Abstract
Ixodid ticks are hematophagous arthropods considered to be prominent ectoparasite vectors that have a negative impact on cattle, either through direct injury or via the transmission of several pathogens. In this study, we investigated the molecular infection rates of numerous tick-borne pathogens in ticks sampled on cattle from the Kabylia region, northeastern Algeria, using a high-throughput microfluidic real-time PCR system. A total of 235 ticks belonging to seven species of the genera Rhipicephalus, Hyalomma, and Ixodes were sampled on cattle and then screened for the presence of 36 different species of bacteria and protozoans. The most prevalent tick-borne microorganisms were Rickettsia spp. at 79.1%, followed by Francisella-like endosymbionts (62.9%), Theileria spp. (17.8%), Anaplasma spp. (14.4%), Bartonella spp. (6.8%), Borrelia spp. (6.8%), and Babesia spp. (2.5%). Among the 80.4% of ticks bearing microorganisms, 20%, 36.6%, 21.7%, and 2.1% were positive for one, two, three, and four different microorganisms, respectively. Rickettsia aeschlimannii was detected in Hyalomma marginatum, Hyalomma detritum, and Rhipicephalus bursa ticks. Rickettsia massiliae was found in Rhipicephalus sanguineus, and Rickettsia monacensis and Rickettsia helvetica were detected in Ixodes ricinus. Anaplasma marginale was found in all identified tick genera, but Anaplasma centrale was detected exclusively in Rhipicephalus spp. ticks. The DNA of Borrelia spp. and Bartonella spp. was identified in several tick species. Theileria orientalis was found in R. bursa, R. sanguineus, H. detritum, H. marginatum, and I. ricinus and Babesia bigemina was found in Rhipicephalus annulatus and R. sanguineus. Our study highlights the importance of tick-borne pathogens in cattle in Algeria.
Keywords: Algeria, ixodid ticks, tick-borne pathogens, co-infection, cattle, high-throughput microfluidic real time PCR
1. Introduction
Ixodid ticks are blood-sucking arthropods, and are considered to be prominent vectors of pathogens for humans as well as domestic and wild animals. They are known to transmit a wide variety of causative agents such as bacteria, protozoa, and viruses that may subsequently infect the mammal host. Globally, the incidence of tick-borne diseases is growing, mostly due to increased interactions between pathogens, vectors, and hosts [1,2]. Furthermore, climate change, including the prolongation of seasons, global warming, and changing precipitation patterns, extends the geographic range of a number of tick species and the pathogens they carry [3].
Ticks are regarded as the primary vectors of pathogens affecting livestock [4] and up to 80% of cattle worldwide are at risk of coming into contact with ticks and contracting diseases caused by transmitted tick-borne pathogens (TBPs) [5]. An individual animal can be infested with hundreds or even thousands of ticks, which clearly magnifies their effect on the host, either by direct injury or by the transmission of pathogens [4]. In addition, the co-transmission of several pathogens may lead to co-infection in animals, which aggravates their vital prognosis or, in some cases, gives rise to atypical forms, thereby complicating diagnosis [6]. Thus, it is very important to detect and identify TBPs, so that veterinarians can predict the risk of infection and subsequently implement appropriate control measures.
Hard ticks belonging to the genera Hyalomma, Rhipicephalus, Ixodes, and Haemaphysalis have been identified feeding on grazing cattle in Algeria [7]. In addition, various studies using classical molecular techniques have detected multiple pathogens in these ticks, e.g., the genera Rickettsia, Anaplasma, Coxiella, and Theileria, in cattle ticks from Algeria [8,9,10,11]. All of these studies used classical molecular methods that can only detect a few pathogens simultaneously, are time-consuming, and require large volumes of DNA for the detection of multiple pathogens. Moreover, evidence of non-pathogenic commensal microorganisms called endosymbionts is poorly documented in Algeria but provides useful information because they may influence the transmission of other tick-borne microorganisms or become pathogenic for humans and/or animals [6,12,13].
To do so, microfluidic-based high-throughput PCR systems have been described by various studies as the most sensitive approach to detect TBPs [6,14,15]. These systems allow the rapid and simultaneous detection of numerous microorganisms using a small volume of DNA, thereby making it possible to carry out large-scale epidemiological investigations on TBPs in ticks [14]. Here, we successfully employed this approach to investigate the distribution of TBPs in bovine ticks from the Kabylia region of Algeria for the first time.
2. Results
2.1. Taxonomical Identification of Collected Tick Species
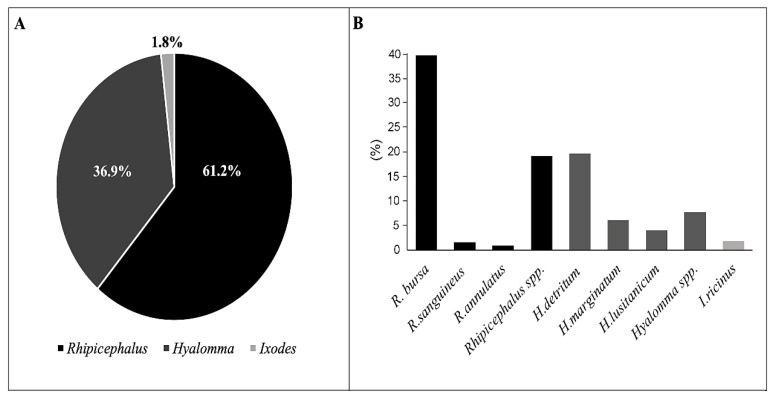
A total of 518 male and 537 female hard ticks were manually detached from bovines. The females varied in size due to different feeding durations. We did not find any immature tick stages in the collected samples. Three common tick genera were identified: Rhipicephalus (646/1055, 61.2%), Hyalomma (390/1055, 36.9%), and Ixodes (19/1055, 1.8%). Among these genera, seven different hard tick species were recognized. R. bursa and H. detritum were the two most common species, followed by H. marginatum and H. lusitanicum and finally I. ricinus, R. sanguineus, and R. annulatus. For morphologically deformed Hyalomma and Rhipicephalus ticks, specimens were determined to the genus level only. The difference in frequencies among identified species is described in Figure 1. In addition, co-infestation with different species of ticks was observed on the sampled bovines.
Figure 1.
Frequencies of ixodid tick species identified in our study: (A) The percentages of the three genera of ticks identified. (B) The frequencies of specific tick species identified.
2.2. Infection Rates of Microorganisms and Their Co-Infection Rates in Ticks
Among all investigated ticks (Table 1), six pathogen genera were identified as follows: Rickettsia (79.1%, 186/235), Theileria (17.8%, 42/235), Anaplasma (14.4%, 34/235), Bartonella (6.8%, 16/235), Borrelia (6.8%, 16/235), and Babesia (2.5%, 6/235). The overall rate of Francisella-like endosymbionts (FLE) was 62.9% (148/235), with a positivity rate of 88.5% (100/113) for Hyalomma spp., followed by 81.3% (11/13) for Ixodes and finally 33.9% (37/109) for the genus Rhipicephalus. Neither Coxiella spp. nor Hepatozoon spp. were detected in any ticks.
Table 1.
Rates of infection with tick-borne pathogens in tick species with 95% confidence intervals (CI).
| Species | Borrelia spp. | A. marginale | A. centrale |
R.
aeschlimannii |
R. massiliae | R. monacensis | R. helvetica | Bartonella spp. | T. orientalis | B. bigemina | FLE |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
R. bursa
(n = 51) |
4 | 2 | 0 | 11 | 0 | 0 | 0 | 3 | 8 | 0 | 15 |
| (7.8%) | (3.9%) | (21.5%) | (5.8%) | (15.6%) | (29.4%) | ||||||
| (0.4–15.2%) | (0–9.2%) | (10.2–32.5%) | (0–12.2%) | (5.7–25.6%) | (16.8–41.9%) | ||||||
|
R. sanguineus
(n = 07) |
1 | 0 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 5 | 1 |
| (14.2%) | (57.1%) | (28.5%) | (71.4%) | (14.2%) | |||||||
| (0–40%) | (20.4–93.8%) | (0–61.9%) | (37.9–100%) | (0–40.2%) | |||||||
|
R. annulatus
(n = 01) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| (100%) | |||||||||||
| (0–100%) | |||||||||||
|
Rhipicephalus spp.
(n = 50) |
1 | 6 | 1 | 5 | 0 | 0 | 0 | 3 | 11 | 0 | 21 |
| (2%) | (12%) | (2%) | (10%) | (6%) | (22%) | (42%) | |||||
| (0–5.8%) | (3–21%) | (0–5.8%) | (1.6–18.3%) | (0–12.5%) | (10.5–33.4%) | (28.3–55.6%) | |||||
|
H. detritum
(n = 41) |
3 | 2 | 0 | 2 | 2 | 0 | 0 | 3 | 6 | 0 | 37 |
| (7.3%) | (4.8%) | (4.8%) | (4.8%) | (7.3%) | (14.3%) | (90.2%) | |||||
| (0–15.2%) | (0–11.3%) | (0–11.3%) | (0–11.3%) | (0–15.2%) | (3.5–25%) | (81.1–99.3%) | |||||
|
H. marginatum
(n = 15) |
0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 12 |
| (6.6%) | (6.6%) | (80%) | |||||||||
| (0–19.1%) | (0–19.1%) | (59.7–100%) | |||||||||
|
H. lusitanicum
(n = 04) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 |
| (25%) | (100%) | ||||||||||
| (0–67.4%) | (25–100%) | ||||||||||
|
Hyalomma spp.
(n = 53) |
6 | 1 | 0 | 8 | 5 | 0 | 0 | 4 | 10 | 0 | 47 |
| (11.3%) | (1.8%) | (15.1%) | (9.4%) | (7.5%) | (18.8%) | (88.6%) | |||||
| (2.7–19.8%) | (0–5.3%) | (5.9–25.6%) | (1.5–17.2%) | (0.2–14.1%) | (8.2–29.3%) | (80–97.1%) | |||||
|
I. ricinus
(n = 13) |
1 | 5 | 0 | 0 | 0 | 5 | 2 | 0 | 6 | 0 | 11 |
| (7.6%) | (38.4%) | (38.4%) | (15.3%) | (46.1%) | (84.6%) | ||||||
| (0–22%) | (12–64.8%) | (12–64.8%) | (0–34.8%) | (19–73.1%) | (64.9–100%) | ||||||
|
Total
(n = 235) |
16 | 16 | 1 | 27 | 11 | 5 | 2 | 16 | 42 | 6 | 148 |
| 6.8% | 6.8% | 0.4% | 11.5% | 4.6% | 2.1% | 0.8% | 6.8% | 17.8% | 2.5% | 62.9% | |
| (3.5–10%) | (3.5–10%) | (0–1.2%) | (7.4–15.5%) | (1.9–7.2%) | (0.2–3.9%) | (0–1.9%) | (3.5–10%) | (13–22.6%) | (0.5–4.4%) | (56.7–69%) |
FLE: Francisella-like endosymbiont.
Among the Rickettsia-tested ticks, 11.5% (27/235) were positive for R. aeschlimannii and 4.8% (11/235) for R. massiliae, and a total of 26.3% (62/235) of ticks were positive for undetermined Rickettsia spp.; 10 Rickettsia specimens were chosen randomly and sequenced. The BLAST search on these 10 specimens (confirmed by gltA gene amplification) revealed that five showed 100% identity with R. monacensis (Accession nos. JX040640.1 and KJ663735.1), two showed 100% identity with R. helvetica (Accession no. KY231199.1), two showed 100% identity with uncultured Rickettsia sp. (Accession no. KU596570.1) and one showed 81.8% identity with another uncultured Rickettsia sp. (Accession no. AP019865.1). For the 36.5% (86/235) of positive samples harboring multiple Rickettsia species at the same time, we sequenced 10 specimens and the BLAST results indicated 100% identity with an uncultured Rickettsia sp. (Accession no. KU596570.1).
R. aeschlimannii was detected in H. marginatum, H. detritum and R. bursa ticks. Four out of six R. sanguineus ticks were positive for R. massiliae. R. massiliae was also amplified in Hyalomma ticks. R. monacensis and R. helvetica were detected only in I. ricinus ticks (Table 1).
DNA of Theileria spp. was detected in 17.8% (42/235) of samples, and nested PCRs followed by sequencing showed an identity of 100% with T. orientalis (Accession no. MH208641.1). This species was detected in H. detritum, H. marginatum, R. bursa, R. sanguineus, and I. ricinus ticks (Table 1)
Anaplasma marginale was the most prevalent species of the genus Anaplasma (16/235, 6.80%), followed by A. centrale detected in 0.4% (1/235) of samples. The remaining 50% (17/34) of samples were confirmed by nested PCR, with the sequencing of nine positive samples revealing identity with unidentified Anaplasma spp. BLAST searches using the 16S rRNA gene sequence showed 99.6% identity with uncultured Anaplasma sp. clone AMCRO1 (Accession no. MN187218.1) in five samples, 98% with uncultured Anaplasma sp. clone AR2-1 (Accession no. MH250195.1) in two samples and 98% with uncultured Anaplasma sp. Oriente CuBov140 clone (Accession no. MK804764.1) in two samples. A. marginale was found in all three identified tick genera (Rhipicephalus, Hyalomma, and Ixodes), and A. centrale was detected only in Rhipicephalus (Table 1).
The genus Borrelia was detected in 6.8% (16/235) of the investigated ticks. None of the eight species-specific primer/probe sets used for high-throughput microfluidic PCR gave a positive signal; therefore, these samples were confirmed by nested PCR followed by sequencing. All PCR-positive samples (16/235) were confirmed by nested PCR: 10/16 were positive on gel and 4/10 were sequenced, from which only two sequences were obtained. The BLAST analysis on the fla gene sequence showed 92.37% identity with an unidentified Borrelia species (Accession no. KR677091.1).
Pathogens belonging to the genus Bartonella were detected in 6.8% (16/235) of the sampled ticks using the high-throughput microfluidic PCR system. Species identification was attempted on all positive samples with conventional PCR target ftsZ gene, but no results were obtained by sequencing.
DNA of Babesia bigemina was found in 6/235 (2.55%). The positive specimens of B. bigemina detected in R. annulatus and R. sanguineus ticks were confirmed by nested PCR and sequencing with an identity of 97.32% (Accession no. MH257721.1).
Among all the ticks analyzed, 80.4% (189/235) were positive for at least one microorganism. The level of single infection was 20% (47/235) with one microorganism; the level of co-infection was 36.6% (86/235) with two, 21.7% (51/235) with three, and 2.1% (5/235) with four microorganisms. Ticks of the genus Ixodes showed the highest rate of co-infection (12/13, 92.3%), followed by ticks of the genus Hyalomma (91/113, 80.5%) and the genus Rhipicephalus with a rate of co-infection of 35.7% (39/109). Double co-infection between FLE and Rickettsia spp. was most common in three tick’s genera identified with the respective frequencies of 48.6% (55/113), 46.2% (6/13), and 14.6% (16/109) in Hyalomma, Ixodes, and Rhipicephalus. Triple co-infections with FLE, Rickettsia spp. and Theileria spp. were identified with a high frequency in ticks of the genus Ixodes 2/13 (15.3%) followed by ticks of the genus Hyalomma (13/113, 11.5%) and the genus Rhipicephalus (3/109, 2.7%). Likewise, the highest rate of co-infection with FLE, Rickettsia spp. and Anaplasma spp. was detected in the Ixodes genus (3/13, 23.1%), followed by the genus Rhipicephalus (5/109, 4.5%) and the genus Hyalomma (5/113, 4.4%). Triple co-infections with FLE, and Rickettsia spp. either with Borrelia spp. or Bartonella spp., were observed primarily in the genus Hyalomma with frequencies of 5.3% (6/113). Finally, quadruple infections were found mostly in the genus Ixodes (1/13, 7.6%), followed by the genus Hyalomma (3/113, 2.6%) and the genus Rhipicephalus (1/109, 0.9%) (details of co-infections between different species of microorganisms are given in Table S1).
3. Discussion
In this study, we identified three tick genera: Rhipicephalus, Hyalomma, and Ixodes. Among these, the thermophilic species R. bursa, H. detritum, and H. marginatum predominated, and the mesophilic species I. ricinus was less abundant (Figure 1). These results corroborate the previous reports from northern Algeria [7,11,16]. In future studies, a combination of morphological and molecular identification of ticks should be performed to identify ticks at the genus level. In North Africa, TBP detection is usually carried out using classic methods, such as PCR or real-time PCRs, which are based on the use of specific primers and/or probes [11,17,18]. This approach is limited by the characterization of a single pathogen. However, recent studies have described the importance of co-infections in the transmission of pathogens and the expression of disease severity. In this study, a new approach, based on high-throughput microfluidic technology, was used to detect 36 different microorganisms (pathogens and symbionts), and to monitor TBP circulation in hard ticks infesting cattle in northeastern Algeria.
It is important to notice that some of the ticks collected on bovines were engorged. Therefore, we cannot conclude that these ticks are vectors for the detected pathogens. The latter may have become infected with the microorganism while feeding on previously infected animals, and/or through co-feeding. Moreover, detection of DNA does not indicate that the pathogen is alive; it simply corresponds to potentially inert traces of the microorganism in the engorged tick. Here, we identified four different genera of bacteria (Rickettsia, Anaplasma, Borrelia, Bartonella) and two genera of protozoans (Theileria and Babesia) with an overall frequency of 80.4% of ticks infected with at least one of these TBPs. For example, Rickettsia spp. had the highest infection rate and four different species (i.e., R. aeschlimannii, R. massiliae, R. monacensis, and R. helvetica) were identified. Belonging to the pathogenic spotted fever group, R. aeschlimannii and R. massiliae cause infections in animals and humans worldwide [19]. The presence of R. aeschlimannii in H. marginatum ticks confirmed previous studies on the association of this vector with this bacteria species [19,20]. Surprisingly, we detected R. aeschlimannii DNA in H. detritum and R. bursa ticks also (Table 1). This species was first isolated from H. marginatum in Morocco and then in other African and Mediterranean countries [10,15,19,21,22,23]. Ticks of the genus Hyalomma have been reported as vectors of R. aeschlimannii, including H. marginatum, H. marginatum rufipes, H. aegyptium, and H. truncatum [19,23]. In addition, this bacteria species has been found in R. appendiculatus in South Africa and Haemaphysalis ticks in Spain [24], and R. turanicus in Greece and China [25,26]. Nevertheless, Rickettsia spp. is considered as an endosymbiont of Hyalomma ticks, and several other tick genera. R. aeschlimannii has been identified in a large percentage of Hyalomma ticks, with unknown clinical relevance [27]. According to this finding and our results, we suggest that tick species other than Hyalomma can also be a suitable carrier for this bacterial species.
In addition, we detected R. massiliae in R. sanguineus. This tick has been described as a vector of this species in the Mediterranean region [8], but we also amplified the DNA of this species for the first time in Hyalomma ticks from Algeria. A similar study in Pakistan also using the microfluidic technique amplified R. massiliae in Hyalomma hussaini and H. anatolicum ticks [28]. The implication of Hyalomma species in the transmission cycle of this pathogen needs to be clarified. In our study, I. ricinus harbored R. monacensis and R. helvetica that were not detected in the other tick species examined (Table 1). This confirms the previous results observed in North Africa as well as Europe [19,20,29,30,31,32].
Regarding the protozoan Theileria and Babesia species, we detected T. orientalis and B. bigemina. Although the primary vectors of T. orientalis are Haemaphysalis spp. ticks [33], in our study, R. bursa, H. detritum, H. marginatum, and I. ricinus ticks were found to be positive for this pathogen. The association of this pathogen with R. bursa and R. annulatus ticks have also been confirmed in Romania and Algeria, respectively [11,34]. Moreover, a strain closely related to T. buffeli has been detected in R. sanguineus, R. bursa, R. annulatus, H. marginatum, Dermacentor marginatum, and Haemaphysalis punctata ticks in Sardinia, Italy [35]. Taking these results together, the transmission of this TBP does not appear to be limited exclusively to Haemaphysalis spp., suggesting that other tick species may be involved in the transmission cycle worldwide. In the present study, we confirmed the presence of B. bigemina in Algerian R. annulatus, which is its principal vector [36]. Furthermore, we also found this protozoan species in R. sanguineus, lending support to previous reports from Sardinia and Iran [35,37]. The biological transmission of A. marginale involves at least 20 species of ticks mainly of the genera Dermacentor and Rhipicephalus [38]. Here, we report the presence of A. marginale in R. bursa, H. detritum, and I. ricinus. Similarly, A. marginale has been reported in R. bursa in Corsica and Portugal [39,40], and in I. ricinus in Hungary [41]. A. centrale, which is transmitted by Rhipicephalus simus [38], was confirmed individually on one Rhipicephalus sp. tick. Bartonella spp. was identified in multiple species of the tick genera Hyalomma and Rhipicephalus. There is little information on Bartonella transmission in cattle, but based on our recent study reporting B. bovis in ticks from Algeria, these ticks may play a critical role in the transmission of this pathogen in this area [42]. We also amplified DNA from Borrelia sp. in 16 specimens of different tick species. Usually, ticks from the genus Ixodes are the vectors of the zoonotic bacteria species B. burgdorferi s.l. from the Lyme disease group, whereas B. theileri, from the relapsing fever group that causes bovine borreliosis, is transmitted by Rhipicephalus ticks [43,44]. In previous research conducted in Algeria, DNA from these bacteria has been amplified in different tick genera in Algeria using quantitative PCR [31].
Coxiella, Francisella, and Rickettsia are the three major endosymbionts reported in ticks [6,45]. In addition to TBPs, we reported for the first time in Algeria a high rate of infection with Francisella-like endosymbionts in all tick species tested except R. annulatus. Furthermore, 60% of investigated ticks harbored unidentified Rickettsia spp., whereas all the specimens were negative for Coxiella-like endosymbionts. It has been reported that symbionts previously considered non-pathogenic may turn out to be pathogenic, as demonstrated for R. helvetica, R. slovaca, and R. monacensis [20,46]. However, the reasons that make one bacterial species become pathogenic while others remain non-pathogenic are still unclear [45]. In future studies, phylogenetic analysis targeting several genes for pathogenic and non-pathogenic microorganisms will allow us to better answer this question. Our study showed co-infection of cattle ticks with a large variety of pathogenic and non-pathogenic microorganisms. High co-infection rates of TBPs in livestock ticks has been reported in other countries [15,23,28,47]. Co-infections represent a significant risk of the cumulative effect of pathogen transfer and subsequent development of the associated diseases [48]. In addition, co-infections can cause severe complications in the treatment of tick-borne illnesses. Therefore, the study of the associations of multiple microorganisms within the same tick is of high importance, and can help better identify potential clinical co-infections to improve the epidemiological knowledge and control of TBPs [6].
4. Materials and Methods
4.1. Ethical Statement
Sample collection for this study was authorized by the National Veterinary School of Algiers, Algeria and by the Veterinary Services Department of the Tizi-Ouzou province, Algeria. All bovines were sampled according to Algerian regulations.
4.2. Tick Collection and Morphological Identification
A total of 1055 ticks were randomly collected from 112 bovines (with an average of nine ticks/bovine) between May 2015 and November 2017 in eight locations in the Kabylia region located in northeastern Algeria. The samples collected from each individual bovine were stored in 70% ethanol. Tick species (or genera) were determined using taxonomic keys developed by Walker et al. [49] and based on morphological characteristics observed under a stereomicroscope.
4.3. DNA Extraction
A random selection of one to three ticks (males and females) from each individual bovine (235 total ticks) were used for genomic DNA extraction. Prior to extraction, ticks were washed three times in sterile distilled water, dried and crushed individually using a sterile scalpel. DNA was then extracted from whole ticks using the NucleoSpin ® Tissue DNA extraction kit (Macherey–Nagel, Düren, Germany) following the manufacturer’s instructions and stored at −20 ℃ until use.
4.4. DNA Pre-Amplification
The Perfecta PreAmp SuperMix (Quanta Biosciences, Beverly, Massachusetts, USA) was used for DNA pre-amplification according to the manufacturer’s instructions. First, all primers pair targeting TBPs were pooled, combining equal volumes with a final concentration of 0.2 μM each.
Then, reactions were carried out in a final volume of 5 μL containing 1 µL of 5× Perfecta Preamp, 1.25 μL of the pooled primer mixture, 1.5 µL of distilled water and 1.25 μL of tick DNA.
The PCR run conditions consisted of a first cycle of 95 ℃ (2 min), followed by 14 cycles of amplification at 95 ℃ (10 s) and 60 ℃ (3 min). The pre-amplified products were diluted in ultra-pure water at 1:10 and kept at −20 ℃ until use.
4.5. High-Throughput Microfluidic Real-Time PCR
The BioMark™ real-time PCR system (Fluidigm, San Francisco, USA) was used for the high-throughput microfluidic system, which can handle 48 real-time PCR reactions simultaneously in one single chip [6,14]. Real-time PCR reactions were performed using 6-carboxyfluorescein (FAM)- and black hole quencher (BHQ1)-labeled TaqMan probes with TaqMan Gene expression master mix in accordance with the manufacturer’s instructions (Applied Biosystems, France). Amplification consisted of 2 min at 50 ℃, 10 min at 95 ℃, followed by 40 cycles of two-step amplification of 15 s at 95 ℃, and 1 min at 60 ℃.
We carried out the high-throughput microfluidic real-time PCR to screen the bacterial and parasitic species known to circulate in ticks. We thus simultaneously targeted 36 different microorganisms belonging to 10 genera (the list of pathogens is shown in Table 2 and the list of each primer set and probes used is given in Table 3). Moreover, two primer/probe sets targeting I. ricinus and R. sanguineus tick species were used as a positive control of tick species identification and one primer/probe set targeting tick species was used to control DNA extractions (primers/probes for other tick species were not available at that time). One negative control (ultra-pure water) and one positive control DNA of Escherichia coli were included in each chip. The results were acquired on the BioMarkTM real-time PCR system and analyzed using the Fluidigm real-time PCR analysis software to obtain crossing point (Cp) values.
Table 2.
Bacteria and parasites targeted in our study.
| Genus | Species | Numbers | |
|---|---|---|---|
| Bacteria | Borrelia | B. burgdorferi senso stricto, B. garinii, B. afzelii, B. valaisiana, B. lusitaniae, B. spielmanii, B. bissettii, B. miyamotoi. | 8 |
| Anaplasma | A. marginale, A. platys, A. phagocytophilum, A.ovis, A. centrale, A. bovis. | 6 | |
| Ehrlichia | E. ruminantium, Neoehrlichia mikurensis. | 2 | |
| Rickettsia | R. conorii, R. slovaca, R. massiliae, R. prowazekii, R. aeschlimannii, R. andeanae, R. typhi, R. akari | 8 | |
| Bartonella | B. henselae | 1 | |
| Francisella | F. tularensis, Francisella-like endosymbionts. | 2 | |
| Coxiella | C. burnettii. | 1 | |
| Parasites | Babesia | B. microti, B. ovis, B. bigemina, B. bovis, B. caballi, B. divergens. | 6 |
| Theileria | T. mutans, T. velifera. | 2 | |
| Hepatozoon | Hepatozoon spp. | ||
| Total | 10 | 36 |
Table 3.
| Pathogen | Target Gene | Primers (F, R; 5′-3′) and Probe (P) | Length (bp) |
|---|---|---|---|
| Borrelia burgdorferi s.s. | rpoB | F-GCTTACTCACAAAAGGCGTCTT | 83 |
| R-GCACATCTCTTACTTCAAATCCT | |||
| P-AATGCTCTTGGACCAGGAGGACTTTCA | |||
| Borrelia garinii | rpoB | F-TGGCCGAACTTACCCACAAAA | 88 |
| R-ACATCTCTTACTTCAAATCCTGC | |||
| P-TCTATCTCTTGAAAGTCCCCCTGGTCC | |||
| Borrelia afzelii | fla | F-GGAGCAAATCAAGATGAAGCAAT | 116 |
| R-TGAGCACCCTCTTGAACAGG | |||
| P-TGCAGCCTGAGCAGCTTGAGCTCC | |||
| Borrelia valaisiana | ospA | F-ACTCACAAATGACAGATGCTGAA | 135 |
| R-GCTTGCTTAAAGTAACAGTACCT | |||
| P-TCCGCCTACAAGATTTCCTGGAAGCTT | |||
| Borrelia lusitaniae | rpoB | F-CGAACTTACTCATAAAAGGCGTC | 87 |
| R-TGGACGTCTCTTACTTCAAATCC | |||
| P-TTAATGCTCTCGGGCCTGGGGGACT | |||
| Borrelia spielmanii | fla | F-ATCTATTTTCTGGTGAGGGAGC | 71 |
| R-TCCTTCTTGTTGAGCACCTTC | |||
| P-TTGAACAGGCGCAGTCTGAGCAGCTT | |||
| Borrelia bissettii | rpoB | F-GCAACCAGTCAGCTTTCACAG | 118 |
| R-CAAATCCTGCCCTATCCCTTG | |||
| P-AAAGTCCTCCCGGCCCAAGAGCATTAA | |||
| Borrelia miyamotoi | glpQ | F-CACGACCCAGAAATTGACACA | 94 |
| R-GTGTGAAGTCAGTGGCGTAAT | |||
| P-TCGTCCGTTTTCTCTAGCTCGATTGGG | |||
| Borrelia spp. | 23S rRNA | F-GAGTCTTAAAAGGGCGATTTAGT | 73 |
| R-CTTCAGCCTGGCCATAAATAG | |||
| P-AGATGTGGTAGACCCGAAGCCGAGT | |||
| Anaplasma marginale | msp1 | F-CAGGCTTCAAGCGTACAGTG | 85 |
| R-GATATCTGTGCCTGGCCTTC | |||
| P-ATGAAAGCCTGGAGATGTTAGACCGAG | |||
| Anaplasma platys | groEL | F-TTCTGCCGATCCTTGAAAACG | 75 |
| R-CTTCTCCTTCTACATCCTCAG | |||
| P-TTGCTAGATCCGGCAGGCCTCTGC | |||
| Anaplasma phagocytophilum | msp2 | F-GCTATGGAAGGCAGTGTTGG | 77 |
| R-GTCTTGAAGCGCTCGTAACC | |||
| P-AATCTCAAGCTCAACCCTGGCACCAC | |||
| Anaplasma ovis | msp4 | F-TCATTCGACATGCGTGAGTCA | 92 |
| R-TTTGCTGGCGCACTCACATC | |||
| P-AGCAGAGAGACCTCGTATGTTAGAGGC | |||
| Anaplasma centrale | groEL | F-AGCTGCCCTGCTATACACG | 79 |
| R-GATGTTGATGCCCAATTGCTC | |||
| P-CTTGCATCTCTAGACGAGGTAAAGGGG | |||
| Anaplasma bovis | groEL | F-GGGAGATAGTACACATCCTTG | 73 |
| R-CTGATAGCTACAGTTAAGCCC | |||
| P-AGGTGCTGTTGGATGTACTGCTGGACC | |||
| Anaplasma spp. | 16S rRNA | F-CTTAGGGTTGTAAAACTCTTTCAG | 160 |
| R-CTTTAACTTACCAAACCGCCTAC | |||
| P-ATGCCCTTTACGCCCAATAATTCCGAACA | |||
| Ehrlichia spp. | 16S rRNA | F-GCAACGCGAAAAACCTTACCA | 98 |
| R-AGCCATGCAGCACCTGTGT | |||
| P-AAGGTCCAGCCAAACTGACTCTTCCG | |||
| Ehrlichia ruminantium | gltA | F-CCAGAAAACTGATGGTGAGTTAG | 116 |
| R-AGCCTACATCAGCTTGAATGAAG | |||
| P-AGTGTAAACTTGCTGTTGCTAAGGTAGCATG | |||
| Neoehrlichia mikurensis | groEL | F-AGAGACATCATTCGCATTTTGGA | 96 |
| R-TTCCGGTGTACCATAAGGCTT | |||
| P-AGATGCTGTTGGATGTACTGCTGGACC | |||
| Rickettsia conorii | 23S-5S ITS | F-CTCACAAAGTTATCAGGTTAAATAG | 118 |
| R-CGATACTCAGCAAAATAATTCTCG | |||
| P-CTGGATATCGTGGCAGGGCTACAGTAT | |||
| Rickettsia slovaca | 23S-5S ITS | F-GTATCTACTCACAAAGTTATCAGG | 138 |
| R-CTTAACTTTTACTACAATACTCAGC | |||
| P-TAATTTTCGCTGGATATCGTGGCAGGG | |||
| Rickettsia massiliae | 23S-5S ITS | F-GTTATTGCATCACTAATGTTATACTG | 128 |
| R-GTTAATGTTGTTGCACGACTCAA | |||
| P-TAGCCCCGCCACGATATCTAGCAAAAA | |||
| Rickettsia prowazekii | gltA | F-CAAGTATCGGTAAAGATGTAATCG | 151 |
| R-TATCCTCGATACCATAATATGCC | |||
| P-ATATAAGTAGGGTATCTGCGGAAGCCGAT | |||
| Rickettsia aeschlimannii | ITS | F-CTCACAAAGTTATCAGGTTAAATAG | 134 |
| R-CTTAACTTTTACTACGATACTTAGCA | |||
| P-TAATTTTTGCTGGATATCGTGGCGGGG | |||
| Rickettsia andeanae | OmpB | F-GGCGGACAGGTAACTTTTGG | 165 |
| R-AAGGATCATAGTATCAGGAACTG | |||
| P- ACACATAGTTGACGTTGGTACAGACGGTAC | |||
| Rickettsia typhi | OmpB | F-CAGGTCATGGTATTACTGCTCA | 133 |
| R-GCAGCAGTAAAGTCTATTGATCC | |||
| P-ACAAGCTGCTACTACAAAAAGTGCTCAAAATG | |||
| Rickettsia akari | OmpB | F-GTGCTGTTGCAGGTGGTAC | 101 |
| R-TAAAGTAATACCGTGTAATGCAGC | |||
| P-ATTACCAGCACCGTTACCTATATCACCGG | |||
| Rickettsia spp. | gltA | F-GTCGCAAATGTTCACGGTACTT | 78 |
| R-TCTTCGTGCATTTCTTTCCATTG | |||
| P-TGCAATAGCAAGAACCGTAGGCTGGATG | |||
| Bartonella henselae | pap31 | F-CCGCTGATCGCATTATGCCT | 107 |
| R-AGCGATTTCTGCATCATCTGCT | |||
| P-ATGTTGCTGGTGGTGTTTCCTATGCAC | |||
| Bartonella spp. | ssrA | F-CGTTATCGGGCTAAATGAGTAG | 118 |
| R-ACCCCGCTTAAACCTGCGA | |||
| P-TTGCAAATGACAACTATGCGGAAGCACGTC | |||
| Francisella tularensis | tul4 | F-ACCCACAAGGAAGTGTAAGATTA | 76 |
| R-GTAATTGGGAAGCTTGTATCATG | |||
| P-AATGGCAGGCTCCAGAAGGTTCTAAGT | |||
| Francisella -like endosymbionts | fopA | F-GGCAAATCTAGCAGGTCAAGC | 91 |
| R-CAACACTTGCTTGAACATTTCTAG | |||
| P-AACAGGTGCTTGGGATGTGGGTGGTG | |||
| Coxiella burnettii | IS1111 | F-TGGAGGAGCGAACCATTGGT | 86 |
| R-CATACGGTTTGACGTGCTGC | |||
| P-ATCGGACGTTTATGGGGATGGGTATCC | |||
| Coxiella burnettii | idc | F-AGGCCCGTCCGTTATTTTACG | 74 |
| R-CGGAAAATCACCATATTCACCTT | |||
| P-TTCAGGCGTTTTGACCGGGCTTGGC | |||
| Babesia microti | CCTeta | F-ACAATGGATTTTCCCCAGCAAAA | 145 |
| R-GCGACATTTCGGCAACTTATATA | |||
| P-TACTCTGGTGCAATGAGCGTATGGGTA | |||
| Babesia ovis | 18SrRNA | F-TCTGTGATGCCCTTAGATGTC | 92 |
| R-GCTGGTTACCCGCGCCTT | |||
| P-TCGGAGCGGGGTCAACTCGATGCAT | |||
| Babesia bigemina | 18SrRNA | F-ATTCCGTTAACGAACGAGACC | 99 |
| R-TTCCCCCACGCTTGAAGCA | |||
| P-CAGGAGTCCCTCTAAGAAGCAAACGAG | |||
| Babesia bovis | CCTeta | F-GCCAAGTAGTGGTAGACTGTA | 100 |
| R-GCTCCGTCATTGGTTATGGTA | |||
| P-TAAAGACAACACTGGGTCCGCGTGG | |||
| Babesia caballi | Rap1 | F-GTTGTTCGGCTGGGGCATC | 94 |
| R-CAGGCGACTGACGCTGTGT | |||
| P-TCTGTCCCGATGTCAAGGGGCAGGT | |||
| Babesia divergens | hsp70 | F-CTCATTGGTGACGCCGCTA | 83 |
| R-CTCCTCCCGATAAGCCTCTT | |||
| P-AGAACCAGGAGGCCCGTAACCCAGA | |||
| Theileria mutans | ITS | F-CCTTATTAGGGGCTACCGTG | 119 |
| R-GTTTCAAATTTGAAGTAACCAAGTG | |||
| P-ATCCGTGAAAAACGTGCCAAACTGGTTAC | |||
| Theileria velifera | 18S rRNA | F-TGTGGCTTATCTGGGTTCGC | 151 |
| R-CCATTACTTTGGTACCTAAAACC | |||
| P-TTGCGTTCCCGGTGTTTTACTTTGAGAAAG | |||
| Theileria spp. | 18S | F-TGAACGAGGAATGCCTAGTATG | 104 |
| R-CACCGGATCACTCGATCGG | |||
| P-TAGGAGCGACGGGCGGTGTGTAC | |||
| Hepatozoon spp. | 18S rRNA | F-ATTGGCTTACCGTGGCAGTG | 175 |
| R-AAAGCATTTTAACTGCCTTGTATTG | |||
| P-ACGGTTAACGGGGGATTAGGGTTCGAT | |||
| Tick species | 16SrRNA | F-AAATACTCTAGGGATAACAGCGT | 99 |
| R-TCTTCATCAAACAAGTATCCTAATC | |||
| P-CAACATCGAGGTCGCAAACCATTTTGTCTA | |||
| Rhipicephalus sanguineus | ITS2 | F-TTGAACGCTACGGCAAAGCG | 110 |
| R-CCATCACCTCGGTGCAGTC | |||
| P-ACAAGGGCCGCTCGAAAGGCGAGA | |||
| Ixodes ricinus | ITS2 | F-CGAAACTCGATGGAGACCTG | 77 |
| R-ATCTCCAACGCACCGACGT | |||
| P-TTGTGGAAATCCCGTCGCACGTTGAAC | |||
| Escherichia coli | eae | F-CATTGATCAGGATTTTTCTGGTGATA | 102 |
| R-CTCATGCGGAAATAGCCGTTA | |||
| P-ATAGTCTCGCCAGTATTCGCCACCAATACC |
F: forward; R: reverse; P: probe; bp: base pairs.
4.6. Standard/Nested PCR and Sequencing
Samples were considered positive for a given microorganism if the Cp value was <30, and if they were positive for a given pathogen species and its corresponding genus and negative for all other species belonging to the same genus. Other positive samples were confirmed using nested PCR or conventional PCR with primers targeting genes or regions different from those of the BioMark™ system (for the primers used, see Table 4). The PCR products were sequenced by Eurofins Genomics (https://Cochin.eurofins.com (accessed on 1 October 2020)) then assembled using BioEdit software (Ibis Biosciences, Carlsbad, CA, USA). Our results were compared in online BLAST (http://www.ncbi.nlm.nih.gov/blast (accessed on 1 October 2020)). searches against sequences publicly available in GenBank (https://www.ncbi.nlm.nih.gov/ (accessed on 1 October 2020)).
Table 4.
List of primers used in this study for confirmation using nested and conventional PCR.
| Pathogen | Target Gene | Primer Name | Sequence (5′-3′) | Amplicon Size (bp) | T | Reference |
|---|---|---|---|---|---|---|
| Borrelia spp. | FlaB | FlaB280 F | GCAGTTCARTCAGGTAACGG | 645 | 55 | [50] |
| FlaL R | GCAATCATAGCCATTGCAGATTGT | |||||
| FlaB_737F | GCATCAACTGTRGTTGTAACATTAACAGG | |||||
| FlaLL R | ACATATTCAGATGCAGACAGAGGT | 407 | ||||
| Anaplasma spp. | 16S rRNA | EHR1 F | GAACGAACGCTGGCGGCAAGC | 693 | 60 | [51] |
| EHR2 R | AGTA(T/C)CG(A/G)ACCAGATAGCCGC | |||||
| EHR3 F | TGCATAGGAATCTACCTAGTAG | |||||
| EHR2 R | AGTA(T/C)CG(A/G)ACCAGATAGCCGC | 629 | 55 | |||
| Rickettsia spp. | gltA | Rsfg877 | GGG GGC CTG CTC ACG GCG G | 381 | 56 | [52] |
| Rsfg1258 | ATT GCA AAA AGT ACA GTG AAC A | |||||
| Bartonella spp. | ftsZ | 257 F | GCCTTCAAGGAGTTGATTTTGTTGTTGCCA | 580 | 55 | [53] |
| 258 R | ACGACCCATTTCATGCATAACAGAAC | |||||
|
Babesia/
Theileria /Hepatozoon spp. |
18S rRNA | BTH 18S 1st F | GTGAAACTGCGAATGGCTCATTAC | 1500 | 58 | [54] |
| BTH 18S 1st R | AAGTGATAAGGTTCACAAAACTTCCC | |||||
| BTH 18S 2nd F | GGCTCATTACAACAGTTATAGTTTATTTG | |||||
| BTH 18S 2nd R | CGGTCCGAATAATTCACCGGAT |
F: forward; R: reverse; bp: base pairs; T: hybridization temperature.
5. Conclusions
We used high-throughput microfluidic real-time PCR to detect TBPs in cattle ticks in Algeria. We confirmed the presence of several bacteria and protozoan species in tick-infested cattle with a fast and highly sensitive molecular method. We detected pathogenic and non-pathogenic (e.g., Francisella-like endosymbionts) microorganisms in ticks feeding on cattle, with a high frequency of co-infections. Further studies on endosymbionts and their possible interactions with pathogens transmitted by ticks are now needed, particularly with regard to the highly predominant ticks Rhipicephalus and Hyalomma in Algeria. Finally, our results highlight the possible involvement of tick species other than those typically reported in the transmission of some pathogens of interest in Algeria; these atypical associations deserve to be investigated further.
Acknowledgments
We would like to acknowledge the farmers who accepted to participate in the study. We thank UMR BIPAR, MiTick team for supporting this research.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/10/3/362/s1; Table S1: Mono and mixed infection between microorganisms detected in cattle ticks.
Author Contributions
Conceptualization, G.B., S.M., H.-J.B. and N.A.; methodology, G.B., H.-J.B., N.A., S.M., C.G. and L.Š.; software, G.B. and C.G., validation, S.M., H.-J.B. and N.A.; formal analysis, G.B.; investigation, G.B.; resources, H.-J.B. and S.M.; data curation, G.B.; writing—original draft preparation, G.B.; writing—review and editing, G.B., N.A., H.-J.B., S.M. and L.Š.; visualization, H.-J.B., N.A. and S.M.; supervision, N.A., H.-J.B.; project administration, N.A., H.-J.B.; funding acquisition, N.A., H.-J.B. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by UMR BIPAR, Maisons-Alfort, France. S.M. Research is supported by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). UMR BIPAR is supported by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (grant no. ANR-10-LABX-62-IBEID).
Data Availability Statement
Data are available under request to corresponding author s and published in GenBank for sequences.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pesquera C., Portillo A., Palomar A.M., Oteo J.A. Investigation of tick-borne bacteria (Rickettsia spp., Anaplasma spp., Ehrlichia spp. and Borrelia spp.) in ticks collected from Andean tapirs, cattle and vegetation from a protected area in Ecuador. Parasit. Vectors. 2015;8:46. doi: 10.1186/s13071-015-0662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehman A., Nijhof A.M., Sauter-Louis C., Schauer B., Staubach C., Conraths F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit. Vectors. 2017;10:190. doi: 10.1186/s13071-017-2138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminade C., McIntyre K.M., Jones A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019;1436:157–173. doi: 10.1111/nyas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De La Fuente J., Estrada-Pena A., Venzal J.M., Kocan K.M., Sonenshine D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 5.Silatsa B.A., Simo G., Githaka N., Mwaura S., Kamga R.M., Oumarou F., Keambou C., Bishop R.P., Djikeng A., Kuiate J.R., et al. A comprehensive survey of the prevalence and spatial distribution of ticks infesting cattle in different agro-ecological zones of Cameroon. Parasit. Vectors. 2019;12:489. doi: 10.1186/s13071-019-3738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moutailler S., Valiente Moro C., Vaumourin E., Michelet L., Tran F.H., Devillers E., Cosson J.F., Gasqui P., Van V.T., Mavingui P., et al. Co-Infection of Ticks: The Rule Rather Than the Exception. PLoS Negl. Trop. Dis. 2016;10:e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benchikh Elfegoun M.C., Kohil K., Gharbi M., Afoutni L., Benachour M.L. Cinétique d’infestation par les tiques des bovins de la région subhumide de Constantine en Algérie. Rev. D’éle. Méd. Vét. Pays Trop. 2019;72:41–45. doi: 10.19182/remvt.31726. [DOI] [Google Scholar]
- 8.Bitam I., Parola P., Matsumoto K., Rolain J.M., Baziz B., Boubidi S.C., Harrat Z., Belkaid M., Raoult D. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann. N. Y. Acad. Sci. 2006;1078:368–372. doi: 10.1196/annals.1374.073. [DOI] [PubMed] [Google Scholar]
- 9.Dib L., Lafri I., Boucheikhchoukh M., Dendani Z., Bitam I., Benakhla A. Seasonal distribution of Rickettsia spp. in ticks in northeast Algeria. New Microbes New Infect. 2019;27:48–52. doi: 10.1016/j.nmni.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelkadir K., Palomar A.M., Portillo A., Oteo J.A., Ait-Oudhia K., Khelef D. Presence of Rickettsia aeschlimannii, ‘Candidatus Rickettsia barbariae’ and Coxiella burnetii in ticks from livestock in Northwestern Algeria. Ticks Tick-Borne Dis. 2019;10:924–928. doi: 10.1016/j.ttbdis.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Sadeddine R., Diarra A.Z., Laroche M., Mediannikov O., Righi S., Benakhla A., Dahmana H., Raoult D., Parola P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick-Borne Dis. 2020;11:101330. doi: 10.1016/j.ttbdis.2019.101330. [DOI] [PubMed] [Google Scholar]
- 12.Noda H., Munderloh U.G., Kurtti T.J. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 1997;63:3926–3932. doi: 10.1128/AEM.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duron O., Noël V., McCoy K.D., Bonazzi M., Sidi-Boumedine K., Morel O., Vavre F., Zenner L., Jourdain E., Durand P., et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 2015;11:e1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Boye Pihl T.P., et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grech-Angelini S., Stachurski F., Vayssier-Taussat M., Devillers E., Casabianca F., Lancelot R., Uilenberg G., Moutailler S. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 2020;67:745–757. doi: 10.1111/tbed.13393. [DOI] [PubMed] [Google Scholar]
- 16.Benchikh Elfegoun M.C., Gharbi M., Djebir S., Kohil K. Dynamique d’activité saisonnière des tiques ixodidés parasites des bovins dans deux étages bioclimatiques du nord-est algérien. Rev. D’éle. Méd. Vét. Pays Trop. 2013;66:117–122. doi: 10.19182/remvt.10150. [DOI] [Google Scholar]
- 17.Sarih M., Socolovschi C., Boudebouch N., Hassar M., Raoult D., Parola P. Spotted fever group Rickettsiae in ticks, Morocco. Emerg. Infect. Dis. 2008;14:1067–1073. doi: 10.3201/eid1407.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Said Y., Lahmar S., Dhibi M., Rjeibi M.R., Jdidi M., Gharbi M. First survey of ticks, tick-borne pathogens (Theileria, Babesia, Anaplasma and Ehrlichia) and Trypanosoma evansi in protected areas for threatened wild ruminants in Tunisia. Parasitol. Int. 2020;81:102275. doi: 10.1016/j.parint.2020.102275. [DOI] [PubMed] [Google Scholar]
- 19.Bitam I. Vectors of Rickettsiae in Africa. Ticks Tick-Borne Dis. 2012;3:382–386. doi: 10.1016/j.ttbdis.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Portillo A., Santibáñez S., García-Álvarez L., Palomar A.M., Oteo J.A. Rickettsioses in Europe. Microbes Infect. 2015;17:834–838. doi: 10.1016/j.micinf.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Beati L., Meskini M., Thiers B., Raoult D. Rickettsia aeschlimannii sp. nov., a new spotted fever group Rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 1997;47:548–554. doi: 10.1099/00207713-47-2-548. [DOI] [PubMed] [Google Scholar]
- 22.Mediannikov O., Diatta G., Fenollar F., Sokhna C., Trape J.F., Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl. Trop. Dis. 2010;4:e821. doi: 10.1371/journal.pntd.0000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehounoud C.B., Yao K.P., Dahmani M., Achi Y.L., Amanzougaghene N., Kacou N’Douba A., N’Guessan J.D., Raoult D., Fenollar F., Mediannikov O. Multiple Pathogens Including Potential New Species in Tick Vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2016;10:e0004367. doi: 10.1371/journal.pntd.0004367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández Soto P., Encinas Grandes A., Pérez Sánchez R. Rickettsia aeschlimannii in Spain: Molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg. Infect. Dis. 2003;9:889–890. doi: 10.3201/eid0907.030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germanakis A., Chochlakis D., Angelakis E., Tselentis Y., Psaroulaki A. Rickettsia aeschlimannii infection in a man, Greece. Emerg. Infect. Dis. 2013;19:1176–1177. doi: 10.3201/eid1907.130232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Q.Q., Guo L.P., Wang A.D., Mu L.M., Zhang K., Chen C.F., Zhang W.J., Wang Y.Z. The first detection of Rickettsia aeschlimannii and Rickettsia massiliae in Rhipicephalus turanicus ticks, in northwest China. Parasit. Vectors. 2015;8:2–5. doi: 10.1186/s13071-015-1242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Socolovschi C., Mediannikov O., Raoult D., Parola P. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Vet. Res. 2009;40:34. doi: 10.1051/vetres/2009017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghafar A., Cabezas-Cruz A., Galon C., Obregon D., Gasser R.B., Moutailler S., Jabbar A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors. 2020;13:1. doi: 10.1186/s13071-019-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dib L., Bitam I., Bensouilah M., Parola P., Raoult D. First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clin. Microbiol. Infect. 2009;15:261–262. doi: 10.1111/j.1469-0691.2008.02277.x. [DOI] [PubMed] [Google Scholar]
- 30.Kernif T., Messaoudene D., Ouahioune S., Parola P., Raoult D., Bitam I. Spotted fever group rickettsiae identified in Dermacentor marginatus and Ixodes ricinus ticks in Algeria. Ticks Tick-Borne Dis. 2012;3:380–381. doi: 10.1016/j.ttbdis.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Boucheikhchoukh M., Laroche M., Aouadi A., Dib L., Benakhla A., Raoult D., Parola P. MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018;57:39–49. doi: 10.1016/j.cimid.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Akl T., Bourgoin G., Souq M.L., Appolinaire J., Poirel M.T., Gibert P., Abi Rizk G., Garel M., Zenner L. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite. 2019;26:20. doi: 10.1051/parasite/2019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFadden A.M.J., Rawdon T.G., Meyer J., Makin J., Morley C.M., Clough R.R., Tham K., Müllner P., Geysen D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naïve cattle. N. Z. Vet. J. 2011;59:79–85. doi: 10.1080/00480169.2011.552857. [DOI] [PubMed] [Google Scholar]
- 34.Andersson M.O., Tolf C., Tamba P., Stefanache M., Radbea G., Rubel F., Waldenström J., Dobler G., Chițimia-Dobler L. Babesia, Theileria, and Hepatozoon species in ticks infesting animal hosts in Romania. Parasitol. Res. 2017;116:2291–2297. doi: 10.1007/s00436-017-5537-4. [DOI] [PubMed] [Google Scholar]
- 35.Chisu V., Alberti A., Zobba R., Foxi C., Masala G. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in ticks from domestic and wild hosts in Sardinia. Acta Trop. 2019;196:60–65. doi: 10.1016/j.actatropica.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Bock R., Jackson L., De Vos A., Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:S247–S269. doi: 10.1017/S0031182004005190. [DOI] [PubMed] [Google Scholar]
- 37.Rajabi S., Esmaeilnejad B., Tavassoli M. A molecular study on Babesia spp. in cattle and ticks in West-Azerbaijan province, Iran. Vet. Res. Forum Int. Q. J. 2017;8:299–306. [PMC free article] [PubMed] [Google Scholar]
- 38.Battilani M., De Arcangeli S., Balboni A., Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017;49:195–211. doi: 10.1016/j.meegid.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Dahmani M., Davoust B., Tahir D., Raoult D., Fenollar F., Mediannikov O. Molecular investigation and phylogeny of Anaplasmataceae species infecting domestic animals and ticks in Corsica, France. Parasit. Vectors. 2017;10:302. doi: 10.1186/s13071-017-2233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrolho J., Antunes S., Santos A.S., Velez R., Padre L., Cabezas-Cruz A., Santos-Silva M.M., Domingos A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick-Borne Dis. 2016;7:443–448. doi: 10.1016/j.ttbdis.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Hornok S., Micsutka A., Fernández de Mera I.G., Meli M.L., Gönczi E., Tánczos B., Mangold A.J., Farkas R., Lutz H., Hofmann-Lehmann R., et al. Fatal bovine anaplasmosis in a herd with new genotypes of Anaplasma marginale, Anaplasma ovis and concurrent haemoplasmosis. Res. Vet. Sci. 2012;92:30–35. doi: 10.1016/j.rvsc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Boularias G., Azzag N., Gandoin C., Bouillin C., Chomel B., Haddad N., Boulouis H.J. Bartonella bovis and Bartonella chomelii infection in dairy cattle and their ectoparasites in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020;70:101450. doi: 10.1016/j.cimid.2020.101450. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S.P., Amanfu W., Losho T.C. Bovine borreliosis in Botswana. Onderstepoort J. Vet. Res. 2000;67:221–223. [PubMed] [Google Scholar]
- 44.Mc Coya B.N., Maïgab O., Schwana T.G. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-Borne Dis. 2014;5:401–403. doi: 10.1016/j.ttbdis.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahantarig A., Trinachartvanit W., Baimai V., Grubhoffer L. Hard ticks and their bacterial endosymbionts (or would be pathogens) Folia Microbiol. 2013;58:419–428. doi: 10.1007/s12223-013-0222-1. [DOI] [PubMed] [Google Scholar]
- 46.Raoult D., Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997;10:694–719. doi: 10.1128/CMR.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gondard M., Delannoy S., Pinarello V., Aprelon R., Devillers E., Galon C., Pradel J., Vayssier-Taussat M., Albina E., Moutailler S. Upscaling surveillance of tick-borne pathogens in the French Caribbean islands. Pathogens. 2020;9:176. doi: 10.3390/pathogens9030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mixson T.R., Campbell S.R., Gill J.S., Ginsberg H.S., Reichard M.V., Schulze T.L., Dasch G.A. Prevalence of Ehrlichia, Borrelia, and Rickettsial Agents in Amblyomma americanum (Acari: Ixodidae) Collected from Nine States. J. Med. Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:POEBAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Walker A.R., Bouattour A., Camicas J., Estrada-Peña A., Horak I., Latif A., Pegram R., Preston P. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Bioscience Reports; Edinburgh, UK: 2003. [Google Scholar]
- 50.Loh S.M., Gofton A.W., Lo N., Gillett A., Ryan U.M., Irwin P.J., Oskam C.L. Novel Borrelia species detected in echidna ticks, Bothriocroton concolor, in Australia. Parasit. Vectors. 2016;9:339. doi: 10.1186/s13071-016-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rar V.A., Fomenko N.V., Dobrotvorsky A.K., Livanova N.H., Rudakova S.A., Fedorov E.G., Astanin V.B., Morozova O.V. Tick borne pathogen detection, Western Siberia, Russia. Emerg. Infect. Dis. 2005;11:1708–1715. doi: 10.3201/eid1111.041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regnery R.L., Spruill C.L., Plikaytis B.D. Genotypic identification of Rickettsiae and estimation of intra species sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/JB.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veikkolainen V., Vesterinen E.J., Lilley T.M., Pulliainen A.T. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg. Infect. Dis. 2014;20:960–967. doi: 10.3201/eid2006.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masatani T., Hayashi K., Andoh M., Tateno M., Endo Y., Asada M., Kusakisako K., Tanaka T., Gokuden M., Hozumi N., et al. Detection and molecular characterization of Babesia, Theileria, and Hepatozoon species in hard ticks collected from Kagoshima, the southern region in Japan. Ticks Tick-Borne Dis. 2017;8:581–587. doi: 10.1016/j.ttbdis.2017.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available under request to corresponding author s and published in GenBank for sequences.