ABSTRACT
The value of vitamin D supplementation in the treatment or prevention of various conditions is often viewed with scepticism as a result of contradictory results of randomised trials. It is now becoming apparent that there is a pattern to these inconsistencies. A recent large trial has shown that high-dose intermittent bolus vitamin D therapy is ineffective at preventing rickets – the condition that is most unequivocally caused by vitamin D deficiency. There is a plausible biological explanation since high-dose bolus replacement induces long-term expression of the catabolic enzyme 24-hydroxylase and fibroblast growth factor 23, both of which have vitamin D inactivating effects. Meta-analyses of vitamin D supplementation in prevention of acute respiratory infection and trials in tuberculosis and other conditions also support efficacy of low dose daily maintenance rather than intermittent bolus dosing. This is particularly relevant during the current COVID-19 pandemic given the well-documented associations between COVID-19 risk and vitamin D deficiency. We would urge that clinicians take note of these findings and give strong support to widespread use of daily vitamin D supplementation.
KEYWORDS: vitamin D, COVID-19, maintenance, dosing, efficacy
Introduction: contradictory results of vitamin D supplementation in clinical trials
Controversy surrounding the possible benefits from taking vitamin D supplements has become more prominent recently with the reports of various associations between vitamin D deficiency and risk of COVID-19.1 Authorities have given only muted support to avoidance of vitamin D deficiency during the current COVID-19 pandemic, concluding that there is insufficient evidence from randomised trials of supplementation to support any protective effect against COVID-19.2 Many randomised trials of vitamin D supplementation have been performed in a wide range of conditions with contradictory and often negative results that have led to considerable scepticism about its value.3,4 This may partly result from optimistic trial of vitamin D supplementation in widely diverse conditions, not always with a plausible mechanism of causation. It is becoming apparent, though, that the use of intermittent high-dose bolus, introduced to achieve high adherence, rather than regular daily maintenance could be an important explanation for the failure of vitamin D supplementation in some trials.
Evidence that low-dose daily vitamin D supplementation is effective but intermittent high-dose bolus is not
Rickets
Rickets is the one condition above all others where there is conclusive and very longstanding evidence, dating back to the 1920s, first in animals and then in children, of response to regular daily vitamin D, either via cod liver oil supplementation or later by food fortification.5 A recent well-conducted trial in 3,046 children has however shown that high-dose bolus vitamin D (100,000 IU every 3 months for 18 months) is ineffective at preventing rickets in children6 – indeed it is not the first trial to show this.7 A smaller study in 72 children with calcium-deficiency rickets showed that oral vitamin D2 50,000 IU monthly for 24 weeks had only a ‘marginally significant effect’ (p=0.06) on recovery compared with calcium supplementation alone.8
Tuberculosis
Tuberculosis is another condition for which there is longstanding evidence suggesting that vitamin D deficiency contributes to illness and susceptibility. As with rickets, this dates back a long way, a successful trial of daily cod liver oil in tuberculosis having been reported in 1848 from London's Brompton Hospital.9 However, although ultraviolet light and vitamin D therapy were well-established therapies, particularly in cutaneous tuberculosis, during the first half of the 20th century, controlled trials were not performed.10 A recent meta-analysis of vitamin D supplementation as an adjunct to chemotherapy for active pulmonary tuberculosis showed significantly accelerated sputum conversion but only in patients with multi-drug-resistant tuberculosis. In this analysis, only one of seven included studies trialled daily vitamin D supplementation.11 No significant difference was seen for daily or weekly supplementation combined versus bolus/2-weekly regimens. However, the single trial of daily supplementation (5,000 IU/day) versus placebo in 288 patients with active pulmonary tuberculosis did show significant benefit across the whole patient population, with 61.3% of patients culture-negative at 4 weeks compared with 42.2% placebo (p=0.032).12
In a 2015 review of vitamin D supplementation as prophylaxis against tuberculosis in high-risk individuals, it was noted that no randomised trials had yet taken place despite consistent evidence that vitamin D deficiency increases risk for developing active tuberculosis.13 There had however been one study looking at the effect of supplementation with 800 IU/day over 6 months in 120 children from Mongolia, all with vitamin D deficiency (<50 nmol/L), which showed a trend towards fewer tuberculin skin test conversions (risk ratio [RR] 0.41, 95% confidence interval [CI] 0.16–1.09; p=0.06) and a significantly greater increase in stature (p<0.003).14 Recently, a much larger trial in 8,851 children from Mongolia, 95.6% with vitamin D deficiency (<50 nmol/L), but this time using a weekly oral dose of 14,000 IU vitamin D3 compared with placebo, showed no impact on subsequent diagnosis of tuberculosis (adjusted RR 0.87, 95% CI 0.49–1.55) or on risk of hospitalisation with acute respiratory infection (adjusted RR 0.86, 95%CI 0.52–1.40).15
Acute respiratory infections
The recent meta-analyses by Martineau and colleagues of the efficacy of vitamin D supplementation in prevention of acute respiratory infections have also reported that low dose daily maintenance supplementation is effective but intermittent high-dose bolus is not.16,17 In their most recent meta-analysis of 42 randomised controlled trials, 47,262 subjects were analysed. A protective effect against risk for acute respiratory infection was seen for trials in which vitamin D supplements were given daily (odds ratio [OR] 0.70, 95% CI 0.61–0.93) but not for trials where vitamin D was given weekly (OR 0.97, 95% CI 0.88–1.06) or monthly to 3-monthly (OR 0.98, 95% CI 0.93–1.03).17
Thus for treatment or prevention of rickets, tuberculosis and acute respiratory infections, there is substantial evidence that it is daily vitamin D supplementation that is effective rather than intermittent high-dose boluses.
A plausible biological mechanism underlies the lack of efficacy of intermittent high-dose (bolus) vitamin D
Vitamin D (cholecalciferol) is synthesised in the skin by the action of ultraviolet B (UVB, wavelength 280–315 nm) on exposed skin, splitting a carbon-to-carbon bond (C9–C10) in the precursor 7-dehydrocholesterol.18 Cholecalciferol is 25-hydroxylated in the liver and subsequently 1α-hydroxylated in the kidneys and in other tissues including immune cells and many epithelia to form the active metabolite 1,25 dihydroxycholecalciferol (1,25(OH)2D). The predominant circulating vitamin D is 25-hydroxycholecalciferol (25(OH)D), which is itself a substrate for local activation in tissues expressing the 1α-hydroxylase enzyme and is present in nanomolar concentrations, whereas circulating active 1,25(OH)2D is only present in picomolar concentrations. Like other lipophilic molecules, 25(OH)D is bound to protein in serum, approximately 80% to the vitamin D binding protein (DBP) and 20% to albumin.19 Given that the vitamin D receptor (VDR) is predominantly a nuclear receptor, it is the intracellular concentration of 1,25(OH)2D that is most relevant and most/all cells that express the VDR, including osteoblasts and immune cells, also express 1α-hydroxylase activity, so circulating concentrations of 1,25(OH)2D may not always reflect intracellular concentrations. Currently, almost all studies of vitamin D rely on the measurement of serum 25(OH)D to determine vitamin D status but, given the complexities of variable protein binding in illness plus its complex metabolism, there is a need to consider other metabolites such as 1,25(OH)2D and 24,25(OH)2D, particularly in the setting of different dosing regimens. The half-life of 25(OH)D in the blood is around 2–3 weeks but the whole-body half-life is around 2–3 months, probably because cholecalciferol, which is lipid-soluble, is conserved in body fat.20 As a result it has been widely presumed that large intermittent boluses, which provide relatively stable 25(OH)D serum concentrations, could provide effective replacement.21
The metabolism of vitamin D is complex, however, and still incompletely understood (Fig 1). A large amount of cholecalciferol can be generated rapidly by sunlight exposure – one full-body UV exposure causing slight skin pinkness is equivalent to 250–625 μg (10,000–25,000 IU) vitamin D3,18 whereas total daily requirement is only around 800–1,000 IU22 – so the level of activated vitamin D in the body could potentially vary enormously without robust regulatory mechanisms.
Fig 1.
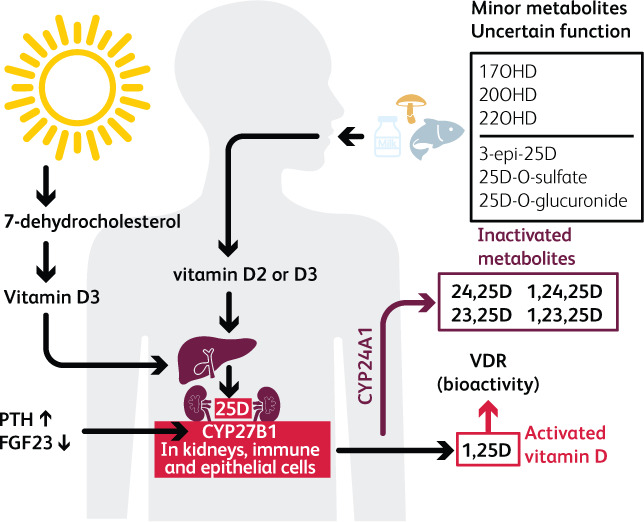
The complexity of vitamin D metabolism. D2 is from plant sources via ultraviolet action on ergosterol and D3 from animal sources via ultraviolet action on 7-dehydrocholesterol. Activation is via 25-hydroxylation in the liver followed by 1α-hydroxylation (CYP27B1) in kidneys, immune cells and many epithelia, to 1,25(OH)2D. Increased FGF23 suppresses 1α-hydroxylation. Free 25(OH)D appears to be preferentially taken up by monocytes,19 so the reduction in DBP in illness may have a protective effect via increased availability of free 25(OH)D. Either 25(OH)D or 1,25(OH)2D can be degraded via 24-hydroxylation (CYP24A1) to 24,25(OH)D or 1,24,25(OH)3D respectively.
It is impossible for vitamin D toxicity to occur through ultraviolet exposure (and extremely high repeated dosing is required for this to occur via supplementation). The principal regulatory check is via 24-hydroxylation forming 24,25(OH)2D or 1,24,25(OH)3D, which are both largely inactive. The inactivating enzyme 24-hydroxylase CYP24A1 catalyses not only 24-hydroxylation but also 23-hydroxylation, generating a series of polyhydroxylated products. Although these may have some biological activity, the importance of this process in inactivating vitamin D is demonstrated by the finding that inborn mutations of CYP24A1 cause idiopathic infantile hypercalcemia.23 CYP24A1 is expressed in cells containing the vitamin D receptor, including kidney, bone, intestine and dendritic cells, and increases markedly in response to rising levels of 25(OH)D.24 The intracellular balance between the activating 1αhydroxylase CYP27B1 and the inactivating 24-hydroxylase CYP24A1 is thought to be critically important, particularly in extra-skeletal tissues, for regulating activity of vitamin D.25
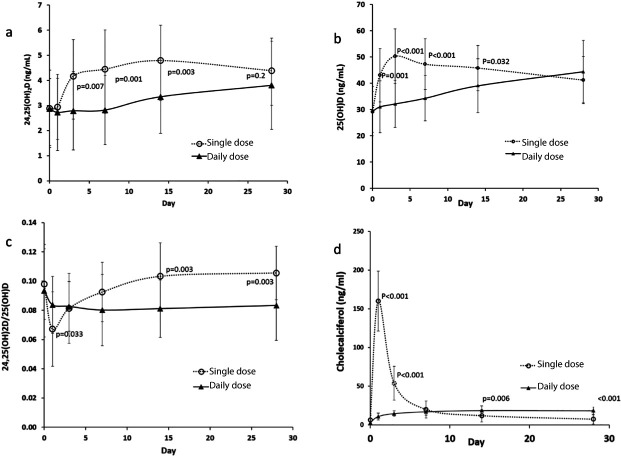
It has only been recognised relatively recently that the increased 24-hydroxylase activity, as a feedback control response to a large bolus of vitamin D, may itself have a long half-life – persisting for several weeks after the bolus dose26 (Fig 2). This implies that a single high-dose bolus of vitamin D could paradoxically lead to intracellular deficiency of activated vitamin D as a rebound phenomenon. This might be particularly important in immune cells such as the dendritic cells that are probably central to the hyperinflammatory state seen in severe COVID-19 and that seem particularly sensitive to this process.24
Fig 2.
Alterations in (a) serum concentration of 24,25(OH)2D, (b) serum concentration of 25(OH)D, (c) ratio of 24,25(OH)2D / 25(OH)D, and (d) serum concentration of cholecalciferol following a single dose of 150,000 IU vitamin D3 (open circles) and daily dose of 5000IU for 28 days (black triangles). The bars represent standard deviations. P values are for comparison of values in daily and single dosing groups at corresponding time points. It can be seen that single high-dose bolus vitamin D induces an increase in the inactivating 24-hydroxylase activity that lasts for around 4 weeks. Reproduced with permission from Ketha et al.26
The role of fibroblast growth factor 23 (FGF23) in vitamin D regulation may be at least equally important in regulating activation of vitamin D. Its expression is increased in the presence of high serum 25(OH)D and 1,25(OH)D concentrations. A meta-analysis of 23 studies has shown that vitamin D supplementation daily or at an equivalent of ≤2,000 IU/day had no significant effect on circulating FGF23 concentrations, whereas higher doses resulted in a significant increase (FGF23 +18 pg/ml, 95% CI 6–30), particularly when circulating 25(OH)D achieved was ≥100 nmol/L (p<0.001).27 The likely importance of this increase in FGF23 in the suppression of vitamin D activation is demonstrated by the condition tumour-associated osteomalacia, in which inappropriate FGF23 secretion by tumour tissue resulting in serum concentrations of FGF23 >30 pg/ml in the context of hypophosphatemia is considered the cut-off for diagnosis.28 Importantly, the rise in FGF23 following vitamin D bolus is very long-lasting. A single large intramuscular bolus of vitamin D2 (300,000 IU) given to 45 subjects induced a progressive 50% increase of circulating FGF23 over at least the following 3 months (p<0.01)29 (Fig 3). FGF23 in-turn markedly suppresses 1α-hydroxylation of 25(OH)D resulting in reduced intracellular activation of vitamin D to 1,25(OH)2D as demonstrated in monocytes.30
Fig 3.
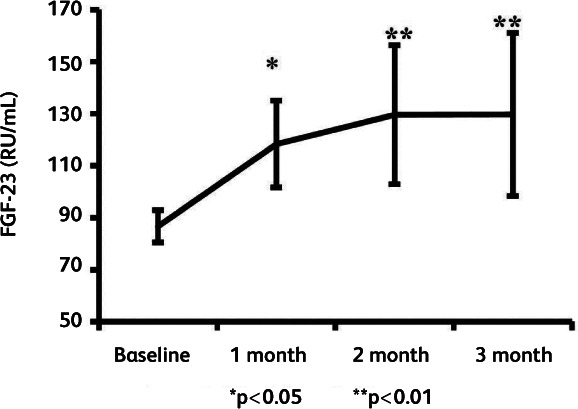
Changes in serum FGF23 after a single bolus of ergocalciferol (vitamin D2) 300,000 IU intramuscularly in 45 subjects with vitamin D deficiency/insufficiency. FGF23, which inhibits 1α-hydroxylase activity, is increased by 50% (P<0.01) 3 months after the bolus. Reproduced with permission from Turner et al.29
In studies of dialysis patients, low serum 25(OH)D combined with high serum FGF23 was shown to associate strongly with infectious and cardiac events and all-cause mortality.31
A simpler explanation might be that high-dose bolus supplementation yields blood concentrations of vitamin D that are harmful. This seems a less likely explanation for the lack of therapeutic effect of bolus supplementation, since potentially harmful blood concentrations are only approached very transiently if at all, eg in the first 24 hours after bolus administration of high-dose 25(OH)D32 and hypercalcaemia is rarely reported. There is also controversial population-based evidence to support a ‘U-shaped curve’ with less benefit at higher mean serum 25(OH)D concentrations.33 This is disputed though34 and seems unlikely to explain the overall negative results of so many trials of bolus supplementation. There are of course other reasons for some of the negative trial results, including vitamin D being treated as a drug rather than a nutrient, often with inclusion of many participants with normal vitamin D status at baseline.35
Implications for COVID-19
There is therefore a clear biological explanation for the lack of efficacy of vitamin D supplementation when delivered as intermittent high-dose boluses. This lack of efficacy of bolus replacement is emphatically reinforced by the recent trial in rickets.6 If the literature on efficacy of vitamin D supplementation in treating or preventing various conditions including respiratory infections is reviewed with this in mind, the efficacy of regular daily maintenance vitamin D supplementation becomes much clearer and more convincing. This is borne out powerfully by the careful meta-analysis by Jolliffe, Martineau and colleagues that shows vitamin D supplementation is associated with significant reduction in risk for a range of acute respiratory infections but only when given as regular daily maintenance and not when given at weekly or 1–3-monthly intervals.17 Given that COVID-19 is also primarily a respiratory infection, this seems highly relevant.
We have pointed out elsewhere the links between vitamin D deficiency, latitude, seasonality, ultraviolet exposure, obesity, ethnicity and COVID-19 risk.1 There, and elsewhere,36 we have also discussed the complex impacts of illness on interpretation of circulating 25(OH)D concentrations in sick patients, the possibility of the ‘healthy user’ effect and the difficulties these present in drawing firm conclusions from association data. Nevertheless, the marked congruence between the risk factors for vitamin D deficiency and the risk factors for severe COVID-19 make the link very plausible. This is supported by the strongly positive results of high-dose 25(OH)D (calcifediol) therapy given three times in the first week then weekly in a small Spanish trial of hospitalised patients.37 It should be noted that high-dose bolus of 25(OH)D will also likely induce potentially deleterious induction of 24-hydroxylase and FGF23, as a consequence of commensurate elevation of serum 1,25(OH)2D.32 It is plausible though that repeated dosing, particularly in the first week of therapy as used in the Spanish trial, could override this. The use of 25(OH)D is interesting and unusual. Sick patients may have reduced hepatic 25-hydroxylation so correction of low serum 25(OH)D may be faster with 25(OH)D supplementation than with standard cholecalciferol.38 The only other randomised trial, published as a pre-print, is a negative trial from Brazil of a bolus of 200,000 IU cholecalciferol given once only to patients admitted to hospital, randomised an average of 10 days after symptom onset.39 Before the pandemic there have also been trials of vitamin D in patients admitted to intensive care but unfortunately these trials (nine to date) have also used high-dose bolus cholecalciferol and have all proved negative.40 There is an ongoing international study (VITDALIZE) targeting vitamin-D-deficient critically ill patients that also has a high frontloading bolus dose (540,000 IU cholecalciferol) but with regular 4,000 IU daily dosing afterwards for 90 days.41
NICE has noted the considerable incidence of vitamin D deficiency in the UK, particularly in winter, and has recommended its supplementation ‘for bone and muscle health’ but has concluded that evidence is currently insufficient to recommend vitamin D supplementation for preventing or treating COVID-19. We have previously pointed out the difficulty of conducting a placebo-controlled trial for a vitamin in the community.42 Waiting until the spring of 2021 at the earliest for the completion of any such trial would moreover lose the opportunity to mitigate COVID-19 risks earlier through correction of population vitamin D deficiency and the appropriate use of calcifediol in COVID-19 illness.
It seems likely to us that much of the current scepticism about the benefits of vitamin D supplementation amongst clinicians, including perhaps those on advisory committees, comes from the contradictory results of trials investigating either bolus or maintenance supplementation. We urge health professionals to note the substantial evidence for efficacy of low-dose daily maintenance vitamin D, rather than intermittent bolus, against various relevant clinical endpoints and to move urgently to encourage the general population to avoid vitamin D deficiency, defined as <50 nmol/L.22 They should be urged to do this to support immune function rather than just bone and muscle health. In the Northern Hemisphere vitamin D deficiency is at peak incidence from February to March and can be readily avoided by a daily supplement of 800–1,000 IU, preferably starting with a higher dose eg 4,000 IU/day for the first four weeks if deficiency is suspected,22 for example in people with dark skin or living in residential care, shielding indoors or working night shifts.
Conflicts of interest
Martin Hewison and David Thickett have received speaking honoraria from Thornton Ross.
References
- 1.Griffin G, Hewison M, Hopkin J, et al. Vitamin D and COVID-19: evidence and recommendations for supplementation. Royal Soc Open Sci 2020; 7:201912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence . COVID-19 rapid guideline: vitamin D. NICE, 2020. www.nice.org.uk/guidance/ng187. [PubMed]
- 3.Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol 2017;5:986–1004. [DOI] [PubMed] [Google Scholar]
- 4.Bolland MJ, Avenell A, Grey A. Should adults take vitamin D supplements to prevent disease? BMJ 2016;355:i6201. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon R, Antonio L. Nutritional rickets: Historic overview and plan for worldwide eradication. J Steroid Biochem Mol Biol 2020;198:105563. [DOI] [PubMed] [Google Scholar]
- 6.Crowe FL, Mughal MZ, Maroof Z, et al. Vitamin D for growth and rickets in stunted children: A randomized trial. Pediatrics 2021;147:e20200815. [DOI] [PubMed] [Google Scholar]
- 7.Chibuzor MT, Graham-Kalio D, Osaji JO, Meremikwu MM. Vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children. Cochrane Database Syst Rev 2020;4:CD012581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thacher TD, Fischer PR, Pettifor JM. Vitamin D treatment in calcium-deficiency rickets: a randomised controlled trial. Arch Dis Child 2014;99:807–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green M. Cod liver oil and tuberculosis. BMJ 2011;343:d7505. [DOI] [PubMed] [Google Scholar]
- 10.Jarrett P, Scragg R. A short history of phototherapy, vitamin D and skin disease. Photochem Photobiol Sci 2017;16:283–90. [DOI] [PubMed] [Google Scholar]
- 11.Jolliffe DA, Ganmaa D, Wejse C, et al. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J 2019;53:1802003. [DOI] [PubMed] [Google Scholar]
- 12.Mily A, Rekha RS, Kamal SM, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: A randomized controlled trial. PLoS One 2015;10:e0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull ER, Drobniewski F. Vitamin D supplementation: a comprehensive review on supplementation for tuberculosis prophylaxis. Expert Rev Respir Med 2015;9:269–75. [DOI] [PubMed] [Google Scholar]
- 14.Ganmaa D, Giovannucci E, Bloom BR, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr 2012;96:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganmaa D, Uyanga B, Zhou X, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med 2020;383:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolliffe D, Camargo CA, Sluyter J, et al. 2020 Vitamin D supplementation to prevent acute respiratory infections: systematic review and meta-analysis of aggregate data from randomised controlled trials. medRxiv 2020.07.14.20152728. [DOI] [PubMed] [Google Scholar]
- 18.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 2013;5:51–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab 2010;95:3368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J Clin Endocrinol Metab 2017;102:3731–8. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Camargo CA, Jr, Reid IR, et al. What factors modify the effect of monthly bolus dose vitamin D supplementation on 25-hydroxyvitamin D concentrations? J Steroid Biochem Mol Biol 2020;201:105687. [DOI] [PubMed] [Google Scholar]
- 22.Griffin G, Hewison M, Hopkin J, et al. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin Med 2021;21:e48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys 2012;523:9–18. [DOI] [PubMed] [Google Scholar]
- 24.Kundu R, Chain BM, Coussens AK, Khoo B, Noursadeghi M. Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur J Immunol 2014;44:1781–90. [DOI] [PubMed] [Google Scholar]
- 25.Adams JS, Chen H, Chun R, et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J Bone Miner Res 2007;22:V20–4. [DOI] [PubMed] [Google Scholar]
- 26.Ketha H, Thacher TD, Oberhelman SS, et al. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3ratio. Bone 2018;110:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zittermann A, Berthold HK, Pilz S. The effect of vitamin D on fibroblast growth factor 23: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 2020. 10.1038/s41430-020-00725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florenzano P, Hartley IR, Jimenez M, et al. Tumor-induced osteomalacia. Calcif Tissue Int 2021;108:128–42. [DOI] [PubMed] [Google Scholar]
- 29.Turner C, Dalton N, Inaoui R, et al. Effect of a 300 000-IU loading dose of ergocalciferol (Vitamin D2) on circulating 1,25(OH)2-vitamin D and fibroblast growth factor-23 (FGF-23) in vitamin D insufficiency. J Clin Endocrinol Metab 2013;98:550–6. [DOI] [PubMed] [Google Scholar]
- 30.Bacchetta J, Sea JL, Chun RF, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 2013;28:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 2016;27:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petkovich M, Melnick J, White J, et al. Modified-release oral calcifediol corrects vitamin D insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol 2015;148:283–9. [DOI] [PubMed] [Google Scholar]
- 33.Amrein K, Quraishi SA, Litonjua AA, et al. Evidence for a U-shaped relationship between prehospital vitamin D status and mortality: a cohort study. J Clin Endocrinol Metab 2014;99:1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan X, Wang J, Song M, et al. Vitamin D status and risk of all-cause and cause-specific mortality in a large cohort: results from the UK Biobank. J. Clin. Endocrinol. Metab 2020;105:e3606–19. [DOI] [PubMed] [Google Scholar]
- 35.Boucher BJ. Why do so many trials of vitamin D supplementation fail? Endocr Connect 2020;9:R195–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med 2021;289:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo ME, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int 2018;29:1697–1711. [DOI] [PubMed] [Google Scholar]
- 39.Murai IH, Fernandes AL, Sales LP, et al. Effect of vitamin D3 supplementation vs placebo on hospital length of stay in patients with severe COVID-19: A multicenter, double-blind, randomized controlled trial. medRxiv 2020.11.16.20232397. [Google Scholar]
- 40.Peng L, Li L, Wang P, Chong W, et al. Association between Vitamin D supplementation and mortality in critically ill patients: A systematic review and meta-analysis of randomized clinical trials. PLoS One 2020;15:e0243768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amrein K, Parekh D, Westphal S, et al. Effect of high-dose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study). BMJ Open 2019;9:e031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin G, Hewison M, Hopkin J, et al. Rapid Response: re: vitamin D and COVID-19 – this is no time for procrastination. BMJ 22 December 2020; www.bmj.com/content/371/bmj.m4912/rapid-responses. [Google Scholar]