/mam.12240. [DOI] [Google Scholar]