Abstract
Fermented soybean products, such as cheonggukjang (Japanese natto), doenjang (soy paste), ganjang (soy sauce), and douchi, are widely consumed in East Asian countries and are major sources of bioactive compounds. The fermentation of cooked soybean with bacteria (Bacillus spp.) and fungi (Aspergillus spp. and Rhizopus spp.) produces a variety of novel compounds, most of which possess health benefits. This review is focused on the preventive and ameliorative potential of fermented soy foods and their components to manage neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases.
Keywords: fermented soybean products, Parkinson’s disease, Alzheimer’s disease, isoflavones, gut microbiota
1. Introduction
Population aging is a global demographic trend. According to the “2019 Revision of World Population Prospects” [1,2], the proportion of people aged 65 years or over worldwide is projected to reach nearly 16% by 2050, and 23% by 2100. However, this extended life expectancy is closely associated with a vulnerability to age-related disorders, such as neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [3]. Unfortunately, there is currently no effective treatment for these devastating diseases. Recently, an increasing number of studies have focused on the development of dietary measures as well as preventive regimens for these conditions [4,5]. For instance, the root of Angelica gigas and Platycodon grandiflorus, as well as Lactobacillus helveticus and phosphatidylserine, have been approved regarding the claim of cognition improvement by the Korean government, which allowed them to be processed and marketed as health functional foods.
The nutritional values and medicinal effects of soybean or its constituents are well documented [6,7,8,9,10,11]. In several Asian countries, including China, Indonesia, Japan, and Korea, fermented soybean products, such as doenjang (soybean paste and Japanese miso), ganjang (soy sauce), natto, and tempeh, have been extensively consumed since ancient times. Numerous studies published in the past decades have revealed that fermented soy products have multifarious health benefits, such as serum cholesterol-lowering, anti-diabetic, anti-hypertensive, anti-cardiovascular, and anti-neuroinflammatory effects [10,12,13,14]. Recently, soybean and its fermented products have received much attention regarding their effects on the gut microbiota, which are linked to the pathogenesis of various neurological disorders, including depression, anxiety, autism, AD, and PD [15,16,17].
This review article discusses the protective effects of popular fermented soy foods and their components in the context of neurodegenerative diseases, with a focus on AD and PD, and describes the possible mechanisms underlying the beneficial effects of these foods.
2. Types of Fermented Soy Products
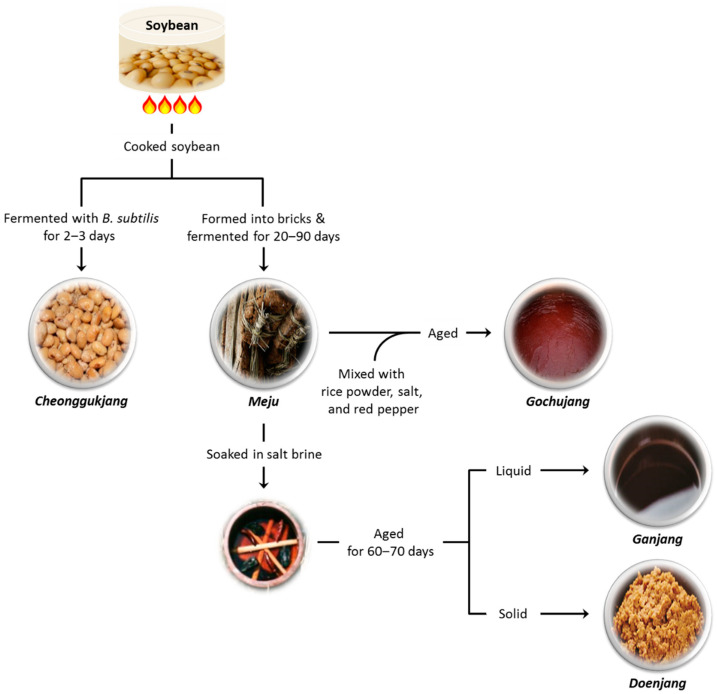
Soybean has been processed into numerous types of products, such as soymilk, tofu, sprouts, and fermented products (Figure 1). The fermented products of soybean include doenjang (soybean paste), ganjang (soy sauce), Korean cheonggukjang, Japanese natto, Korean gochujang, Indonesian tempeh, sieng (Cambodia, Laos), pepok (Myanmar), thua nao (Thailand), and knema (India, Nepal, and Bhutan) [18].
Figure 1.
Examples of Korean fermented soy products.
Korean cheonggukjang and Japanese natto are both produced via two main steps, i.e., cooking and fermentation. In the first step, soybean is soaked in water at room temperature for 18 h, followed by steaming at 121 °C for 30 min. The second step consists in the fermentation of cooked soybean with airborne Bacillus species, including Bacillus subtilis, originating from the ambient environment or from inoculation for 48 h (Figure 1) [19].
While most of these products, including cheonggukjang and natto, are fermented with Bacillus spp., some products, such as doenjang, soy sauce, and tempeh, are manufactured by fermenting cooked soybean with fungi, such as Aspergillus and Rhizopus, resulting in the extensive breakdown of soy components and the production of novel bioactive compounds [18].
Douchi, which is a traditional Chinese food that is prepared using fermented and salted black soybeans, has been a popular seasoning in foods and a folk medicine in China for centuries [20]. Sufu or furu is one of the fermented soybean products, which has been consumed as a side dish in China over the centuries [21,22]. Sufu is a cheese-like product that is made by Aspergillus oryzae in solid-state fermentation of salted and ripened tofu through activities of hydrolytic enzymes, such as protease, α-amylase, β-amylase, and lipase [21,22].
3. Bioactive Components of Fermented Soy Products
Soybean contains a variety of biologically functional components that can be grouped into isoflavones, soyasaponins, lignans, cinnamic acid derivatives, terpenes, and sterols. The fermentation process results in the chemical modification and reduction of soy components. Although soybean is known to contain anti-nutritional factors, such as phytates, trypsin inhibitors and lectins [23], most fermented soy products have been analyzed to contain very small amounts of these factors, when compared with raw soybean [24]. In particular, lactic acid bacteria (LAB)-mediated fermentation can reduce phytates and trypsin inhibitors [25], and hydrolyze tannic acid via their tannase activities [26,27]. Almost all lectins in soybeans are destroyed during fermentation processes over 72 h [28]. Moreover, various novel compounds are generated during the fermentation process not originally present in raw soybean (Table 1).
Table 1.
Fermented soy products and phytochemicals.
| Fermented Soy Products | Phytochemicals | Biological Functions | References |
|---|---|---|---|
| Cheonggukjang (natto) | Free isoflavones, levan, γPGA, natto kinase, vitamin K | Antioxidant, anti-hypertension, fibrinolysis, bone health | [29,30,31] |
| Doenjang (miso) | Free isoflavones, non-DDMP-conjugated soyasaponins (I, III, Be), peptides, amino acids, MRPs, kojic acid | Antioxidant, anti-obesity, anti-tumorigenic, anti-hypertension, anti-sarcopenia, skin whitening, immune modulation, sympathetic nerve activity, anti-diabetic activity | [32,33,34,35,36,37,38,39,40,41,42,43] |
| Ganjang | Amino acids, peptides, MRPs, 1-methyl-1,2,3,4-tetrahydro-β-carboline and 1-methyl-β-carboline | Anti-platelet activity, anti-allergenicity, anti-hypertension | [34,44,45,46,47] |
| Douchi | Subtilisin DFE, isoflavones, peptides | Antioxidant, fibrinolysis, α-amylase inhibition, ACE inhibition, anti-acetylcholine esterase | [20,48,49,50,51] |
| Tempeh | 6,7,4’-trihydroxyisoflavone, isoflavones, peptides | Antioxidant, cognitive improvement, BACE1 inhibition, | [52,53,54] |
| Gochujang | Capsaicin, free isoflavones | Antioxidant, anti-obesity | [55,56,57] |
| Fermented soymilk | Free isoflavones, water-soluble vitamins (B2, B6, folate, and B12), vitamin K2 (menaquinone-7) | Antioxidant, anti-obesity, increased mineral bioavailability, anti-mutagenic, hypocholesterolemic effects | [58,59,60,61,62,63] |
γPGA, gamma-polyglutamate; MRPs, Maillard reaction products, DDMP, 2,3-Dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one; ACE, angiotensin-converting enzyme.
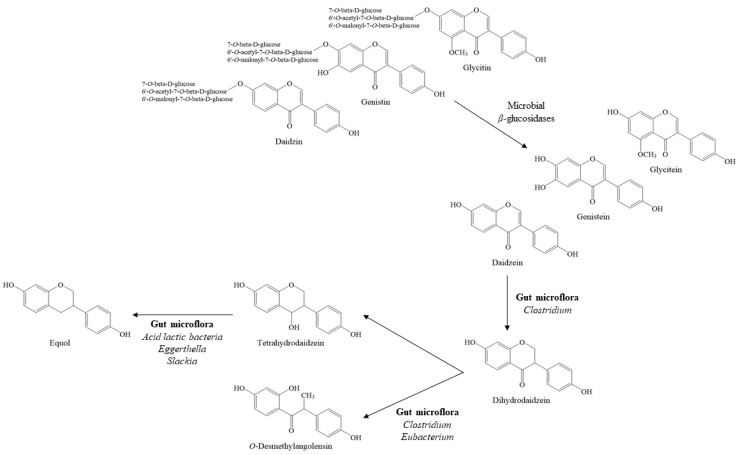
The fermentation of cooked soybean with Aspergillus and other microorganisms, as performed during the manufacture of meju (a brick of dried fermented soybeans), generates novel compounds as well as extensively converts isoflavone glycosides into aglycones (Figure 2). For instance, free isoflavones account for 2.67% of the total isoflavones of soy flour, whereas aglycones represent more than 75% of the total isoflavones present in the product after fermentation for 48 h with Aspergillus oryzae [32]. In addition, extended fermentation was reported to reduce the amount of both aglycones and glycosides, although the relative ratio of aglycones increases with fermentation [64].
Figure 2.
The biotransformation of soy isoflavones by microorganisms during food fermentation or in the gut.
Further, daidzein may be converted to O-desmethylangolensin and equol by gut microflora (Figure 2), although these metabolites are rarely found in fermented soy foods [65]. Similarly, genistein is metabolized to dihydrogenistein, 6′-hydroxy-O-desmethylangolensin, and 4-hydroxyphenyl-2-propionic acid by lactic acid bacteria and Bifidobacteria [66].
The recommended daily dose of soy isoflavones varies, and ranges from 40 to 120 mg according to different studies [67,68,69]. A recent study reported that the intake of 900 mg unconjugated soy isoflavones per day was safe and well tolerated in healthy postmenopausal women [67]. Significant increases in physiologically active isoflavone aglycone levels have been reported during fermentation processes, ranging from 16.74 µg/g soy when soaked to 31.44 µg/g soy at fermentation [68,69]. Furthermore, genistein levels in the fermented soybean products, miso and natto, ranged between 38.5 to 229.1 µg/g food and were higher than the soybean products, soy milk (1.9 to 13.9 µg/g food), and tofu (94.8 to 137.7 µg/g food), suggesting the β-glycosyl bonds of genistin were cleaved and transformed to genistein during fermentation [70].
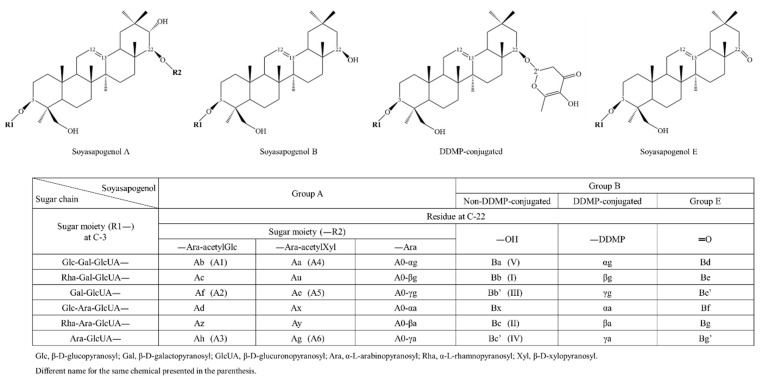
Soyasaponins, a group of distinctive compounds present in soybean, also undergo an extensive change during fermentation by fungi in the course of the doenjang manufacturing process. Moreover, 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP)-conjugated soyasaponins are the molecular forms present in unprocessed soybeans, whereas unconjugated soyasaponins are mainly detected in processed soy products (Figure 3) [33]. The DDMP group in conjugated soyasaponins is easily removed from the parent compounds upon changes in temperature, pH, and solvent conditions [71]. After the fermentation of soybean using naturally occurring microorganisms, as in the preparation of meju and doenjang, the levels of unconjugated soyasaponins are increased, while the content of DDMP-conjugated soyasaponins is reduced by steaming [72]. The meju fermentation and brining steps have been reported to increase several unconjugated soyasaponins and decrease DDMP-conjugated soyasaponins. Fermentation of meju results in the conversion of most DDMP-conjugated soyasaponins to unconjugated soyasaponins I, III, and Be (Figure 3) [71].
Figure 3.
Chemical structure and nomenclature of soyasaponins (adapted from the literature with minor modifications [73,74]).
During the cheonggukjang or natto manufacturing process, the fermentation of cooked soybean by bacteria, such as Bacillus subtilis, produces various metabolites, including peptones, peptides, amino acids, sugars, organic acids, natto kinase, levan, and polyglutamic acid, which is responsible for the sticky and slimy texture of these foods; moreover, these secondary metabolites dramatically affect the organoleptic and biological properties of the resultant products [75]. The contents of free amino acids and fatty acids were increased by protease and lipase activities, respectively, during sufu ripening period [21,76]. Eight biogenic amines, such as putrescine, cadaverine, spermidine, spermine, tyramine, 2-phenethylamine, histamine, and tryptamine, have been reported to be formed by the decarboxylation of free amino acids [76].
Maillard reaction products (MRPs), which are a group of well-known brownish compounds, are newly formed through chemical reaction between amino acids and sugars during the manufacturing process of fermented food products. Several MRPs, including fructose-lysine, were identified in soy sauce and miso [34]. MRPs have been reported to have antioxidant activity in vitro, cancer-preventive activity, and beneficial effects on gut health [77].
Many other compounds were also reported in different soy products. The anti-platelet alkaloids 1-methyl-1,2,3,4-tetrahydro-β-carboline and 1-methyl-β-carboline were detected in soy sauce. These two compounds suppress the maximal aggregation response induced by adenosine 5′-diphosphate, epinephrine, collagen, platelet-activating factor, and thrombin, respectively [34,78]. In addition, the asperparaline A, B, and C alkaloids were identified in the insoluble residue of whole soybean (called okara) fermented with Aspergillus japonicus JV-23, and were reported to have paralytic activity in silkworms [34,79]. Because these alkaloids are not present in raw soybean, the compounds were most likely to have been newly generated or introduced in the final product during the fermentation process.
Tempeh, which is a traditional Indonesian soy food made of fermented soybean, is popular because of its umami taste. Recently, a novel 15-amino-acid peptide (GENEEEDSGAIVTVK) that mainly contributes to the umami taste was identified [80].
In addition, soymilk contains low levels of water-soluble vitamins, such as riboflavin (vitamin B2) and cobalamin (vitamin B12). When it is fermented, the nutritional value of soymilk is enhanced by the high-level production of fat-soluble vitamin K2 (menaquinone-7) and water-soluble B vitamins, such as vitamins B2, B6, and B12 and folate [81,82].
Furanones, such as 4-hydroxy-2(or 5)-ethyl-5(or2)-methyl-3(2H)-furanone (HEMF), 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF), and 4-hydroxy-5-methyl-3(2H)-furanone (HMF), are probably formed from the Maillard reaction during yeast fermentation in the production of Japanese and Korean soy sauces [44,83,84]. HEMF is considered a key flavor compound in soy sauce, and HDMF and HMF are reported to have antioxidant activities and anti-carcinogenic effects [44,84].
4. Isoflavones and Neurodegenerative Diseases
AD and PD, the two most common neurodegenerative disorders, are characterized by a series of events encompassing abnormal protein aggregation, oxidative stress, neuroinflammation, and neuronal death. The canonical molecular changes of AD include the formation of insoluble amyloid beta peptide (Aβ) aggregates and neurofibrillary tangles (NFTs) primed by the hyperphosphorylated tau protein. PD is characterized by the intracellular accumulation of insoluble α-synuclein and the formation of Lewy bodies in neurons and glial cells [85]. This abnormal protein deposition contributes to neuronal dysfunction and degeneration, and further impairs the architecture and function of neural circuits in specific areas of the brain [85,86,87]. As oxidative stress and neuroinflammation are widely believed to be critical events in the pathological development of AD and PD, compounds with antioxidative and/or anti-inflammatory activity are expected to retard the progression of these two neurodegenerative diseases.
4.1. Isoflavones and AD
Soy isoflavones have been reported to have neuroprotective effects in various animal studies. In particular, the compounds were shown to attenuate AD-related pathology and reduce its progression. These effects of isoflavones are most likely associated with their antioxidative activity and their affinity for estrogen receptors [88].
In an experiment using a mouse model, soy isoflavones significantly attenuated galactose-induced oxidative stress, as evidenced by the reversal of the oxidative stress- and AD-related parameters, such as increased serum levels of thiobarbituric-acid-reactive substances in the brain and serum; increased levels of protein-bound carbonyls in the brain, kidney and liver; increased serum levels of advanced glycation end products; and increased expression of caspase-3 and Bax in splenocytes and of Aβ, β-amyloid precursor protein-cleaving enzyme-1 (BACE-1), and presenilin-1 (a subunit of γ-secretase) in the brain [89]. In addition, dietary isoflavones improved cognitive function in an ovariectomized rat model of AD [90].
Among the isoflavones, genistein was reported to ameliorate the Aβ-induced impairments responsible for neuronal death in AD animal models by exerting antioxidant activity, abating Aβ toxicity, inhibiting nitric oxide (NO) generation, and reducing tau pathology [90,91]. Another study also demonstrated that soy isoflavones reduced neuronal death and prevented degeneration of the nervous system through anti-inflammatory activity, regulation of cell signaling pathways, and antioxidant activity [92].
4.2. Isoflavones and PD
Genistein has been reported to protect dopaminergic neurons against lipopolysaccharide (LPS)-induced neuroinflammation in a PD model [93]. Moreover, it suppresses the production of superoxide, tumor necrosis factor alpha (TNFα), and nitric oxide (NO) in microglia and mesencephalic neuron–glia cultures [88]. Microglial cells in the brain are triggered by infection or injury, thereby releasing proinflammatory mediators, such as cytokines and reactive oxygen species (ROS) [94,95]. These cytokines and ROS may facilitate the formation of complexes with proteins, thus altering the function of crucial proteins and eventually causing cell death [95].
Interestingly, genistein was found to inhibit the accumulation and production of ROS and NO, thus protecting dopaminergic neurons from oxidative neuronal injury [88,94]. In addition, genistein exerted a protective effect on dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice, which was likely attributable to the suppression of apoptotic neuronal cell death in midbrain via the upregulation of the Bcl 2 gene [96].
Daidzein was also reported to attenuate the LPS-induced expression of inflammatory mediators in a murine microglial BV-2 cell line. More specifically, pre-exposure of cells to daidzein significantly suppressed the expression of the proinflammatory factors NO and interleukin 6 (IL-6), with dampening of p38 mitogen-activated protein kinase (MAPK) phosphorylation, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, and ROS production [97].
A recent report demonstrated that soy isoflavones attenuated the oxidative stress and inflammation induced by atrazine, as indicated by malondialdehyde accumulation and glutathione depletion, and increased TNFα and IL-6 release, respectively, in the substantia nigra. In addition, atrazine downregulated LC3-II and Beclin-1 and upregulated p62 in the substantial nigra, suggesting autophagy inhibition. In contrast, these effects were reversed by pre-treatment with soy isoflavones, suggesting that the compounds can restore the autophagy function of dopaminergic neurons in the substantia nigra. In fact, the dysregulation of autophagy is emerging as a major etiology of PD as reported by a number of studies [92,98,99,100]. In particular, restoring mitochondria-specific autophagy (termed mitophagy) in PD neurons has been demonstrated to prevent oxidative stress and dopaminergic neuronal damage in in vivo models and in patient-derived cells [98,101,102,103].
Furthermore, isoflavones (daidzein, genistein, biochanin A, and formononetin) induce mitochondrial biogenesis in myoblasts and renal cells through the activation of the NAD-dependent deacetylase sirtuin-1 (SIRT1)/peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) pathway [104,105]. In turn, genistein upregulates the estrogen-related receptor alpha (ERR-α), ERR-β, PGC-1α, SIRT3, and the nuclear factor erythroid 2-related factor 2 (Nrf2) downstream enzymes, thus enhancing mitochondrial biogenesis and antioxidant responses [106].
Considering the effects of isoflavones on mitochondrial biogenesis and mitophagy in several tissues, it is highly plausible that isoflavones regulate mitochondrial homeostasis in the central nervous system (CNS) [107,108]. As several studies reported that PD is associated with dysregulated mitophagy, isoflavones in soy products offer a good therapeutic and/or preventive potential for PD [92,98,99,100].
5. Other Components in Fermented Soy Products and Neurodegenerative Diseases
As mentioned previously, it is most likely that antioxidants have a beneficial effect on neurodegenerative diseases, which are intimately related to oxidative stress. Fermented soy products have been reported to contain not only isoflavones, but also other antioxidant molecules.
5.1. Amino Acids and Peptides with Antioxidant Activity
Recent studies reported that the free amino acids, such as alanine, glycine, histidine, leucine, methionine, phenylalanine, tryptophan, tyrosine, and valine, present in peptides have antioxidant activity [109,110,111]. For instance, the radical scavenging activities of a peptide can be attributed to imidazole, indole, and phenol groups in histidine, tryptophan, and tyrosine, respectively [110,112], in which those chemical groups can easily donate protons to electron-deficient radicals [111].
Watanabe and coworkers also claimed that the amino acids and peptides formed during fermentation are responsible for antioxidant activity in the water-soluble fraction of Rhizopus-fermented tempeh [38]; the contents of free amino acids and peptides were found to increase during the aerobic fermentation with Rhizopus, with concomitant increase in antioxidant activity in the water-soluble fraction.
5.2. Soyasaponins
Soyasaponins have been reported to significantly inhibit NF-κB activation in LPS-treated microglial BV-2 cells. In particular, soyasapogenol B (SB) recovered LPS-induced cognitive deficit in a mouse model [113]. Furthermore, SB significantly increased cAMP response element-binding protein phosphorylation and brain-derived neurotrophic factor expression in LPS-treated mice and corticosterone-stimulated SH-SY5Y cells, and inhibited NF-κB activation in LPS-treated mice. Soyasaponin subclasses A1, A2, and I also inhibited the LPS-induced cyclooxygenase 2 (COX-2) expression in a dose-dependent manner through negative regulation of NF-kB. These studies consistently suggest that soyasaponins attenuate memory deficits by suppressing NF-κB-mediated inflammation [113,114].
6. Effect of Fermented Soy Products and Gut Microbiota on Neurodegenerative Diseases
It is well established that even the short-term dietary intake intake of fermented soy products can affect the composition of the human gut microbiota. For instance, an animal-based diet decreases the levels of Firmicutes, which metabolize dietary plant polysaccharides (Eubacterium rectale, Roseburia, and Ruminococcus bromii), while increasing the abundance of bile-tolerant microorganisms (Alistipes, Bacteroides, and Bilophila) [115,116]. A large-scale genome-wide analysis of human fecal samples demonstrated that the consumption of LAB-containing foods is reflected in the gut microbial balance [117].
6.1. Fermented Soy Products and Gut Microbiota
The fermentation of soybean confers unique sensory attribute, extends shelf life, modifies the nutritional quality and phytochemical profile, and enhances digestibility. In addition, the microorganisms used in fermentation themselves are a good source of prebiotics as well as probiotics.
Several recent studies have reported that the composition and structure of the gut microbiota could be changed by the consumption of fermented soy foods, such as fermented soymilk (yogurt), fermented tofu, soy paste (doenjang), and soy sauce (ganjang).
Fermented soymilk manufactured using Lactobacillus and Bifidobacterium affects populations of human fecal microbiota [118] in a desirable way, inducing effects that include alleviation of menopausal symptoms [119], control of hypercholesterolemia [120], modulation of mitogen-stimulated splenocyte proliferation, and TNFα production [121]. Fermented soy foods prepared with Enterococci and Lactobacilli were shown to increase these bacterial population in the gut. Similarly, water-soluble extracts of soybean fermented with Lactobacillus helveticus and Enterococcus faecium were reported to significantly increase the populations of Enterococci, Lactobacilli, and Bifidobacteria in the gut, and decrease the level of Enterobacteriaceae. The consumption of soymilk fermented by Enterococcus faecium or Lactobacillus plantarum significantly increased the populations of Bifidobacterium, Enterococcus, and Lactobacillus in the gut, while their effect on the abundance of gut Clostridium and Bacteroides was inconsistent. Tempeh, an Indonesian traditional fermented soy product, has been shown to increase the relative abundance of Bifidobacterium, Lactobacillus, Escherichia coli, and Enterococcus in an in vitro gut simulator model. In contrast, the consumption of natto was shown to increase the abundance of Bacillus and Bifidobacterium and decrease Clostridia and Enterobacteriaceae in the gut microbiota [122].
Fermented soy products manufactured by traditional methods in Korea are reported to contain high levels of Bacillus species, such as Bacillus amyloliquefaciens, Bacillus subtilis, and Bacillus licheniformis [123]. Nam and coworkers analyzed over 12,000 bacterial pyrosequences in a commercial brand of cheonggukjang and found that the vast majority of bacteria were assigned to the phylum Firmicutes (>95%), followed by Proteobacteria (<5%). Most of the Firmicutes were Bacillus species, although the levels of Bacillus subtilis (1.1–45.2%), Bacillus licheniformis (3.2–33.6%), and Bacillus amyloliquefaciens (0.2–9.2%) varied greatly according to brand. In some cheonggukjang samples, specific unclassified Bacillus species and lactic acid bacteria were the dominant microbes [124]. Kim and coworkers examined the bacterial communities in meju and also found that the predominant phylum was Firmicutes (93.6%) [125].
Recent studies demonstrated that the consumption of cheonggukjang fermented by Bacillus subtilis or Bacillus amyloliquefaciens increased the abundance of Bifidobacteriales and Lactobacillales in the gut. However, the population of Enterobacteriales, which are considered harmful bacteria, were lowered by a cheonggukjang-containing diet [126,127].
6.2. Gut Microbiota and Neurodegenerative Diseases
Emerging evidence strongly supports the notion that gut microbial composition and balance is closely associated with the risk of neurodegenerative diseases. The human gastrointestinal (GI) tract is estimated to harbor 100 trillion microorganisms, generally called the gut microbiota, which is determined by both host genetics and environmental factors [128,129]. An increasing number of studies have shown that the gut microbiota critically affects the function and development of the CNS [15].
6.2.1. Gut Microbiota and PD
PD is a multifactorial neurodegenerative disease that is believed to be caused by both genetic changes and environmental factors. It is characterized by the deposition of toxic α-synuclein inclusions that lead to the death of dopaminergic neurons in the striatum and, consequently, motor dysfunction [130,131].
The pathogenesis of PD has been speculated to be associated with the GI tract as α-synuclein deposition was observed in the peripheral nervous system, especially in the enteric and pelvic plexus, of patients with PD [132]. A subsequent study suggested that the PD pathology originates from the peripheral organs in which α-synuclein is seeded, such as the GI tract and nasal cavity, before being retrograde transported to the cerebral cortex through the vagal nerve [132,133]. Furthermore, many patients with PD experience hyposmia and GI problems prior to the manifestation of classical PD symptoms, and patients with inflammatory bowel disease are also at a higher risk of developing PD [134]. Thus, the microbiota present in the GI tract are most likely involved in the pathogenesis of PD, in a direct or indirect manner.
The apoptotic death of dopaminergic neuronal cells in the substantia nigra has been widely believed to be triggered by oxidative stress [135,136]. Excessive production of ROS can cause oxidative damage in the brain of patients with PD, as shown by increased DNA damage and lipid peroxidation in the substantia nigra [18]. Increase in protein oxidation is also observed in many areas of the brain, with the substantia nigra being particularly susceptible [137,138]. Therefore, it is expected that antioxidants will attenuate and/or prevent the progression of PD. As mentioned above, naturally occurring antioxidants have a good potential to attenuate and/or prevent the progression of PD, which is associated with neuronal apoptosis triggered by excessive ROS production and a diminished capability to handle oxidative stress by dopaminergic neurons and/or neighboring tissues.
The fermentation of soybean produces several antioxidative compounds, such as peptides, aglycone forms of isoflavones, and soyasaponins; thus, it is most likely that fermented soy products alleviate the progression and aggravation of PD. Soy protein, which usually represents approximately 40% of the seed content, is degraded into peptides by microbial proteases during fermentation. The peptides produced from soy proteins exhibit various beneficial effects, including antioxidant activity, which regulate the redox balance in the gut and subsequently influence the gut microbiota in a positive manner [139,140].
Gut microbiota have been reported to preferentially ferment peptides over free amino acids [141], and some peptides possess high resistance against gastrointestinal digestion [142]; therefore, these peptides can affect the composition of gut microbiota and can be utilized by the gut microbiota to produce neurotransmitters, such as butyrate [141], which may improve the negative symptoms of neurodegenerative diseases [143]. In fact, butyrate greatly regulates immune functions and energy metabolism of hosts, and mediates host–microbe crosstalk through transporters (MCT1/SLC16A1; SMCT1/SLC5A8) and specific receptors (GPR43/FFAR2; GPR41/FFAR3; GPR109a/HCAR2). The effect of butyrate may also be mediated by the β-oxidation pathway and the inhibition of histone deacetylases (HDACs), leading to enhanced histone acetylation and gene expression in host cells. Butyrate is also widely used as an experimental pharmacological compound and, more recently, in neuroscience research [144,145]. Thus, this compound has been in the spotlight in research into the microbiota–gut–brain axis, to understand how gut-derived butyrate affects brain functions and behaviors, ranging from depression to neurodegenerative diseases and cognitive impairment [146].
Recent studies have demonstrated that a probiotic mixture of Lactobacillus rhamnosus GG, Bifidobacterium animalis lactis, and Lactobacillus acidophilus increases butyrate and subsequently rescues the nigral dopaminergic neurons from MPTP-and rotenone-induced neurotoxicity in a mouse model [147]. The neuroprotective effect of butyrate may be mediated by the upregulation of occludins, zonula occludens-1, and Bcl-2, and, in particular, the stimulation of the colonic glucagon-like peptide-1 (GLP-1) and the upregulation of brain GLP-1R [51].
6.2.2. Gut Microbiota and AD
Disturbances in the composition of gut microbiota are related to immune activation and increased permeability of the gut barrier, thus leading to systemic inflammation, which, in turn, may compromise the blood–brain barrier and trigger neuroinflammation, neural damage, and neurodegeneration. More specifically, age-related alterations in the gut microbiota characterized by lowered diversity and stability may lead to an incessant inflammatory state of the gut mucosa, ultimately resulting in chronic systemic inflammation, including neuroinflammation [148,149,150].
It has been reported that the gut microbial composition of patients with AD is hallmarked by a decreased abundance of Firmicutes and Actinobacteria, and an increased abundance of Bacteroidetes and Proteobacteria. More specifically, the families that were reduced within the Firmicutes phylum include Clostridiaceae, Mogibacteriaceae, Peptostreptococcaceae, Ruminococcaceae, and Turicibacteraceae. The Acinetobacteria and Bifidobacteriaceae families were reduced in the gut of patients with AD. In contrast, Bacteroidaceae and Rikenellaceae within the Bacteroidetes phylum were increased in these individuals. In general, patients with AD harbor an increased number of proinflammatory bacteria, such as Bacteroidetes and Proteobacteria (Escherichia and Shigella), and have decreased anti-inflammatory bacteria (Firmicutes, Bifidobacterium, and Eubacterium rectale). However, additional research is required to establish a solid correlation between gut microbiota and AD, as the alterations in the gut microbiota of patients with AD were not consistent among studies [151].
A plant-based salutary foods diet containing probiotics, soybeans, nuts, omega-3 polyunsaturated fatty acids, and antioxidants, as well as a low intake of saturated fats, animal-derived foods, and refined sugar, has been reported to inhibit the inflammatory response, attenuate insulin resistance, and lower the risk of cognitive impairment and AD [152,153].
The intake of cheonggukjang fermented with Bacillus species prevents and alleviates the memory impairment observed in patients with AD and cerebral ischemic condition. In particular, cheonggukjang, which contains a high poly-L-γ-glutamic acid (γ-PGA), exhibited better efficacy for improving glucose metabolism and neuronal cell survival than did a low level of γ-PGA [126], although the neuroprotective effect and related mechanism(s) of γ-PGA remain unclear.
Yang and colleagues reported that soybeans fermented with Bacillus licheniformis enhanced cognitive function in diabetic rats with AD-type dementia [154]. Several proteinases produced by Bacillus pumilus and Bacillus subtilis and present in fermented soy products possess amyloid-degrading activity; therefore, they can be developed into anti-aggregation drugs [155,156], although many hurdles in the delivery of the proteases to target sites are anticipated. Another study found that Bacillus subtilis, a microorganism that is predominant in traditionally made cheonggukjang, restored the lifespan of Caenorhabditis elegans strains that expressing Aβ to values similar to the life expectancy of the wild-type strain [157]. The direct effects of microorganism in AD models are believed to be associated with the ability of Bacillus subtilis to biosynthesize quorum-sensing peptides (i.e., the competence and sporulation factor) and form a gut-associated biofilm, which is associated with the anti-aging effect.
7. Conclusions
The accumulation of toxic unique proteins or peptides characterized by abnormal conformational properties inside neuronal cells in the brain is a common feature of AD and PD, which are the two most prevalent neurodegenerative diseases with an incidence that keeps increasing globally. These peptides or proteins usually exert deleterious effects on the CNS through the generation of ROS, exacerbation of inflammation, alteration of mitochondrial homeostasis, and their combinations.
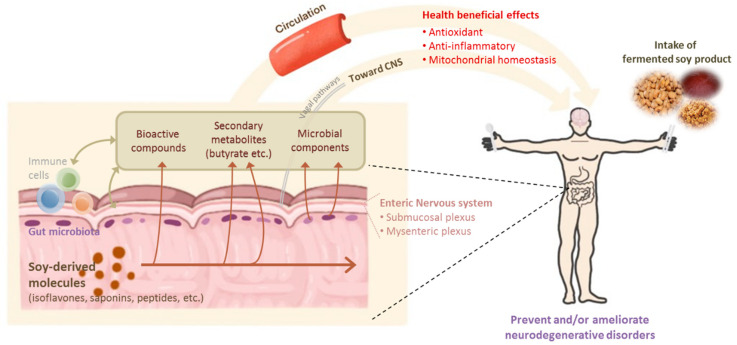
Fermented soybean products have well-known beneficial effects on neurodegenerative diseases and afford a variety of health benefits, such as the prevention of several chronic diseases. In particular, the free isoflavones generated during the fermentation of cooked soybean may attenuate the progression of AD and PD via antioxidant activity and the restoration of ROS-mediated mitochondrial dysfunction, as illustrated in Figure 4.
Figure 4.
Health-beneficial effects of fermented soy products on neurodegenerative disorders.
Recent studies also suggested that the regulation of the gut microbiome by fermented soy products can modulate neurodegenerative diseases through metabolites produced by microbial fermentation, such as butyrate, or by changing the gut microbial composition in a beneficial fashion.
However, clinical data regarding the therapeutic or preventive effects of fermented soybean products in neurodegenerative diseases are limited. Further research using large, long-term clinical trials to evaluate fermented soybean products and their components would be helpful in making specific dietary recommendations to patients with AD and PD.
Author Contributions
Conceptualization, J.-S.K. and C.H.J.; writing—original draft preparation, J.-S.K. and J.O. and C.H.J.; writing—review and editing, J.S.L. and H.J.K.; visualization, J.O. and C.H.J.; supervision, J.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bio industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea (Grant No. 319103042HD020).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations Department of Economic and Social Affairs Population Division World Population Prospects 2019: Highlights. ST/ESA/SER.A/423. [(accessed on 8 February 2021)];2019 Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf.
- 2.Selected Results of the 2019 UN World Population Projections. [(accessed on 8 February 2021)];Popul. Dev. Rev. 2019 45:689–694. doi: 10.1111/padr.12288. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/padr.12288. [DOI] [Google Scholar]
- 3.Mariani E., Polidori M.C., Cherubini A., Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Fifel K., Videnovic A. Circadian alterations in patients with neurodegenerative diseases: Neuropathological basis of underlying network mechanisms. Neurobiol. Dis. 2020;144:105029. doi: 10.1016/j.nbd.2020.105029. [DOI] [PubMed] [Google Scholar]
- 5.Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad A., Hayat I., Arif S., Masud T., Khalid N., Ahmed A. Mechanisms Involved in the Therapeutic Effects of Soybean (Glycine Max) Int. J. Food Prop. 2014;17:1332–1354. doi: 10.1080/10942912.2012.714828. [DOI] [Google Scholar]
- 7.Isanga J., Zhang G.N. Soybean bioactive components and their implications to health—A review. Food Rev. Int. 2008;24:252–276. doi: 10.1080/87559120801926351. [DOI] [Google Scholar]
- 8.Wang Q., Ge X., Tian X., Zhang Y., Zhang J., Zhang P. Soy isoflavone: The multipurpose phytochemical (Review) Biomed. Rep. 2013;1:697–701. doi: 10.3892/br.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko J.W., Chung Y.S., Kwak C.S., Kwon Y.H. Doenjang, a Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients. 2019;11:1702. doi: 10.3390/nu11081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayachandran M., Xu B.J. An insight into the health benefits of fermented soy products. Food Chem. 2019;271:362–371. doi: 10.1016/j.foodchem.2018.07.158. [DOI] [PubMed] [Google Scholar]
- 11.Cao Z.H., Green-Johnson J.M., Buckley N.D., Lin Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019;37:223–238. doi: 10.1016/j.biotechadv.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Kim B., Hong V.M., Yang J., Hyun H., Im J.J., Hwang J., Yoon S., Kim J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016;21:297–309. doi: 10.3746/pnf.2016.21.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.C., Quang T.H., Yoon C.S., Ngan N.T.T., Lim S.I., Lee S.Y., Kim Y.C., Oh H. Anti-neuroinflammatory activities of indole alkaloids from kanjang (Korean fermented soy source) in lipopolysaccharide-induced BV2 microglial cells. Food Chem. 2016;213:69–75. doi: 10.1016/j.foodchem.2016.06.068. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri R., Sawada N., Goto A., Yamaji T., Iwasaki M., Noda M., Iso H., Tsugane S., Japan Public Health Center-based Prospective Study Group Association of soy and fermented soy product intake with total and cause specific mortality: Prospective cohort study. BMJ. 2020;368:m34. doi: 10.1136/bmj.m34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez B., Delgado S., Blanco-Miguez A., Lourenco A., Gueimonde M., Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017;61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 17.Lee H.J., Hwang Y.H., Kim D.H. Lactobacillus plantarum C29-Fermented Soybean (DW2009) Alleviates Memory Impairment in 5XFAD Transgenic Mice by Regulating Microglia Activation and Gut Microbiota Composition. Mol. Nutr. Food Res. 2018;62:e1800359. doi: 10.1002/mnfr.201800359. [DOI] [PubMed] [Google Scholar]
- 18.Shin D., Jeong D. Korean traditional fermented soybean products: Jang. J. Ethn. Foods. 2015;2:2–7. doi: 10.1016/j.jef.2015.02.002. [DOI] [Google Scholar]
- 19.Lee Y.J., Kim N.Y., Kim U.S., Han M.J. Development of Lentil Cheonggukjang Fermented by Bacillus subtilis Isolated from Traditional Soy Sauce. J. Korean Soc. Food Cult. 2017;32:566–575. [Google Scholar]
- 20.Wang D., Wang L.J., Zhu F.X., Zhu J.Y., Chen X.D., Zou L., Saito M., Li L.T. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food) Food Chem. 2008;107:1421–1428. doi: 10.1016/j.foodchem.2007.09.072. [DOI] [Google Scholar]
- 21.Li Y.Y., Yu R.C., Chou C.C. Some biochemical and physical changes during the preparation of the enzyme-ripening sufu, a fermented product of soybean curd. J. Agric. Food Chem. 2010;58:4888–4893. doi: 10.1021/jf904600a. [DOI] [PubMed] [Google Scholar]
- 22.Han B.-Z., Rombouts F.M., Nout M.J.R. A Chinese fermented soybean food. Int. J. Food Microbiol. 2001;65:1–10. doi: 10.1016/S0168-1605(00)00523-7. [DOI] [PubMed] [Google Scholar]
- 23.Licandro H., Ho P.H., Nguyen T.K.C., Petchkongkaew A., Nguyen H.V., Chu-Ky S., Nguyen T.V.A., Lorn D., Wache Y. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control. 2020;110:106957. doi: 10.1016/j.foodcont.2019.106957. [DOI] [Google Scholar]
- 24.Anderson R.L., Wolf W.J. Compositional Changes in Trypsin-Inhibitors, Phytic Acid, Saponins and Isoflavones Related to Soybean Processing. J. Nutr. 1995;125:S581–S588. doi: 10.1093/jn/125.3_Suppl.581S. [DOI] [PubMed] [Google Scholar]
- 25.Sindhu S.C., Khetarpaul N. Probiotic fermentation of indigenous food mixture: Effect on antinutrients and digestibility of starch and protein. J. Food Compost. Anal. 2001;14:601–609. doi: 10.1006/jfca.2001.1022. [DOI] [Google Scholar]
- 26.Osawa R., Kuroiso K., Goto S., Shimizu A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl. Environ. Microbiol. 2000;66:3093–3097. doi: 10.1128/AEM.66.7.3093-3097.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez H., Curiel J.A., Landete J.M., de las Rivas B., de Felipe F.L., Gomez-Cordoves C., Mancheno J.M., Munoz R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009;132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Cuadrado C., Hajos G., Burbano C., Pedrosa M.M., Ayet G., Muzquiz M., Pusztai A., Gelencser E. Effect of natural fermentation on the lectin of lentils measured by immunological methods. Food Agric. Immunol. 2002;14:41–49. doi: 10.1080/09540100220137655. [DOI] [Google Scholar]
- 29.Sumi H., Hamada H., Tsushima H., Mihara H., Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43:1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda Y., Iki M., Morita A., Kajita E., Kagamimori S., Kagawa Y., Yoneshima H. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese population-based osteoporosis (JPOS) study. J. Nutr. 2006;136:1323–1328. doi: 10.1093/jn/136.5.1323. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa Y., Yamaguchi F., Yuasa K., Tahara Y. Efficient production of gamma-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci. Biotechnol. Biochem. 1997;61:1684–1687. doi: 10.1271/bbb.61.1684. [DOI] [PubMed] [Google Scholar]
- 32.da Silva L.H., Celeghini R.M.S., Chang Y.K. Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chem. 2011;128:640–644. doi: 10.1016/j.foodchem.2011.03.079. [DOI] [Google Scholar]
- 33.Hu J., Lee S.O., Hendrich S., Murphy P.A. Quantification of the group B soyasaponins by high-performance liquid chromatography. J. Agric. Food Chem. 2002;50:2587–2594. doi: 10.1021/jf0114740. [DOI] [PubMed] [Google Scholar]
- 34.Kang J., Badger T.M., Ronis M.J., Wu X. Non-isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010;58:8119–8133. doi: 10.1021/jf100901b. [DOI] [PubMed] [Google Scholar]
- 35.Coward L., Barnes N.C., Setchell K.D.R., Barnes S. Genistein, Daidzein, and Their Beta-Glycoside Conjugates—Antitumor Isoflavones in Soybean Foods from American and Asian Diets. J. Agric. Food Chem. 1993;41:1961–1967. doi: 10.1021/jf00035a027. [DOI] [Google Scholar]
- 36.Okouchi R., Sakanoi Y., Tsuduki T. Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise. Nutrients. 2019;11:560. doi: 10.3390/nu11030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baggott J.E., Ha T., Vaughn W.H., Juliana M.M., Hardin J.M., Grubbs C.J. Effect of miso (Japanese soybean paste) and NaCl on DMBA-induced rat mammary tumors. Nutr. Cancer. 1990;14:103–109. doi: 10.1080/01635589009514083. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N., Fujimoto K., Aoki H. Antioxidant activities of the water-soluble fraction in tempeh-like fermented soybean (GABA-tempeh) Int. J. Food Sci. Nutr. 2007;58:577–587. doi: 10.1080/09637480701343846. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi F., Hashimoto Y., Kaji A., Sakai R., Kawate Y., Okamura T., Kitagawa N., Okada H., Nakanishi N., Majima S., et al. Habitual Miso (Fermented Soybean Paste) Consumption Is Associated with a Low Prevalence of Sarcopenia in Patients with Type 2 Diabetes: A Cross-Sectional Study. Nutrients. 2020;13:72. doi: 10.3390/nu13010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentley R. From miso, sake and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006;23:1046–1062. doi: 10.1039/b603758p. [DOI] [PubMed] [Google Scholar]
- 41.Kumazawa T., Nishimura A., Asai N., Adachi T. Isolation of immune-regulatory Tetragenococcus halophilus from miso. PLoS ONE. 2018;13:e0208821. doi: 10.1371/journal.pone.0208821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito K. Review of the health benefits of habitual consumption of miso soup: Focus on the effects on sympathetic nerve activity, blood pressure, and heart rate. Environ. Health Prev. Med. 2020;25:45. doi: 10.1186/s12199-020-00883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon D.Y., Daily J.W., Kim H.J., Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Kataoka S. Functional effects of Japanese style fermented soy sauce (shoyu) and its components. J. Biosci. Bioeng. 2005;100:227–234. doi: 10.1263/jbb.100.227. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M. Immunological functions of soy sauce: Hypoallergenicity and antiallergic activity of soy sauce. J. Biosci. Bioeng. 2005;100:144–151. doi: 10.1263/jbb.100.144. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara T., Sano A., Yamaguchi H., Sugimoto K.R.I., Chikata H., Kinoshita E., Uchida R. Antihypertensive Effect of Peptide-Enriched Soy Sauce-Like Seasoning and Identification of Its Angiotensin I-Converting Enzyme Inhibitory Substances. J. Agric. Food Chem. 2010;58:821–827. doi: 10.1021/jf903261h. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Zhao M., Parkin K.L. beta-carboline derivatives and diphenols from soy sauce are in vitro quinone reductase (QR) inducers. J. Agric. Food Chem. 2011;59:2332–2340. doi: 10.1021/jf104653n. [DOI] [PubMed] [Google Scholar]
- 48.Peng Y., Huang Q., Zhang R.H., Zhang Y.Z. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003;134:45–52. doi: 10.1016/S1096-4959(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 49.Chen J., Cheng Y.Q., Yamaki K., Li L.T. Anti-alpha-glucosidase activity of Chinese traditionally fermented soybean (douchi) Food Chem. 2007;103:1091–1096. doi: 10.1016/j.foodchem.2006.10.003. [DOI] [Google Scholar]
- 50.Zhang J.H., Tatsumi E., Ding C.H., Li L.T. Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product. Food Chem. 2006;98:551–557. doi: 10.1016/j.foodchem.2005.06.024. [DOI] [Google Scholar]
- 51.Liu J.M., Wang F.Y., Liu S.Z., Du J.M., Hu X.Z., Xiong J.J., Fang R.C., Chen W.Q., Sun J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 52.Esaki H., Onozaki H., Kawakishi S., Osawa T. New antioxidant isolated from tempeh. J. Agric. Food Chem. 1996;44:696–700. doi: 10.1021/jf950454t. [DOI] [Google Scholar]
- 53.Handajani Y.S., Turana Y., Yogiara Y., Widjaja N.T., Sani T.P., Christianto G.A.M., Suwanto A. Tempeh Consumption and Cognitive Improvement in Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2020:1–6. doi: 10.1159/000510563. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad A., Ramasamy K., Majeed A.A., Mani V. Enhancement of beta-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm. Biol. 2015;53:758–766. doi: 10.3109/13880209.2014.942791. [DOI] [PubMed] [Google Scholar]
- 55.Shin H.W., Jang E.S., Moon B.S., Lee J.J., Lee D.E., Lee C.H., Shin C.S. Anti-obesity effects of gochujang products prepared using rice koji and soybean meju in rats. J. Food Sci. Tech. Mys. 2016;53:1004–1013. doi: 10.1007/s13197-015-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang H.J., Lee Y.S., Choi I.S. Comparison of physicochemical properties and antioxidant activities of fermented soybean-based red pepper paste, Gochujang, prepared with five different red pepper (Capsicum annuum L.) varieties. J. Food Sci. Tech. Mys. 2018;55:792–801. doi: 10.1007/s13197-017-2992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Son H.K., Shin H.W., Jang E.S., Moon B.S., Lee C.H., Lee J.J. Gochujang prepared using rice and wheat koji partially alleviates high-fat diet-induced obesity in rats. Food Sci. Nutr. 2020;8:1562–1574. doi: 10.1002/fsn3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y.C., Yu R.C., Chou C.C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128–135. doi: 10.1016/j.fm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y.Y., Thakur K., Feng J.Y., Cai J.S., Zhang J.G., Hu F., Russo P., Spano G., Wei Z.J. Riboflavin-overproducing lactobacilli for the enrichment of fermented soymilk: Insights into improved nutritional and functional attributes. Appl. Microbiol. Biotechnol. 2020;104:5759–5772. doi: 10.1007/s00253-020-10649-1. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X.L., Wu Y.F., Wang Y.S., Wang X.Z., Piao C.H., Liu J.M., Liu Y.L., Wang Y.H. The protective effects of probiotic-fermented soymilk on high-fat diet-induced hyperlipidemia and liver injury. J. Funct. Foods. 2017;30:220–227. doi: 10.1016/j.jff.2017.01.002. [DOI] [Google Scholar]
- 61.Rekha C.R., Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010;109:1198–1208. doi: 10.1111/j.1365-2672.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- 62.Hsieh M.L., Chou C.C. Mutagenicity and antimutagenic effect of soymilk fermented with lactic acid bacteria and bifidobacteria. Int. J. Food Microbiol. 2006;111:43–47. doi: 10.1016/j.ijfoodmicro.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi M., Hirahata R., Egusa S., Fukuda M. Hypocholesterolemic Effects of Lactic Acid-Fermented Soymilk on Rats Fed a High Cholesterol Diet. Nutrients. 2012;4:1304–1316. doi: 10.3390/nu4091304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nam D.H., Kim H.J., Lim J.S., Kim K.H., Park C.S., Kim J.H., Lim J., Kwon D.Y., Kim I.H., Kim J.S. Simultaneous Enhancement of Free Isoflavone Content and Antioxidant Potential of Soybean by Fermentation with Aspergillus oryzae. J. Food Sci. 2011;76:H194–H200. doi: 10.1111/j.1750-3841.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y.M., Shih T.W., Chiu C.P., Pan T.M., Tsai T.Y. Effects of lactic acid bacteria-fermented soy milk on melanogenesis in B16F0 melanocytes. J. Funct. Foods. 2013;5:395–405. doi: 10.1016/j.jff.2012.11.012. [DOI] [Google Scholar]
- 66.Peiroten A., Gaya P., Alvarez I., Landete J.M. Production of O-desmethylangolensin, tetrahydrodaidzein, 6′-hydroxy-O-desmethylangolensin and 2-(4-hydroxyphenyl)-propionic acid in fermented soy beverage by lactic acid bacteria and Bifidobacterium strains. Food Chem. 2020;318:126521. doi: 10.1016/j.foodchem.2020.126521. [DOI] [PubMed] [Google Scholar]
- 67.Pop E.A., Fischer L.M., Coan A.D., Gitzinger M., Nakamura J., Zeisel S.H. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: A phase I clinical trial. Menopause. 2008;15:684–692. doi: 10.1097/gme.0b013e318167b8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang T.S., Ding H.Y., Tai S.S., Wu C.Y. Metabolism of the soy isoflavones daidzein and genistein by fungi used in the preparation of various fermented soybean foods. Biosci. Biotechnol. Biochem. 2007;71:1330–1333. doi: 10.1271/bbb.60573. [DOI] [PubMed] [Google Scholar]
- 69.Piao Y.Z., Eun J.B. Physicochemical characteristics and isoflavones content during manufacture of short-time fermented soybean product (cheonggukjang) J. Food Sci. Technol. 2020;57:2190–2197. doi: 10.1007/s13197-020-04255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukutake M., Takahashi M., Ishida K., Kawamura H., Sugimura T., Wakabayashi K. Quantification of Genistein and Genistin in Soybeans and Soybean Products. Food Chem. Toxicol. 1996;34:457–461. doi: 10.1016/0278-6915(96)87355-8. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W., Tang F.Y., Yeo M.C., Popovich D.G. Fermentation of Group B Soyasaponins with Probiotic Lactobacillus Rhamnosus. J. Food Biochem. 2012;36:179–188. doi: 10.1111/j.1745-4514.2010.00524.x. [DOI] [Google Scholar]
- 72.Lee S.Y., Lee S., Lee S., Oh J.Y., Jeon E.J., Ryu H.S., Lee C.H. Primary and secondary metabolite profiling of doenjang, a fermented soybean paste during industrial processing. Food Chem. 2014;165:157–166. doi: 10.1016/j.foodchem.2014.05.089. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W., Popovich D.G. Chemical and Biological Characterization of Oleanane Triterpenoids from Soy. Molecules. 2009;14:2959–2975. doi: 10.3390/molecules14082959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krishnamurthy P., Tsukamoto C., Takahashi Y., Hongo Y., Singh R.J., Lee J.D., Chung G. Comparison of saponin composition and content in wild soybean (Glycine soja Sieb. and Zucc.) before and after germination. Biosci. Biotechnol. Biochem. 2014;78:1988–1996. doi: 10.1080/09168451.2014.946389. [DOI] [PubMed] [Google Scholar]
- 75.Jeon H.L., Yang S.J., Son S.H., Kim W.S., Lee N.K., Paik H.D. Evaluation of probiotic Bacillus subtilis P229 isolated from cheonggukjang and its application in soybean fermentation. Lebensm. Wiss. Technol. 2018;97:94–99. doi: 10.1016/j.lwt.2018.06.054. [DOI] [Google Scholar]
- 76.Guan R.-F., Liu Z.-F., Zhang J.-J., Wei Y.-X., Wahab S., Liu D.-H., Ye X.-Q. Investigation of biogenic amines in sufu (furu): A Chinese traditional fermented soybean food product. Food Control. 2013;31:345–352. doi: 10.1016/j.foodcont.2012.10.033. [DOI] [Google Scholar]
- 77.Somoza V. Five years of research on health risks and benefits of Maillard reaction products: An update. Mol. Nutr. Food Res. 2005;49:663–672. doi: 10.1002/mnfr.200500034. [DOI] [PubMed] [Google Scholar]
- 78.Tsuchiya H., Sato M., Watanabe I. Antiplatelet activity of soy sauce as functional seasoning. J. Agric. Food Chem. 1999;47:4167–4174. doi: 10.1021/jf990147d. [DOI] [PubMed] [Google Scholar]
- 79.Hayashi H., Nishimoto Y., Akiyama K., Nozaki H. New paralytic alkaloids, asperparalines A, B and C, from Aspergillus japonicus JV-23. Biosci. Biotechnol. Biochem. 2000;64:111–115. doi: 10.1271/bbb.64.111. [DOI] [PubMed] [Google Scholar]
- 80.Amin M.N.G., Kusnadi J., Hsu J.L., Doerksen R.J., Huang T.C. Identification of a novel umami peptide in tempeh (Indonesian fermented and its mechanism to the umami T1R. Food Chem. 2020;333:127411. doi: 10.1016/j.foodchem.2020.127411. [DOI] [PubMed] [Google Scholar]
- 81.Kamao M., Suhara Y., Tsugawa N., Uwano M., Yamaguchi N., Uenish K., Ishida H., Sasaki S., Okano T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J. Nutr. Sci. Vitaminol. 2007;53:464–470. doi: 10.3177/jnsv.53.464. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y.-Y., Thakur K., Feng J.-Y., Cai J.-S., Zhang J.-G., Hu F., Wei Z.-J. B-vitamin enriched fermented soymilk: A novel strategy for soy-based functional foods development. Trends Food Sci. Technol. 2020;105:43–55. doi: 10.1016/j.tifs.2020.08.019. [DOI] [Google Scholar]
- 83.Zhang Y.F., Tao W.Y. Flavor and taste compounds analysis in Chinese solid fermented soy sauce. Afr. J. Biotechnol. 2009;8:673–681. [Google Scholar]
- 84.Kataoka S., Liu W., Albright K., Storkson J., Pariza M. Inhibition of Benzo[a]pyrene-induced Mouse Fore, stomach Neoplasia and Reduction of H202 Concentration in Human Polymorphonuclear Leucocytes by Flavour Components of Japanese-style Fermented Soy Sauce. Food Chem. Toxicol. 1997;35:449–457. doi: 10.1016/S0278-6915(97)00009-4. [DOI] [PubMed] [Google Scholar]
- 85.Spires-Jones T.L., Attems J., Thal D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017;134:187–205. doi: 10.1007/s00401-017-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dugger B.N., Dickson D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie A.M., Gao J., Xu L., Meng D.M. Shared Mechanisms of Neurodegeneration in Alzheimer’s Disease and Parkinson’s Disease. Biomed Res. Int. 2014;2014:648740. doi: 10.1155/2014/648740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soni M., Rahardjo T.B., Soekardi R., Sulistyowati Y., Lestariningsih, Yesufu-Udechuku A., Irsan A., Hogervorst E. Phytoestrogens and cognitive function: A review. Maturitas. 2014;77:209–220. doi: 10.1016/j.maturitas.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh H.M., Wu W.M., Hu M.L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem. Toxicol. 2009;47:625–632. doi: 10.1016/j.fct.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 90.Sarkaki A., Amani R., Badavi M., Moghaddam A.Z., Aligholi H., Safahani M., Haghighizadeh M.H. Pre-treatment effect of different doses of soy isoflavones on spatial learning and memory in an ovariectomized animal model of Alzheimer’s disease. Pak. J. Biol. Sci. 2008;11:1114–1119. doi: 10.3923/pjbs.2008.1114.1119. [DOI] [PubMed] [Google Scholar]
- 91.Uddin M.S., Kabir M.T. Emerging Signal Regulating Potential of Genistein Against Alzheimer’s Disease: A Promising Molecule of Interest. Front. Cell Dev. Biol. 2019;7:197. doi: 10.3389/fcell.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu Y., An Y., Lv C., Ma W., Xi Y., Xiao R. Dietary soybean isoflavones in Alzheimer’s disease prevention. Asia Pac. J. Clin. Nutr. 2018;27:946–954. doi: 10.6133/apjcn.052018.01. [DOI] [PubMed] [Google Scholar]
- 93.Wang X.J., Chen S.D., Ma G.Z., Ye M., Lu G.Q. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport. 2005;16:267–270. doi: 10.1097/00001756-200502280-00013. [DOI] [PubMed] [Google Scholar]
- 94.Gao H.M., Jiang J., Wilson B., Zhang W., Hong J.S., Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson’s disease. J. Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 95.Hussain G., Zhang L.B., Rasul A., Anwar H., Sohail M.U., Razzaq A., Aziz N., Shabbir A., Ali M., Sun T. Role of Plant-Derived Flavonoids and Their Mechanism in Attenuation of Alzheimer’s and Parkinson’s Diseases: An Update of Recent Data. Molecules. 2018;23:814. doi: 10.3390/molecules23040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L.X., Chen W.F., Xie J.X., Wong M.S. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci. Res. 2008;60:156–161. doi: 10.1016/j.neures.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Chinta S.J., Ganesan A., Reis-Rodrigues P., Lithgow G.J., Andersen J.K. Anti-Inflammatory Role of the Isoflavone Diadzein in Lipopolysaccharide-Stimulated Microglia: Implications for Parkinson’s Disease. Neurotox. Res. 2013;23:145–153. doi: 10.1007/s12640-012-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Liu N., Lu B.W. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci. Ther. 2019;25:859–875. doi: 10.1111/cns.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Killackey S.A., Philpott D.J., Girardin S.E. Mitophagy pathways in health and disease. J. Cell Biol. 2020;219:e202004029. doi: 10.1083/jcb.202004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montava-Garriga L., Ganley I.G. Outstanding Questions in Mitophagy: What We Do and Do Not Know. J. Mol. Biol. 2020;432:206–230. doi: 10.1016/j.jmb.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 101.Scorziello A., Borzacchiello D., Sisalli M.J., Di Martino R., Morelli M., Feliciello A. Mitochondrial Homeostasis and Signaling in Parkinson’s Disease. Front. Aging Neurosci. 2020;12:100. doi: 10.3389/fnagi.2020.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez-Vicente M. Neuronal Mitophagy in Neurodegenerative Diseases. Front. Mol. Neurosci. 2017;10:64. doi: 10.3389/fnmol.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamano K., Matsuda N., Tanaka K. The ubiquitin signal and autophagy: An orchestrated dance leading to mitochondrial degradation. EMBO Rep. 2016;17:300–316. doi: 10.15252/embr.201541486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Apelt J., Schliebs R. beta-Amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. doi: 10.1016/S0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- 105.Valles S.L., Dolz-Gaiton P., Gambini J., Borras C., Lloret A., Pallardo F.V., Vina J. Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res. 2010;1312:138–144. doi: 10.1016/j.brainres.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 106.Zhou X., Yuan L., Zhao X., Hou C., Ma W., Yu H., Xiao R. Genistein antagonizes inflammatory damage induced by beta-amyloid peptide in microglia through TLR4 and NF-kappaB. Nutrition. 2014;30:90–95. doi: 10.1016/j.nut.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Davinelli S., De Stefani D., De Vivo I., Scapagnini G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020;31:536–550. doi: 10.1016/j.tem.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Yessenkyzy A., Saliev T., Zhanaliyeva M., Masoud A.R., Umbayev B., Sergazy S., Krivykh E., Gulyayev A., Nurgozhin T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients. 2020;12:1344. doi: 10.3390/nu12051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ajibola C.F., Fashakin J.B., Fagbemi T.N., Aluko R.E. Effect of Peptide Size on Antioxidant Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Protein Hydrolysate Fractions. Int. J. Mol. Sci. 2011;12:6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo H., Kouzuma Y., Yonekura M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009;113:238–245. doi: 10.1016/j.foodchem.2008.06.081. [DOI] [Google Scholar]
- 111.Sanjukta S., Rai A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016;50:1–10. doi: 10.1016/j.tifs.2016.01.010. [DOI] [Google Scholar]
- 112.Nam K.A., You S.G., Kim S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008;73:C249–C255. doi: 10.1111/j.1750-3841.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 113.Lee H.J., Lim S.M., Ko D.B., Jeong J.J., Hwang Y.H., Kim D.H. Soyasapogenol B and Genistein Attenuate Lipopolysaccharide-Induced Memory Impairment in Mice by the Modulation of NF-kappaB-Mediated BDNF Expression. J. Agric. Food Chem. 2017;65:6877–6885. doi: 10.1021/acs.jafc.7b02569. [DOI] [PubMed] [Google Scholar]
- 114.Zha L.Y., Chen J.D., Sun S.X., Mao L.M., Chu X.W., Deng H., Cai J.W., Li X.F., Liu Z.Q., Cao W.H. Soyasaponins Can Blunt Inflammation by Inhibiting the Reactive Oxygen Species-Mediated Activation of PI3K/Akt/NF-kB Pathway. PLoS ONE. 2014;9:e107655. doi: 10.1371/journal.pone.0107655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muegge B.D., Kuczynski J., Knights D., Clemente J.C., Gonzalez A., Fontana L., Henrissat B., Knight R., Gordon J.I. Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pasolli E., De Filippis F., Mauriello I.E., Cumbo F., Walsh A.M., Leech J., Cotter P.D., Segata N., Ercolini D. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat. Commun. 2020;11:2610. doi: 10.1038/s41467-020-16438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng I.C., Shang H.F., Lin T.F., Wang T.H., Lin H.S., Lin S.H. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J. Gastroenterol. 2005;11:1225–1227. doi: 10.3748/wjg.v11.i8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiang S.S., Pan T.M. Antiosteoporotic Effects of Lactobacillus-Fermented Soy Skim Milk on Bone Mineral Density and the Microstructure of Femoral Bone in Ovariectomized Mice. J. Agric. Food Chem. 2011;59:7734–7742. doi: 10.1021/jf2013716. [DOI] [PubMed] [Google Scholar]
- 120.Cavallini D.C.U., Manzoni M.S.J., Bedani R., Roselino M.N., Celiberto L.S., Vendramini R.C., de Valdez G.F., Abdalla D.S.P., Pinto R.A., Rosetto D., et al. Probiotic Soy Product Supplemented with Isoflavones Improves the Lipid Profile of Moderately Hypercholesterolemic Men: A Randomized Controlled Trial. Nutrients. 2016;8:52. doi: 10.3390/nu8010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Appukutty M., Ramasamy K., Rajan S., Vellasamy S., Ramasamy R., Radhakrishnan A.K. Effect of orally administered soy milk fermented with Lactobacillus plantarum LAB12 and physical exercise on murine immune responses. Benef. Microbes. 2015;6:491–496. doi: 10.3920/BM2014.0129. [DOI] [PubMed] [Google Scholar]
- 122.Dimidi E., Cox S.R., Rossi M., Whelan K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients. 2019;11:1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwon G.H., Lee H.A., Park J.Y., Kim J.S., Lim J., Park C.S., Kwon D.Y., Kim Y.S., Kim J.H. Development of a RAPD-PCR method for identification of Bacillus species isolated from Cheonggukjang. Int. J. Food Microbiol. 2009;129:282–287. doi: 10.1016/j.ijfoodmicro.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 124.Nam Y.D., Yi S.H., Lim S.I. Bacterial diversity of cheonggukjang, a traditional Korean fermented food, analyzed by barcoded pyrosequencing. Food Control. 2012;28:135–142. doi: 10.1016/j.foodcont.2012.04.028. [DOI] [Google Scholar]
- 125.Kim Y.S., Kim M.C., Kwon S.W., Kim S.J., Park I.C., Ka J.O., Weon H.Y. Analyses of Bacterial Communities in Meju, a Korean Traditional Fermented Soybean Bricks, by Cultivation-Based and Pyrosequencing Methods. J. Microbiol. 2011;49:340–348. doi: 10.1007/s12275-011-0302-3. [DOI] [PubMed] [Google Scholar]
- 126.Jeong D.Y., Ryu M.S., Yang H.J., Park S. gamma-PGA-Rich Chungkookjang, Short-Term Fermented Soybeans: Prevents Memory Impairment by Modulating Brain Insulin Sensitivity, Neuro-Inflammation, and the Gut-Microbiome-Brain Axis. Foods. 2021;10:221. doi: 10.3390/foods10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeong D.Y., Daily J.W., Lee G.H., Ryu M.S., Yang H.J., Jeong S.Y., Qiu J.Y., Zhang T., Park S. Short-Term Fermented Soybeans with Bacillus amyloliquefaciens Potentiated Insulin Secretion Capacity and Improved Gut Microbiome Diversity and Intestinal Integrity To Alleviate Asian Type 2 Diabetic Symptoms. J. Agric. Food Chem. 2020;68:13168–13178. doi: 10.1021/acs.jafc.9b07962. [DOI] [PubMed] [Google Scholar]
- 128.Boulange C.L., Neves A.L., Chilloux J., Nicholson J.K., Dumas M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giguere N., Nanni S.B., Trudeau L.E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018;9:455. doi: 10.3389/fneur.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Surmeier D.J., Obeso J.A., Halliday G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017;18:101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Bjorklund T., Wang Z.Y., Roybon L., Melki R., Li J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 133.Ma L.Y., Liu G.L., Wang D.X., Zhang M.M., Kou W.Y., Feng T. Alpha-Synuclein in Peripheral Tissues in Parkinson’s Disease. ACS Chem. Neurosci. 2019;10:812–823. doi: 10.1021/acschemneuro.8b00383. [DOI] [PubMed] [Google Scholar]
- 134.De Rui M., Inelmen E.M., Trevisan C., Pigozzo S., Manzato E., Sergi G. Parkinson’s disease and the non-motor symptoms: Hyposmia, weight loss, osteosarcopenia. Aging Clin. Exp. Res. 2020;32:1211–1218. doi: 10.1007/s40520-020-01470-x. [DOI] [PubMed] [Google Scholar]
- 135.Levy O.A., Malagelada C., Greene L.A. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo J.D., Zhao X., Li Y., Li G.R., Liu X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review) Int. J. Mol. Med. 2018;41:1817–1825. doi: 10.3892/ijmm.2018.3406. [DOI] [PubMed] [Google Scholar]
- 137.Trist B.G., Hare D.J., Double K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell. 2019;18:e13031. doi: 10.1111/acel.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jenner P., Olanow C.W. Understanding cell death in Parkinson’s disease. Ann. Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 139.Xu H., Deng R.X., Li E.T.S., Shen J.G., Wang M.F. Pinosylvin provides neuroprotection against cerebral ischemia and reperfusion injury through enhancing PINK1/Parkin mediated mitophagy and Nrf2 pathway. J. Funct. Foods. 2020;71:104019. doi: 10.1016/j.jff.2020.104019. [DOI] [Google Scholar]
- 140.Zhou J.J., Chen M.F., Wu S.J., Liao X.Y., Wang J., Wu Q.P., Zhuang M.Z., Ding Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020;134:109230. doi: 10.1016/j.foodres.2020.109230. [DOI] [PubMed] [Google Scholar]
- 141.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 142.Singh B.P., Vij S. In vitro stability of bioactive peptides derived from fermented soy milk against heat treatment, pH and gastrointestinal enzymes. LWT–Food Sci. Technol. 2018;91:303–307. doi: 10.1016/j.lwt.2018.01.066. [DOI] [Google Scholar]
- 143.Chakraborty A., Banerjee S., Mukherjee B., Poddar M.K. Calorie restriction improves aging-induced impairment of cognitive function in relation to deregulation of corticosterone status and brain regional GABA system. Mech. Ageing Dev. 2020;189:111248. doi: 10.1016/j.mad.2020.111248. [DOI] [PubMed] [Google Scholar]
- 144.Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fischer A., Sananbenesi F., Mungenast A., Tsai L.H. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol. Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 146.Stilling R.M., van de Wouw M., Clarke G., Stanton C., Dinan T.G., Cryan J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 147.Srivastav S., Neupane S., Bhurtel S., Katila N., Maharjan S., Choi H., Hong J.T., Choi D.Y. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019;69:73–86. doi: 10.1016/j.jnutbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 148.Kowalski K., Mulak A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019;25:48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dinan T.G., Cryan J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Quigley E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017;17:94. doi: 10.1007/s11910-017-0802-6. [DOI] [PubMed] [Google Scholar]
- 151.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K., et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pistollato F., Cano S.S., Elio I., Vergara M.M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 153.Pistollato F., Iglesias R.C., Ruiz R., Aparicio S., Crespo J., Lopez L.D., Manna P.P., Giampieri F., Battino M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018;131:32–43. doi: 10.1016/j.phrs.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 154.Yang H.J., Kwon D.Y., Kim H.J., Kim M.J., Jung D.Y., Kang H.J., Kim D.S., Kang S., Moon N.R., Shin B.K., et al. Fermenting soybeans with Bacillus licheniformis potentiates their capacity to improve cognitive function and glucose homeostaisis in diabetic rats with experimental Alzheimer’s type dementia. Eur. J. Nutr. 2015;54:77–88. doi: 10.1007/s00394-014-0687-y. [DOI] [PubMed] [Google Scholar]
- 155.Hsu R.L., Lee K.T., Wang J.H., Lee L.Y.L., Chen R.P.Y. Amyloid-Degrading Ability of Nattokinase from Bacillus subtilis Natto. J. Agric. Food Chem. 2009;57:503–508. doi: 10.1021/jf803072r. [DOI] [PubMed] [Google Scholar]
- 156.Novik G., Savich V. Beneficial microbiota. Probiotics and pharmaceutical products in functional nutrition and medicine. Microb. Infect. 2020;22:8–18. doi: 10.1016/j.micinf.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 157.Cogliati S., Clementi V., Francisco M., Crespo C., Arganaraz F., Grau R. Bacillus Subtilis Delays Neurodegeneration and Behavioral Impairment in the Alzheimer’s Disease Model Caenorhabditis Elegans. J. Alzheimers Dis. 2020;73:1035–1052. doi: 10.3233/JAD-190837. [DOI] [PubMed] [Google Scholar]