Abstract
Simple Summary
Associations between thyroid cancer and breast cancer have been elucidated, in that patients with breast cancer have a greater risk of developing subsequent thyroid cancer. However, not much is known about the relationship other primary cancers and subsequent thyroid cancer. In this review, we completed a thorough review of the existing literature to understand the relationship between primary cancers and second primary thyroid cancer (SPTC). Our findings suggest that surveillance protocols should be considered for patients at a higher risk of SPTC, including those with primary breast, renal cell, basal cell, and ovarian cancers who are female and/or Caucasian.
Abstract
Background: It is critical to understand factors that may contribute to an increased risk of SPTC in order to develop surveillance protocols in high-risk individuals. This systematic review and meta-analysis will assess the association between primary malignancy and SPTC. Methods: A search of PubMed and Embase databases was completed in April 2020. Inclusion criteria included studies that reported the incidence or standardized incidence ratio of any primary malignancy and SPTC, published between 1980–2020. The PRISMA guidelines were followed and the Newcastle–Ottawa Scale was used to assess quality of studies. Results: 40 studies were included, which were comprised of 1,613,945 patients and 15 distinct types of primary cancers. In addition, 4196 (0.26%) patients developed SPTC following a mean duration of 8.07 ± 4.39 years. Greater risk of developing SPTC was found following primary breast (56.6%, 95%CI, 44.3–68.9, p < 0.001), renal cell (12.2%, 95%CI, 7.68–16.8, p < 0.001), basal cell (7.79%, 95%CI, 1.79–13.7, p = 0.011), and ovarian cancer (11.4%, 95%CI, 3.4–19.5, p = 0.005). SPTC patients were more likely to be females (RR = 1.58, 95%CI, 1.2–2.01, p < 0.001) and Caucasians (p < 0.001). Conclusions: Surveillance protocols should be considered for patients at a higher risk of SPTC, including those with primary breast, renal cell, basal cell and ovarian cancers who are female and/or Caucasian.
Keywords: thyroid cancer, SPTC, breast cancer, systematic review
1. Introduction
There is a rising incidence of thyroid cancer in the United States [1]. This incidence has tripled in the last few decades and continues to rise at a rate of 4% per year [2]. Elevated rates of secondary primary thyroid cancer (SPTC) have been reported across a spectrum of primary cancer survivors [3,4]. By understanding the factors that may contribute to an increased SPTC risk, the underlying etiology of SPTC can be elucidated. Furthermore, the efficacy of surveillance protocols for high-risk individuals on the outcome of disease may be considered.
One emerging association with SPTC is breast cancer (BC) [5,6]. An et al. completed a retrospective case-control study demonstrating that the overall risk of SPTC or BC is increased in patients with prior BC or thyroid cancer, respectively [5]. Although the cause of this relationship remains unclear, potential factors for this association include chemotherapy and radiotherapy of the primary tumor, detection bias, genetic susceptibility, hormonal signaling, and environmental factors [6]. Connections between other primary malignancies such as stomach, liver, and colorectal cancers and SPTC have also been reported [7,8]. However, to date, there is no comprehensive review on the primary cancers associated with SPTC.
The primary aim of this meta-analysis is to evaluate the risk of developing SPTC following various primary malignancies. The secondary aim is to determine whether certain factors such as age, gender, race, and history of radiation and chemotherapy increase the risk of developing SPTC.
2. Materials and Methods
2.1. Literature Search Strategy
A comprehensive search was completed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Titles and abstracts between 1980–2020, in the English language were searched across PubMed and Embase in April 2020. The medical subjects’ headings (MeSH) terms were used: (“primary malignancy” OR “cancer” OR “primary cancer”) AND (“secondary thyroid cancer” OR “thyroid cancer” OR “second primary thyroid cancer”). Articles including papillary, medullary, and follicular thyroid cancers were included. References of retrieved articles were also assessed for relevant studies.
2.2. Study Selection
Inclusion criteria included (1) any prospective or retrospective cohort study investigating the association of any primary malignancy and development of SPTC, (2) studies that reported the incidence or standardized incidence ratios (SIR) of cancers, and (3) studies published in a peer-reviewed journal. The major exclusion criteria were (1) abstract-only articles, conference articles, editorials, expert opinions, and (2) studies that did not stratify characteristics of various primary tumors.
2.3. Data Abstraction and Synthesis
Two (LNT and ARC) independent reviewers screened all citations for relevance and reviewed full-text articles. Any disagreement was resolved by discussion with another researcher (EAT). Data extraction was performed including relevant demographics, study design, publication year, and outcomes. The quality of each study was appraised by two independent reviewers (LNT and ARC) using the Newcastle–Ottawa Scale (NOS) [9].
2.4. Statistical Analysis
STATA Software version 16.0 (Stata Corporation, College Station, TX, USA) was used to perform a random-effects meta-analysis examining the SPTC risk based on incidence or SIR with the 95% confidence interval (CI) published in each study. Reported log risk ratio was converted to risk ratio. Hedges’ g was used to measure effect size. Potential heterogeneity across studies was examined using the Cochran’s Q-statistic and I2 statistic. Heterogeneity p < 0.05 or I2 > 50% indicates significant heterogeneity across studies. Meta-regression and subgroup analysis were employed to identify the source of heterogeneity.
3. Results
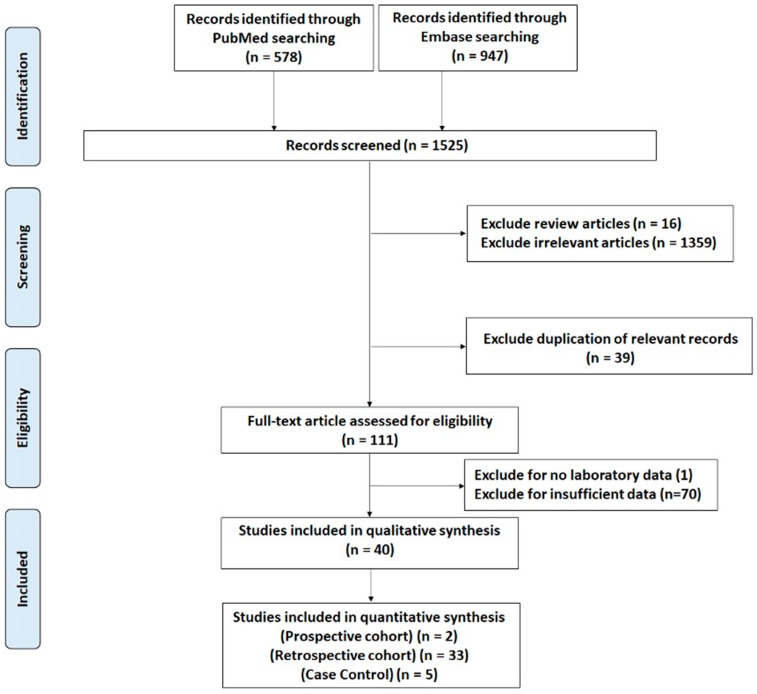
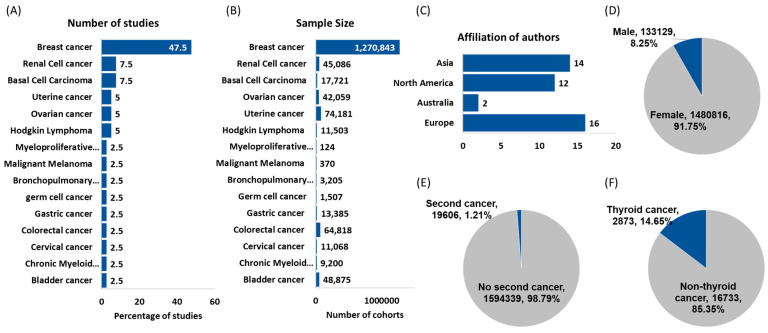
The search strategy identified 1525 citations yielding 1486 unique citations after exclusion of duplicates. Following exclusion of irrelevant articles, 111 full-text articles were reviewed. Workflow for study selection is demonstrated in Figure 1. Characteristics of studies are patients are depicted in Figure 2.
Figure 1.
Systematic review process according to PRISMA guidelines.
Figure 2.
Features of studies and demographics of cancer patients. (A) distribution of studies according to the site of primary tumors; (B) sample size for each type of primary cancer; (C) geographical distribution of studies. Three studies were affiliated by authors from multiple countries; (D) distribution of cancer cohorts according to their sex; (E) frequency of a second primary cancer following another malignancy; (F) frequency of second primary thyroid cancer.
3.1. Characteristics of the Included Studies
A total of 40 articles (33 retrospective cohorts, 5 retrospective case-controls and 2 prospective cohorts) including 1,613,945 cancer patients with 15 distinct primary cancers were included in the analysis [5,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. The study characteristics are shown in Table 1.
Table 1.
Characteristics of the study population.
| Source | Country | Study Type | Study Period | Sample Size | Female | Male | Primary Cancer | Mean Age at Primary Diagnosis, Years | Follow-Up Duration, Years | SPTC ( + ) | SPTC (−) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schlosser S [10] (2020) | Israel | R | 1991–2012 | 266 | 266 | 0 | Breast | 45.9 | 17 | 1 | 265 |
| Ciftciler R [21] (2019) | Turkey | R | 2001–2015 | 124 | 49 | 75 | MPN | 53.0 | 12 | 1 | 123 |
| Li S [32] (2019) | USA | R | 1992–2013 | 333,266 | 333,266 | 0 | Breast | 54.0 | N/A | 842 | 332,424 |
| Bryk S [42] (2018) | Finland | R | 1968–2013 | 986 | 986 | 0 | Ovarian | 40.0 | N/A | 6 | 980 |
| Kwon W [43] (2018) | Korea | R | 1993–2013 | 48,875 | 9524 | 39,351 | Bladder | 65.3 | 3.1 | 107 | 48,768 |
| Corso G [44] (2018) | Italy | R | 1994–2010 | 21,527 | 21,527 | 0 | Breast | N/A | N/A | 78 | 21,449 |
| Joung J [45] (2018) | Korea | R | 1993–2013 | 40,347 | 12,483 | 27,864 | RCC | 57.0 | 3.08 | 311 | 40,036 |
| Kumar V [46] (2018) | USA | R | 2002–2014 | 9200 | 3772 | 5428 | CML | N/A | 4.2 | 16 | 9184 |
| Chen T [47] (2017) | Germany/Sweden | R | 1997–2012 | 65,575 | 65,575 | 0 | Uterine | 68.5 | 3.9 | 42 | 65,533 |
| Sud A [11] (2017) | Sweden | R | 1965–2012 | 9522 | 4034 | 5488 | HL | 49.0 | 12.6 | 20 | 9502 |
| Kanninen T [12] (2017) | USA | R | 1992–2012 | 41,073 | 41,073 | 0 | Ovarian | 59.9 | N/A | 71 | 41,002 |
| Lococo F [13] (2017) | Italy | R | 1975–2011 | 3205 | 1069 | 2136 | Lung | 61.7 | N/A | 16 | 3189 |
| Liao Z [14] (2017) | USA | R | 1980–2011 | 1507 | 1507 | 0 | Germ Cell | 33.0 | 14.3 | 6 | 1501 |
| Liu Y [15] (2017) | China | R | 2008–2015 | 28 | 28 | 0 | Breast | 44.5 | 10.8 | 5 | 23 |
| Murray K [16] (2016) | USA | P | 1989–2010 | 3066 | 1972 | 1094 | RCC | 60.9 | 2.7 | 12 | 3054 |
| Krilaviciute A [17] (2016) | Lithuania | R | 1998–2007 | 12,584 | 8074 | 4510 | BCC | N/A | 14 | 30 | 12,554 |
| Silverman B [18] (2016) | Israel | R | 1990–2006 | 46,090 | 46,090 | 0 | Breast | N/A | 8.3 | 155 | 45,935 |
| Marcheselli, R [19] (2015) | Italy | R | 1996–2007 | 1830 | 1830 | 0 | Breast | 64.0 | 6.3 | 11 | 1819 |
| An J [5] (2015) | Korea | R | 1970–2009 | 6833 | 6833 | 0 | Breast | 43.4 | 4.4 | 81 | 6752 |
| Michaelson E [20] (2014) | USA | R | 1969–2008 | 1981 | 912 | 1069 | HL | 27.0 | 20.3 | 28 | 1953 |
| Koivisto-Korander R [22] (2012) | Europe | R | 1943–2000 | 8606 | 8606 | 0 | Uterine | N/A | 6.6 | 12 | 8594 |
| Antonelli A [23] (2012) | Italy | R | 1983–2009 | 1673 | 622 | 1051 | RCC | 61.6 | 5.9 | 15 | 1658 |
| Tabuchi T [7] (2012) | Japan | R | 1985–2004 | 13,385 | 4194 | 9191 | Gastric | N/A | 3.9 | 31 | 13,354 |
| Yadav B [24] (2009) | India | R | 1985–1995 | 1084 | 1084 | 0 | Breast | N/A | 12 | 1 | 1083 |
| Lee K [25] (2008) | China | R | 1979–2003 | 53,783 | 53,783 | 0 | Breast | 50.3 | 5.41 | 45 | 53,738 |
| Kirova Y [26] (2008) | France | R | 1981–1997 | 16,705 | 16,705 | 0 | Breast | 56.2 | 10.5 | 20 | 16,685 |
| Mellemkjaer L [27] (2006) | Europe, Australia, Canada, Singapore | R | 1943–2000 | 525,527 | 525,527 | 0 | Breast | N/A | 7.2 | 552 | 524,975 |
| Sadetzki S [28] (2003) | Israel | R | 1960–1998 | 49,207 | 49,207 | 0 | Breast | N/A | 7.1 | 72 | 49,135 |
| Adjadj É [29] (2003) | France | R | 1954–1983 | 200 | 200 | 0 | Breast | 48.0 | 9 | 8 | 192 |
| Tanaka H [30] (2001) | Japan | R | 1970–1995 | 2786 | 2786 | 0 | Breast | 50.9 | 8.6 | 7 | 2779 |
| Huang J [31] (2001) | USA | R | 1973–1993 | 194,798 | 194,798 | 0 | Breast | N/A | N/A | 140 | 194,658 |
| Li C [33] (2000) | USA | R | 1974–1994 | 2189 | 2189 | 0 | Breast | 59.8 | 4.2 | 20 | 2169 |
| Friedman, G [34] (2000) | USA | R | 1974–1997 | 3164 | 1516 | 1648 | BCC | N/A | 11.3 | 4 | 3160 |
| McCredie M [35] (1997) | Australia | R | 1972–1991 | 64,818 | 31,661 | 33,157 | Colorectal | 66.6 | 3.8 | 16 | 64,802 |
| Volk N [36] (1997) | Slovenia | R | 1961–1994 | 8791 | 8791 | 0 | Breast | 57.0 | 7.3 | 10 | 8781 |
| Lindelöf B [37] (1991) | Sweden | R | 1973–1983 | 1973 | 1039 | 934 | BCC | 68.0 | 6.5 | 5 | 1968 |
| Gutman M [38] (1991) | Israel | R | 1974–1986 | 370 | 237 | 133 | MM | 49.0 | 3.4 | 2 | 368 |
| Boice J [39] (1988) | USA, Denmark, Sweden, UK | R | N/A | 11,068 | 11,068 | 0 | Cervical | 52.0 | N/A | 43 | 11,025 |
| Murakami R [40] (1987) | Japan | P | 1965–1982 | 2786 | 2786 | 0 | Breast | 50.9 | 8.6 | 7 | 2779 |
| Ron E [41] (1984) | USA | R | 1935–1978 | 3147 | 3147 | 0 | Breast | 58.4 | N/A | 24 | 3123 |
R: Retrospective study, P: Prospective, SPTC: Second primary thyroid cancer, N/A: not applicable; MPN: Myeloproliferative neoplasms; CML: Chronic myelogenous leukemia; Lung: includes bronchopulmonary carcinoid HL: Hodgkin’s lymphoma; BCC: Basal cell carcinoma; MM: Malignant melanoma; RCC: Renal cell cancer.
Breast cancer (BC) was the most frequent primary cancer (19 studies, 47.5%), followed by renal cell cancer (RCC) (3 studies, 7.5%) and basal cell carcinoma (BCC) (3 studies, 7.5%).
In total, 1,270,843 (78.74%) of the study population had breast cancer as the primary tumor site. Included studies were published in many countries (United States, India, China, Japan, Israel, etc.) in the last four decades (1984–2020). In addition, 1,480,816 (91.75%) women and 133,129 (8.25%) men were included in our study analysis. Quality assessment scores by the Newcastle–Ottawa Scale (NOS) are depicted in Table 2. Most of the included studies were comprised of low risk of bias, although some confounding was possible for studies that did not include any multivariable or otherwise adjusted analyses.
Table 2.
Quality index scoring based on Newcastle–Ottawa Scale (NOS).
| Source | Selection (4 Max) | Comparability (1 Max) | Outcome (3 Max) | Overall Rating (8 Max) |
|---|---|---|---|---|
| Schlosser S [10] (2020) | 2 | 1 | 2 | 5 |
| Ciftciler R [21] (2019) | 3 | 1 | 3 | 7 |
| Li S [32] (2019) | 3 | 1 | 2 | 6 |
| Bryk S [42] (2018) | 3 | 1 | 2 | 6 |
| Kwon W [43] (2018) | 3 | 1 | 3 | 7 |
| Corso G [44] (2018) | 3 | 1 | 2 | 7 |
| Joung J [45] (2018) | 3 | 1 | 3 | 7 |
| Kumar V [46] (2018) | 3 | 1 | 2 | 6 |
| Chen T [47] (2017) | 3 | 1 | 3 | 7 |
| Sud A [11] (2017) | 3 | 1 | 2 | 6 |
| Kanninen T [12] (2017) | 3 | 1 | 3 | 7 |
| Lococo F [13] (2017) | 3 | 1 | 3 | 7 |
| Liao Z [14] (2017) | 3 | 1 | 3 | 7 |
| Liu Y [15] (2017) | 3 | 1 | 2 | 6 |
| Murray K [16] (2016) | 3 | 1 | 3 | 7 |
| Krilaviciute A [17] (2016) | 3 | 1 | 3 | 7 |
| Silverman B [18] (2016) | 2 | 1 | 3 | 6 |
| Marcheselli, R [19] (2015) | 4 | 1 | 3 | 8 |
| An J [5] (2015) | 2 | 1 | 3 | 6 |
| Michaelson E [20] (2014) | 3 | 1 | 3 | 7 |
| Koivisto-Korander R [22] (2012) | 4 | 1 | 3 | 8 |
| Antonelli A [23] (2012) | 3 | 1 | 3 | 7 |
| Tabuchi T [7] (2012) | 3 | 1 | 2 | 6 |
| Yadav B [24] (2009) | 3 | 1 | 3 | 7 |
| Lee K [25] (2008) | 3 | 1 | 3 | 7 |
| Kirova Y [26] (2008) | 3 | 1 | 3 | 7 |
| Mellemkjaer L [27] (2006) | 3 | 1 | 3 | 7 |
| Sadetzki S [28] (2003) | 2 | 1 | 3 | 6 |
| Adjadj É [29] (2003) | 3 | 1 | 3 | 7 |
| Tanaka H [30] (2001) | 3 | 1 | 3 | 7 |
| Huang J [31] (2001) | 3 | 1 | 3 | 7 |
| Li C [33] (2000) | 3 | 1 | 2 | 6 |
| Friedman, G [34] (2000) | 3 | 1 | 3 | 7 |
| McCredie M [35] (1997) | 3 | 1 | 3 | 7 |
| Volk N [36] (1997) | 4 | 1 | 3 | 8 |
| Lindelöf B [37] (1991) | 3 | 1 | 3 | 7 |
| Gutman M [38] (1991) | 3 | 1 | 3 | 7 |
| Boice J [39] (1988) | 3 | 1 | 3 | 7 |
| Murakami R [40] (1987) | 4 | 1 | 2 | 7 |
| Ron E [41] (1984) | 3 | 1 | 2 | 6 |
3.2. Characteristics and Risks of Any Second Primary Cancer Following a Primary Cancer
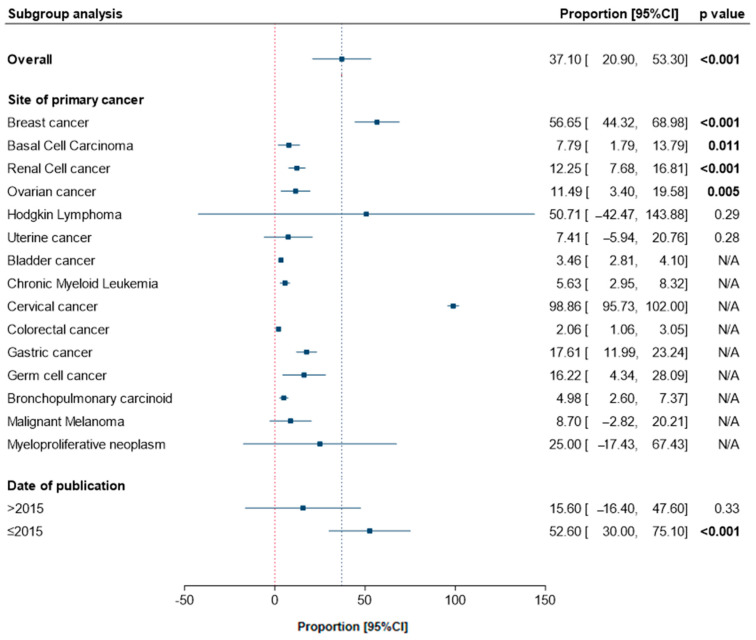
Across 40 studies, 96,675 (5.99%) survivors developed a second cancer different from their original primary cancer. In those who developed a second primary cancer, the most prevalent types of cancer were thyroid (37.1%, 95%CI, 20.8–53.2), lung (21.7%, 95%CI, 19.1–24.4), colon (21.2%, 95%CI, 15.7–26.6), and ovarian (14.8%, 95%CI, 9.6–20.0).
3.3. Characteristics and Risks of SPTC Following a Primary Cancer
SPTC was reported following 15 different types of primary cancer. 4196 (0.26%) survivors developed SPTC following a mean duration of 8.07 ± 4.39 years. Subgroup analysis by primary site revealed significant prevalence of thyroid cancer following BC (56.6%, 95%CI, 44.3–68.9, p < 0.001), RCC (12.2%, 95%CI, 7.68–16.8, p < 0.001), ovarian cancer (OC) (11.4%, 95%CI, 3.4–19.5, p = 0.005), and BCC of the skin (7.79%, 95%CI, 1.79–13.7, p = 0.011), Figure 3. Categorization of studies by date of publication revealed a high frequency of thyroid cancer in studies prior to 2015 (52.6%, 95%CI, 30.0–75.1, p < 0.001) compared to more recent articles (15.6%, 95%CI, 16.4–47.6, p = 0.33).
Figure 3.
Pooled frequency of SPTC following a primary cancer. Overall and subgroup analysis are shown.
3.4. Characteristics of SPTC Patients
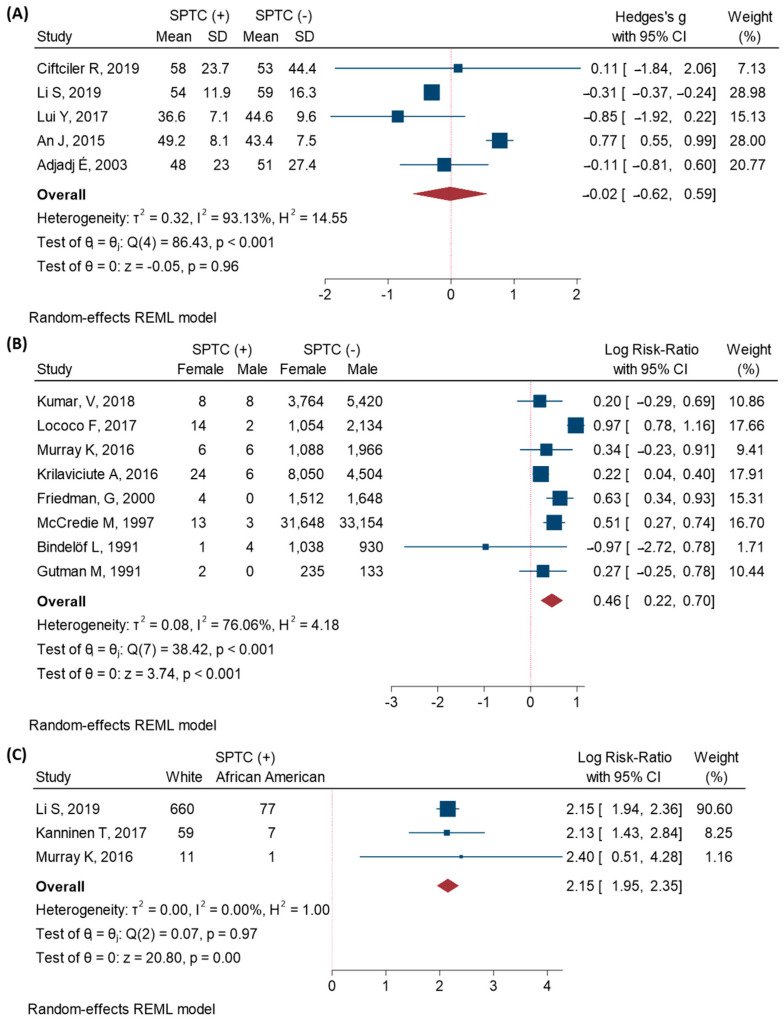
There was no significant difference between the age of patients who developed SPTC and those who were cancer free (Standard mean difference (SMD) = −0.02, 95%CI, −0.62–0.59, p = 0.96), Figure 4A. However, females were more likely to develop a SPTC (RR = 1.58, 95%CI, 1.2–2.01, p < 0.001), Figure 4B. Among the SPTC group, 81.3% were Caucasian and 9.2% were African Americans. Caucasian primary cancer patients were more than eight times more at risk to develop a second primary thyroid cancer (RR = 8.58, 95%CI, 7.02–10.48, p < 0.001), Figure 4C. In contrast, those who received chemotherapy (RR = 0.63, 95%CI, 0.26–1.47, p = 0.28) or radiotherapy (RR = 1.7, 95%CI, 0.03–81.8, p = 0.78) did not have a greater risk of developing SPTC.
Figure 4.
Characteristics of the SPTC patients. Random-effects model with restricted maximum-likelihood method was used. (A) Age, reported as standardized mean difference; (B) Gender, reported as log risk ratio; (C) Race, reported as log risk ratio.
4. Discussion
This current study is the largest multi-cohort analysis evaluating the risk of SPTC following any primary cancer. SPTC developed in 4196 (0.26%) of primary cancer patients following a mean duration of 8.07 ± 4.39 years. Patients were more likely to develop SPTC following primary BC, RCC, OC, and BCC of the skin. The highest risk of SPTC was found in patients with prior breast cancer. Caucasians and females were more likely to develop SPTC. Lastly, those who received chemotherapy or radiotherapy did not have a greater risk of developing thyroid cancer.
The most common primary cancer associated with SPTC was breast cancer. The relationship between breast and thyroid cancer has been previously studied. [6,32] However, the specific relevant risk factors and mechanisms remain unclear. One possible explanation for this relationship includes the hypothalamic-pituitary axis (HPA), which regulates both the mammary glands in breast and the thyroid gland. The increased actions of estrogen receptors in both glands have also been implicated [47]. As both thyroid and breast cancer are the most common cancers in women, a hormonal relationship between these cancers is not unsurprising. Of note, our study population comprised of 91.75% women with 78.74% of patients with primary breast cancer. Thus, the strong relationship between these cancers may be due to the bias of our study sample.
Our comprehensive study is the first review to report on various primary cancers associated with SPTC besides breast cancer. In particular, SPTC was associated with primary renal cell, ovarian, and basal cell skin cancers. The relationship between RCC and thyroid cancer has been questioned [48,49]. In a retrospective, population-based study, Fossen et al. found that female thyroid cancer patients had a 2-fold increase prevalence of subsequent RCC, whereas males had a 4.5-fold increase in subsequent RCC [48]. Key regulators such as circular RNA RAPGEF5 have been associated with both RCC and thyroid cancer [50]. Current literature on the relationship between these cancers sparse and more studies on their association should be further evaluated.
Existing data on the shared etiology of ovarian and thyroid cancer are limited. However, studies on this topic are emerging. In 2018, Weingarten et al. demonstrated that various transcription factors involved in the thyroid hormone axis may be associated with ovarian cancer metastasis [49]. In addition, thyroid hormones have been shown to bind to integrin αvβ3, which has oncogenic effects in cancers such as breast, ovarian, and melanoma [51]. Similar to breast cancer, there may be a hormonal pathway associated with these two cancers. The third primary cancer that showed a greater risk of SPTC was BCC of the skin [24]. Molecular studies have established that thyroid hormone action plays a critical role of multiple oncogenic pathways related to BCC tumorigenesis, specifically involving the miR21/GRHL3/D3 axis [24].
While not significant, other primary cancers associated with SPTC in this study includes leukemia and lymphoma, gastric cancer, and malignant melanoma. In a review by Schonfeld et al., patients who had chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL) had an elevated risk of SPTC [52]. The authors suggest that thyroid dysfunction secondary to targeted therapy for lymphoid malignancies may be a potential explanation. Furthermore, studies have demonstrated shared genetic relationship between thyroid hormone and gastric and colorectal cancers. For example, Tamandani et al. demonstrated an association between promoter methylation and expression of thyroid hormone receptor beta (THRβ) gene in patients with gastric cancer in an Iranian population [53]. In addition, oncogenic mutations in BRAF, an oncoprotein, has been found in melanoma, thyroid cancer, and colorectal cancers [53].
Radiotherapy (RT) and chemotherapy during primary cancer treatment in childhood have been associated with the development of SPTC [4]. However, this association has not been supported in the adult population [20,54]. Sun et al. studied 55,318 women with breast cancer and found that the risk of thyroid cancer among women who received RT was not significantly higher than that of women who received no RT [54]. In addition, in a retrospective study involving 127,563 head and neck cancer survivors, those who received radiation were not at greater risk of developing SPTC [55]. Our study confirms that adults have a low risk of radiation-induced thyroid cancers. Studies involving the long-term effects of chemotherapy on SPTC are limited. In summary, many potential factors are related to the development of SPTC such as detection bias, genetic susceptibility, hormonal signaling, and environmental factors. Further studies on the relevant risk factors are needed to better understand these associations.
The finding of greater rates of thyroid cancer in certain populations must be evaluated in a broader context. One potential explanation for the higher risk of SPTC is detection bias. That is, since cancer patients are subject to increased surveillance following their first cancer diagnosis, they are more likely to be detected with a second primary cancer. Moreover, detection bias is associated with access to healthcare. Minority patients are less likely to have insurance and access to health care compared to Caucasian patients [56]. The more robust follow up of Caucasian patients may reflect the higher detection and thus association of SPTC in Caucasians compared with other races in our study. Finally, familial non-medullary thyroid cancer (FNMTC) accounts for approximately 5% of all thyroid cancers [57]. Patients with syndromes associated with FNMTC have a greater risk of developing cancers in addition to thyroid cancer. For example, familial adenomatous polyposis (FAP), a condition with multiple polyps in the colon, is associated with papillary thyroid cancer. Other examples include Cowden’s syndrome (associated with benign lesions of breast, colon, and endometrium, and brain) and Carney syndrome (associated with testicular, adrenal, or pituitary tumors) [57]. Due to our large study sample, our study may have included patients with such syndromes.
Strengths and Limitations
This study has some strengths, including a comprehensive database search involving studies from all countries. In addition, we performed independent reviews of citations and articles in the selection of eligible studies, data abstraction, and critical appraisal of included studies.
There are a few limitations to this study. Due to the heterogeneity of study groups, there was a lack of data stratification by tumor type (i.e., papillary, follicular, medullary), staging, and recurrence of malignancy. Additionally, patient mortality was not included in our analysis. Other confounding factors such as smoking history, obesity, environment exposure, and family history of cancer were not reported in many of the included studies. Future studies should include an evaluation of these various factors when examining subsequent cancer risk.
5. Conclusions
An increased risk of SPTC was observed following a broad range of primary cancers. Surveillance protocols should be considered for patients at a greater risk for developing SPTC, including those with primary breast, renal cell, basal cell, and ovarian cancers who are female and/or Caucasian. Future studies should evaluate factors such as cancer staging and patient mortality.
Acknowledgments
A sincere thank you to Loula Burton from Tulane’s Research Proposal Development Office for her diligent editing and proofreading of this paper.
Author Contributions
Conceptualization, L.N.T., A.R.C., E.A.T., E.K.; methodology, L.N.T., A.R.C.; software, M.H.H., M.Z.; validation, E.A.T., E.K., G.W.R.; formal analysis, M.H.H., M.Z.; investigation, L.N.T., A.R.C.; writing—original draft preparation, L.N.T., A.R.C.; writing—review and editing, L.N.T., A.R.C., E.A.T., E.K., G.W.R.; supervision, E.A.T., E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Carol Lavin Bernick Grant” from Tulane University, New Orleans to Eman Toraih (PI) 1 July 2020–31 June 2021, and by a pilot grant from Tulane University Health Sciences Center to Emad Kandil.
Institutional Review Board Statement
The overall process of the meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Analysis involved data of published studies and not included individual study subjects, thus exempted from the Institutional Review Board approval.
Informed Consent Statement
Patient consent was waived since it is a meta-analysis of published articles.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies L., Welch H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 2.Kilfoy B.A., Zheng T., Holford T.R., Han X., Ward M.H., Sjodin A., Zhang Y., Bai Y., Zhu C., Guo G.L., et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lal G., Groff M., Howe J.R., Weigel R.J., Sugg S.L., Lynch C.F. Risk of subsequent primary thyroid cancer after another malignancy: Latency trends in a population-based study. Ann. Surg. Oncol. 2012;19:1887–1896. doi: 10.1245/s10434-011-2193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor A.J., Croft A.P., Palace A.M., Winter D.L., Reulen R.C., Stiller C.A., Stevens M.C.G., Hawkins M., M. Risk of thyroid cancer in survivors of childhood cancer: Results from the British Childhood Cancer Survivor Study. Int. J. Cancer. 2009;125:2400–2405. doi: 10.1002/ijc.24581. [DOI] [PubMed] [Google Scholar]
- 5.An J.H., Hwangbo Y., Ahn H.Y., Keam B., Lee K.E., Han W., Park D.J., Park I.A., Noh D.-Y., Youn Y.-K., et al. A Possible Association Between Thyroid Cancer and Breast Cancer. Thyroid. 2015;25:1330–1338. doi: 10.1089/thy.2014.0561. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S.M., White M.G., Hong S., Aschebrook-Kilfoy B., Kaplan E.L., Angelos P., Kulkarni S.A., Olopade O.I., Grogan R.H. The breast-thyroid cancer link: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016;25:231–238. doi: 10.1158/1055-9965.EPI-15-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabuchi T., Ito Y., Ioka A., Miyashiro I., Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: Update of analyses using population-based cancer registry data. Cancer Sci. 2012;103:1111–1120. doi: 10.1111/j.1349-7006.2012.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C., Bi X., Pan D., Chen Y., Carling T., Ma S., Udelsman R., Zhang Y. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid. 2013;23:575–582. doi: 10.1089/thy.2011.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessingthe Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 4 September 2020)]; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 10.Schlosser S., Rabinovitch R., Shatz Z., Galper S., Shahadi-Dromi I., Finkel S., Jacobson G., Rasco A., Friedman E., Laitman Y., et al. Radiation-Associated Secondary Malignancies in BRCA Mutation Carriers Treated for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020;107 doi: 10.1016/j.ijrobp.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Ciftciler R., Aksu S., Malkan U.Y., Buyukasik Y., Haznedaroglu I.C. Fibrosis development, leukemic transformation and secondary malignancies complicating the clinical course of essential thrombocythemia. Int. J. Hematol. Oncol. 2019;29:14–21. doi: 10.4999/uhod.193371. [DOI] [Google Scholar]
- 12.Liu Y., Dong C., Chen L. The clinicopathological features of second primary cancer in patients with prior breast cancer. Medicine. 2017;96 doi: 10.1097/MD.0000000000006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryk S., Pukkala E., Färkkilä A., Heikinheimo M., Unkila-Kallio L., Riska A. Other Primary Malignancies among Women with Adult-Type Ovarian Granulosa Cell Tumors. Int. J. Gynecol. Cancer. 2018;28:1529–1534. doi: 10.1097/IGC.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 14.Kwon W.A., Joung J.Y., Lim J., Oh C.M., Jung K.W., Kim S.H., Seo H.K., Park W.S., Chung J., Lee K.H., et al. Risk of second primary Cancer among bladder Cancer patients: A population-based cohort study in Korea. BMC Cancer. 2018;18:617. doi: 10.1186/s12885-018-4530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corso G., Veronesi P., Santomauro G.I., Maisonneuve P., Morigi C., Peruzzotti G., Intra M., Sacchini V., Galimberti V. Multiple primary non-breast tumors in breast cancer survivors. J. Cancer Res. Clin. Oncol. 2018;144:979–986. doi: 10.1007/s00432-018-2621-9. [DOI] [PubMed] [Google Scholar]
- 16.Joung J.Y., Kwon W.A., Lim J., Oh C.M., Jung K.W., Kim S.H., Seo H.K., Park W.S., Chung J., Lee K.H., et al. Second primary cancer risk among kidney cancer patients in Korea: A population-based cohort study. Cancer Res. Treat. 2018;50:293–301. doi: 10.4143/crt.2016.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar V., Garg M., Chaudhary N., Chandra A.B. An observational study on risk of secondary cancers in chronic myeloid leukemia patients in the TKI era in the United States. Peer J. 2018;2018 doi: 10.7717/peerj.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T., Brenner H., Fallah M., Jansen L., Castro F.A., Geiss K., Holleczek B., Katalinic A., Luttmann S., Sundquist K., et al. Risk of second primary cancers in women diagnosed with endometrial cancer in German and Swedish cancer registries. Int. J. Cancer. 2017;141:2270–2280. doi: 10.1002/ijc.30930. [DOI] [PubMed] [Google Scholar]
- 19.Sud A., Thomsen H., Sundquist K., Houlston R.S., Hemminki K. Risk of second cancer in Hodgkin lymphoma survivors and influence of family history. J. Clin. Oncol. 2017;35:1584–1590. doi: 10.1200/JCO.2016.70.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanninen T.T., Nasioudis D., Sisti G., Holcomb K., Di Tommaso M., Khalil S., Gojayev A., Witkin S.S. Epidemiology of second primary tumors in women with ovarian cancer. Int. J. Gynecol. Cancer. 2017;27:659–667. doi: 10.1097/IGC.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 21.Lococo F., Galeone C., Sacchettini C., Leuzzi G., Cesario A., Paci M., Mangone L. Second malignancy risk in patients with bronchopulmonary carcinoids: Epidemiological results from Italian Network of Cancer Registries. Tumori. 2017;103:e15–e20. doi: 10.5301/tj.5000598. [DOI] [PubMed] [Google Scholar]
- 22.Liao Z., Rodrigues M.C., Poynter J.N., Amatruda J.F., Rodriguez-Galindo C., Frazier A.L. Risk of second malignant neoplasms in women and girls with germ cell tumors. Ann. Oncol. 2017;28:329–332. doi: 10.1093/annonc/mdw609. [DOI] [PubMed] [Google Scholar]
- 23.Murray K.S., Zabor E.C., Spaliviero M., Russo P., Bazzi W.M., Musser J.E., Ari Hakimi A., Bernstein M.L., Dalbagni G., Coleman J.A., et al. Second primary malignancies in renal cortical neoplasms: An updated evaluation from a single institution. World J. Urol. 2016;34:1667–1672. doi: 10.1007/s00345-016-1832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krilaviciute A., Vincerzevskiene I., Smailyte G. Basal cell skin cancer and the risk of second primary cancers: A cancer registry–based study in Lithuania. Ann. Epidemiol. 2016;26:511–514. doi: 10.1016/j.annepidem.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Silverman B.G., Lipshitz I., Keinan-Boker L. Second Primary Cancers After Primary Breast Cancer Diagnosis in Israeli Women, 1992 to 2006. J. Glob. Oncol. 2017;3:135–142. doi: 10.1200/JGO.2016.003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcheselli R., Marcheselli L., Cortesi L., Bari A., Cirilli C., Pozzi S., Ferri P., Napolitano M., Federico M., Sacchi S. Risk of second primary malignancy in breast cancer survivors: A nested population-based case-control study. J. Breast Cancer. 2015;18:378–385. doi: 10.4048/jbc.2015.18.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaelson E.M., Chen Y.H., Silver B., Tishler R.B., Marcus K.J., Stevenson M.A., Ng. A.K. Thyroid malignancies in survivors of hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:636–641. doi: 10.1016/j.ijrobp.2013.11.237. [DOI] [PubMed] [Google Scholar]
- 28.Koivisto-Korander R., Scélo G., Ferro G., Mellemkjaer L., Hemminki K., Weiderpass E., Tamaro S., Pompe-Kirn V., Tracey E., Brewster D.H., et al. Second primary malignancies among women with uterine sarcoma. Gynecol. Oncol. 2012;126:30–35. doi: 10.1016/j.ygyno.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Antonelli A., Calza S., Arrighi N., Zani D., Corti S., Cozzoli A., Zanotelli T., Cosciani Cunico S., Simeone C. Clinical features and prognosis of patients with renal cancer and a second malignancy. Urol. Oncol. Semin. Orig. Investig. 2012;30:294–300. doi: 10.1016/j.urolonc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Yadav B.S., Sharma S.C., Patel F.D., Ghoshal S., Kapoor R., Kumar R. Nonbreast Second Malignancies After Treatment of Primary Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:1489–1492. doi: 10.1016/j.ijrobp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Lee K.-D., Chen S.-C., Chan C.H., Lu C.-H., Chen C.-C., Lin J.-T., Chen M.-F., Huang S.-H., Yeh C.-M., Chen M.-C. Increased Risk for Second Primary Malignancies in Women with Breast Cancer Diagnosed at Young Age: A Population-Based Study in Taiwan. Cancer Epidemiol. Biomark. Prev. 2008;17:2647–2655. doi: 10.1158/1055-9965.EPI-08-0109. [DOI] [PubMed] [Google Scholar]
- 32.Kirova Y.M., De Rycke Y., Gambotti L., Pierga J.Y., Asselain B., Fourquet A. Second malignancies after breast cancer: The impact of different treatment modalities. Br. J. Cancer. 2008;98:870–874. doi: 10.1038/sj.bjc.6604241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellemkjær L., Friis S., Olsen J.H., Scélo G., Hemminki K., Tracey E., Andersen A., Brewster D.H., Pukkala E., McBride M.L., et al. Risk of second cancer among women with breast cancer. Int. J. Cancer. 2006;118:2285–2292. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 34.Sadetzki S., Calderon-Margalit. R., Peretz C., Novikov I., Barchana M., Papa M.Z. Second primary breast and thyroid cancers (Israel) Cancer Causes Control. 2003;14:367–375. doi: 10.1023/A:1023908509928. [DOI] [PubMed] [Google Scholar]
- 35.Adjadj É., Rubino C., Shamsaldim A., Lê M.G., Schlumberger M., De Vathaire F. The risk of multiple primary breast and thyroid carcinomas: Role of the radiation dose. Cancer. 2003;98:1309–1317. doi: 10.1002/cncr.11626. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H., Tsukuma H., Koyama H., Kinoshita Y., Kinoshita N., Oshima A. Second primary cancers following breast cancer in the Japanese female population. Jpn. J. Cancer Res. 2001;92:1–8. doi: 10.1111/j.1349-7006.2001.tb01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J., Walker R., Groome P.G., Shelley W., Mackillop W.J. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer. 2001;92:1411–1418. doi: 10.1002/1097-0142(20010915)92:6<1411::AID-CNCR1464>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Li C.I., Rossing M.A., Voigt L.F., Daling J.R. Multiple primary breast and thyroid cancers: Role of age at diagnosis and cancer treatments (United States) Cancer Causes Control. 2000;11:805–811. doi: 10.1023/A:1008942616092. [DOI] [PubMed] [Google Scholar]
- 39.Friedman G.D., Tekawa I.S. Association of basal cell skin cancers with other cancers (United States) Cancer Causes Control. 2000;11:891–897. doi: 10.1023/A:1026591016153. [DOI] [PubMed] [Google Scholar]
- 40.McCredie M., Macfarlane G.J., Bell J., Coates M. Second primary cancers after cancers of the colon and rectum in New South Wales, Australia, 1972–1991. [(accessed on 29 February 2020)];Cancer Epidemiol. Biomark. Prev. 1997 6:155–160. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9138657. [PubMed] [Google Scholar]
- 41.Volk N., Pompe-Kirn V. Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control. 1997;8:764–770. doi: 10.1023/A:1018487506546. [DOI] [PubMed] [Google Scholar]
- 42.Lindelöf B., Sigurgeirsson B., Wallberg P., Eklund G. Occurrence of other malignancies in 1973 patients with basal cell carcinoma. J. Am. Acad Dermatol. 1991;25:245–248. doi: 10.1016/0190-9622(91)70189-9. [DOI] [PubMed] [Google Scholar]
- 43.Gutman M., Shafir R., Rozin R.R., Klausner J.M., Cnaan A., Inbar M., Chaitchik S. Are malignant melanoma patients at higher risk for a second cancer? Cancer. 1991;68:660–665. doi: 10.1002/1097-0142(19910801)68:3<660::AID-CNCR2820680337>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Boice J., Engholm G., Kleinerma R., Bletterner M., Stovall M., Lisco H. Radiation dose and second cancer risk in patients treated for cancer of the cervix. [(accessed on 1 September 2020)];Radiat. Res. 1988 116:3–55. doi: 10.2307/3577477. Available online: https://pubmed.ncbi.nlm.nih.gov/3186929/ [DOI] [PubMed] [Google Scholar]
- 45.Murakami R., Hiyama T., Hanai A., Fujimoto I. Second primary cancers following female breast cancer in Osaka, Japan—A population-based cohort study. [(accessed on 11 January 2020)];Jpn. J. Clin. Oncol. 1987 17:293–302. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3694826. [PubMed] [Google Scholar]
- 46.Ron E., Curtis R., Hoffman D.A., Flannery J.T. Multiple primary breast and thyroid cancer. Br. J. Cancer. 1984;49:87–92. doi: 10.1038/bjc.1984.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark O.H., Gerend P.L., Davis M., Goretzki P.E., Hoffman P.G. Estrogen and thyroid-stimulating hormone (TSH) receptors in neoplastic and nonneoplastic human thyroid tissue. BMC Public Health. 2019;19 doi: 10.1016/0022-4804(85)90012-5. [DOI] [PubMed] [Google Scholar]
- 48.Van Fossen V.L., Wilhelm S.M., Eaton J.L., McHenry C.R. Association of thyroid, breast and renal cell cancer: A population-based study of the prevalence of second malignancies. Ann. Surg. Oncol. 2013;20:1341–1347. doi: 10.1245/s10434-012-2718-3. [DOI] [PubMed] [Google Scholar]
- 49.Weingarten C., Jenudi Y., Tshuva R.Y., Moskovich D., Alfandari A., Hercbergs A., Davis P.J., Ellis M., Ashur-Fabian O. The Interplay between Epithelial-Mesenchymal Transition (EMT) and the Thyroid Hormones-αvβ3 Axis in Ovarian Cancer. Horm. Cancer. 2018;9:22–32. doi: 10.1007/s12672-017-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Q., Liu T., Bao Y., Zhao T., Wang J., Wang H., Wang A., Gan X., Wu Z., Wang L. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 2020;469:68–77. doi: 10.1016/j.canlet.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Shinderman-Maman E., Cohen K., Moskovich D., Hercbergs A., Werner H., Davis P.J., Ellis M., Ashur-Fabian O. Thyroid hormones derivatives reduce proliferation and induce cell death and DNA damage in ovarian cancer. Sci. Rep. 2017;7:16475. doi: 10.1038/s41598-017-16593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schonfeld S.J., Morton L.M., de González A.B., Curtis R.E., Kitahara C.M. Risk of second primary papillary thyroid cancer among adult cancer survivors in the United States, 2000-2015. Cancer Epidemiol. 2020;64:101664. doi: 10.1016/j.canep.2019.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman M., Salajegheh A., Smith R., Lam A. BRAF Inhibitor Therapy for Melanoma, Thyroid and Colorectal Cancers: Development of Resistance and Future Prospects. Curr. Cancer Drug Targets. 2014;14:128–143. doi: 10.2174/1568009614666140121150930. [DOI] [PubMed] [Google Scholar]
- 54.Sun L.-M., Lin C.-L., Liang J.-A., Huang W.-S., Kao C.-H. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int. J. Cancer. 2015;137:2896–2903. doi: 10.1002/ijc.29667. [DOI] [PubMed] [Google Scholar]
- 55.Polednik K.M., Simpson M.C., Adjei Boakye E., Mohammed K.A., Dombrowski J.J., Varvares M.A., Osazuwa-Peters N. Radiation and Second Primary Thyroid Cancer Following Index Head and Neck Cancer. Laryngoscope. 2019;129:1014–1020. doi: 10.1002/lary.27467. [DOI] [PubMed] [Google Scholar]
- 56.Doty M.M., Holmgren A.L. Unequal access: Insurance instability among low-income workers and minorities. [(accessed on 11 October 2020)];Issue Brief. 2004 :1–6. Available online: https://pubmed.ncbi.nlm.nih.gov/15077607/ [PubMed] [Google Scholar]
- 57.Randolph G. Surgery of the Thyroid and Parathyroid Glands. 3rd ed. Elsevier; Phildelphia, PA, USA: 2020. [(accessed on 11 October 2020)]. Available online: https://www.elsevier.com/books/surgery-of-the-thyroid-and-parathyroid-glands/randolph/978-0-323-66127-0. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.