ABSTRACT
Iron deficiency anaemia (IDA) currently affects 1.2 billion people and iron deficiency without anaemia (IDWA) is at least twice as common. IDWA is poorly recognised by clinicians despite its high prevalence, probably because of suboptimal screening recommendations. Diagnosing IDWA relies on a combination of tests, including haemoglobin and ferritin levels, as well as transferrin saturation. Although the causes of iron deficiency may sometimes be obvious, many tend to be overlooked. Iron sufficiency throughout pregnancy is necessary for maternal and foetal health. Preoperative IDWA must be corrected to reduce the risk of transfusion and postoperative anaemia. Oral iron is the first-line treatment for managing IDWA; however, intravenous supplementation should be used in chronic inflammatory conditions and when oral therapy is poorly tolerated or ineffective. This review considers the causes and clinical features of IDWA, calls for greater awareness of the condition, and proposes diagnostic and management algorithms.
KEYWORDS: anaemia, iron deficiency, preoperative, pregnancy, transfusion
Introduction
Iron deficiency (ID) is the most prevalent nutritional deficiency and a major precipitant of anaemia. According to a major international study, nearly 1.2 billion people suffer from iron deficiency anaemia (IDA) and iron deficiency without anaemia (IDWA) is estimated to be at least twice as common.1,2 IDA is the most frequent presentation of ID; hence, there is an ongoing misconception that the two terms are synonymous. ID is a broader term and refers to low iron stores that do not meet the body's iron requirements, regardless of whether anaemia is present or not.2 Although ID decreases haemoglobin synthesis, it is only classed as anaemia once haemoglobin levels fall below certain cut-off values. The World Health Organization (WHO) has set these at 130 g/L in males, 120 g/L in non-pregnant females and 110g/L in pregnant females.3 Nevertheless, symptoms of anaemia such as fatigue can be present without anaemic haemoglobin levels.4 Recognising IDWA as a clinical diagnosis is crucial to ensuring adequate management, especially for patients with chronic conditions such as heart failure (HF) where IDWA can increase long-term mortality.5
Diagnostic definition of iron deficiency
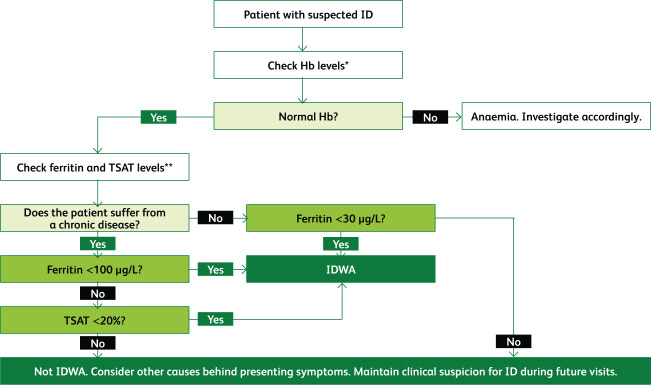
Ferritin is an indicator of iron stores and is the most sensitive and specific biomarker for assessing ID. The WHO defines low ferritin as levels <15 μg/L for adults and <12 μg/L for children.6 However, in clinical practice, when ferritin levels dip below 30 μg/L, ID can be ascertained.7 Ferritin is an acute-phase reactant that is increased in serum during chronic inflammation. Cut-off values for ferritin in ID are increased to 100 μg/L in states of chronic inflammation. Transferrin saturation (TSAT) levels below 20% are also diagnostic of ID. In chronic inflammatory conditions when ferritin levels are 100–300 μg/L, TSAT should be used to diagnose ID.6,8 Serum iron levels fluctuate throughout the day and should not be used for diagnosis.9 Fig 1 proposes an algorithm for diagnosing ID based on the current literature.
Fig 1.
An algorithm for the diagnosis of iron deficiency based on the best current evidence. This represents how tests are interpreted and not the order of requesting them.3,8,10 Hb = haemoglobin; ID = iron deficiency; IDWA = iron deficiency without anaemia; TSAT = transferrin saturation. *Normal levels of haemoglobin are ≥130 g/L for males, ≥120 g/L for females and ≥110 g/L for a pregnant female. **Ferritin levels below 30 μg/L indicate ID; in chronic inflammatory conditions ferritin levels may be elevated and so the threshold is raised to 100 μg/L. Ferritin levels can be raised to 100–300 μg/L in chronic inflammation; in such cases TSAT levels must be used. Normal ranges can slightly vary according to the laboratory.
Other useful tests include hepcidin, soluble transferrin receptor (sTFR) and reticulocyte haemoglobin content (RHC); however, they are not widely used. Although hepcidin is usually low or normal in absolute ID (AID), it helps distinguish AID from functional ID (FID).10 The sTFR is a valuable indicator of ID as, unlike ferritin, it is unaffected by inflammation. Unfortunately, performing this test takes too long and it is not widely available. When haemoglobin levels are normal, a low RHC indicates early ID in functional stores and hints at iron need, pre-anaemia and a risk of developing IDA.11
The distinction between IDA and IDWA relies on the use of strict haemoglobin cut-offs. However, clinicians should consider the fact that normal haemoglobin ranges have been set using population data. Essentially, what may be a normal haemoglobin level for one person may be abnormal for another, especially if a patient has a haemoglobin level in the low normal range but their usual haemoglobin levels are higher. Patients suffering from ID should be treated regardless of whether they are explicitly defined as anaemic. Cut-off haemoglobin ranges are useful, but their limitations should be kept in mind and patients should be assessed on a case-by-case basis.
Causes of iron deficiency
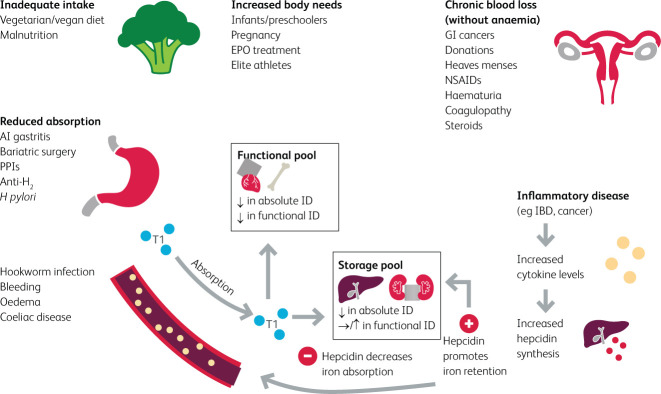
Iron has both a storage pool and a functional pool. The storage pool is the reticuloendothelial system which consists of the liver, spleen and lymph nodes. The functional pool consists of red blood cells, bone marrow and cardiac and skeletal muscle. Iron is absorbed in the duodenum via specific transporters and is carried by transferrin molecules to the storage and functional pools. Iron deficiency can be absolute or functional. AID is when the storage pool is iron-deficient due to reduced intake, increased needs, reduced absorption or excessive loss. AID also causes low iron levels within the functional pool. In FID the burden is the chronic inflammation, causing cytokine and hepcidin release. Hepcidin causes iron deficiency via the blockage of an iron exporter known as ferroportin. There are two ways in which this blockage causes ID. First, it reduces iron absorption in the duodenum; second, it causes iron retention within the storage pools. This means that despite normal iron levels within the storage pools, functional pools are iron deficient and cannot utilise the stored iron for vital body processes.2,10,12
Causes of iron deficiency can be grouped into the following categories: inadequate dietary intake, increased body needs, reduced absorption, chronic inflammation and chronic blood loss.
Inadequate intake can result from iron-deficient diets, such as the increasingly popular vegan diets, or having higher iron requirements, as seen in growing children and pregnant women.2,13,14 Athletes and those performing in demanding sports have increased iron needs and are at a higher risk of developing ID, mainly due to chronic inflammation and increased losses. Hepcidin levels are elevated in chronic inflammation, resulting in blockage of the only known iron exporter, ferroportin. Additionally, athletes have greater iron losses through urine and sweat during vigorous activity.15–17
Iron absorption occurs mainly in the proximal small intestine. The presence of sufficient gastric acid is required for the reduction of Fe3+ to Fe2+, which is more readily absorbed.18 Bariatric surgery patients are highly susceptible to ID due to a decreased absorptive surface area and/or reduced gastric acid secretion.2,18 A lower postoperative intake of dietary iron further increases the risk of ID.13 Less commonly, Helicobacter pylori infection may cause ID due to reduced iron absorption and blood loss.2 Patients with autoimmune gastritis also incur a loss of gastric acid secretion, thus failing to absorb iron effectively.19 Moreover, chronic use of proton pump inhibitors or histamine-2 receptor antagonists can increase the risk of ID via a similar mechanism.2 The consumption of coffee, tea or calcium (in supplements or dairy products) has been reported to reduce iron absorption.15,20 The significance of these dietary components as causes of ID is sometimes overlooked.
Chronic inflammation, such as in coeliac disease, inflammatory bowel disease (IBD) and HF, increases hepcidin production, blocking iron transporters and reducing absorption, and causes iron entrapment within storage pools. This ultimately results in FID.8,10,13
ID may also result from major or chronic occult blood losses. This is common in women with menorrhagia, and is further amplified in obesity and during rapid growth in adolescence, which may deplete iron stores.13,21,22 ID is also common in frequent blood donors and pregnant women.13 Other causes include nosebleeds, GI bleeds (eg angiodysplasia), surgical procedures, injuries, accidents, and the use of intrauterine devices, anticoagulants or antiplatelets.2,12,13,23–25 Fig 2 summarises the causes of ID and provides a clinically relevant background of the pathophysiology at play.
Fig 2.
Causes of iron deficiency.2,10,25 AI gastritis = autoimmune gastritis; anti H2 = anti histamine-2 receptor (H2 receptor antagonist); EPO = erythropoietin; GI cancers = gastrointestinal cancers; H pylori = Helicobacter pylori; IBD = inflammatory bowel disease; NSAIDs = non-steroidal anti-inflammatory drugs; PPIs = proton pump inhibitors; Tf = transferrin.
Clinical features
It is well established that iron plays an indispensable role in haemoglobin and myoglobin synthesis. Less appreciated, however, is its role in mitochondrial functioning, including the synthesis of cofactors and enzymes necessary for cellular respiration.26,27 Consequently, highly metabolic cells such as cardiomyocytes and skeletal muscle cells are dependent on iron for optimum functioning.10 A randomised controlled trial (RCT) by Melenovsky et al showed that ID reduces aerobic respiration and citric acid cycle enzyme activity in advanced HF.28 Similarly, several studies have demonstrated the negative impact of ID on cellular metabolism.28–31 A recent RCT of 40 chronic HF patients with ID demonstrated enhanced skeletal muscle energetics following iron supplementation.31 Although many cellular processes depend on iron, it is likely that they are only impacted in severe ID, where anaemia would likely be present. More research is needed to further clarify the effects of IDWA on cellular processes and how such effects would relate to the clinical presentation.
Symptoms of ID such as fatigue and exercise intolerance are nonspecific, making it difficult to identify whether ID is the culprit or a chronic disease such as HF, which may present with similar symptoms.32 Other causes such as hypothyroidism, depression and burnout may also be responsible. Additionally, it is difficult to distinguish IDWA from IDA based solely on symptoms given their overlap; the main clinical difference is that symptoms are more severe in IDA. A recent systematic review concluded that iron supplementation in IDWA improves subjective measures of fatigue.4 In IDWA patients with coexisting HF or IBD, intravenous (IV) supplementation improves symptom control and quality of life, respectively.32,33 However, evidence on the effect of iron supplementation on physical activity, often assessed by maximal oxygen consumption tests (VO2 max), is mixed.15,34
Severe ID may also cause cardiac and skeletal myopathy, which is detrimental in HF.35 This is due to impaired clearance of reactive oxygen species, increasing oxidative stress and thereby weakening cardiac muscle. Ultimately, the atrophy of cardiac, peripheral, and respiratory muscles leads to reduced exercise tolerance and dyspnoea on exertion.36 In HF, mitochondrial function is already impaired, and the superimposed effect of ID can be deleterious.37 The clinical features of IDWA are summarised in Fig 3.
Fig 3.
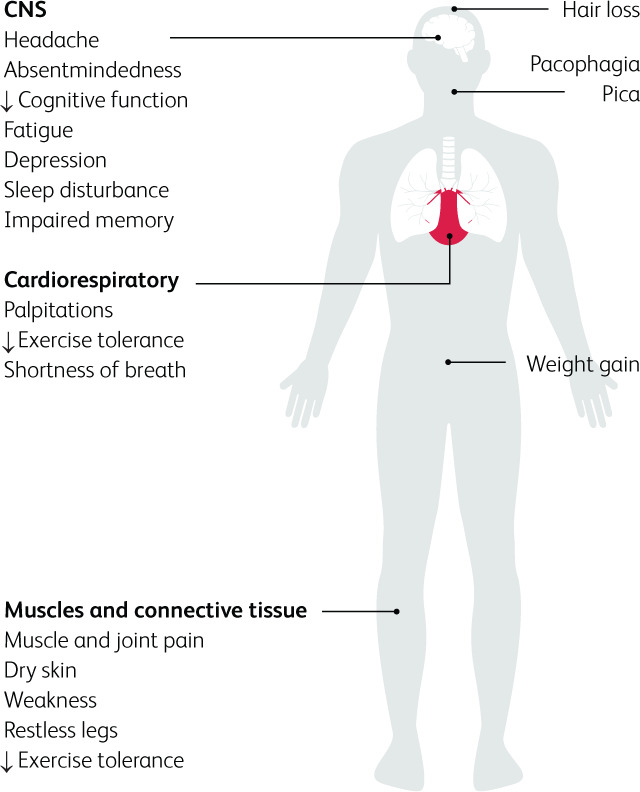
Effects of iron deficiency on the human body.13
Iron deficiency in pregnancy
IDA increases maternal morbidity and the risk of poor pregnancy outcomes, including intrauterine growth restriction, prematurity and low birth weight.38 Compared to non-iron-deficient women, gravidas with depleted iron stores or IDWA at the start of pregnancy are more likely to develop pre- and postnatal ID and have a newborn with a lower birth weight.39 Anaemia is a late manifestation of ID. During foetal growth and when iron is scarce, iron is directed primarily to erythropoietic tissues over the rest of the body. Therefore, ID may exist in other organs such as the brain, despite normal haemoglobin levels. ID has been associated with mental illness and impaired neurocognitive functions, such as poor memory and slower neural processing, which may be due to ID irrespective of anaemia.40 Postnatal ID is linked to neonatal iron status and foetal iron loading, and is associated with permanent cognitive and behavioural effects that are measurable until up to 19 years of age, even with postnatal iron repletion.41 Iron sufficiency is vital throughout the entire pregnancy. It is especially crucial from week 32 of gestation when rapid myelination of the brain begins and throughout infancy.42 Consequently, mothers should be screened and treated for ID before conception. Iron can be replaced throughout pregnancy using oral iron every other day in the first trimester to improve maternal absorption. If ID persists, then IV iron is safe to use in the second and third trimesters. Furthermore, newborns should be screened and treated for ID after birth to avoid permanent neurocognitive damage.38
Preoperative iron deficiency
Unlike preoperative IDA, the effects of preoperative IDWA on surgical outcomes have not received sufficient attention. Patient blood management aims to detect iron disturbances ahead of surgery, thereby reducing transfusions and post-operative complications.43 Nevertheless, recommendations often understate the importance of preoperative IDWA.
Preoperative IDWA in abdominal or cardiac surgery increases the risk of postoperative infection, fatigue, transfusion and anaemia.44 A recent RCT of 252 IDWA patients found that providing a short term combination therapy of IV iron, erythropoietin alpha, vitamin B12 and folic acid reduced blood transfusions in patients with preoperative IDWA undergoing elective cardiac surgery.45 Furthermore, pre-operative supplementation of non-anaemic patients undergoing orthopaedic surgery with oral iron, vitamin C, and folic acid reduced blood transfusions.46 The British Committee for Standards in Haematology recommends iron supplementation in patients with IDWA (ferritin <100 μg/L and TSAT <20%) who are planned to undergo surgery with a predicted haemoglobin loss of >30 g/L.47
An international consensus statement on perioperative ID and anaemia emphasised the importance of screening for and managing IDWA prior to surgery. Elective surgery with significant expected blood loss should be postponed until ID and/or anaemia is corrected to reduce the risk of postoperative anaemia. The recommendation is that oral iron is used when surgery is more than 6 weeks away; otherwise intravenous supplementation is best.48 Collaboration between primary and secondary care practitioners is vital to effective management of preoperative IDWA. Ideally, testing should occur in primary care when a referral is first made to avoid delays to surgery.47,48
In the future, raising awareness of IDWA among clinicians, especially in primary care, could reduce the prevalence of undiagnosed IDWA. This would minimise the risk of preoperative IDWA and IDA and improve patient blood management.
Directions for management
IDWA should be treated when identified, with a target ferritin of 100 mg/L.7 Treatment should be continued until ferritin levels have normalised and symptoms have resolved. Patients should be offered dietary advice and oral iron replacement.15 IV replacement should be considered for symptomatic patients with treatment-resistant IDWA. Furthermore, ferritin levels should be checked every 6–12 months following treatment, especially in heavily menstruating women and those considering pregnancy.13
Diet is important in the management of IDWA. Patients should aim to consume meat, poultry, or fish at least five times a week, with complementary wholemeal products, legumes, and vegetables.15 They may also be referred to a dietician for detailed assessment and advice. Nevertheless, dietary supplementation alone may not be enough to correct the deficiency, thus necessitating medicinal replacement.49
Oral iron is associated with gastrointestinal side effects such as constipation, diarrhoea, dyspepsia, and nausea, which have been associated with poor adherence.50 Using single doses on alternate days as opposed to multiple doses on consecutive days has been shown to result in higher absorption and better regulation of hepcidin levels in iron-depleted women.51 The recommended oral dose is 28–50 mg iron daily or 100 mg on alternate days for 25 days.15,49,52 Ideally, patients should be contacted 1 week following the start of treatment to assess drug tolerance, with changes to formulation or dose agreed if necessary.49 Patients’ haemoglobin, ferritin, and CRP levels in addition to red blood cell indices should be checked 6–8 weeks following the start of treatment to assess treatment response.15 If oral supplementation is inadequate, patients should be considered for IV replacement and referral to a specialist. Once ferritin levels have corrected, patients should be followed up with blood tests every 6–12 months, with replacement reintroduced if necessary.15
Where oral iron is ineffective or poorly tolerated, patients should be offered IV supplementation.53 It should also be used in IDWA to circumvent diminished absorption following gastric bypass, in IBD where the intestinal mucosa is damaged, and also in other chronic inflammatory conditions, including HF, where hepcidin is elevated.53–55 IV iron has proven to be effective and well-tolerated in IDWA, and may be more efficacious than oral preparations.52,56 Around 1% of patients experience minor infusion reactions, which should prompt cessation of infusion and management of symptoms (see Gómez-Ramírez et al for detailed guidance).57 Severe reactions have traditionally been overstated due to the risks associated with high molecular weight iron dextran (HMWID), which has now been replaced by substantially safer formulations.58–60 In the UK, these are low molecular weight dextran, iron sucrose, ferric carboxymaltose and iron isomaltoside (known in the US as ferric derisomaltose).61 Ferumoxytol, which was withdrawn from European markets in 2015, continues to be available in the USA, and has been shown to be just as safe.58,62 Excluding HMWID, the risk of severe reactions is very low (1:250,000 administrations).57,63 Nevertheless, it is essential to administer IV iron in a setting where such adverse effects can be adequately managed.7 As with oral iron, follow up is essential.44 The suggested management pathway for IDWA is summarised in Fig 4.
Fig 4.
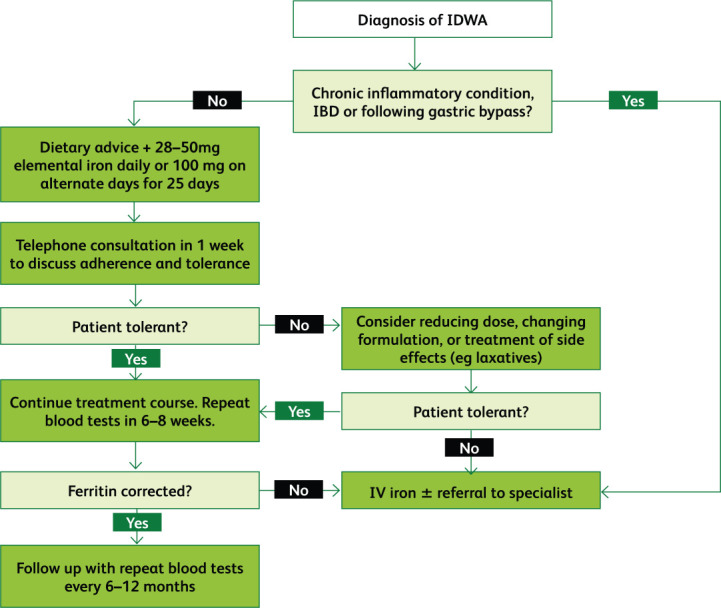
Flowchart summarising the management of iron deficiency without anaemia.15,49,52–55,58
Conclusion
Despite the simplicity of pathogenesis and treatment, IDWA often falls outside the scope of clinical suspicion. It is important to not only spread awareness about the condition in the medical community, but to also develop guidelines and pathways to assist clinicians. Doing so requires greater collaboration between specialists and general practitioners to identify and treat IDWA, especially preoperatively and in the context of pregnancy. In this paper, we have attempted to develop a concise guide for clinicians. Nonetheless, further research is needed to identify the best diagnostic serum markers, treatment targets, and optimal oral iron dosing regimens for IDWA.
Key points
Within the body, iron has roles other than haemoglobin synthesis.
Iron deficiency (ID) and anaemia are not synonymous; patients with ID can present with symptoms without having anaemia. Therefore iron deficiency without anaemia (IDWA) must be recognised as a clinical diagnosis on its own.
Haemoglobin ranges are based on averages and should not be used as strict cut-offs. Haemoglobin levels in the low normal range may not be normal for some people as they may be accustomed to higher levels.
Diagnosis currently depends on haemoglobin levels, ferritin levels and transferrin saturation. However, further research into the use of newer biomarkers is needed.
Many causes of ID are overlooked and clinicians need to maintain high clinical suspicion.
Females with IDWA must be treated before the start of their pregnancy to avoid the risks of perinatal and postnatal ID or IDA. These include permanent neurocognitive effects on children and having a child with a low birth weight.
ID with or without anaemia should be ruled out in the preoperative setting as failure to do so may have a negative effect on patient outcomes.
Preoperative IDWA must be studied in more depth, as the literature on this topic is sparse. There is a need for more universal pre-operative screening and treatment.
IDWA should be treated when identified. Specific guidelines on whom to treat and how to treat must be developed.
Oral iron is the first line treatment for IDWA.
Newer formulations of IV iron are much safer than traditionally thought, and should be used where oral iron is poorly tolerated or ineffective.
Conflicts of interest
Jecko Thachil has received honoraria from Norgine pharmaceuticals.
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camaschella C. Iron deficiency. Blood 2019;133:30–9. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. WHO, 2011. Available from www.who.int/vmnis/indicators/haemoglobin/en/ [Accessed 6 February 2020].
- 4.Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open 2018;8:e019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote Beverborg N, Klip IjT, Meijers WC, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018;11:e004519. [DOI] [PubMed] [Google Scholar]
- 6.Daru J, Allotey J, Peña-Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review: Serum ferritin for defining iron deficiency in pregnancy. Transfusion Med 2017;27:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soppi ET. Iron deficiency without anemia – a clinical challenge. Clin Case Rep 2018;6:1082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappellini MD, Comin-Colet J, de Francisco A, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol 2017;92:1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Solal A, Leclercq C, Mebazaa A, et al. Diagnosis and treatment of iron deficiency in patients with heart failure: Expert position paper from French cardiologists. Arch Cardiovasc Dis 2014;107:563–71. [DOI] [PubMed] [Google Scholar]
- 10.Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018;138:80–98. [DOI] [PubMed] [Google Scholar]
- 11.Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr 2017:1606S–1614S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolaou M, Chrysohoou C, Georgilas TA, et al. Management of iron deficiency in chronic heart failure: Practical considerations for clinical use and future directions. Eur J Int Med 2019;65:17–25. [DOI] [PubMed] [Google Scholar]
- 13.Soppi E. Iron deficiency without anemia – common, important, neglected. Clin Case Rep 2019;5:2–7. [Google Scholar]
- 14.Haider LM, Schwingshackl L, Hoffmann G, Ekmekcioglu C. The effect of vegetarian diets on iron status in adults: A systematic review and meta-analysis. Crit Rev Food Sci Nutr 2018;58:1359–74. [DOI] [PubMed] [Google Scholar]
- 15.Clénin G. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med Wkly 2017;147:w14434. [DOI] [PubMed] [Google Scholar]
- 16.Suedekum NA, Dimeff RJ. Iron and the athlete. Curr Sports Med Rep 2005;4:199–202. [DOI] [PubMed] [Google Scholar]
- 17.Clénin G, Cordes M, Huber A, et al. Iron deficiency in sports – definition, influence on performance and therapy. Swiss Med Wkly 2015;145:w14196. [DOI] [PubMed] [Google Scholar]
- 18.Steenackers N, Van der Schueren B, Mertens A, et al. Iron deficiency after bariatric surgery: what is the real problem? Proc Nutr Soc 2018;77:445–55. [DOI] [PubMed] [Google Scholar]
- 19.Kulnigg-Dabsch S. Autoimmune gastritis. Wien Med Wochenschr 2016;166:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lönnerdal B. Calcium and iron absorption - mechanisms and public health relevance. Int J Vitam Nutr Res 2010;80:293–9. [DOI] [PubMed] [Google Scholar]
- 21.Johnson S, Lang A, Sturm M, O'Brien SH. Iron deficiency without anemia: a common yet under-recognized diagnosis in young women with heavy menstrual bleeding. J Pediatr Adolesc Gynecol 2016;29:628–31. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson WS. Iron deficiency in adolescence. J Paediatr 2017;187:2. [DOI] [PubMed] [Google Scholar]
- 23.Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med 2013;3:a011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemingway AB. Angiodysplasia as a cause of iron deficiency anaemia. Blood Rev 1989;3:147–51. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370:511–20. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein TP, Kirk SR, Meyer SC, Holman KLM. Myoglobin structure and function: A multiweek biochemistry laboratory project. Biochem Mol Biol Educ 2015;43:181–8. [DOI] [PubMed] [Google Scholar]
- 27.Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001;131:676S–690S. [DOI] [PubMed] [Google Scholar]
- 28.Melenovsky V, Petrak J, Mracek T, et al. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail 2017;19:522–30. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Reports 2015;13:533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoes MF, Grote Beverborg N, Kijlstra JD, et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018;20:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charles-Edwards G, Amaral N, Sleigh A, et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency: FERRIC-HF II randomized mechanistic trial. Circulation 2019;139:2386–98. [DOI] [PubMed] [Google Scholar]
- 32.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. J Am Coll Cardiol 2008;51:103–12. [DOI] [PubMed] [Google Scholar]
- 33.Çekiç C, ipek S, Aslan F, et al. The effect of intravenous iron treatment on quality of life in inflammatory bowel disease patients with nonanemic iron deficiency. Gastroenterol Res Pract 2015;2015:582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burden RJ, Morton K, Richards T, et al. Is iron treatment beneficial in, iron-deficient but non-anaemic (IDNA) endurance athletes? A systematic review and meta-analysis. Br J Sports Med 2015;49:1389–97. [DOI] [PubMed] [Google Scholar]
- 35.Stugiewicz M, Tkaczyszyn M, Kasztura M, et al. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications: Iron deficiency and skeletal muscles. Eur J Heart Fail 2016;18:762–73. [DOI] [PubMed] [Google Scholar]
- 36.van der Meer P, van der Wal HH, Melenovsky V. Mitochondrial function, skeletal muscle metabolism, and iron deficiency in heart failure. Circulation 2019;139:2399–402. [DOI] [PubMed] [Google Scholar]
- 37.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest 2018;128:3716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology 2019;115:269–74. [DOI] [PubMed] [Google Scholar]
- 39.Ribot B, Aranda N, Viteri F, et al. Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birthweight even when supplemented daily with moderate iron. Hum Reprod 2012;27:1260–6. [DOI] [PubMed] [Google Scholar]
- 40.Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol 2020;223:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 2006;160:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastian TW, von Hohenberg WC, Mickelson DJ, et al. Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism, and dendrite complexity. Dev Neurosci 2016;38:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franchini M, Marano G, Veropalumbo E, et al. Patient blood management: a revolutionary approach to transfusion medicine. Blood Transfusion [Internet]. 2019 Jun 19 [cited 2020 Sep 16]; Available from: 10.2450/2019.0109-19. [DOI] [PMC free article] [PubMed]
- 44.Muñoz M, Gómez-Ramírez S, Besser M, Pavía J, Gomollón F, Liumbruno GM, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus 2019;17:191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019;393:2201–12. [DOI] [PubMed] [Google Scholar]
- 46.Cuenca J, García-Erce JA, Martínez F, et al. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg 2007;5:89–94. [DOI] [PubMed] [Google Scholar]
- 47.Kotzé A, Harris A, Baker C, et al. British Committee for standards in haematology guidelines on the identification and management of pre-operative anaemia. Br J Haematol 2015;171:322–31. [DOI] [PubMed] [Google Scholar]
- 48.Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233–47. [DOI] [PubMed] [Google Scholar]
- 49.Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med 2008;121:943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baird-Gunning J, Bromley J. Correcting iron deficiency. Aust Prescr 2016;39:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol 2017;4:e524–33. [DOI] [PubMed] [Google Scholar]
- 52.Muñoz M, Gómez-Ramírez S, Bhandari S. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf 2018;17:149–59. [DOI] [PubMed] [Google Scholar]
- 53.Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematology Am Soc Hematol Educ Program 2016;2016:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Haehling S, Ebner N, Evertz R, et al. Iron deficiency in heart failure. JACC Heart Fail 2019;7:36–46. [DOI] [PubMed] [Google Scholar]
- 55.Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol 2015;6:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krayenbuehl P-A, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011;118:3222–7. [DOI] [PubMed] [Google Scholar]
- 57.Gómez-Ramírez S, Shander A, Spahn DR, et al. Prevention and management of acute reactions to intravenous iron in surgical patients. Blood Transfus 2019;17:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auerbach M, Macdougall I. The available intravenous iron formulations: History, efficacy, and toxicology: The available intravenous iron formulations. Hemodial Int 2017;21:S83–92. [DOI] [PubMed] [Google Scholar]
- 59.Auerbach M, Adamson J, Bircher A, et al. On the safety of intravenous iron, evidence trumps conjecture. Haematologica 2015;100:e214–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szebeni J, Fishbane S, Hedenus M, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management: Hypersensitivity reactions to intravenous iron. B J Pharmacol 2015;172:5025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joint Formulary Committee . British National Formulary. https://about.medicinescomplete.com/publication/british-national-formulary/.
- 62.Abdulrehman J, Tang GH, Auerbach M, et al. The safety and efficacy of ferumoxytol in the treatment of iron deficiency: a systematic review and meta-analysis. Transfusion 2019;59:3646–56. [DOI] [PubMed] [Google Scholar]
- 63.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplantation 2006;21:378–82. [DOI] [PubMed] [Google Scholar]