Abstract
Six new prenylated indole diketopiperazine alkaloids, asperthrins A–F (1–6), along with eight known analogues (7–14), were isolated from the marine-derived endophytic fungus Aspergillus sp. YJ191021. Their planar structures and absolute configurations were elucidated by HR-ESI-MS, 1D/2D NMR data, and time-dependent density functional theory (TDDFT)/ECD calculation. The isolated compounds were assayed for their inhibition against three agricultural pathogenic fungi, four fish pathogenic bacteria, and two agricultural pathogenic bacteria. Compound 1 exhibited moderate antifungal and antibacterial activities against Vibrio anguillarum, Xanthomonas oryzae pv. Oryzicola, and Rhizoctonia solani with minimal inhibitory concentration (MIC) values of 8, 12.5, and 25 μg/mL, respectively. Furthermore, 1 displayed notable anti-inflammatory activity with IC50 value of 1.46 ± 0.21 μM in Propionibacterium acnes induced human monocyte cell line (THP-1).
Keywords: indole diketopiperazine alkaloids, endophytic fungus, Aspergillus sp., antimicrobial
1. Introduction
Endophytic fungi refer to microorganisms that spend their entire or part of their life cycle in plant tissues, animals, and environments without causing any obvious infection or visible disease to the host [1]. Endophytic fungi are prolific microbial resources for the production ability of many biologically active secondary metabolites, which can help the host to resist pathogenic microorganisms [2]. Various endophytic fungi have drawn substantial attention due to their potential to produce chemically diverse and biologically active secondary metabolites with anti-cancer, anti-microbial, anti-viral, and insecticidal activities [3,4,5,6]. In our continuous searching for novel bioactive secondary metabolites from marine endophytic fungi [7,8,9], the Aspergillus sp. YJ191021 attracted our attention, not only for the characteristic indole diketopiperazine ultraviolet (UV) absorptions of the crude extracts, but also for their potent antimicrobial activities against agricultural pathogenic fungi.
Diketopiperazine alkaloids are valued not only for their properties and functions in fungal self-biology, but also for niche establishment to defend abiotic and biotic stress in nature. They are cyclodipeptides formed by condensation of two amino acids under the control of NRPS genes [10], especially those isolated from the genera Aspergillus and Penicillium [11]. Among them, those derived from tryptophan and proline are the most popular types in the current study, especially in structural diversity, chemical synthesis, and pharmacological activity [12,13,14]. Besides, the substitution of the isopentenyl group enriches the variability of their structures. Prenylated indole alkaloids have been reported to show a wide array of biological activities including antimicrobial, insecticidal, and cytotoxic activities [14,15]. The fascinating structural and biological properties of prenylated indole alkaloids make it possible for them to be developed into our armor and weaponry: Natural agrochemicals and drugs.
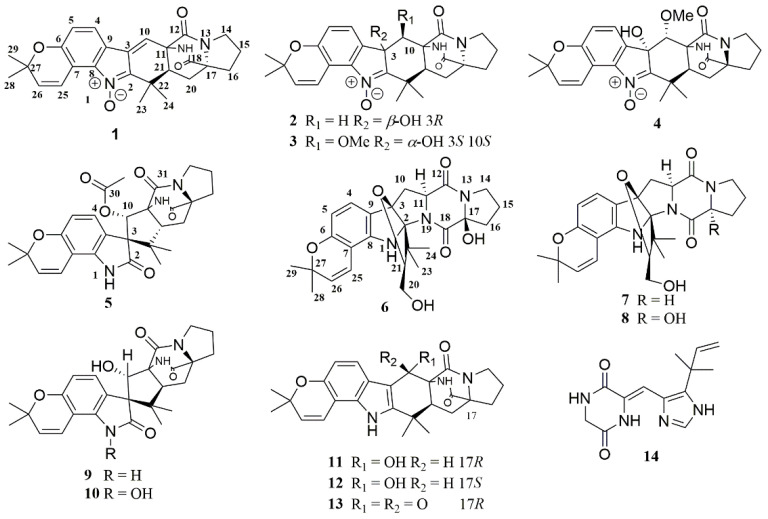
In this study, we described the isolation and structure identification of six new prenylated indole diketopiperazine alkaloids (1–6), together with eight known analogues: Gartryprostatin A (7) [15], gartryprostatin B (8) [15], sclerotiamide (9) [16], notoamide H (10) [17], 6-epi-notoamide R (11) [18], notoamide R (12) [19], (-)-notoamide I (13) [20], and gartryprostatin C (14) [15] (Figure 1) from the marine-derived endophytic fungus Aspergillus sp. YJ191021. All compounds were assayed for their inhibition against three agricultural pathogenic fungi, Rhizoctonia solani, Fusarium oxysporum, Colletotrichum gloeosporioides penz, and two agricultural pathogenic bacteria Xanthomonas oryzae pv. Oryzae, and X. oryzae pv. oryzicola. Furthermore, part of the compounds was evaluated for their anti-inflammatory activity in Propionibacterium acnes-stimulated THP-1 human monocytic cell line. Herein, we described the isolation, structural identification, and biological evaluation of the isolated diketopiperazine alkaloids.
Figure 1.
Structures of 1–14.
2. Results and Discussion
Asperthrin A (1) was isolated as brilliant yellowish powders. Based on the [M + H]+ ion peak at m/z 446.2071 (calcd. for C26H28N3O4, 446.2074) in the HR-ESI-MS data and 13C NMR data, its molecular formula was determined as C26H27N3O4, indicating 15 degrees of unsaturation. For the NMR data (Table 1), the characteristic signals were attributed based on careful analyses of 1H NMR, 13C NMR, and HSQC spectra (Figures S1–S4) as follows: Four methyl groups at δH 1.22 (3H, s, Me-23), 1.55 (3H, s, Me-24), 1.42 (3H, s, Me-28), 1.41 (3H, s, Me-29), one methine proton signal at δH 2.31 (1H, dd, J = 10.2, 5.6 Hz, H-21), five olefinic protons at δH 7.80 (1H, d, J = 8.0 Hz, H-4), 6.88 (1H, d, J = 8.0 Hz, H-5), 7.05 (1H, s, H-10), 7.74 (1H, d, J = 10.2 Hz, H-25), 5.96 (1H, d, J = 10.2 Hz, H-26), one exchangeable proton at δH 8.82 (1H, s, NH-19), as well as eight aliphatic protons at δH 1.83–3.40 (8H, m, H2-14, H2-15, H2-16, H2-20) attributable to four methylene groups. The 13C NMR and DEPT data disclosed 26 carbon signals, including four sp3 non-protonated carbons at δC 60.3 (C-11), 66.5 (C-17), 35.6 (C-22) [one oxygen-bearing sp3 carbon at δC 77.1 (C-27)], and eight sp2 non-protonated carbons (δC 145.4, 132.7, 155.1, 111.4, 139.6, 117.6, 168.2, and 172.1). There were odd numbers of olefinic carbon signals in the 13C NMR spectrum, which implied that the double bond in the indole ring was connected between the carbon atom and nitrogen atom. According to the previously introduced molecular formula, there were four oxygen atoms in compound 1. Except for two carbonyl groups and one cyclic ether group, the last oxygen atom was deducted to form the imine-oxide group located at 1-N. The presence of six double bonds and two carbonyls accounted for eight of the 15 degrees of unsaturation, indicating the existence of a heptatomic ring system for 1.
Table 1.
1H (600 MHz) NMR Data of 1–6.
| Position | 1 a | 2 a | 3 a | 4 a | 5 a | 6 b |
|---|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 1(NH) | 10.69 (s) | |||||
| 4 | 7.80 (d, 8.0) | 7.32 (d, 8.0) | 7.43 (d, 8.0) | 7.36 (d, 8.1) | 6.89 (d, 8.1) | 6.97 (d, 8.1) |
| 5 | 6.88 (d, 8.0) | 6.85 (d, 8.0) | 6.83 (d, 8.0) | 6.88 (d, 8.1) | 6.36 (d, 8.1) | 6.20 (d, 8.1) |
| 10 | 7.05 (s) | a 2.64 (d, 15.2) | 4.72 (s) | 4.12 (d, 1.2) | 5.75 (s) | a 2.72 (dd, 12.9, 7.0) |
| b 2.05 (d, 15.2) | b 2.59 (m) | |||||
| 11 | 4.59 (dd, 11.9, 7.0) | |||||
| 14 | a 3.40 (m) | a 3.34 (m) | 3.36 (m) | 3.40 (t, 6.5) | a 3.40 (m) | 3.56 (m) |
| b 3.34 (m) | b 3.29 (m) | b 3.30 (m) | ||||
| 15 | a 2.00 (m) | a 1.97 (m) | a 1.99 (m) | a 2.02 (m) | a 1.99 (m) | a 1.99 (m) |
| b 1.84 (m) | b 1.81 (m) | b 1.81 (m) | b 1.85 (m) | b 1.86 (m) | b 1.90 (m) | |
| 16 | a 2.53 (m) | a 2.50 (m) | a 2.54 (m) | a 2.54 (m) | a 2.48 (m) | a 2.33 (m) |
| b 1.83 (m) | b 1.83 (m) | b 1.81 (m) | b 1.85 (m) | b 1.80 (m) | b 2.10 (m) | |
| 19(NH) | 8.82 (s) | 7.52 (s) | 7.74 (s) | 7.87 (s) | 8.54 (s) | |
| 20 | a 2.21 (dd, 13.4, 10.2) | a 2.13 (m) | a 2.10 (dd, 13.2, 10.3) | a 2.02 (m) | a 2.04 (m) | a 3.64 (m) |
| b 1.89 (m) | b 1.85 (m) | b 1.75 (m) | b 1.85 (m) | b 1.79 (m) | b 3.56 (m) | |
| 21 | 2.31 (dd, 10.2, 5. 6) | 2.13 (m) | 3.08 (dd, 10.3, 6.5) | 3.53 (dd, 10.1, 7.8) | 2.68 (dd, 10.2, 6.5) | 3.81 (dd, 7.6, 2.8) |
| 23 | 1.22 (s) | 1.34 (s) | 1.31 (s) | 1.15 (s) | 0.52 (s) | 1.29 (s) |
| 24 | 1.55 (s) | 1.54 (s) | 1.32 (s) | 1.30 (s) | 1.12 (s) | 0.82 (s) |
| 25 | 7.74 (d, 10.2) | 7.83 (d, 10.2) | 7.78 (d, 10.2) | 7.76 (d, 10.1) | 6.57 (d, 9.8) | 6.15 (d, 9.9) |
| 26 | 5.96 (d, 10.2) | 5.90 (d, 10.2) | 5.92 (d, 10.2) | 5.93 (d, 10.1) | 5.75 (d, 9.8) | 5.50 (d, 9.9) |
| 28 | 1.42 (s) | 1.39 (s) | 1.41 (s) | 1.42 (s) | 1.38 (s) | 1.37 (s) |
| 29 | 1.41 (s) | 1.39 (s) | 1.39 (s) | 1.40 (s) | 1.35 (s) | 1.35 (s) |
| 31 | 2.03 (s) | |||||
| 3-OH | 6.39 (s) | 6.31 (s) | 6.27 (d, 1.2) | |||
| OMe-10 | 3.03 (s) | 3.31 (s) |
a Measured at 600 MHz (1H) in CDCl3; b measured at 600 MHz (1H) in DMSO-d6.
The connection fragment of 1 was further confirmed by analysis of the HMBC spectrum (Figure S5). The HMBC correlations from H-4 (δH 7.80) to C-3 (δC 132.7)/C-6 (δC 155.1)/C-8 (δC 139.6), and H-26 (δH 5.96) to C-7 (δC 111.4)/C-27 (δC 77.1)/C-28 (δC 27.9) indicated the presence of the isopentenyl-substituted indole motif (Figure 2). The bicyclo [2.2.2]diazaoctane ring, biosynthetically derived from a diketopiperazine ring and an isoprenyl group, was indicated by key HMBC correlations from H-20a (δH 2.21) to C-11 (δC 60.3)/C-17 (δC 66.5)/C-18 (δC 172.1)/C-21 (δC 46.3) and from H-21 (δH 2.31) to C-12 (δC 168.2)/C-22 (δC 35.6)/C-23 (δC 17.5). Besides, the HMBC correlations from H-10 (δH 7.05) to C-2 (δC 145.4)/C-21 (δC 46.3) and from H-23 (δH 1.22) to C-2 (δC 145.4)/C-21 (δC 46.3) proved the existence of a conjugated exo-double bond-bearing cyclohexene, which was formed by the connection between prenylated indole and diazaoctane moieties. Based on the spectral analysis, the planar structure of 1 was the same as 6-epi-avrainvillamide, isolated from A. taichungensis [18].
Figure 2.
1H-1H COSY (bold blue lines) and key HMBC (red arrows) correlations for 1–6.
The ROESY spectrum (Figure 3 and Figure S6) exhibited correlations between 19-NH (δH 8.82) and H-23 (δH 1.22), between H-21 (δH 2.31) and H-24 (δH 1.55), supporting that H-21 and H-24 were co-facial and assigned as α-oriented whereas H-23 is β-oriented, respectively. Additionally, the absence of a cross peak between H-21 (δH 2.31) and 19-NH (δH 8.82) indicated that the relative configuration between N13-C17 and C21-22 was anti [21]. Williams reported that the Cotton effect at λ = 200–250 nm arising from an n–π* transition of the diketopiperazine moiety is diagnostic of the bicyclo[2.2.2]diazaoctane ring system [21,22]. The negative Cotton effect at 225 nm in ECD spectrum (Figure 4A and Figure S8), which was opposite to that of 6-epi-avrainvillamide [18], empirically indicated that the absolute configurations of C-11 and C-17 in 1 were 11R, and 17R. Combined with the analysis of the ROESY spectrum, the absolute configuration of C-21 was assigned as 21S. To further verify the aforementioned absolute configuration deduction of 1, the calculated ECD spectrum was conducted. The absolute configurations of 11R, 17R, and 21S were determined for the well match between the calculated and the experimental ECD spectra (Figure 4A).
Figure 3.
Key ROESY correlations for 1–6.
Figure 4.
Experimental and calculated ECD spectra for 1–6. (A) for 1 and 5; (B) for 2; (C) for 3 and 4; (D) for 6.
Asperthrin B (2) was obtained as white powders. The molecular formula C26H29N3O5, which was determined by the [M + Na]+ ion at m/z 486.2002 (calcd. for C26H29N3O5Na, 486.1999) from the HR-ESI-MS and 13C NMR data was 18 amu higher than the molecular mass of 1, implying the presence of an additional hydroxy group in its structure. A careful comparison of the 13C NMR data of 2 with those of 1 (Table 2) showed significant upfield shifts of C-3 (δC 75.8) and C-10 (δC 36.0), indicating a saturation of the double bond between C-3 and C-10. The HMBC correlations from H-4 (δH 7.32) to C-3 (δC 75.8) (Figure 2 and Figure S14) confirmed that the hydroxyl group was attached to C-3. The ROESY correlations (Figure 3 and Figure S15) from 19-NH (δH 7.52) to H-23 (δH 1.34) and H-10a (δH 2.64) to 3-OH (δH 6.39)/19-NH (δH 7.52)/H-23 (δH 1.34) indicated these protons were co-facial and β-oriented. Accordingly, the ROESY correlations between H-21 (δH 2.13) and H-24 (δH 1.54) revealed that H-21 and H-24 were α-oriented. The absolute configurations of C-3, C-11, C-17, and C-21 in 2 were assigned as 3R, 11R, 17R, and 21S based on the negative Cotton effect at 225 nm in ECD spectra (Figure 4B and Figure S17) and calculated ECD spectra (Figure 4B).
Table 2.
13C (150 MHz) NMR Data of 1–6.
| Position | 1 a | 2 a | 3 a | 4 a | 5 a | 6 b |
|---|---|---|---|---|---|---|
| δc, Type | δc, Type | δc, Type | δc, Type | δc, Type | δc, Type | |
| 2 | 145.4, C | 151.2, C | 153.3, C | 152.4, C | 176.3, C | 98.6, C |
| 3 | 132.7, C | 75.8, C | 78.5, C | 78.0, C | 64.3, C | 97.1, C |
| 4 | 121.7, CH | 121.9, CH | 123.1, CH | 124.2, CH | 125.0, CH | 125.2, CH |
| 5 | 116.3, CH | 116.7, CH | 116.1, CH | 116.9, CH | 108.7, CH | 108.0, CH |
| 6 | 155.1, C | 154.3, C | 154.2, C | 154.4, C | 152.5, C | 155.1, C |
| 7 | 111.4, C | 112.3, C | 112.0, C | 111.9, C | 104.8, C | 103.7, C |
| 8 | 139.6, C | 140.0, C | 140.9, C | 140.1, C | 138.0, C | 146.8, C |
| 9 | 117.6, C | 131.2, C | 129.3, C | 129.4, C | 124.6, C | 118.8, C |
| 10 | 121.8, CH | 36.0, CH2 | 76.9, CH | 76.1, CH | 74.1, CH | 38.9, CH2 |
| 11 | 60.3, C | 61.7, C | 62.1, C | 62.3, C | 68.9, C | 63.4, CH |
| 12 | 168.2, C | 168.4, C | 168.7, C | 168.5, C | 168.1, C | 167.1, C |
| 14 | 44.2, CH2 | 44.0, CH2 | 44.3, CH2 | 44.4, CH2 | 43.9, CH2 | 45.3, CH2 |
| 15 | 24.4, CH2 | 24.3, CH2 | 24.6, CH2 | 24.5, CH2 | 24.9, CH2 | 21.0, CH2 |
| 16 | 29.0, CH2 | 28.9, CH2 | 29.0, CH2 | 29.1, CH2 | 28.1, CH2 | 31.6, CH2 |
| 17 | 66.5, C | 66.8, C | 66.9, C | 66.8, C | 69.3, C | 93.6, C |
| 18 | 172.1, C | 172.4, C | 172.4, C | 172.2, C | 172.5, C | 165.4, C |
| 20 | 30.5, CH2 | 31.8, CH2 | 29.2, CH2 | 30.5, CH2 | 27.9, CH2 | 61.8, CH2 |
| 21 | 46.3, CH | 48.7, CH | 42.0, CH | 50.0, CH | 51.1, CH | 91.1, CH |
| 22 | 35.6, C | 38.6, C | 36.8, C | 36.6, C | 47.2, C | 46.7, C |
| 23 | 17.5, CH3 | 21.2, CH3 | 14.3, CH3 | 13.5, CH3 | 25.6, CH3 | 21.2, CH3 |
| 24 | 23.4, CH3 | 27.0, CH3 | 22.9, CH3 | 22.8, CH3 | 21.3, CH3 | 18.2, CH3 |
| 25 | 115.8, CH | 116.3, CH | 116.0, CH | 115.9, CH | 117.2, CH | 116.6, CH |
| 26 | 133.7, CH | 133.2, CH | 133.3, CH | 133.5, CH | 130.8, CH | 129.1, CH |
| 27 | 77.1, C | 76.5, C | 76.6, C | 76.7, C | 76.3, C | 76.0, C |
| 28 | 27.9, CH3 | 27.8, CH3 | 27.9, CH3 | 27.9, CH3 | 28.8, CH3 | 28.2, CH3 |
| 29 | 27.9, CH3 | 27.8, CH3 | 27.8, CH3 | 27.9, CH3 | 27.9, CH3 | 27.6, CH3 |
| 30 | 170.0, C | |||||
| 31 | 21.0, CH3 | |||||
| 10-OMe | 61.8, CH3 | 60.0, CH3 |
a Measured at 150 MHz (13C) in CDCl3; b measured at 150 MHz (13C) in DMSO-d6.
Asperthrin C (3) was isolated as white powders. Based on the [M + Na]+ ion at m/z 516.2102 (calcd. for C27H31N3O6Na, 516.2105) in the (+)- HR-ESI-MS and 13C NMR data, the molecular formula was determined as C27H31N3O6, which was 30 amu more than 2. Comparation of the 1H NMR spectrum (Figure S19) of 3 with that of 2 indicated that there was a methoxy group in 3. The HMBC correlation from OMe-10 (δH 3.03) to C-10 (δC 76.9) (Figure 2 and Figure S23) suggested that the methoxy group was attached to C-10. The ROESY correlations (Figure 3 and Figure S24) between 19-NH (δH 7.74) and OMe-10 (δH 3.03)/H-23 (δH 1.31) indicated that these protons were co-facial and assigned as β-oriented. The ROESY correlations between 3-OH (δH 6.31) and H-10 (δH 4.72)/H-21 (δH 3.08), and between H-21 (δH 3.08) to H-24 (δH 1.32), indicated that they were in the α-orientation. Based on the negative Cotton effect at 225 nm in ECD spectra (Figure 4C and Figure S26) and the well match result between experimental and calculated ECD spectra (Figure 4C), the absolute configurations of C-3, C-10, C-11, C-17, and C-21 in 3 were assigned as 3S, 10S, 11S, 17R, and 21S.
Asperthrin D (4) was isolated as white powders. The molecular formula was determined as C27H31N3O6 by the (+)-HRESIMS data from the [M + Na]+ ion at m/z 516.2106 (calcd. for C27H31N3O6Na, 516.2105) as same as that of 3. Detailed analyses of the 1D NMR and 2D NMR spectra (Figures S28–S32) indicated that the planar structure of 4 was the same as that of 3. However, the chemical shifts of H-10 (δH 4.12) and OMe-10 (δH 3.31) in 4 differed from those of 3, implying that the configuration of C-10 was opposite to that of 3. The relative configuration was assigned by the ROESY correlations from 19-NH (δH 7.87) to OMe-10 (δH 3.31)/H-21 (δH 3.53), 3-OH (δH 6.27) to H-21 (δH 3.53)/H-24 (δH 1.30), and H-10 (δH 4.12) to H-23 (δH 1.15) (Figure 3 and Figure S33). The absolute configurations of C-3, C-10, C-11 C-17, and C-21 in 4 were assigned as 3S, 10R, 11R, 17S, 21S based on the positive Cotton effect at 225 nm and the calculated ECD spectra results (Figure 4C and Figure S35).
Asperthrin E (5) was obtained as white powders. The molecular formula was determined as C28H31N3O6 by the [M + H]+ ion at m/z 528.2102 (calcd. for C28H31N3O6Na, 528.2105) from HR-ESI-MS and 13C NMR data. The proton signal at δH 2.03 (3H, s) in 1H NMR spectrum (Figure S37) indicated the presence of an acetoxy group. The HMBC correlations from NH-1 (δH 10.69) to C-2 (δC 176.3) and H-4 (δH 6.89)/H-10 (δH 5.75)/H-23 (δH 0.52)/NH-1 (δH 10.69) to C-3 (δC 64.3) (Figure 2 and Figure S41) indicated the presence of an indoxyl core with a spiro-quaternary center at C-3. Detailed analyses of 1D NMR and 2D NMR spectra (Figures S37–S41) indicated that the planar structure of 5 was the same as that of 10-O-acetylsclerotiamide [23]. The ROESY correlations (Figure 3 and Figure S42) from 19-NH (δH 8.54) to H-24 (δH 1.12) indicated that NH-19 and H-24 were located on the α-face of the cyclopentane ring. The ROESY correlations from H-21 (δH 2.68) to H-10 (δH 5.75)/H-23 (δH 0.52) indicated that these protons located on the β-orientation of the cyclopentane ring. Besides, the ROESY correlations from H-21 (δH 2.68) to H-4 (δH 6.89) indicated that the cyclopentane ring was orthogonal to the plane of the indoxyl ring. Based on the positive Cotton effect at 225 nm and the calculated ECD spectra results (Figure 4A and Figure S44), the absolute configurations of C-3, C-10, C-11, C-17, and C-21 in 5 were assigned as 3R, 10S, 11R, 17S, 21R.
Asperthrin F (6) was isolated as white powders, and the molecular formula was determined as C26H31N3O6 by (+)-HRESIMS [M + Na]+ ion at m/z 504.2103 (calcd. for C26H31N3O6Na, 504.2105) and 13C NMR data, indicating 13 degrees of unsaturation. The 1H NMR, 13C NMR, and HSQC spectra (Table 1, Table 2 and Figures S46–S49) showed four methyl groups, five sp3-methylenes (including one oxygen-bearing methylene), two sp3-methine carbon signals (including one oxygenated carbon), five sp3 non-protonated carbons (including three oxygen-bearing carbons), four olefinic methines, and six sp2 non-protonated carbons. The 1H and 13C NMR data of 6 resembled those of gartryprostatin B [15], with the exception that C-17 oxygen-bearing sp3-methine signal (δC 93.6) and C-16 sp3-methylene signal (δH 2.10, 2.33; δC 31.6) had a clear difference. With six degrees of unsaturation accounting for the eight aromatic carbons and two carbonyls, there must be a heptatomic ring system to meet the 13 degrees of unsaturation in 6. The HMBC correlations from H-21 (δH 3.81) to C-2 (δC 98.6), H-23 (δH 1.29) to C-2 (δC 98.6)/C-21 (δC 91.1)/C-24 (δC 18.2) (Figure 2 and Figure S50), and the deshielded shifts of C-3 (δC 97.1)/C-21 (δC 91.1) indicated that the furan ring was formed by an oxygen bridge between C-3 and C-21. Further detailed 1D and 2D NMR spectral analysis revealed that a planar structure of 6 was the same as that of gartryprostatin B. The relative configuration of C-2/C-3/C-21 in the furan ring in 6 was determined by ROESY correlations from H-21 (δH 3.81) to H-23 (δH 1.29), H-10b (δH 2.72) to H-4 (δH 6.97), and H-24 (δH 0.82) to H-10a (δH 2.59). Besides, the ROESY correlation from H-10b (δH 2.72) to H-11 (δH 4.59) determined the relative configurations of C-11. Based on the chemical shift differences of C-17 in 13C NMR data, the relative configurations of C-17 should be opposite to that of gartryprostatin B. The absolute configurations of C-2, C-3, C-11, C-17, and C-21 in 6 were determined as 2S, 3R, 11S, 17S, 21S by the comparison between calculated and experimental ECD spectra (Figure 4D and Figure S53).
Compounds 1–14 were assayed for their anti-agricultural pathogenic and anti-inflammatory activities. As shown in Table 3, 1 displayed both antibacterial and antifungal activities with minimal inhibitory concentration (MIC) values of 50, 12.5, and 100 μg/mL against X. oryzae pv. oryzae, X. oryzae pv. oryzicola, and R. solani, respectively. Furthermore, 1 also exhibited moderate antibacterial activity against four fish pathogens, Edwardsiella tarda, Vibrio anguillarum, Aeromonas hydrophilia, and V. parahaemolyticus, with MIC values of 16, 8, 32, and 16 μg/mL, respectively. Compounds 5, 9, and 10 showed antifungal activities with the MIC values of 25 μg/mL against R. solani. The results showed that 1, 5, 6, 9, 10, and 12 displayed moderate anti-inflammatory activity with IC50 values of 1.5, 30.5, 37.2, 41.6, 46.2, and 34.3 μM, respectively, by measuring the inhibitory effects in P. acnes-induced THP-1 cells (Table 4).
Table 3.
Antimicrobial activities of compounds 1–14 (MIC, μg/mL).
| No. | Bacteria | Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Xe | Xa | Et | Va | Ah | Vp | Rs | Fo | Cg | |
| 1 | 50 | 12.5 | 16 | 8 | 32 | 16 | 100 | >100 | >100 |
| 5 | >100 | >100 | — | — | — | — | 25 | >100 | >100 |
| 9 | >100 | >100 | — | — | — | — | 25 | >100 | >100 |
| 10 | >100 | >100 | — | — | — | — | 25 | >100 | >100 |
| Chloromycetin | 12.5 | 12.5 | 2 | 0.5 | 2 | 2 | — | — | — |
| Ketoconazole | — | — | — | — | — | — | 0.78 | 100 | 12.5 |
Xe: Xanthomonas oryzae pv. oryzae; Xa: Xanthomonas oryzae pv. oryzicola; Et: Edwardsiella tarda; Va: Vibrio anguillarum; Ah: Aeromonas hydrophilia; Vp: Vibrio parahaemolyticus; Rs: Rhizoctonia solani; Fo: Fusarium oxysporum; Cg: Colletotrichum gloeosporioides.
Table 4.
Anti-inflammatory activities of tested compounds.
| No. | THP-1 Cells | P. acnes | |
|---|---|---|---|
| IC50 (μM) | SC (μM) | MIC (μM) | |
| 1 | 1.46 ± 0.21 | 0–5 | >5 |
| 5 | 30.5 ± 0.2 | 0–40 | >40 |
| 6 | 37.2 ± 3.1 | 0–50 | >50 |
| 9 | 41.6 ± 1.3 | 0–50 | >50 |
| 10 | 46.2 ± 2.2 | 0–50 | >50 |
| 12 | 34.3 ± 1.6 | 0–50 | >50 |
| Tretinoin | 3.38 ± 0.28 | 0–50 | >50 |
SC: Safe concentration, indicating the concentration range of THP-1 cells viability over 80% treated by tested compounds.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured using a JASCO P-1020 polarimeter (JASCO Corporation, Tokyo, Japan) in MeOH at 25 °C. UV spectra were recorded on a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation, Tokyo, Japan) in MeOH. ECD spectra were obtained by Chirascan circular dichroism spectrometers (Applied Photophysics Ltd., Leatherhead, UK). Both 1D and 2D NMR spectra were recorded on a Bruker AVIII-600 NMR spectrometer, using TMS as an internal standard. High-resolution electrospray ionization (HR-ESI-MS) was carried out with an Agilent 6529B Q-TOF instrument (Agilent Technologies, Santa Clara, CA, USA). Column chromatography was performed on silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) and ODS (50 µm, YMC, Kyoto, Japan) on a Flash Chromatograph System (SepaBen machine, Santai Technologies, Changzhou, China). Preparative high-performance liquid chromatography (Pre-HPLC) was performed on a Shimadzu LC-20 system (Shimadzu, Tokyo, Japan) equipped with a Shim-pack RP-C18 column (20 × 250 mm i.d., 10 µm, Shimadzu, Tokyo, Japan) with a flow rate at 10 mL/min at 25 °C.
3.2. Fungal Material
The fungal strain A. sp. YJ191021 was isolated from a soil sample, which was collected from the intertidal zone of Zhoushan, Zhejiang, China, in June 2018. The fungal strain was identified according to their morphological characteristics and by sequencing the fungal ITS region in rDNA. The fungal strain is stored in State Key Laboratory of Bioreactor Engineering laboratory of Shanghai at −80 °C.
3.3. Fermentation, Extraction, and Isolation
The fungus was incubated on potato dextrose agar (PDA) medium at 28 °C for 3 days. Then the grown strain was inoculated to a 250 mL Erlenmeyer flask containing 50 mL of potato dextrose broth (PDB). After 2 days of fermentation, the seed cultures were added to Erlenmeyer flasks (100 × 1000 mL), each containing 100 g of dry rice and 120 mL of distilled water, which was previously sterilized at 121 °C for 30 min. All flasks were incubated at room temperature for 30 days. After incubation, whole fermented rice medium was extracted three times using ethyl acetate (EtOAc), and then solvents were concentrated under reduced pressure to give a crude extract (193.4 g). Next, the crude extract was subjected to a macroporous resin column eluting by a gradient EtOH-H2O (from 30%, 50%, 70% to 100% EtOH). The 50% fraction (33.6 g) was then separated on a silica gel column eluting with a stepwise gradient of CH2Cl2-MeOH (from 25:1 to 5:1) to yield five subfractions (A–E). Fraction D (4.3 g) was further purified by an ODS column (MeCN-H2O, 35:65) and a semi-preparative HPLC eluting with 60% MeOH/H2O to yield compounds 1 (22.3 mg, tR 30.4 min), 14 (31.4 mg, tR 10.4 min). Fraction C (3.2 g) was further purified by an ODS column (MeCN-H2O, 30:70) and a semi-preparative HPLC eluting with 55% MeOH/H2O to yield compounds 2 (13.4 mg, tR 34.2 min), 3 (11.2 mg, tR 30.6 min), and 4 (9.2 mg, tR 28.1 min). Fraction B (3.6 g) was further purified by an ODS column (MeCN-H2O, 40:60) and a semi-preparative HPLC eluting with 60% MeOH/H2O to yield compounds 6 (16.7 mg, tR 26.8 min), 7 (4.2 mg, tR 23.4 min), and 8 (18.3 mg, tR 19.3 min). The fraction A was separated on an ODS column (MeCN-H2O, 55:45) to yield four subfractions (A1–A4). The subfraction A3 was further purified by a semi-preparative HPLC eluting with 75% MeOH/H2O to yield compounds 5 (6.4 mg, tR 22.6 min), 9 (12.3 mg, tR 24.2 min), and 10 (10.1 mg, tR 25.6 min). The subfraction A1 was further purified by a semi-preparative HPLC eluting with 65% MeOH/H2O to yield compounds 11 (8.4 mg, tR 18.4 min), 12 (7.3 mg, tR 16.2 min), and 13 (7.6 mg, tR 19.6 min).
Asperthrin A (1): Brilliant yellowish powder; [α − 75.4 (c 0.1, MeOH); IR vmax 3448, 1708, 1409, 1368, 1192, 1123 cm−1; UV (MeOH) λmax (log ε) 217 (4.00), 306 (3.65) nm; ECD (2.00 mM, MeOH) λmax (Δε) 225 (−4.33), 242 (+0.70) nm; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS at m/z 446.2071 [M + H]+ (calcd. for C26H28N3O4, 446.2074).
Asperthrin B (2): White powder; [α − 40.2 (c 0.1, MeOH); IR vmax 3398, 1693, 1404, 1117, cm−1; UV (MeOH) λmax (log ε) 203 (4.05), 266 (3.70) nm; ECD (2.00 mM, MeOH) λmax (Δε) 224 (−4.63), 242 (+0.77) nm; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS at m/z 486.2002 [M + Na]+ (calcd. for C26H29N3O5Na, 486.1999).
Asperthrin C (3): White powder; [α + 16.6 (c 0.1, MeOH); IR vmax 3417, 2983, 1689, 1538, 1496, 1121 cm−1; UV (MeOH) λmax (log ε) 203 (4.10), 267 (3.74) nm; ECD (2.00 mM, MeOH) λmax (Δε) 224 (−4.63), 242 (+2.23) nm; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS at m/z 516.2102 [M + Na]+ (calcd. for C27H31N3O6Na, 516.2105).
Asperthrin D (4): White powder; [α + 32.2 (c 0.1, MeOH); IR vmax 3428, 2934, 1695, 1490, 1112 cm−1; UV (MeOH) λmax (log ε) 206 (4.09), 267 (3.76) nm; ECD (2.00 mM, MeOH) λmax (Δε) 225 (+4.21), 242 (−1.79) nm; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS at m/z 516.2106 [M + H]+ (calcd. for C27H31N3O6Na, 516.2105).
Asperthrin E (5): White powder; [α + 53.4 (c 0.1, MeOH); IR vmax 3437, 2981, 1696, 1463, 1123 cm−1; UV (MeOH) λmax (log ε) 203 (3.96), 247 (4.03) nm; ECD (2.00 mM, MeOH) λmax (Δε) 236 (+4.17), 253 (−2.16) nm; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS at m/z 528.2102 [M + Na]+ (calcd. for C28H31N3O6Na, 528.2105).
Asperthrin F (6): White powder; [α − 157.4 (c 0.1, MeOH); IR vmax 3498, 2980, 1689, 1418, 1072 cm−1; UV (MeOH) λmax (log ε) 237 (4.16), 288 (3.64), 338 (3.50) nm; ECD (2.00 mM, MeOH) λmax (Δε) 226 (−4.68), 241 (−2.26) nm, 257 (−4.94); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS at m/z 504.2103 [M + Na]+ (calcd. for C26H31N3O6Na, 504.2105).
3.4. Antimicrobial Assays
Minimum Inhibitory Concentration (MIC) assays were used to assess antimicrobial activities of all isolated compounds against two agricultural pathogenic bacteria (Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola) and three agricultural fungi (Colletotrichum gloeosporioides penz, Fusarium oxysporum and Rhizoctonia solani). Furthermore, Compound 1 was tested for antibacterial activities against four fish pathogens, Edwardsiella tarda, Vibrio anguillarum, Aeromonas hydrophilia, and Vibrio parahaemolyticus. Chloromycetin was used as a positive antibacterial control and ketoconazole was used as a positive antifungal control. The experimental procedure is detailed in the (Supporting Information (SI). All the experiments were performed in three independent replicates.
3.5. Anti-Inflammatory Assays
The human monocyte cell line THP-1 (Cell Bank of China Science Academy, Shanghai, China) and Propionibacterium acnes (ATCC6919, Xiangfu biotech, Shanghai, China) were used in anti-inflammatory experiments. The P. acnes in logarithmic growth phase was used to induce inflammation in THP-1 cells. MTT method was carried out for tested compounds to determine their safe concentration to THP-1 cells. Besides, antimicrobial assays were performed to exclude false anti-inflammatory activity of these compounds raised from their inhibition to P. acnes. The inhibitory activity of the test compounds on the secretion of inflammatory factor 1L-1β by THP-1 cells was assayed by ELISA experiment [24,25]. The experimental procedure is detailed in the SI. All the experiments were performed in three independent replicates.
4. Conclusions
In summary, six new prenylated indole diketopiperazine alkaloids, asperthrins A-F (1–6), along with eight known analogues (7–14), were isolated from the solid rice cultures of the marine-derived fungus Aspergillus sp. YJ191021. Asperthrin A (1) showed potent anti-bacterial, anti-fungal, and anti-inflammatory activities at micromolar level. These results expand the chemical diversity of prenylated indole alkaloids and provide a basis for further development of prenylated indole alkaloids into natural agrochemicals and drug leads.
Acknowledgments
Authors thank Wei Wang (State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology) for proving X. oryzae pv. oryzae, X. oryzae pv. oryzicola, and R. solani.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/19/3/157/s1, Figures S1–S54: 1H, 13C, HSQC, HMBC, ROESY, UV, IR, ECD, and HRESIMS spectra of the new compounds 1–6, Tables S1–S6: Computational data of 1 and 4.
Author Contributions
J.Y., Y.D., and Z.W. performed the isolation, purification, and identification of all compounds. L.G. and Y.J. tested the anti-inflammatory activities, and supervised the laboratory work. M.G. and X.X. edited the manuscript. F.A. supervised the laboratory work, designed the experiments, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key R&D Program of China (2018YFC1706206, 2019YFC0312504), and the National Natural Science Foundation of China (41876189, 81703388). This work was also supported by State Key Laboratory of Bioreactor Engineering and the Open Research Fund Program of Institute of regulatory science, Beijing Technology and Business University (CRS-2020-02), the Open Research Fund Program of Key Laboratory of Cosmetic (Beijing Technology and Business University), China National Light Industry (KLC-2019-ZD2).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou Z.X., Wei D.F., Lu Y.H. Polyhexamethylene guanidine hydrochloride shows bactericidal advantages over chlorhexidine digluconate against ESKAPE bacteria. Biotechnol. Appl. Bioc. 2015;62:268–274. doi: 10.1002/bab.1255. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Yao L.Y., Lu Y.H. Ceriporia lacerata DMC1106, a new endophytic fungus: Isolation, identification, and optimal medium for 2’,4’-dihydroxy-6’-methoxy-3’,5’-dimethylchalcone production. Biotechnol. Bioproc. Eng. 2013;18:669–678. doi: 10.1007/s12257-012-0846-z. [DOI] [Google Scholar]
- 3.Farooq S., Qayum A., Nalli Y., Lauro G., Chini M.G., Bifulco G., Chaubey A., Singh S.K., Riyaz-Ul-Hassan S., Ali A. Discovery of a Secalonic Acid Derivative from Aspergillus aculeatus, an Endophyte of Rosa damascena Mill., Triggers Apoptosis in MDA-MB-231 Triple Negative Breast Cancer Cells. ACS Omega. 2020;5:24296–24310. doi: 10.1021/acsomega.0c02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao D.L., Han X.B., Wang M., Zeng Y.T., Li Y.Q., Ma G.Y., Liu J., Zheng C.J., Wen M.X., Zhang Z.F., et al. Herbicidal and Antifungal Xanthone Derivatives from the Alga-Derived Fungus Aspergillus versicolor D5. J. Agric. Food Chem. 2020;68:11207–11214. doi: 10.1021/acs.jafc.0c04265. [DOI] [PubMed] [Google Scholar]
- 5.Peyrat L.A., Eparvier V., Eydoux C., Guillemot J.C., Litaudon M., Stien D. Carneic Acids from an Endophytic Phomopsis sp. as Dengue Virus Polymerase Inhibitors. J. Nat. Prod. 2020;83:2330–2336. doi: 10.1021/acs.jnatprod.9b01169. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X.L., Wang X.F., Xu K., Li W., Chen D., Zhang P. Characterization of a New Insecticidal Anthraquinone Derivative from an Endophyte of Acremonium vitellinum against Helicoverpa armigera. J. Agric. Food Chem. 2020;68:11480–11487. doi: 10.1021/acs.jafc.0c05680. [DOI] [PubMed] [Google Scholar]
- 7.Liu W.H., Ding Y., Ji X., An F.L., Lu Y.H. Curvulaide A, a bicyclic polyketide with anti-anaerobic bacteria activity from marine-derived Curvularia sp. J. Antibiot. 2018;72:111–113. doi: 10.1038/s41429-018-0110-7. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y., Zhu X., Hao L., Zhao M., Hua Q., An F. Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus versicolor DY180635. Mar. Drugs. 2020;18:338. doi: 10.3390/md18070338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han W.B., Lu Y.H., Zhang A.H., Zhang G.F., Mei Y.N., Jiang N., Lei X., Song Y.C., Ng S.W., Tan R.X. Curvulamine, a New Antibacterial Alkaloid Incorporating Two Undescribed Units from a Curvularia Species. Org. Lett. 2014;16:5366–5369. doi: 10.1021/ol502572g. [DOI] [PubMed] [Google Scholar]
- 10.Borthwick A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012;112:3641–3716. doi: 10.1021/cr200398y. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y.M., Liang X.A., Kong Y., Jia B. Structural Diversity and Biological Activities of Indole Diketopiperazine Alkaloids from Fungi. J. Agric. Food Chem. 2016;64:6659–6671. doi: 10.1021/acs.jafc.6b01772. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Xu D., Sun W., Yang B., Li F., Liu M., Wang J., Xue Y., Hu Z., Zhang Y. HPLC-DAD-Directed Isolation of Linearly Fused Prenylated Indole Alkaloids from a Soil-Derived Aspergillus versicolor. J. Nat. Prod. 2019;82:2181–2188. doi: 10.1021/acs.jnatprod.9b00183. [DOI] [PubMed] [Google Scholar]
- 13.Levinson A.M. Total Synthesis of Aspeverin via an Iodine(III)-Mediated Oxidative Cyclization. Org. Lett. 2014;16:4904–4907. doi: 10.1021/ol5024163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P., Yuan X.L., Du Y.M., Zhang H.B., Shen G.M., Zhang Z.F., Liang Y.J., Zhao D.-L., Xu K. Angularly Prenylated Indole Alkaloids with Antimicrobial and Insecticidal Activities from an Endophytic Fungus Fusarium sambucinum TE-6L. J. Agric. Food Chem. 2019;67:11994–12001. doi: 10.1021/acs.jafc.9b05827. [DOI] [PubMed] [Google Scholar]
- 15.He W., Xu Y., Fu P., Zuo M., Liu W., Jiang Y., Wang L., Zhu W. Cytotoxic Indolyl Diketopiperazines from the Aspergillus sp. GZWMJZ-258, Endophytic with the Medicinal and Edible Plant Garcinia multiflora. J. Agric. Food Chem. 2019;67:10660–10666. doi: 10.1021/acs.jafc.9b04254. [DOI] [PubMed] [Google Scholar]
- 16.Whyte A.C., Gloer J.B., Wicklow D.T., Dowd P.F. Sclerotiamide: A New Member of the Paraherquamide Class with Potent Antiinsectan Activity from the Sclerotia of Aspergillus sclerotiorum. J. Nat. Prod. 1996;59:1093–1095. doi: 10.1021/np960607m. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto S., Kato H., Samizo M., Nojiri Y., Onuki H., Hirota H., Ohta T. Notoamides F-K, Prenylated Indole Alkaloids Isolated from a Marine-Derived Aspergillus sp. J. Nat. Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]
- 18.Cai S.X., Luan Y.P., Kong X.L., Zhu T.J., Gu Q.Q., Li D.H. Isolation and Photoinduced Conversion of 6-epi-Stephacidins from Aspergillus taichungensis. Org. Lett. 2013;15:2168–2171. doi: 10.1021/ol400694h. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto S., Umaoka H., Yoshikawa K., Ikeda T., Hirota H. Notoamide O, a Structurally Unprecedented Prenylated Indole Alkaloid, and Notoamides P-R from a Marine-Derived Fungus, Aspergillus sp. J. Nat. Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto K., Sadahiro Y., Kagiyama I., Kato H., Sherman D.H., Williams R.M., Tsukamoto S. Isolation of amoenamide A and five antipodal prenylated alkaloids from Aspergillus amoenus NRRL 35600. Tetrahedron Lett. 2017;58:2797–2800. doi: 10.1016/j.tetlet.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato H., Yoshida T., Tokue T., Nojiri Y., Hirota H., Ohta T., Williams R.M., Tsukamoto S. Notoamides A–D: Prenylated Indole Alkaloids Isolated from a Marine-Derived Fungus, Aspergillus sp. Angew. Chem. Int. Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 22.Qian J., Wu J.Y., Yao B.B., Lu Y.H. Preparation of a polyclonal antibody against hypericin synthase and localization of the enzyme in red-pigmented Hypericum perforatum L. plantlets. Acta Biochim. Pol. 2012;59:639–645. doi: 10.18388/abp.2012_2104. [DOI] [PubMed] [Google Scholar]
- 23.Afiyatullov S.S., Zhuravleva O.I., Antonov A.S., Berdyshev D.V., Pivkin M.V., Denisenko V.A., Popov R.S., Gerasimenko A.V., von Amsberg G., Dyshlovoy S.A., et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018;71:846–853. doi: 10.1038/s41429-018-0072-9. [DOI] [PubMed] [Google Scholar]
- 24.Guo M.M., An F.L., Wei X., Hong M.H., Lu Y.H. Comparative Effects of Schisandrin A, B, and C on Acne-Related Inflammation. Inflammation. 2017;40:2163–2172. doi: 10.1007/s10753-017-0656-8. [DOI] [PubMed] [Google Scholar]
- 25.An F.L., Wang X.B., Yang M.H., Luo J., Kong L. Bioactive A-ring rearranged limonoids from the root barks of Walsura robusta. Acta Pharm. Sin. B. 2019;9:545–556. doi: 10.1016/j.apsb.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.