Abstract
Multidrug resistant (MDR) bacteria are increasingly observed in nosocomial and community-acquired settings. Anaerobes are no exception to this rule, but there are fewer reports of MDR in the scientific literature on anaerobes than there are for other bacteria. In this short case report, we describe the first case of bacteraemia caused by a multidrug-resistant Bacteroides faecis, which produces a carbapenemase encoded by the blaCfiA gene. This bacteraemia followed a digestive surgery operation. Surprisingly, these findings did not lead to a change in antibiotic therapy, probably because the patient’s clinical state had improved. Nevertheless this report calls for better knowledge of anaerobic bacteria and for a systematic antimicrobial stewardship procedure following bacteraemia.
Keywords: bacteraemia, anaerobic bacteria, Bacteroides fragilis group, carbapenemase, antimicrobial resistance, blaCfiA
1. Introduction
The World Health Organization recognizes antibiotic resistance as “one of the biggest threats to global health” [1]. Concerns have focused on aerobic and aero-anaerobic bacteria (especially on Staphylococcus aureus, Enterobacterales, Enterococcus faecium, Pseudomonas aeruginosa and Acinetobacter baumannii). Nevertheless, increasing antibiotic resistance is also readily observed in anaerobes, especially in bacteria of the Bacteroides fragilis group [2]. Indeed, resistance to several antibiotics is observed, including carbapenems. This latter resistance can be mediated by a metallo-β-lactamase, encoded by the blaCfiA gene [3].
Anaerobic bacteria are an important cause of bloodstream infections. Bacteria of the B. fragilis group are more frequently isolated than other anaerobes (for example, Gram-negative bacilli, Fusobacterium spp. Peptostreptococcus, Clostridium spp.), but remain poorly known to clinicians [4]. As a result, this group may not be taken into account when choosing an antibiotic for treatment, resulting in a high degree of mortality, especially in bloodstream infections [5]. In this clinical case report, we describe the first case of bacteraemia caused by a carbapenemase producing Bacteroides faecis.
2. Case Report
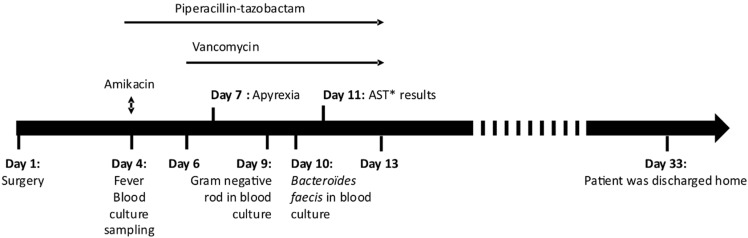
A 57-year-old man was admitted to hospital for an abdominoperineal resection and placement of an end colostomy. He had a history of perineal hidradenitis suppurativa and had been successively treated by multiple antibiotics (notably, ertapenem and doxycycline) and TNF-alpha inhibitors for years. Multiple fistulas and sphincter destruction later complicated his initial pathology, requiring several surgical treatments, including abdominoperineal amputation, followed by a perineal reconstruction using surgical flaps that was conducted on day 1 (Figure 1).
Figure 1.
Chronology of the clinical case. (* AST: Antimicrobial Susceptibility Testing).
Post-operative history was marked by repeated vomiting, some of which was inhaled. An abscess on the laparotomy scar was also noted. Four days after this surgery, he was febrile which led to the sampling of two blood culture pairs. A blood sample for routine lab testing including blood count, C-reactive protein and liver and kidney function tests, was also taken with blood cultures. Blood analysis revealed an elevated C-reactive protein level at 215 mg/L (normal < 5mg/L) and white blood cell count at 12,980/mm3. He then received empirical antibiotic therapy based on piperacillin-tazobactam (4 g three times a day) combined with a dose of amikacin (1.4 g). Local care was provided, the scar abscess was flattened, and sessions of hyperbaric oxygen therapy were prescribed. Two days later, vancomycin (2 g per day) was added since he still had a fever. The fever sharply declined following vancomycin introduction.
While he was still apyretic, on day 9 after the surgery, one of the blood-culture bottles sampled during his febrile episode was detected as positive. Direct examination revealed a Gram-negative rod. The next day, B. faecis was identified using MALDI-TOF MS (Bruker Daltonic, Bremen, Germany). Antimicrobial susceptibility testing showed a resistance to beta-lactams, including carbapenems and clindamycin. This strain remained susceptible to metronidazole.
Since his clinical condition had improved, the antibiotic regimen was stopped after 10 days of piperacillin-tazobactam administration. He left hospital 4 weeks later. Figure 1 shows the chronology of the clinical case.
The B. faecis strain was sent to the French National Reference Centre for Anaerobic Bacteria and Botulism at the Pasteur Institute. Identification of B. faecis was confirmed by sequencing the 16s rDNA and of the hsp60 genes. The blacfiA gene, encoding a metallo-β-lactamase which conferred the resistance to carbapenem, was detected. The additional antibiogram showed that the strain was susceptible to rifampicin, trimethoprim-sulfamethoxazole and moxifloxacin. Table 1 shows the minimum inhibitory concentrations (MIC) of different antimicrobial drugs, obtained after strain analysis.
Table 1.
Minimum inhibitory concentrations (MIC) and clinical categorization obtained for different antibiotics (Technic: Sensititre™ Anaerobe MIC Plate). EUCAST: European Committee on Antimicrobial Susceptibility Testing-CASFM: Comité de l’antibiogramme de la Société Française de Microbiologie.
| Antibiotic | EUCAST Breakpoints |
CASFM Breakpoints |
MIC (mg/L) | Interpretation |
|---|---|---|---|---|
| Penicillin | 0.25–0.5 | - | >8 | Resistant |
| Amoxicillin | 0.5–2 | - | >32 | Resistant |
| Amoxicillin/clavulanic acid | 4–8 | 4–8 | >32 | Resistant |
| Piperacillin/tazobactam | 8–16 | 8–16 | >128 | Resistant |
| Piperacillin | 16 | - | >128 | Resistant |
| Cefoxitin | - | - | >64 | Resistant |
| Imipenem | 2–4 | 2–4 | >128 | Resistant |
| Meropenem | 2–8 | 2–8 | >8 | Resistant |
| Chloramphenicol | 8 | 8 | 8 | Susceptible-standard dosing regimen |
| Erythromycin | - | - | >128 | Resistant |
| Clindamycin | 4 | 4 | >64 | Resistant |
| Metronidazole | 4 | 4 | 2 | Susceptible-standard dosing regimen |
| Moxifloxacin | - | 1–2 | 2 | Susceptible-increased exposure |
| Tetracycline | - | - | >16 | Resistant |
| Vancomycin | - | - | 4 | Resistant |
One year later, the patient was still alive, and his medical condition had improved.
3. Discussion
Here, we report the first case of bacteraemia caused by carbapenem-resistant B. faecis. This resistance was mediated by a metallo-β-lactamase, encoded by the blaCfiA gene.
Anaerobes are part of different normal flora, especially prevalent in the respiratory tract, in the vaginal cavity, on the skin, and foremost, in the digestive tract. In the latter, the Bacteroidetes phylum represents approximately 23% of the gut microbiota and includes several species of the Bacteroides genus, in particular, B. fragilis and B. thetaiotaomicron [6]. B. faecis belongs to the B. fragilis group and was isolated for the first time in 2010 by Kim et al. from human faeces [6]. Although anaerobes are commensal bacteria, they can cause severe infections, including bacteraemia [4]. They are isolated from 0.5 to 12% of positive blood cultures and members of the B. fragilis group are the most prevalent anaerobic bacteria involved in bacteraemia, with the primary disease usually being an intra-abdominal infection [4]. Up to now, three cases of B. faecis infections have been described (Table 2), all were due to intra-abdominal infections and strains were susceptible to piperacillin-tazobactam and carbapenems. However, B. faecis infections may be underdiagnosed due to identification difficulties and their possible misidentification as other Bacteroides species, such as B. thetaiotaomicron [6].
Table 2.
Cases of human infections due to Bacteroides faecis reported in the literature.
| Reference | Type of Infection | Risk Factors | Resistance | Treatment | Outcome |
|---|---|---|---|---|---|
| Lee et al. 2015 [7] | Post-operative peritonitis | Sigmoid colon cancer | Piperacillin (SIE), cefoxitin, cefotetan | Piperacillin-tazobactam | Favorable |
| Lee et al. 2015 [7] | Bacteremia secondary to post-operative peritonitis | Rectal cancer | Piperacillin (SIE), cefoxitin, cefotetan | Piperacillin-tazobactam | Favorable |
| Garcia et al. 2016 [8] | Bacteremia secondary to colonic ischemia | Epicardic electrodes | Amoxicillin, piperacillin, clindamycin | Metronidazole | Death |
Carbapenem resistance is increasingly observed in bacteria of the B. fragilis group, notably strains that produce metallo-β-lactamases, encoded by the cfiA gene [2,9]. The expression of the blaCfiA gene is linked to the presence of an insertion sequence which provides its promoter [10]. For this strain, it is clear that the blaCfiA gene was expressed, since this B. faecis strain was resistant to imipenem and all beta-lactams, even those associated with beta-lactamase inhibitors. A study by Justesen et al. demonstrated that 10.2% of the B. fragilis group strains isolated in blood cultures from several Danish hospitals carried the blaCfiA gene [11]. In contrast, a Hungarian study did not find strains carrying the blaCfiA gene in blood culture isolates [2]. Other mechanisms causing carbapenem resistance have been described, such as penicillin-binding proteins modification [12] and over-expression of bmeABC pumps [13].
On the one hand, antibiotic resistance reduces the rate of appropriate empirical antibiotic therapy administration. On the other hand, without effective treatment, bacteraemia caused by anaerobes of the B. fragilis group has a high mortality rate. This rate varies according to the species isolated (24 to 31% for B. fragilis, 50% for B. distasonis and almost 100% for B. thetaiotaomicron) [4,6]. Moreover, an increase in mortality has been well documented in the literature in the absence of appropriate antibiotic therapy for Bacteroides spp. bacteraemia [5]. More surprisingly antimicrobial susceptibility testing results are not always taken into account in the antibiotherapy choice. As a consequence, an increase in mortality (from 18% when the antibiotic is adapted, to 55% when the antimicrobial is not adapted) was evidenced by Salonen et al. [5]. In this present case, the identification of a multi-drug resistant B. faecis responsible for the bloodstream infection did not result in a therapeutic change. This was probably because the patient’s clinical condition had improved after the empirical antibiotic administration. Despite the strain’s resistance to the administrated antibiotic treatments, the patient’s condition improved. We can hypothesize that this bacteraemia results from a digestive translocation with a very low inoculum, which is corroborated by the time of positivity of the blood culture which took a long time (5 days). In addition, the source of infection could have been controlled thanks to hyperbaric oxygen therapy sessions and to the surgical treatment of scar abscess.
Nevertheless, this clinical case illustrates the lack of medical knowledge on anaerobes, which calls for strong interactions between physicians, infectious disease specialists and clinical microbiologists in the form of an antimicrobial stewardship programs. Such strategies have demonstrated a decrease in mortality and a better management for S. aureus bacteraemia [14].
4. Conclusions
To our knowledge this is the first report of a bacteraemia caused by a carbapem-resistant, blaCfiA producer B. faecis. This bacterium is rarely reported in humans, and may cause severe diseases. In this clinical case, digestive translocation probably caused the bacteraemia, which explains why the infectious situation was controlled without the use of efficient antibiotics.
Anaerobes are poorly known by physicians who not involved in infectious diseases, thus, increasing knowledge of these clinical cases is crucial to improve the treatment of patients.
Author Contributions
C.K., T.R.; writing—original draft preparation and strain analysis, L.D.; strain analysis, B.R.; patient management, Y.R.; writing—review and editing, B.J.; writing—review and editing, P.H.B.; conceptualization and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received for this case report.
Institutional Review Board Statement
For case reports ethical review and approval are not required.
Informed Consent Statement
Informed consent was obtained from the patient involved in the case report.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abadi A.T.B., Rizvanov A.A., Haertlé T., Blatt N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience. 2019;9:778–788. doi: 10.1007/s12668-019-00658-4. [DOI] [Google Scholar]
- 2.Nagy E., Urbán E., Nord C.E. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin. Microbiol. Infect. 2011;17:371–379. doi: 10.1111/j.1469-0691.2010.03256.x. [DOI] [PubMed] [Google Scholar]
- 3.Ogane K., Tarumoto N., Kodana M., Onodera A., Imai K., Sakai J., Kawamura T., Takeuchi S., Murakami T., Mitsutake K., et al. Antimicrobial susceptibility and prevalence of resistance genes in Bacteroides fragilis isolated from blood culture bottles in two tertiary care hospitals in Japan. Anaerobe. 2020;64:102215. doi: 10.1016/j.anaerobe.2020.102215. [DOI] [PubMed] [Google Scholar]
- 4.Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16:183–189. doi: 10.1016/j.anaerobe.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Salonen J.H., Eerola E., Meurman O. Clinical Significance and Outcome of Anaerobic Bacteremia. Clin. Infect. Dis. 1998;26:1413–1417. doi: 10.1086/516355. [DOI] [PubMed] [Google Scholar]
- 6.Kim M.-S., Roh S.W., Bae J.-W. Bacteroides faecis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2010;60:2572–2576. doi: 10.1099/ijs.0.020024-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y., Kim H.S., Yong D., Jeong S.H., Lee K., Chong Y. Bacteroides faecisandBacteroides intestinalisRecovered from Clinical Specimens of Human Intestinal Origin. Yonsei Med. J. 2015;56:292–294. doi: 10.3349/ymj.2015.56.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia M., Bouvet P., Petitpas F., Jayle C., Legeay C., Sautereau J., Michaud A., Burucoa C., Plouzeau C. First case report of a human sepsis involving a recently identified anaerobic agent: Bacteroides faecis. Anaerobe. 2016;42:74–77. doi: 10.1016/j.anaerobe.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Falagas M.E., Siakavellas E. Bacteroides, Prevotella, and Porphyromonas species: A review of antibiotic resistance and therapeutic options. Int. J. Antimicrob. Agents. 2000;15:1–9. doi: 10.1016/S0924-8579(99)00164-8. [DOI] [PubMed] [Google Scholar]
- 10.Edwards R. Expression of the carbapenemase gene (cfiA) in Bacteroides fragilis. J. Antimicrob. Chemother. 2000;46:1009–1012. doi: 10.1093/jac/46.6.1009. [DOI] [PubMed] [Google Scholar]
- 11.Justesen U.S., Hansen F., Østergaard C., Schønheyder H.C., Hansen D.S., Lemming L.E., Schumacher H., Heltberg O., Knudsen J.D., Dzajic E., et al. High rates of reduced susceptibility in the Bacteroides fragilis group isolated from blood cultures—The first national survey in Denmark. Int. J. Antimicrob. Agents. 2013;42:188–190. doi: 10.1016/j.ijantimicag.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Ayala J., Quesada A., Vadillo S., Criado J., Píriz S. Penicillin-binding proteins of Bacteroides fragilis and their role in the resistance to imipenem of clinical isolates. J. Med. Microbiol. 2005;54:1055–1064. doi: 10.1099/jmm.0.45930-0. [DOI] [PubMed] [Google Scholar]
- 13.Pumbwe L., Ueda O., Yoshimura F., Chang A., Smith R.L., Wexler H.M. Bacteroides fragilis BmeABC efflux systems additively confer intrinsic antimicrobial resistance. J. Antimicrob. Chemother. 2006;58:37–46. doi: 10.1093/jac/dkl202. [DOI] [PubMed] [Google Scholar]
- 14.Vogel M., Schmitz R.P., Hagel S., Pletz M.W., Gagelmann N., Scherag A., Schlattmann P., Brunkhorst F.M. Infectious disease consultation for Staphylococcus aureus bacteremia—A systematic review and meta-analysis. J. Infect. 2016;72:19–28. doi: 10.1016/j.jinf.2015.09.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.