Abstract
Endometriosis is a common gynecological disorder characterized by the ectopic growth of endometrial-like tissue outside the uterine cavity. Etiopathogenesis of endometriosis is poorly understood; it is plausible, however, that the disease may be associated with oxidative stress related to local heme and iron metabolism. Therefore, the aim of the study was to reveal a possible association of endometriosis with a stress-inducible heme oxygenase 1 (HMOX1). For this purpose, 228 patients with clinically confirmed endometriosis and 415 control parous women from general Polish population were examined for functional –413A>T (rs2071746) single-nucleotide polymorphism (SNP) and (GT)n dinucleotide repeat length polymorphism in the promoter of HMOX1 gene. In addition, –413A>T SNP was assessed by the specific TaqMan® SNP Genotyping Assay, and (GT)n polymorphism was determined by PCR product size analysis. We found that endometriosis is associated with an increased frequency of −413A(GT)31,32 haplotype (OR (95%CI) = 1.27 (1.01–1.60), p = 0.0381) and −413A(GT)31,32 homozygous genotype [OR (95%CI) = 1.51 (1.06–2.17), p = 0.0238]. These data suggest that endometriosis is associated with functional polymorphism of HMOX1 gene, and this gene may play a part in the pathogenesis of this disorder.
Keywords: endometriosis, heme oxygenase 1, inflammatory response, single-nucleotide polymorphism
1. Introduction
Endometriosis is a common gynecological disorder affecting from 2% up to 22% (approximately 10%) women in reproductive age [1]. It is associated with ectopic implantation and growth of endometrial-like tissue (endometrial glands and stroma) in the pelvic cavity. The disease manifests with pelvic inflammatory reactions and pain and is one of the major causes of female infertility [2,3]. Etiopathogenesis of endometriosis is still poorly understood. It appears to be a multifactorial trait with the involvement of a variety of genetic and environmental factors [3,4,5].
There exist several theories of development of endometriosis [2,5,6]. However, the most accepted one postulates that ectopic endometriotic tissue originates from retrograde menstruation [6,7]. However, it is still unclear what facilitates survival and heterotopic implantation of endometrial epithelial and stromal cells. This may be related to abrogated elimination of endometriotic cells by NK cells, their increased resistance to apoptosis, and increased expression of adhesion molecules, as well as facilitation of local angiogenic response [8,9,10,11,12]. The possible mechanisms of these phenomena, however, still remain to be elucidated.
There is a growing body of evidence that the mechanisms involved in etiopathogenesis of endometriosis may include the pathways responsible for regulation of local oxidative stress and detoxification [13,14,15]. In particular, the pathways involved in heme and iron metabolism may be of interest, especially since these agents were found to be increased in the peritoneal fluid of women with endometriosis and may be involved in the pathogenesis of this disorder [16,17,18,19,20,21]. Heme and iron metabolism depend on activity of heme oxygenases (HMOXs), rate-limiting enzymes responsible for heme degradation leading to generation of equimolar amounts of biliverdin, carbon monoxide (CO), and free iron [22,23,24]. A key enzyme is stress-inducible heme oxygenase 1 (HMOX1), a 32.8-kDa enzyme encoded in humans by HMOX1 gene located on chromosome 22q12 [24,25,26].
Factors involved in the induction of HMOX1 include various oxidative agents and inflammatory cytokines, which strongly suggests that activity of this enzyme represents an adaptive response mechanism to cellular oxidative stress [26,27]. Expression of HMOX1 has been reported to depend on the functional polymorphisms in the 5′ UTR region promoter of HMOX1 gene, namely –413A>T (rs2071746) single-nucleotide polymorphism (SNP) [28,29] and (GT)n dinucleotide repeat length polymorphism [30]. This strongly implies that regulation and biological significance of HMOX1 expression at least partially depends on the host genetic background.
HMOX1 has been found to be expressed in endometriotic lesions [17]. However, a relationship between this enzyme and development of endometriosis has not been established, so far. Therefore, the aim of the present study was to assess whether endometriosis is associated with known functional polymorphisms of HMOX1 gene.
2. Materials and Methods
2.1. Patients and Control Subjects
All women with endometriosis and control subjects included in the present study were diagnosed at I and II Department of Obstetrics and Gynecology, Medical University of Warsaw and the Department of Gynecology, Military Institute of Medicine, Warsaw, Poland. The disease has been confirmed and classified on the basis of laparoscopic and histopathological examinations according to the revised criteria of the American Fertility Society (rAFS) [31].
The genetic association study included 228 Polish Caucasian women with endometriosis (mean age 33.1 ± 7.5, range 22–58 years). This group consisted of 11 (4.8%) patients with minimal/mild (rAFS stage I/II) and 217 (95.2%) patients with moderate/severe (rAFS stage III/IV) disease. Ovarian endometriotic cysts were present in 217 (95.2%) cases, and peritoneal lesions were found in 110 (48.2%) women. Both type of lesions was detected in 189 (82.9%) patients, 28 (12.3%) patients had only ovarian cysts, and 11 (4.8%) patients had only peritoneal lesions.
The control group for the genetic study consisted of 415 anonymous unrelated parous women randomly selected from the cohort representative of background population of Central Poland which has been described elsewhere [32].
The study has been approved and was conducted according to strict guidelines of the ethical committee at the Medical University of Warsaw and all patients gave an informed consent to the study.
2.2. Typing for −413A>T and (GT)n Polymorphisms
Genomic DNA was isolated from 5 mL of the peripheral blood by the salting-out method [33]. The quality of isolated DNA was checked spectrophotometrically and the optical 260/280 nm density ratio at of the samples was always >1.7. DNA samples were tested for the −413A>T SNP (rs2071746) of HMOX1 gene using specific TaqMan® SNP Genotyping Assay and 7500 real-time PCR system with sequence-detection software (Applied Biosystems, Foster City, CA, USA).
The (GT)n dinucleotide length polymorphism was determined by amplifying the 5′ flanking region of HMOX1 gene containing the (GT)n repeats using the FAM-labeled sense 5′-AGAGCCTGCAGCTTCTCAGA-3′ and an antisense 5′-ACAAAGTCTGGCCATAGGAC-3′ primers, as described by Kimpara et al. [34]. The amplicon size was determined by the 3130 Genetic Analyzer (Applied Biosystems) and Gene Mapper software (Applied Biosystems).
2.3. Statistical Analysis
Continues variables were analyzed by Mann–Whitney U test using Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA). Allele, haplotype, and genotype distributions were analyzed by Pearson’s chi-squared (χ2) test or, as appropriate, two-tail Fisher exact probability test using VassarStats statistical calculator available at http://vassarstats.net, accessed on 21 March 2021. Hardy-Weinberg equilibrium (HWE), linkage disequilibrium, and haplotype analysis was performed using PLINK [35] or Arlequin [36] software. Odds ratios (OR) and their 95% confidence intervals (95% CI) for the indicated genotype were calculated versus a pool of all other genotypes. The differences between the tested variables were considered significant at least at p < 0.05.
3. Results
The distribution of HMOX1 –413 SNP A and T alleles and genotypes in patients with endometriosis and control population is shown in Table 1. The genotype distribution in control subjects was in Hardy–Weinberg equilibrium (p = 0.987). On the contrary, genotype distribution in women with endometriosis did not fulfill criteria for Hardy–Weinberg equilibrium (p = 0.004). An analysis of allele distribution did not reveal any differences between women with endometriosis and control group [OR (95%CI) = 1.16 (0.92–1.47), p = 0.20]. However, the frequency of –413AA genotype was significantly higher in endometriosis group (OR (95%CI) = 1.48 (1.06–2.07), p = 0.0228). Endometriosis was also associated with a significantly decreased frequency of –413AT genotype (OR (95%CI) = 0.66 (0.48–0.92), p = 0.0132) (Table 1).
Table 1.
Allele and genotype distribution of −413A>T SNP (single-nucleotide polymorphism) and two most frequent (GT)n dinucleotide repeat variants of HMOX1 gene in patients with endometriosis and control women.
| Allele/Genotype | Endometriosis (n = 228) | Control (n = 415) | p-Value * | OR (95% CI) | |
|---|---|---|---|---|---|
| -413A>T | A T |
269 (59.0%) 187 (41.0%) |
459 (55.3%) 371 (44.7%) |
0.2017 | 1.16 (0.92–1.47) |
| AA AT TT |
90 (39.5%) 89 (39.0%) 49 (21.5%) |
127 (30.6%) 205 (49.4%) 83 (20.0%) |
0.0228 0.0132 0.6547 |
1.48 (1.06–2.07) 0.66 (0.48–0.92) 1.09 (0.74–1.63) |
|
| HWE P | 0.004 | 0.987 | |||
| (GT)n | (GT)23,24 (GT)31,32 |
141 (30.8%) 240 (52.4%) |
262 (31.6%) 392 (47.2%) |
0.777 0.0754 |
0.96 (0.75–1.23) 1.23 (0.98–1.55) |
| (GT)23,24(GT)23,24 (GT)23,24(GT)31,32 (GT)31,32(GT)31,32 |
23 (10.1%) 62 (27.2%) 72 (31.6%) |
41 (9.1%) 119 (28.7%) 101 (24.3%) |
0.9203 0.6891 0.0480 |
1.02 (0.60–1.75) 0.93 (0.65–1.33) 1.43 (1.01–2.05) |
|
| HWE P | 0.09 ** | 0.39 ** |
* p-values were calculated by Pearson χ2 test. ** HWE analysis was performed with all (GT)n genotypes found in endometriosis and control group (for details see Table 2). OR (95% CI): odds ratio (95% confidence intervals). n: number of cases. HWE. p: Probability for Hardy–Weinberg equilibrium.
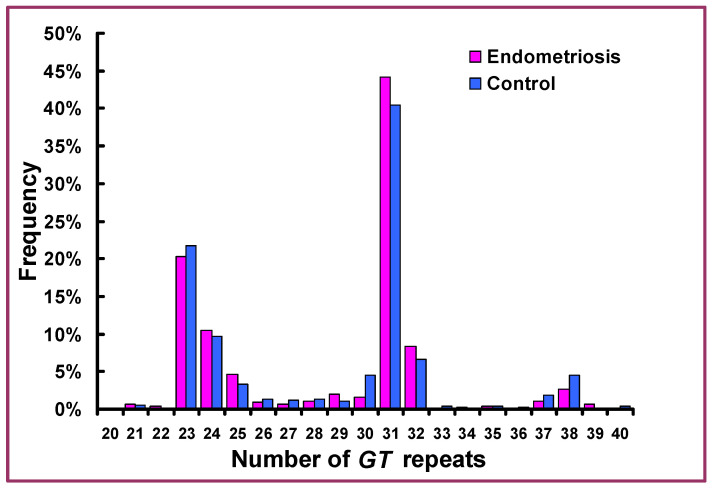
Distribution of the number of GT repeats in the promoter of HMOX1 gene in patients with endometriosis and control women is shown in Figure 1. As can be seen, the number of repeats ranged from 21 to 40, and the frequency distribution of (GT)n alleles was uneven with many rare variants. When alleles with frequencies >5% were considered, two clearly different groups displaying 23 and 24 repeats as well as 31 and 32 repeats, respectively, could be distinguished. These two variant groups accounted for ca. 80% of all endometriosis and control cases, and therefore, only these groups were considered for specific analyses. They will be further referred to as (GT)23,24 and (GT)31,32 variants. The detailed results of distribution of (GT)n alleles and genotypes in relation to −413A>T SNP genotypes are given in Table 2.
Figure 1.
Frequency distribution of the length of GT repeats in the promoter of HMOX1 gene in patients with endometriosis and control women.
Table 2.
Distribution of (GT)n variants in relation to −416A>T genotypes of HMOX1 gene in women with endometriosis and control subjects.
| (GT)n Genotype | 416A>T SNP Genotype | ||||||
|---|---|---|---|---|---|---|---|
| AA | AT | TT | |||||
| Endometriosis n = 90 |
Control n = 127 |
Endometriosis n = 89 |
Control n = 205 |
Endometriosis n = 49 |
Control n = 83 |
||
| 21 | 23 | 0 | 0 | 1 (0.4%) | 0 | 0 | 1 (0.2%) |
| 21 | 24 | 0 | 0 | 0 | 1 (0.2%) | 1 (0.4%) | 0 |
| 21 | 26 | 0 | 0 | 0 | 0 | 1 (0.4%) | 0 |
| 21 | 31 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 21 | 32 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 22 | 27 | 0 | 0 | 0 | 0 | 1 (0.4%) | 0 |
| 22 | 31 | 0 | 0 | 1 (0.4%) | 1 (0.2%) | 0 | 0 |
| 23 | 23 | 0 | 0 | 0 | 1 (0.2%) | 11 (4.8%) | 16 (3.9%) |
| 23 | 24 | 0 | 0 | 1 (0.4%) | 0 | 6 (2.6%) | 21 (5.1%) |
| 23 | 25 | 0 | 0 | 3 (1.3%) | 2 (0.5%) | 2 (0.9%) | 6 (1.4%) |
| 23 | 26 | 0 | 0 | 0 | 1 (0.2%) | 1 (0.4%) | 2 (0.5%) |
| 23 | 27 | 0 | 0 | 0 | 1 (0.2%) | 0 | 1 (0.2%) |
| 23 | 28 | 0 | 0 | 0 | 2 (0.5%) | 0 | 1 (0.2%) |
| 23 | 29 | 0 | 0 | 0 | 0 | 2 (0 (1.9%) | 1 (0.2%) |
| 23 | 30 | 0 | 0 | 1 (0.4%) | 10 (2.4%) | 0 | 0 |
| 23 | 31 | 2 (0.9%) | 1 (0.2%) | 34 (14.9%) | 74 (17.8%) | 1 (0.4%) | 0 |
| 23 | 32 | 0 | 0 | 8 (3.5%) | 9 (2.1%) | 0 | 0 |
| 23 | 33 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 23 | 35 | 0 | 0 | 0 | 0 | 1 (0.4%) | 2 (0.5%) |
| 23 | 37 | 0 | 0 | 0 | 0 | 2 (0.9%) | 3 (0.7%) |
| 23 | 38 | 0 | 0 | 0 | 0 | 4 (1.7%) | 8 (1.9%) |
| 23 | 39 | 0 | 0 | 0 | 0 | 1 (0.4%) | 0 |
| 24 | 24 | 0 | 0 | 0 | 0 | 5 (2.2%) | 3 (0.7%) |
| 24 | 25 | 0 | 0 | 0 | 1 (0.2%) | 5 (2.2%) | 1 (0.2%) |
| 24 | 26 | 0 | 0 | 0 | 1 (0.2%) | 0 | 2 (0.5%) |
| 24 | 28 | 0 | 0 | 1 (0.4%) | 1 (0.2%) | 0 | 0 |
| 24 | 29 | 0 | 0 | 1 (0.4%) | 1 (0.2%) | 0 | 0 |
| 24 | 30 | 0 | 0 | 1 (0.4%) | 4 (1%) | 1 (0.4%) | 0 |
| 24 | 31 | 0 | 0 | 15 (6.6%) | 30 (7.2%) | 0 | 0 |
| 24 | 32 | 0 | 0 | 2 (0.9%) | 5 (1.2%) | 0 | 0 |
| 24 | 38 | 0 | 0 | 0 | 0 | 3 (1.3%) | 6 (1.4%) |
| 24 | 39 | 0 | 0 | 0 | 0 | 1 (0.4%) | 0 |
| 24 | 40 | 0 | 0 | 0 | 0 | 0 | 1 (0.2%) |
| 25 | 27 | 0 | 0 | 0 | 0 | 0 | 1 (0.2%) |
| 25 | 28 | 0 | 0 | 0 | 0 | 0 | 1 (0.2%) |
| 25 | 30 | 0 | 0 | 1 (0.4%) | 2 (0.5%) | 0 | 0 |
| 25 | 31 | 3 (1.3%) | 2 (0.5%) | 3 (1.3%) | 5 (1.2%) | 0 | 0 |
| 25 | 32 | 2 (0.9%) | 0 | 1 (0.4%) | 1 (0.2%) | 0 | 0 |
| 25 | 34 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 25 | 37 | 0 | 0 | 0 | 1 (0.2%) | 0 | 2 (0.5%) |
| 25 | 39 | 0 | 0 | 1 (0.4%) | 0 | 0 | 1 (0.2%) |
| 26 | 29 | 1 (0.4%) | 0 | 0 | 0 | 0 | 0 |
| 26 | 31 | 1 (0.4%) | 1 (0.2%) | 0 | 3 (0.7%) | 0 | 0 |
| 26 | 32 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 27 | 31 | 1 (0.4%) | 2 (0.5%) | 1 (0.4%) | 4 (1%) | 0 | 0 |
| 27 | 32 | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| 28 | 31 | 2 (0.9%) | 4 (1%) | 2 | 1 (0.2%) | 0 | 0 |
| 28 | 32 | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| 29 | 29 | 1 (0.4%) | 0 | 0 | 0 | 0 | 0 |
| 29 | 30 | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| 29 | 31 | 2 (0.9%) | 5 (1.2%) | 1 (0.4%) | 0 | 0 | 0 |
| 29 | 38 | 0 | 0 | 0 | 0 | 0 | 1 (0.2%) |
| 30 | 30 | 0 | 5 (1.2%) | 0 | 0 | 0 | 0 |
| 30 | 31 | 1 (0.4%) | 3 (0.7%) | 1 (0.4%) | 1 (0.2%) | 0 | 0 |
| 30 | 32 | 1 (0.4%) | 2 (0.5%) | 0 | 0 | 0 | 0 |
| 30 | 35 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 30 | 38 | 0 | 0 | 0 | 4 (1%) | 0 | 0 |
| 31 | 31 | 50 (21.9%) | 72 (17.3%) | 0 | 3 (0.7%) | 0 | 0 |
| 31 | 32 | 22 (9.6%) | 19 (4.6%) | 0 | 1 (0.2%) | 0 | 0 |
| 31 | 33 | 0 | 2 (0.5%) | 0 | 0 | 0 | 0 |
| 31 | 34 | 1 (0.4%) | 0 | 0 | 0 | 0 | 0 |
| 31 | 35 | 0 | 0 | 1 (0.4%) | 0 | 0 | 0 |
| 31 | 36 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 31 | 37 | 0 | 0 | 2 (0.9%) | 9 (2.2%) | 0 | 0 |
| 31 | 38 | 0 | 0 | 4 | 15 (3.6%) | 0 | 0 |
| 31 | 40 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 32 | 32 | 0 | 6 (1.4%) | 0 | 0 | 0 | 0 |
| 32 | 37 | 0 | 0 | 1 (0.4%) | 1 (0.2%) | 0 | 0 |
| 32 | 38 | 0 | 0 | 1 (0.4%) | 0 | 0 | 0 |
| 32 | 40 | 0 | 0 | 0 | 1 (0.2%) | 0 | 0 |
| 36 | 38 | 0 | 0 | 0 | 0 | 0 | 1 (0.2%) |
| 38 | 38 | 0 | 0 | 0 | 0 | 0 | 1 (0.2%) |
Highlighted are the most frequent genotypes. n: number of cases.
(GT)n genotype distribution was in Hardy–Weinberg equilibrium both among endometriosis patients (p = 0.09) and control subjects (p = 0.39). Distribution of (GT)23,24 and (GT)31,32 variants and their genotypes are shown in Table 1. As can be seen, the frequency of (GT)23,24 in endometriosis and control groups was 30.8% and 31.6%, respectively, whereas the respective frequency of (GT)31,32 was 52.4% and 47.2%. The difference in distribution of (GT)23,24 in endometriosis and control group was not significant (OR (95%CI) = 0.96 (0.75–1.23), p = 0.777), whereas the difference in the frequency of (GT)31,32 had a borderline significance (OR (95%CI) = 1.23 (0.98–1.55), p = 0.0754). Analysis of distribution of (GT)23,24 and (GT)31,32 genotypes has revealed that endometriosis is associated with increased frequency of (GT)31,32 homozygotes (OR (95%CI) = 1.43 (1.01–2.05), p = 0.0480). There were no differences in distribution of (GT)23,24/(GT)31,32 heterozygotes and (GT)23,24 homozygotes in endometriosis group as compared with control (Table 1).
Haplotype analysis has revealed that HMOX1 –413A>T SNP alleles and (GT)n repeats are in linkage disequilibrium. In addition, –413A allele is associated with (GT)31,32 allele (r2 = 0.71), whereas –413T allele is associated with (GT)23,24 (r2 = 0.57).
Analysis of distribution of these two major HMOX1 haplotypes and their related genotypes is presented in Table 3. As shown, endometriosis was significantly associated with –413A(GT)31,32 haplotype (OR (95%CI) = 1.27 (1.01–1.60), p = 0.0381). There was no difference in the frequency of –413T(GT)23,24 haplotype between endometriosis and control group (OR (95%) = 0.95 (0.67–1.35), p = 0.6954). Frequency of the other haplotypes was relatively low, and none of them showed any significant association with endometriosis (data not shown).
Table 3.
Distribution of two major HMOX1 −413A>T (GT)n haplotypes and genotypes in patients with endometriosis and control women.
| Haplotype/Genotype | Endometriosis (n = 228) | Control (n = 415) | p-Value * | OR (95%CI) |
|---|---|---|---|---|
| −413A(GT)31,32 −413T(GT)23,24 |
238 (52.1%) 138 (30.2%) |
388 (46.8%) 260 (31.3%) |
0.0381 0.6954 |
1.27 (1.01–1.60) 0.95 (0.74–1.15) |
| −413A(GT)31,32/413A(GT)31,32 −413A(GT)other/−413A(GT)other |
72 (31.6%) 18 (7.9%) |
97 (23.4%) 30 (7.2%) |
0.0238 0.7641 |
1.51 (1.06–2.17) 1.10 (0.60–2.02) |
* p-Values were calculated by Pearson χ2 test or Fisher exact probability test where applicable. OR (95% CI): odds ratio (95% confidence intervals). n: number of cases.
Analysis of distribution of –413A alleles in combination with (GT)31,32 variant is shown in Table 3. As demonstrated, –413A(GT)31,32/–413A(GT)31,32 homozygous genotype was significantly more frequent in women with endometriosis (OR (95%CI) = 1.51 (1.06–2.17), p = 0.0238). On the other hand, analysis of other combinations between –413AA homozygotes and other (GT)n variants, as well as (GT)31,32(GT)31,32 homozygotes and –413AT or –413TT did not reveal any difference between endometriosis and control group (Table 3). None of the other HMOX1 genotypes including combinations of –413T and (GT)23,24 alleles were significantly associated with endometriosis (data not shown).
4. Discussion
The results of our study show that endometriosis is related to functional polymorphism in the promoter of HMOX1 gene. The disease was found to be associated with an increased frequency of both –413AA and (GT)31,32(GT)31,32 homozygotes. In addition, we observed that the distribution of –413 HMOX genotypes among patients (but not controls) significantly departed from Hardy–Weinberg equilibrium (HWE). Since departure from HWE among cases has been proposed as an independent test detecting an association [37,38], this observation further confirms that the HMOX locus is not neutral in endometriosis.
It should be mentioned, however, that both alleles are in linkage disequilibrium, and, indeed, endometriosis was associated with increased frequency of –413A(GT)31,32 haplotypes as well as –413A(GT)31,32/–413A(GT)31,32 genotypes. This indicates that disease may be related to homozygosity in either –413A or (GT)31,32 allele or may be linked to their combination. However, it was not possible to distinguish between the effect of –413AA or (GT)31,32(GT)31,32 homozygosity because of the very low frequency of subjects in whom these two alleles were not found together (Table 2).
Distribution of –413A>T HMOX1 SNP alleles in endometriosis and control group subjects was found to be similar to distribution reported for other populations [28,29] and is consistent with data presented in HapMap database (http://hapmap.ncbi.nlm.nih.gov/, accessed on 21 March 2021). Analysis of the frequency of HMOX1 (GT)n alleles has revealed two clearly different groups displaying 23–24 repeats or 31–32 (GT) repeats which account for ca. 80% of all studied subjects. This observation is also consistent with other reports on (GT)n allele distribution [30]. It should be stressed, that in a majority of these studies (GT)n alleles were classified into short or long and sometimes medium variants. However, criteria for such classification were arbitrary, and therefore, to avoid a bias related to a great diversity of (GT)n variants, we decided to limit our analysis only to specific long (GT)31,32 and short (GT)23,24 alleles.
Our present result in women with endometriosis is similar to the previous reports of Ono et al. [28,29] who found that prevalence of –413A HMOX1 homozygote is associated with a higher risk of women’s’ hypertension and coronary artery disease. In respect to the (GT)n HMOX1 polymorphism, our results are also similar to results reported for a variety of other diseases including some cardiovascular disorders and transplanted organ dysfunction [30] as well as idiopathic recurrent miscarriage [39], where longer (GT)n variants (n>30) were associated with increased susceptibility to the disease.
The biological significance of HMOX1 promoter polymorphism still remains a matter of controversy. It is generally accepted that short (GT)n variants are associated with an increased HMOX1 expression and activity. This is based on both the biological observations of cell lines isolated from subjects carrying different (GT)n variants [40,41] and cloned HMOX1 promoter/luciferase assays [42,43]. It should be stressed, however, that these studies were focused on (GT)n variants and did not take into consideration a possible influence of −413A>T SNP polymorphism and possibly other yet-unknown factors. In contrast to these reports, luciferase reporter assays performed by Ono et al. [29] have revealed that transcriptional activity of −413A(GT)30-harboring HMOX1 promoter constructs was about six times higher than that of −413T(GT)23 ones. This strongly implies that the relationship between the length of (GT)n variants and prevalence of −413A>T alleles needs further elucidation.
Expression of HMOX1 may be stimulated by inflammatory cytokines as well as oxidative stress agents [22,23,24] which are known to be involved in the pathogenesis of endometriosis [12,13,14,15]. Oxidative stress agents may include heme [17] which may be released into the peritoneal cavity and the lumen of endometriotic ovarian cysts (chocolate cysts) from erythrocytes originating from retrograde menstruation and shedding of endometriotic tissue in course of menstrual cycle. It should be stressed, however, that final elucidation of the regulatory mechanism(s) of HMOX1 expression in endometriosis requires more extensive studies.
The specific role of HMOX1 in etiopathogenesis of endometriosis also remains to be established. There is a growing evidence that this enzyme may exert a variety of cytoprotective effects [22,23,24]. HMOX1 was reported to protect cells from apoptosis [44,45,46] and may exert anti-inflammatory effects [47,48,49,50]. This effect may be mediated by degradation of toxic and pro-oxidant heme, as well as in generation of products of heme metabolism such as biliverdin and bilirubin which were also reported to exert anti-inflammatory and immunoregulatory activity [51,52,53]. Therefore, it is possible that an increased HMOX1 activity in endometriotic cells might protect them from heme toxicity and oxidative stress and thus facilitate their survival and adhesion in ectopic sites. Furthermore, HMOX1 plays an important role in angiogenesis [24,54,55], thus further supporting its possible role in development of endometriotic lesions.
Clinical significance of HMOX1 promoter polymorphism in endometriosis also remains obscure. It would be of interest to correlate HMOX1 polymorphism with some clinical features of endometriosis such as stage of the disease, lesion type and their location. However, the patients’ cohort recruited in the present study consisted mostly (95%) of women with moderate/severe (III/IV) stage of the disease. Furthermore, 95% of patients had endometriotic ovarian cysts, and only 5% of them had peritoneal lesions. Such patients’ stratification made any statistical correlation analysis meaningless. Therefore, further studies on a group of patients including significantly more cases with minimal/mild stage of endometriosis and higher prevalence of only peritoneal lesions would be necessary.
In summary, our present results show that endometriosis is associated with functional polymorphism of HMOX1 gene promoter. This suggests that HMOX1 plays an important role in etiopathogenesis of this disorder, possibly by exerting a protective effect and facilitating survival of endometriotic cells in ectopic sites. This, however, needs further extensive studies.
Author Contributions
Ł.M., A.Ś. and M.S. were involved in nucleic acid isolation, SNP and STR typing and RT-PCR evaluations, data analysis, and manuscript drafting and editing and critical discussion. J.P. was involved in STR typing and analysis. E.B., P.I.R. and P.K. were involved in clinical material collection and clinical data analysis. P.W. was involved in manuscript drafting and critical discussion. R.P. was involved in statistical analysis, manuscript drafting, and critical discussion. J.M. was involved in study conception and design, data analysis, and drafting the article and its final edition. All authors participated in critical reading of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Faculty of Medicine, Medical University of Warsaw grants 1M15/NK1W/09 and 1M15/N/10.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Medical University of Warsaw.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are included in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guo S.W., Wang Y. Sources of heterogeneities in estimating the prevalence of endometriosis in infertile and previously fertile women. Fertil. Steril. 2006;86:1584–1595. doi: 10.1016/j.fertnstert.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Giudice L.C., Kao L.C. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Zondervan K.T., Becker C.M., Missmer S.A. Endometriosis. N. Engl. J. Med. 2020;382:1244–1256. doi: 10.1056/NEJMra1810764. [DOI] [PubMed] [Google Scholar]
- 4.Zondervan K.T., Cardon L.R., Kennedy S.H. The genetic basis of endometriosis. Curr. Opin. Obstet. Gynecol. 2001;13:309–314. doi: 10.1097/00001703-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Koninckx P.R., Ussia A., Adamyan L., Wattiez A., Gomel V., Martin D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019;111:327–340. doi: 10.1016/j.fertnstert.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Nisolle M., Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997;68:585–596. doi: 10.1016/S0015-0282(97)00191-X. [DOI] [PubMed] [Google Scholar]
- 7.Sampson J.A. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obst. Gynecol. 1927;14:442–469. doi: 10.1016/S0002-9378(15)30003-X. [DOI] [Google Scholar]
- 8.Sciezynska A., Komorowski M., Soszynska M., Malejczyk J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019;8:1468. doi: 10.3390/jcm8091468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Velasco J.A., Arici A. Apoptosis and the pathogenesis of endometriosis. Semin. Reprod. Med. 2003;21:165–172. doi: 10.1055/s-2003-41323. [DOI] [PubMed] [Google Scholar]
- 10.Becker C.M., D’Amato R.J. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc. Res. 2007;74:121–130. doi: 10.1016/j.mvr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Harada T., Kaponis A., Iwabe T., Taniguchi F., Makrydimas G., Sofikitis N., Paschopoulos M., Paraskevaidis E., Terakawa N. Apoptosis in human endometrium and endometriosis. Hum. Reprod. Update. 2004;10:29–38. doi: 10.1093/humupd/dmh007. [DOI] [PubMed] [Google Scholar]
- 12.Gazvani R., Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 13.Van Langendonckt A., Casanas-Roux F., Donnez J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002;77:861–870. doi: 10.1016/S0015-0282(02)02959-X. [DOI] [PubMed] [Google Scholar]
- 14.Ngo C., Chereau C., Nicco C., Weill B., Chapron C., Batteux F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009;175:225–234. doi: 10.2353/ajpath.2009.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajihara H., Yamada Y., Kanayama S., Furukawa N., Noguchi T., Haruta S., Yoshida S., Sado T., Oi H., Kobayashi H. New insights into the pathophysiology of endometriosis: From chronic inflammation to danger signal. Gynecol. Endocrinol. 2011;27:73–79. doi: 10.3109/09513590.2010.507292. [DOI] [PubMed] [Google Scholar]
- 16.Van Langendonckt A., Casanas-Roux F., Donnez J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil. Steril. 2002;78:712–718. doi: 10.1016/S0015-0282(02)03346-0. [DOI] [PubMed] [Google Scholar]
- 17.Van Langendonckt A., Casanas-Roux F., Dolmans M.M., Donnez J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil. Steril. 2002;77:561–570. doi: 10.1016/S0015-0282(01)03211-3. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H., Yamada Y., Kanayama S., Furukawa N., Noguchi T., Haruta S., Yoshida S., Sakata M., Sado T., Oi H. The role of iron in the pathogenesis of endometriosis. Gynecol. Endocrinol. 2009;25:39–52. doi: 10.1080/09513590802366204. [DOI] [PubMed] [Google Scholar]
- 19.Woo J.H., Choi Y.S., Choi J.H. Iron-Storage Protein Ferritin Is Upregulated in Endometriosis and Iron Overload Contributes to a Migratory Phenotype. Biomedicines. 2020;8:454. doi: 10.3390/biomedicines8110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Defrere S., Gonzalez-Ramos R., Lousse J.C., Colette S., Donnez O., Donnez J., Van Langendonckt A. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol. Histopathol. 2011;26:1083–1092. doi: 10.14670/HH-26.1083. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Zeng X., Lu D., Yin M., Shan M., Gao Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2020 doi: 10.1093/humrep/deaa363. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal A., Nick H.S. Renal response to tissue injury: Lessons from heme oxygenase-1 GeneAblation and expression. J. Am. Soc. Nephrol. 2000;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Dulak J., Deshane J., Jozkowicz A., Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keyse S.M., Tyrrell R.M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse D., Choi A.M. Heme oxygenase-1: The “emerging molecule” has arrived. Am. J. Respir. Cell Mol. Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 27.Farombi E.O., Surh Y.J. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J. Biochem. Mol. Biol. 2006;39:479–491. doi: 10.5483/BMBRep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 28.Ono K., Goto Y., Takagi S., Baba S., Tago N., Nonogi H., Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Ono K., Mannami T., Iwai N. Association of a promoter variant of the haeme oxygenase-1 gene with hypertension in women. J. Hypertens. 2003;21:1497–1503. doi: 10.1097/00004872-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Exner M., Minar E., Wagner O., Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Andrews W.C., Buttram V.C., Jr., Weed J.C., Hammond C.B., Thomas H.H. Revised American Fertility Society classification of endometriosis: 1985. Fertil. Steril. 1985;43:351–352. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 32.Skorka A., Bednarczuk T., Bar-Andziak E., Nauman J., Ploski R. Lymphoid tyrosine phosphatase (PTPN22/LYP) variant and Graves’ disease in a Polish population: Association and gene dose-dependent correlation with age of onset. Clin. Endocrinol. 2005;62:679–682. doi: 10.1111/j.1365-2265.2005.02279.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimpara T., Takeda A., Watanabe K., Itoyama Y., Ikawa S., Watanabe M., Arai H., Sasaki H., Higuchi S., Okita N., et al. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum. Genet. 1997;100:145–147. doi: 10.1007/s004390050480. [DOI] [PubMed] [Google Scholar]
- 35.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Excoffier L., Laval G., Schneider S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2007;1:47–50. doi: 10.1177/117693430500100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen D.M., Ehm M.G., Weir B.S. Detecting marker-disease association by testing for Hardy-Weinberg disequilibrium at a marker locus. Am. J. Hum. Genet. 1998;63:1531–1540. doi: 10.1086/302114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balding D.J. A tutorial on statistical methods for population association studies. Nat. Rev. Genet. 2006;7:781–791. doi: 10.1038/nrg1916. [DOI] [PubMed] [Google Scholar]
- 39.Denschlag D., Marculescu R., Unfried G., Hefler L.A., Exner M., Hashemi A., Riener E.K., Keck C., Tempfer C.B., Wagner O. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol. Hum. Reprod. 2004;10:211–214. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- 40.Hirai H., Kubo H., Yamaya M., Nakayama K., Numasaki M., Kobayashi S., Suzuki S., Shibahara S., Sasaki H. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 41.Taha H., Skrzypek K., Guevara I., Nigisch A., Mustafa S., Grochot-Przeczek A., Ferdek P., Was H., Kotlinowski J., Kozakowska M., et al. Role of heme oxygenase-1 in human endothelial cells: Lesson from the promoter allelic variants. Arterioscler. Thromb. Vasc. Biol. 2010;30:1634–1641. doi: 10.1161/ATVBAHA.110.207316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada N., Yamaya M., Okinaga S., Nakayama K., Sekizawa K., Shibahara S., Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y.H., Lin S.J., Lin M.W., Tsai H.L., Kuo S.S., Chen J.W., Charng M.J., Wu T.C., Chen L.C., Ding Y.A., et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum. Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 44.Brouard S., Berberat P.O., Tobiasch E., Seldon M.P., Bach F.H., Soares M.P. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J. Biol. Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 45.Silva G., Cunha A., Gregoire I.P., Seldon M.P., Soares M.P. The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. J. Immunol. 2006;177:1894–1903. doi: 10.4049/jimmunol.177.3.1894. [DOI] [PubMed] [Google Scholar]
- 46.Was H., Cichon T., Smolarczyk R., Rudnicka D., Stopa M., Chevalier C., Leger J.J., Lackowska B., Grochot A., Bojkowska K., et al. Overexpression of heme oxygenase-1 in murine melanoma: Increased proliferation and viability of tumor cells, decreased survival of mice. Am. J. Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee T.S., Chau L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 48.Lee T.S., Tsai H.L., Chau L.Y. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Delta 12,14-prostaglandin J2. J. Biol. Chem. 2003;278:19325–19330. doi: 10.1074/jbc.M300498200. [DOI] [PubMed] [Google Scholar]
- 49.Freitas A., Alves-Filho J.C., Secco D.D., Neto A.F., Ferreira S.H., Barja-Fidalgo C., Cunha F.Q. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br. J. Pharmacol. 2006;149:345–354. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piantadosi C.A., Withers C.M., Bartz R.R., MacGarvey N.C., Fu P., Sweeney T.E., Welty-Wolf K.E., Suliman H.B. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 2011;286:16374–16385. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y., Li P., Lu J., Xiong W., Oger J., Tetzlaff W., Cynader M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:1887–1897. doi: 10.4049/jimmunol.181.3.1887. [DOI] [PubMed] [Google Scholar]
- 52.Wegiel B., Baty C.J., Gallo D., Csizmadia E., Scott J.R., Akhavan A., Chin B.Y., Kaczmarek E., Alam J., Bach F.H., et al. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 2009;284:21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu H., Wang J., Jiang H., Ma Y., Pan S., Reddy S., Sun X. Bilirubin protects grafts against nonspecific inflammation-induced injury in syngeneic intraportal islet transplantation. Exp. Mol. Med. 2010;42:739–748. doi: 10.3858/emm.2010.42.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grochot-Przeczek A., Dulak J., Jozkowicz A. Heme oxygenase-1 in neovascularisation: A diabetic perspective. Thromb. Haemost. 2010;104:424–431. doi: 10.1160/TH09-12-0825. [DOI] [PubMed] [Google Scholar]
- 55.Soares M.P., Seldon M.P., Gregoire I.P., Vassilevskaia T., Berberat P.O., Yu J., Tsui T.Y., Bach F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the paper.