Abstract
The effectiveness of somatic cell nuclear transfer (SCNT) in mammals seems to be still characterized by the disappointingly low rates of cloned embryos, fetuses, and progeny generated. These rates are measured in relation to the numbers of nuclear-transferred oocytes and can vary depending on the technique applied to the reconstruction of enucleated oocytes. The SCNT efficiency is also largely affected by the capability of donor nuclei to be epigenetically reprogrammed in a cytoplasm of reconstructed oocytes. The epigenetic reprogrammability of donor nuclei in SCNT-derived embryos appears to be biased, to a great extent, by the extranuclear (cytoplasmic) inheritance of mitochondrial DNA (mtDNA) fractions originating from donor cells. A high frequency of mtDNA heteroplasmy occurrence can lead to disturbances in the intergenomic crosstalk between mitochondrial and nuclear compartments during the early embryogenesis of SCNT-derived embryos. These disturbances can give rise to incorrect and incomplete epigenetic reprogramming of donor nuclei in mammalian cloned embryos. The dwindling reprogrammability of donor nuclei in the blastomeres of SCNT-derived embryos can also be impacted by impaired epigenetic rearrangements within terminal ends of donor cell-descended chromosomes (i.e., telomeres). Therefore, dysfunctions in epigenetic reprogramming of donor nuclei can contribute to the enhanced attrition of telomeres. This accelerates the processes of epigenomic aging and replicative senescence in the cells forming various tissues and organs of cloned fetuses and progeny. For all the above-mentioned reasons, the current paper aims to overview the state of the art in not only molecular mechanisms underlying intergenomic communication between nuclear and mtDNA molecules in cloned embryos but also intrinsic determinants affecting unfaithful epigenetic reprogrammability of telomeres. The latter is related to their abrasion within somatic cell-inherited chromosomes.
Keywords: cloned mammalian embryo, SCNT-derived progeny, mtDNA, nuclear–mitochondrial interaction, epigenetic reprogrammability, telomere shortening/attrition
1. Biotechnological Possibilities of Applying the Techniques of Somatic Cell Nuclear Transfer (SCNT) to Produce Cloned Mammalian Species
The somatic cell cloning technique is a method of embryonic genome engineering. Unlike animal transgenesis, it involves micromanipulation not of individual nuclear DNA genes but of the whole nuclear and/or mitochondrial genome of both interphase nuclear donor somatic cells and female germ cells (in vitro- or in vivo-matured oocytes arrested at metaphase II), which are used as recipients of exogenous genetic material. Of all mammalian cloning techniques, somatic cell cloning can result in producing the largest numbers of genetically identical individuals that are designated as clones. In the somatic cell cloning of mammals, nuclear donor cells are available in practically unlimited quantities. Tissue samples obtained by biopsy from adult animals or fetuses are composed of hundreds of thousands cells, which can be further multiplied/expanded in vitro. Furthermore, when cloning certain adult animals, tissue may be biopsied repeatedly to produce identical clones every time [1,2,3,4,5,6,7].
Animal cloning by somatic cell nuclear transfer (SCNT), which avoids the sexual reproduction pathway, offers the opportunity to obtain monogenetic offspring derived not only from adult animals of high genetic merit but also from genetically transformed (transgenic) specimens. Over the last 24 years, intra- and interspecies cloning via SCNT resulted in a fairly large number of transgenic and non-transgenic offspring, not only in various species or infertile interspecific hybrids (bastards) of domesticated animals, such as
- (1)
- (2)
- (3)
- (4)
- (5)
-
(6)
water buffaloes—Chinese swamp buffaloes [48,49] and Indian river/riverine buffaloes [50,51,52,53];
- (7)
-
(8)
two-humped or Bactrian camels [57];
- (9)
- (10)
-
(11)
polecat-ferrets [72];
- (12)
- (13)
-
(14)
rats [85]; but also in several species of endangered or non-endangered wild mammals, such as
- (15)
-
(16)
mouflon [88];
-
(17)
European red deer [89];
-
(18)
African wild cat [90];
-
(19)
Arabian sand cat [91];
- (20)
-
(21)
coyote or prairie wolf [94];
-
(22)
cynomolgus monkey, also known as Java macaque, crab-eating macaque, or long-tailed macaque—a catarrhine monkey from the family Cercopithecidae [95]; and even in the extinct subspecies of the Spanish/Iberian ibex:
-
(23)
Pyrenean ibex, a wild goat known as bucardo [96].
Explanation of the mechanisms underlying intergenomic communication between nuclear and mitochondrial DNA molecules in cloned embryos and recognition/identification of the determinants affecting aberrant epigenetic reprogrammability of chromosomal telomeres will be suitable and reliable for resolving or reducing the imperfections in the generation of cloned embryos, conceptuses, and offspring by using SCNT technology. Moreover, the development of efficient strategies applied to the cryopreservation of nuclear donor somatic cells, nuclear-transferred oocytes reconstructed with somatic cells, and somatic cell-cloned embryos seems to be an inevitable progressive step contributing to the expedition of future large-scale attempts aimed to more successfully produce and multiply mammalian cloned offspring. The latter seems to be a sine qua non condition that allows one to more efficiently use SCNT-based assisted reproductive technology not only for transgenic, biotechnological, biomedical, and biopharmaceutical research but also for the ex situ conservation of biodiversity in both anthropogenic (agricultural) and non-anthropogenic (wild) ecosystems.
2. Dependence of Epigenetic Mechanisms Underlying Somatic Cell Nuclear Reprogramming and Intergenomic Communication between Nuclear and Mitochondrial DNA Fractions in Cloned Embryos on Various Approaches to Reconstruction of Enucleated Oocytes
In the reconstruction of enucleated oocytes (cytoplasts/ooplasts) by SCNT, the original genetic material is replaced with the somatic cell-inherited nuclear genome. Different approaches to SCNT are used to generate nuclear-transferred oocytes, i.e., oocytes reconstructed with somatic cell nuclei and the resultant cloned embryos (Table 1). The most common procedure is a relatively low-invasive method of SCNT based on the fusion of cytoplast–nuclear donor cell couplets that is induced by electric pulses [34,97,98,99,100,101] (Table 1). An alternative reconstruction method is a much more invasive microsurgical procedure, in which whole nuclear donor cells [102,103] or somatic-cell-derived karyoplasts [29,44,82,104,105] are microinjected directly into the cytoplasm of enucleated oocytes (Table 1). The karyoplast is a live membrane-bound structure formed as a result of mechanically induced lysis of the whole somatic cell. It contains the interphase cell nucleus or metaphase chromosomes that are surrounded only by a thin layer of the perinuclear cytoplasm (the so-called perikaryon) [27,105,106,107,108].
Table 1.
Comparative characterization of the approaches to reconstruction of enucleated mammalian oocytes at the biotechnical, cytological, molecular, and epigenetic levels.
| Method Used for Reconstruction of Enucleated Metaphase II-Stage Oocytes | Characterization at the Biotechnical and Cytological Levels |
Characterization at the Molecular Level |
Characterization at the Epigenetic Level |
|---|---|---|---|
| Electrofusion of ooplast–somatic cell couplets | Relatively low invasiveness of the method: - the generated electrostatic field interferes with ultrastructure and functions of the oolemma of nuclear recipient cells and the plasmalemma of nuclear donor cells through: • transient formation in the plasma membrane phospholipid bilayer of micropores (microchannels) that facilitate fusion of ooplast–somatic cell complexes and are the pathway for passive intracellular transport of calcium ions under the conditions of simultaneous fusion and electrical activation (F/A) of reconstituted oocytes |
Relatively high probability of the occurrence in the obtained clonal cybrids of: - cellular mtDNA heteroplasmy; - abnormal nuclear–cytoplasmic interactions; - abnormal intergenomic communication between allogeneic nuclear DNA, somatic cell-inherited mtDNA molecules, and mtDNA molecules of ooplasmic origin |
Relatively high probability of the occurrence of abnormalities in: - structural and epigenetic remodeling of nuclear chromatin; - epigenetic reprogramming of transcriptional activity of the nuclear genome, including rearrangement of telomeres of somatic cell chromosomes in the reconstructed oocytes and in cloned embryos that develop as a result of their activation |
|
Direct intraooplasmic
microinjection of whole somatic cells |
High invasiveness of the method: - interferes with the ultrastructure of the plasmalemma and the membrane and cytoskeleton of enucleated oocytes through: • direct microsurgical transfer and deposition in their ooplasm of tiny (small-diameter) somatic cells displaying intact integrity of the plasma membrane |
Relatively high probability of the occurrence in the obtained clonal cybrids of: - cellular mtDNA heteroplasmy; - abnormal nuclear–cytoplasmic interactions; - abnormal intergenomic communication between allogeneic nuclear DNA, somatic cell-inherited mtDNA molecules, and mtDNA molecules of ooplasmic origin |
Relatively high probability of the occurrence of abnormalities in: - structural and epigenetic remodeling of nuclear chromatin; - epigenetic reprogramming of transcriptional activity of the nuclear genome, including rearrangement of telomeres of somatic cell chromosomes in the reconstructed oocytes and in cloned embryos that develop as a result of their activation |
| Direct intraooplasmic microinjection of karyoplasts | The highest invasiveness of the method: - interferes with the ultrastructure of the plasmalemma and the membrane and cytoskeleton of nuclear donor cells through: • their mechanically induced cytolysis to isolate karyoplasts - interferes with the ultrastructure of the plasmalemma and the membrane and cytoskeleton of enucleated oocytes through: • direct microsurgical transfer and deposition in their ooplasm of karyoplasts |
Relatively low probability of the occurrence in the obtained clonal cybrids of: - cellular mtDNA heteroplasmy; - abnormal nuclear–cytoplasmic interactions; - abnormal intergenomic communication between allogeneic nuclear DNA, somatic cell-inherited mtDNA molecules, and mtDNA molecules of ooplasmic origin |
Relatively low probability of the occurrence of abnormalities in: - structural and epigenetic remodeling of nuclear chromatin; - epigenetic reprogramming of transcriptional activity of the nuclear genome, including rearrangement of telomeres of somatic cell chromosomes in the reconstructed oocytes and in cloned embryos that develop as a result of their activation |
Whatever the method used, the reconstruction of ooplasts results in the combination and mingling (hybridization) of cytoplasmic environments of the ooplast and intact somatic cell or karyoplast isolated from the whole nuclear donor cell. As a result, a nuclear–cytoplasmic/nuclear–ooplasmic hybrid (i.e., cloned cybrid) is formed. This hybrid cell, formed by the hybridization of cytoplasmic microenvironments of the cells derived from two different developmental lines: gametogenic (germinal) and somatogenic (somatic), is referred to as a reconstructed or reconstituted oocyte or cybrid cloned zygote. As the mitotic cycle of nuclear donor somatic cells (artificially arrested at the G0 phase) is characterized by “latent” transcriptional activity, inhibited proliferative growth, and a slower metabolism of all organelles, the meiotic cycle of nuclear recipient oocytes also undergoes transient and reversible arresting at the metaphase II (MII) stage. At this stage of meiosis, the processes of advanced transcriptional suppression of genomic DNA take place as a result of attaining nuclear and ooplasmic maturity states. Proper coordination of the cytophysiological state of somatic cells or the karyoplasts isolated from them, and of the cytophysiological state of ooplasts during the reconstruction of cloned cybrids, results from the hybridization of the cytoplasmic environments of nuclear donor cells at the G0 phase of mitosis and of enucleated nuclear recipient oocytes at the MII stage of meiosis [27,98,101,109,110].
Techniques of enucleated oocyte reconstruction may largely affect molecular mechanisms of nuclear chromatin rearrangement, which include both its structural remodeling and epigenetic reprogramming of genomic DNA [99,102,104,107,111,112,113]. Hybridizing the cytoplasmic environment of two cells at different stages of the division cycle interferes with the cell cycle controlling mechanisms and carries the risk of abnormalities further into the development of the cybrid cloned zygote. However, not only does the proper selection of the cytophysiological states of somatic cells/karyoplasts and ooplasts during the reconstruction of cloned cybrids reduce genomic instability, rendering the genome less vulnerable to mutations, but it also reduces the degree of asynchrony in nuclear–cytoplasmic interactions and decreases the frequency of abnormal epigenome-dependent rearrangements of exogenous nuclear chromatin [114,115,116,117,118,119,120].
In contrast to electrofusion, intraooplasmic microinjection of karyoplasts allows for the selective removal of a large part of the cytoplasm of nuclear donor cells, thus enabling relative thinning of the remnants of the somatic cell cytoplasm in a cytosolic microenvironment of the ooplast and early zygote. The direct consequence of this is that the adverse effect of cytoplasmic components of the somatic cell on remodeling and reprogramming of the transferred somatic cell nucleus, and thereby on the development of the reconstituted embryo, is avoided. Where nuclei of relatively small-diameter somatic cells are transplanted (e.g., cumulus oophorus cells, mural granulosa cells, and serum-starved fibroblast cells), the method of choice is the intraooplasmic microinjection of karyoplasts or whole nuclear donor cells [80,98,102,103,104,112,121,122]. Taking into account the above-mentioned finding, the in vitro developmental potential of cloned pig embryos that had been reconstructed by direct intraooplasmic microinjection of somatic cell-descended karyoplasts or whole tiny somatic cells was shown to be relatively higher in relation to cloned embryos produced by the electrofusion of somatic cell–ooplast couplets [29,102,107]. The small diameter of the above types of somatic cells is the reason for a considerably reduced contact surface area with the plasmalemma of enucleated oocytes (oolemma), which reduces the percentage of fused ooplast–nuclear donor cell complexes. In turn, the direct microinjection of karyoplasts or whole small-diameter somatic cells into the cytoplasm of enucleated oocytes avoids technical problems (resulting from inadequate adhesion of plasma membranes), which have the greatest limiting effect on the efficiency of electrofusion of nuclear donor cells with cytoplasts [102,103,104,122].
The direct microinjection of somatic cell nuclei into the cytoplasm of enucleated oocytes has the added advantage of being the “cleanest” of all nuclear transplantation methods. It requires no physicochemical transducers, which often have adverse effects by reducing the in vitro developmental potential of mammalian cloned embryos. For the cell electrofusion technique, all components of the donor cell (both nuclear and cytoplasmic components: organelles and cytoskeletal elements) become an integral part of the oocyte. In contrast, for intraooplasmic microinjection of karyoplasts, plasmalemma and the vast majority of the cytoplasmic material of the nuclear donor cell is rejected following cell lysis. Therefore, only trace amounts of residual cytoplasm, in the form of a narrow rim of membrane-bound protoplasm around the cell nucleus, are introduced as a small karyoplast into the enucleated oocyte. This is of prime importance in some studies that examine nuclear–cytoplasmic interactions in mammalian cloned cybrids [29,44,81,82,104,107,111,122].
The basic paradigm underlying the somatic cell cloning of mammals is the scientific thesis that the donor cell nucleus has to be completely reprogrammed epigenetically by specific factors of the oocyte’s origin in order to support the development of the cybrid cloned zygote to term. A considerable portion of the protein nucleoplasmic (karyolymphatic) factors and cytosolic factors of the somatic cell, which are engaged directly or indirectly in the mechanisms underlying epigenetic reprogramming of donor cell genome, is associated with nuclear chromatin. The qualitative and quantitative composition of these factors within the somatic cell changes together with progressing cytodifferentiation. When the whole donor cell is fused with the enucleated oocyte, those specific factors of somatic cell are also transferred into the cytoplasm of the nuclear recipient oocyte. As a result of this, they may block the endogenous oocyte factors from supporting proper remodeling and reprogramming the epigenetic profile, which is characteristic of a foreign nucleus of a terminally differentiated somatic cell, toward an epigenetic status typical of the nucleus of totipotent stem cells such as the zygote [28,102,123,124,125,126,127]. Exogenous nucleoplasmic and cytoplasmic factors derived from the nuclear donor cell, which are responsible for modulating the epigenetic status of genomic DNA, are incorporated together with oocyte mRNA transcripts and proteins, into the remodeled nucleus of the somatic cell (the so-called pseudo-pronucleus). The pseudo-pronucleus is formed following artificial activation of the embryonic developmental program of the reconstructed oocyte [1,128,129,130,131,132,133,134,135,136,137]. In turn, an overabundance of the somatic cell-derived agents modulating the epigenetic profile of the donor nucleus may remarkably reduce the concentration and activity of the oocyte’s epigenetic factors. Thus, it may diminish the incidence of complete epigenetic reprogramming of transcriptional activity of the somatic cell nucleus in the developing cloned embryo [112,132,133,134,135,136,137,138,139,140,141].
3. Inheritance of the Mitochondrial Genome and Intergenomic Communication between Mitochondrial and Nuclear DNA Fractions during the Development of Cloned Embryos
The increased competence of the oocyte cytoplasm for epigenetic remodeling and reprogramming the somatic cell-inherited nuclear and mitochondrial genomes in cybrid cloned zygotes is a sine qua non condition for correctly inducing the developmental program specific for mammalian SCNT embryos [142,143,144,145,146,147,148,149,150,151,152,153,154].
Mitochondria are semiautonomous organelles that contain their own genetic material in the form of double-stranded (α-helix) circular DNA molecules (mtDNAs) of about 16,300–16,500 base pairs (bp). The mitochondrial DNA encodes 13 proteins, 22 tRNAs, and 2 rRNAs. Up to 95% of proteins, which are the products of the cytoplasmic translation system encoded by nuclear DNA, are involved in biogenesis and cytophysiological functions of mitochondria [155,156,157]. The copy number of mitochondrial genome in a typical mammalian somatic cell is approximately 2–5 × 103, whereas the number of mtDNA molecules in a meiotically matured (MII-stage) oocyte is about 1.6 × 105 in mice, 2.5 × 105 in cattle, 3–5 × 105 in pigs, and 3–8 × 105 in humans. The number of mitochondria in the somatic cell averages 1 × 103, and one organelle harbors between 1 and 10 mtDNA molecules. In turn, a single mitochondrion in the meiotically matured oocyte contains from one to two copies of the mitochondrial genome, which confirms that the abundance of the intraooplasmic population of these organelles is generally equivalent to the total pool of mtDNA molecules of an unfertilized mammalian oocyte [106,139,158,159].
In the procedure of cloning by SCNT, mitochondria of nuclear donor cells are transplanted with the nuclear genetic apparatus into the cytoplasm of enucleated recipient oocytes. Irrespective of the method used for the reconstruction of enucleated oocytes (Table 1), this step of the SCNT procedure always results in the conjunction and mingling (hybridization) of cytoplasmic environments of the ooplast and somatic cell or karyoplast. After its intraooplasmic microinjection, the karyoplast may also be a source of mitochondria (mitochondrial genome) of heteroplasmic origin. Therefore, a reconstructed cloned embryo, which from a cytological viewpoint is a cytoplasmic hybrid (cybrid), harbors the mitochondrial genome of both maternal (oocyte’s) and exogenous origin (i.e., introduced together with the nuclear donor cell) [107,111,160,161,162]. In cloned embryos, fetuses, and offspring, mitochondria are primarily inherited with ooplasmic material. In turn, probably during the first few mitotic cleavage divisions, mitochondria derived from nuclear donor cells are rapidly eliminated from the cytoplasm of embryonic cells at the anaphase stage. The removal of somatic cell-inherited mitochondria largely depends on the polyubiquitination of specific protein substrates. For that reason, the presence of the somatogenic mitochondrial genome in the cells of cloned blastocysts is difficult to detect by genetic engineering techniques [133,158,163,164]. As a consequence, the uniparental inheritance of extranuclear genetic information in dividing cybrid cloned zygotes is regulated by the biodegradation of ubiquitin-labeled mitochondrial proteins (including ribonucleoproteins) and the nucleolysis of mtDNA molecules that are deprived of histones and non-histone proteins. The proteolytic degradation of mitochondria of heteroplasmic (allogeneic) origin is catalyzed by a complex proteasomal system in each blastomere of cloned embryos. This system is characterized by a Svedberg sedimentation coefficient of 26 and designated as a 26S proteasome. The mechanism of nucleolytic biodestruction of all the somatic-cell-derived mtDNA copies is determined by normal function of the intracellular lysosomal cycle, which is related to the exocytosis of endosomal vesicles. The ultimate outcome of this reaction is the removal from embryonic cells of the exogenous mtDNA fractions, which had previously been subjected to internucleosomal fragmentation into short oligonucleotide segments. The preimplantation-stage selective segregation of the mitochondrial genome stemming from nuclear donor cells that is indirectly induced by the anaphase-promoting complex/cyclosome (APC/C) gradually leads to the establishment of cellular mtDNA homoplasmy in cloned embryos reconstituted with somatic cell nuclei. It is noteworthy that APC/C undergoes the heterodimerization with cyclin-dependent kinase cdc20 and is an integral part of the polysubunit enzymatic complex of ubiquitin ligase. Only occasionally could the lasting hybridization of allogeneic mtDNA copies (the so-called mtDNA heteroplasmy) be identified in the pre- and postnatal period of ontogenetic development of mammalian cloned specimens. This phenomenon of intracellular mtDNA heteroplasmy resulted from synergism/complementarity in the intergenomic communication between mtDNA molecules inherited with both nuclear donor cell cytoplasm and nuclear recipient cell ooplasm [155,165,166,167,168].
There are several species-specific epigenetic factors present in the oocyte cytoplasm that may contribute to nuclear–cytoplasmic incompatibilities either immediately after somatic cell nuclear transfer or at later stages of cloned embryo development [105,136,169,170]. In turn, this potential lack of coordination in the interactions of nuclear and cytosolic factors of cybrid cloned zygotes is probably one of the reasons for the limited practical application of the somatic cell cloning technique. It has been demonstrated that maternally inherited mtDNA molecules accumulated in the mitochondrial reservoirs of the oocyte cytosol play an important role in nuclear–ooplasmic asynchrony. This asynchrony involves incompatibilities in both the epigenetic modifications of the somatic genome supporting the developmental program of reconstituted cybrids and a lack of synergy in the molecular mechanisms controlling the karyokinesis and cytokinesis restriction points. These restriction points related to the anaphase segregation of somatic cell-derived chromosomes and asymmetrical telophase division of the cloned cybrid (nuclear–ooplasmic hybrid) that encompasses the expulsion of the pseudo-polar body into perivitelline space are collectively responsible for coordinated pseudomeiotic to mitotic cycle transition following activation of the reconstituted oocyte [134,156].
Moreover, the presence of an oocyte-derived mitochondrial genetic apparatus has been shown to influence the implantation of cloned embryos in the endometrium of a recipient female’s uteri. For that reason, the deleterious effect, on the preimplantation development of cloned embryos, of heterogeneous mtDNA sources as a result of possible mitochondrial heteroplasmy in the reconstructed nuclear–cytoplasmic hybrids should not be discounted [139,160,161,169]. That is why the production of nuclear-transferred embryos, fetuses, and offspring with a precisely defined profile of nucleotide sequences in regulatory or coding segments of the nuclear and/or mitochondrial genome seems to be valuable tool. This tool can be suitable for experimentally dissecting the effects of not only nuclear and cytoplasmic genetic/epigenetic components but also the intrauterine environment of recipient females on embryonic, fetal, and postnatal development of cloned specimens [2,155,158,171].
Therefore, in the hybrid cytoplasmic environment of cloned zygotes, genetically different fractions of mitochondrial DNA of maternal (oocyte’s) origin were found to coexist with those derived from the cytoplasm of allogeneic somatic cells. Although this extranuclear (mitochondrial) genetic apparatus of cloned nuclear–ooplasmic hybrids contains small (approximately 0.01%) amounts of a cell’s genetic information, this mtDNA-dependent genetic information is completely different from information recorded in the nucleotide sequences of nuclear DNA. The latter provides approximately 99.99% of the cellular genome. In this respect, nuclear transplantation of allogeneic somatic cells into enucleated recipient oocytes (where nuclear donor cells and oocytes are derived from genetically different animals of the same species) gives rise to generating nuclear–cytoplasmic hybrids, which are characterized by heterogeneous mtDNA copies. In view of the fact that such heteroplasmic cloned cybrids develop into embryos with cellular mtDNA heteroplasmy, this may lead to apparent genotypic and phenotypic identity/compatibility of the cloned offspring (only in terms of traits determined by nuclear genome-dependent inheritance). Such cloned offspring exhibits a degree of variation/incompatibility with regard to phenotypic traits determined by cytoplasmic (extranuclear) inheritance. The latter is dependent on the mitochondrial genotype known as the mitotype [105,157,159,160,167].
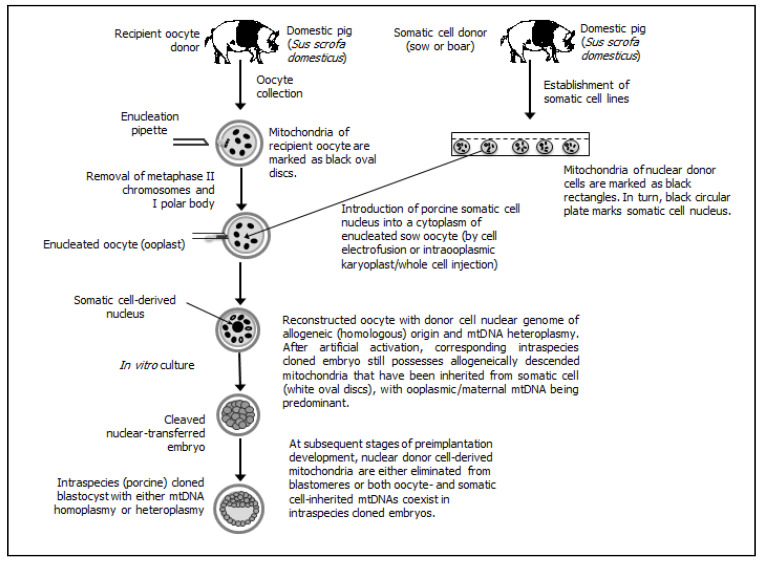
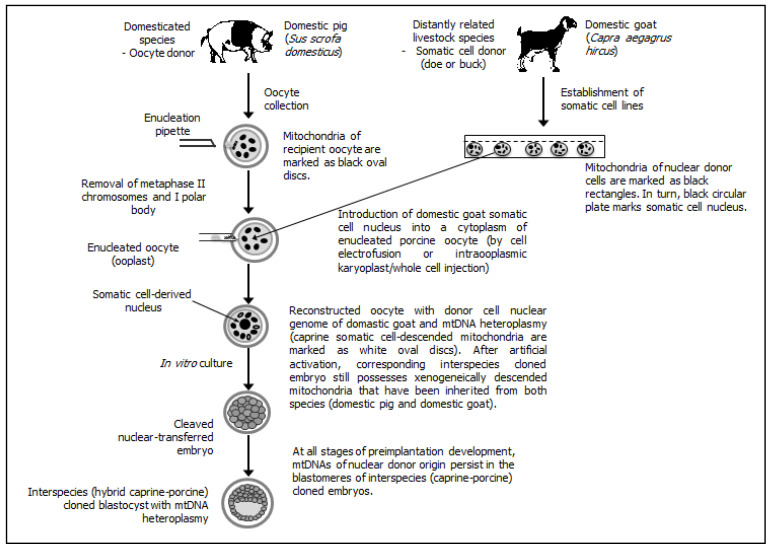
Different possible patterns/scenarios of extranuclear (cytoplasmic) inheritance of mtDNA fractions have been presented (see Figure 1 (for intraspecies cloning by SCNT) [2,158,165,172], Figure 2 (for interspecies SCNT using nuclear donor cells and recipient oocytes derived from closely related mammalian species) [163,170,171,173,174], and Figure 3 (for interspecies SCNT using nuclear donor cells and recipient oocytes derived from phylogenetically distant mammalian species) [162,164,175,176]).
Figure 1.
Intraspecies somatic cell cloning, in which the inheritance of allogeneic (homologous) mtDNAs stemming from genetically different nuclear recipient oocytes and nuclear donor cells is still incompletely recognized during preimplantation development of nuclear-transferred pig embryos. In the vast majority of intraspecies (porcine) cloned embryos, mitochondrial genome primarily arises from the nuclear recipient oocytes, whereas in their other counterparts, mtDNA copies appear to be inherited heteroplasmically (i.e., both from nuclear donor cells and from recipient ooplasm).
Figure 2.
Interspecies (intrafamily and intragenus) somatic cell cloning, in which nuclear donor cells and recipient oocytes are recovered from phylogenetically consanguineous species (i.e., wild boar and domestic pig, respectively). Porcine oocyte-derived mitochondrial DNA (mtDNA) is inherited predominantly during preimplantation development of interspecies (wild boar→pig) cloned embryos, leading to intracellular mtDNA homoplasmy at the blastocyst stage.
Figure 3.
Interspecies (interfamily and intergenus) somatic cell cloning, in which nuclear donor cells and recipient oocytes are recovered from phylogenetically non-consanguineous species (i.e., domestic goat and domestic pig, respectively). Xenogeneic (heterologous) mitochondria that have been inherited from caprine nuclear donor cells and porcine recipient oocytes coexist during preimplantation development of interspecies (goat→pig) cloned embryos, leading to intracellular mtDNA heteroplasmy at the blastocyst stage.
The “ideal” clone can be generated only in a situation where the nuclei of its own (autogeneic) somatic cells are transferred into enucleated recipient oocytes. Put another way, such a cloned specimen can be produced when nuclear donor cells and oocytes originate from genetically identical individuals of a mammalian species, i.e., from monosexual (female) individuals. It is necessary to stress that completely homoplasmic cybrid cloned zygotes can only be created from the oocytes reconstructed in such a manner. The latter are characterized by homogeneous fractions of mtDNA molecules. The artificial activation of such nuclear–cytoplasmic hybrids results in the development of cloned embryos displaying cellular mtDNA homoplasmy. This naturally results in complete genotypic and phenotypic identity/compatibility of somatic cell-cloned fetuses and the resultant offspring. Taking into consideration the previously mentioned findings, only in the case of mammalian cloned females does the mitotype exhibit a homogeneous pattern of coding and regulatory sequences in all mtDNA copies of the somatic and germ cell lines. This condition can only be met assuming that during ontogenesis, the mitochondrial genome will not undergo spontaneous point mutations or those induced by reactive oxygen species [2,42,106,133,166,168,172,177].
Among the reasons for genetic diversification between the cloned specimens generated (somatic clones) and individuals subjected to somatic cell cloning (i.e., donors of somatic cells for SCNT procedure), mention should be made of the effect of mitochondrial (extranuclear/extrachromosomal) inheritance and the impact of intrauterine environment of recipient females receiving cloned embryos. Extranuclear inheritance of genetic material results from the microsurgical, random introduction of foreign mtDNA copies with the nuclear donor cell cytoplasm into the cytoplasmic environment of recipient oocyte. The mismatch of the mitochondrial genome molecules of maternal (oocyte’s) origin and of somatogenic (nuclear donor cell) origin, i.e., mtDNA heteroplasmy, leads to inter-specimen diversification within the mitotype. This results in intra-population and inter-population genetic and phenotypic variability dependent on the mitochondrial genome [134,155,156,157,161]. The phenotypic differences between somatic clones and specimens undergoing SCNT are also contributed by different morphological, anatomotopographical, histological, physiological, endocrinological, embryotrophic, and immunological considerations associated with the reproductive system of recipient surrogates. Moreover, transplacental leakage of leukocyte and erythroblast mitochondria from the blood stream of recipient surrogates to the blood stream of cloned fetuses is often observed. This type of leukocyte–erythroblast chimerism results both from mtDNA heteroplasmy in peripheral blood cells and from genetic mosaicism within subpopulations of nucleated hematopoietic cells (i.e., hematopoietic karyocytes). Such chimerism may also have a certain effect on differences in the mitotype of cloned progeny [139,160,165,169].
4. Epigenetic Reprogramming of Telomeres in Chromosomes Inherited from Somatic Cell Nuclei throughout Development of Cloned Embryos, Fetuses, and Progeny
One of the essential prerequisites for epigenetic reprogramming of the cellular memory dependent on somatic cell-derived nuclear genome (nuclear DNA; nDNA) in the ontogenesis of mammals produced by SCNT is the structural–functional rearrangement of nuclear chromatin. The latter is associated with conformational changes in the length of terminal ends of chromosomes known as telomeres [178,179,180,181,182]. In turn, epigenomic biochemical alterations within telomeric chromatin are related to the biocatalytic activity of the telomerase enzyme [3,183,184,185]. One unresolved problem is the “epigenetic age” of cloned animals, which seems to be correlated to the length of terminal DNA fragments, i.e., the telomeres [186,187,188,189]. The telomeres are deoxyribonucleoprotein structures involved in the stabilization of the structure and conformation of nuclear chromatin during the division period of the mitotic cell cycle. This is necessary for the replication of mutation-free genomic DNA and karyokinetic segregation of chromosomes [190,191,192]. The replication of linear DNA in eukaryotic nuclear chromatin encounters the problem that the 5′-end of the lagging strand cannot replicate, as there is no space for the replication initiating RNA primer. An RNA primer is synthesized on the lagging strand template by primase or RNA polymerase, whose role is played by DNA polymerase α. This creates the risk that somatic cell chromosomes will shorten with every replication round, thus losing genetic information. In mammalian somatic cells, the classical α isoform of DNA polymerase is not capable of semiconservative replication of the 5′-end synthesized in fragments of the DNA chain, whose replication is delayed in relation to the 3′-end of the continuously copied leading strand [182,193]. As a result, in each cell division cycle, unreplicated telomere DNA sequences are gradually lost. For this reason, telomere length is a specific “physiological mitotic clock” of the cell. The shortening of chromosome telomeric regions is positively correlated with the number of cell divisions. Therefore, when the telomere length reaches a critical restriction/control point in a karyokinetically active somatic cell, this is signalized by the loss of nuclear chromatin stability, which is epigenetically programmed in the spatial structure/configuration and telomere functions. This is also signalized by triggering replicative senescence in the cell [187,194,195,196]. The characteristics of cells that undergo progressive replicative senescence include a considerable increase in diameter and a flattened shape caused by a drastic increase in cytosol volume. All of the above-mentioned epigenetic, genetic, physiological, morphological, and ultrastructural transformations, which occur in aging cells, lead in the first place to a rapid slowdown of both intracellular anabolic processes and the kinetics of mitotic divisions. At a later stage, these transformations bring about the irreversible inhibition of metabolic and proliferative activity. As a consequence of single doubling in the population of mammalian adult dermal fibroblasts cultured in vitro, telomere length decreases by about 48 DNA nucleotide pairs [180,183,188,197,198].
Telomerase is a ribonucleoprotein enzyme complex that displays the total activities of RNA reverse transcriptase and DNA integrase only in germ and embryonic cells, while its partial activity is observed in fetal somatic cells undergoing tissue-specific cytodifferentiation. However, the biocatalytic activity of this enzyme completely ceases in terminally differentiated somatic cells of adult specimens [184,185,193,199]. The function of telomerase is to restore the primary length of DNA telomeres by reverse transcription of its own RNA template. This gives rise to the de novo synthesis (reduplication) of tandem repeats within noncoding telomere DNA sequences (5′-TTAGGG-3′) that were lost as a result of terminating either consecutive mitotic and meiotic divisions of gametogenic (germinal) cells or mitotic cycles of blastomeres, leading to consecutive cleavage divisions of embryos. In the last phase of semiconservative DNA replication, the 3′-end of the leading strand extends beyond the 5′-end of the lagging strand. Telomerase contains an RNA molecule that is partially complementary to the tandem repeat of the short 5′-TTAGGG-3′ sequence at the 3′-end of the leading DNA strand, thus elongating the leading strand of telomeric DNA region using RNA as the template. Next, the enzyme detaches and binds to a new telomeric end to extend the leading DNA strand. The extension process may occur hundreds of times before telomerase finally dissociates. Then, the extended, replicated leading strand serves as a template for replication of the 5′-end of the lagging strand that is catalyzed by DNA polymerase α. These two processes, where the 5′-ends of DNA are shortened during basic semiconservative replication and subsequently elongated due to telomerase activity, are mutually balanced, whereby the total chromosomal length remains more or less the same [180,192,199,200]. In contrast, a lack of telomerase activity and, as a consequence, a lack of elongating the temporally and spatially restricted length of nuclear DNA telomeric sequences are epigenomically determined factors specific for adult somatic cells that provide a source of nuclear donors for the SCNT procedure. These factors limit the survival rate, proliferative activity, and the number of division cycles of a cell before the cell reaches the critical point of the maximum telomere shortening. The latter is simultaneously the mitotic control point that signals the initiation and irreversibility of the replicative senescence of terminally differentiated somatic cells [188,190,195,201].
The problem of telomere shortening/attrition and replicative senescence of somatic cells was observed in chromosomes of Dolly the sheep, the first cloned mammal [114,178,189,196]. The telomeres in the chromosomes of Dolly the cloned ewe (at the age of 3 years) were much shorter than the telomeres in the chromosomes of control animals, which were of the same age and were born through natural reproduction. Moreover, the telomere length in Dolly’s chromosomes was similar to that in the chromosomes of a 6-year-old sheep, which was used as a donor of somatic cells for the cloning procedure. At the time of molecular analysis of telomeres, Dolly was 3 years old, and her epigenetic age corresponded to the actual age of a 9-year-old sheep. Put differently, Dolly’s somatic cells were epigenetically older by 6 years than herself. Born on 5 July 1996, Dolly the sheep lived above 6.5 years and was euthanized on 14 February 2003 after being diagnosed with a malignant lung cancer known as Jaagsiekte (ovine pulmonary adenocarcinoma). The etiologic agent of this chronic, contagious, and fatal lung cancer in sheep is Jaagsiekte sheep retrovirus (JSRV), which is responsible for the oncogenic transformation of bronchial exocrine epithelial cells, i.e., type II pneumocytes and bronchiolar club (Clara) cells. By 2000, Dolly produced a total of 6 lambs (including twins and triplets). Therefore, the cloned ewe was reproductively sound and displayed high fertility and prolificacy, which means that her reproductive capacity upon reaching sexual and breeding maturity was not impaired. However, in 2001, the hind legs of 5-year-old Dolly exhibited the first symptoms of an autoimmune chronic degenerative joint disease (osteoarthritis), namely rheumatoid arthritis. It should be noted that this disease is relatively frequent in different breeds of sheep, but generally, it does not affect animals younger than 10 years of age. Two questions arise: Could Dolly live 6–9 years less than the expected lifespan of 12–15 years (which is the average lifespan of Finn Dorset sheep, represented by the somatic cell donor ewe in the SCNT procedure)? As a result of somatic cell cloning, did she exhibit rapidly progressing symptoms of premature (anatomical and physiological) aging of the entire body or of some of its parts, tissues, and organs? The results of experiments performed to determine the telomeric age of Dolly the sheep suggest that animals cloned by transferring adult somatic cell nucleus into the enucleated oocyte are epigenetically compromised. For this reason, they have a genetic age of a specimen playing the role of somatic cell donor for SCNT. This means that at birth, they are epigenetically and genetically much older than their real-time birth date [178,184,189,196]. However, the evidence for cloned sheep was not reflected in the studies focused on the analyses of chromosomes isolated from somatic cells derived from cloned cattle. A study by Lanza et al. [194] on the chromosomes of cloned calves produced by using long-term cultured fibroblast cells for SCNT showed that the telomere length of these young animals is even slightly greater than that of control animals, despite the fact that the chromosomes of nuclear donor cells were almost completely depleted of telomeres. These analyses confirmed that the terminal ends of chromosomes are efficiently resynthesized in blastomeres of bovine cloned embryos, with a contribution from highly active telomerases. Analogously, Tian et al. [179] demonstrated that telomere length in chromosomes of four live (about 15.4 kbp) and six dead cloned calves (about 15.9 kbp) that had been generated using dermal fibroblast cells or cumulus cells derived from a 13-year-old cow not only did not differ considerably from the telomere length characteristic of control chromosomes (about 14.7 kbp) but also significantly exceeded (by about 3–3.5 kbp) the telomere length in chromosomes of the aging cow (about 12.4 kbp), which served as the donor of somatic cells for SCNT-based cloning. Finally, Kato et al. [202] provided evidence that in cloned cattle, the telomere length is shortened only in the tissues matching those from the biopsy specimens of which the primary cell cultures were established. In turn, the latter provided the somatic cell lines that were nuclear donors used for SCNT procedures.
Telomere length in chromosomes of the dermal fibroblast cells originating from six transgenic cloned pigs matched the telomere length of the chromosomes of dermal fibroblast cells originating from control animals of the same age and produced by natural reproduction. In turn, two cloned piglets that died 3 to 7 days after birth displayed the same length of terminal ends of chromosomes as the telomeres of chromosomes in fetuses at the third trimester of pregnancy [185]. The terminal restriction fragment (TRF) assay of genomic DNA isolated from the cells stemming from the biopsy specimens retrieved from different organs/tissues of cloned fetuses (gonads, heart, liver, lungs, kidneys, and skin) has confirmed that telomere length in chromosomes remains constant in all the cell lines arising from cytodifferentiation that takes place throughout fetogenesis. The reason for this is the high efficiency of restoring the primary length of terminal chromosome ends via the active telomerase isoform throughout the interphase replication cycle of nuclear DNA in differentiating somatic cells that occupy new tissue niches and are engaged in multi-stage histo- and organogenesis processes. During the postnatal period, telomeres are gradually shortened with each mitotic division of somatic cells, and the reduction of telomere length is tissue-specific. This reflects inhibition of the biocatalytic activity of telomerase in differentiated lines of somatic cells derived from skin tissue explants and various internal organs harvested from gilts and boars both before and after attainment of sexual maturity [180,181,185,192,198].
5. Comprehensive Summary and Future Goals
Cloning by SCNT is currently used in assisted reproductive technologies (ARTs) of many mammalian species, including various species of farm animals. The application of this technology in experimental embryology and in molecular population genetics is of great importance for livestock breeding.
Somatic cell cloning as a method of asexual reproduction offers the opportunity for production and/or multiplication of monogenetic and monosexual progeny of high breeding worth, whose genotypic and phenotypic identity with progenitor donor of transcriptional mitochondrial and nuclear apparatus of the somatic cell only concerns genomic DNA. Animals produced by SCNT differ in phenotypic traits determined by the random segregation of oocyte-derived/maternal and somatic cell-derived/somatogenic mitochondrial genome (mtDNA) as a result of cytoplasmic (extranuclear) inheritance of genetic material [106,133,159,161]. Nevertheless, the particularly high application value of somatic cell cloning technology is related to the possibility of generating genotypically and phenotypically identical transgenic animals, i.e., animals with transformed nuclear genomes that are valuable due to the expression product of modified genes [4,28,38,203]. The yield of recombinant transgenic protein synthesis by genetically transformed cloned specimens is, to a certain extent, dependent on the effect of heteroplasmic sources of mitochondrial genotype (mitotype) on the transcriptional activity profile of modified nuclear DNA genes. This correlation may be negative with a high coefficient of heritability and regressive repeatability of a given quantitative and qualitative trait resulting from the transgenization of a breeding herd [2,133,158]. Therefore, an important problem in the production and multiplication of transgenic cloned specimens (the so-called clonal founder animals) is to generate offspring with an identical mitochondrial genome. These offspring carry only homoplasmic copies of mtDNA derived either from recipient oocytes or from somatic donor cells of genetically modified nuclei. Not without significance is the effect of inheritance of extranuclear genetic information that is accumulated in mitochondrial reservoirs of both somatic (somatogenic) and germinal (gametogenic) cell lines on the transcriptional activity of quantitative trait loci (QTLs). The latter encompass loci for such traits of transgenic cloned specimens as reproductive traits (e.g., fertility and prolificacy), productive traits, including meatiness (e.g., loin eye area, contents of striated muscle tissue, intramuscular and intermuscular connective tissue, adipose tissue in different carcass, and half-carcass cuts) and milk yield traits (e.g., volume of milk synthesis, and milk secretion and ejection per day and per lactation period) [2,4,106,108,136,157,159,203]. In turn, genetic determinants of prolificacy or fertility traits from the heteroplasmic or homoplasmic pattern of mitochondrial genome segregation may influence the processes of intergenerational transmission of the transgene in germ cell lines of the descendant generations of cloned animals with the transformed nuclear genotype. On the one hand, a negative or positive genetic correlation between milk yield or dressing percentage traits (inherited with genomic DNA) and the transcriptional activity profile of mitochondrial DNA genes may be responsible for different extents or patterns of tissue-specific or organ-specific expression of xenogeneic (e.g., human) gene constructs in transgenic cloned animals. The expression extents or patterns of these gene constructs may be characterized by the inhibition or onset of their transcriptional suppression. On the other hand, the above-mentioned negative or positive correlation may also affect the expression profile of xenogeneic gene constructs (transgenes) in different cells, tissues, and organs of genetically modified cloned specimens. This profile of transcriptional activity of the transgenes integrated with the nuclear genome may be homogenous or heterogeneous, resulting in the induction or absence of transgenic mosaicism/chimerism in cloned animals [4,5,136,158,168,169]. The xenogeneic expressive gene constructs that have been incorporated into genomic DNA of cells localized in different tissues and organs of transgenic cloned animals can encode, for example, recombinant human therapeutic proteins. The synthesis and exo- or endocrine secretion of these proteins can be targeted at secretory cells of the mammary gland or smooth and striated muscle tissue found in all the corporeal organs, organ systems, and parts of farm animals [35,139,204,205,206,207].
Intergenomic communication between mitochondrial DNA and the transgene stably integrated with nuclear DNA may also create differences in the efficiency of transgenesis, which induces targeted mutagenesis, i.e., monoallelic deletion or the insertional inactivation of the gene coding for myostatin. Myostatin is a muscle-tissue-specific hormonal protein that paracrinally inhibits the gain (hypetrophy and hyperplasia) of skeletal and smooth muscles [18,208]. The presence of one or two knockout alleles of the myostatin gene or the presence of one or two posttranscriptionally silenced mRNA copies encoded by the myostatin gene in heterozygous or homozygous transgenic cloned beef cattle increases meatiness in cows and bulls. This results from the hypertrophy and hyperplasia of not only striated but also smooth muscle tissue [18,204].
The attractiveness of SCNT-based cloning of transgenic mammals, including various species of domesticated animals, is decided by the applicability of the hormonal or enzymatic product of the modified gene expression. This applicability first of all determines the scale and scope of the research. Although the first cloned mammal was a sheep, research targeted at the somatic cell cloning of other livestock species had a much wider span. The mammary glands (udders) of transgenic cloned cows [11,12,14,206,209,210], transgenic cloned sheep [23,24], and transgenic cloned goats [17,19] may become live bioreactors for producing humanized milk, easy-to-digest milk, or milk containing recombinant human therapeutic proteins (biopharmaceuticals or nutraceuticals). The latter may find clinical application in the treatment of patients afflicted with genetically determined diseases [12,211,212,213].
Compared to other ARTs in mammals (including livestock species), the efficiency of somatic cell cloning in domesticated animals, which is measured by the percentage of offspring born in relation to the number of reconstructed oocytes, remains low and oscillates between 0.3% and 2% on average. Nonetheless, the biotechnological possibilities of the somatic cell cloning in different mammalian species is far ahead of our understanding of the biological determinants, in particular the molecular and epigenetic aspects, of this method [108,132,192,203]. Yet, the biological foundations that have been laid for embryonic genome engineering of domesticated animals, especially over the last 24 years, made feasible the development of an innovative technology of in vitro embryo production using the somatic cell cloning procedure, which may meet the requirements for application in laboratories or, in some cases, only for limited practical purposes [127,214,215]. The assisted reproductive technology that encompasses SCNT could be used on a larger practical scale only after the efficiency of somatic cell cloning in various mammalian species, including livestock, is increased to match the efficiency of in vitro fertilization (IVF) or artificial insemination (AI) as part of multiple ovulation and embryo transfer (MOET) programs in cattle. However, due to the relatively high incidence of lethal or sublethal developmental anomalies or anatomo-histological defects in cloned fetuses and progeny, it is not possible to use the somatic cell cloning of farm animals on a commercial scale, at least at the present level of sophistication of the relevant research performed in Europe and the world. Furthermore, it is also worth noting that the elaboration and optimization of efficient approaches applied to cryopreserving nuclear donor somatic cells, nuclear-transferred oocytes reconstructed with somatic cells, and somatic cell-cloned embryos appear to be important milestones that can help cryogenically protect these valuable types of biological materials. This can bring the investigators closer to the perspective of progression in the outcome of producing mammalian SCNT progeny. In turn, future large-scale attempts undertaken to more successfully generate mammalian cloned offspring can expedite their practical use for the purposes of not only agricultural, transgenic, biotechnological, biomedical, and biopharmaceutical research fields but also ex situ conservation of biological diversity in different anthropogenic and unspoiled natural ecosystems.
To sum up, it seems that after making the transition from basic to applied research, the techniques for intra- and interspecies somatic cell cloning of mammals could contribute to (1) the conservation of genetic resources and the establishment of the genetic reserves of threatened mammalian species and breeds, (2) the restoration and multiplication of the subpopulations of endangered or vulnerable wild and domesticated species of mammals in order to maintain biodiversity and to increase the level of intra-population and inter-specimen genetic variability, and (3) revival (“resurrection”) and reintroduction into the wild of extinct, free-living species of mammals. Moreover, the practically applied research into the cloning of domesticated animals could serve to achieve other tangible benefits, including (4) the improvement of the breeding (genetic) and productive value of different farm animal breeds, e.g., increasing their milk and meat yields and reproductive ability (prolificacy and fertility), and (5) the implementation of basic research into interdisciplinary sciences aimed at the generation of animal biotechnological (transgenic) products for the biomedical, biopharmaceutical, nutraceutical, and food technology industries. One classic example of this is the permanent and highly heritable targeted transgenization of the mammary glands of domesticated species of small and large ruminants (i.e., sheep, goats, and cattle, respectively) and their use as animal bioreactors for producing humanized milk or milk containing recombinant human therapeutic proteins such as biopharmaceuticals and nutraceuticals.
Abbreviations
| AI | Artificial insemination |
| APC/C | Anaphase-promoting complex/cyclosome |
| ARTs | Assisted reproductive technologies |
| IVF | In vitro fertilization |
| JSRV | Jaagsiekte sheep retrovirus |
| MII | Metaphase II |
| MOET | Multiple ovulation and embryo transfer |
| mtDNA | Mitochondrial DNA |
| nDNA | Nuclear DNA |
| QTLs | Quantitative trait loci |
| SCNT | Somatic cell nuclear transfer |
Author Contributions
Conceptualization, M.S. (Marcin Samiec); Writing the article—original draft, M.S. (Marcin Samiec) and M.S. (Maria Skrzyszowska); Writing the article—review and editing, M.S. (Marcin Samiec); Supervision and funding acquisition, M.S. (Marcin Samiec); Graphic documentation, M.S. (Marcin Samiec). All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Science and Higher Education in Poland as a statutory activity No. 04-19-05-00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gao R., Wang C., Gao Y., Xiu W., Chen J., Kou X., Zhao Y., Liao Y., Bai D., Qiao Z., et al. Inhibition of aberrant DNA re-methylation improves post-implantation development of somatic cell nuclear transfer embryos. Cell Stem Cell. 2018;23:426–435.e5. doi: 10.1016/j.stem.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Liu H.J., Xue J., Li K., Ying Z.Z., Zheng Z., Wang R. Improvement of the nuclear transfer efficiency by using the same genetic background of recipient oocytes as the somatic donor cells in goats. Cell Biol. Int. 2012;36:555–560. doi: 10.1042/CBI20110287. [DOI] [PubMed] [Google Scholar]
- 3.Liu H.J., Peng H., Hu C.C., Li X.Y., Zhang J.L., Zheng Z., Zhang W.C. Effects of donor cells’ sex on nuclear transfer efficiency and telomere lengths of cloned goats. Reprod. Domest. Anim. 2016;51:789–794. doi: 10.1111/rda.12752. [DOI] [PubMed] [Google Scholar]
- 4.Samiec M., Skrzyszowska M. Transgenic mammalian species, generated by somatic cell cloning, in biomedicine, biopharmaceutical industry and human nutrition/dietetics—recent achievements. Pol. J. Vet. Sci. 2011;14:317–328. doi: 10.2478/v10181-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 5.Samiec M., Skrzyszowska M. The possibilities of practical application of transgenic mammalian species generated by somatic cell cloning in pharmacology, veterinary medicine and xenotransplantology. Pol. J. Vet. Sci. 2011;14:329–340. doi: 10.2478/v10181-011-0051-6. [DOI] [PubMed] [Google Scholar]
- 6.Gavin W., Buzzell N., Blash S., Chen L., Hawkins N., Miner K., Pollock D., Porter C., Bonzo D., Meade H. Generation of goats by nuclear transfer: A retrospective analysis of a commercial operation (1998–2010) Transgenic Res. 2020;29:443–459. doi: 10.1007/s11248-020-00207-w. [DOI] [PubMed] [Google Scholar]
- 7.Moulavi F., Asadi-Moghadam B., Omidi M., Yarmohammadi M., Ozegovic M., Rastegar A., Hosseini S.M. Pregnancy and calving rates of cloned dromedary camels produced by conventional and handmade cloning techniques and in vitro and in vivo matured oocytes. Mol. Biotechnol. 2020;62:433–442. doi: 10.1007/s12033-020-00262-y. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg E.J., Strelchenko N.S., Augenstein M.L., Betthauser J.M., Childs L.A., Eilertsen K.J., Enos J.M., Forsythe T.M., Golueke P.J., Koppang R.W., et al. Production of cloned cattle from in vitro systems. Biol. Reprod. 2002;67:327–333. doi: 10.1095/biolreprod67.1.327. [DOI] [PubMed] [Google Scholar]
- 9.Green A.L., Wells D.N., Oback B. Cattle cloned from increasingly differentiated muscle cells. Biol. Reprod. 2007;77:395–406. doi: 10.1095/biolreprod.106.058164. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino Y., Hayashi N., Taniguchi S., Kobayashi N., Sakai K., Otani T., Iritani A., Saeki K. Resurrection of a bull by cloning from organs frozen without cryoprotectant in a −80 °C freezer for a decade. PLoS ONE. 2009;4:e4142. doi: 10.1371/journal.pone.0004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Wang Y., Guo W., Chang B., Liu J., Guo Z., Quan F., Zhang Y. Zinc-finger nickase-mediated insertion of the lysostaphin gene into the beta-casein locus in cloned cows. Nat. Commun. 2013;4:2565. doi: 10.1038/ncomms3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y., Wang Y., Liu J., Lan H., Shao M., Yu Y., Quan F., Zhang Y. Production of transgenic cattle highly expressing human serum albumin in milk by phiC31 integrase-mediated gene delivery. Transgenic Res. 2015;24:875–883. doi: 10.1007/s11248-015-9898-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang M., Sun Z., Yu T., Ding F., Li L., Wang X., Fu M., Wang H., Huang J., Li N., et al. Large-scale production of recombinant human lactoferrin from high-expression, marker-free transgenic cloned cows. Sci. Rep. 2017;7:10733. doi: 10.1038/s41598-017-11462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Ding F., Wang T., Liu W., Lindquist S., Hernell O., Wang J., Li J., Li L., Zhao Y., et al. Purification and characterization of recombinant human bile salt-stimulated lipase expressed in milk of transgenic cloned cows. PLoS ONE. 2017;12:e0176864. doi: 10.1371/journal.pone.0176864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keefer C.L., Keyston R., Lazaris A., Bhatia B., Begin I., Bilodeau A.S., Zhou F.J., Kafidi N., Wang B., Baldassarre H., et al. Production of cloned goats after nuclear transfer using adult somatic cells. Biol. Reprod. 2002;66:199–203. doi: 10.1095/biolreprod66.1.199. [DOI] [PubMed] [Google Scholar]
- 16.Lan G.C., Chang Z.L., Luo M.J., Jiang Y.L., Han D., Wu Y.G., Han Z.B., Ma S.F., Tan J.H. Production of cloned goats by nuclear transfer of cumulus cells and long-term cultured fetal fibroblast cells into abattoir-derived oocytes. Mol. Reprod. Dev. 2006;73:834–840. doi: 10.1002/mrd.20443. [DOI] [PubMed] [Google Scholar]
- 17.Meng L., Wan Y., Sun Y., Zhang Y., Wang Z., Song Y., Wang F. Generation of five human lactoferrin transgenic cloned goats using fibroblast cells and their methylation status of putative differential methylation regions of IGF2R and H19 imprinted genes. PLoS ONE. 2013;8:e77798. doi: 10.1371/journal.pone.0077798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z.R., Zhong B.S., Jia R.X., Wan Y.J., Zhang Y.L., Fan Y.X., Wang L.Z., You J.H., Wang Z.Y., Wang F. Production of myostatin-targeted goat by nuclear transfer from cultured adult somatic cells. Theriogenology. 2013;79:225–233. doi: 10.1016/j.theriogenology.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Feng X., Cao S., Wang H., Meng C., Li J., Jiang J., Qian Y., Su L., He Q., Zhang Q. Production of transgenic dairy goat expressing human α-lactalbumin by somatic cell nuclear transfer. Transgenic Res. 2015;24:73–85. doi: 10.1007/s11248-014-9818-8. [DOI] [PubMed] [Google Scholar]
- 20.He Z., Lu R., Zhang T., Jiang L., Zhou M., Wu D., Cheng Y. A novel recombinant human plasminogen activator: Efficient expression and hereditary stability in transgenic goats and in vitro thrombolytic bioactivity in the milk of transgenic goats. PLoS ONE. 2018;13:e0201788. doi: 10.1371/journal.pone.0201788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCreath K.J., Howcroft J., Campbell K.H.S., Colman A., Schnieke A.E., Kind A.J. Production of gene targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000;405:1066–1069. doi: 10.1038/35016604. [DOI] [PubMed] [Google Scholar]
- 22.Loi P., Clinton M., Barboni B., Fulka J., Jr., Cappai P., Feil R., Moor R.M., Ptak G. Nuclei of nonviable ovine somatic cells develop into lambs after nuclear transplantation. Biol. Reprod. 2002;67:126–132. doi: 10.1095/biolreprod67.1.126. [DOI] [PubMed] [Google Scholar]
- 23.Deng S., Li G., Zhang J., Zhang X., Cui M., Guo Y., Liu G., Li G., Feng J., Lian Z. Transgenic cloned sheep overexpressing ovine toll-like receptor 4. Theriogenology. 2013;80:50–57. doi: 10.1016/j.theriogenology.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P., Liu P., Dou H., Chen L., Chen L., Lin L., Tan P., Vajta G., Gao J., Du Y., et al. Handmade cloned transgenic sheep rich in omega-3 fatty acids. PLoS ONE. 2013;8:e55941. doi: 10.1371/journal.pone.0055941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C., Shang X., Cheng L., Yang L., Liu X., Bai C., Wei Z., Hua J., Li G. DNMT 1 maintains hypermethylation of CAG promoter specific region and prevents expression of exogenous gene in fat-1 transgenic sheep. PLoS ONE. 2017;12:e0171442. doi: 10.1371/journal.pone.0171442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Y., Liu R., Zhang X., Zhang J., Zheng Z., Huang C., Cao G., Liu H., Zhang X. Effects of recipient oocyte source, number of transferred embryos and season on somatic cell nuclear transfer efficiency in sheep. Reprod. Domest. Anim. 2019;54:1443–1448. doi: 10.1111/rda.13546. [DOI] [PubMed] [Google Scholar]
- 27.Onishi A., Iwamoto M., Akita T., Mikawa S., Takeda K., Awata T., Hanada H., Perry A.C.F. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- 28.Lee G.S., Kim H.S., Hyun S.H., Lee S.H., Jeon H.Y., Nam D.H., Jeong Y.W., Kim S., Kim J.H., Han J.Y., et al. Production of transgenic cloned piglets from genetically transformed fetal fibroblasts selected by green fluorescent protein. Theriogenology. 2005;63:973–991. doi: 10.1016/j.theriogenology.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe S., Iwamoto M., Suzuki S.I., Fuchimoto D., Honma D., Nagai T., Hashimoto M., Yazaki S., Sato M., Onishi A. A novel method for the production of transgenic cloned pigs: Electroporation-mediated gene transfer to non-cultured cells and subsequent selection with puromycin. Biol. Reprod. 2005;72:309–315. doi: 10.1095/biolreprod.104.031591. [DOI] [PubMed] [Google Scholar]
- 30.Brunetti D., Perota A., Lagutina I., Colleoni S., Duchi R., Calabrese F., Seveso M., Cozzi E., Lazzari G., Lucchini F., et al. Transgene expression of green fluorescent protein and germ line transmission in cloned pigs derived from in vitro transfected adult fibroblasts. Cloning Stem Cells. 2008;10:409–419. doi: 10.1089/clo.2008.0036. [DOI] [PubMed] [Google Scholar]
- 31.Deng W., Yang D., Zhao B., Ouyang Z., Song J., Fan N., Liu Z., Zhao Y., Wu Q., Nashun B., et al. Use of the 2A peptide for generation of multi-transgenic pigs through a single round of nuclear transfer. PLoS ONE. 2011;6:e19986. doi: 10.1371/journal.pone.0019986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter A., Kurome M., Kessler B., Zakhartchenko V., Klymiuk N., Nagashima H., Wolf E., Wuensch A. Potential of primary kidney cells for somatic cell nuclear transfer mediated transgenesis in pig. BMC Biotechnol. 2012;12:84. doi: 10.1186/1472-6750-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurome M., Geistlinger L., Kessler B., Zakhartchenko V., Klymiuk N., Wuensch A., Richter A., Baehr A., Kraehe K., Burkhardt K., et al. Factors influencing the efficiency of generating genetically engineered pigs by nuclear transfer: Multi-factorial analysis of a large data set. BMC Biotechnol. 2013;13:43. doi: 10.1186/1472-6750-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z., He X., Chen L., Shi J., Zhou R., Xu W., Liu D., Wu Z. Bone marrow mesenchymal stem cells are an attractive donor cell type for production of cloned pigs as well as genetically modified cloned pigs by somatic cell nuclear transfer. Cell. Reprogram. 2013;15:459–470. doi: 10.1089/cell.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ju H., Zhang J., Bai L., Mu Y., Du Y., Yang W., Li Y., Sheng A., Li K. The transgenic cloned pig population with integrated and controllable GH expression that has higher feed efficiency and meat production. Sci. Rep. 2015;5:10152. doi: 10.1038/srep10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu D., Liu S., Shang S., Wu F., Wen X., Li Z., Li Y., Hu X., Zhao Y., Li Q., et al. Production of transgenic-cloned pigs expressing large quantities of recombinant human lysozyme in milk. PLoS ONE. 2015;10:e0123551. doi: 10.1371/journal.pone.0123551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozawa M., Himaki T., Ookutsu S., Mizobe Y., Ogawa J., Miyoshi K., Yabuki A., Fan J., Yoshida M. Production of cloned miniature pigs expressing high levels of human apolipoprotein(a) in plasma. PLoS ONE. 2015;10:e0132155. doi: 10.1371/journal.pone.0132155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J., Li Q., Li Y., Wen X., Li Z., Zhang Z., Zhang J., Yu Z., Li N. Expression of recombinant human α-lactalbumin in milk of transgenic cloned pigs is sufficient to enhance intestinal growth and weight gain of suckling piglets. Gene. 2016;584:7–16. doi: 10.1016/j.gene.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Kwon D.J., Kim D.H., Hwang I.S., Kim D.E., Kim H.J., Kim J.S., Lee K., Im G.S., Lee J.W., Hwang S. Generation of α-1,3-galactosyltransferase knocked-out transgenic cloned pigs with knocked-in five human genes. Transgenic Res. 2017;26:153–163. doi: 10.1007/s11248-016-9979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J., Wang A., Huang C., Sun Y., Song B., Zhou R., Li L. Generation of marker-free pbd-2 knock-in pigs using the CRISPR/Cas9 and Cre/loxP systems. Genes. 2020;11:951. doi: 10.3390/genes11080951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H., Li Y., Wiriyahdamrong T., Yuan Z., Qing Y., Li H., Xu K., Guo J., Jia B., Zhang X., et al. Improved production of GTKO/hCD55/hCD59 triple-gene-modified Diannan miniature pigs for xenotransplantation by recloning. Transgenic Res. 2020;29:369–379. doi: 10.1007/s11248-020-00201-2. [DOI] [PubMed] [Google Scholar]
- 42.Galli C., Lagutina I., Crotti G., Colleoni S., Turini P., Ponderato N., Duchi R., Lazzari G. Pregnancy: A cloned horse born to its dam twin. Nature. 2003;424:635. doi: 10.1038/424635a. [DOI] [PubMed] [Google Scholar]
- 43.Lagutina I., Lazzari G., Duchi R., Colleoni S., Ponderato N., Turini P., Crotti G., Galli C. Somatic cell nuclear transfer in horses: Effect of oocyte morphology, embryo reconstruction method and donor cell type. Reproduction. 2005;130:559–567. doi: 10.1530/rep.1.00772. [DOI] [PubMed] [Google Scholar]
- 44.Hinrichs K., Choi Y.H., Love C.C., Chung Y.G., Varner D.D. Production of horse foals via direct injection of roscovitine-treated donor cells and activation by injection of sperm extract. Reproduction. 2006;131:1063–1072. doi: 10.1530/rep.1.01095. [DOI] [PubMed] [Google Scholar]
- 45.Hinrichs K., Choi Y.H., Varner D.D., Hartman D.L. Production of cloned horse foals using roscovitine-treated donor cells and activation with sperm extract and/or ionomycin. Reproduction. 2007;134:319–325. doi: 10.1530/REP-07-0069. [DOI] [PubMed] [Google Scholar]
- 46.Olivera R., Moro L.N., Jordan R., Pallarols N., Guglielminetti A., Luzzani C., Miriuka S.G., Vichera G. Bone marrow mesenchymal stem cells as nuclear donors improve viability and health of cloned horses. Stem Cells Cloning. 2018;11:13–22. doi: 10.2147/SCCAA.S151763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods G.L., White K.L., Vanderwall D.K., Li G.P., Aston K.I., Bunch T.D., Meerdo L.N., Pate B.J. A mule cloned from fetal cells by nuclear transfer. Science. 2003;301:1063. doi: 10.1126/science.1086743. [DOI] [PubMed] [Google Scholar]
- 48.Shi D., Lu F., Wei Y., Cui K., Yang S., Wei J., Liu Q. Buffalos (Bubalus bubalis) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–291. doi: 10.1095/biolreprod.107.060210. [DOI] [PubMed] [Google Scholar]
- 49.Lu F., Luo C., Li N., Liu Q., Wei Y., Deng H., Wang X., Li X., Jiang J., Deng Y., et al. Efficient generation of transgenic buffalos (Bubalus bubalis) by nuclear transfer of fetal fibroblasts expressing enhanced green fluorescent protein. Sci. Rep. 2018;8:6967. doi: 10.1038/s41598-018-25120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang B.Z., Yang C.Y., Li R.C., Qin G.S., Zhang X.F., Pang C.Y., Chen M.T., Huang F.X., Li Z., Zheng H.Y., et al. An inter-subspecies cloned buffalo (Bubalus bubalis) obtained by transferring of cryopreserved embryos via somatic cell nuclear transfer. Reprod. Domest. Anim. 2010;45:e21–e25. doi: 10.1111/j.1439-0531.2009.01510.x. [DOI] [PubMed] [Google Scholar]
- 51.Madheshiya P.K., Sahare A.A., Jyotsana B., Singh K.P., Saini M., Raja A.K., Kaith S., Singla S.K., Chauhan M.S., Manik R.S., et al. Production of a cloned buffalo (Bubalus bubalis) calf from somatic cells isolated from urine. Cell. Reprogram. 2015;17:160–169. doi: 10.1089/cell.2014.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saini M., Selokar N.L., Palta P., Chauhan M.S., Manik R.S., Singla S.K. An update: Reproductive handmade cloning of water buffalo (Bubalus bubalis) Anim. Reprod. Sci. 2018;197:1–9. doi: 10.1016/j.anireprosci.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Selokar N.L., Sharma P., Saini M., Sheoran S., Rajendran R., Kumar D., Sharma R.K., Motiani R.K., Kumar P., Jerome A., et al. Successful cloning of a superior buffalo bull. Sci. Rep. 2019;9:11366. doi: 10.1038/s41598-019-47909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wani N.A., Wernery U., Hassan F.A., Wernery R., Skidmore J.A. Production of the first cloned camel by somatic cell nuclear transfer. Biol. Reprod. 2010;82:373–379. doi: 10.1095/biolreprod.109.081083. [DOI] [PubMed] [Google Scholar]
- 55.Wani N.A., Hong S.B. Source, treatment and type of nuclear donor cells influences in vitro and in vivo development of embryos cloned by somatic cell nuclear transfer in camel (Camelus dromedarius) Theriogenology. 2018;106:186–191. doi: 10.1016/j.theriogenology.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Wani N.A., Hong S., Vettical B.S. Cytoplast source influences development of somatic cell nuclear transfer (SCNT) embryos in vitro but not their development to term after transfer to synchronized recipients in dromedary camels (Camelus dromedarius) Theriogenology. 2018;118:137–143. doi: 10.1016/j.theriogenology.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Wani N.A., Vettical B.S., Hong S.B. First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: A step towards preserving the critically endangered wild Bactrian camels. PLoS ONE. 2017;12:e0177800. doi: 10.1371/journal.pone.0177800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin T., Kraemer D., Pryor J., Liu L., Rugila J., Howe I., Buck S., Murphy K., Westhusin M. A cat cloned by nuclear transplantation. Nature. 2002;415:859. doi: 10.1038/nature723. [DOI] [PubMed] [Google Scholar]
- 59.Choi E.G., Yin X.J., Lee H.S., Kim L.H., Shin H.D., Kim N.H., Kong I.K. Reproductive fertility of cloned male cats derived from adult somatic cell nuclear transfer. Cloning Stem Cells. 2007;9:281–290. doi: 10.1089/clo.2006.0069. [DOI] [PubMed] [Google Scholar]
- 60.Yin X.J., Lee H.S., Lee Y.H., Seo Y.I., Jeon S.J., Choi E.G., Cho S.J., Cho S.G., Min W., Kang S.K., et al. Cats cloned from fetal and adult somatic cells by nuclear transfer. Reproduction. 2005;129:245–249. doi: 10.1530/rep.1.00403. [DOI] [PubMed] [Google Scholar]
- 61.Yin X.J., Lee H.S., Yu X.F., Kim L.H., Shin H.D., Cho S.J., Choi E.G., Kong I.K. Production of second-generation cloned cats by somatic cell nuclear transfer. Theriogenology. 2008;69:1001–1006. doi: 10.1016/j.theriogenology.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin X.J., Lee H.S., Yu X.F., Choi E., Koo B.C., Kwon M.S., Lee Y.S., Cho S.J., Jin G.Z., Kim L.H., et al. Generation of cloned transgenic cats expressing red fluorescence protein. Biol. Reprod. 2008;78:425–431. doi: 10.1095/biolreprod.107.065185. [DOI] [PubMed] [Google Scholar]
- 63.Song S.H., Lee K.L., Xu L., Joo M.D., Hwang J.Y., Oh S.H., Kong I.K. Production of cloned cats using additional complimentary cytoplasm. Anim. Reprod. Sci. 2019;208:106125. doi: 10.1016/j.anireprosci.2019.106125. [DOI] [PubMed] [Google Scholar]
- 64.Lee B.C., Kim M.K., Jang G., Oh H.J., Yuda F., Kim H.J., Shamim M.H., Kim J.J., Kang S.K., Schatten G., et al. Dogs cloned from adult somatic cells. Nature. 2005;436:641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- 65.Jang G., Kim M.K., Oh H.J., Hossein M.S., Fibrianto Y.H., Hong S.G., Park J.E., Kim J.J., Kim H.J., Kang S.K., et al. Birth of viable female dogs produced by somatic cell nuclear transfer. Theriogenology. 2007;67:941–947. doi: 10.1016/j.theriogenology.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Jang G., Oh H.J., Kim M.K., Fibrianto Y.H., Hossein M.S., Kim H.J., Kim J.J., Hong S.G., Park J.E., Kang S.K., et al. Improvement of canine somatic cell nuclear transfer procedure. Theriogenology. 2008;69:146–154. doi: 10.1016/j.theriogenology.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 67.Jang G., Hong S.G., Oh H.J., Kim M.K., Park J.E., Kim H.J., Kim D.Y., Lee B.C. A cloned toy poodle produced from somatic cells derived from an aged female dog. Theriogenology. 2008;69:556–563. doi: 10.1016/j.theriogenology.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Hong S.G., Jang G., Kim M.K., Oh H.J., Park J.E., Kang J.T., Koo O.J., Kim D.Y., Lee B.C. Dogs cloned from fetal fibroblasts by nuclear transfer. Anim. Reprod. Sci. 2009;115:334–339. doi: 10.1016/j.anireprosci.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Hossein M.S., Jeong Y.W., Park S.W., Kim J.J., Lee E., Ko K.H., Kim H.S., Kim Y.W., Hyun S.H., Shin T., et al. Cloning Missy: Obtaining multiple offspring of a specific canine genotype by somatic cell nuclear transfer. Cloning Stem Cells. 2009;11:123–130. doi: 10.1089/clo.2008.0029. [DOI] [PubMed] [Google Scholar]
- 70.Ock S.A., Choi I., Im G.S., Yoo J.G. Whole blood transcriptome analysis for lifelong monitoring in elite sniffer dogs produced by somatic cell nuclear transfer. Cell. Reprogram. 2019;21:301–313. doi: 10.1089/cell.2019.0056. [DOI] [PubMed] [Google Scholar]
- 71.Eun K., Hong N., Jeong Y.W., Park M.G., Hwang S.U., Jeong Y.I.K., Choi E.J., Olsson P.O., Hwang W.S., Hyun S.H., et al. Transcriptional activities of human elongation factor-1α and cytomegalovirus promoter in transgenic dogs generated by somatic cell nuclear transfer. PLoS ONE. 2020;15:e0233784. doi: 10.1371/journal.pone.0233784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z., Sun X., Chen J., Liu X., Wisely S.M., Zhou Q., Renard J.P., Leno G.H., Engelhardt J.F. Cloned ferrets produced by somatic cell nuclear transfer. Dev. Biol. 2006;293:439–448. doi: 10.1016/j.ydbio.2006.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chesné P., Adenot P.G., Viglietta C., Baratte M., Boulanger L., Renard J.P. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat. Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- 74.Skrzyszowska M., Smorąg Z., Słomski R., Kątska-Książkiewicz L., Kalak R., Michalak E., Wielgus K., Lehmann J., Lipiński D., Szalata M., et al. Generation of transgenic rabbits by the novel technique of chimeric somatic cell cloning. Biol. Reprod. 2006;74:1114–1120. doi: 10.1095/biolreprod.104.039370. [DOI] [PubMed] [Google Scholar]
- 75.Li S., Chen X., Fang Z., Shi J., Sheng H.Z. Rabbits generated from fibroblasts through nuclear transfer. Reproduction. 2006;131:1085–1090. doi: 10.1530/rep.1.01065. [DOI] [PubMed] [Google Scholar]
- 76.Li S., Guo Y., Shi J., Yin C., Xing F., Xu L., Zhang C., Liu T., Li Y., Li H., et al. Transgene expression of enhanced green fluorescent protein in cloned rabbits generated from in vitro-transfected adult fibroblasts. Transgenic Res. 2009;18:227–235. doi: 10.1007/s11248-008-9227-y. [DOI] [PubMed] [Google Scholar]
- 77.Meng Q., Polgar Z., Liu J., Dinnyes A. Live birth of somatic cell-cloned rabbits following trichostatin A treatment and cotransfer of parthenogenetic embryos. Cloning Stem Cells. 2009;11:203–208. doi: 10.1089/clo.2008.0072. [DOI] [PubMed] [Google Scholar]
- 78.Yin M., Jiang W., Fang Z., Kong P., Xing F., Li Y., Chen X., Li S. Generation of hypoxanthine phosphoribosyltransferase gene knockout rabbits by homologous recombination and gene trapping through somatic cell nuclear transfer. Sci. Rep. 2015;5:16023. doi: 10.1038/srep16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ono Y., Shimozawa N., Ito M., Kono T. Cloned mice from fetal fibroblast cells arrested at metaphase by a serial nuclear transfer. Biol. Reprod. 2001;64:44–50. doi: 10.1095/biolreprod64.1.44. [DOI] [PubMed] [Google Scholar]
- 80.Wakayama T., Perry A.C., Zuccotti M., Johnson K.R., Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 81.Wakayama S., Ohta H., Hikichi T., Mizutani E., Iwaki T., Kanagawa O., Wakayama T. Production of healthy cloned mice from bodies frozen at −20 °C for 16 years. Proc. Natl. Acad. Sci. USA. 2008;105:17318–17322. doi: 10.1073/pnas.0806166105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizutani E., Oikawa M., Kassai H., Inoue K., Shiura H., Hirasawa R., Kamimura S., Matoba S., Ogonuki N., Nagatomo H., et al. Generation of cloned mice from adult neurons by direct nuclear transfer. Biol. Reprod. 2015;92:81. doi: 10.1095/biolreprod.114.123455. [DOI] [PubMed] [Google Scholar]
- 83.Tanabe Y., Kuwayama H., Wakayama S., Nagatomo H., Ooga M., Kamimura S., Kishigami S., Wakayama T. Production of cloned mice using oocytes derived from ICR-outbred strain. Reproduction. 2017;154:859–866. doi: 10.1530/REP-17-0372. [DOI] [PubMed] [Google Scholar]
- 84.Azuma R., Miyamoto K., Oikawa M., Yamada M., Anzai M. Combinational treatment of trichostatin A and vitamin C improves the efficiency of cloning mice by somatic cell nuclear transfer. J. Vis. Exp. 2018;134:57036. doi: 10.3791/57036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Q., Renard J.P., Le Friec G., Brochard V., Beaujean N., Cherifi Y., Fraichard A., Cozzi J. Generation of fertile cloned rats by regulating oocyte activation. Science. 2003;302:1179. doi: 10.1126/science.1088313. [DOI] [PubMed] [Google Scholar]
- 86.Lanza R.P., Cibelli J.B., Diaz F., Moraes C.T., Farin P.W., Farin C.E., Hammer C.J., West M.D., Damiani P. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning. 2000;2:79–90. doi: 10.1089/152045500436104. [DOI] [PubMed] [Google Scholar]
- 87.Srirattana K., Imsoonthornruksa S., Laowtammathron C., Sangmalee A., Tunwattana W., Thongprapai T., Chaimongkol C., Ketudat-Cairns M., Parnpai R. Full-term development of gaur-bovine interspecies somatic cell nuclear transfer embryos: Effect of trichostatin a treatment. Cell. Reprogram. 2012;14:248–257. doi: 10.1089/cell.2011.0099. [DOI] [PubMed] [Google Scholar]
- 88.Loi P., Ptak G., Barboni B., Fulka J., Jr., Cappai P., Clinton M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 2001;19:962–964. doi: 10.1038/nbt1001-962. [DOI] [PubMed] [Google Scholar]
- 89.Berg D.K., Li C., Asher G., Wells D.N., Oback B. Red deer cloned from antler stem cells and their differentiated progeny. Biol. Reprod. 2007;77:384–394. doi: 10.1095/biolreprod.106.058172. [DOI] [PubMed] [Google Scholar]
- 90.Gómez M.C., Pope C.E., Giraldo A., Lyons L.A., Harris R.F., King A.L., Cole A., Godke R.A., Dresser B.L. Birth of African Wildcat cloned kittens born from domestic cats. Cloning Stem Cells. 2004;6:247–258. doi: 10.1089/clo.2004.6.247. [DOI] [PubMed] [Google Scholar]
- 91.Gómez M.C., Pope C.E., Kutner R.H., Ricks D.M., Lyons L.A., Ruhe M., Dumas C., Lyons J., López M., Dresser B.L., et al. Nuclear transfer of sand cat cells into enucleated domestic cat oocytes is affected by cryopreservation of donor cells. Cloning Stem Cells. 2008;10:469–483. doi: 10.1089/clo.2008.0021. [DOI] [PubMed] [Google Scholar]
- 92.Kim M.K., Jang G., Oh H.J., Yuda F., Kim H.J., Hwang W.S., Hossein M.S., Kim J.J., Shin N.S., Kang S.K., et al. Endangered wolves cloned from adult somatic cells. Cloning Stem Cells. 2007;9:130–137. doi: 10.1089/clo.2006.0034. [DOI] [PubMed] [Google Scholar]
- 93.Oh H.J., Kim M.K., Jang G., Kim H.J., Hong S.G., Park J.E., Park K., Park C., Sohn S.H., Kim D.Y., et al. Cloning endangered gray wolves (Canis lupus) from somatic cells collected postmortem. Theriogenology. 2008;70:638–647. doi: 10.1016/j.theriogenology.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 94.Hwang I., Jeong Y.W., Kim J.J., Lee H.J., Kang M., Park K.B., Park J.H., Kim Y.W., Kim W.T., Shin T., et al. Successful cloning of coyotes through interspecies somatic cell nuclear transfer using domestic dog oocytes. Reprod. Fertil. Dev. 2013;25:1142–1148. doi: 10.1071/RD12256. [DOI] [PubMed] [Google Scholar]
- 95.Liu Z., Cai Y., Wang Y., Nie Y., Zhang C., Xu Y., Zhang X., Lu Y., Wang Z., Poo M., et al. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell. 2018;172:881–887.e7. doi: 10.1016/j.cell.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 96.Folch J., Cocero M.J., Chesné P., Alabart J.L., Domínguez V., Cognié Y., Roche A., Fernández-Arias A., Martí J.I., Sánchez P., et al. First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology. 2009;71:1026–1034. doi: 10.1016/j.theriogenology.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Niemann H., Wrenzycki C., Lucas-Hahn A., Brambrink T., Kues W.A., Carnwath J.W. Gene expression patterns in bovine in vitro-produced and nuclear transfer-derived embryos and their implications for early development. Cloning Stem Cells. 2002;4:29–38. doi: 10.1089/153623002753632020. [DOI] [PubMed] [Google Scholar]
- 98.Kurome M., Fujimura T., Murakami H., Takahagi Y., Wako N., Ochiai T., Miyazaki K., Nagashima H. Comparison of electro-fusion and intracytoplasmic nuclear injection methods in pig cloning. Cloning Stem Cells. 2003;5:367–378. doi: 10.1089/153623003772032862. [DOI] [PubMed] [Google Scholar]
- 99.Martinez-Diaz M.A., Che L., Albornoz M., Seneda M.M., Collis D., Coutinho A.R., El-Beirouthi N., Laurin D., Zhao X., Bordignon V. Pre- and postimplantation development of swine-cloned embryos derived from fibroblasts and bone marrow cells after inhibition of histone deacetylases. Cell. Reprogram. 2010;12:85–94. doi: 10.1089/cell.2009.0047. [DOI] [PubMed] [Google Scholar]
- 100.Samiec M., Opiela J., Lipiński D., Romanek J. Trichostatin A-mediated epigenetic transformation of adult bone marrow-derived mesenchymal stem cells biases the in vitro developmental capability, quality, and pluripotency extent of porcine cloned embryos. Biomed Res. Int. 2015;2015:814686. doi: 10.1155/2015/814686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samiec M., Romanek J., Lipiński D., Opiela J. Expression of pluripotency-related genes is highly dependent on trichostatin A-assisted epigenomic modulation of porcine mesenchymal stem cells analysed for apoptosis and subsequently used for generating cloned embryos. Anim. Sci. J. 2019;90:1127–1141. doi: 10.1111/asj.13260. [DOI] [PubMed] [Google Scholar]
- 102.Lee J.W., Wu S.C., Tian X.C., Barber M., Hoagland T., Riesen J., Lee K.H., Tu C.F., Cheng W.T.K., Yang X. Production of cloned pigs by whole-cell intracytoplasmic microinjection. Biol. Reprod. 2003;69:995–1001. doi: 10.1095/biolreprod.103.015917. [DOI] [PubMed] [Google Scholar]
- 103.Jiang M.X., Yang C.X., Zhang L.S., Zheng Y.L., Liu S.Z., Sun Q.Y., Chen D.Y. The effects of chemical enucleation combined with whole cell intracytoplasmic injection on panda-rabbit interspecies nuclear transfer. Zygote. 2004;12:315–320. doi: 10.1017/S0967199404002941. [DOI] [PubMed] [Google Scholar]
- 104.Kawano K., Kato Y., Tsunoda Y. Comparison of in vitro development of porcine nuclear-transferred oocytes receiving fetal somatic cells by injection and fusion methods. Cloning Stem Cells. 2004;6:67–72. doi: 10.1089/1536230041372337. [DOI] [PubMed] [Google Scholar]
- 105.Samiec M., Skrzyszowska M. Biological transcomplementary activation as a novel and effective strategy applied to the generation of porcine somatic cell cloned embryos. Reprod. Biol. 2014;14:128–139. doi: 10.1016/j.repbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Samiec M. The role of mitochondrial genome (mtDNA) in somatic and embryo cloning of mammals. A review. J. Anim. Feed Sci. 2005;14:213–233. doi: 10.22358/jafs/67008/2005. [DOI] [Google Scholar]
- 107.Samiec M., Skrzyszowska M. Microsurgical nuclear transfer by intraooplasmic karyoplast injection as an alternative embryo reconstruction method in somatic cloning of pigs and other mammal species; application value of the method and its technical advantages: A review. Czech J. Anim. Sci. 2005;50:235–242. doi: 10.17221/4163-CJAS. [DOI] [Google Scholar]
- 108.Samiec M., Skrzyszowska M. Intrinsic and extrinsic molecular determinants or modulators for epigenetic remodeling and reprogramming of somatic cell-derived genome in mammalian nuclear-transferred oocytes and resultant embryos. Pol. J. Vet. Sci. 2018;21:217–227. doi: 10.24425/119040. [DOI] [PubMed] [Google Scholar]
- 109.Lacham-Kaplan O., Diamente M., Pushett D., Lewis I., Trounson A. Developmental competence of nuclear transfer cow oocytes after direct injection of fetal fibroblast nuclei. Cloning. 2000;2:55–62. doi: 10.1089/152045500436078. [DOI] [PubMed] [Google Scholar]
- 110.Galli C., Lagutina I., Vassiliev I., Duchi R., Lazzari G. Comparison of microinjection (piezo-electric) and cell fusion for nuclear transfer success with different cell types in cattle. Cloning Stem Cells. 2002;4:189–196. doi: 10.1089/15362300260339476. [DOI] [PubMed] [Google Scholar]
- 111.Samiec M., Skrzyszowska M. Molecular conditions of the cell nucleus remodelling/reprogramming process and nuclear-transferred embryo development in the intraooplasmic karyoplast injection technique: A review. Czech J. Anim. Sci. 2005;50:185–195. doi: 10.17221/4142-CJAS. [DOI] [Google Scholar]
- 112.Samiec M., Skrzyszowska M. Assessment of in vitro developmental capacity of porcine nuclear-transferred embryos reconstituted with cumulus oophorus cells undergoing vital diagnostics for apoptosis detection. Ann. Anim. Sci. 2013;13:513–529. doi: 10.2478/aoas-2013-0035. [DOI] [Google Scholar]
- 113.Skrzyszowska M., Karasiewicz J., Bednarczyk M., Samiec M., Smorąg Z., Waś B., Guszkiewicz A., Korwin-Kossakowski M., Górniewska M., Szablisty E., et al. Generation of cloned and chimeric embryos/offspring using the new methods of animal biotechnology. Reprod. Biol. 2006;6(Suppl. 1):119–135. [PubMed] [Google Scholar]
- 114.Wilmut I., Beaujean N., de Sousa P.A., Dinnyes A., King T.J., Paterson L.A., Wells D.N., Young L.E. Somatic cell nuclear transfer. Nature. 2002;419:583–586. doi: 10.1038/nature01079. [DOI] [PubMed] [Google Scholar]
- 115.Dean W., Santos F., Reik W. Epigenetic reprogramming in early mammalian development and following somatic nuclear transfer. Semin. Cell Dev. Biol. 2003;14:93–100. doi: 10.1016/S1084-9521(02)00141-6. [DOI] [PubMed] [Google Scholar]
- 116.Kang Y.K., Yeo S., Kim S.H., Koo D.B., Park J.S., Wee G., Han J.S., Oh K.B., Lee K.K., Han Y.M. Precise recapitulation of methylation change in early cloned embryos. Mol. Reprod. Dev. 2003;66:32–37. doi: 10.1002/mrd.10330. [DOI] [PubMed] [Google Scholar]
- 117.Beaujean N., Taylor J., Gardner J., Wilmut I., Meehan R., Young L. Effect of limited DNA methylation reprogramming in the normal sheep embryo on somatic cell nuclear transfer. Biol. Reprod. 2004;71:185–193. doi: 10.1095/biolreprod.103.026559. [DOI] [PubMed] [Google Scholar]
- 118.Samiec M., Skrzyszowska M., Bochenek M. In vitro development of porcine nuclear-transferred embryos derived from fibroblast cells analysed cytometrically for apoptosis incidence and accuracy of cell cycle synchronization at the G0/G1 stages. Ann. Anim. Sci. 2013;13:735–752. doi: 10.2478/aoas-2013-0049. [DOI] [Google Scholar]
- 119.Samiec M., Skrzyszowska M., Opiela J. Creation of cloned pig embryos using contact-inhibited or serum-starved fibroblast cells analysed intra vitam for apoptosis occurrence. Ann. Anim. Sci. 2013;13:275–293. doi: 10.2478/aoas-2013-0009. [DOI] [Google Scholar]
- 120.Opiela J., Samiec M., Romanek J. In vitro development and cytological quality of inter-species (porcine→bovine) cloned embryos are affected by trichostatin A-dependent epigenomic modulation of adult mesenchymal stem cells. Theriogenology. 2017;97:27–33. doi: 10.1016/j.theriogenology.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 121.Cheong H.T., Ikeda K., Martinez Diaz M.A., Katagiri S., Takahashi Y. Development of reconstituted pig embryos by nuclear transfer of cultured cumulus cells. Reprod. Fertil. Dev. 2000;12:15–20. doi: 10.1071/RD00051. [DOI] [PubMed] [Google Scholar]
- 122.Roh S., Hwang W.S. In vitro development of porcine parthenogenetic and cloned embryos: Comparison of oocyte-activating techniques, various culture systems and nuclear transfer methods. Reprod. Fertil. Dev. 2002;14:93–99. doi: 10.1071/RD01090. [DOI] [PubMed] [Google Scholar]
- 123.Cezar G.G., Bartolomei M.S., Forsberg E.J., First N.L., Bishop M.D., Eilertsen K.J. Genome-wide epigenetic alterations in cloned bovine fetuses. Biol. Reprod. 2003;68:1009–1014. doi: 10.1095/biolreprod.102.010181. [DOI] [PubMed] [Google Scholar]
- 124.Lee J., Inoue K., Ono R., Ogonuki N., Kohda T., Kaneko-Ishino T., Ogura A., Ishino F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2003;129:1807–1817. doi: 10.1242/dev.129.8.1807. [DOI] [PubMed] [Google Scholar]
- 125.Shi W., Zakhartchenko V., Wolf E. Epigenetic reprogramming in mammalian nuclear transfer. Differentiation. 2003;71:91–113. doi: 10.1046/j.1432-0436.2003.710201.x. [DOI] [PubMed] [Google Scholar]
- 126.Seki Y., Hayashi K., Itoh K., Mizugaki M., Saitou M., Matsui Y. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev. Biol. 2005;278:440–458. doi: 10.1016/j.ydbio.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 127.Jin L., Guo Q., Zhang G.L., Xing X.X., Xuan M.F., Luo Q.R., Luo Z.B., Wang J.X., Yin X.J., Kang J.D. The histone deacetylase inhibitor, CI994, improves nuclear reprogramming and in vitro developmental potential of cloned pig embryos. Cell. Reprogram. 2018;20:205–213. doi: 10.1089/cell.2018.0001. [DOI] [PubMed] [Google Scholar]
- 128.Campbell K.H., Alberio R. Reprogramming the genome: Role of the cell cycle. Reprod. Suppl. 2003;61:477–494. doi: 10.1530/biosciprocs.5.035. [DOI] [PubMed] [Google Scholar]
- 129.Bang J.I., Yoo J.G., Park M.R., Shin T.S., Cho B.W., Lee H.G., Kim B.W., Kang T.Y., Kong I.K., Kim J.H., et al. The effects of artificial activation timing on the development of SCNT-derived embryos and newborn piglets. Reprod. Biol. 2013;13:127–132. doi: 10.1016/j.repbio.2013.01.181. [DOI] [PubMed] [Google Scholar]
- 130.Rybouchkin A., Kato Y., Tsunoda Y. Role of histone acetylation in reprogramming of somatic nuclei following nuclear transfer. Biol. Reprod. 2006;74:1083–1089. doi: 10.1095/biolreprod.105.047456. [DOI] [PubMed] [Google Scholar]
- 131.Nashun B., Hill P.W., Hajkova P. Reprogramming of cell fate: Epigenetic memory and the erasure of memories past. EMBO J. 2015;34:1296–1308. doi: 10.15252/embj.201490649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saini M., Selokar N.L. Approaches used to improve epigenetic reprogramming in buffalo cloned embryos. Indian J. Med. Res. 2018;148:S115–S119. doi: 10.4103/ijmr.IJMR_2096_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Samiec M. The effect of mitochondrial genome on architectural remodeling and epigenetic reprogramming of donor cell nuclei in mammalian nuclear transfer-derived embryos. J. Anim. Feed Sci. 2005;14:393–422. doi: 10.22358/jafs/67034/2005. [DOI] [Google Scholar]
- 134.Srirattana K., Matsukawa K., Akagi S., Tasai M., Tagami T., Nirasawa K., Nagai T., Kanai Y., Parnpai R., Takeda K. Constant transmission of mitochondrial DNA in intergeneric cloned embryos reconstructed from swamp buffalo fibroblasts and bovine ooplasm. Anim. Sci. J. 2011;82:236–243. doi: 10.1111/j.1740-0929.2010.00827.x. [DOI] [PubMed] [Google Scholar]
- 135.Narbonne P., Miyamoto K., Gurdon J.B. Reprogramming and development in nuclear transfer embryos and in interspecific systems. Curr. Opin. Genet. Dev. 2012;22:450–458. doi: 10.1016/j.gde.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takeda K. Functional consequences of mitochondrial mismatch in reconstituted embryos and offspring. J. Reprod. Dev. 2019;65:485–489. doi: 10.1262/jrd.2019-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mann M.R.W., Chung Y.G., Nolen L.D., Verona R.I., Latham K.E., Bartolomei M.S. Disruption of imprinted gene methylation and expression in cloned preimplantation stage mouse embryos. Biol. Reprod. 2003;69:902–914. doi: 10.1095/biolreprod.103.017293. [DOI] [PubMed] [Google Scholar]
- 138.Mann M.R.W., Lee S.S., Doherty A.S., Verona R.I., Nolen L.D., Schultz R.M., Bartolomei M.S. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- 139.Czernik M., Anzalone D.A., Palazzese L., Oikawa M., Loi P. Somatic cell nuclear transfer: Failures, successes and the challenges ahead. Int. J. Dev. Biol. 2019;63:123–130. doi: 10.1387/ijdb.180324mc. [DOI] [PubMed] [Google Scholar]
- 140.Lai L., Tao T., Macháty Z., Kühholzer B., Sun Q.Y., Park K.W., Day B.N., Prather R.S. Feasibility of producing porcine nuclear transfer embryos by using G2/M-stage fetal fibroblasts as donors. Biol. Reprod. 2001;65:1558–1564. doi: 10.1095/biolreprod65.5.1558. [DOI] [PubMed] [Google Scholar]
- 141.Lai L., Park K.W., Cheong H.T., Kühholzer B., Samuel M., Bonk A., Im G.S., Rieke A., Day B.N., Murphy C.N., et al. Transgenic pig expressing the enhanced green fluorescent protein produced by nuclear transfer using colchicine-treated fibroblasts as donor cells. Mol. Reprod. Dev. 2002;62:300–306. doi: 10.1002/mrd.10146. [DOI] [PubMed] [Google Scholar]
- 142.Lorthongpanich C., Solter D., Lim C.Y. Nuclear reprogramming in zygotes. Int. J. Dev. Biol. 2010;54:1631–1640. doi: 10.1387/ijdb.103201cl. [DOI] [PubMed] [Google Scholar]
- 143.Esteves T.C., Balbach S.T., Pfeiffer M.J., Araúzo-Bravo M.J., Klein D.C., Sinn M., Boiani M. Somatic cell nuclear reprogramming of mouse oocytes endures beyond reproductive decline. Aging Cell. 2011;10:80–95. doi: 10.1111/j.1474-9726.2010.00644.x. [DOI] [PubMed] [Google Scholar]
- 144.Kungulovski G., Jeltsch A. Epigenome editing: State of the art, concepts, and perspectives. Trends Genet. 2016;32:101–113. doi: 10.1016/j.tig.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 145.Eilertsen K.J., Power R.A., Harkins L.L., Misica P. Targeting cellular memory to reprogram the epigenome, restore potential, and improve somatic cell nuclear transfer. Anim. Reprod. Sci. 2007;98:129–146. doi: 10.1016/j.anireprosci.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 146.Whitworth K.M., Prather R.S. Somatic cell nuclear transfer efficiency: How can it be improved through nuclear remodeling and reprogramming? Mol. Reprod. Dev. 2010;77:1001–1015. doi: 10.1002/mrd.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mason K., Liu Z., Aguirre-Lavin T., Beaujean N. Chromatin and epigenetic modifications during early mammalian development. Anim. Reprod. Sci. 2012;134:45–55. doi: 10.1016/j.anireprosci.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 148.Armstrong L.M., Lako W., Dean W., Stojkovic M. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells. 2006;24:805–814. doi: 10.1634/stemcells.2005-0350. [DOI] [PubMed] [Google Scholar]
- 149.Corry G.N., Tanasijevic B., Barry E.R., Krueger W., Rasmussen T.P. Epigenetic regulatory mechanisms during preimplantation development. Birth Defects Res. C Embryo Today. 2009;87:297–313. doi: 10.1002/bdrc.20165. [DOI] [PubMed] [Google Scholar]
- 150.Prather R.S., Ross J.W., Isom S.C., Green J.A. Transcriptional, posttranscriptional and epigenetic control of porcine oocyte maturation and embryogenesis. Soc. Reprod. Fertil. Suppl. 2009;66:165–176. [PMC free article] [PubMed] [Google Scholar]
- 151.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 152.Yang X., Smith S.L., Tian X.C., Lewin H.A., Renard J.P., Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- 153.Buganim Y., Faddah D.A., Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet. 2013;14:427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodriguez-Osorio N., Urrego R., Cibelli J.B., Eilertsen K., Memili E. Reprogramming mammalian somatic cells. Theriogenology. 2012;78:1869–1886. doi: 10.1016/j.theriogenology.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 155.Bowles E.J., Campbell K.H., St. John J.C. Nuclear transfer: Preservation of a nuclear genome at the expense of its associated mtDNA genome(s) Curr. Top. Dev. Biol. 2007;77:251–290. doi: 10.1016/S0070-2153(06)77010-7. [DOI] [PubMed] [Google Scholar]
- 156.Lagutina I., Fulka H., Brevini T.A., Antonini S., Brunetti D., Colleoni S., Gandolfi F., Lazzari G., Fulka J., Jr., Galli C. Development, embryonic genome activity and mitochondrial characteristics of bovine-pig inter-family nuclear transfer embryos. Reproduction. 2010;140:273–285. doi: 10.1530/REP-09-0578. [DOI] [PubMed] [Google Scholar]
- 157.St. John J.C., Srirattana K., Tsai T.S., Sun X. The mitochondrial genome: How it drives fertility. Reprod. Fertil. Dev. 2017;30:118–139. doi: 10.1071/RD17408. [DOI] [PubMed] [Google Scholar]
- 158.Hiendleder S. Mitochondrial DNA inheritance after SCNT. Adv. Exp. Med. Biol. 2007;591:103–116. doi: 10.1007/978-0-387-37754-4_8. [DOI] [PubMed] [Google Scholar]
- 159.Takeda K. Mitochondrial DNA transmission and confounding mitochondrial influences in cloned cattle and pigs. Reprod. Med. Biol. 2013;12:47–55. doi: 10.1007/s12522-012-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Burgstaller J.P., Schinogl P., Dinnyes A., Müller M., Steinborn R. Mitochondrial DNA heteroplasmy in ovine fetuses and sheep cloned by somatic cell nuclear transfer. BMC Dev. Biol. 2007;7:141. doi: 10.1186/1471-213X-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hua S., Lu C., Song Y., Li R., Liu X., Quan F., Wang Y., Liu J., Su F., Zhang Y. High levels of mitochondrial heteroplasmy modify the development of ovine-bovine interspecies nuclear transferred embryos. Reprod. Fertil. Dev. 2012;24:501–509. doi: 10.1071/RD11091. [DOI] [PubMed] [Google Scholar]
- 162.Huang X., Song L., Zhan Z., Gu H., Feng H., Li Y. Factors affecting mouse somatic cell nuclear reprogramming by rabbit ooplasms. Cell. Reprogram. 2017;19:344–353. doi: 10.1089/cell.2017.0021. [DOI] [PubMed] [Google Scholar]
- 163.Sansinena M.J., Lynn J., Bondioli K.R., Denniston R.S., Godke R.A. Ooplasm transfer and interspecies somatic cell nuclear transfer: Heteroplasmy, pattern of mitochondrial migration and effect on embryo development. Zygote. 2011;19:147–156. doi: 10.1017/S0967199410000419. [DOI] [PubMed] [Google Scholar]
- 164.Kwon D., Koo O.J., Kim M.J., Jang G., Lee B.C. Nuclear-mitochondrial incompatibility in interorder rhesus monkey-cow embryos derived from somatic cell nuclear transfer. Primates. 2016;57:471–478. doi: 10.1007/s10329-016-0538-y. [DOI] [PubMed] [Google Scholar]
- 165.Takeda K., Kaneyama K., Tasai M., Akagi S., Takahashi S., Yonai M., Kojima T., Onishi A., Tagami T., Nirasawa K., et al. Characterization of a donor mitochondrial DNA transmission bottleneck in nuclear transfer derived cow lineages. Mol. Reprod. Dev. 2008;75:759–765. doi: 10.1002/mrd.20837. [DOI] [PubMed] [Google Scholar]
- 166.Yan Z.H., Zhou Y.Y., Fu J., Jiao F., Zhao L.W., Guan P.F., Huang S.Z., Zeng Y.T., Zeng F. Donor-host mitochondrial compatibility improves efficiency of bovine somatic cell nuclear transfer. BMC Dev. Biol. 2010;10:31. doi: 10.1186/1471-213X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yan H., Yan Z., Ma Q., Jiao F., Huang S., Zeng F., Zeng Y. Association between mitochondrial DNA haplotype compatibility and increased efficiency of bovine intersubspecies cloning. J. Genet. Genom. 2011;38:21–28. doi: 10.1016/j.jcg.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 168.Srirattana K., St. John J.C. Manipulating the mitochondrial genome to enhance cattle embryo development. G3 (Bethesda) 2017;7:2065–2080. doi: 10.1534/g3.117.042655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hiendleder S., Prelle K., Brüggerhoff K., Reichenbach H.D., Wenigerkind H., Bebbere D., Stojkovic M., Müller S., Brem G., Zakhartchenko V., et al. Nuclear-cytoplasmic interactions affect in utero developmental capacity, phenotype, and cellular metabolism of bovine nuclear transfer fetuses. Biol. Reprod. 2004;70:1196–1205. doi: 10.1095/biolreprod.103.023028. [DOI] [PubMed] [Google Scholar]
- 170.Ma L.B., Yang L., Hua S., Cao J.W., Li J.X., Zhang Y. Development in vitro and mitochondrial fate of interspecies cloned embryos. Reprod. Domest. Anim. 2008;43:279–285. doi: 10.1111/j.1439-0531.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 171.Imsoonthornruksa S., Srirattana K., Phewsoi W., Tunwattana W., Parnpai R., Ketudat-Cairns M. Segregation of donor cell mitochondrial DNA in gaur-bovine interspecies somatic cell nuclear transfer embryos, fetuses and an offspring. Mitochondrion. 2012;12:506–513. doi: 10.1016/j.mito.2012.07.108. [DOI] [PubMed] [Google Scholar]
- 172.Choi Y.H., Ritthaler J., Hinrichs H. Production of a mitochondrial-DNA identical cloned foal using oocytes recovered from immature follicles of selected mares. Theriogenology. 2014;82:411–417. doi: 10.1016/j.theriogenology.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 173.Gómez M.C., Pope C.E., Ricks D.M., Lyons J., Dumas C., Dresser B.L. Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer. Reprod. Fertil. Dev. 2009;21:76–82. doi: 10.1071/RD08222. [DOI] [PubMed] [Google Scholar]
- 174.Magalhães L.C., Cortez J.V., Bhat M.H., Sampaio A.C.N.P.C., Freitas J.L.S., Duarte J.M.B., Melo L.M., Freitas V.J.F. In vitro development and mitochondrial gene expression in brown brocket deer (Mazama gouazoubira) embryos obtained by interspecific somatic cell nuclear transfer. Cell Reprogram. 2020;22:208–216. doi: 10.1089/cell.2019.0069. [DOI] [PubMed] [Google Scholar]
- 175.Yamochi T., Kida Y., Oh N., Ohta S., Amano T., Anzai M., Kato H., Kishigami S., Mitani T., Matsumoto K., et al. Development of interspecies cloned embryos reconstructed with rabbit (Oryctolagus cuniculus) oocytes and cynomolgus monkey (Macaca fascicularis) fibroblast cell nuclei. Zygote. 2013;21:358–366. doi: 10.1017/S0967199412000019. [DOI] [PubMed] [Google Scholar]
- 176.Amarnath D., Choi I., Moawad A.R., Wakayama T., Campbell K.H. Nuclear-cytoplasmic incompatibility and inefficient development of pig-mouse cytoplasmic hybrid embryos. Reproduction. 2011;142:295–307. doi: 10.1530/REP-11-0044. [DOI] [PubMed] [Google Scholar]
- 177.Lee J.H., Peters A., Fisher P., Bowles E.J., St. John J.C., Campbell K.H. Generation of mtDNA homoplasmic cloned lambs. Cell. Reprogram. 2010;12:347–355. doi: 10.1089/cell.2009.0096. [DOI] [PubMed] [Google Scholar]
- 178.Shiels P.G., Kind A.J., Campbell K.H.S., Waddington D., Wilmut I., Colman A., Schnieke A.E. Analysis of telomere lengths in cloned sheep. Nature. 1999;399:316–317. doi: 10.1038/20580. [DOI] [PubMed] [Google Scholar]
- 179.Tian X.C., Xu J., Yang X. Normal telomere lengths found in cloned cattle. Nat. Genet. 2000;26:272–273. doi: 10.1038/81559. [DOI] [PubMed] [Google Scholar]
- 180.Jeon H.Y., Hyun S.H., Lee G.S., Kim H.S., Kim S., Jeong Y.W., Kang S.K., Lee B.C., Han J.Y., Ahn C., et al. The analysis of telomere length and telomerase activity in cloned pigs and cows. Mol. Reprod. Dev. 2005;71:315–320. doi: 10.1002/mrd.20279. [DOI] [PubMed] [Google Scholar]
- 181.Kurome M., Hisatomi H., Matsumoto S., Tomii R., Ueno S., Hiruma K., Saito H., Nakamura K., Okumura K., Matsumoto M., et al. Production efficiency and telomere length of the cloned pigs following serial somatic cell nuclear transfer. J. Reprod. Dev. 2008;54:254–258. doi: 10.1262/jrd.20038. [DOI] [PubMed] [Google Scholar]
- 182.Gomes N.M., Ryder O.A., Houck M.L., Charter S.J., Walker W., Forsyth N.R., Austad S.N., Venditti C., Pagel M., Shay J.W., et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu J., Yang X. Telomerase activity in early bovine embryos derived from parthenogenetic activation and nuclear transfer. Biol. Reprod. 2001;64:770–774. doi: 10.1095/biolreprod64.3.770. [DOI] [PubMed] [Google Scholar]
- 184.Cui W., Wylie D., Aslam S., Dinnyes A., King T., Wilmut I., Clark A.J. Telomerase-immortalized sheep fibroblasts can be reprogrammed by nuclear transfer to undergo early development. Biol. Reprod. 2003;69:15–21. doi: 10.1095/biolreprod.102.013250. [DOI] [PubMed] [Google Scholar]
- 185.Jiang L., Carter D.B., Xu J., Yang X., Prather R.S., Tian X.C. Telomere lengths in cloned transgenic pigs. Biol. Reprod. 2004;70:1589–1593. doi: 10.1095/biolreprod.103.022616. [DOI] [PubMed] [Google Scholar]
- 186.Miyashita N., Shiga K., Yonai M., Kaneyama K., Kobayashi S., Kojima T., Goto Y., Kishi M., Aso H., Suzuki T., et al. Remarkable differences in telomere lengths among cloned cattle derived from different cell types. Biol. Reprod. 2002;66:1649–1655. doi: 10.1095/biolreprod66.6.1649. [DOI] [PubMed] [Google Scholar]
- 187.Kishigami S., Wakayama S., Hosoi Y., Iritani A., Wakayama T. Somatic cell nuclear transfer: Infinite reproduction of a unique diploid genome. Exp. Cell Res. 2008;314:1945–1950. doi: 10.1016/j.yexcr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 188.Le R., Kou Z., Jiang Y., Li M., Huang B., Liu W., Li H., Kou X., He W., Rudolph K.L., et al. Enhanced telomere rejuvenation in pluripotent cells reprogrammed via nuclear transfer relative to induced pluripotent stem cells. Cell Stem Cell. 2014;14:27–39. doi: 10.1016/j.stem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 189.Huili J., Haosheng L., Dengke P. Epigenetic reprogramming by somatic cell nuclear transfer: Questions and potential solutions. Yi Chuan. 2014;36:1211–1218. doi: 10.3724/SP.J.1005.2014.1211. [DOI] [PubMed] [Google Scholar]
- 190.Bekaert S., Derradji H., Baatout S. Telomere biology in mammalian germ cells and during development. Dev. Biol. 2004;274:15–30. doi: 10.1016/j.ydbio.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 191.Schaetzlein S., Rudolph K.L. Telomere length regulation during cloning, embryogenesis and ageing. Reprod. Fertil. Dev. 2005;17:85–96. doi: 10.1071/RD04112. [DOI] [PubMed] [Google Scholar]
- 192.Kong Q., Ji G., Xie B., Li J., Mao J., Wang J., Liu S., Liu L., Liu Z. Telomere elongation facilitated by trichostatin A in cloned embryos and pigs by somatic cell nuclear transfer. Stem Cell Rev. 2014;10:399–407. doi: 10.1007/s12015-014-9499-y. [DOI] [PubMed] [Google Scholar]
- 193.Betts D.H., Perrault S., Harrington L., King W.A. Quantitative analysis of telomerase activity and telomere length in domestic animal clones. Methods Mol. Biol. 2006;325:149–180. doi: 10.1385/1-59745-005-7:149. [DOI] [PubMed] [Google Scholar]
- 194.Lanza R.P., Cibelli J.B., Blackwell C., Cristofalo V.J., Francis M.K., Baerlocher G.M., Mak J., Schertzer M., Chavez E.A., Sawyer N., et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- 195.Kühholzer-Cabot B., Brem G. Aging of animals produced by somatic cell nuclear transfer. Exp. Gerontol. 2002;37:1317–1323. doi: 10.1016/S0531-5565(02)00176-6. [DOI] [PubMed] [Google Scholar]
- 196.Burgstaller J.P., Brem G. Aging of cloned animals: A mini-review. Gerontology. 2017;63:417–425. doi: 10.1159/000452444. [DOI] [PubMed] [Google Scholar]
- 197.Kim H.M., Cho Y.S., Kim H., Jho S., Son B., Choi J.Y., Kim S., Lee B.C., Bhak J., Jang G. Whole genome comparison of donor and cloned dogs. Sci. Rep. 2013;3:2998. doi: 10.1038/srep02998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jeon H.Y., Jeong Y.W., Kim Y.W., Jeong Y.I., Hossein S.M., Yang H., Hyun S.H., Jeung E.B., Hwang W.S. Senescence is accelerated through donor cell specificity in cloned pigs. Int. J. Mol. Med. 2012;30:383–391. doi: 10.3892/ijmm.2012.1003. [DOI] [PubMed] [Google Scholar]
- 199.Dang-Nguyen T.Q., Haraguchi S., Akagi S., Somfai T., Kaneda M., Watanabe S., Kikuchi K., Tajima A., Nagai T. Telomere elongation during morula-to-blastocyst transition in cloned porcine embryos. Cell. Reprogram. 2012;14:514–519. doi: 10.1089/cell.2012.0045. [DOI] [PubMed] [Google Scholar]
- 200.Betts D., Bordignon V., Hill J., Winger Q., Westhusin M., Smith L., King W. Reprogramming of telomerase activity and rebuilding of telomere length in cloned cattle. Proc. Natl. Acad. Sci. USA. 2001;98:1077–1082. doi: 10.1073/pnas.98.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Imsoonthornruksa S., Sangmalee A., Srirattana K., Parnpai R., Ketudat-Cairns M. Development of intergeneric and intrageneric somatic cell nuclear transfer (SCNT) cat embryos and the determination of telomere length in cloned offspring. Cell. Reprogram. 2012;14:79–87. doi: 10.1089/cell.2011.0054. [DOI] [PubMed] [Google Scholar]
- 202.Kato Y., Tani T., Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J. Reprod. Fertil. 2000;120:231–237. doi: 10.1530/jrf.0.1200231. [DOI] [PubMed] [Google Scholar]
- 203.Samiec M., Skrzyszowska M. Can reprogramming of overall epigenetic memory and specific parental genomic imprinting memory within donor cell-inherited nuclear genome be a major hindrance for the somatic cell cloning of mammals?—A review. Ann. Anim. Sci. 2018;18:623–638. doi: 10.2478/aoas-2018-0015. [DOI] [Google Scholar]
- 204.Tessanne K., Golding M.C., Long C.R., Peoples M.D., Hannon G., Westhusin M.E. Production of transgenic calves expressing an shRNA targeting myostatin. Mol. Reprod. Dev. 2012;79:176–185. doi: 10.1002/mrd.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Y., Zhao S., Bai L., Fan J., Liu E. Expression systems and species used for transgenic animal bioreactors. Biomed Res. Int. 2013;2013:580463. doi: 10.1155/2013/580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu D., Liu S., Ding F., Wang H., Li J., Li L., Dai Y., Li N. Large-scale production of functional human lysozyme from marker-free transgenic cloned cows. Sci. Rep. 2016;6:22947. doi: 10.1038/srep22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guo Y., Li H., Wang Y., Yan X., Sheng X., Chang D., Qi X., Wang X., Liu Y., Li J., et al. Screening somatic cell nuclear transfer parameters for generation of transgenic cloned cattle with intragenomic integration of additional gene copies that encode bovine adipocyte-type fatty acid-binding protein (A-FABP) Mol. Biol. Rep. 2017;44:159–168. doi: 10.1007/s11033-016-4094-8. [DOI] [PubMed] [Google Scholar]
- 208.Proudfoot C., Carlson D.F., Huddart R., Long C.R., Pryor J.H., King T.J., Lillico S.G., Mileham A.J., McLaren D.G., Whitelaw C.B., et al. Genome edited sheep and cattle. Transgenic Res. 2015;24:147–153. doi: 10.1007/s11248-014-9832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang B., Wang J., Tang B., Liu Y., Guo C., Yang P., Yu T., Li R., Zhao J., Zhang L., et al. Characterization of bioactive recombinant human lysozyme expressed in milk of cloned transgenic cattle. PLoS ONE. 2011;6:e17593. doi: 10.1371/journal.pone.0017593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang J., Yang P., Tang B., Sun X., Zhang R., Guo C., Gong G., Liu Y., Li R., Zhang L., et al. Expression and characterization of bioactive recombinant human α-lactalbumin in the milk of transgenic cloned cows. J. Dairy Sci. 2008;91:4466–4476. doi: 10.3168/jds.2008-1189. [DOI] [PubMed] [Google Scholar]
- 211.Jang G., Bhuiyan M.M., Jeon H.Y., Ko K.H., Park H.J., Kim M.K., Kim J.J., Kang S.K., Lee B.C., Hwang W.S. An approach for producing transgenic cloned cows by nuclear transfer of cells transfected with human alpha 1-antitrypsin gene. Theriogenology. 2006;65:1800–1812. doi: 10.1016/j.theriogenology.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 212.Salamone D., Barañao L., Santos C., Bussmann L., Artuso J., Werning C., Prync A., Carbonetto C., Dabsys S., Munar C., et al. High level expression of bioactive recombinant human growth hormone in the milk of a cloned transgenic cow. J. Biotechnol. 2006;124:469–472. doi: 10.1016/j.jbiotec.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 213.Monzani P.S., Sangalli J.R., De Bem T.H., Bressan F.F., Fantinato-Neto P., Pimentel J.R., Birgel-Junior E.H., Fontes A.M., Covas D.T., Meirelles F.V. Breeding of transgenic cattle for human coagulation factor IX by a combination of lentiviral system and cloning. Genet. Mol. Res. 2013;12:3675–3688. doi: 10.4238/2013.February.28.25. [DOI] [PubMed] [Google Scholar]
- 214.Loi P., Iuso D., Czernik M., Ogura A. A new, dynamic era for somatic cell nuclear transfer? Trends Biotechnol. 2016;34:791–797. doi: 10.1016/j.tibtech.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 215.Niemann H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology. 2016;86:80–90. doi: 10.1016/j.theriogenology.2016.04.021. [DOI] [PubMed] [Google Scholar]