Abstract
The anaerobic bacterium Cutibacterium acnes has been increasingly linked to the development of degenerative disc disease (DDD), although causality is yet to be conclusively proven. To better study how this organism could contribute to the aetiology of DDD, improved animal models that are more reflective of human disc anatomy, biology and mechanical properties are required. Against this background, our proof-of concept study aimed to be the first demonstration that C. acnes could be safely administered percutaneously into sheep intervertebral discs (IVDs) for in vivo study. Following our protocol, two sheep were successfully injected with a strain of C. acnes (8.3 × 106 CFU/disc) previously recovered from a human degenerative disc. No adverse reactions were noted, and at one-month post inoculation all triplicate infected discs in our first animal grew C. acnes, albeit at a reduced load (5.12 × 104 to 6.67 × 104 CFU/disc). At six months, no growth was detected in discs from our second animal indicating bacterial clearance. This pilot study has demonstrated the feasibility of safe percutaneous injection of C. acnes into sheep IVDs under fluoroscopic guidance. The design of follow-up sheep studies to investigate the potential of C. acnes to drive pathological changes within infected discs should now be pursued.
Keywords: Cutibacterium acnes, sheep model, spinal intervertebral discs, percutaneous injections, bacterial discitis
1. Introduction
According to the Global Burden of Disease study of 2017, low back pain (LBP) is the world’s leading cause of disability [1]. It is estimated that 80% of Americans will experience LBP during their lifetime, and this incidence is predicted to increase due to ageing populations and current demographics such as sedentary lifestyles and obesity [2]. Although the etiology of low back pain is multifaceted, there is a strong correlation with degeneration of the intervertebral disc (IVD) [3]. Curative treatments for degenerative disc disease (DDD) are not available, as the complex pathologic mechanisms, including disc nutritional factors, cytokines, biomechanical factors, and genetic factors are not fully understood.
Over the last 20 years, evidence has accumulated to suggest that infection of IVDs especially with the anaerobic bacterium Cutibacterium acnes (previously Propionibacterium acnes) may have an important role to play in the development of DDD and discogenic pain [4,5,6,7,8,9,10,11,12,13,14]. This organism, which is a substantial component of the normal microbiota on human skin, is a low-grade opportunistic pathogen most famously described for its association with the inflammatory skin condition acne vulgaris [15]. It is, however, also a cause of osteomyelitis, eye, dental and indwelling medical device-related infections, and has been linked to prostate disease and sarcoidosis [16,17,18,19,20,21,22]. C. acnes has been observed in IVDs with a biofilm-like morphology that precludes it presence in such samples due to surgical contamination [23,24]. It has also been reported that human IVDs associated with C. acnes are more likely to be characterised by type I modic changes (MC) in the endplates on magnetic resonance imaging (MRI) scans [25,26,27]. Animal studies have also shown that C. acnes infection of rabbit discs induces degeneration and inflammation of the endplate region, presenting as MCs [28,29]. Despite these observations, the role that C. acnes may play in DDD remains controversial, and continued studies are required, especially using appropriate animal models, to help establish causality. More generally, the development of accurate animal models of DDD would also provide more meaningful insight into the pathophysiology of DDD, as well as a vehicle for testing potential therapeutics [30].
Commonly used animal models for disc research include rabbits, dogs, and sheep, as well as the caudal discs of rats and cows. The majority of these models (rats, pigs, rabbits, and cows) are, however, flawed in their application to DDD in humans as they differ from human lumbar discs in terms of cell composition, size, and mechanical loading [31]. For example, many species (rats, pigs, cats, rabbits, and some dogs) retain notochordal cells into adulthood, and these appear to help maintain the IVD [32]. In contrast, humans and chondrodysplastic canines lose notochordal cells postnatally, and both develop spontaneous DDD [32].
Sheep discs closely approximate to human discs in size, experience similar biomechanical stresses, and lose notochord cells in early adulthood [30,31,32,33,34]. Furthermore, sheep recover well from surgical interventions and are relatively easy to maintain long term [30]. For these reasons, sheep have frequently served as models for DDD as well as device implantation and cellular therapy investigations [30]. Additionally, the use of intermediate or large sized animals helps to decrease errors associated with “scaling up” of medication doses and therapies for use in subsequent human clinical studies [34].
As a sheep model of C. acnes infection of IVDs could be extremely valuable to study the role of this bacterium in DDD, the aim of this proof-of-concept investigation was to be the first to demonstrate that C. acnes could be safely administered percutaneously into sheep IVDs to establish a short term or chronic infection.
2. Materials and Methods
2.1. Animals
Mature Dorset cross ewes (approximately 60 kg; 2.5 years of age) were included in this study, each bearing a permanent United States Department of Agriculture (USDA) tag to prevent any animal misidentification (#USDA 1283 and 1286). Sheep were individually housed in 5 × 5 foot pens, and fed grass hay twice per day; no other foods or supplements were utilised. This study was approved by Purdue University’s Institutional Animal Care and Use Committee [IACUC] and all experiments were performed in accordance with relevant guidelines and regulations.
2.2. Anesthesia
A standard anesthesia protocol was used for percutaneous intradiscal injections and MRI. Sheep were premedicated with xylazine (AnaSed; Akorn, Lake Forest, IL, USA; 0.03 mg/kg IV) and butorphanol (Torbugesic; Zoetis, Kalamazoo, MI USA, 0.01 mg/kg IV) and induced with a combination of ketamine (VetaKet; Akorn, 2.2 mg/kg IV) and diazepam (Hospira, Lake Forest, IL USA, 0.06 mg/kg IV). After local application of a few drops of a 2% lidocaine (AnaSed; Akorn) solution on the vocal folds, sheep were intubated and anesthesia was maintained using isoflurane. Throughout anesthesia, lactated Ringer’s solution was administered (10 mL/kg/hr). Post-operatively, buprenorphine (Par Pharmaceutical, Chestnut Ridge, NY USA, 0.005 mg/k IV) and flunixin meglumine (Merck & Co., Kenilworth, NJ, USA, 1.1 mg/kg IM) were administered for analgesia.
2.3. MRI
All scans were performed using a 1.5 Tesla GE Signa LX (General Electric, Milwaukee, WI, USA) magnet with the sheep in dorsal (supine) recumbancy; at each session, all scans were reviewed by three physician board certified radiologists. Under general anesthesia, a baseline MRI scan of their spine was performed, and during the same anesthetic period, selected lumbar discs of each sheep were percutaneously injected by a board certified, physician neuroradiologist (BH). Once a month, again under general anesthesia, an MRI scan of the lumbar spine was repeated. MRIs were not performed at three and five months for sheep #1286 due to very cold weather, which prohibited the transfer of the sheep.
2.4. Bacterial Injectate
The C. acnes strain selected for this study belonged to the type IA1 phylogroup (C. acnes subsp. acnes) (PD271) and was previously cultured from human pathologic disc material; this was provided by the Central European Institute of Technology (Brno, Czech Republic (OS)). The bacterium was cultured anaerobically in brain heart infusion broth at 37 °C until logarithmic growth phase. The bacterial cells were then washed and resuspended in phosphate buffered saline (PBS) (Fisher Scientific, Waltham, MA, USA). Bacterial counts were carried out on modified Clostridial Reinforced Agar (CRA) (Becton, Dickinson and Company, Cockeysville, MD, USA). Each IVD was injected with inoculum (~0.1 mL) containing ~ 8 × 107 colony forming units (CFU)/mL which was similar to that utilised in previously published rabbit animal model studies with this bacterium [28,29].
2.5. Injection Protocol
The intra-discal injection protocol described below, which was previously developed on rabbits [28,29,35], was practiced on a sheep from an unrelated study. Instead of bacterial injectate, a radiopaque contrast medium (Omnipaque 180 Iohexol, GE Healthcare/McKesson Medical-Surgical, Marlborough, MA, USA) was utilised, then the location of the injections was verified using computerised tomography (GE VCT 64 slice; General Electric), to ensure that the discograms were anatomic, as desired (Figure S1).
Sheep were anesthetised and their lumbar areas clipped and scrubbed with chlorhexidine using standard techniques. After the sheep was placed in perfectly symmetric ventral (prone) recumbancy, sterile surgical drapes were applied. Then, needles were percutaneously inserted in selected discs under fluoroscopic guidance. Using antero-posterior and lateral “C”-arm (GE OEC 9900 Elite, GE OEC Medical Systems, Inc., Salt Lake City, UT, USA) fluoroscopic radiographic verification, five separate six inch 25/20 gauge coaxial Quincke spinal needles (#183109; Halyard Health, Apharetta, GA, USA) were inserted from a right posterolateral approach. The 20-gauge hollow introducer needle was started approximately 5.5 cm lateral to the mid-line and angled medially (toward the spine) at an approximate 30° angle to the vertical, exactly in line with the disc itself, and stopped at the outer edge of the disc annulus. A six inch, 25-gauge Quincke hollow needle (also Halyard Health) was then passed through the introducer needle and into the centre of the nucleus pulposus region of the disc. A 1 mL Hamilton Gastight syringe (#81301; Hamilton Company, Reno, NV, USA) was used to hold the inoculum, which was kept under anaerobic conditions until the injection step. Discs L2-3, L4-5, and L6-7 were injected with the C. acnes inoculum in all animals, whereas L3-4 had a needle insertion only (no injectate) and L5-6 was injected with saline (0.12 mL).
2.6. Euthanasia and Post Mortem Procedures
Sheep were euthanised with sodium pentobarbital (Euthanasia Solution; MWI, Boise, ID, USA, or Euthasol; Virbac AH, Inc., Fort Worth, TX, USA) 390 mg/mL IV (15 mL) according to the 2020 AVMA Guidelines for the Euthanasia of Animals [36]. One sheep (USDA#1283) was euthanised 1 month after injection to investigate if infection was still present in the relative short term, while the other was sacrificed (USAD#1286) at 6 months to determine if a long term or chronic infection had established. Immediately after euthanasia the lumbar spine was isolated, and the surrounding muscles and tendons were removed. Then each individual disc was aseptically retrieved in its totality.
2.7. Disc Tissue Sampling
Harvested discs were extensively homogenised in sterile PBS using an Omni Tissue Homogenizer (Omni International, Keenesaw, GA, USA) under anaerobic conditions for both anaerobic and aerobic bacterial culture. To determine the bacterial load in the discs, the homogenate was serially diluted in sterile PBS and aliquots (4 L) of each dilution plated on modified CRA plates for incubation anaerobically at 37 °C for an extended period of time before bacterial enumeration. For our sheep USAD #1286 at six months, disc tissue was also evaluated by Gram staining.
2.8. Statistical Analysis
Statistical analysis was performed using the Wilcoxon-matched-pairs signed rank test (2-tailed).
3. Results
3.1. Percutaneous Injection of C. acnes into Sheep IVDs under Fluoroscopic Guidance
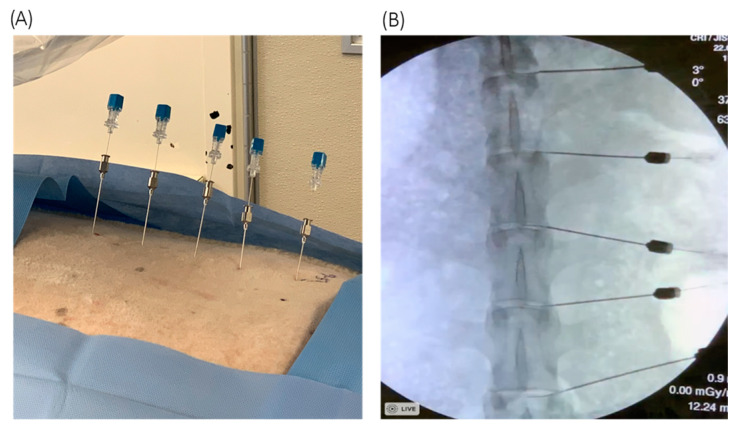
Under fluoroscopic guidance, six inch 25/20-gauge hollow coaxial spinal needles were able to accurately penetrate percutaneously into the nucleus pulposus of sheep discs prior to the introduction of a C. acnes inoculum via a Hamilton syringe (Figure 1). The percutaneous injection technique, in the hands of a highly experienced neuroradiologist, was straightforward and easily documented with biplane C-arm fluoroscopy (Figure 1B).
Figure 1.
(A) 25-gauge needles within the 20-gauge introducer needles, serially spaced into the lumbar discs. (B) Fluoroscopic radiograph verifying needle placement in the AP plane.
3.2. Animal Welfare
No adverse reactions were observed with either animal upon intradiscal injection, and the general anesthesia and MRIs were uneventful with no complications. There was no sign of constricted mobility after injection and the sheep were independently mobile and eating shortly after the procedure. In the first sheep used for a one-month infection (USDA#1283), a single disc (L5-6) did generate a “giving way” sensation when inoculated with sterile saline, possibly due to internal acute annular fissure formation.
3.3. Evidence of Short-Term C. acnes Colonisation
The sheep sacrificed at one-month post inoculation (USDA#1283) demonstrated the presence of C. acnes in all three IVD tissues after CRA plate culture. Although the total numbers of C. acnes cultured (5.12 × 104–6.67 × 104 CFU per disc) were less than the original 8 × 106 CFU per disc inoculums, this was not statistically significant (Figure 2).
Figure 2.
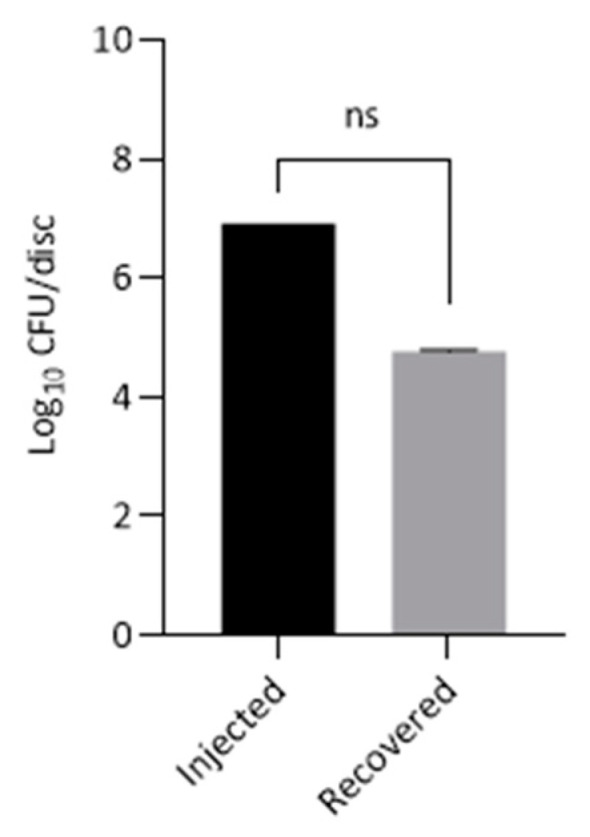
Growth of C. acnes from 3 × discs of the same sheep (USDA#1283) four weeks after initial inoculation. Data is presented as mean ± SEM of log10 CFU per disc. Statistical analysis was performed for paired samples.
With our second animal sacrificed at six-months post inoculation (USDA#1286), no bacteria were detected in any of the infected IVD tissues by anaerobic and aerobic culture or Gram staining. For both animals, control discs injected with sterile saline or needle insertion only were all culture-negative.
3.4. MRI
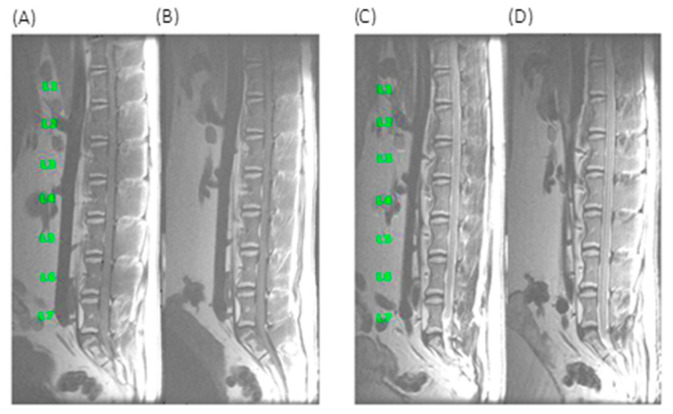
The one month MRI scan for the animal sacrificed at this endpoint remained unchanged from the pre-injection baseline (Figure 3).
Figure 3.
One month MRI scans for sheep USDA#1283. (A) T1 weighted baseline (B) One month T1 weighted (C) T2 weighted FS baseline (D) one month T2 weighted FS.
Similarly, for the second sheep sacrificed at 6 months (USDA#1286) no MRI changes were observed from baseline at 1,2,4 and 6-month endpoints within the disc space and/or adjacent vertebral bodies (data not shown).
4. Discussion
The objective of this proof-of-concept study was to demonstrate that percutaneous inoculation of sheep lumbar IVDs with C. acnes can be performed safely and is a feasible approach for future investigations. In this regard, our work was successful and the infected sheep suffered no adverse reactions during the procedures. Due to the pilot nature of this study, and the use of large animals that were required to be sacrificed at each endpoint for disc analyses, we made the decision that we would only utilize two sheep in this phase of our investigations which we note is a limitation of the work. This was based on ethical grounds and to minimize the stress and cost to the animals, and to keep to the principle of the accepted 3Rs (Replacement, Reduction, Refinement). While this meant that only one animal was used for each time point, we balanced this with the infection of three discs per animal for triplicate observations. While the latter could be considered ‘technical replicates’ rather than true biological replicates, we feel this approach was the right balance given we had no prior knowledge on how the sheep would react to bacterial infection or indeed the viability of the experiments.
Our ability to perform percutaneous injections into the nucleus pulposus of the sheep lumbar discs is consistent with the findings of Elliott and colleagues in their very thorough study of needle diameter effects in animal models of disc degeneration [37]. They found that if a needle’s diameter to disc height was less than 40% there should be minimal annulus damage, although variable but nonsignificant minor effects were observed with needle:height ratios between 25–40%. Due to the stiffness required in our study, we felt that a 25-gauge needle would be necessary for our experiments to transition through the skin, fascia, and musculature to reach the surface of the annulus and continued entry into the nucleus pulposus. While a 27-gauge needle was used with no appreciable damage in sheep by Elliott et al. [37], a 25-gauge needle was not specifically evaluated. However, as 25-gauge = 0.455 mm diameter, and the average disc height of the sheep used in their study was 3.93 mm, this would only equate to 0.455/3.93 = 12%. On this basis, we believed a 25-gauge needle insertion would not cause any appreciable harm to our discs and our MRI scans of puncture alone seem to validate that this was correct. Moving forward, we recommend the use of 25-gauge needles since they are malleable and strong enough to penetrate the ovine AF whilst causing minimal damage.
Beyond the demonstration that successful inoculation of sheep IVDs with C. acnes was possible without significant tissue disruption, our results also revealed that once injected, C. acnes can survive within the disc for at least one month, although the bacterial load measured after this period did reduce. At the six-month endpoint, however, no bacteria were evident based on culture or Gram staining of tissue. The apparent absence of C. acnes after this period likely reflects its clearance from the IVD tissue. While inadequate tissue processing to vigorously disrupt any potential biofilm or bacterial cells existing intracellularly within disc tissue may also lead to reduced or false-negative culture counts, this was not the case in our study as all tissue was extensively homogenised prior to agar plate culture. The absence of bacterial growth at six months may also reflect our use of only a single animal for this endpoint. It is very possible that with other sheep displaying a different biological response to infection, or indeed a different C. acnes strain, the results may well be different. We note, however, that in previous studies where C. acnes has been used to investigate disc changes in rabbits and rats over prolonged time intervals, bacterial loads after initial inoculation were not studied [28,29,38]. As a consequence, it is unclear the extent to which, if any, the bacterium was still present in these animals at completion of the experiments. We can speculate that in humans, DDD due to C. acnes may occur based on cycles of infection, clearance and reinfection in compromised discs of susceptible individuals, or an initial infection may stimulate a chronic inflammatory response that persists after the organism has disappeared. Interestingly, a previously described mouse model of prostate infection with C. acnes found that while the bacteria could still be detected in the dorsal prostate at weeks one and two post-inoculation, at week eight no organisms were present based on immunohistochemistry analysis despite the persistence of chronic inflammation in most animals [39]. Also, in a rat model of prostate infection with C. acnes, bacterial counts were similarly found to fall over time to very low levels at six months [40]. On the basis of these previous small animal data, albeit in the context of prostate disease, our bacterial count results at six months do not appear unusual. In future experiments, shorter monthly follow ups to monitor changes in bacterial levels and associated pathological changes will be more informative.
Now that we have established a method for successful infection of sheep discs with C. acnes, our results will aid in the planning and design of future experiments with a greater number of animals. Further work should immediately focus on inoculum concentration, comparison of different strains, post-infection incubation periods, host inflammatory responses and analysis of IVD tissue for biofilm-like colonization and intracellular forms similar to that seen in other chronic diseases associated with C. acnes (e.g., sarcoidosis; prostate disease).
5. Conclusions
This proof-of-concept study demonstrates that sheep discs can be safely injected with C. acnes for in vivo study. Further work is now warranted with an increased number of animals and outcome measures to fully clarify the growth pattern and duration of C. acnes in ovine discs, and to further elucidate its role in the aetiology of disc degeneration. It is hoped that sheep will ultimately prove a good model to study the role of C. acnes in the pathophysiology of DDD, and to investigate the use of antibiotics and other novel therapeutic strategies to treat the condition in humans.
Acknowledgments
We thank Molly Miller and Melanie Glover for their assistance in data and information management.
Supplementary Materials
The following are available online at https://www.mdpi.com/2306-7381/8/3/48/s1, Figure S1: Discograms verifying the accuracy of this percutaneous intradiscal lumbar spine injection technique. Figure S2: 25 gauge needles within the 20 gauge introducer needles, serially spaced, extending into the lumbar discs.
Author Contributions
Conceptualization, M.F.C.; methodology, N.S.A., B.H. and M.N.S.; investigation, N.S.A., B.H., M.N.S., C.J.C., S.K.N., M.F.C.; resources, M.N.C. and O.S.; writing—original draft preparation, E.C.C., A.M., M.N.C., F.S.A. and M.F.C.; writing-review and editing, E.C.C.; N.S.A., B.H., M.N.S., C.J.C.; O.S.; M.N.C.; A.M.; F.S.A.; V.V.; S.K.N. and M.F.C.; project administration, G.J.B. and R.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Indiana Orthopaedic Hospital Research Foundation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Purdue University (protocol number 1905001889 approved on 29 May 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute for Health Metrics and Evaluation (IHME) Findings from the Global Burden of Disease Study. IHME; Seattle, WA, USA: 2018. [Google Scholar]
- 2.Urban J., Roberts S. Degeneration of the intervertebral disc. Arthritis Res. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Stirling A., Worthington T., Rafiq M., Lambert P.A., Elliot T.S. Association between sciatica and Propionibacterium acnes. Lancet. 2001;357:2024–2025. doi: 10.1016/S0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- 5.Coscia M., Denys G., Wack M. Propionibacterium acnes, coagulase-negative staphylococcus, and the “biofilm-like” intervertebral disc. Spine. 2016;41:1860–1865. doi: 10.1097/BRS.0000000000001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salehpour F., Aghazadeh J., Mirzaei F., Ziaeii E., Alavi S.A.N. Propionibacterium acnes infection in disc material and different antibiotic susceptibility in patients with lumbar disc herniation. Int. J. Spine Surg. 2019;13:146–152. doi: 10.14444/6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajasekaran S., Tangavel C., Aiyer S.N., Nayagam S.M., Raveendran M., Demonte N.L., Subbaiah P., Kanna R., Shetty A.P., Dharmalingam K. ISSLS PRIZE IN CLINICAL SCIENCE 2017: Is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur. Spine J. 2017;26:1384–1400. doi: 10.1007/s00586-017-4972-3. [DOI] [PubMed] [Google Scholar]
- 8.Bivona L.J., Camacho J.E., Usmani F., Nash A., Bruckner J.J., Hughes M., Bhandutia A.K., Koh E.Y., Banagan K.E., Gelb D.E., et al. The prevalence of bacterial infection in patients undergoing elective ACDF for degenerative cervical spine conditions: A prospective cohort study with contaminant control. Glob. Spine J. 2021;11:13–20. doi: 10.1177/2192568219888179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y., Jiao Y., Yuan Y., Zhou Z., Zheng Y., Xiao J., Li C., Chen Z., Cao P. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg. Microbes Infect. 2018;7:1. doi: 10.1038/s41426-017-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Y., Chen Y., Zhou Z., Jiao Y., Li C., Zheng Y., Lin Y., Xiao J., Chen Z., Cao P. Association between chronic inflammation and latent infection of Propionibacterium acnes in non-pyogenic degenerated intervertebral discs: A pilot study. Eur. Spine J. 2018;27:2506–2517. doi: 10.1007/s00586-017-5363-5. [DOI] [PubMed] [Google Scholar]
- 11.Tang G., Wang Z., Chen J., Zhang Z., Qian H., Chen Y. Latent infection of low-virulence anaerobic bacteria in degenerated lumbar intervertebral discs. BMC Musculoskelet. Disord. 2018;19:445. doi: 10.1186/s12891-018-2373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollason J., McDowell A., Albert H.B., Barnard E., Worthington T., Hilton A.C., Vernallis A., Patrick S., Elliott T., Lambert P. Genotypic and antimicrobial characterization of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. BioMed Res. Int. 2013;2013:530382. doi: 10.1155/2013/530382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang G., Chen Y., Chen J., Wang Z., Jiang W. Higher proportion of low-virulence anaerobic bacterial infection in young patients with intervertebral disc herniation. Exp. Ther. Med. 2019;18:3085–3089. doi: 10.3892/etm.2019.7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudli S., Liebenberg E., Magnitsky S., Miller S., Demir-Deviren S., Lotz J.C. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with modic changes. J. Orthop. Res. 2016;34:1447–1455. doi: 10.1002/jor.23265. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin J., Watterson S., Layton A.M., Bjourson A.J., Barnard E., McDowell A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms. 2019;7:128. doi: 10.3390/microorganisms7050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole K.A., Banerjee A., Dipreta J.A. Propionibacterium acnes osteomyelitis after intraosseous cannulation in a child. Case Rep. Orthop. 2019:7170154. doi: 10.1155/2019/7170154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ely A.L., Neely K. Propionibacterium acnes endophthalmitis following Baerveldt glaucoma device implantation. J. Glaucoma. 2020;29:e71–e73. doi: 10.1097/IJG.0000000000001526. [DOI] [PubMed] [Google Scholar]
- 18.Niazi S.A., Clarke D., Do T., Gilbert S.C., Mannocci F., Beighton D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J. Clin. Microbiol. 2010;48:3859–3869. doi: 10.1128/JCM.01326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper K.E., Jacobson M.J., Cofield R.H., Sperling J.W., Sanchez-Sotelo J., Osmon D.R., McDowell A., Patrick S., Steckelberg J.M., Mandrekar J.N., et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 2009;47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaver M., Lagundzin D., Thapa I., Lee J., Ali H., Kielian T., Skar G.L. C. acnes central nervous system catheter infection induces long-term changes in the CSF proteome. Infect. Immun. 2020:IAI.00531-20. doi: 10.1128/IAI.00531-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen R.J., Shannon B.A., McNeal J.E., Shannon T., Garrett K.L. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: A possible link to cancer evolution? J. Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 22.Eishi Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir. Investig. 2013;51:56–68. doi: 10.1016/j.resinv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Capoor M.N., Ruzicka F., Schmitz J.E., James G.A., Machackova T., Jancalek R., Smrcka M., Lipina R., Ahmed F.S., Alamin T.F., et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS ONE. 2017;12:e0174518. doi: 10.1371/journal.pone.0174518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohrt-Nissen S., Fritz B.G., Walbom J., Kragh K.N., Bjarnsholt T., Dahl B., Manniche C. Bacterial biofilms: A possible mechanism for chronic infection in patients with lumbar disc herniation—A prospective proof-of-concept study using fluorescence in situ hybridization. APMIS. 2018;126:440–447. doi: 10.1111/apm.12841. [DOI] [PubMed] [Google Scholar]
- 25.Georgy M.M., Vaida F., Stern M., Murphy K. Association between type I modic changes and Propionibacterium acnes infection in the cervical spine: An observational study. AJNR. 2018;39:1764–1767. doi: 10.3174/ajnr.A5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert H.B., Lambert P., Rollason J., Sorensen J.S., Worthington T., Pedersen M.B., Nørgaard H.S., Vernallis A., Busch F., Manniche C., et al. Does nuclear tissue infected with bacteria following disc herniations lead to modic changes in the adjacent vertebrae? Eur. Spine J. 2013;22:690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aghazadeh J., Salehpour F., Ziaeii E., Javanshir N., Samadi A., Sadeghi J., Mirzaei F., Naseri Alavi S.A. Modic changes in the adjacent vertebrae due to disc material infection with Propionibacterium acnes in patients with lumbar disc herniation. Eur. Spine J. 2017;26:3129–3134. doi: 10.1007/s00586-016-4887-4. [DOI] [PubMed] [Google Scholar]
- 28.Shan Z., Zhang X., Li S., Yu T., Liu J., Zhao F. Propionibacterium acnes incubation in the discs can result in time-dependent modic changes: A long-term rabbit model. Spine (Phila Pa 1976) 2017;42:1595–1603. doi: 10.1097/BRS.0000000000002192. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z., Zheng Y., Yuan Y., Jiao Y., Xiao J., Zhou Z., Cao P. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: A pilot study. Biomed. Res. Int. 2016;2016:9612437. doi: 10.1155/2016/9612437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly C., Ghosh P., Jenkin G., Oehme D., Goldschlager T. A review of animal models of intervertebral disc degeneration: Pathophysiology, regeneration, and translation to the clinic. Biomed. Res. Int. 2016;2016:5952165. doi: 10.1155/2016/5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connell G.D., Vresilovic E.J., Elliott D.M. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 32.Cappello R., Bird J.L., Pfeiffer D., Bayliss M.T., Dudhia J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976) 2006;31:873–882; discussion 883. doi: 10.1097/01.brs.0000209302.00820.fd. [DOI] [PubMed] [Google Scholar]
- 33.Jin L., Balian G., Li X.J. Animal models for disc degeneration-an update. Histol. Histopathol. 2018;33:543–554. doi: 10.14670/HH-11-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilke H.J., Kettler A., Wenger K.H., Claes L.E. Anatomy of the sheep spine and its comparison to the human spine. Anat. Rec. 1997;247:542–555. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Lipp C., Kirker K., Agostinho A., James G., Stewart P. Testing wound dressings using an in vitro wound model. J. Wound Care. 2010;19:220–226. doi: 10.12968/jowc.2010.19.6.48468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. [(accessed on 1 December 2020)]; Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 37.Elliott D.M., Yerramalli C.S., Beckstein J.C., Boxberger J.I., Johannessen W., Vresilovic E.J. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine (Phila Pa 1976) 2008;33:588–596. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- 38.Zamora T., Palma J., Andia M., Garcia P., Wozniak A., Solar A., Campos M. Effect of Propionibacterium acnes (PA) injection on intervertebral disc degeneration in a rat model: Does it mimic modic changes? Orthop. Traumatol. Surg. Res. 2017;103:795–799. doi: 10.1016/j.otsr.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Shinohara D.B., Vaghasia A.M., Yu S.H., Mak T.N., Brüggemann H., Nelson W.G., De Marzo A.M., Yegnasubramanian S., Sfanos K.S. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate. 2013;73:1007–1105. doi: 10.1002/pros.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsson J., Drott J.B., Laurantzon L., Laurantzon O., Bergh A., Elgh F. Chronic prostatic infection and inflammation by Propionibacterium acnes in a rat prostate infection model. PLoS ONE. 2012;7:e51434. doi: 10.1371/journal.pone.0051434. Erratum in: PLoS ONE2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and Supplementary Material.