Abstract
This study aims to evaluate potentially appropriate antiplatelet therapy in patients with chronic kidney disease. A systematic analysis was conducted to identify the clinical outcomes of available antiplatelet therapy regimens with enhanced platelet inhibition activity (intervention of 5 regimens) over the standard dose of clopidogrel-based dual antiplatelet therapy in patients with renal insufficiency. An electronic keyword search was performed on Pubmed, Embase, and Cochrane Library per PRISMA guidelines. We performed a prespecified net clinical benefit analysis (a composite of the rates of all-cause or cardiac-related death, myocardial infarction, major adverse cardiac outcomes, and minor and major bleeding), and included 12 studies. The intervention substantially lowered the incidence of all-cause mortality (RR 0.67; p = 0.003), major adverse cardiac outcomes (RR 0.79; p < 0.00001), and myocardial infarction (RR 0.28; p = 0.00007) without major bleeding (RR 1.14; p = 0.33) in patients with renal insufficiency, but no significant differences were noticed with cardiac-related mortality and stent thrombosis. The subgroup analysis revealed substantially elevated bleeding risk in patients with severe renal insufficiency or on hemodialysis (RR 1.68; p = 0.002). Our study confirmed that the intervention considerably enhances clinical outcomes in patients with renal insufficiency, however, a standard dose of clopidogrel-based antiplatelet therapy is favorable in patients with severe renal insufficiency.
Keywords: acute coronary syndrome, antiplatelet, dual antiplatelet therapy, P2Y12 inhibitors, chronic kidney disease, hemodialysis, clopidogrel resistance, high on-treatment of platelet reactivity
1. Introduction
The current guidelines published by the American Heart Association in conjunction with the College of Cardiology (AHA/ACC) and the European Society of Cardiology (ESC) highly recommend dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor in patients with acute coronary syndromes (ACS) to avoid the increased risk of platelet aggregation associated with ACS progression and prognosis [1,2]. Appropriate use of DAPT offers intense platelet inhibition compared to single antiplatelet therapy, subsequently providing secondary prevention of stent thrombosis and new-onset myocardial infarction (MI) and decreased mortality after a percutaneous coronary intervention (PCI) or ACS. However, DAPT may be associated with elevated bleeding risk [2,3]. The optimal DAPT regimen and duration are tailored to patients’ risk indicators of bleeding and clinical presentation during therapy. The guidelines suggest a 12-month DAPT treatment in patients with a low risk of bleeding and one month in patients with a very high risk of bleeding [2]. Active bleeding disorders, a low body weight, old age, concomitant drug use with potential bleeding risk, reduced estimated glomerular filtration rate (eGFR), and poor cytochrome P450 metabolizers may alter the pharmacokinetic and pharmacodynamic profiles of antiplatelets in the DAPT regimen and can influence clinical outcomes of DAPT [4,5]. Nonetheless, precise estimation of bleeding risk is impracticable in patients with multiple comorbidities, especially those with renal insufficiency [6,7].
Chronic kidney disease (CKD), defined as an eGFR < 60 mL/min per 1.73 m2, has several disease-specific features affecting ACS prognosis [8]. Almost 30% of patients diagnosed with ST-elevation myocardial infarction (STEMI) and 40% of patients with non-ST elevation myocardial infarctions (NSTEMI) are diagnosed with CKD. ACS-related mortality is reportedly substantially elevated in relation to renal insufficiency, predisposing patients on dialysis to the most pronounced mortality [5,8,9]. Another concern is that CKD patients are at elevated risk for bleeding regardless of antiplatelet treatment, as indicated by a study that demonstrated a 1.7-fold increased bleeding risk in CKD patients without a history of stroke, MI, or peripheral arterial disease [10]. The 2020 ESC guidelines identified CKD as a risk factor for bleeding after PCI in patients with ACS, and the Academic Research Consortium for High Bleeding Risk (ARC-HBR) classified severe or end-stage CKD (eGFR < 30 mL/min) as a major risk factor for bleeding and moderate CKD (eGFR 30–59 mL/min) as a minor risk factor for bleeding [2,11]. However, dose adjustments based on the degree of renal insufficiency are not required for a DAPT regimen in patients with ACS and CKD considering the elevated thrombotic risks. Moreover, a suitable antiplatelet regimen in these patient populations remains unclear.
The current ESC guideline recommends the use of potent P2Y12 inhibitors, ticagrelor or prasugrel, over clopidogrel, which has response variability associated with genetic mutation and drug interactions. However, the clear recommendation regarding selection of P2Y12 inhibitors in CKD patients including those with severe CKD (eGFR < 15 mL/min) is unavailable [2,9,12,13]. Another interesting aspect of clopidogrel is associated with variable platelet reactivity in relation to the degree of renal insufficiency; platelet reactivity increases with decreased renal function, which subsequently increases the risk of major adverse cardiovascular events (MACEs), ischemic events, and hospitalization in patients with CKD regardless of appropriate standard doses of clopidogrel-based DAPT dose and duration [14]. Recent studies have demonstrated improved clinical outcomes, such as MACE, with other potent oral P2Y12 receptor inhibitors over clopidogrel in ACS patients. However, the risks and benefits remain unclear as these agents significantly increase bleeding, which those with CKD are already susceptible to [10,15,16]. Besides, the efficacy and safety of DAPT with potent P2Y12 inhibitors in patients with renal sufficiency including patients on dialysis who have the most pronounced ACS-related mortality and bleeding risk are not fully determined because of limited numbers of clinical trials in these populations [14,15,16]. As a substantial portion of the population diagnosed with ACS has CKD, and these patients may have thrombotic events and an elevated risk for bleeding, appropriate antiplatelet treatment cannot be neglected in this population. Therefore, the aim of this study is to investigate potentially appropriate antiplatelet therapy in patients with CKD including those on dialysis by evaluating the safety and efficacy of different antiplatelet therapy regimens with enhanced antiplatelet activity to overcome clopidogrel response variability [13], over the standard clopidogrel-based DAPT regimen.
2. Materials and Methods
2.1. Data Sources and Search Strategy
We searched PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL), without year and language restrictions. The initial database search involved a combination of keywords and Medical Subject Headings (MeSH) of ‘renal,’ ‘renal dialysis,’ ‘renal replacement therapy,’ ‘chronic renal disease,’ ‘chronic kidney failure,’ ‘renal insufficiency,’ ‘clopidogrel,’ ‘prasugrel,’ ‘ticagrelor,’ ‘P2Y12 inhibitor,’ ‘cilostazol,’ and ‘antiplatelet’ in the title/abstract. Search filters were set as ‘clinical trials,’ ‘randomized controlled trials,’ and ‘humans’. The last search was updated in October of 2020. We also manually searched the references of eligible review articles to identify additional research for analysis. Trials were potentially eligible regardless of the study phase, dose schedule, and study region. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines regarding the search strategy and selection process [17].
2.2. Study Selection and Data Extraction
Two reviewers (Park and Choi) independently screened the titles and abstracts of all studies that were identified from the literature search to verify eligibility. Disagreements related to the study selection were further discussed until a consensus was reached. Any disagreements not meeting consensus were resolved by a third researcher.
From each eligible clinical trial, we extracted the study and article characteristics (including the name of the first author, year of publication, study design, study period, and study region), study population (including the number of randomly assigned patients, the types of concomitant cardiovascular disease, and the status of renal impairment), study intervention (including comparator, drug names and dosage, schedule, and intervention duration), outcomes related to efficacy (including mortality, MACE, MI, and stent thrombosis) and safety (including major and minor bleeding), and intermediate measurements of platelet function (including inhibition of platelet aggregation (IPA) and platelet reactivity unit (PRU)). Duplicated studies, review articles, commentaries, editorials, conference abstracts, case reports, and protocols were excluded. The PICOS (Patients, Intervention, Comparator, Outcomes, and Study design) summary of our study is shown in Table 1 [18].
Table 1.
PICOS of this study.
| Component | Definition |
|---|---|
| P (patients) | Patients in ACS with CKD, including HD |
| I (intervention) | DAPT regimen with enhanced antiplatelet activity (or reduced platelet reactivity) to overcome response variability of clopidogrel and is composed with [13]:
|
| C (comparator) | Standard dose of clopidogrel-based DAPT |
| O (outcomes) | Efficacy outcomes: all-cause or cardiac-related mortality, MACE, MI, stent thrombosis; safety outcomes: major or minor bleeding; intermediate outcomes: IPA and PRU |
| S (study design) | RCTs, observational studies, and prospective studies |
Abbreviations. ACS: acute coronary syndrome, CKD: chronic kidney disease, DAPT: dual antiplatelet therapy, HD: hemodialysis, IPA: inhibition of platelet aggregation, LD: loading dose, MD: maintenance dose, PRU: platelet reactivity unit, RCT: randomized clinical trial.
2.3. End Points
The efficacy outcomes of interest were all-cause mortality, cardiac-related mortality, MACE, MI, and stent thrombosis. The safety outcomes were major and minor bleeding events and the intermediate outcomes were IPA and PRU.
2.4. Assessment of Bias Risk and Evidence
Two independent reviewers (Park and Choi) assessed the methodological quality of randomized controlled trials (RCTs) based on Cochrane’s Risk of Bias (RoB) [19]. The studies were scored as low, unclear, or high in the following domains: randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential bias. The Risk of Bias Assessment Tool for Nonrandomized Study (ROBINS-I) was adapted to evaluate the study quality of non-RCTs regarding the selection of participants, confounding variables, measurement interventions, blinding for assessment, incomplete outcome data, and selective outcome reporting [20,21]. Any disagreements on the risk of bias and quality of evidence were resolved by a third reviewer (Rhie). Egger’s test and funnel plots were utilized to detect possible publication bias; a p-value >0.05 and a symmetric funnel plot imply a low risk of publication bias.
2.5. Statistical Methods
Dichotomous data for each eligible study, including all-cause mortality, cardiac-related mortality, MACE, MI, stent thrombosis, and major or minor bleeding events, were analyzed with relative risks (RR) and 95% confidence intervals (CI) to estimate the risks in patients with ACS receiving various interventions (doubled loading dose (LD) of clopidogrel-based DAPT, doubled maintenance dose (MD) of clopidogrel-based DAPT, prasugrel-based DAPT, ticagrelor-based DAPTs, and triple antiplatelet therapy with cilostazol) versus a control (standard dose of clopidogrel-based DAPT). We reversely calculated the number of patients in the study when the results were reported as a percentage (%) in the original work.
Heterogeneity across the included studies was first evaluated by Cochran’s Q test (considered significant for p < 0.10) [22] and quantified I2 index [23]. The study outcomes with high heterogeneity (I2 > 50%) were analyzed by a random-effect model (Mantel-Haenszel), while those with low heterogeneity (I2 < 50%) were evaluated by a fixed-effect model.
Subgroup analysis was performed to explore the high heterogeneities observed in the bleeding analysis. After the overall bleeding analysis was conducted to compare the interventions and control in patients with CKD, including those on hemodialysis, the risk of major and minor bleeding according to the degree of renal insufficiency was further analyzed via a comparative analysis on bleeding risks between patients with CKD of moderate renal insufficiency (eGFR < 60 mL/min) and those with severe CKD (eGFR < 30 mL/min) or on hemodialysis. p-values were estimated by two-sided tests. Any p-values less than 0.05 were considered statistically significant. The outcomes of interest were evaluated by pooling raw data from individual clinical trials. All statistical analyses were performed using Review Manager (RevMan) Version 5. 4 (The Cochrane Collaboration, 2020) [24].
3. Results
3.1. Study Search and Selection
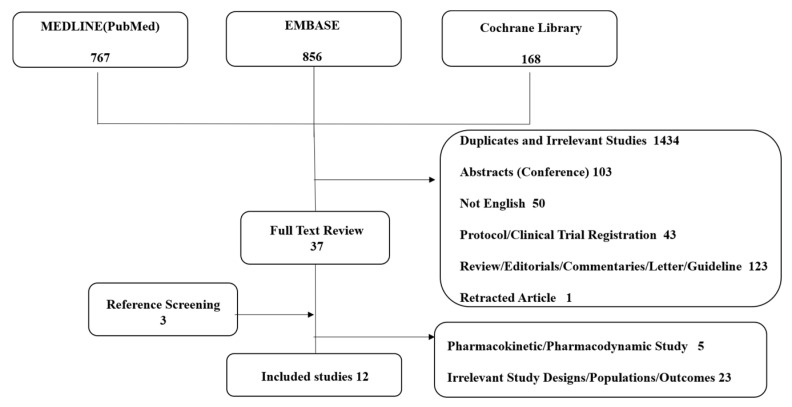
The primary literature search from MEDLINE, Embase, and Cochrane yielded 1,791 articles (Figure 1). A total of 37 articles were eligible for full-text review after excluding duplicated or irrelevant studies (1434), abstracts and conference posters (103), study protocols or clinical trial registrations (43), reviews, editorials, commentaries, letters, and guidelines (123), retracted articles (1), and studies not written in English (50). A manual reference screening identified three articles eligible for full-text review. After excluding irrelevant study designs and outcomes (28), a total of 12 clinical trials [5,25,26,27,28,29,30,31,32,33,34,35] were included in this study, including 33,658 patients with CKD on antiplatelet therapy.
Figure 1.
Study selection diagram (Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)).
3.2. Study Characteristics
Table 2 provides details on the characteristics of included studies. All patients had ACS with CKD, including those on hemodialysis. This study included six randomized trials [5,30,32,33,34,35], two post hoc analyses from previous clinical trials [28,31], and four observational studies [25,26,27,29]. The study regions included China [30], Japan [31], Korea [26,29,32,33,34,35], Sweden [27], and the United States [25], along with two multinational studies [5,28]. Eight studies [5,25,26,27,28,29,30,31] recruited ACS patients with creatinine clearance (CrCl) or eGFR less than 60 mL/min, and four studies [32,33,34,35] recruited patients on hemodialysis. In the original studies, the patients’ characteristics (such as age, ACS status, and level of renal impairment) were well balanced between the arms.
Table 2.
Study characteristics.
| Author Name | Study Region | Study Design | Patient Population | Intervention | Comparator (Control) | Duration | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| CKD Populations (eGFR < 60 mL/min) | ||||||||
| Barber et al. (2017) [25] | U.S. | Multicenter, observational study | ACS patients with CKD undergoing PCI (n = 5613) | Prasugrel + ASA (n = 617) |
Clopidogrel +ASA (n = 4996) |
1 year | No significant difference in MACE, death, and MI | No significant difference in bleeding |
| Choi et al. (2012) [26] | Korea | Prospective, multicenter, online registry of Korea (KAMIR) | AMI patients with renal dysfunction (n = 2288) | Triple therapy (ASA+clopidogrel+cilostazol) (n = 701) |
Clopidogrel +ASA (n = 1587) |
Not available | Significantly lower rates of in-hospital death (6.7% vs. 11.3%, p = 0.001) and 1-month MACE (11.1% vs. 16.3% p = 0.002) in triple therapy group but no difference in 12-month MACE | No significant difference in bleeding, in-hospital major bleeding (p = 0.870) |
| Edfors et al. (2018) [27] | Sweden | Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry study | NSTEMI and STE15MI patients discharged with DAPT and eGFR less than 60 mL/min (n = 11,538) | Ticagrelor 180 mg LD followed by 90 mg BID +ASA (n = 2196) |
Clopidogrel + ASA (n = 9342) |
12 months | Ticagrelor as compared with clopidogrel was associated with a lower 1-year risk of the composite outcome (eGFR 30–60: 0.82 (0.70 to 0.97), eGFR < 30: 0.95 (0.69 to 1.29), p = 0.55)across the eGFR strata. | Ticagrelor as compared with clopidogrel was associated with a higher risk of bleeding (eGFR 30–60: 1.13 (0.84 to 1.51), eGFR < 30: 1.79 (1.00 to 3.21), p for interaction = 0.30) across the eGFR strata. |
| James et al. (2010) [28] | Multinational | Post hoc analysis of a multicenter, randomized, double-blind trial | ACS patients with chronic kidney disease (n = 3237) | Ticagrelor 180 mg LD followed by 90 mg BID+ Aspirin (75–100 mg) | Clopidogrel 300 mg LD followed by 75 mg daily + Aspirin (75–100 mg) |
12 months | Significantly reduced primary end points (CV death, MI, stroke) of HR 0.77 [0.65–0.9], p = 0.03 and mortality of HR 0.72 [0.58–0.89], p = 0.02 in ticagrelor | No significant difference in major bleeding rates (HR: 1.07 [0.88–1.30]), fatal bleedings (HR: 0.48 [0.15–1.54]), and non-CABG-related major bleedings (HR:1.29 [0.97–1.68]) |
| Kim et al. (2012) [29] | Korea | Prospective, open, observational, multicenter on-line registry of Korea (KAMIR) | STEMI patients undergoing PCI with CKD within 24 h of onset (n = 1457) | Clopidogrel 600 mg LD+75 mg MD + ASA 100mg | Clopidogrel 300mg LD+ 75mg MD + ASA 100mg | Not available | No difference in MACE at 1 month (15.6 vs. 16.4%, p = 0.700) and 12 months (19.0% vs. 21.3%, p = 0.32) | In-hospital major bleeding rate was similar (0.8% vs. 0.2%, p = 0.09). |
| Liang et al. (2015) [30] | China | Prospective, randomized, open-label, parallel-group, single-center study | CAD patients with CKD undergoing PCI with DES (n = 370) | Clopidogrel 300 mg LD followed by 150 mg daily + ASA 100 mg daily (n = 186) | Clopidogrel 300 mg LD followed by 75 mg daily + ASA 100mg daily (n = 184) | 30 days | Significantly lower rates of stent thrombosis (1.1% vs. 4.9%, p = 0.03) and MACE (2.7% vs. 7.6%, p = 0.03) in patients who received 150 mg | No significant difference in major(1.6% vs. 1.1%, p = 1.00) or minor (5.4% vs. 2.2%, p = 0.11) bleeding |
| Melloni et al. (2015) [5] | Multinational | Phase 3, randomized, double-blinded, double-dummy, active-control study | ACS patients enrolled in TRIOLOGY-ACS study (n = 8953) | Prasugrel 30 mg LD followed by 10 mg daily (5 mg daily for patients older than 75 years or if < 60 kg +ASA | Clopidogrel 300 mg LD followed by 75 mg daily+ ASA | 30 months | Substantially reduced PRU in prasugrel in all three CKD stages, Difference in PRU between (prasugrel-clopidogrel): severe CKD: −81.8 [−130.6, −33.1] moderate CKD: −70.8 [−84.8, −56.8) normal/mild CKD −101.4 [−110.1, −92.7] | Not available |
| Nishi et al. (2017) [31] | Japan | Post hoc analysis of a single-center, prospective, crossover study | Japanese patients undergoing PCI (n = 53 total, n = 15 for CKD and n = 38 for non-CKD) | Prasugrel 3.75 mg daily + ASA 100 mg | Clopidogrel 300 mg LD followed by 75 mg daily+ ASA 100 mg | 28 days (crossover at day 14) | Significantly lower PRU in prasugrel treated patients (165.3 ± 61.8 vs. 224.3 ± 57.0, p = 0.002) | Not available |
| Patients on HD | ||||||||
| Jeong et al. (2015) [32] | Korea | Single-center, prospective, randomized, crossover study | Patients with kidney failure with HTPR on HD (n = 25) | Ticagrelor 180 mg LD followed by 90 mg BID +ASA 100 mg | Clopidogrel 300 mg LD followed by 75 mg daily + ASA 100 mg | 10 weeks | More rapid and greater platelet inhibition in ticagrelor treated group (p < 0.05) | Two clinically relevant cases of minor bleeding in ticagrelor-treated group (1 arteriovenousfistula bleeding and 1 oral bleeding) |
| Kim et al. (2017) [33] | Korea | Prospective, randomized, single-center study | ESRD patients on regular HD (n = 52) | (1) Ticagrelor 90 mg LD followed by 90 mg daily +ASA (n = 13) OR (2) Ticagrelor 180 mg LD followed by 90 mg BID+ ASA(n = 21)/ | Clopidogrel 300 mg LD followed by 75 mg daily +ASA(n = 18) | 14 days | Significant difference in IPA in low ticagrelor group compared to clopidogrel treated group. Standard ticagrelor group showed the highest IPA (ANCOVA < 0.001) | No bleeding events in low-dose ticagrelor BARC Type I (gum bleeding) events: clopidogrel (5.9%) standard ticagrelor (5.6%) BARC Type 2 events (arteriovenous fistula bleeding) standard ticagrelor (5.6%) |
| Park et al. (2009) [34] | Korea | Prospective, randomized, open-label single-center study | Patients with CRF (75% patients on HD) (n = 36) | Clopidogrel 600 mg LD followed by 150 mg daily +ASA 100 mg | Clopidogrel 300 mg LD followed by 75 mg daily + ASA 100 mg | 4 weeks/30 days | No significant difference in PRU (302 ± 81 vs. 308 ± 70, p = 0.824) and mean percentage of inhibition (23.4 ± 14.4 vs. 21.3 ± 16.0, p = 0.808) | Gastrointestinal ulcer bleeding in 1 patient who received clopidogrel at 150 mg |
| Woo et al. (2011) [35] | Korea | Prospective, open, randomized platelet function study | CKD patients undergoing HD who received PCI (n = 74) | (1) Clopidogrel 150 mg/day +ASA 100 mg (n = 25) (2) Triple therapy (clopidogrel 75 mg + cilostazol+ ASA 100 mg) (n = 25) | Clopidogrel 75 mg + ASA 100 mg (n = 24) | 14 days | The rate of high on-treatment platelet activity was significantly lower in triple therapy (10% vs. 43% vs. 32% p < 0.05) | Gastrointestinal ulcer bleeding in 1 patient who received clopidogrel at 150 mg |
By reviewing the original studies, five different antiplatelet therapies were found to assess the efficacy and safety of this treatment. One was doubled LD clopidogrel-based DAPT [29], three were doubled MD clopidogrel-based DAPT [30,34,35], three were prasugrel-based DAPT [5,25,31], four were ticagrelor-based DAPT [27,28,32,33], and two were triple therapy (a standard dose of clopidogrel-based DAPT with cilostazol, and one was doubled MD clopidogrel-based DAPT) [26,35]. All 12 trials had a control of a standard dose of clopidogrel-based DAPT. Two trials [31,33] demonstrated efficacy with a reduced dosage considering the decreased renal function of one with prasugrel [31] and one with ticagrelor [33].
The intervention outcomes were expressed either in efficacy outcomes, such as mortality, MACE, MI, and stent thrombosis [25,26,27,28,29,30], or intermediate outcomes, such as platelet reactivity [5,31,32,33,34,35]. The safety outcomes were evaluated by the incidence of major and minor bleeding events [25,26,27,28,29,30,32,33,34,35]. The original studies assessing the interventional efficacy in patients on hemodialysis showed intermediate and safety outcomes [32,33,34,35]. The quality assessments of each study are described in Supplementary Tables S1 and S2. The risk of bias was generally acceptable as inferred by Egger’s test (p > 0.05) and the symmetric funnel plots (Supplementary Figure S1).
3.3. Outcomes in Patients with CKD
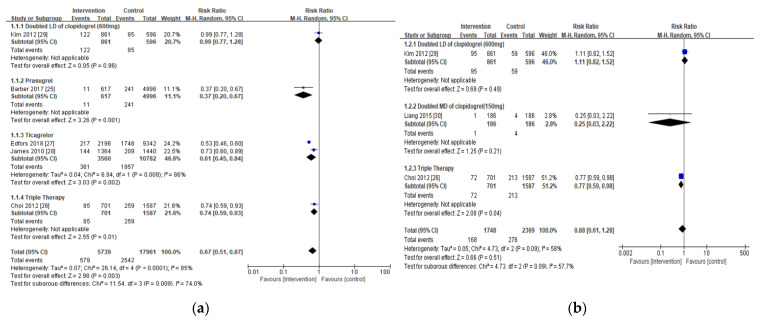
Compared with the standard dose of clopidogrel-based DAPT (control), the intervention substantially lowered the risk of all-cause mortality in patients with CKD and ACS (RR 0.67; 95% CI 0.51, 0.87; p = 0.003), but no significant difference was noted for cardiac-related death (RR 0.88; 95% CI 0.61, 1.28, p = 0.51) (Figure 2a,b).
Figure 2.
Forest plots of antiplatelet therapy effects on mortality in chronic kidney disease CKD patients: (a) all-cause mortality; (b) cardiac-related mortality. Blue indicates the risk ratio evaluated from each study and black indicates the overall risk ratio of the antiplatelet regimen.
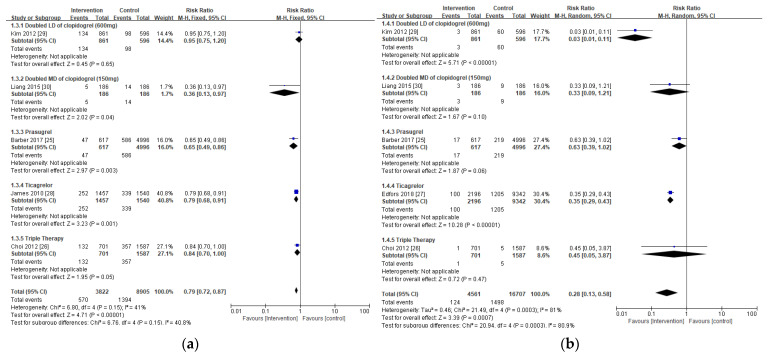
Furthermore, the intervention considerably decreased the risk of MACE (RR 0.79; 95% CI 0.72, 0.87; p < 0.00001, Figure 3a) and MI (RR 0.28; 95% CI 0.13, 0.58; p = 0.00007, Figure 3b) in subjects with CKD compared to the controls without increased risk for major bleeding (RR 1.14; 95% CI 0.87, 1.50; p = 0.33, Figure 3d). There was no significant difference in the risk of stent thrombosis between the intervention and the control (RR 0.71; 95% CI 0.38, 1.32; p = 0.28, Figure 3c).
Figure 3.
Forest plots of clinical outcomes in CKD patients from antiplatelet therapy: (a) major adverse cardiovascular events (MACE); (b) myocardial infarction (MI); (c) stent thrombosis; and (d) major bleeding risks. Blue indicates the risk ratio evaluated from each study and black indicates the overall risk ratio of the antiplatelet regimen.
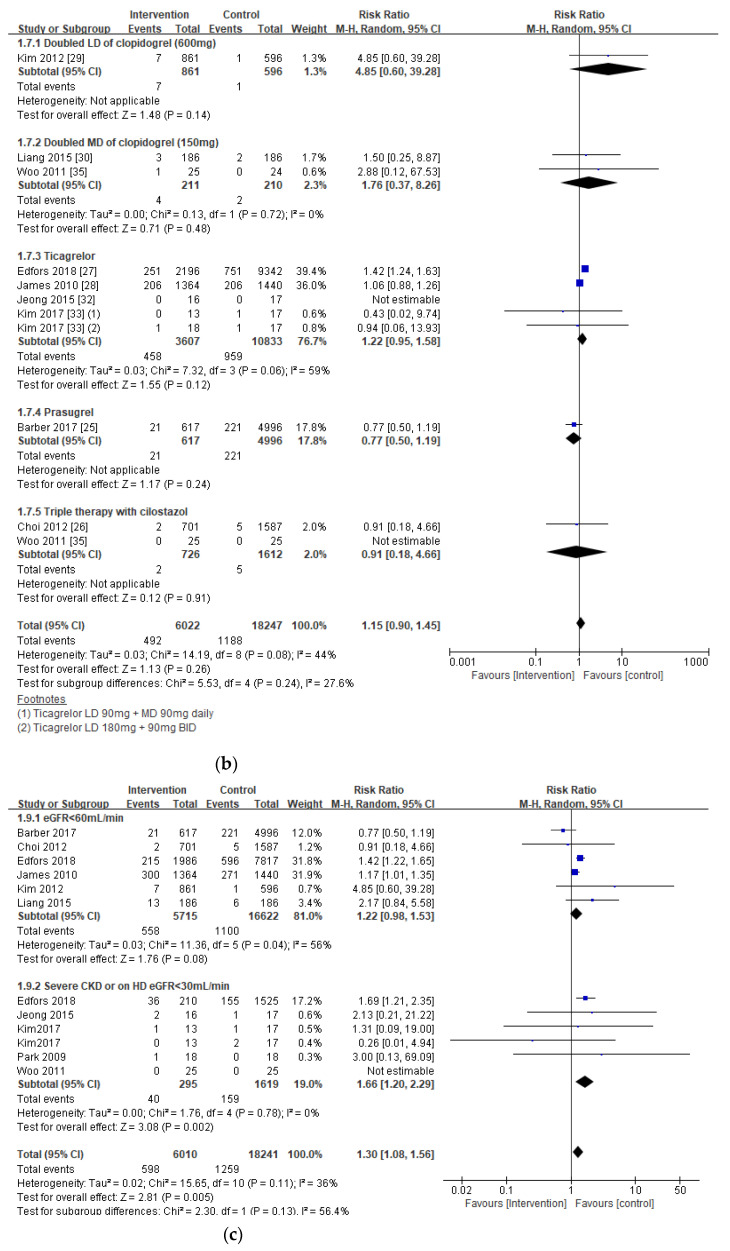
3.4. Safety Outcomes in Patients with CKD, Including Those on Hemodialysis
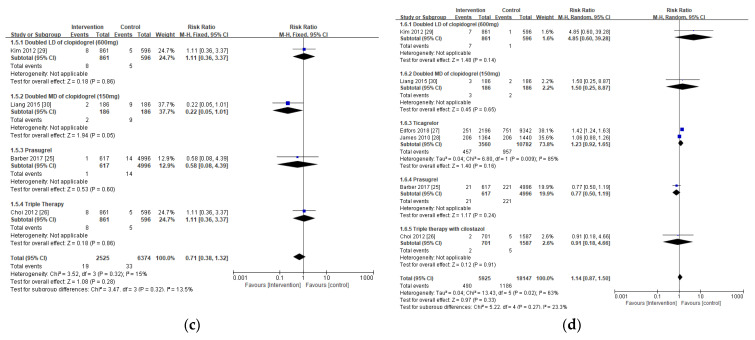
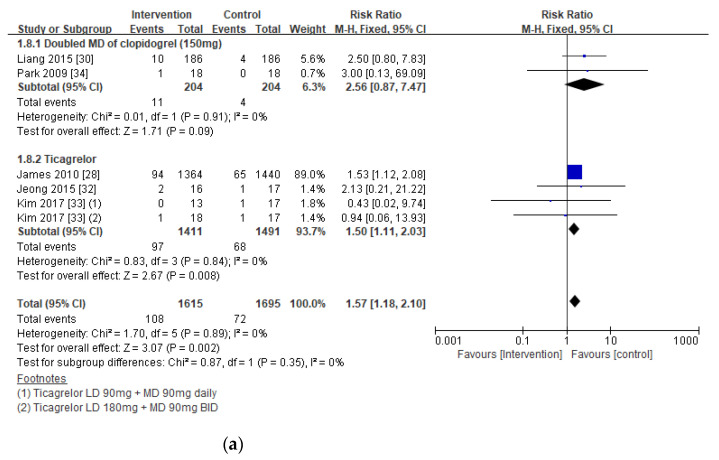
The risk of major bleeding events in patients with CKD, including those on hemodialysis, was similar between the intervention and the control groups (RR 1.15; 95% CI 0.90, 1.45; p = 0.26), but the risk of minor bleeding events was substantially higher in the intervention group (RR 1.57; 95% CI 1.18, 2.10; p = 0.002) (Figure 4a,b).
Figure 4.
Forest plots of bleeding risks associated with antiplatelet therapy in all CKD patients, including those on hemodialysis: (a) major bleeding; (b) minor bleeding; and (c) subgroup analysis of major and minor bleeding between estimated glomerular filtration rate (eGFR) < 60 mL/min and eGFR < 30 mL/min or hemodialysis (HD). Blue indicates the risk ratio evaluated from each study and black indicates the overall risk ratio of the antiplatelet regimen.
Subgroup Analysis in Bleeding Risks Associated with the Extent of Renal Insufficiency
A subgroup analysis revealed that the risk of both major and minor bleeding was substantially elevated with the intervention in patients with severe CKD (eGFR < 30 mL/min) or on hemodialysis (RR 1.30; 95% CI 1.09, 1.55; p = 0.002), but not in CKD patients with moderate renal insufficiency eGFR (RR 1.22; 95% CI 0.98, 1.53; p = 0.08) (Figure 4c).
3.5. Intermediate Outcomes in Patients with CKD
The intermediate outcome, expressed as a measurement of platelet function, was assessed in six studies [5,31,32,33,34,35], two [5,31] in patients with CKD and four [32,33,34,35] in patients on hemodialysis (Table 2). Platelet activity was significantly reduced with DAPT with prasugrel or ticagrelor and triple therapy, even with a reduced dose of prasugrel and ticagrelor. Nevertheless, doubled clopidogrel LD or MD failed to decrease platelet reactivity.
4. Discussion
In this study, we investigated the clinical efficacy and safety of different antiplatelet therapy regimens with enhanced platelet-inhibition activity over a standard dose of clopidogrel-based DAPT in subjects with CKD. We found that the interventions, antiplatlet therapy regimens with enhanced antiplatelet activity over standard clopdigorel-based DAPT, substantially improved clinical outcomes, including all-cause mortality (RR 0.67, p = 0.003), MACE (RR 0.79, p < 0.00001), and MI (RR 0.28, p = 0.0007) without major bleeding (RR 1.14, p = 0.33) in patients with ACS and CKD. Also, the risk of bleeding was substantially greater in patients with severe CKD (eGFR < 30 mL/min) than those with moderate renal insufficiency. Platelet activity is significantly inhibited with prasugrel, ticagrelor, and triple therapy, but not with double-dose clopidogrel in patients with CKD, including those on hemodialysis.
The current guidelines highly recommend DAPT as a part of ACS management to improve patient outcomes and prognosis, as platelet aggregation plays a pivotal role in ACS progression. However, the appropriate antiplatelet therapy regimen in those with CKD remains ambiguous [1,2]. According to an observational study, the majority of people with CKD are discharged with a standard dose of clopidogrel-based regimen with LD of 300 mg and MD of 75 mg in addition to low-dose aspirin. The percentage of patients discharged on this regimen increases according to the degree of renal dysfunction [16]. However, people with CKD have complex hemostatic properties that may hinder the clinical benefits of ACS management. This is implied by an elevated ischemic risk despite proper antiplatelet therapy and consequent increasing mortality [5,8].
Previous studies have reported a 6–12% intraindividual variability of the clopidogrel response secondary to multiple factors, including genetic polymorphism of cytochrome (CYP) 2C19, as clopidogrel is mainly metabolized into its active ingredient by CYP 2C19. [36,37,38]. However, the underlying mechanism of clopidogrel-response variability in CKD patients including those on dialysis remains unclear, as the degree of reduced clopidogrel response increases with renal insufficiency, and studies report patients on dialysis have poor responses to clopidogrel regardless of having the 2C19*2 alleles, the loss-of-function genotype [9,14]. Considering the marked increased risk of ischemic events and mortality in patients with CKD, and the fact that a substantial number of the ACS populations has CKD as an underlying comorbidity, the clinical outcomes of antiplatelet therapy should be clarified to determine a suitable DAPT regimen for these populations [8].
We evaluated the efficacy of intervention composed of five different antiplatelet regimens with enhanced platelet inhibition activity to overcome clopidogrel-response variability in CKD patients: doubled LD of clopidogrel, doubled MD of clopidogrel, prasugrel, ticagrelor-based DAPT, and triple antiplatelet therapy with cilostazol. Although the intervention provided considerable clinical benefits over a standard dose of clopidogrel-based DAPT, each intervention must be assessed before clinical application in CKD patients. The clopidogrel dose has been doubled in patients with diminished clopidogrel response, but our results indicate that the clinical benefits of this are relatively insignificant. Furthermore, the clinical benefits of triple therapy with cilostazol, a phosphodiesterase III inhibitor, must also be clarified because mortality was the only improved clinical outcome, despite the addition of medication. Similar to the result of previous studies, this study revealed that replacing clopidogrel with a potent oral P2Y12 inhibitor such as prasugrel or ticagrelor provides beneficial clinical outcomes in CKD patients, as implied by a substantially lowered all-cause mortality risk, MACE, and MI without elevated bleeding risks [15].
We also found that the interventions did not increase the risk of major bleeding in those with CKD despite enhanced antiplatelet activity, but the risk of bleeding was markedly increased in patients with CKD and renal function severely decreased, including those on hemodialysis. Our analysis indicated that renal insufficiency may play a crucial role in increasing bleeding risks. An observational study revealed the most substantial incidence of bleeding events occur in those with stage 4 CKD [16]. Additionally, a large proportion of stage 4 CKD patients discharged with potent P2Y12 inhibitors switched from a DAPT regimen to a clopidogrel-based regimen secondary to bleeding events. Considering the risks and benefits of antiplatelet use, a standard dose of clopidogrel-based DAPT can be preferred in those with severe CKD, including those on dialysis. Further research needs to elucidate a clear mechanism of bleeding associated with renal dysfunction and an approach to attenuate bleeding risks from potent P2Y12 inhibitors.
Recently, a real-world observational study comparing clinical efficacy and bleeding risk between clopidogrel and ticagrelor in patients with a risk of high bleeding, including those with renal insufficiency, demonstrated substantially reduced risk of net adverse clinical endpoints (NACE), composites of all-cause death, MI, stroke, and major bleeding events at one year, and MACE in patients treated with ticagrelor when compared to those with clopidogrel [39]. Nonetheless, we cannot affirm that ticagrelor is a drug of choice over other regimens in patients with mild to moderate renal insufficiency at this point because the authors suggested DAPT duration is associated with the risk of ischemic and bleeding events at one year after MI based on the results of no difference in the risk of NACE and MACE between ticagrelor and clopidogrel treated patients after adjusting factors increasing bleeding risk such as age, PCI, smoking status, creatinine clearance, previous MI and number of high-risk bleeding criteria [39]. Another study, however, demonstrated controversial results; prolonged use of low dose ticagrelor (60 mg twice daily) after 12 months of DAPT provided long-term efficacy without major bleeding events [40]. Summing up, DAPT with potent P2Y12 inhibitors may be a plausible treatment option for CKD patients, but further controlled studies on the impact of duration and type of DAPT, along with disease-related factors on ischemic and bleeding outcomes, in CKD patients are warranted. This is because CKD patients possess multiple underlying factors that may subsequently affect hemodynamics and ischemic/bleeding risk, and the clear mechanism of these effects are yet to be determined.
This is the first meta-analysis investigating five different and potentially appropriate antiplatelet therapy regimens with enhanced antiplatelet activity over standard clopidogrel-based DAPT in patients with CKD. We assessed the clinical safety of different antiplatelet therapy regimens in patients on hemodialysis. Based on our study results, patients with moderate renal insufficiency may clinically benefit from the antiplatelet therapy regimen with enhanced antiplatelet activity (intervention) without bleeding risks. However, uncertainty remains regarding the use of alternative antiplatelet therapy regimens in patients on hemodialysis secondary to the most significant bleeding risk and unavailable clinical outcomes, such as mortality and MACE. Although the current guidelines do not recommend adjusting the dose of P2Y12 inhibitors, dosage adjustment should be considered in these patient populations based on the results which displayed significantly lowered platelet function with low doses of prasugrel and ticagrelor when compared to a standard and doubled clopidogrel dose [1,2,31,33,41]. Additionally, a PEGASUS-TIMI trial has revealed that ticagrelor treatment of 60 mg twice daily in patients with high-risk features such as old age, diabetes mellitus requiring medication, or renal insufficiency (eGFR < 60 mL/min) provided similar cardiovascular protection with better safety profiles when compared to ticagrelor 90 mg twice daily [42]. Nonetheless, treatment with ticagrelor 60 mg is recommended in patients with extended DAPT beyond 12 months, implying validated clinical efficacy of low-dose ticagrelor-based DAPT for ACS management in CKD is limited [40]. Hence, cautious selection of antiplatelet therapy regimens with specific monitoring plans are encouraged, and further studies assessing clinical outcomes with reduced doses of prasugrel or ticagrelor should be considered in CKD patients.
This study has some limitations. The included studies had different study designs and outcome measurements, which may hinder their clinical application. Moreover, fewer patients with CKD received the intervention than a standard dose of clopidogrel-based DAPT. Also, a small number of studies evaluated each intervention, which may attenuate the validity of these study results. These issues may be justified because CKD patients are a vulnerable population that is under-represented in RCTs. This accentuates the importance of this study as it provides insight on potential antiplatelet therapies in clopidogrel-resistant patients with CKD who have been diagnosed with ACS. As CKD patients are at considerable risk for ischemic and bleeding events, further research that determines personalized factors associated with the beneficial clinical outcomes of antiplatelet therapy is needed. This will help provide appropriate antiplatelet therapy in clopidogrel-resistant CKD patients without increasing bleeding events.
5. Conclusions
In conclusion, the interventions composed of five different antiplatelet activities with enhanced antiplatelet activities over standard clopidogrel-based DAPT considerably enhanced clinical outcomes regarding all-cause mortality, MACE, and MI without bleeding risks in CKD patients diagnosed with ACS. This suggests that the interventions are plausible treatment alternatives in CKD patients who may have reduced clopidogrel response. However, standard doses of clopidogrel-based DAPT is recommended in those with severe CKD, including those on hemodialysis, based on the studies available with unclear clinical outcomes and increased bleeding risk. Further studies investigating personalized factors associated with improved clinical outcomes are warranted to implement the most appropriate antiplatelet therapy in this population.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4426/11/3/222/s1, Figure S1: Funnel plots of (a) all-cause mortality; and (b) major bleeding, Table S1: Quality assessment of randomized controlled trials, Table S2: Quality assessment of non-randomized controlled trials.
Author Contributions
Conceptualization, S.J.R. and S.P.; methodology, S.J.R., S.P., and Y.J.C.; software, S.P., Y.J.C., M.G.K., and S.D.K.; validation, Y.J.C., J.E.K., and M.G.K.; formal analysis, S.P., Y.J.C., and J.E.K.; investigation, S.P., Y.J.C., and S.J.R.; resources, S.P.; data curation, S.P., Y.J.C., and J.E.K.; writing—original draft preparation, S.P., and Y.J.C.; writing—review and editing, J.E.K., M.G.K., M.J.G., S.D.K., and S.J.R.; visualization, S.P., and Y.J.C.; supervision, S.J.R.; project administration, S.J.R.; funding acquisition, S.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea Government Ministry of Science and ICT (2020R1A2C1009224) and the Ministry of Education (2020R1A6A1A03043528).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levine G.N., Bates E.R., Bittl J.A., Brindis R.G., Fihn S.D., Fleisher L.A., Granger C.B., Lange R.A., Mack M.J., Mauri L., et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 2.Collet J.P., Thiele H., Barbato E., Barthelemy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020:ehaa575. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 3.Becker R.C., Bassand J.P., Budaj A., Wojdyla D.M., James S.K., Cornel J.H., French J., Held C., Horrow J., Husted S., et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur. Heart J. 2011;32:2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- 4.Angiolillo D.J. Variability in responsiveness to oral antiplatelet therapy. Am. J. Cardiol. 2009;103(Suppl. 3):27a–34a. doi: 10.1016/j.amjcard.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Melloni C., Cornel J.H., Hafley G., Neely M.L., Clemmensen P., Zamoryakhin D., Prabhakaran D., White H.D., Fox K.A., Ohman E.M., et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: Insights from the TRILOGY ACS Trial. Eur. Heart J. Acute Cardiovasc. Care. 2016;5:443–454. doi: 10.1177/2048872615598631. [DOI] [PubMed] [Google Scholar]
- 6.Wiviott S., Braunwald E., McCabe C., McCabe B., Montalescot G., Ruzyllo W., Gottlieb S., Neumann F., Ardissino D., De Servi S., et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 7.Wallentin L., Becker R.C., Budaj A., Cannon C.P., Emanuelsson H., Held C., Horrow J., Husted S., James S., Katus H., et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 8.Marenzi G., Cabiati A., Assanelli E. Chronic kidney disease in acute coronary syndromes. World J. Nephrol. 2012;1:134–145. doi: 10.5527/wjn.v1.i5.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi A., Shinke T., Otake H., Takaya T., Osue T., Kinutani H., Kuroda M., Takahashi H., Terashita D., Hirata K. Impact of residual platelet reactivity under clopidogrel treatment for lesions and the clinical outcome after drug-eluting stent implantation in patients with hemodialysis. J. Cardiol. 2016;67:531–537. doi: 10.1016/j.jjcc.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Ocak G., Rookmaaker M.B., Algra A., de Borst G.J., Doevendans P.A., Kappelle L.J., Verhaar M.C., Visseren F.L., Group S.S. Chronic kidney disease and bleeding risk in patients at high cardiovascular risk: A cohort study. J. Thromb. Haemost. 2018;16:65–73. doi: 10.1111/jth.13904. [DOI] [PubMed] [Google Scholar]
- 11.Urban P., Mehran R., Colleran R., Angiolillo D.J., Byrne R.A., Capodanno D., Cuisset T., Cutlip D., Eerdmans P., Eikelboom J., et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation. 2019;140:240–261. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreiro J.L., Angiolillo D.J. Clopidogrel response variability: Current status and future directions. Thromb. Haemost. 2009;102:7–148. doi: 10.1160/TH09-03-0185. [DOI] [PubMed] [Google Scholar]
- 13.Park K.W., Kim H. Options to overcome clopidogrel response variability. Circ. J. 2012;76:287–292. doi: 10.1253/circj.CJ-11-1494. [DOI] [PubMed] [Google Scholar]
- 14.Muller C., Caillard S., Jesel L., Ghannudi S.E., Ohlmann P., Sauleau E., Hannedouche T., Gachet C., Moulin B., Morel O. Association of estimated GFR with platelet inhibition in patients treated with clopidogrel. Am. J. Kidney Dis. 2012;59:777–785. doi: 10.1053/j.ajkd.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Bonello L., Laine M., Lemesle G., Puymirat E., Darby T., Thuny F., Paganelli F., Aradi D., Frere C., Burtey S., et al. Meta-analysis of potent P2Y12-ADP receptor antagonist therapy compared to clopidogrel therapy in acute coronary syndrome patients with chronic kidney disease. Thromb. Haemost. 2018;118:1839–1846. doi: 10.1055/s-0038-1669426. [DOI] [PubMed] [Google Scholar]
- 16.Rymer J.A., Kaltenbach L.A., Doll J.A., Messenger J.C., Peterson E.D., Wang T.Y. Safety of Dual-Antiplatelet Therapy After Myocardial Infarction Among Patients With Chronic Kidney Disease. J. Am. Heart Assoc. 2019;8:e012236. doi: 10.1161/JAHA.119.012236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Methley A.M., Campbell S., Chew-Graham C., McNally R., Cheraghi-Sohi S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Higgins J.P.T., Elbers RG Reeves BC and the development group for ROBINSI Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I): Detailed guidance, updated 12 October 2016. [(accessed on 1 November 2020)]; Available online: http://www.riskofbias.info.
- 21.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen, Denmark: [(accessed on 22 January 2021)]. Review Manager (RevMan) [Computer Program]. Version 5.4. Available online: https://training.cochrane.org/ [Google Scholar]
- 25.Baber U., Chandrasekhar J., Sartori S., Aquino M., Kini A.S., Kapadia S., Weintraub W., Muhlestein J.B., Vogel B., Faggioni M., et al. Associations Between Chronic Kidney Disease and Outcomes With Use of Prasugrel Versus Clopidogrel in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: A Report From the PROMETHEUS Study. JACC Cardiovasc. Interv. 2017;10:2017–2025. doi: 10.1016/j.jcin.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y.H., Suh S.H., Choi J.S., Kim C.S., Sim D.S., Bae E.H., Lim S.Y., Ma S.K., Jeong M.H., Kim S.W., et al. Triple vs. dual antiplatelet therapy in patients with acute myocardial infarction and renal dysfunction. Circ. J. 2012;76:2405–2411. doi: 10.1253/circj.CJ-12-0236. [DOI] [PubMed] [Google Scholar]
- 27.Edfors R., Sahlen A., Szummer K., Renlund H., Evans M., Carrero J.J., Spaak J., James S.K., Lagerqvist B., Varenhorst C., et al. Outcomes in patients treated with ticagrelor versus clopidogrel after acute myocardial infarction stratified by renal function. Heart. 2018;104:1575–1582. doi: 10.1136/heartjnl-2017-312436. [DOI] [PubMed] [Google Scholar]
- 28.James S., Budaj A., Aylward P., Buck K.K., Cannon C.P., Cornel J.H., Harrington R.A., Horrow J., Katus H., Keltai M., et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122:1056–1067. doi: 10.1161/CIRCULATIONAHA.109.933796. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.Y., Jeong M.H., Moon J.H., Ahn Y.K., Chae S.C., Hur S.H., Hong T.J., Kim Y.J., Seong I.W., Chae I.H., et al. Impact of Clopidogrel loading dose in patients with chronic kidney disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am. J. Cardiol. 2012;110:1598–1606. doi: 10.1016/j.amjcard.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Liang J., Wang Z., Shi D., Liu Y., Zhao Y., Han H., Li Y., Liu W., Zhang L., Yang L., et al. High clopidogrel dose in patients with chronic kidney disease having clopidogrel resistance after percutaneous coronary intervention. Angiology. 2015;66:319–325. doi: 10.1177/0003319714538804. [DOI] [PubMed] [Google Scholar]
- 31.Nishi T., Ariyoshi N., Nakayama T., Fujimoto Y., Sugimoto K., Wakabayashi S., Hanaoka H., Kobayashi Y. Impact of chronic kidney disease on platelet inhibition of clopidogrel and prasugrel in Japanese patients. J. Cardiol. 2017;69:752–755. doi: 10.1016/j.jjcc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Jeong K.H., Cho J.H., Woo J.S., Kim J.B., Kim W.S., Lee T.W., Kim K.S., Ihm C.G., Kim W. Platelet reactivity after receiving clopidogrel compared with ticagrelor in patients with kidney failure treated with hemodialysis: A randomized crossover study. Am. J. Kidney Dis. 2015;65:916–924. doi: 10.1053/j.ajkd.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.S., Woo J.S., Kim J.B., Kim W.S., Lee T.W., Kim K.S., Ihm C.G., Kim W., Jeong K.H. The pharmacodynamics of low and standard doses of ticagrelor in patients with end stage renal disease on hemodialysis. Int. J. Cardiol. 2017;238:110–116. doi: 10.1016/j.ijcard.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Park S.H., Kim W., Park C.S., Kang W.Y., Hwang S.H., Kim W. A comparison of clopidogrel responsiveness in patients with versus without chronic renal failure. Am. J. Cardiol. 2009;104:1292–1295. doi: 10.1016/j.amjcard.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Woo J.S., Kim W., Lee S.R., Jung K.H., Kim W.S., Lew J.H., Lee T.W., Lim C.K. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: Results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. Am. Heart J. 2011;162:1018–1025. doi: 10.1016/j.ahj.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Shuldiner A.R., O’Connell J.R., Bliden K.P., Gandhi A., Ryan K., Horenstein R.B., Damcott C.M., Pakyz R., Tantry U.S., Gibson Q., et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma T.K., Lam Y.Y., Tan V.P., Yan B.P. Variability in response to clopidogrel: How important are pharmacogenetics and drug interactions? Br. J. Clin. Pharmacol. 2011;72:697–706. doi: 10.1111/j.1365-2125.2011.03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochholzer W., Trenk D., Fromm M.F., Valina C.M., Stratz C., Bestehorn H.P., Büttner H.J., Neumann F.J. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 2010;55:2427–2434. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Gragano F., Moscarella E., Calabro P., Arturo C., Pafundi P.C., Lelasi A., Patti G., Cavallari I., Antonucci E., Cirillo P., et al. Clopidogrel versus ticagrelor in high-bleeding risk patients presenting with acute coronary syndromes:insights from the multicenter STAR-ANTIPLATELET registry. Intern. Emerg. Med. 2020;16:379–387. doi: 10.1007/s11739-020-02404-1. [DOI] [PubMed] [Google Scholar]
- 40.Cesaro A., Talialatela V., Gragnano F., Moscarella E., Fimiani F., Conte M., Barletta V., Monda E., Limongelli G., Severino S., et al. Low-dose ticagrelor in patients with high ichemic risk and previous myocaridal infarction: A multicenter prospective real-world observational study. J. Cardiovasc. Pharmacol. 2020;76:173–180. doi: 10.1097/FJC.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos D., Panagiotou A., Xanthopoulou I., Komninakis D., Kassimis G., Davlouros P., Fourtounas C., Goumenos D. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on-treatment platelet reactivity. J. Thromb. Haemost. 2011;9:2379–2385. doi: 10.1111/j.1538-7836.2011.04531.x. [DOI] [PubMed] [Google Scholar]
- 42.Bonaca M.P., Bhatt D.L., Cohen M., Steg P.G., Storey R.F., Jensen E.C., Magnani G., Bansilal S., Fish M.P., Im K., et al. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infaction. N. Engl. J. Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.