Abstract
The resilience of high-grade gliomas (HGGs) against conventional chemotherapies is due to their heterogeneous genetic landscape, adaptive phenotypic changes, and immune escape mechanisms. Innovative immunotherapies have been developed to counteract the immunosuppressive capability of gliomas. Nevertheless, further research is needed to assess the efficacy of the immuno-based approach. The aim of this study is to review the newest immunotherapeutic approaches for glioma, focusing on the drug types, mechanisms of action, clinical pieces of evidence, and future challenges. A PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis)-based literature search was performed on PubMed/Medline and ClinicalTrials.gov databases using the keywords “active/adoptive immunotherapy,” “monoclonal antibodies,” “vaccine,” and “engineered T cell.”, combined with “malignant brain tumor”, “high-grade glioma.” Only articles written in English published in the last 10 years were selected, filtered based on best relevance. Active immunotherapies include systemic temozolomide, monoclonal antibodies, and vaccines. In several preclinical and clinical trials, adoptive immunotherapies, including T, natural killer, and natural killer T engineered cells, have been shown to be potential treatment options for relapsing gliomas. Systemic temozolomide is considered the backbone for newly diagnosed HGGs. Bevacizumab and rindopepimut are promising second-line treatments. Adoptive immunotherapies have been proven for relapsing tumors, but further evidence is needed.
Keywords: bevacizumab, CAR T cell, cell-based therapy, glioblastoma, immunotherapy, malignant brain tumor, temozolomide
1. Introduction
High-grade gliomas (HGGs) are the most common malignant brain tumors with an incidence of 6/100,000 per year [1,2,3,4,5]. The current standard treatment protocol provides maximum surgical resection, adjuvant systemic chemotherapy, and whole-brain radiation [6,7]. The prognosis still remains extremely dismal, with a 5-year survival rate of less than 10% and a median survival of 14–16 months from diagnosis [8]. Intrinsic glioma cell heterogenicity, high mitotic activity, abnormal angiogenesis, and early local recurrence are responsible for the resilience of these tumors toward standard treatments [9,10,11]. Despite the biological complexity and adaptive nature of these lethal neoplasms, recent studies have identified some molecular and epigenetic markers, such as isocitrate dehydrogenase and O6-methylguanine-DNA-methyltransferase (MGMT) gene promoter, which are useful for predicting the prognosis and planning new targeted therapeutic options [12,13,14,15,16,17,18,19]. Moreover, the detection of glioma immunogenomics, alongside immunosuppressive mechanisms within the tumor microenvironment, has prompted the development of new immunotherapies [20,21,22,23,24,25,26,27].
The emerging immune-based technologies exploit manipulated molecules, purified tumor-specific neoantigens, and autologous/allogeneic lymphocytes, engineered for specific therapeutic purposes especially to counteract glioma-mediated immune suppression.
Therefore, the aim of the present study is to provide an overview of the classification, mechanisms of antitumor immune response, evidence from clinical trials, limitations, and future challenges of the immunotherapeutic approach for the treatment of malignant brain gliomas.
2. Materials and Methods
A comprehensive online literature review was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The PubMed/Medline (https://pubmed.ncbi.nlm.nih.gov, accessed on 30 January 2021) and ClinicalTrials.gov (https://clinicaltrials.gov, accessed on 30 January 2021) databases were used, with combinations of Medical Subject Headings (MeSH) terms and text words. The main MeSH terms and key words were “malignant brain tumor,” “high-grade glioma,” and “glioblastoma”, further merged with “immunotherapy, active”; “immunotherapy, adoptive”. Supplementary research was conducted with additional MeSH terms: “chemotherapy”; “vaccine”; “alkylating agents”; “monoclonal antibodies”; “engineered T cell”; and “allogenic NK cell”, in order to restrict the field of interest to the novel immunotherapeutic strategies. The eligibility criteria included only articles written in English or translated, published in the last 10 years, and related to neuro-oncology. Review articles and editorials were included and filtered according to best match and relevance based on title and abstract.
On the ClinicalTrials.gov database, the search terms used to identify clinical trials were as follows: “malignant brain tumor,” “high-grade glioma” “central nervous system”, “immunotherapy, active”, and “immunotherapy, adoptive”. Interventional studies and clinical trials were included. No limits for the study phase or recruitment status were applied. Duplicates and titles with no English language translation available were removed. Trials related to innovative therapies for high-grade gliomas were chosen.
A descriptive analysis on the classification criteria, therapeutic mechanisms, and concluded and ongoing clinical trials was conducted. All the inclusion and exclusion criteria are outlined in Table 1.
Table 1.
Inclusion and exclusion criteria for systematic review.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Reviews, peer-reviews, editorials | Case reports, abstracts, and dissertations |
| Clinical, pre-clinical Trials | Withdrawn or abandoned clinical trials |
| English language, or translated | Non-English language |
| Publications from 2010–2020 | Studies not from 2010–2020 |
| Studies on humans or human products | Animal studies |
| Publications related to neuro-oncology | Publications not related to neuro-oncology |
| Publications related to high-grade glioma | Publications not related to high-grade glioma |
| Adult and pediatric patients |
3. Results
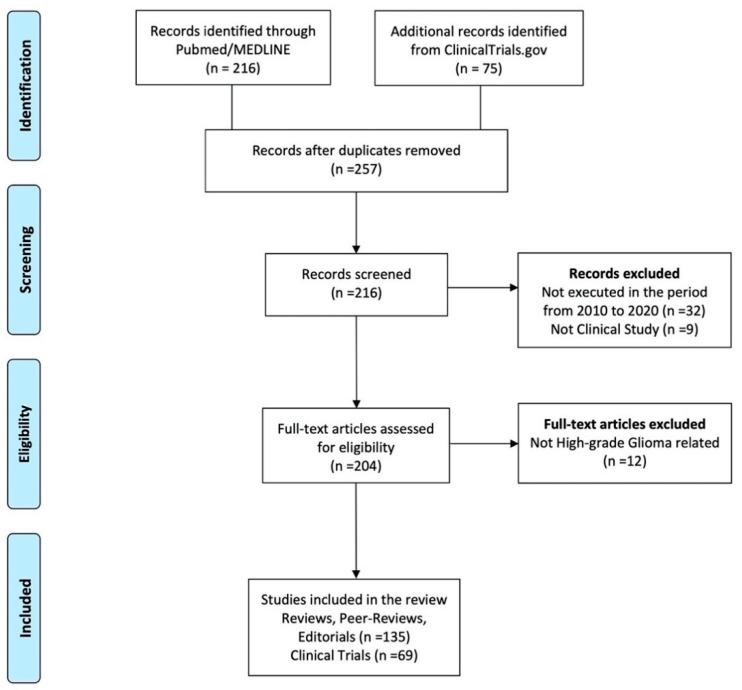
The literature search returned a total of 216 articles and 75 clinical trials. After the removal of duplicates and implementation of the exclusion criteria, a total of 135 articles and 69 clinical trials were assessed for eligibility and included in the review. Figure 1 presents the PRISMA flow chart for the literature selection process (Figure 1) and Table 2 summarizes the main clinical trials on immunotherapies for HGGs (Table 2).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow-chart.
Table 2.
Main clinical trials on immunotherapies for high-grade gliomas.
| # | ClinicalTrials.gov Identifier | Title | Status | Phase | Diseases | # of Pts. Enrolled | Treatment | Locations |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT03011671 | Study of Acetazolamide With Temozolomide in Adults With Newly Diagnosed or Recurrent Malignant Glioma | Suspended | I | Malignant Glioma of Brain | 24 | Acetazolamide, Temozolomide | USA |
| 2 | NCT02416999 | Ultra-low Dose Bevacizumab Plus Temozolomide for Recurrent High-grade Gliomas | Unknown | NA | Recurrent High-grade Glioma | 30 | Ultra-low dose Bevacizumab, Temozolomide | CHN |
| 3 | NCT01891747 | A Phase I Study of High-dose L-methylfolate in Combination With Temozolomide and Bevacizumab in Recurrent High Grade Glioma | Active, not recruiting | I | Malignant Glioma | 12 | Bevacizumab, Temozolomide, Vitamin C | USA |
| 4 | NCT04267146 | Nivolumab in Combination With Temozolomide and Radiotherapy in Children and Adolescents With Newly Diagnosed High-grade Glioma | Recruiting | I, II | High Grade Glioma | 40 | Nivolumab, Temozolomide, Radiotherapy | FR |
| 5 | NCT00782756 | Bevacizumab, Temozolomide and Hypofractionated Radiotherapy for Patients With Newly Diagnosed Malignant Glioma | Completed | II | Brain Cancer, Malignant Glioma | 40 | Radiotherapy, Temozolomide, Bevacizumab | USA |
| 6 | NCT04547621 | HSRT and IMRT Chemoradiotherapy for Newly Diagnosed GBM | Active, not recruiting | I, II | Glioma, Malignant | 50 | Radiation, Temozolomide | CHN |
| 7 | NCT01390948 | A Study of Bevacizumab (Avastin) in Combination With Temozolomide and Radiotherapy in Paediatric and Adolescent Participants With High-Grade Glioma | Completed | II | High Grade Glioma | 124 | Bevacizumab, Radiotherapy, Temozolomide | A |
| 8 | NCT00660621 | A Phase II Study Of Gliadel, Concomitant Temozolomide And Radiation, Followed By Dose Dense Therapy With Temozolomide Plus Bevacizumab For Newly Diagnosed Malignant High Grade Glioma | Unknown | II | Glioma | 40 | Temozolomide, Bevacizumab | USA |
| 9 | NCT03633552 | Efficacy of Two Temozolomide Regimens in Adjuvant Treatment of Patients With Brain High Grade Glioma | Recruiting | III | Glioblastoma Multiforme Anaplastic Astrocytoma | 62 | Temozolomide | IR |
| 10 | NCT01105702 | Temodar (Temozolomide), Bevacizumab, Lithium and Radiation for High Grade Glioma | Terminated | II | Brain Cancer | 28 | Temozolomide, Bevacizumab, Lithium Carbonate, Radiation | USA |
| 11 | NCT01740258 | Bevacizumab Beyond Progression (BBP) | Completed | II | Malignant Glioma, Glioblastoma, Gliosarcoma | 68 | Radiation Therapy, Temozolomide, Bevacizumab | USA |
| 12 | NCT01478321 | Efficacy of Hypofractionated XRT w/Bev. + Temozolomide for Recurrent Gliomas | Terminated | II | Adult Anaplastic Astrocytoma Ependymoma Oligodendroglioma/Glioblastoma | 54 | Temozolomide, Bevacizumab Hypofractionated radiation therapy | USA |
| 13 | NCT00943826 | A Study of Bevacizumab (Avastin®) in Combination With Temozolomide and Radiotherapy in Participants With Newly Diagnosed Glioblastoma | Completed | III | Glioblastoma | 921 | Bevacizumab, Temozolomide Radiation therapy | USA |
| 14 | NCT00884741 | Temozolomide and Radiation Therapy With or Without Bevacizumab in Treating Patients With Newly Diagnosed Glioblastoma | Completed | III | Glioblastoma, Gliosarcoma, Supratentorial Glioblastoma | 637 | Radiation Therapy, Temozolomide, Bevacizumab | USA |
| 15 | NCT01046279 | Hypertension Monitoring in Glioma Patients Treated With Bevacizumab | Terminated | NA | Glioma | 40 | Bevacizumab | ZH |
| 16 | NCT00271609 | Bevacizumab for Recurrent Malignant Glioma | Completed | II | Recurrent High-Grade Gliomas | 88 | Bevacizumab | USA |
| 17 | NCT02833701 | Bevacizumab and Ascorbic Acid in Patients Treating With Recurrent High Grade Glioma | Terminated | I | Glioblastoma, Glioma | 9 | Ascorbic Acid, Bevacizumab | USA |
| 18 | NCT00595322 | Bevacizumab in the Radiation Treatment of Recurrent Malignant Glioma | Completed | NA | Recurrent Malignant Gliomas, Primary Brain Tumor | 25 | Bevacizumab, Radiation | USA |
| 19 | NCT00337207 | Bevacizumab in Treating Patients With Recurrent or Progressive Glioma | Completed | II | Central Nervous System Tumors | 55 | Bevacizumab | USA |
| 20 | NCT01091792 | Exploratory Study of the Modulation of the Immune System by VEGF Blockade in Patients With Glioblastoma Multiforme (GBM) | Completed | I | Glioblastoma Multiforme | 13 | Bevacizumab | USA |
| 21 | NCT00883298 | Bi-weekly Temozolomide Plus Bevacizumab for Adult Patients With Recurrent Glioblastoma Multiforme | Completed | II | Recurrent Glioblastoma Multiforme Recurrent Gliosarcoma | 30 | Temozolomide, Bevacizumab | USA |
| 22 | NCT01811498 | Repeated Super-Selective Intraarterial Cerebral Infusion of Bevacizumab (Avastin) for Treatment of Newly Diagnosed GBM | Active, not recruiting | I, II | Glioblastoma Multiforme, Brain Tumor | 25 | Bevacizumab | USA |
| 23 | NCT01730950 | Bevacizumab With or Without Radiation Therapy in Treating Patients With Recurrent Glioblastoma | Active, not recruiting | II | Adult Giant Cell Glioblastoma, Glioblastoma, Adult Gliosarcoma Recurrent Adult Brain Tumor | 182 | Bevacizumab, Radiation therapy | USA |
| 24 | NCT02761070 | Bevacizumab Alone Versus Dose-dense Temozolomide Followed by Bevacizumab for Recurrent Glioblastoma, Phase III | Recruiting | III | Recurrent Glioblastoma | 146 | Temozolomide, Bevacizumab | J |
| 25 | NCT01209442 | Hypofractionated Intensity-Modulated Radiation Therapy With Temozolomide and Bevacizumab for Glioblastoma Multiforme | Completed | II | Glioblastoma Multiforme | 30 | Bevacizumab, Temozolomide Radiation Therapy | USA |
| 26 | NCT01125046 | Bevacizumab in Treating Patients With Recurrent or Progressive Meningiomas | Completed | II | Central Nervous System Tumors | 50 | Bevacizumab | USA |
| 27 | NCT01526837 | Bevacizumab (Avastin) Into the Tumor Resection Cavity in Subjects With Glioblastoma Multiforme at First Recurrence | Terminated | I | Glioblastoma Multiforme | 1 | Bevacizumab | USA |
| 28 | NCT01443676 | Avastin Plus Radiotherapy in Elderly Patients With Glioblastoma | Completed | II | Glioblastoma | 75 | Bevacizumab, Radiation therapy | USA |
| 29 | NCT00590681 | Bevacizumab and Temozolomide Following Radiation and Chemotherapy for Newly Diagnosed Glioblastoma Multiforme | Completed | II | Glioblastoma Multiforme | 62 | Bevacizumab, Temozolomide | USA |
| 30 | NCT03925246 | Efficacy of Nivolumab for Recurrent IDH Mutated High-Grade Gliomas | Active, not recruiting | II | High Grade Glioma, Brain Cancer | 43 | Nivolumab | FR |
| 31 | NCT00345163 | A Study to Evaluate Bevacizumab Alone or in Combination With Irinotecan for Treatment of Glioblastoma Multiforme (BRAIN) | Completed | II | Glioblastoma | 167 | Bevacizumab, Irinotecan | NA |
| 32 | NCT03890952 | Translational Study of Nivolumab in Combination With Bevacizumab for Recurrent Glioblastoma | Recruiting | II | Recurrent Adult Brain Tumor | 40 | Nivolumab, Bevacizumab | DNK |
| 33 | NCT01498328 | A Study of Rindopepimut/GM-CSF in Patients With Relapsed EGFRvIII-Positive Glioblastoma | Completed | II | Glioblastoma | 127 | Rindopepimut (CDX-110) with GM-CSF Bevacizumab, KLH | USA |
| 34 | NCT03743662 | Nivolumab With Radiation Therapy and Bevacizumab for Recurrent MGMT Methylated Glioblastoma | Recruiting | II | Glioblastoma | 94 | Re-irradiation, Bevacizumab Nivolumab, Re-resection | USA |
| 35 | NCT03452579 | Nivolumab Plus Standard Dose Bevacizumab Versus Nivolumab Plus Low Dose Bevacizumab in GBM | Active, not recruiting | II | Glioblastoma | 90 | Nivolumab, Standard/Reduced Dose Bevacizumab | USA |
| 36 | NCT02550249 | Neoadjuvant Nivolumab in Glioblastoma | Completed | II | Glioblastoma Multiforme | 29 | Nivolumab | ES |
| 37 | NCT04195139 | Nivolumab and Temozolomide Versus Temozolomide Alone in Newly Diagnosed Elderly Patients With GBM | Recruiting | II | Glioblastoma Multiforme | 102 | Nivolumab, Temozolomide | A |
| 38 | NCT02667587 | An Investigational Immuno-therapy Study of Temozolomide Plus Radiation Therapy With Nivolumab or Placebo, for Newly Diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer) | Active, not recruiting | III | Brain Neoplasms | 693 | Nivolumab, Temozolomide, Radiotherapy | USA |
| 39 | NCT02617589 | An Investigational Immuno-therapy Study of Nivolumab Compared to Temozolomide, Each Given With Radiation Therapy, for Newly-diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer) | Active, not recruiting | III | Brain Cancer | 560 | Nivolumab, Temozolomide, Radiotherapy | USA |
| 40 | NCT01213407 | Dendritic Cell Cancer Vaccine for High-grade Glioma | Completed | II | Glioblastoma Multiforme | 87 | Trivax, Temozolomide, Surgery, Radiotherapy | AU |
| 41 | NCT02529072 | Nivolumab With DC Vaccines for Recurrent Brain Tumors | Completed | I | Malignant Glioma, Astrocytoma | 6 | Nivolumab | USA |
| 42 | NCT03718767 | Nivolumab in Patients With IDH-Mutant Gliomas With and Without Hypermutator Phenotype | Recruiting | II | Malignant Glioma of Brain | 95 | Nivolumab | USA |
| 43 | NCT01480479 | Phase III Study of Rindopepimut/GM-CSF in Patients With Newly Diagnosed Glioblastoma | Completed | III | Malignant Glioma of Brain | 745 | Rindopepimut (CDX-110) with GM-CSF Temozolomide, KLH | USA |
| 44 | NCT01058850 | Phase I Rindopepimut After Conventional Radiation in Children w/Diffuse Intrinsic Pontine Gliomas | Terminated | I | Brain Cancer, Brain Stem Tumors | 3 | Rindopepimut | USA |
| 45 | NCT00045968 | Study of a Drug [DCVax®-L] to Treat Newly Diagnosed GBM Brain Cancer | Unknown | III | Glioblastoma | 348 | Dendritic cell immunotherapy | USA |
| 46 | NCT02808364 | Personalized Cellular Vaccine for Recurrent Glioblastoma (PERCELLVAC2) | Active, not recruiting | I | Glioblastoma | 10 | Personalized cellular vaccine | CHN |
| 47 | NCT02709616 | Personalized Cellular Vaccine for Glioblastoma (PERCELLVAC) | Active, not recruiting | 10 | Glioblastoma | 10 | Personalized cellular vaccine | CHN |
| 48 | NCT02209376 | Autologous T Cells Redirected to EGFRVIII-With a Chimeric Antigen Receptor in Patients With EGFRVIII+ Glioblastoma | Terminated | I | Patients With Residual or Reccurent EGFRvIII+ Glioma | 11 | CART-EGFRvIII T cells | USA |
| 49 | NCT03726515 | CART-EGFRvIII + Pembrolizumab in GBM | Active, not recruiting | I | Glioblastoma | 7 | CART-EGFRvIII T cells, Pembrolizumab | USA |
| 50 | NCT02664363 | EGFRvIII CAR T Cells for Newly-Diagnosed WHO Grade IV Malignant Glioma | Terminated | I | Glioblastoma, Gliosarcoma | 3 | EGFRvIII CAR T cells | USA |
| 51 | NCT01109095 | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients With GBM | Completed | I | Glioblastoma Multiforme | 16 | HER.CAR CMV-specific CTLs | USA |
| 52 | NCT02208362 | Genetically Modified T-cells in Treating Patients With Recurrent or Refractory Malignant Glioma | Recruiting | I | Glioblastoma, Recurrent Malignant Glioma | 92 | IL13Ralpha2-specific Hinge-optimized 41BB-co-stimulatory CAR Truncated CD19-expressing Autologous T-Lymphocytes | USA |
| 53 | NCT04003649 | IL13Ralpha2-Targeted Chimeric Antigen Receptor (CAR) T Cells With or Without Nivolumab and Ipilimumab in Treating Patients With Recurrent or Refractory Glioblastoma | Recruiting | I | Recurrent/Refractory Glioblastoma | 60 | IL13Ralpha2-specific Hinge-optimized 4-1BB-co-stimulatory CAR/Truncated CD19-expressing Autologous TN/MEM Cells, Ipilimumab, Nivolumab | USA |
| 54 | NCT03383978 | Intracranial Injection of NK-92/5.28.z Cells in Patients With Recurrent HER2-positive Glioblastoma | Recruiting | I | Glioblastoma | 30 | NK-92/5.28.z | DE |
| 55 | NCT03392545 | Combination of Immunization and Radiotherapy for Malignant Gliomas (InSituVac1) | Recruiting | I | High Grade Glioma, Glioblastoma, Glioma of Brainstem | 30 | Combined immune adjuvants and radiation | CHN |
| 56 | NCT03389230 | Memory-Enriched T Cells in Treating Patients With Recurrent or Refractory Grade III-IV Glioma | Recruiting | I | High Grade Glioma, Glioblastoma | 42 | HER2(EQ)BBζ/CD19t+ Tcm | USA |
| 57 | NCT03347097 | Adoptive Cell Therapy of Autologous TIL and PD1-TIL Cells for Patients With Glioblastoma Multiforme | Active, not recruiting | I | Glioblastoma Multiforme | 40 | TIL | CHN |
| 58 | NCT03344250 | Phase I EGFR BATs in Newly Diagnosed Glioblastoma | Recruiting | I | Glioblastoma Multiforme | 18 | EGFR BATs with SOC RT and TMZ | USA |
| 59 | NCT03170141 | Immunogene-modified T (IgT) Cells Against Glioblastoma Multiforme | Enrolling by invitation | I | Glioblastoma Multiforme | 20 | Antigen-specific IgT cells | CHN |
| 60 | NCT02937844 | Pilot Study of Autologous Chimeric Switch Receptor Modified T Cells in Recurrent Glioblastoma Multiforme | Unknown | I | Glioblastoma Multiforme | 20 | Anti-PD-L1 CSR T cells, Cyclophosphamide, Fludarabine | CHN |
| 61 | NCT02799238 | Autologuos Lymphoid Effector Cells Specific Against Tumour (ALECSAT) as Add on to Standard of Care in Patients With Glioblastoma | Completed | II | Glioblastoma | 62 | ALECSAT, Radiotherapy, Temozolomide | SE |
| 62 | NCT02060955 | Randomized Phase 2 Study to Investigate Efficacy of ALECSAT in Patients With GBM Measured Compared to Avastin/Irinotecan | Terminated | II | Glioblastoma Multiforme | 25 | ALECSAT, Bevacizumab/Irinotecan | DK |
| 63 | NCT01588769 | A Phase I Study to Investigate Tolerability and Efficacy of ALECSAT Administered to Glioblastoma Multiforme Patients | Completed | I | Glioblastoma Multiforme | 23 | ALECSAT cell based immunotherapy | DK |
| 64 | NCT01454596 | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients With Malignant Gliomas Expressing EGFRvIII | Completed | I, II | Malignant Glioma, Glioblastoma, Gliosarcoma | 18 | Epidermal growth factor receptor(EGFRv)III Chimeric antigen receptor (CAR) transduced PBL, Aldesleukin, Fludarabine, Cyclophosphamide | USA |
| 65 | NCT01144247 | Cellular Immunotherapy Study for Brain Cancer | Completed | I | Malignant Glioma of Brain | 10 | Alloreactive CTL | USA |
| 66 | NCT01082926 | Phase I Study of Cellular Immunotherapy for Recurrent/Refractory Malignant Glioma Using Intratumoral Infusions of GRm13Z40-2, An Allogeneic CD8+ Cytolytic T-Cell Line Genetically Modified to Express the IL 13-Zetakine and HyTK and to be Resistant to Glucocorticoids, in Combination With Interleukin-2 | Completed | I | Malignant Glioma of Brain | 6 | Therapeutic allogeneic lymphocytes, Aldesleukin | USA |
| 67 | NCT00730613 | Cellular Adoptive Immunotherapy Using Genetically Modified T-Lymphocytes in Treating Patients With Recurrent or Refractory High-Grade Malignant Glioma | Completed | I | Central Nervous System Tumors | 3 | Therapeutic autologous lymphocytes | NA |
| 68 | NCT00331526 | Cellular Adoptive Immunotherapy in Treating Patients With Glioblastoma Multiforme | Completed | II | Central Nervous System Tumors | 83 | Aldesleukin, Therapeutic autologous lymphocytes, Adjuvant therapy, Surgery | USA |
| 69 | NCT00004024 | Biological Therapy Following Surgery and Radiation Therapy in Treating Patients With Primary or Recurrent Astrocytoma or Oligodendroglioma | Completed | II | Central Nervous System Tumors | 60 | Aldesleukin, Autologous tumor cell vaccine, Muromonab-CD3, Sargramostim, Therapeutic autologous lymphocytes, Surgical procedure, Radiation therapy | USA |
A: Australia; ALECSAT: Autologous Lymphoid Effector Cells Specific Against Tumor; AU: Austria; CHN: China; CTL: cytotoxic T-lymphocytes; DE: Denmark; EGFR BATs: EGFR Bi-armed Activated T-cells; EGFR: epidermal growth factor; EGFRvIII: epidermal growth factor receptor variant III; ES: Spain; FR: France; GBM: Glioblastoma; HER2(EQ)BBζ/CD19t+ Tcm: preparation of genetically modified autologous central memory enriched T-cells (Tcm) expressing a chimeric antigen receptor consisting of an anti-human epidermal growth factor 2 (HER2) variable fragment that is linked to the signaling domain of the T-cell antigen receptor complex zeta chain (BBζ), and truncated cluster of differentiation (CD)19; IL-13Rα2: interleukin-13 receptor α2; IR: Iran; J: Japan; NA: Not Available; PBL: peripheral blood lymphocytes; PD-L1 CSR: programmed death Ligand 1 chimeric switch receptor; RT: Radiotherapy; SE: Sweden; TIL: Tumor-infiltrating T-Lymphocyte; TMZ: temozolomide; USA: United States; ZH: Zurich.
3.1. Classification of Immunotherapies
Immunotherapies for brain gliomas can be classified based on the molecular mechanism and type of drug involved [27,28,29,30,31]. Two potential strategies were outlined: active immunotherapy, aimed at directly inducing the host antitumoral immune response, and adoptive (or passive) immunotherapy, resulting in the transduction of autologous/allogeneic immune cells aimed at the introduction or restoration of immune functions [29,32,33]. Alkylating agents, monoclonal antibodies, and vaccines are used for active immunotherapy, whereas engineered T cells, natural killer (NK) cells, and natural killer T (NKT) cells are used for adoptive immune strengthening. Table 3 reports the classification of novel immunotherapies for HGGs.
Table 3.
Classification of immunotherapies for malignant brain tumors.
| Immunotherapies | |||
|---|---|---|---|
| Active | Checkpoint Inhibitors | Alkylating agent | TMZ |
| MAbs | BVZ, Nivolumab | ||
| Vaccine | Rindopepimut DCVax-Brain PerCellVac2 |
||
| Adoptive | T cell | TCR transgenic T | |
| CAR T | EGFRIII IL-13Ra2 HER2 EphA2 |
||
| NK cell | Allogenic NK | ||
| Anti-KIR Abs | |||
| ADCC | |||
| DNRII | |||
| NK exosomes | |||
| NKT | Autologous NKT, autologous mature DC cultured with NKT ligand α-galactosyl ceramide | ||
| Hybrid | ALECSAT | ||
ADCC: antibody-dependent cellular cytotoxicity; ALECSAT: Autologous Lymphoid Effector Cells Specific Against Tumor; Anti-KIR Abs: Antibody-mediated blocking of KIR; BVZ: Bevacizumab; CAR T: chimeric antigen receptor; DC: Dendritic Cell; DNRII: dominant-negative receptor II; EGFRIII: epidermal growth factor receptor variant III; EphA2: erythropoietin-producing hepatocellular carcinoma A2; HER2: human epidermal growth factor 2; IL-13Ra2: interleukin-13 receptor α2; MAbs: Monoclonal Antibodies; NK: natural killer cells; NKT: T lymphocyte-natural killer cells; T: T lymphocyte; TMZ: Temozolomide.
3.2. Active Immunotherapies
3.2.1. Alkylating Agents
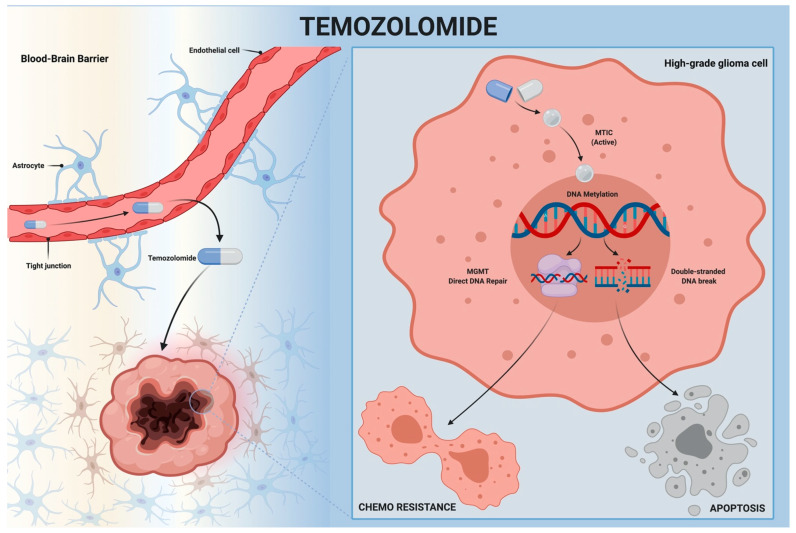
Temozolomide (TMZ) (Temodar®, Schering-Plough Research Institute, Kenilworth, NJ, USA), an oral alkylating agent, is the backbone of systemic therapy for HGGs [6,7]. Alkylating agents are basically chemotherapy drugs. They target tumor cells and block the cell cycle by damaging DNA double strands [34,35]. In prodrug status, TMZ crosses the blood–brain barrier (BBB) owing to its lipophilic properties and it reaches the tumor cells at therapeutic-relevant concentrations [36,37,38]. It is spontaneously converted into the active compound 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC), which can methylate DNA bases. MTIC transfers alkyl groups to guanine or adenine at the N7-/O6- or N3-position, respectively. The methylation results in base pair mismatch and chromatin structure remodeling, induces G2/M cell cycle arrest, and finally leads to cell apoptosis [37,39,40,41]. As a result, TMZ therapeutic success is closely dependent on DNA repair pathways, which leads to glioma therapeutic resistance [42,43]. The demethylating enzyme MGMT acts directly in repairing O6-methylguanine, consequently avoiding gene mutation and glioma cell death [44,45]. The methylation status of the MGMT promoter and the enzyme expression predict the response to TMZ and are also prognostic factors of survival [46,47,48,49]. Figure 2 describes the TMZ mechanism of action and its effects in the glioma cells (Figure 2).
Figure 2.
Temozolomide mechanism of action. Temozolomide crosses the blood–brain barrier and reaches high-grade glioma cells. It is spontaneously converted to the active compound (MTIC), which methylates DNA bases, breaks double-strand DNA, and leads to apoptosis. MTIC action is opposed to the DNA repair pathways, including MGMT. MGMT: O6-methylguanine-DNA methyltransferase; MTIC: 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide; TMZ: Temozolomide.
3.2.2. Monoclonal Antibodies
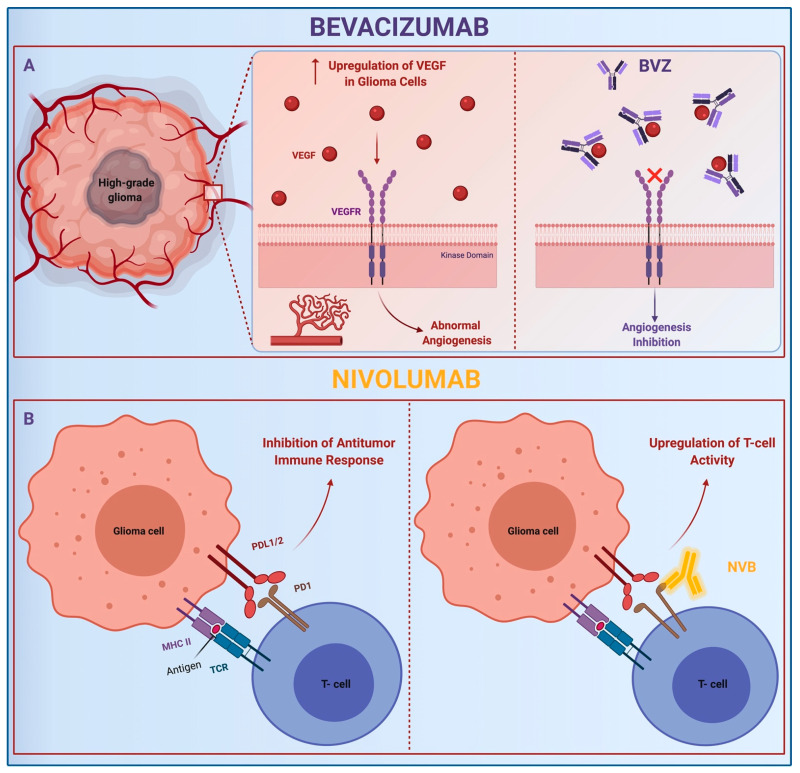
Monoclonal antibodies (MAbs) are purified immunoglobulin with monovalent affinity. MAbs bind specific molecular epitopes and enlist the host immune system against tumors. Bevacizumab (BVZ) is the most widely tested, especially for the treatment of recurrent glioblastoma [50,51,52,53,54,55,56,57]. It directly targets the vascular endothelial growth factor A (VEGF-A) inhibiting the interaction with VEGF tyrosine kinase receptor, which is overexpressed on the surface of endothelial cells as a result of tissue hypoxia, and interrupts aberrant tumor angiogenesis (Figure 3A). The antiangiogenic effect exerted by BVZ extends to the reduction of microvessel density and vascular permeability, resulting in the antiedema effect [51,58,59]. Furthermore, recent studies have investigated the potential role of BVZ in modulating the immunosuppressive tumor microenvironment [60,61,62]. Two phase III clinical trials, the Avastin in Glioblastoma (AVAglio92) [63] (#NCT00943826) and the Radiation Therapy Oncology Group (RTOG)-082593 [64] (#NCT00884741), studied the combination of BVZ with standard therapy, demonstrating promising results for recurrent HGGs.
Figure 3.
Mechanism of action of MAbs, bevacizumab and nivolumab. BVZ: (A) Bevacizumab; MHC: Major Histocompatibility Complex; NVB: (B) Nivolumab; PD-1: Programmed Cell Death Protein. Abbreviations: PDL-1/2: Programmed Cell Death Protein Ligand 1/2; TCR: Transgenic T Cell Receptor; VEGF-A: Vascular Endothelial Growth Factor A; VEGFR: Vascular Endothelial Growth Factor Tyrosine Kinases Receptor.
The AVAglio92 documented a significant improvement in the median progression free survival (PFS) to 10.6 months in the BVZ group, versus 6.2 months in the placebo one (p < 0.001). Overall survival (OS) proved to be higher only during the first two years of treatment in the BVZ group (72.4% versus 66.3%) [63]. The RTOG-082593 reported an increase in PFS in the BVZ group compared to the control group (10.7 months versus 7.3 months, p = 0.007), but equally failed to demonstrate a better OS [64].
Nivolumab, a human IgG4, directly binds programmed cell death protein 1 (PD-1), exposed on activated T cells. Physiologically, PD-1 interacts with its ligands, namely PD-L1 and PD-L2, downregulating the immune cascade. PD-Ls are overexpressed on HGG cells as a mechanism of immune escape. Nivolumab impounds PD-1 and boosts the antitumoral activity of CD4+ and CD8+ cells (Figure 3B) [65,66,67]. Despite BVZ and nivolumab being studied for glioma therapy in several clinical trials and also as a combined protocol (#NCT03890952, #NCT03743662, #NCT03452579) [68,69,70], the administration route remains still a concern. Because the BBB physiologically blocks the antibody access to the brain, several studies are focusing on innovative mAb-delivering routes, to improve the drug efficacy and intratumoral uptake. Intra-arterial administration, intracranial injection, and nanoparticle and liposomal carriers are currently potential strategies [56,71,72,73,74].
3.2.3. Vaccine
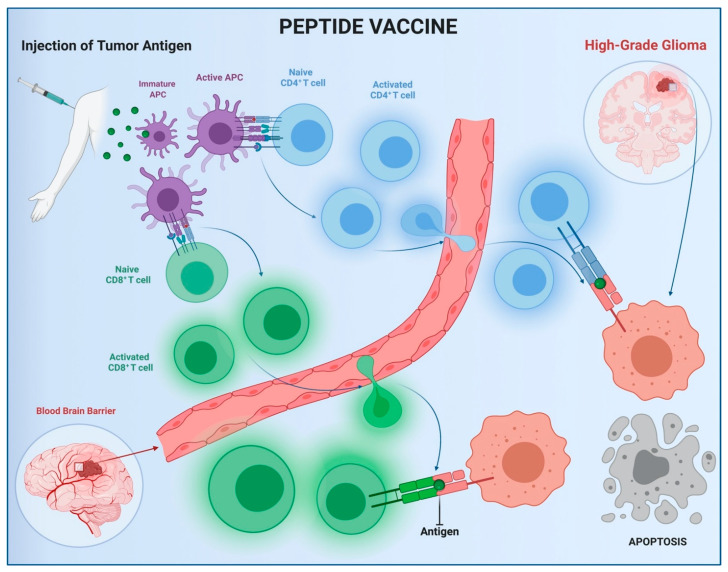
Anticancer vaccination was designed to strengthen host immune response by stimulating the production of self-antibodies against tumor antigens. Advanced research in translational medicine has allowed the isolation of specific glioma immunogens, i.e., epidermal growth factor receptor variant III (EGFRvIII). EGFRvIII is an active splice variant, found in 30% of primary glioblastoma (GBM), which enhances cell proliferation and survival [75,76,77,78,79,80]. Rindopepimut (Rintega®, Celldex Therapeutics, Inc., Phillipsburg, NJ, USA), a 14-mer injectable peptide vaccine against EGFRvIII, was projected to activate CD4+ and CD8+ T cells against malignant brain tumor cells (Figure 4). It was also evaluated in some preclinical studies. The first phase I trial, VICTORI, showed an excellent safety profile, a PFS of 10.2 months and an OS of 22.8 months. Some phase II trials (ACTIVATE, ACT II–III, and ReACT) confirmed the low toxicity and demonstrated an increase in median progression-free and overall survival for recurrent gliomas. The ACTIVATE phase II trial reported a PFS of 14.2 and 6.4 months, and an OS of 26 and 15.2 months in the vaccinated patients and control group, respectively. In ACT II and III studies, the PFS was 15.2 and 12.3 for the rindopepimut group, respectively, while the PFS was 6.4 months in both control groups. The OS was 23.6 and 24.6 months in ACT II and III trials, respectively, compared to the control groups (15.2 months). ReACT showed a PFS at 6 months of 28% for rindopepimut, compared to 16% in the control group [79,81,82,83,84].
Figure 4.
Peptide vaccine mechanism of action. APC: Antigen-Presenting Cell.
ACT IV, a multicentric phase III trial, tested the combination of rindopepimut and standard chemotherapy with TMZ in newly diagnosed GBM, showing no significant difference in OS between the two groups (#NCT01480479) [85].
Several other multiple-epitope vaccines are also under development for glioma treatment [86]. Among these vaccines, the dendritic cell vaccine (DCVax-Brain) consists of purified dendritic cells and tumor antigens (#NCT00045968) [87,88], whereas the personalized cellular vaccine (PerCellVac2) is made up of allogeneic peripheral blood cells combined with autologous glioma antigens (#NCT02808364, #NCT02709616) [89].
3.3. Adoptive Immunotherapies
3.3.1. Engineered T Cells
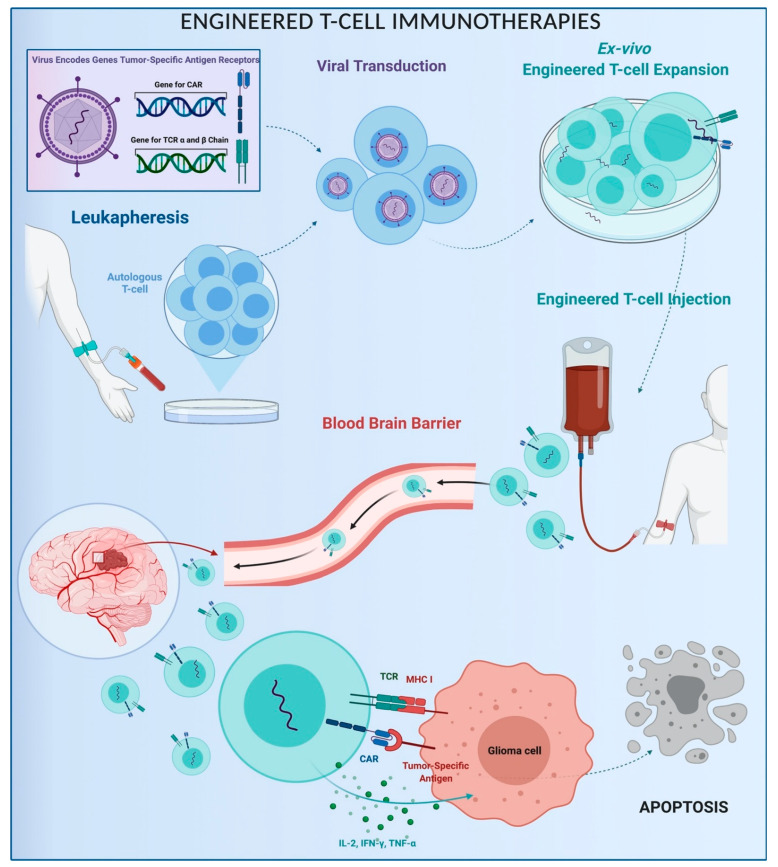
In the field of adoptive immunotherapies, T-cell-based strategies are the most promising. Protocols begin with patient leukapheresis, followed by the engineering of autologous T cells via viral vectors. The viral transduction integrates autologous T cells with tumor-specific antigen receptor genes, specifically the chimeric antigen receptor (CAR) and the transgenic T cell receptor (TCR) genes [33,90]. CARs have an extracellular domain that binds tumor-specific ligands, whereas the intracellular domain activates T cell pathways [91,92,93,94,95,96,97]. The specificity of CAR T depends on the transinfected CAR genes. Several CAR genes were investigated: EGFRvIII (#NCT02209376, #NCT02664363, #NCT03726515), human epidermal growth factor receptor 2 (HER2) (#NCT01109095, #NCT03389230), interleukin-13 receptor A2 (IL13Ra2) (#NCT00730613, #NCT02208362, #NCT04003649), and erythropoietin-producing hepatocellular carcinoma A2 (EphA2). EGFRvIII-, HER2-, and IL13Ra2-targeted CAR T cells were just tested for HGG therapy in preclinical and clinical trials. In 2017, O’Rourke and colleagues treated 10 patients with a single-dose of EGFRvIII CAR T cells (#NCT02209376), reporting no dose-related side effects and a good safety profile, but neither the OS nor the PFS was shown to increase. In 2017, Ahmed et al. tested HER2-specific CAR T cells for treatment of 17 recurrent HER2+ high-grade gliomas (#NCT01109095) and showed similar results. Further studies are needed to determine their feasibility and safety [98,99,100,101,102,103,104,105,106,107].
The TCR complex is exposed on the surface of T cells and interacts with the major histocompatibility complex (MHC), leading to activation of the cellular immune cascade. TCR transgenic T cells are genetically engineered to express receptors against specific tumor MHC and stimulate the immune response against malignant brain tumors [108,109,110,111]. CAR and TCR engineered T cells are expanded ex vivo and injected, cross the BBB, bind the tumor cells, and activate the antitumoral immune cascade, thus promoting apoptosis (Figure 5) [94,112,113,114,115].
Figure 5.
Schematic representation of engineered T-cell immunotherapy. CAR: Chimeric Antigen Receptor; MHC: Major Histocompatibility Complex; TCR: Transgenic T Cell Receptor.
3.3.2. NK Cells
NK cells are involved in the treatment of malignant brain tumors by means of different strategies [116]. The first strand can exploit allogeneic NK cells and target tumor cells by the lack of MHC and, without being inactivated, exert oncolytic effects [117,118,119,120]. Another approach includes the NK immunoglobulin-like receptor antibodies (anti-KIR Abs). They block the link between NK cells and MHC class I, avoid the immunosuppression response, and increase the NK-mediated tumor apoptosis [116,121]. Furthermore, the antibody-dependent cell-mediated cytotoxicity (ADCC) is a mechanism wherein specific antibodies, directed to HGG antigens, interact with NK cells, promoting tumor lyses. The Fab portion binds antigens on the tumor cell surface, whereas the fragment crystallizable (Fc) region recognizes the NK cell receptors, such as CD16/FcγRIII, mediating the antitumoral immune response. Among ADCC, EGFR tumor antigen and CD16 (FcγIIIA), KIR2DS2, and NK Group 2D (NKG2D) receptors are the most researched [121].
In 2017, Yvon and colleagues proved the efficacy of cord blood NK cells that were retrovirally transduced and manipulated to express the TGF-β-dominant-negative receptor II (DNRII) [121,122]. DNRII makes NK cells immune to the TGF-β that is overexpressed in the tumor microenvironment as an immune evasion promoter. Other techniques under development involve the exosomes as a vector for NK cells and a new sort of CAR (CAR-KHYG-1) targeting EGFRvIII [123,124,125].
3.3.3. NKT Cells and Hybrid Therapies
NKT cells are a subgroup of T cells with concomitant properties of T and NK cells, both expressing the type of molecular surfaces. Particularly, CD1d-restricted NKT cells were found to play an important role as immunoregulatory cells within the glioma microenvironment [126]. Strategies to overcome the immune tolerance of glioma include the expansion in vitro of autologous mature dendritic cells (DCs) with NKT ligand α-galactosyl ceramide, which enhances NKT cell cytotoxic activity [127,128]. Another integrated approach is the autologous lymphoid effector cells specific against tumor cell (ALECSAT) therapy (Cytovac A/S, Hørsholm, Denmark). ALECSAT is a 26-day immunization protocol. Autologous lymphocytes and monocytes were isolated and differentiated in vitro. The mature DCs and nonactivated lymphocytes are cultured with CD4+ T cells and 5-aza-2′-deoxycytidine, a DNA-demethylation agent, which induces the expression of tumor antigens. The activated CD4+ T cell and CD8+ cytotoxic lymphocyte selectively target tumor cells, whereas the glioma cells that do not express the antigen are targeted by activated NK cells, reducing the chance of cancer immune evasion (#NCT02060955) [129].
4. Discussion
The present study reviews current immunotherapeutic approaches to malignant brain tumors, specifically focusing on classification, immunomechanisms, evidence from clinical trials, limitations, and future challenges.
The current first-line multimodal treatment involves the combination of gross total surgical resection, followed by the use of adjuvant alkylating agents and whole-brain radiotherapy [6,7,130]. Despite TMZ being the only systemic agent approved as of now, glioma cells have often been found to be resistant [131,132,133]. One of the major disadvantages of TMZ is the rapid in vivo hydrolytic degradation. Several studies seek to overcome the quick drug clearance through polymer scaffold molecules, simultaneously upgrading therapeutic efficacy and reducing adverse events [134,135]. TMZ also frequently induces lymphopenia and myelosuppression, as do many other chemotherapy drugs. The immunodepletion leads to less immune surveillance and inhibition of antitumor immune response [136,137,138]. Another downside is the tumor expression of DNA repair enzymes, such as MGMT, which nullify the alkylating agents’ therapeutic effects. The MGMT promoter status heavily influences overall survival, which is almost 21 months for methylated MGMT HGGs rather than 13 months for the unmethylated ones [46,49,139,140].
The failure of current therapeutic approaches, combined with advances in translational medicine, has led to the development of new strategies. The first turning point was the use of MAbs and BVZ. The Food and Drug Administration approved the use of BVZ based on the encouraging results achieved by the phase II BRAIN study (#NCT00345163), conducted on 167 patients affected by recurrent GBMs [141]. The initial enthusiasm around BVZ approval waned due to the lack of an effective overall survival improvement. The decrease of enhancement on MRI T1, which occurred in up to 60% of BVZ patients and was defined as a radiographic pseudo-response, is nothing more than an antiangiogenic effect without real tumor shrinkage. BVZ treatment stops blood vessel proliferation and reduces BBB permeability, resulting in an immediate contrast-enhancement decrease. However, these microvascular changes do not affect tumor proliferation or overall survival [142,143,144,145]. The third therapeutic option, within active immunotherapies, is the antitumoral vaccination. Peptide vaccines are designed to activate host immunity versus tumor-specific antigens. They have proven to be feasible and safe, but are still ineffective [80,146]. HGGs’ intrinsic heterogeneity, mechanisms of immune escape, and loss of antigenic variants mediate patient immunosuppression and invalidate the potency of the vaccine. Personalized multipeptide vaccines that are customized to target more than one tumor antigen should be developed in the future [147,148].
The glioma microenvironment, populated by cancer stem cells, immunosuppressive molecules, and interleukins, plays a pivotal role in tumor growth and immune resistance [20,149,150,151,152,153,154]. The rationale of adoptive immunotherapies is to modulate the tumor setting through the activation of cell immunity. These technologies are based on the manipulation of autologous/allogenic immunological cells and the engineering of CD8+ and CD4+ T lymphocytes [155]. Preliminary studies have considered CAR T cells as a valid and safe tool, which are tested intravenously and through intracranial infusion. However, the limited survival of T CAR, mechanisms of antigen escape, and ineffective homing to tumor cells do not make this a strategy yet achievable as a second-line treatment. The emerging NK-cell-based approach still has its limitations, such as the high expression of MHC I and HLA type A on glioma cells, which tie the KIRs and impede NK cells’ antitumoral activity.
Limitations and Future Perspectives
Several aspects are responsible for the resilience of glioma, such as the lack of tumor antigen, loss of immunological phenotype, and immune evasive molecule production, which all together still limit the success of both active and adoptive immunotherapies.
Furthermore, the main limitation lies in the need to overcome the BBB, the reason why the administration route is pivotal. Several studies have proposed innovative methods, such as the implanted intracerebral convection-enhanced deliveries or the superselective intra-arterial cerebral infusion [156,157,158,159,160,161]. The intra-arterial administration is additionally facilitated by the previous destruction of BBB, carried out through osmotic agents, hypertonic solutions and mannitol, or ultrasounds [156,162,163]. Low frequency ultrasounds are a non-invasive strategy which lead to BBB disruption resulting in increased drug penetration and faster time to reach the tumor site [162]. An innovative emerging technique exploits the vascularized temporoparietal fascial flaps with the aim to bypass the BBB. These flaps are vascularized by the external carotid artery system, free from the BBB system. They can be transposed into the surgical cavity, after glioma resection, and could allow effective drug penetration and residual tumor cell targeting [164].
Another key aspect is the engineering of biocompatible and non-toxic small carriers able to improve drug diffusion and tissue distribution. Viral vehicles, nanoparticles, and liposomes are currently the most investigated [165,166,167,168].
The development of new administration routes and the advances in engineering more efficient and safe carriers will allow the implementation of standard therapeutic protocols with concomitant tailored immunotherapies.
5. Conclusions
Immunotherapeutic strategies are designed to overcome the immune escape pathways and the immunosuppressive glioma microenvironment, which are responsible for the resilience of HGGs.
Antitumoral immunotherapies involve active immune products along with adoptive engineered T, NK, and NKT cells.
BVZ is indicated in the treatment of recurrent HGGs, but not yet within the upfront protocol for newly diagnosed HGGs. Vaccines have been proven to be safe and feasible, although their treatment efficacy needs to be evaluated further. CAR T cells against EGFRvIII and engineered TCR T, NK, and hybrid cells have demonstrated a promising potential in several preclinical studies.
Although they are still not considered to be the first-line treatment against malignant gliomas, immunotherapies have shown excellent results in improving PFS.
Further clinical studies are focusing on validating antitumoral immunotherapeutic approaches such as personalized second-line strategies.
Author Contributions
Conceptualization, A.G.L.; methodology, A.G.L.; validation, S.L.; formal analysis, A.G.L.; investigation, S.L.; resources, S.L.; data curation, A.G.L.; writing—original draft preparation, A.G.L.; writing—review and editing, S.L.; visualization, A.G.L.; supervision, S.L.; project administration, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in the study were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data Availability Statement
All data are included in the main text.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16:iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J., Comber H., Forman D., Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Soerjomataram I., Lortet-Tieulent J., Parkin D.M., Ferlay J., Mathers C., Forman D., Bray F. Global burden of cancer in 2008: A systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 4.Ladomersky E., Genet M., Zhai L., Gritsina G., Lauing K.L., Lulla R.R., Fangusaro J., Lenzen A., Kumthekar P., Raizer J.J., et al. Improving vaccine efficacy against malignant glioma. Onco Immunol. 2016;5:e1196311. doi: 10.1080/2162402X.2016.1196311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom Q.T., Gittleman H., Stetson L., Virk S.M., Barnholtz-Sloan J.S. Epidemiology of Gliomas. Cancer Treat. Res. 2014;163:1–14. doi: 10.1007/978-3-319-12048-5_1. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R., Hegi M.E., Mason W.P., Bent M.J.V.D., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Johnson D.R., O’Neill B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neuro Oncol. 2012;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 9.Eagan R.T., Scott M. Evaluation of prognostic factors in chemotherapy of recurrent brain tumors. J. Clin. Oncol. 1983;1:38–44. doi: 10.1200/JCO.1983.1.1.38. [DOI] [PubMed] [Google Scholar]
- 10.Wen P.Y., Kesari S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 11.Perry J.R., Laperriere N., O’Callaghan C.J., Brandes A.A., Menten J., Phillips C., Fay M., Nishikawa R., Cairncross J.G., Roa W., et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017;376:1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 12.Chen R., Smith-Cohn M., Cohen A.L., Colman H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics. 2017;14:284–297. doi: 10.1007/s13311-017-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel-Passow J.E., Lachance D.H., Molinaro A.M., Walsh K.M., Decker P.A., Sicotte H., Pekmezci M., Rice T.W., Kosel M.L., Smirnov I.V., et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig K., Kornblum H.I. Molecular markers in glioma. J. Neuro-Oncol. 2017;134:505–512. doi: 10.1007/s11060-017-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F., Li G., Liu H., Zhao Z., Chai R., Liu Y., Jiang H., Zhai Y., Feng Y., Li R., et al. Molecular subtyping reveals immune alterations in IDH wild-type lower-grade diffuse glioma. J. Pathol. 2020;251:272–283. doi: 10.1002/path.5468. [DOI] [PubMed] [Google Scholar]
- 16.Wesseling P., Capper D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2017;44:139–150. doi: 10.1111/nan.12432. [DOI] [PubMed] [Google Scholar]
- 17.Louis D.N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 18.Lucifero A.G., Luzzi S., Brambilla I., Guarracino C., Mosconi M., Foiadelli T., Savasta S. Gene therapies for high-grade gliomas: From the bench to the bedside. Acta Biomed. 2020;91:32–50. doi: 10.23750/abm.v91i7-S.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campanella R., Guarnaccia L., Cordiglieri C., Trombetta E., Caroli M., Carrabba G., La Verde N., Rampini P., Gaudino C., Costa A., et al. Tumor-Educated Platelets and Angiogenesis in Glioblastoma: Another Brick in the Wall for Novel Prognostic and Targetable Biomarkers, Changing the Vision from a Localized Tumor to a Systemic Pathology. Cells. 2020;9:294. doi: 10.3390/cells9020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gieryng A., Pszczolkowska D., Walentynowicz K.A., Rajan W.D., Kaminska B. Immune microenvironment of gliomas. Lab. Investig. 2017;97:498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox J.A., Ramakrishna R., Magge R. Immunotherapy in Glioblastoma. World Neurosurg. 2018;116:518–528. doi: 10.1016/j.wneu.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Grabowski M.M., Sankey E.W., Ryan K.J., Chongsathidkiet P., Lorrey S.J., Wilkinson D.S., Fecci P.E. Immune suppression in gliomas. J. Neuro Oncol. 2021;151:3–12. doi: 10.1007/s11060-020-03483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanaei S., Afshari K., Hirbod-Mobarakeh A., Mohajer B., Dastmalchi D.A., Rezaei N. Therapeutic efficacy of specific immunotherapy for glioma: A systematic review and meta-analysis. Rev. Neurosci. 2018;29:443–461. doi: 10.1515/revneuro-2017-0057. [DOI] [PubMed] [Google Scholar]
- 24.Lucifero A.G., Luzzi S., Brambilla I., Trabatti C., Mosconi M., Savasta S., Foiadelli T. Innovative therapies for malignant brain tumors: The road to a tailored cure. Acta Biomed. 2020;91:5–17. doi: 10.23750/abm.v91i7-S.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehcordi S.R., Ricci A., Di Vitantonio H., De Paulis D., Luzzi S., Palumbo P., Cinque B., Tempesta D., Coletti G., Cipolloni G., et al. Stemness Marker Detection in the Periphery of Glioblastoma and Ability of Glioblastoma to Generate Glioma Stem Cells: Clinical Correlations. World Neurosurg. 2017;105:895–905. doi: 10.1016/j.wneu.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 26.Campanella R., Guarnaccia L., Caroli M., Zarino B., Carrabba G., La Verde N., Gaudino C., Rampini A., Luzzi S., Riboni L., et al. Personalized and translational approach for malignant brain tumors in the era of precision medicine: The strategic contribution of an experienced neurosurgery laboratory in a modern neurosurgery and neuro-oncology department. J. Neurol. Sci. 2020;417:117083. doi: 10.1016/j.jns.2020.117083. [DOI] [PubMed] [Google Scholar]
- 27.Luzzi S., Lucifero A.G., Brambilla I., Mantelli S.S., Mosconi M., Foiadelli T., Savasta S. Targeting the medulloblastoma: A molecular-based approach. Acta Biomed. 2020;91:79–100. doi: 10.23750/abm.v91i7-S.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luzzi S., Crovace A.M., Del Maestro M., Lucifero A.G., Elbabaa S.K., Cinque B., Palumbo P., Lombardi F., Cimini A., Cifone M.G., et al. The cell-based approach in neurosurgery: Ongoing trends and future perspectives. Heliyon. 2019;5:e02818. doi: 10.1016/j.heliyon.2019.e02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mount N.M., Ward S.J., Kefalas P., Hyllner J. Cell-based therapy technology classifications and translational challenges. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20150017. doi: 10.1098/rstb.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everson R.G., Antonios J.P., Liau L.M. Cell-Based Immunotherapy of Gliomas. Prog. Neurol. Surg. 2018;32:90–100. doi: 10.1159/000469683. [DOI] [PubMed] [Google Scholar]
- 31.Lucifero A.G., Luzzi S., Brambilla I., Schena L., Mosconi M., Foiadelli T., Savasta S. Potential roads for reaching the summit: An overview on target therapies for high-grade gliomas. Acta Biomed. 2020;91:61–78. doi: 10.23750/abm.v91i7-S.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han S.J., Zygourakis C., Lim M., Parsa A.T. Immunotherapy for Glioma: Promises and challenges. Neurosurg. Clin. N. Am. 2012;23:357–370. doi: 10.1016/j.nec.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Luzzi S., Lucifero A.G., Brambilla I., Magistrali M., Mosconi M., Savasta S., Foiadelli T. Adoptive immunotherapies in neuro-oncology: Classification, recent advances, and translational challenges. Acta Biomed. 2020;91:18–31. doi: 10.23750/abm.v91i7-S.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyatt M.D., Pittman D.L. Methylating Agents and DNA Repair Responses: Methylated Bases and Sources of Strand Breaks. Chem. Res. Toxicol. 2006;19:1580–1594. doi: 10.1021/tx060164e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman H.S., Kerby T., Calvert H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 36.Schreck K.C., Grossman S.A. Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology. 2018;32:555–560. [PubMed] [Google Scholar]
- 37.Agarwala S.S., Kirkwood J.M. Temozolomide, a Novel Alkylating Agent with Activity in the Central Nervous System, May Improve the Treatment of Advanced Metastatic Melanoma. Oncologist. 2000;5:144–151. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 38.Portnow J., Badie B., Chen M., Liu A., Blanchard S., Synold T.W. The Neuropharmacokinetics of Temozolomide in Patients with Resectable Brain Tumors: Potential Implications for the Current Approach to Chemoradiation. Clin. Cancer Res. 2009;15:7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denny B.J., Wheelhouse R.T., Stevens M.F.G., Tsang L.L.H., Slack J.A. NMR and Molecular Modeling Investigation of the Mechanism of Activation of the Antitumor Drug Temozolomide and Its Interaction with DNA. Biochemistry. 1994;33:9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- 40.Moody C.L., Wheelhouse R.T. The Medicinal Chemistry of Imidazotetrazine Prodrugs. Pharmaceuticals. 2014;7:797–838. doi: 10.3390/ph7070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strobel H., Baisch T., Fitzel R., Schilberg K., Siegelin M.D., Karpel-Massler G., Debatin K.-M., Westhoff M.-A. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines. 2019;7:69. doi: 10.3390/biomedicines7030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hombach-Klonisch S., Mehrpour M., Shojaei S., Harlos C., Pitz M., Hamai A., Siemianowicz K., Likus W., Wiechec E., Toyota B.D., et al. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol. Ther. 2018;184:13–41. doi: 10.1016/j.pharmthera.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Stevens M.F., Bradshaw T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012;5:102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 44.Hegi M.E., Diserens A.-C., Gorlia T., Hamou M.-F., De Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., et al. MGMTGene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 45.Rao A.M., Quddusi A., Shamim M.S. The significance of MGMT methylation in Glioblastoma Multiforme prognosis. J. Pak. Med. Assoc. 2018;68:1137–1139. [PubMed] [Google Scholar]
- 46.Binabaj M.M., Bahrami A., ShahidSales S., Joodi M., Mashhad M.J., Hassanian S.M., Anvari K., Avan A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell. Physiol. 2018;233:378–386. doi: 10.1002/jcp.25896. [DOI] [PubMed] [Google Scholar]
- 47.Thon N., Kreth S., Kreth F.W. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. OncoTargets Ther. 2013;6:1363–1372. doi: 10.2147/OTT.S50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermisson M., Klumpp A., Wick W., Wischhusen J., Nagel G., Roos W., Kaina B., Weller M. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J. Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 49.Wick W., Weller M., Bent M.V.D., Sanson M., Weiler M., Von Deimling A., Plass C., Hegi M.E., Platten M., Reifenberger G. MGMT testing—The challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014;10:372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 50.Kim M.M., Umemura Y., Leung D. Bevacizumab and Glioblastoma: Past, Present, and Future Directions. Cancer J. 2018;24:180–186. doi: 10.1097/PPO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 51.Diaz R.J., Ali S., Qadir M.G., De La Fuente M.I., Ivan M.E., Komotar R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neuro Oncol. 2017;133:455–467. doi: 10.1007/s11060-017-2477-x. [DOI] [PubMed] [Google Scholar]
- 52.Grill J., Massimino M., Bouffet E., Azizi A.A., McCowage G., Cañete A., Saran F., Le Deley M.-C., Varlet P., Morgan P.S., et al. Phase II, Open-Label, Randomized, Multicenter Trial (HERBY) of Bevacizumab in Pediatric Patients with Newly Diagnosed High-Grade Glioma. J. Clin. Oncol. 2018;36:951–958. doi: 10.1200/JCO.2017.76.0611. [DOI] [PubMed] [Google Scholar]
- 53.Salmaggi A., Gaviani P., Botturi A., Lamperti E., Simonetti G., Ferrari D., Silvani A. Bevacizumab at recurrence in high-grade glioma. Neurol. Sci. 2011;32:251–253. doi: 10.1007/s10072-011-0799-6. [DOI] [PubMed] [Google Scholar]
- 54.Bent M.J.V.D., Klein M., Smits M., Reijneveld J.C., French P.J., Clement P., De Vos F.Y.F., Wick A., Mulholland P.J., Taphoorn M.J.B., et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): A randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018;19:1170–1179. doi: 10.1016/S1470-2045(18)30362-0. [DOI] [PubMed] [Google Scholar]
- 55.Su J.M.-F., Murray J.C., McNall-Knapp R.Y., Bowers D.C., Shah S., Adesina A.M., Paulino A.C., Jo E., Mo Q., Baxter P.A., et al. A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr. Blood Cancer. 2020;67:e28283. doi: 10.1002/pbc.28283. [DOI] [PubMed] [Google Scholar]
- 56.Khasraw M., Simeonovic M., Grommes C. Bevacizumab for the treatment of high-grade glioma. Expert Opin. Biol. Ther. 2012;12:1101–1111. doi: 10.1517/14712598.2012.694422. [DOI] [PubMed] [Google Scholar]
- 57.Khasraw M., Ameratunga M., Grommes C. Bevacizumab for the treatment of high-grade glioma: An update after Phase III trials. Expert Opin. Biol. Ther. 2014;14:729–740. doi: 10.1517/14712598.2014.898060. [DOI] [PubMed] [Google Scholar]
- 58.Im S.A., Gomez-Manzano C., Fueyo J., Liu T.J., Ke L.D., Kim J.S., Lee H.Y., Steck P.A., Kyritsis A.P., Yung W.K. Antiangiogenesis treatment for gliomas: Transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999;59:895–900. [PubMed] [Google Scholar]
- 59.Von Baumgarten L., Brucker D., Tirniceru A., Kienast Y., Grau S., Burgold S., Herms J., Winkler F. Bevacizumab Has Differential and Dose-Dependent Effects on Glioma Blood Vessels and Tumor Cells. Clin. Cancer Res. 2011;17:6192–6205. doi: 10.1158/1078-0432.CCR-10-1868. [DOI] [PubMed] [Google Scholar]
- 60.Gabrilovich D.I., Chen H.L., Girgis K.R., Cunningham H.T., Meny G.M., Nadaf S., Kavanaugh D., Carbone D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 61.Terme M., Pernot S., Marcheteau E., Sandoval F., Benhamouda N., Colussi O., Dubreuil O., Carpentier A.F., Tartour E., Taieb J. VEGFA-VEGFR Pathway Blockade Inhibits Tumor-Induced Regulatory T-cell Proliferation in Colorectal Cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 62.Shrimali R.K., Yu Z., Theoret M.R., Chinnasamy D., Restifo N.P., Rosenberg S.A. Antiangiogenic Agents Can Increase Lymphocyte Infiltration into Tumor and Enhance the Effectiveness of Adoptive Immunotherapy of Cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chinot O.L., Rouge T.D.L.M., Moore N., Zeaiter A., Das A., Phillips H., Modrusan Z., Cloughesy T. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv. Ther. 2011;28:334–340. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 64.Gilbert M.R., Dignam J.J., Armstrong T.S., Wefel J.S., Blumenthal D.T., Vogelbaum M.A., Colman H., Chakravarti A., Pugh S., Won M., et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schalper K.A., Rodriguez-Ruiz M.E., Diez-Valle R., López-Janeiro A., Porciuncula A., Idoate M.A., Inogés S., De Andrea C., De Cerio A.L.-D., Tejada S., et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 2019;25:470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J., Chen A.X., Gartrell R.D., Silverman A.M., Aparicio L., Chu T., Bordbar D., Shan D., Samanamud J., Mahajan A., et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019;25:462–469. doi: 10.1038/s41591-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito H., Nakashima H., Chiocca E.A. Molecular responses to immune checkpoint blockade in glioblastoma. Nat. Med. 2019;25:359–361. doi: 10.1038/s41591-019-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Omuro A., Vlahovic G., Lim M., Sahebjam S., Baehring J., Cloughesy T., Voloschin A., Ramkissoon S.H., Ligon K.L., Latek R., et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20:674–686. doi: 10.1093/neuonc/nox208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filley A.C., Henriquez M., Dey M. Recurrent glioma clinical trial, CheckMate-143: The game is not over yet. Oncotarget. 2017;8:91779–91794. doi: 10.18632/oncotarget.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reardon D.A., Brandes A.A., Omuro A., Mulholland P., Lim M., Wick A., Baehring J., Ahluwalia M.S., Roth P., Bähr O., et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abrishami M., Zarei-Ghanavati S., Soroush D., Rouhbakhsh M., Jaafari M.R., Malaekeh-Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29:699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- 72.Burkhardt J.-K., Riina H., Shin B.J., Christos P., Kesavabhotla K., Hofstetter C.P., Tsiouris A.J., Boockvar J.A. Intra-Arterial Delivery of Bevacizumab after Blood-Brain Barrier Disruption for the Treatment of Recurrent Glioblastoma: Progression-Free Survival and Overall Survival. World Neurosurg. 2012;77:130–134. doi: 10.1016/j.wneu.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong E.T., Gautam S., Malchow C., Lun M., Pan E., Brem S. Bevacizumab for recurrent glioblastoma multiforme: A meta-analysis. J. Natl. Compr. Cancer Netw. 2011;9:403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y.-X., Liu W.-J., Zhang H.-R., Zhang Z.-W. Delivery of bevacizumab by intracranial injection: Assessment in glioma model. OncoTargets Ther. 2018;11:2673–2683. doi: 10.2147/OTT.S159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Del Vecchio C.A., Li G., Wong A.J. Targeting EGF receptor variant III: Tumor-specific peptide vaccination for malignant gliomas. Expert Rev. Vaccines. 2012;11:133–144. doi: 10.1586/erv.11.177. [DOI] [PubMed] [Google Scholar]
- 76.Li G., Mitra S., Wong A.J. The Epidermal Growth Factor Variant III Peptide Vaccine for Treatment of Malignant Gliomas. Neurosurg. Clin. N. Am. 2010;21:87–93. doi: 10.1016/j.nec.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Elsamadicy A.A., Chongsathidkiet P., Desai R., Woroniecka K., Farber S.H., Fecci P.E., Sampson J.H. Prospect of rindopepimut in the treatment of glioblastoma. Expert Opin. Biol. Ther. 2017;17:507–513. doi: 10.1080/14712598.2017.1299705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sampson J.H., Archer G.E., Mitchell D.A., Heimberger A.B., Bigner D.D. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin. Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sampson J.H., Archer G.E., Mitchell D.A., Heimberger A.B., Herndon J.E., Lally-Goss D., McGehee-Norman S., Paolino A., Reardon D.A., Friedman A.H., et al. An epidermal growth factor receptor variant III–targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol. Cancer Ther. 2009;8:2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swartz A.M., Batich K.A., Fecci P.E., Sampson J.H. Peptide vaccines for the treatment of glioblastoma. J. Neuro Oncol. 2014;123:433–440. doi: 10.1007/s11060-014-1676-y. [DOI] [PubMed] [Google Scholar]
- 81.Sampson J.H., Heimberger A.B., Archer G.E., Aldape K.D., Friedman A.H., Friedman H.S., Gilbert M.R., Ii J.E.H., McLendon R.E., Mitchell D.A., et al. Immunologic Escape After Prolonged Progression-Free Survival with Epidermal Growth Factor Receptor Variant III Peptide Vaccination in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sampson J.H., Aldape K.D., Archer G.E., Coan A., Desjardins A., Friedman A.H., Friedman H.S., Gilbert M.R., Herndon J.E., McLendon R.E., et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2010;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai R., Recht L., Reardon D., Paleologos N., Groves M., Rosenfeld M., Davis T., Archer G., Green J., Heimberger A., et al. Long-term Follow-up of ACT III: A Phase II Trial of Rindopepimut (CDX-110) in Newly Diagnosed Glioblastoma. Neuro-Oncology. 2011;13:iii34–iii40. [Google Scholar]
- 84.Gatson N.T.N., Weathers S.-P.S., De Groot J.F. ReACT Phase II trial: A critical evaluation of the use of rindopepimut plus bevacizumab to treat EGFRvIII-positive recurrent glioblastoma (retracted) CNS Oncol. 2016;5:11–26. doi: 10.2217/cns.15.38. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Platten M. EGFRvIII vaccine in glioblastoma—InACT-IVe or not ReACTive enough? Neuro-Oncology. 2017;19:1425–1426. doi: 10.1093/neuonc/nox167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lowenstein P.R., Castro M.G. Multiple Expressed Endogenous Glioma Epitopes as Novel Vaccines for Gliomas. Clin. Cancer Res. 2016;22:4760–4762. doi: 10.1158/1078-0432.CCR-16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phuphanich S., Wheeler C.J., Rudnick J.D., Mazer M., Wang H., Nuño M.A., Richardson J.E., Fan X., Ji J., Chu R.M., et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2012;62:125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Polyzoidis S., Ashkan K. DCVax®-L—Developed by Northwest Biotherapeutics. Hum. Vaccines Immunother. 2014;10:3139–3145. doi: 10.4161/hv.29276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan Y., Zeng S., Gong Z., Xu Z. Clinical implication of cellular vaccine in glioma: Current advances and future prospects. J. Exp. Clin. Cancer Res. 2020;39:257. doi: 10.1186/s13046-020-01778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartmann J., Schüßler-Lenz M., Bondanza A., Buchholz C.J. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Srivastava S., Riddell S.R. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y.-H., Kim C.H. Evolution of chimeric antigen receptor (CAR) T cell therapy: Current status and future perspectives. Arch. Pharmacal Res. 2019;42:607–616. doi: 10.1007/s12272-019-01136-x. [DOI] [PubMed] [Google Scholar]
- 93.Petersen C.T., Krenciute G. Next Generation CAR T Cells for the Immunotherapy of High-Grade Glioma. Front. Oncol. 2019;9:69. doi: 10.3389/fonc.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chuntova P., Downey K.M., Hegde B., Almeida N.D., Okada H. Genetically Engineered T-Cells for Malignant Glioma: Overcoming the Barriers to Effective Immunotherapy. Front. Immunol. 2018;9:3062. doi: 10.3389/fimmu.2018.03062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi B.D., Curry W.T., Carter B.S., Maus M.V. Chimeric antigen receptor T-cell immunotherapy for glioblastoma: Practical insights for neurosurgeons. Neurosurg. Focus. 2018;44:E13. doi: 10.3171/2018.2.FOCUS17788. [DOI] [PubMed] [Google Scholar]
- 96.Bagley S.J., Desai A.S., Linette G.P., June C.H., O’Rourke D.M. CAR T-cell therapy for glioblastoma: Recent clinical advances and future challenges. Neuro Oncol. 2018;20:1429–1438. doi: 10.1093/neuonc/noy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chow K.K.H., Naik S., Kakarla S., Brawley V.S., Shaffer D.R., Yi Z., Rainusso N., Wu M.-F., Liu H., Kew Y., et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol. Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A., et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmed N., Brawley V., Hegde M., Bielamowicz K., Kalra M., Landi D., Robertson C., Gray T.L., Diouf O., Wakefield A., et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017;3:1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kwatra M.M. A Rational Approach to Target the Epidermal Growth Factor Receptor in Glioblastoma. Curr. Cancer Drug Targets. 2017;17:290–296. doi: 10.2174/1568009616666161227091522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Padfield E., Ellis H.P., Kurian K.M. Current Therapeutic Advances Targeting EGFR and EGFRvIII in Glioblastoma. Front. Oncol. 2015;5:5. doi: 10.3389/fonc.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren P.-P., Li M., Li T.-F., Han S.-Y. Anti-EGFRvIII Chimeric Antigen Receptor-Modified T Cells for Adoptive Cell Therapy of Glioblastoma. Curr. Pharm. Des. 2017;23:2113–2116. doi: 10.2174/1381612823666170316125402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.-C., Naranjo A., Starr R., Wagner J.R., Wright C., et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown C.E., Aguilar B., Starr R., Yang X., Chang W.-C., Weng L., Chang B., Sarkissian A., Brito A., Sanchez J.F., et al. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol. Ther. 2018;26:31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krenciute G., Prinzing B.L., Yi Z., Wu M.-F., Liu H., Dotti G., Balyasnikova I.V., Gottschalk S. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells but Results in Antigen Loss Variants. Cancer Immunol. Res. 2017;5:571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmed N., Salsman V.S., Kew Y., Shaffer D., Powell S., Zhang Y.J., Grossman R.G., Heslop H.E., Gottschalk S. HER2-Specific T Cells Target Primary Glioblastoma Stem Cells and Induce Regression of Autologous Experimental Tumors. Clin. Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heemskerk M.H. T-cell receptor gene transfer for the treatment of leukemia and other tumors. Haematologica. 2010;95:15–19. doi: 10.3324/haematol.2009.016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kessels H.W.H.G., Wolkers M.C., Boom M.D.V.D., Valk M.A.V.D., Schumacher T.N.M. Immunotherapy through TCR gene transfer. Nat. Immunol. 2001;2:957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 110.Park T.S., Rosenberg S.A., Morgan R.A. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29:550–557. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith T.W., Nishimura M.I. Targeting Cancer with Genetically Engineered TCR T Cells. Methods Mol. Biol. 2019;214:129–151. doi: 10.1007/978-3-030-23765-3_4. [DOI] [PubMed] [Google Scholar]
- 112.Sermer D., Brentjens R. CAR T-cell therapy: Full speed ahead. Hematol. Oncol. 2019;37:95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- 113.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 114.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mohanty R., Chowdhury C.R., Arega S., Sen P., Ganguly P., Ganguly N. CAR T cell therapy: A new era for cancer treatment (Review) Oncol. Rep. 2019;42:2183–2195. doi: 10.3892/or.2019.7335. [DOI] [PubMed] [Google Scholar]
- 116.Golán I., De La Fuente L.R., Costoya J.A. NK Cell-Based Glioblastoma Immunotherapy. Cancers. 2018;10:522. doi: 10.3390/cancers10120522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karantalis V., Schulman I.H., Balkan W., Hare J.M. Allogeneic cell therapy: A new paradigm in therapeutics. Circ. Res. 2015;116:12–15. doi: 10.1161/CIRCRESAHA.114.305495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geller M.A., Miller J.S. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3:1445–1459. doi: 10.2217/imt.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W.D., Tosti A., Posati S., Rogaia D., Frassoni F., Aversa F., et al. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 120.Ishikawa E., Tsuboi K., Saijo K., Harada H., Takano S., Nose T., Ohno T. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer. Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
- 121.Navarro A.G., Kmiecik J., Leiss L., Zelkowski M., Engelsen A., Bruserud Ø., Zimmer J., Enger P., Chekenya M. NK Cells with KIR2DS2 Immunogenotype Have a Functional Activation Advantage to Efficiently Kill Glioblastoma and Prolong Animal Survival. J. Immunol. 2014;193:6192–6206. doi: 10.4049/jimmunol.1400859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yvon E.S., Burga R., Powell A., Cruz C.R., Fernandes R., Barese C., Nguyen T., Abdel-Baki M.S., Bollard C.M. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19:408–418. doi: 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 123.Murakami T., Nakazawa T., Natsume A., Nishimura F., Nakamura M., Matsuda R., Omoto K., Tanaka Y., Shida Y., Park Y.-S., et al. Novel Human NK Cell Line Carrying CAR Targeting EGFRvIII Induces Antitumor Effects in Glioblastoma Cells. Anticancer. Res. 2018;38:5049–5056. doi: 10.21873/anticanres.12824. [DOI] [PubMed] [Google Scholar]
- 124.Kmiecik J., Navarro A.G., Poli A., Planagumà J.P., Zimmer J., Chekenya M. Combining NK cells and mAb9.2.27 to combat NG2-dependent and anti-inflammatory signals in glioblastoma. OncoImmunology. 2014;3:e27185. doi: 10.4161/onci.27185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu L., Oh J.M., Gangadaran P., Kalimuthu S., Baek S.H., Jeong S.Y., Lee S.-W., Lee J., Ahn B.-C. Targeting and Therapy of Glioblastoma in a Mouse Model Using Exosomes Derived from Natural Killer Cells. Front. Immunol. 2018;9:824. doi: 10.3389/fimmu.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Seino K.-I., Motohashi S., Fujisawa T., Nakayama T., Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dhodapkar K.M., Cirignano B., Chamian F., Zagzag D., Miller D.C., Finlay J.L., Steinman R.M. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int. J. Cancer. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 128.Giaccone G., Punt C.J.A., Ando Y., Ruijter R., Nishi N., Peters M., Von Blomberg B.M.E., Scheper R.J., Van Der Vliet H.J.J., Eertwegh A.J., et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 129.Wenger A., Werlenius K., Hallner A., Thorén F.B., Farahmand D., Tisell M., Smits A., Rydenhag B., Jakola A.S., Carén H. Determinants for Effective ALECSAT Immunotherapy Treatment on Autologous Patient-Derived Glioblastoma Stem Cells. Neoplasia. 2018;20:25–31. doi: 10.1016/j.neo.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harter D.H., Wilson T.A., Karajannis M.A. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014;5:64. doi: 10.4103/2152-7806.132138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giese A., Bjerkvig R., Berens M., Westphal M. Cost of Migration: Invasion of Malignant Gliomas and Implications for Treatment. J. Clin. Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 132.Bocangel D.B., Finkelstein S., Schold S.C., Bhakat K.K., Mitra S., Kokkinakis D.M. Multifaceted resistance of gliomas to temozolomide. Clin. Cancer Res. 2002;8:2725–2734. [PubMed] [Google Scholar]
- 133.Rabé M., Dumont S., Álvarez-Arenas A., Janati H., Belmonte-Beitia J., Calvo G.F., Thibault-Carpentier C., Séry Q., Chauvin C., Joalland N., et al. Identification of a transient state during the acquisition of temozolomide resistance in glioblastoma. Cell Death Dis. 2020;11:1–14. doi: 10.1038/s41419-019-2200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiapaer S., Furuta T., Tanaka S., Kitabayashi T., Nakada M. Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med. Chir. 2018;58:405–421. doi: 10.2176/nmc.ra.2018-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fang C., Wang K., Stephen Z.R., Mu Q., Kievit F.M., Chiu D.T., Press O.W., Zhang M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces. 2015;7:6674–6682. doi: 10.1021/am5092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sengupta S., Marrinan J., Frishman C., Sampath P. Impact of Temozolomide on Immune Response during Malignant Glioma Chemotherapy. Clin. Dev. Immunol. 2012;2012:1–7. doi: 10.1155/2012/831090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alvino E., Pepponi R., Pagani E., Lacal P.M., Nunziata C., Bonmassar E., D’Atri S. O(6)-benzylguanine enhances the in vitro immunotoxic activity of temozolomide on natural or antigen-dependent immunity. J. Pharmacol. Exp. Ther. 1999;291:1292–1300. [PubMed] [Google Scholar]
- 138.Heimberger A.B., Sun W., Hussain S.F., Dey M., Crutcher L., Aldape K., Gilbert M., Hassenbusch S.J., Sawaya R., Schmittling B., et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: Case study. Neuro-Oncology. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fukushima T., Takeshima H., Kataoka H. Anti-glioma therapy with temozolomide and status of the DNA-repair gene MGMT. Anticancer. Res. 2009;29:4845–4854. [PubMed] [Google Scholar]
- 140.Bell E.H., Zhang P., Fisher B.J., Macdonald D.R., McElroy J.P., Lesser G.J., Fleming J., Chakraborty A.R., Liu Z., Becker A.P., et al. Association of MGMT Promoter Methylation Status with Survival Outcomes in Patients with High-Risk Glioma Treated with Radiotherapy and Temozolomide: An Analysis from the NRG Oncology/RTOG 0424 Trial. JAMA Oncol. 2018;4:1405–1409. doi: 10.1001/jamaoncol.2018.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Friedman H.S., Prados M.D., Wen P.Y., Mikkelsen T., Schiff D., Abrey L.E., Yung W.A., Paleologos N., Nicholas M.K., Jensen R., et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 142.Arevalo O.D., Soto C., Rabiei P., Kamali A., Ballester L.Y., Esquenazi Y., Zhu J.-J., Riascos R.F. Assessment of Glioblastoma Response in the Era of Bevacizumab: Longstanding and Emergent Challenges in the Imaging Evaluation of Pseudoresponse. Front. Neurol. 2019;10:460. doi: 10.3389/fneur.2019.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Castro B.A., Aghi M.K. Bevacizumab for glioblastoma: Current indications, surgical implications, and future directions. Neurosurg. Focus. 2014;37:E9. doi: 10.3171/2014.9.FOCUS14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kothari P., White N.S., Farid N., Chung R., Kuperman J.M., Girard H.M., Shankaranarayanan A., Kesari S., McDonald C.R., Dale A.M. Longitudinal Restriction Spectrum Imaging Is Resistant to Pseudoresponse in Patients with High-Grade Gliomas Treated with Bevacizumab. Am. J. Neuroradiol. 2013;34:1752–1757. doi: 10.3174/ajnr.A3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Auer T.A., Breit H.-C., Marini F., Renovanz M., Ringel F., Sommer C.J., Brockmann M.A., Tanyildizi Y. Evaluation of the apparent diffusion coefficient in patients with recurrent glioblastoma under treatment with bevacizumab with radiographic pseudoresponse. J. Neuroradiol. 2019;46:36–43. doi: 10.1016/j.neurad.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 146.Swartz A.M., Li Q.-J., Sampson J.H. Rindopepimut: A promising immunotherapeutic for the treatment of glioblastoma multiforme. Immunotherapy. 2014;6:679–690. doi: 10.2217/imt.14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Londhe V.Y., Date V. Personalized neoantigen vaccines: A glimmer of hope for glioblastoma. Expert Rev. Vaccines. 2020;19:407–417. doi: 10.1080/14760584.2020.1750376. [DOI] [PubMed] [Google Scholar]
- 148.Neagu M.R., Reardon D.A. An Update on the Role of Immunotherapy and Vaccine Strategies for Primary Brain Tumors. Curr. Treat. Options Oncol. 2015;16:54. doi: 10.1007/s11864-015-0371-3. [DOI] [PubMed] [Google Scholar]
- 149.Ma Q., Long W., Xing C., Chu J., Luo M., Wang H.Y., Liu Q., Wang R.-F. Cancer Stem Cells and Immunosuppressive Microenvironment in Glioma. Front. Immunol. 2018;9:2924. doi: 10.3389/fimmu.2018.02924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tamura R., Tanaka T., Akasaki Y., Murayama Y., Yoshida K., Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med Oncol. 2019;37:2. doi: 10.1007/s12032-019-1329-2. [DOI] [PubMed] [Google Scholar]
- 151.Luzzi S., Lucifero A.G., Brambilla I., Trabatti C., Mosconi M., Savasta S., Foiadelli T. The impact of stem cells in neuro-oncology: Applications, evidence, limitations and challenges. Acta Biomed. 2020;91:51–60. doi: 10.23750/abm.v91i7-S.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Palumbo P., Lombardi F., Siragusa G., Dehcordi S.R., Luzzi S., Cimini A., Cifone M.G., Cinque B. Involvement of NOS2 Activity on Human Glioma Cell Growth, Clonogenic Potential, and Neurosphere Generation. Int. J. Mol. Sci. 2018;19:2801. doi: 10.3390/ijms19092801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palumbo P., Lombardi F., Augello F.R., Giusti I., Luzzi S., Dolo V., Cifone M.G., Cinque B. NOS2 inhibitor 1400W Induces Autophagic Flux and Influences Extracellular Vesicle Profile in Human Glioblastoma U87MG Cell Line. Int. J. Mol. Sci. 2019;20:3010. doi: 10.3390/ijms20123010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Antonosante A., Brandolini L., D’Angelo M., Benedetti E., Castelli V., Del Maestro M., Luzzi S., Giordano A., Cimini A., Allegretti M. Autocrine CXCL8-dependent invasiveness triggers modulation of actin cytoskeletal network and cell dynamics. Aging. 2020;12:1928–1951. doi: 10.18632/aging.102733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kwok D., Okada H. T-Cell based therapies for overcoming neuroanatomical and immunosuppressive challenges within the glioma microenvironment. J. Neuro Oncol. 2020;147:281–295. doi: 10.1007/s11060-020-03450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.D’Amico R.S., Khatri D., Reichman N., Patel N.V., Wong T., Fralin S.R., Li M., Ellis J.A., Ortiz R., Langer D.J., et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood–brain barrier disruption: Where are we now, and where we are going. J. Neuro Oncol. 2020;147:261–278. doi: 10.1007/s11060-020-03435-6. [DOI] [PubMed] [Google Scholar]
- 157.D’Amico R.S., Neira J.A., Yun J., Alexiades N.G., Banu M., Englander Z.K., Kennedy B.C., Ung T.H., Rothrock R.J., Romanov A., et al. Validation of an effective implantable pump-infusion system for chronic convection-enhanced delivery of intracerebral topotecan in a large animal model. J. Neurosurg. 2020;133:614–623. doi: 10.3171/2019.3.JNS1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elleaume H., Barth R.F., Rousseau J., Bobyk L., Balosso J., Yang W., Huo T., Nakkula R. Radiation therapy combined with intracerebral convection-enhanced delivery of cisplatin or carboplatin for treatment of the F98 rat glioma. J. Neuro-Oncol. 2020;149:193–208. doi: 10.1007/s11060-020-03600-x. [DOI] [PubMed] [Google Scholar]
- 159.Sonabend A.M., Stuart R.M., Yun J., Yanagihara T., Mohajed H., Dashnaw S., Bruce S.S., Brown T., Romanov A., Sebastian M., et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro-Oncology. 2011;13:886–893. doi: 10.1093/neuonc/nor051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chakraborty S., Filippi C.G., Wong T., Ray A., Fralin S., Tsiouris A.J., Praminick B., Demopoulos A., McCrea H.J., Bodhinayake I., et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neuro-Oncol. 2016;128:405–415. doi: 10.1007/s11060-016-2099-8. [DOI] [PubMed] [Google Scholar]
- 161.Riina H., Knopman J., Greenfield J., Fralin S., Gobin Y.P., Tsiouris A., Souweidane M., Boockvar J. Balloon-Assisted Superselective Intra-Arterial Cerebral Infusion of Bevacizumab for Malignant Brainstem Glioma: A Technical Note. Interv. Neuroradiol. 2010;16:71–76. doi: 10.1177/159101991001600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Etame A.B., Diaz R.J., Smith C.A., Mainprize T.G., Hynynen K., Rutka J.T. Focused ultrasound disruption of the blood-brain barrier: A new frontier for therapeutic delivery in molecular neurooncology. Neurosurg. Focus. 2012;32:E3. doi: 10.3171/2011.10.FOCUS11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carpentier A., Canney M., Vignot A., Reina V., Beccaria K., Horodyckid C., Karachi C., Leclercq D., Lafon C., Chapelon J.-Y., et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016;8:343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 164.Patel N.V., Khatri D., D’Amico R., Abrams M., Reichman N., Filippi C.G., Anderson T., Ratzon F., Wong T., Fralin S., et al. Vascularized Temporoparietal Fascial Flap: A Novel Surgical Technique to Bypass the Blood-Brain Barrier in Glioblastoma. World Neurosurg. 2020;143:38–45. doi: 10.1016/j.wneu.2020.07.132. [DOI] [PubMed] [Google Scholar]
- 165.Jiang W., Kim B.Y., Rutka J.T., Chan W.C. Advances and challenges of nanotechnology-based drug delivery systems. Expert Opin. Drug Deliv. 2007;4:621–633. doi: 10.1517/17425247.4.6.621. [DOI] [PubMed] [Google Scholar]
- 166.Yoshida J., Mizuno M. Clinical gene therapy for brain tumors. Liposomal delivery of anticancer molecule to glioma. J. Neuro-Oncol. 2003;65:261–267. doi: 10.1023/B:NEON.0000003655.03671.fa. [DOI] [PubMed] [Google Scholar]
- 167.Lachmann R.H. Herpes simplex virus-based vectors. Int. J. Exp. Pathol. 2004;85:177–190. doi: 10.1111/j.0959-9673.2004.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jabir N.R., Tabrez S., Ashraf G.M., Shakil S., Damanhouri G.A., Kamal M.A. Nanotechnology-based approaches in anticancer research. Int. J. Nanomed. 2012;7:4391. doi: 10.2147/IJN.S33838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the main text.