Abstract
Insulin was first isolated almost a century ago, yet commercial formulations of insulin and its analogues for hormone replacement therapy still fall short of appropriately mimicking endogenous glycemic control. Moreover, the controlled delivery of complementary hormones (such as amylin or glucagon) is complicated by instability of the pharmacologic agents and complexity of maintaining multiple infusions. In this review, we highlight the advantages and limitations of recent advances in drug formulation that improve protein stability and pharmacokinetics, prolong drug delivery or enable alternative dosage forms for the management of diabetes. With controlled delivery, these formulations could improve closed-loop glycemic control.
Single sentence summary:
A review of developments in formulation engineering and delivery strategies of insulin for the treatment of diabetes.
Engineering opportunities to improve diabetes management
Over the last century, insulin replacement therapy has been imperative to saving lives and improving diabetes treatment outcomes. The administration of exogenous insulin prevents ketoacidosis which was once a universally fatal condition. Though insulin has historically been the focus of diabetes management, metabolic signaling from the endocrine pancreas is more complex than solely glucose-mediated insulin secretion. In people without diabetes, insulin, amylin and glucagon are metabolic hormones that work in concert to maintain glucose homeostasis (Fig. 1A) (1, 2). Insulin and amylin are co-secreted from beta cells at a fixed ratio and with similar diurnal patterns, where upregulation of both hormones occurs at mealtimes (2). These two hormones act synergistically at mealtimes: insulin promotes glucose uptake into the hepatic and peripheral tissues, while amylin acts centrally to slow gastric emptying and increase satiety to slow glucose release (1–4). In contrast, glucagon acts as an opposing force to insulin, mobilizing endogenous glucose production through glycogenolysis and gluconeogenesis to prevent hypoglycemia. Both insulin and amylin suppress glucagon secretion at mealtimes, when endogenous glucose production is unnecessary (2, 5).
Fig. 1. Metabolism and current delivery challenges.
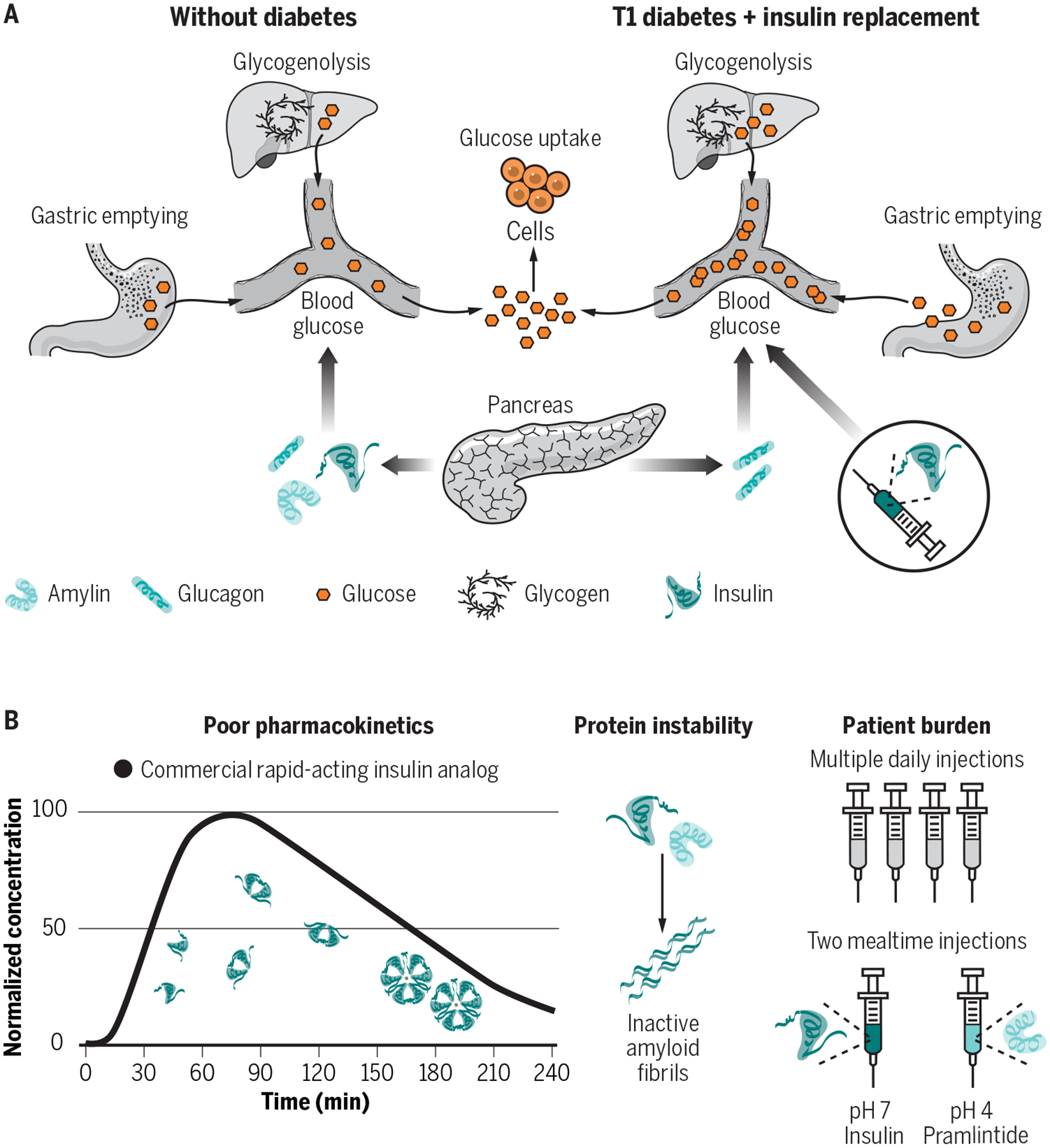
(A) In patients without diabetes, insulin, amylin and glucagon are secreted from the endocrine pancreas and work in tandem to maintain glucose homeostasis. Insulin and amylin work synergistically, where insulin promotes glucose uptake by cells and amylin slows gastric emptying and increases satiety. Glucagon, responsible for promoting glucose mobilization through glycogenolysis, is suppressed at mealtimes through paracrine signaling. (B) In patients with type 1 diabetes, subcutaneous delivery of insulin analogues (e.g., lispro, aspart, glulisine) can restore glucose uptake at mealtimes, but in the absence of replacement of amylin or its analogues (e.g., pramlintide) the effects of slowed gastric emptying and post-prandial glucagon suppression are lost – exacerbating prandial glucose excursions. (C) Therapies to deliver insulin, amylin analogues (pramlintide) and glucagon exist, but ideal use is highly burdensome, and the high costs preclude their use for many patients. Current drug delivery challenges include protein instability, burdensome treatment administration, and pharmacokinetics that do not sufficiently mimic endogenous hormone secretion to allow for optimal glucose control.
In type 1 diabetes (T1D), an autoimmune response destroys the pancreatic beta cells that produce and secrete insulin and amylin. Glucagon secretion by the alpha cells is not lost in T1D. In fact, glucagon becomes upregulated at mealtimes in patients with T1D, compounding mealtime glucose excursions arising due to the absence of paracrine insulin and amylin signaling (Fig. 1B) (1, 2, 6). As such, approaches to deliver insulin and amylin or their analogues have been developed. Clinical rapid-acting mealtime insulin, long-acting basal insulins, mealtime amylin, and rescue glucagon (used to counteract severe hypoglycemia where oral glucose intake is insufficient) have been developed to maintain glucose homeostasis. Yet, effective treatment with insulin alone is already highly burdensome and costly – requiring frequent glucose monitoring, mealtime insulin boluses, basal insulin delivery through infusion pumps or long-acting analogues, and routine carbohydrate counting – and still does not truly recapitulate the complexities of metabolic control in nondiabetic individuals (Fig. 1C) (7). The inclusion of additional therapeutics like amylin into routine treatment regimens has been shown to improve glucose management and better mimic endogenous metabolic control, but patient adoption has unfortunately remained low due to the increased patient burden associated with these treatments (8–10). Thus, innovation is needed to engineer the delivery of complementary therapeutics like amylin and glucagon for compatibility with existing treatment regimens, such as infusion pumps, to promote wider patient adoption.
Drug delivery strategies that reduce patient burden, increase patient compliance, and improve access to these critical drugs are crucial to change the diabetes treatment landscape. In this review, we highlight engineering approaches to develop new formulations to address current challenges in diabetes management. We focus on how excipients, the “inactive” ingredients of drug formulations, are critical components that determine the pharmacokinetics, pharmacodynamics, and stability of formulations (11, 12). Stabilizing excipients enable shelf-stable formulations of more rapid-acting insulins or liquid glucagon, and improve the compatibility of biopharmaceuticals for co-delivery, which can be used in insulin pumps or closed-loop systems. Further, biomaterials can be used to facilitate strategies for sustained delivery to improve basal insulins, glucose-responsive materials for “smart” (i.e. autonomous) delivery systems, and alternative routes of administration. These active areas of research hold tremendous potential to reduce patient burden by decreasing needle use while improving diabetes management.
Strategies for insulin stability
Insulin stability is the foundation for innovative formulation design, especially for pump-compatible insulins, because of the requirement to remain stable over extended periods without refrigeration. Further, improved insulin stability that increases cold chain resilience could improve global access to insulin, especially in hot climates with limited access to refrigeration and temperature-controlled transport. In most formulations, the predominant insulin association state is the stable hexamer, which is at equilibrium with a small number of insulin monomers (the biologically active form) and dimers (13, 14). The excipients in current insulin formulations have been carefully chosen to preserve the activity of insulin over the duration of its shelf-life. Insulin formulation design is hindered by the propensity for insulin to self-aggregate and form amyloid fibrils, which are insoluble, inactive, and immunogenic (15, 16). Insulin aggregation arises at hydrophobic interfaces, such as the air-water or vial-water interfaces, where monomers partially unfold and expose hydrophobic regions that can nucleate amyloid fibril formation (Fig. 2A) (17, 18). Insulin monomers are most susceptible to aggregation because hydrophobic portions of the insulin chain that are usually protected in the dimeric or hexameric state become exposed after adsorption to the air-water interface and induce aggregation (17, 18). Although there are strategies to increase insulin stability through engineering new insulin analogues, excipients that minimize the number of monomers in formulation, or which reduce nucleation events at the interface, can greatly improve formulation stability as well (17, 19).
Fig. 2. Formulation excipients, insulin aggregation, and stabilization techniques.
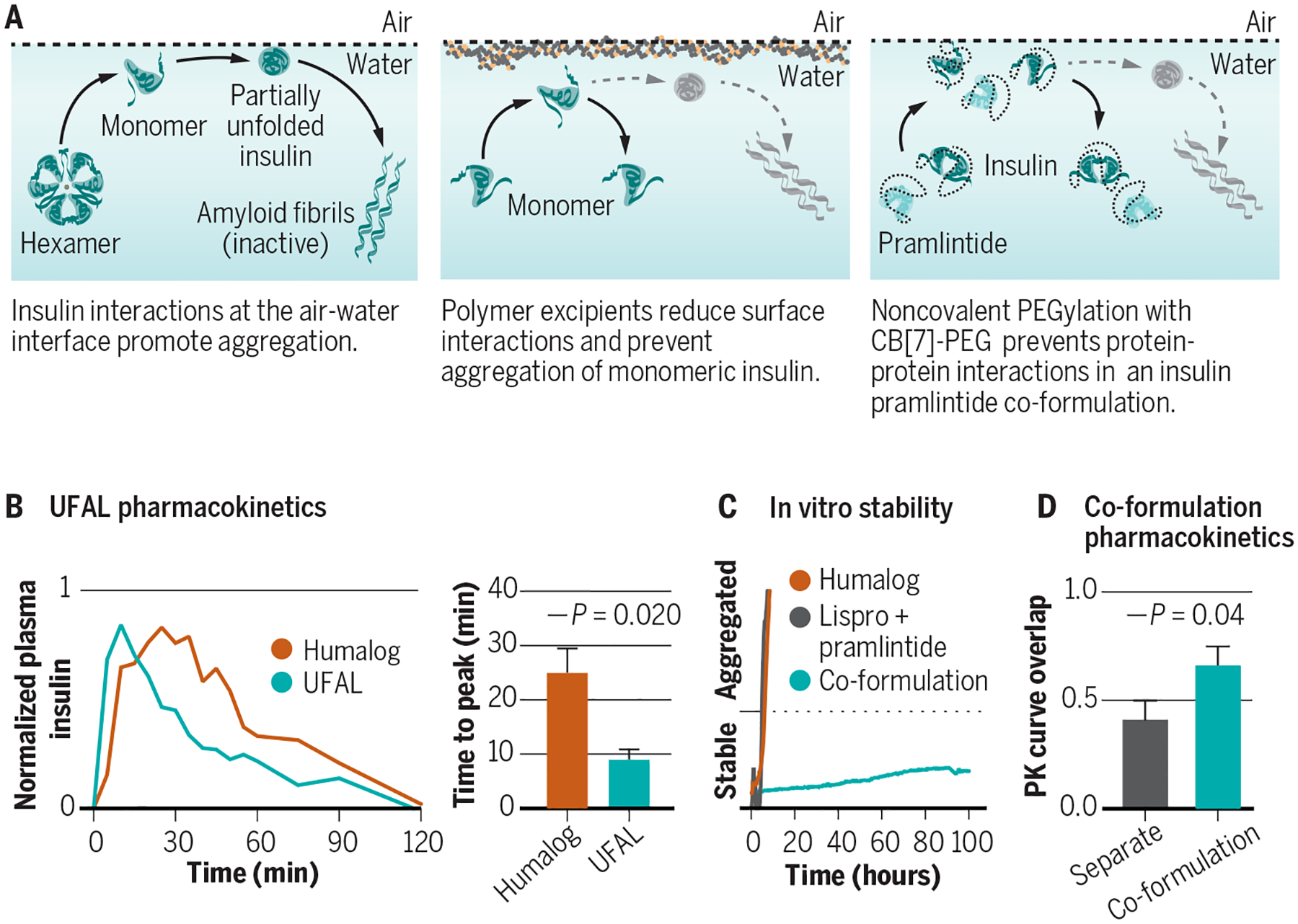
(A) Aggregation of biopharmaceuticals such as insulin typically occurs as a result of protein-protein interactions at a hydrophobic interface (e.g., the air-water or vial-water interfaces) that nucleate aggregation events. (B) Excipients, such as tonicity modifiers, antimicrobial preservatives, and stabilizing agents can affect insulin association state and are carefully chosen to balance stability in the vial and absorption upon subcutaneous administration. (C) Ultrafast acting insulins aim to shift the equilibrium of insulin association states from the insulin hexamer towards the insulin monomer to promote more rapid absorption and commensurate onset of action, as well as to reduce the duration of action. New excipient platforms look at displacing insulin from the air-water and vial-water interfaces using amphiphilic copolymers to prevent protein-protein aggregation. (D) Judicious design of polyacrylamide-based copolymer excipients can generate an ultrafast absorbing lispro (UFAL) formulation comprising mostly monomeric insulin that is significantly more stable even than current commercial fast-acting insulin formulations (e.g., Humalog). (E) Pharmacokinetic exposure curve and time to peak exposure for this UFAL formulation in diabetic pigs indicates a 2.8-fold decrease in the time-to-peak when compared to Humalog. (F) Simultaneous non-covalent PEGylation of insulin and pramlintide using cucurbit[7]uril-poly(ethylene glycol) (CB[7]-PEG) provides a protective “wrapper” on each protein that allows for stable co-formulation of the two historically incompatible therapeutics at pH=7. (G) Supramolecular PEGylation with CB[7]-PEG enables insulin-pramlintide co-formulations to be more stable to stressed aging than even commercial Humalog. (H) Pharmacokinetic curves for a CB[7]-PEG stabilized insulin-pramlintide co-formulation in diabetic pigs demonstrating increased overlap of insulin and pramlintide action compared to the current clinical approach of separate administrations. Adapted from (24, 38).
Most commercially available insulins are injectable liquid formulations and must include tonicity agents (i.e. salts or sugars used to achieve isotonicity with the blood) and antimicrobial preservatives in addition to stabilizing agents (Fig. 2B) (20, 21). In most formulations, zinc acts as the primary stabilizing agent, promoting the T6 insulin hexamer aggregation state. In addition to their antimicrobial properties, the inclusion of phenol and/or meta-cresol also increases stability by forming hydrogen bonds between insulin dimers that promotes the R6 hexamer aggregation state (13, 22). Even the tonicity agents are chosen with stability in mind, whereby glycerol is the most commonly used tonicity agent and has been shown to increase stability of insulin formulations (23).
Surfactants have been used to displace insulin from interfaces and improve stability through reducing the number of insulin-insulin interactions, thus lowering the probability of a nucleation event (17, 20, 21). Insuman U400 (Sanofi Aventis) uses Poloxamer 171 to enable a stable concentrated formulation for use with implantable intraperitoneal pumps. Apidra (insulin glulisine, Sanofi Aventis) is a zinc-free formulation that makes use of polysorbate 20 to improve formulation stability. Recent research has identified amphiphilic polyacrylamide-based copolymers as another strategy to displace insulin from interfaces and improve stability (Fig. 2C) (24). These copolymer excipients can improve long-term stability of recombinant insulin under stressed aging conditions and enable the stable formulation of monomeric insulin (Fig. 2D–E).
Another strategy used to prevent insulin aggregation is through conjugation of protective hydrophilic polymers. Covalent and non-covalent polymer conjugation has been used as a strategy for shielding protein-protein interactions (Fig. 2F) (14, 25, 26). While covalent conjugation is effective for insulin stabilization, it presents concerns of altered activity and pharmacokinetics upon administration in the body. Non-covalent modification achieved via host-guest binding of cucurbit[7]uril-poly(ethylene glycol) (CB[7]-PEG) with the N-terminal phenylalanine on insulin presents an alternative to covalent conjugation that improves insulin stability but leaves the protein unmodified after almost immediate dissociation upon dilution in the body following injection (14, 25, 26). In addition to over 10-fold increased stability, non-covalent insulin modification with CB[7]-PEG demonstrates the ability to modulate pharmacokinetics through alteration of PEG length, increase protein solubility, and co-formulate otherwise incompatible proteins (25).
Innovations in pump compatible formulations
Presently, insulin infusion pumps allow for subcutaneous delivery of rapid-acting insulin without the requirement for multiple daily injections. A continuous basal infusion is provided throughout the day and insulin boluses are used to compensate for carbohydrates consumed at mealtimes and to correct hyperglycemia. Formulations used in pumps must remain stable without refrigeration for days in conventional subcutaneous pumps, and months in implanted intraperitoneal pumps.
Automated insulin delivery presents an opportunity to reduce patient burden and provide improved glucose management. Closed loop systems use data from a continuous glucose monitor (CGM) to modulate delivery of continuously infused subcutaneous insulin using an algorithm. Current hybrid closed-loop devices require users to input carbohydrate quantities at mealtimes and are not ideal for managing glucose variation associated with changes in physiologic state (such as illness and exercise). Effective fully autonomous closed-loop control is still elusive. Current attempts are limited by the delayed absorption and extended duration of action of subcutaneously delivered rapid-acting insulin formulations. Delayed insulin absorption kinetics mean that the algorithm cannot rapidly reduce glucose spikes and the extended duration of action can enhance the risks of hypoglycemia as a result of insulin stacking (i.e. the additive effects of residual insulin boluses given close together) (27, 28). Therefore, there is a need for formulation engineering that can better mimic endogenous insulin secretion and allow for more rapid response to changing glucose concentrations.
Ultrafast acting insulins
The advent of insulin analogues (e.g., lispro, aspart, glulisine) provided insulins with kinetics that can more closely mimic endogenous meal-time insulin secretion (12); however, there is still room for improvement. Insulin delivery for the treatment of diabetes remains burdensome, requiring careful attention to timing, dosing, and carbohydrate consumption by the patient. Endogenous insulin secretion from the healthy pancreas is directed immediately to the portal vein, whereas subcutaneous delivery of current rapid-acting insulin formulations results in a delayed onset of action of ~20–30 min, peak action at ~60–90 min, duration of action of ~3–4 hours and tail out until 6 hours (12, 29). The delayed time to onset in current rapid-acting formulations is a result of the mixed association states of the insulin molecules in formulation and the kinetics of subcutaneous administration.
Ultrafast acting insulins with more rapid onset and reduced duration of action would allow for more rapid adaptation to changing glucose concentrations in closed-loop systems. Traditional approaches have focused on amino acid modification of regular human insulin to more rapidly dissociate the stable insulin hexamer, while recent advances focused on increasing subcutaneous absorption rate and isolating the insulin monomer (14, 30–35). Yet, the balance between speed and stability remains a challenge, and the requirement for formulations to be shelf-stable has thus far limited commercial advancements to only incremental increases in kinetics.
Excipients play a critical role in insulin association state, absorption and stability. Niacinamide (vitamin B3), treprostinil (prostacyclin analogue), sodium citrate and hyaluronidase promote more rapid absorption of insulin after subcutaneous administration (30, 31, 36). Niacinamide increases insulin monomer content, both niacinamide and treprostinil act as vasodilators to improve insulin absorption, and hyaluronidase is an enzyme which acts by depolymerizing hyaluronan in the subcutaneous space to enable increased insulin dispersion and absorption (30, 31). Zinc-free formulations disrupt the insulin hexamer and skew insulin association states towards the insulin monomer. Yet, phenol and meta-cresol still promote hexamer formation, which ultimately results in similar pharmacokinetics between zinc-free glulisine formulations (Apidra, Sanofi Aventis) and other zinc-containing fast-acting insulin formulations (37). Citric acid has been used in combination with Ethylenediaminetetraacetic acid (EDTA) to mask insulin surface charges and prevent hexamer re-association after the chelation of zinc ions (32–35). Most recently, the antimicrobial preservative phenoxyethanol has been identified as a potential substitute for meta-cresol and phenol that promotes monomers (70–80%) in zinc-free formulations with lispro or aspart (14, 24).
The caveat to a higher fraction of insulin monomers in formulation is that additional excipients are required to attain stable shelf-life. In Fiasp (insulin aspart, Novo Nordisk), the higher monomeric content achieved with niacinamide is balanced with the addition of amino acid L-arginine to increase stability (31). BioChaperone Lispro (Adocia) combines oligosaccharides (BioChaperone) modified with anionic charges and amino acid moieties with insulin to promote both rapid absorption and increased stability. Fiasp shows modest improvements over first-generation insulin aspart (Novolog), with a reduction in time to peak action by only 10 minutes and a reduction in duration of action of about 15 minutes (39). Unfortunately, small differences in pharmacokinetics do not necessarily translate into improved clinical outcomes, as demonstrated in three different studies of Fiasp use in pumps and closed-loop systems (40–42). Initial clinical trial data from Adocia’s ultra-rapid BioChaperone Lispro formulations suggest that this formulation will surpass the improved kinetics of Fiasp (43, 44). Most recently, clinical trials for Ultra Rapid Lispro (URLi, Eli Lilly) demonstrated a combination of treprostinil and sodium citrate promote rapid insulin absorption to reduce time to onset by 13 minutes, and peak exposure by 12 minutes compared to Humalog (36). Yet, whether these improvements in pharmacokinetics will translate into improved clinical outcomes with current technology remains to be determined.
Recent work has also identified CB[7]-PEG and amphiphilic polyacrylamide-based copolymers as promising polymeric excipients to stabilize monomeric insulin (14, 24). In particular, preclinical studies using amphiphilic polyacrylamide-based copolymers in combination with phenoxyethanol zinc-free lispro have been used to generate an ultrafast absorbing lispro (UFAL) formulation (Fig. 2, A to C) that is more than twice as stable to stressed aging as commercial Humalog and displays absorption kinetics that are almost three-fold faster than Humalog in diabetic pigs (24).
Insulin-pramlintide co-formulations
A true hormone replacement therapy for type 1 diabetes would combine delivery of an insulin and amylin analogue (pramlintide). Studies have shown that treatment with insulin and pramlintide at mealtimes results in improved glucose control compared to treatment with insulin alone, observed as a 0.3% decrease in hemoglobin A1c (HbA1c) concentrations (8, 9). Pramlintide delivery is also critical to restoring post-prandial glucagon suppression, which is not possible using subcutaneously delivered insulin alone (45, 46). Despite the improved glycemic outcomes, pramlintide therapy has not been widely adopted for clinical use due to the burdensome requirement for separate administrations of insulin and pramlintide, and symptoms of nausea associated with higher dosages. Pramlintide is not stable at pH~7, which is the typical pH of insulin formulations (38). Moreover, when delivered separately under current formulation conditions, pramlintide has disparate pharmacokinetics from rapid-acting insulin, which reduces their synergistic effects.
Co-formulations of insulin and pramlintide offer the possibility for a dual-hormone replacement therapy that more closely mimics hormone secretion from beta cells. A pump-compatible co-formulation would enable adoption of pramlintide by pump users without the need to introduce mealtime injections. In closed-loop systems, the action of pramlintide to slow glucose appearance at mealtimes would permit algorithms and insulin additional time to react to mealtime glucose spikes. Indeed, initial pump and closed-loop studies delivering fixed ratios of insulin and pramlintide (from separate pumps) demonstrate improved glycemic control after meals (46–48). The stability of insulin and pramlintide in a single formulation is the foremost challenge to incorporating pramlintide into closed-loop control, yet a formulation with matched insulin and pramlintide pharmacokinetics has the potential to mimic endogenous secretion.
Initial exploratory work has shown that without additional excipients insulin analogues and pramlintide can be stably co-formulated at pH=5, whereas the inclusion of stabilizing excipients can enable co-formulation at physiological pH (38, 49, 50). Commercial efforts at advancing an insulin pramlintide co-formulation are ongoing with phase I/II clinical trials of Adocia’s M1Pram underway (50). M1Pram stabilizes insulin and pramlintide together at physiological pH using Adocia’s BioChaperone oligosaccharide system. Recently, simultaneous supramolecular PEGylation of insulin and pramlintide with CB[7]-PEG was shown to enable a stable insulin-pramlintide co-formulation at pH=7 (38). Further, non-covalent PEGylation results in more similar diffusion rates between zinc-free lispro and pramlintide in the co-formulation, which translates to increased pharmacokinetic overlap and a commensurate improvement in post-prandial glucagon suppression in diabetic pigs when compared to separate injections of lispro and pramlintide (Fig. 2, D to F). These studies highlight the importance of appropriately tuning the pharmacokinetics of these two hormones to maximize their synergy in diabetes management.
Stable liquid glucagon formulations
Exogenous glucagon is often prescribed as a rescue drug for severe hypoglycemia with the inability to ingest carbohydrates (such as loss of consciousness). This rescue therapy is effective with adequate glycogen stores but remains burdensome due to the instability of glucagon. Glucagon is highly insoluble and unstable, requiring reconstitution from a lyophilized powder in a pH~2 buffer immediately prior to administration (51). This formulation is extremely unstable and begins to form amyloid fibrils within hours of reconstitution. This burdensome delivery method adds stress in an emergency situation and can result in delayed treatment (51). Recent developments in stable liquid glucagon formulations include glucagon analogues, solvent changes, changes in formulation pH, and excipients to improve solubility and reduce aggregation (52–57). This is critical not just for pumps but also to reduce patient burden during emergency glucagon administration. In 2019, the United States Food and Drug Administration (FDA) approved the first stable liquid glucagon, GVoke, developed by Xeris Pharmaceuticals, which uses dimethyl sulfoxide (DMSO) as a solvent to reduce glucagon degradation. Initial clinical trials in subcutaneous infusion pumps demonstrated similar pharmacodynamics between the stable liquid glucagon and reconstituted glucagon, showing promise for translation to closed-loop systems (52). Yet, there is still room for improvement: patients reported increased pain and erythema at the injection site in these initial pump studies, which may prevent the formulation’s use for chronic infusion (52).
Dual-hormone closed-loop systems that separately deliver insulin and glucagon have potential to improve glucose homeostasis by increasing the aggressiveness of the insulin controller due to the ability to treat hypoglycemia. Glucagon infusions could be used to prevent exercise-associated hypoglycemia, counteract overestimates in insulin delivery, and enable more aggressive insulin dosing at mealtimes (58). In clinical trials, these dual-hormone closed-loop systems have shown reduced incidences of hypoglycemia when used with reconstituted glucagon (58). However, pump-stable liquid glucagon formulations remain the biggest hurdle for the translation of dual-hormone closed-loop pumps. An ideal glucagon formulation for use in pumps would remain stable at 30 °C for over 7 days.
Sustained delivery strategies
Extensive efforts have been taken to develop commercial formulations of insulin analogues with a range of pharmacokinetics that allow for effective coverage of mealtime and basal insulin requirements. Long-acting insulin analogues are intended to mimic physiological basal insulin secretion and control blood glucose during fasting periods (59). These formulations provide an alternative to continuous insulin infusion from a pump. Typically, long-acting insulin analogues, such as insulin glargine, detemir, and degludec, are absorbed more slowly with an onset effect in 1.5–2 hours that plateaus and remains relatively flat for the duration of action (59, 60). The duration of action varies for different analogues, with a duration of about 12–24 hours for insulin detemir, 24 hours for insulin glargine, and greater than 24 hours for insulin degludec (59). The ongoing goal of basal insulin analogue delivery is the creation of formulations with a long-acting, peak-less kinetic profile. Advances that further reduce patient burden by combining mealtime and basal action, dynamically deliver insulin in response to glucose load, or more closely mimic natural hormone secretion to improve restoration of normal metabolic function would be valuable tools for improved glucose control.
New approaches to long-acting insulins
Long-acting insulin formulations in current clinical use achieve their prolonged action through a variety of mechanisms, including poorly soluble insulin–protamine (NPH insulin), pH-dependent precipitation (insulin glargine) and local albumin binding (insulin detemir). New approaches to the prolonged delivery of insulin analogues have aimed to achieve more stable pharmacokinetic and pharmacodynamic profiles, extended duration of action, and reduced day-to-day variability (61). Approaches to long-acting insulins have included covalent and supramolecular PEGylation (25, 62), self-association or aggregation (63, 64), covalent modifications (65), hydrogels (66), and stimuli-responsive materials (67–70) (Fig. 3). Among these approaches, modification of insulin either covalently or non-covalently has shown to be effective in prolonging insulin circulation time owing to the increased hydrodynamic size of the protein after modification. Supramolecular PEGylation demonstrates an opportunity for both increased stability and extended duration of action after a fast-acting mealtime response (Fig. 3A). In diabetic mice, non-covalent PEGylation of insulin with CB[7]-PEG has shown similar time to onset of action compared to insulin alone, but the duration of insulin was tunable with increasing PEG length (25). This technology is now being exploited to develop next-generation supramolecular excipients that enable rapid-acting and long-acting insulin formulations.
Fig. 3. Sustained insulin delivery strategies.
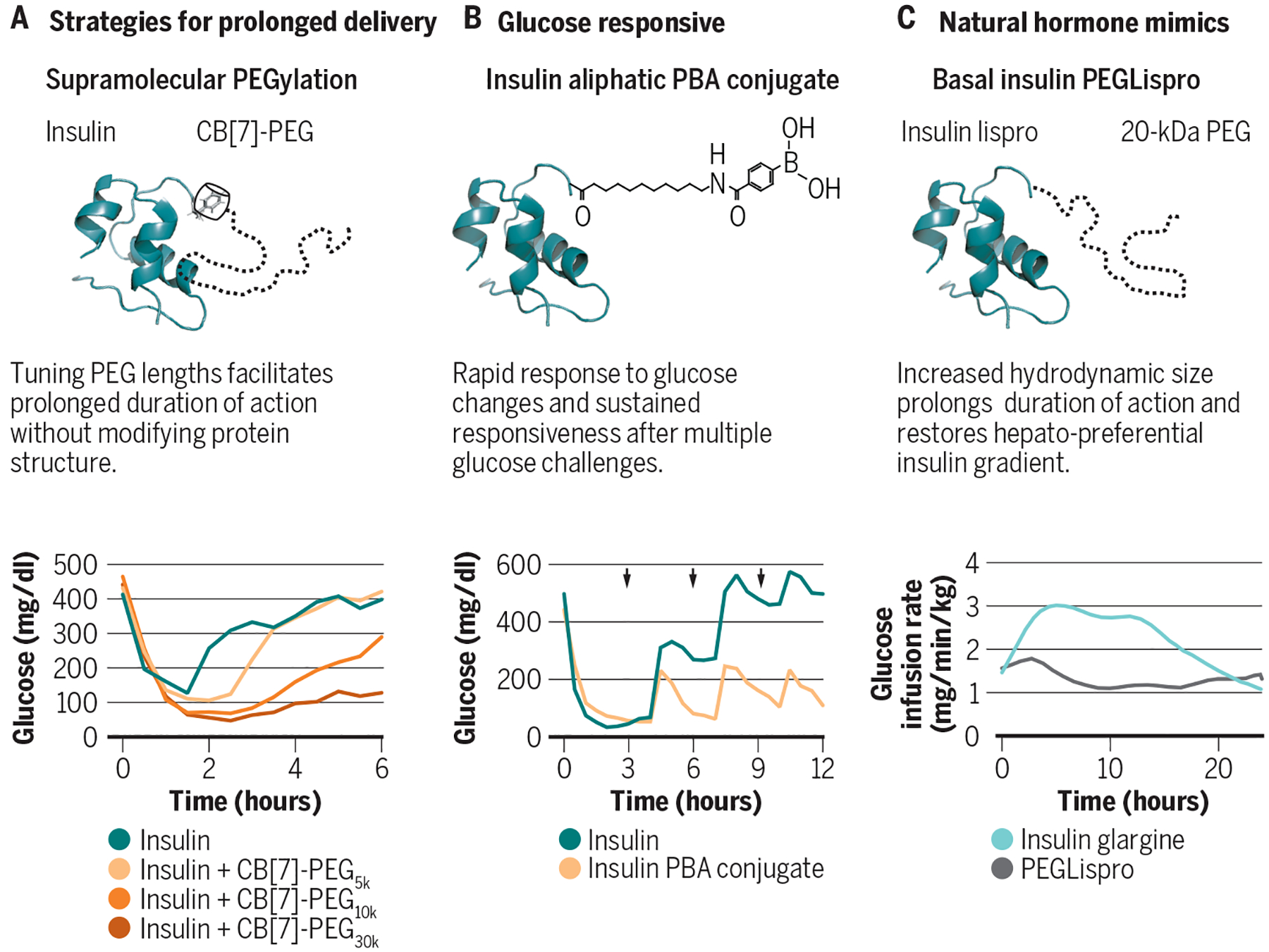
Long-acting insulin analogues aim to mimic physiological basal insulin secretion and control blood glucose concentrations during fasting periods. Long-acting insulin analogues, such as insulin glargine, detemir, and degludec, are absorbed more slowly with an onset effect in 1.5–2 hours that plateaus and remains relatively flat for the entire duration of action. (A) Supramolecular PEGylation of insulin with CB[7]-PEG demonstrates an opportunity for both rapid initial insulin response and tunable duration of action in diabetic mice by changing the length of the PEG chain attached to the CB[7]-PEG excipient. (B) A glucose-responsive insulin with a phenylboronic acid (PBA) pendant group on an aliphatic chain allows for both prolonged circulation in the blood and rapid response to glucose changes in diabetic mice. (C) Basal insulin PEGLispro (BIL) is a long-acting insulin analogue where insulin lispro is covalently modified on the B28 lysine with a 20‐kDa PEG chain, increasing the hydrodynamic radius of the insulin that results in a hepato-preferential insulin biodistribution with flat pharmacokinetic and pharmacodynamic profiles in human patients. Adapted from (25, 62, 69).
Glucose-responsive, prolonged delivery systems are also of great interest in the drug delivery community (67, 71, 72). The ideal glucose-responsive delivery system would approximate an artificial pancreas system, initiating and suspending insulin delivery in response to glucose loads (72). There has been extensive research in developing these systems, however there are several challenges that have limited advances (71, 72). Material responsiveness, in part due to diffusion-limited materials, pose a challenge because the lag between glucose detection and insulin release limits meal-time responsiveness and can increase risk of hypoglycemia (67). Similarly, most glucose detection moieties have cross-reactivity with fructose and other diols which have lower glycemic indices than glucose and can increase the risk of hypoglycemia from non-specific insulin release (72). Progress has been made in addressing these challenges and promising studies include work with glucose-responsive insulin analogues, charge-switchable polymeric complexes, and microneedles (68, 69, 73–75).
Glucose-responsive insulin analogues have demonstrated both long-acting and glucose-responsive behavior in diabetic mice (69, 73). Aliphatic phenylboronic acid (PBA)-modified insulin conjugates demonstrate rapid glucose depletion with a mealtime (oral glucose tolerance test) glucose response comparable to that of a healthy mouse, and continued responsiveness over the course of three consecutive challenges (Fig. 3B) (69). The use of an albumin binding chain, similar to determir, allows for prolonged circulation in the blood and the direct contact with blood glucose allows for the rapid activation or deactivation of insulin action (69). Another promising approach uses a transdermal insulin patch bearing microneedles loaded with insulin in a glucose-responsive polymeric matrix made of PBA groups (68). This microneedle patch is small, discrete and exploits the faster absorption associated with intradermal delivery to rapidly release glucose. The patch rapidly responded to oral glucose tolerance tests in diabetic mice and pigs, and demonstrated continued responsiveness for over 20 hours in diabetic pigs (68). These studies address the need for rapid insulin release in response to glucose stimuli, and the PBA insulin conjugate demonstrates reduced hypoglycemia compared to high doses of insulin alone; however, the lower limit glucose concentrations reported in both works are still unacceptably low for safety when translated to humans. To improve upon these technologies, a suspend threshold must be established closer to the euglycemic range in order to reduce the risk of hypoglycemia.
Hepato-preferential long-acting insulins
Current long-acting insulins have been effective at restoring basal insulin delivery, but the balance between hepatic and peripheral insulin uptake remains disproportionate. After secretion from the healthy pancreas, insulin travels directly through the portal vein where about 40–80% of the insulin is absorbed into the liver, with the remainder circulating to the peripheral tissues (76, 77). However, when insulin is delivered subcutaneously to treat T1D, this biodistribution ratio is skewed towards the peripheral tissues, resulting in lower hepatic insulin delivery (78). The absence of hepatically delivered insulin results in decreased metabolic flexibility - the switch from glucose oxidation to fat oxidation overnight (61). Moreover, over delivery of insulin to the peripheral tissues contributes to the risk of hypoglycemia, because the peripheral tissues lack the hepatic mechanisms to clear insulin during low glucose events (78). As such, efforts have been focused on the development and delivery of hepato‐preferential insulin analogues to restore normal metabolic function.
Basal insulin PEGLispro (BIL) is a long-acting insulin analogue where insulin lispro is covalently conjugated with a 20‐kDa polyethylene glycol polymer chain on the B28 lysine, making it significantly larger than native insulin or most insulin analogues. The large hydrodynamic radius of BIL results in a flat pharmacokinetic and pharmacodynamic profile with duration of action over 24-hours (Fig. 3C) (62, 78–80). BIL demonstrates low day-to-day variability and provides dosing flexibility, with similar performance when delivered within an 8–40 hour window after the last dose (62). During clinical trials, reduced incidences of nocturnal hypoglycemia, lower HbA1c, and reduced glucose variability were observed in patients receiving BIL compared to insulin glargine (62). Importantly, BIL has preferential hepatic action compared to conventional insulin, and improves metabolic flexibility by promoting greater lipid metabolism in the post-absorptive period than treatment with insulin glargine (61, 62, 78, 81). Ultimately, Eli Lilly discontinued the development of BIL after their Phase 3 trials indicated that further study would be needed to better understand the potential effects of increased liver fat observed with BIL treatment compared with insulin glargine. Regardless, these studies demonstrated the importance of restoring hepatic insulin delivery to holistically enhancing diabetes management. Future research would need to elucidate the cause and effect of increased liver fat content and identify if alternative delivery approaches could restore the hepatic insulin gradient without negative effects.
Basal amylin formulations
In addition to the need for basal insulin, patients with T1D could benefit from basal amylin replacement, which would more closely mimic the diurnal secretion patterns from the healthy pancreas. Unfortunately, the current kinetics of pramlintide (Symlin, Astra Zeneca) only support mealtime boluses and are not suited for long-term delivery. A combination insulin-amylin basal formulation could be a powerful strategy to reduce insulin requirements and mediate glucagon-stimulated glycemic excursions for restored metabolic control. One approach to address this challenge was reported by Nascimento et. al (65), describing a novel N-terminal PEGylated human amylin analogue, BZ043, and its potential to improve the control of glycemia using lower doses of insulin. The authors demonstrated that BZ043 exhibited a prolonged anti-hyperglycemic effect and, together with glargine, promoted long-lasting normoglycemia in diabetic rats. These studies indicated that combining BZ043 and glargine in a fixed-ratio co-formulation might conveniently improve diabetes management. There remains a great need for new technologies for delivery of basal amylin formulations with an emphasis on developing approaches to co-deliver insulin and amylin analogues. Future research could include the use of supramolecular excipients or hydrogels to achieve stable co-formulation and delivery of these therapeutic proteins.
Alternative dosage forms to reduce patient burden
Alternative dosage forms present an opportunity to reduce patient burden by reducing or eliminating the injections required for hormone replacement. These alternative delivery forms include oral, transdermal and inhalable insulin delivery, which are less invasive than current insulin injections or infusion pumps. Advances in drug delivery have overcome the challenges associated with protein stability and permeability to enable these delivery routes; however, alternative dosage forms still suffer from limitations in dosing options for the precise control required by patients with T1D. Predefined doses and inconsistent bioactivity may limit the use of these therapies to applications in basal insulin delivery or insulin replacement in patients with type 2 diabetes (T2D) where less precision is necessary. Here we discuss the advances in inhalable, oral and transdermal delivery routes and the prospects of these routes for clinical application.
Oral delivery
Oral drug delivery is preferred by patients and has higher compliance rates due to ease of administration (82, 83). Yet, orally delivered insulin requires stabilization to survive exposure to enzymes and the acidic environment of the stomach (83, 84), and moving insulin past the intestinal epithelial barrier to enter the blood poses a significant challenge (84). Nanocarriers and enterically coated capsules – carrying either insulin itself or mucoadhesive intestinal patches – have been successfully employed in preclinical animal models to stabilize insulin and can be used to deliver cargo to the small intestine, but can generally only safely achieve low bioavailability (85–87). Recent advances have identified zwitterionic micelles that promote transcellular insulin transport through the intestinal epithelium as well as promising excipients such as ionic liquids (e.g., choline and geranate) and anionic nanoparticles (<100 nm) that can increase the permeabilization of epithelial tight junctions to enhance insulin absorption from the intestine (88–90). Anionic nanoparticles bind to surface receptors on the intestinal wall and temporarily mediate the opening of tight junctions (Fig. 4A). Initial biocompatibility studies have shown that these effects on permeability last about 4 hours and high molecular weight species (i.e., bacteria) remain unable to pass through the tight junctions (89).
Fig. 4. Design challenges for alternative dosage forms.
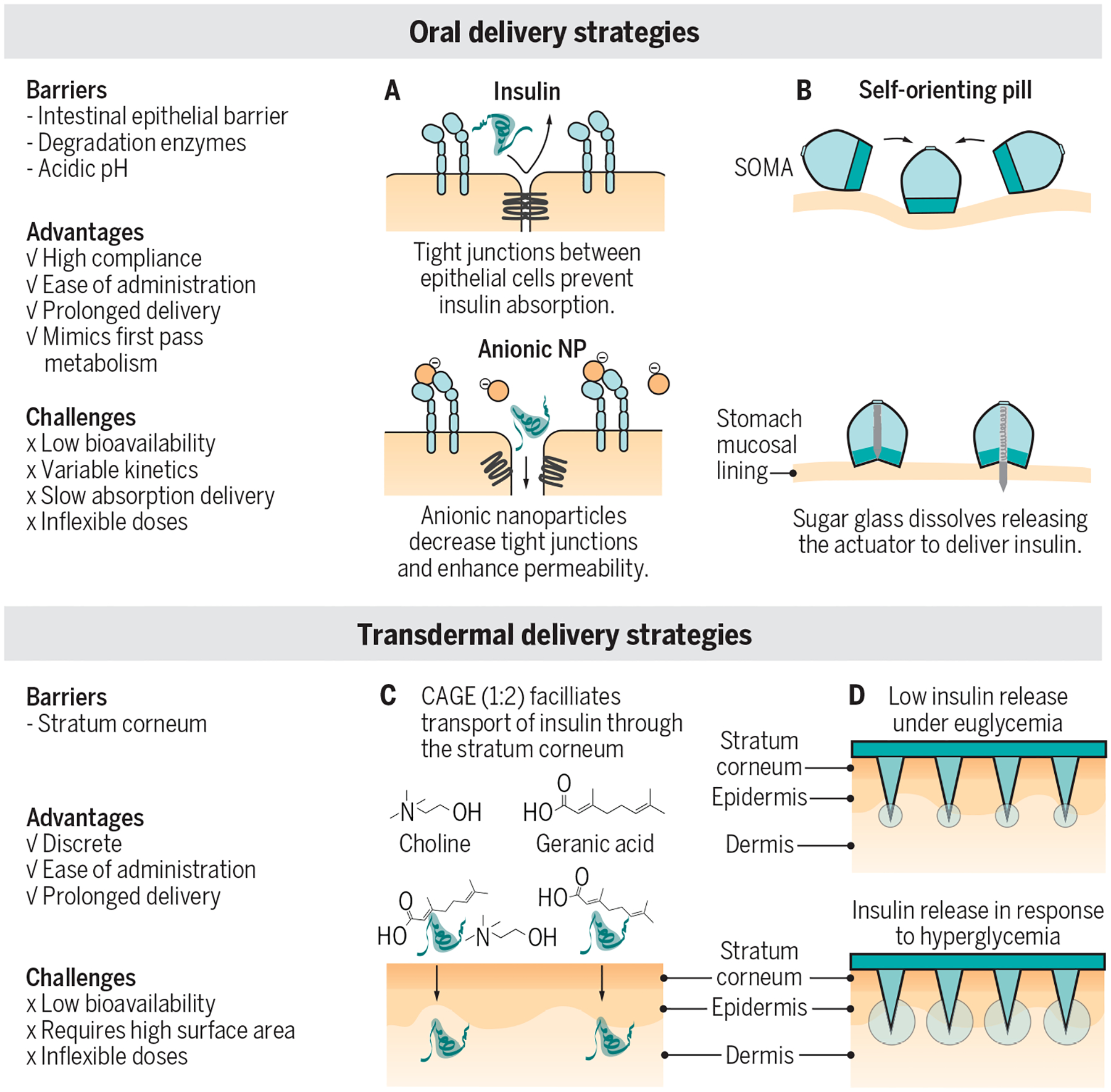
Alternatives to insulin injections have the potential to reduce patient burden and increase patient compliance. Oral and transdermal insulin delivery systems are in the early stages of development. but there are currently no clinically approved treatments available. These technologies transport peptides like insulin and glucagon past barriers that typically block the absorption of biologics. To this end, two main strategies are being pursued: increased permeability to facilitate absorption and penetration of a tissue barrier. (A) Anionic nanoparticles (NP) are used as a formulation excipient to decrease tight junctions and increase permeability to allow insulin absorption in the small intestine (data shown in mice). (B) Self-orienting millimeter-scale applicator (SOMA) is a pill that can be swallowed and then activates in the stomach (a spring is released after a sugar disc dissoves) to inject an insulin millipost past the stomach mucosal lining, where the insulin can be absorbed into systemic circulation (data shown in pigs). S.C., subcutaneous. (C) Choline and geranate (CAGE) ionic liquids facilitate transdermal delivery by enhancing insulin permeability through the stratum corneum and into the dermis (data shown in rats). (D) Glucose-responsive microneedles penetrate the stratum corneum and allow for insulin release into the dermis in response to hyperglycemia (data shown in pigs). Blue arrows indicate meals. An oral or transdermal insulin would present a major advance in reducing the number of required injections. Current intermediate kinetics observed in (A, B and D) preclinical animal studies may suggest that oral and transdermal insulin may be best suited for prolonged basal insulin delivery or for use in helping to manage type 2 diabetes. Clinical pharmacokinetic and pharmacodynamic data in humans will be necessary to understand the true translational potential of these formulations for diabetes management. Figures are adapted from (68, 89, 91–93).
Other studies take a mechanical approach to penetrate the epithelial barrier for insulin absorption, including the use of a self-actuating pill to inject either microneedles into the gastrointestinal lining or a millipost of compressed insulin into the gastric mucosa of the stomach (Fig. 4B) (91, 94). These strategies all show promise in their ability to overcome the barriers of stability and degradation and succeed in moving insulin out of the gastrointestinal tract and into the bloodstream with high bioavailability for protein therapeutics. They will, however, still face challenges of specific dosing and rapid pharmacokinetics, which may limit them to basal applications or use in treatment of T2D.
Intradermal delivery
Transdermal insulin delivery is another non-invasive alternative to subcutaneous insulin administration and requires penetrating the stratum corneum - the barrier of the skin that protects against pathogens and moisture loss. Typically only small lipophilic drugs (<500 Da) are able to pass this layer; however, drug delivery strategies using hydrogels, ionic liquids and microneedles have been successful in transporting insulin across this barrier to the dermis (92, 93, 95–99). Ionic liquids comprising choline and geranate have been shown to be synergistic permeation enhancers that interact with the lipid barrier of the stratum corneum to mediate the transport of insulin to the dermis (Fig. 4C) (92, 93, 100). Topically applied insulin was active and showed prolonged glucose lowering compared to subcutaneous insulin in healthy rats (92). A challenge of transdermal delivery lies in the poor insulin bioavailability, resulting in the need for high insulin doses and a large surface area for topical application. Similar to oral delivery, transdermal delivery approaches struggle with precise dosing and prolonged duration of action, which may be better suited for basal delivery or treatment of T2D. Transdermal delivery has the potential to reduce the number of needles required but is not yet a viable solution to replace mealtime insulin injections.
Insulin microneedle patches have also been extensively explored for both traditional and glucose-responsive intradermal insulin delivery (68, 75, 97, 98, 101). Intradermal microneedles increase insulin absorption compared to subcutaneous administration. Beyond insulin, transdermal delivery of glucagon is also being developed. Recently, a glucose-responsive microneedle patch using glucose-sensitive microgels that release glucagon in response to hypoglycemia was reported (Fig. 4D) (99). In the microneedles, PBA moieties can cross-link with glucose, forming secondary crosslinks that result in the contraction of the microgels in response to low glucose conditions and commensurate release of glucagon into the dermis (99). In rats, this microneedle patch prevented hypoglycemia for over 1.5 hours after injection of a high dose of insulin (99), demonstrating the potential for this patch to act as protective treatment against hypoglycemia. Although this system suffers from low non-specific cargo release characteristic of PBA-based glucose-responsive materials, unlike insulin delivery systems, low glucagon secretion does not pose a safety concern and is therefore less likely to hinder the translatability of this system.
Concern regarding the safety and long-term effects of microneedles for intradermal delivery has been raised, particularly in light of the requirement for routine use of these technologies for diabetes management (98, 102, 103). In particular, early design strategies for microneedles made from silicon or metal raised concerns about increased risk of infection and risk of needle fragments remaining in the skin (98, 102, 103). More recently, microneedle designs have shifted towards using dissolvable or degradable polymeric materials which can improve device safety as any material deposited in the skin could be metabolized (103); however, further long-term study of the safety and effects of microneedle use is required before broad commercial translation.
Inhaled insulin
Inhaled insulin is the alternative dosage form that has seen the most success in translation to the clinic, but the challenges of this dosage form have resulted in limited commercial success. Some concern has been raised regarding the safety of pulmonary delivered insulin including treatment associated cough, insulin antibody production, cancer risk and long-term consequences on lung function (84, 104). The first generation of inhaled insulins suffered from poor bioavailability, variable kinetics in patient populations, and required bulky inhalers for delivery (84). Exubera (Pfizer), the first FDA approved inhaled insulin, was released in 2006 but was pulled from the market by 2007 due to poor sales (104). In 2014, inhaled insulin returned to the market with the launch of Afrezza (MannKind, Sanofi). Afrezza is composed of insulin adsorbed onto technosphere particles, which are self-assembled fumaryl diketopiperazine microparticles. These particles (2–2.5 μm in diameter) are delivered to the alveolar region of the lung where they take advantage of the high surface area and rapid absorption; this constituted the first truly ultrafast absorbing insulin (105). Afrezza demonstrated kinetics that approach the ultrafast action of intravenous administration (106). When Afrezza was taken in place of a mealtime bolus in closed-loop system trials, reduced hyperglycemic excursions were observed, suggesting that an ultrafast pump-compatible insulin could advance closed-loop control. Afrezza has made improvements with a smaller inhaler design, ultra-rapid kinetics and easier dosing options (104, 106). The inhaled form makes it incompatible with pump use or closed-loop systems. Further, the ultrafast kinetics can be too fast for mixed meals (i.e. balanced of carbohydrates, protein, and fat) and may not extend throughout the entire meal period.
Translational models for formulation engineering
Several diabetes animal models exist, including autoimmune models (Non-obese diabetic (NOD) mice, BioBreeding (BB) Rat), non-insulin dependent diabetes models (Zucker Diabetic Fatty rats, Goto-Kakizaki, Ossabaw pigs) and chemically-induced insulin deficient models (107). Individual rodent models have been reviewed in detail, and their use depends on the application and goal of the study (108). For formulation development, especially for insulin delivery, chemically induced diabetes presents the most straightforward option. Streptozotocin (STZ) and alloxan are both used to induce an insulin deficient phenotype as the result of selective beta cell destruction. STZ-induced type 1-like models of diabetes are well established in mice, rats and pigs (107, 109).
Mouse models present challenges for pharmacokinetics experiments because the low blood volume in mice limits the number and volume of samples that can be taken. Moreover, mice often experience stress-related glucose spikes with injection that can skew blood glucose measurements at early timepoints. In contrast, rats can be trained to receive subcutaneous injections and will cooperate for blood collection with minimal stress. The larger blood volume of the rat also makes it possible to obtain high resolution pharmacokinetic data.
A challenge faced by rodent models includes difficulty conducting a meal challenge, as rodents are nocturnal and graze on food constantly during their waking hours (108). Oral or intraperitoneal glucose tolerance tests can be used to simulate a meal challenge (107). Another challenge with rodent models is that size often necessitates dilution of formulations to facilitate accurate dosing. For insulin, dilution favors the monomeric and dimeric forms of the insulin instead of the insulin hexamer, which can mask differences in absorption kinetics between formulations. Further, rodents have loose skin which increases the available surface area for more rapid intradermal absorption. Rodent model studies have shown consistent time to peak onset between rapid-acting insulin analogues and regular insulin, which is in contrast to human studies where rapid-acting insulin analogues can decrease time-to-onset by about half (110).
Pigs present an important translational model because they have skin that closely resembles human skin and thus exhibit many similarities with regard to pharmacokinetics of compounds delivered by subcutaneous administration (111). The larger size of the pigs also allows for the delivery of neat (undiluted) commercial insulin (100 U/mL), which is important to preserve the solution equilibrium of insulin multimers to accurately assess pharmacokinetics. Moreover, pigs can be fed discrete meals, which enables the researcher to probe the efficacy of formulations or treatments in improving mealtime glucose management. Overall, pigs represent a translational model that is most scalable to humans, however, insulin pharmacokinetics remain roughly twice as fast in pigs as those observed in humans.
Future drug delivery opportunities in diabetes management
In normal physiology, insulin is released into the portal circulation along with carefully titrated quantities of amylin and glucagon. Current state-of-the-art commercial closed-loop technology uses interstitial glucose sensing and slow subcutaneous insulin delivery tied together with control strategies originally intended for industrial processes. Without technological breakthroughs it is unlikely that these commercial systems can match the innate biological control systems that have been finely tuned by billions of years of evolution. Multiple converging technologies are required to overcome the limits of the insulin-only approach to glucose control for management of diabetes. Computational approaches to automated insulin delivery can refine current systems but they rely on outside information or assumptions about individual behaviors. Formulation and excipient design to develop an insulin that reduces blood sugar instantaneously without residual hypoglycemic effect would allow for the simplest of control strategies. Combination therapies with amylin to slow and reduce glycemic excursions or glucagon to allow hypoglycemia rescue are possible but currently cumbersome given the need for separate wearable and coordinated devices. Co-formulations can overcome this weakness. Existing formulations and technologies, when used optimally, allow sufficient glycemic control to avoid more prominent microvascular complications. Unfortunately, these tools and their optimal use is a luxury afforded primarily to the socioeconomically privileged and those engaged in disease management (i.e. clinicians, diabetes technology developers). Using advances in formulation engineering and drug delivery technology to develop accessible and easy to use formulations and delivery platforms for diabetes management would improve outcomes and reduce burden for many patients. The development of glucose-responsive delivery strategies with increased safety profiles could be an attractive alternative to closed-loop control. Moreover, mealtime alternative dosage forms, like an oral insulin suited for prandial glucose coverage, could reduce patient burden and improve patient compliance. By fostering accessible technologies that allow for insulin delivery that more closely mimics endogenous delivery, people with diabetes could live safe from complications with much reduced effort.
ACKNOWLEDGEMENTS
FUNDING
This work was funded in part by an National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 (NIH grant #R01DK119254) and a Pilot & Feasibility seed grant from the Stanford Diabetes Research Center (NIH grant #P30DK116074) and the Stanford Child Health Research Institute, as well as the American Diabetes Association Grant (1-18-JDF-011) and a Research Starter Grant from the PhRMA Foundation. C.L.M. was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship and the Stanford BioX Bowes Graduate Student Fellowship.
Footnotes
COMPETING INTERESTS
E.A.A. and C.L.M. are inventors on provisional patent applications 63/011,928 and 62/948,159 filed by Stanford University describing some of the technology reported in this review. E.A.A., B.A.B, and C.L.M are inventors on a provisional patent application 62/804,357 filed by Stanford University describing some of the technology reported in this manuscript.
REFERENCES
- 1.Heptulla RA, Rodriguez LM, Bomgaars L, Haymond MW, The Role of Amylin and Glucagon in the Dampening of Glycemic Excursions in Children With Type 1 Diabetes. Diabetes 54, 1100 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Martin C, The Physiology of Amylin and Insulin: Maintaining the Balance Between Glucose Secretion and Glucose Uptake. Diabetes Educ 32, 101S–104S (2006). [DOI] [PubMed] [Google Scholar]
- 3.Hinshaw L, Schiavon M, Dadlani V, Mallad A, Dalla Man C, Bharucha A, Basu R, Geske JR, Carter RE, Cobelli C, Basu A, Kudva YC, Effect of Pramlintide on Postprandial Glucose Fluxes in Type 1 Diabetes. J. Clin. Endocrinol. Metab 101, 1954–1962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebovitz HE, Adjunct therapy for type 1 diabetes mellitus. Nature Reviews Endocrinology 6, 326–334 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Aronoff SL, Berkowitz K, Shreiner B, Want L, Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectrum 17, 183 (2004). [Google Scholar]
- 6.Campbell JE, Drucker DJ, Islet α cells and glucagon—critical regulators of energy homeostasis. Nature Reviews Endocrinology 11, 329–338 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Dutta T, Kudva YC, Persson X-MT, Schenck LA, Ford GC, Singh RJ, Carter R, Nair KS, Impact of Long-Term Poor and Good Glycemic Control on Metabolomics Alterations in Type 1 Diabetic People. J Clin Endocrinol Metab 101, 1023–1033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratner R, Whitehouse MS Fau - Fineman F, Fineman S Fau - Strobel Ms, Strobel L Fau - Shen S, Shen DG Fau - Maggs L, Maggs OG Fau - Kolterman Dg, Kolterman C Fau - Weyer Og, Weyer C, Adjunctive therapy with pramlintide lowers HbA1c without concomitant weight gain and increased risk of severe hypoglycemia in patients with type 1 diabetes approaching glycemic targets. Exp Clin Endocrinol Diabetes 113, 199–204 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, Weyer C, Kolterman OG, Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet. Med 21, 1204–1212 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Hampp C, Borders-Hemphill DG Fau - Moeny V, Moeny DK, Fau - Wysowski Dg Wysowski DK, Use of antidiabetic drugs in the U.S, 2003–2012. [DOI] [PubMed]
- 11.Mitragotri S, Burke PA, Langer R, Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nature Reviews Drug Discovery 13, 655–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu C, Gillard P, Benhalima K, Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat. Rev. Endocrinol 13, 385–399 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Gast K, Schüler A, Wolff M, Thalhammer A, Berchtold H, Nagel N, Lenherr G, Hauck G, Seckler R, Rapid-Acting and Human Insulins: Hexamer Dissociation Kinetics upon Dilution of the Pharmaceutical Formulation. Pharm Res 34, 2270–2286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maikawa CL, Smith AAA, Zou L, Meis CM, Mann JL, Webber MJ, Appel EA, Stable Monomeric Insulin Formulations Enabled by Supramolecular PEGylation of Insulin Analogues. Adv. Ther 75, 1900094 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods RJ, Alarcon J, McVey E, Pettis RJ, Intrinsic fibrillation of fast-acting insulin analogs. Journal of diabetes science and technology 6, 265–276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura M, Misumi Y, Nomura T, Oka W, Isoguchi A, Kanenawa K, Masuda T, Yamashita T, Inoue Y, Ando Y, Ueda M, Extreme Adhesion Activity of Amyloid Fibrils Induces Subcutaneous Insulin Resistance. Diabetes 68, 609 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Sluzky V, Klibanov AM, Langer R, Mechanism of insulin aggregation and stabilization in agitated aqueous solutions. Biotechnol. Bioeng 40, 895–903 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Sluzky V, Tamada JA, Klibanov AM, Langer R, Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. U. S. A 88, 9377–9381 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong X, Blakely A, Karra P, VandenBerg MA, Ghabash G, Whitby F, Zhang YW, Webber MJ, Holland WL, Hill CP, Chou DH-C, Novel four-disulfide insulin analog with high aggregation stability and potency. Chemical Science 11, 195–200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rayaprolu BM, Strawser JJ, Anyarambhatla G, Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Development and Industrial Pharmacy 44, 1565–1571 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Akers MJ, Excipient-drug interactions in parenteral formulations. J. Pharm. Sci 91, 2283–2300 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Teska BM, Alarcón J, Pettis RJ, Randolph TW, Carpenter JF, Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature. J. Pharm. Sci 103, 2255–2267 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Saha S, Deep S, Glycerol inhibits the primary pathways and transforms the secondary pathway of insulin aggregation. Physical Chemistry Chemical Physics 18, 18934–18948 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Mann JL, Maikawa CL, Smith AAA, Grosskopf AK, Baker SW, Roth GA, Meis CM, Gale EC, Liong CS, Correa S, Chan D, Stapleton LM, Yu AC, Muir B, Howard S, Postma A, Appel EA, An Ultra-fast Insulin Formulation Enabled by High Throughput Screening of Polymeric Excipients. Science Translational Medicine 12, eaba6676 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webber MJ, Appel EA, Vinciguerra B, Cortinas AB, Thapa LS, Jhunjhunwala S, Isaacs L, Langer R, Anderson DG, Supramolecular PEGylation of biopharmaceuticals. Proc. Natl. Acad. Sci. U. S. A 113, 14189–14194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinai JM, Taylor AB, Ryno LM, Hargreaves ND, Morris CA, Hart PJ, Urbach AR, Molecular recognition of insulin by a synthetic receptor. J. Am. Chem. Soc 133, 8810–8813 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R, The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes. Metab 20, 245–256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cengiz E, Undeniable need for ultrafast-acting insulin: the pediatric perspective. J. Diabetes Sci. Technol 6, 797–801 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polonsky KS, Given BD, Van Cauter E, Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Invest 81, 442–448 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow L, Muchmore DB, Hompesch M, Ludington EA, Vaughn DE, Comparative pharmacokinetics and insulin action for three rapid-acting insulin analogs injected subcutaneously with and without hyaluronidase. Diabetes Care 36, 273–275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kildegaard J, Buckley ST, Nielsen RH, Povlsen GK, Seested T, Ribel U, Olsen HB, Ludvigsen S, Jeppesen CB, Refsgaard HHF, Bendtsen KM, Kristensen NR, Hostrup S, Sturis J, Elucidating the Mechanism of Absorption of Fast-Acting Insulin Aspart: The Role of Niacinamide. Pharm. Res 36, 49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner S, Hompesch M, Pohl R, Simms P, Flacke F, Mohr T, Pfützner A, Heinemann L, A novel insulin formulation with a more rapid onset of action. Diabetologia 51, 1602–1606 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinemann L, Nosek L, Flacke F, Albus K, Krasner A, Pichotta P, Heise T, Steiner S, U-100, pH-Neutral formulation of VIAject®: faster onset of action than insulin lispro in patients with type 1 diabetes. Diabetes Obes. Metab 14, 222–227 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Krasner A, Pohl R, Simms P, Pichotta P, Hauser R, De Souza E, A review of a family of ultra-rapid-acting insulins: formulation development. J. Diabetes Sci. Technol 6, 786–796 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pohl R, Hauser R, Li M, De Souza E, Feldstein R, Seibert R, Ozhan K, Kashyap N, Steiner S, Ultra-rapid absorption of recombinant human insulin induced by zinc chelation and surface charge masking. J. Diabetes Sci. Technol 6, 755–763 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiramoto M, Nasu R, Oura T, Imori M, Ohwaki K, Ultra-Rapid Lispro results in accelerated insulin lispro absorption and faster early insulin action in comparison with Humalog® in Japanese patients with type 1 diabetes. Journal of Diabetes Investigation 11, 672–680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker RHA, Frick AD, Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin. Pharmacokinet 47, 7–20 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Maikawa CL, Smith AAA, Zou L, Roth GA, Gale EC, Stapleton LM, Baker SW, Mann JL, Yu AC, Correa S, Grosskopf AK, Liong CS, Meis CM, Chan D, Troxell MD, Maahs DM, Buckingham BA, Webber MJ, Appel EA, A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs. Nature Biomedical Engineering 4, 507–517 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haahr H, Heise T, Fast-Acting Insulin Aspart: A Review of its Pharmacokinetic and Pharmacodynamic Properties and the Clinical Consequences. Clin. Pharmacokinet 59, 155–172 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bode BW, Johnson JA, Hyveled L, Tamer SC, Demissie M, Improved Postprandial Glycemic Control with Faster-Acting Insulin Aspart in Patients with Type 1 Diabetes Using Continuous Subcutaneous Insulin Infusion. Diabetes technology & therapeutics 19, 25–33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dovc K, Piona C, Yeşiltepe Mutlu G, Bratina N, Jenko Bizjan B, Lepej D, Nimri R, Atlas E, Muller I, Kordonouri O, Biester T, Danne T, Phillip M, Battelino T, Faster Compared With Standard Insulin Aspart During Day-and-Night Fully Closed-Loop Insulin Therapy in Type 1 Diabetes: A Double-Blind Randomized Crossover Trial. Diabetes Care, dc190895 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Lal R, Hsu L, Wang J, Basina M, Buckingham B, Fiasp® (Fast-Acting Insulin Aspart) Use with a MedtronicTM 670G System. Journal of diabetes science and technology 14, 361–492 (2020). [Google Scholar]
- 43.Andersen G, Meiffren G, Lamers D, DeVries JH, Ranson A, Seroussi C, Alluis B, Gaudier M, Soula O, Heise T, Ultra-rapid BioChaperone Lispro improves postprandial blood glucose excursions vs insulin lispro in a 14-day crossover treatment study in people with type 1 diabetes. Diabetes Obes. Metab, (2018). [DOI] [PubMed] [Google Scholar]
- 44.Heise T, Meiffren G, Alluis B, Seroussi C, Ranson A, Arrubla J, Correia J, Gaudier M, Soula O, Soula R, DeVries JH, Klein O, Bode B, BioChaperone Lispro versus faster aspart and insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion: A randomized euglycemic clamp study. Diabetes Obes. Metab, (2018). [DOI] [PubMed] [Google Scholar]
- 45.Riddle MC, Yuen KCJ, de Bruin TW, Herrmann K, Xu J, Öhman P, Kolterman OG, Fixed ratio dosing of pramlintide with regular insulin before a standard meal in patients with type 1 diabetes. Diabetes Obes. Metab 17, 904–907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riddle MC, Nahra R, Han J, Castle J, Hanavan K, Hompesch M, Huffman D, Strange P, Öhman P, Control of Postprandial Hyperglycemia in Type 1 Diabetes by 24-Hour Fixed-Dose Coadministration of Pramlintide and Regular Human Insulin: A Randomized, Two-Way Crossover Study. Diabetes Care, (2018). [DOI] [PubMed] [Google Scholar]
- 47.Haidar A, Tsoukas M, Sarah T, Strauss N, Yale JF, Rutkowski J, Bossy A, Pytka E, Nguyen HT, Legault L, Insulin-plus-Pramlintide Artificial Pancreas in Type 1 Diabetes—Randomized Controlled Trial. Diabetes 67, 210–OR (2018). [Google Scholar]
- 48.Haidar A, Tsoukas MA, Bernier-Twardy S, Yale J-F, Rutkowski J, Bossy A, Pytka E, El Fathi A, Strauss N, Legault L, A Novel Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care 43, 597 (2020). [DOI] [PubMed] [Google Scholar]
- 49.da Silva DC, Lima LMTR, Physico-chemical properties of co-formulated fast-acting insulin with pramlintide. Int. J. Pharm, (2018). [DOI] [PubMed] [Google Scholar]
- 50.Meiffren G, Seroussi C, Ranson A, Charvet R, Gaudier M, Andersen G, Zijlstra E, Devries JH, Heise TIM, Soula R, Soula O, 150-OR: BioChaperone Pramlintide Insulin (BCPramIns), a New Co-Formulation of Pramlintide (PRAM) and Human Insulin (INS), Improves Postprandial Blood Glucose (BG) vs. Both Separate Injections of PRAM+INS and Insulin Lispro (LIS) in Subjects with T1D. Diabetes 68, 150–OR (2019). [Google Scholar]
- 51.Patil M, Deshmukh NJ, Patel M, Sangle GV, Glucagon-based therapy: Past, present and future. Peptides 127, 170296 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Castle JR, Youssef JE, Branigan D, Newswanger B, Strange P, Cummins M, Shi L, Prestrelski S, Comparative Pharmacokinetic/Pharmacodynamic Study of Liquid Stable Glucagon Versus Lyophilized Glucagon in Type 1 Diabetes Subjects. Journal of diabetes science and technology 10, 1101–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caputo N, Castle JR, Bergstrom CP, Carroll JM, Bakhtiani PA, Jackson MA, Roberts CT Jr., David LL, Ward WK, Mechanisms of glucagon degradation at alkaline pH. Peptides 45, 40–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caputo N, Jackson MA, Castle JR, El Youssef J, Bakhtiani PA, Bergstrom CP, Carroll JM, Breen ME, Leonard GL, David LL, Roberts CT Jr., Ward WK, Biochemical stabilization of glucagon at alkaline pH. Diabetes Technol. Ther 16, 747–758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pohl R, Li M, Krasner A, De Souza E, Development of stable liquid glucagon formulations for use in artificial pancreas. J. Diabetes Sci. Technol 9, 8–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steiner SS, Li M, Hauser R, Pohl R, Stabilized Glucagon Formulation for Bihormonal Pump Use. Journal of diabetes science and technology 4, 1332–1337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taleb N, Coriati A, Khazzaka C, Bayonne J, Messier V, Rabasa-Lhoret R, Stability of Commercially Available Glucagon Formulation for Dual-Hormone Artificial Pancreas Clinical Use. Diabetes Technol. Ther 19, 589–594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haidar A, Insulin-and-Glucagon Artificial Pancreas Versus Insulin-Alone Artificial Pancreas: A Short Review. Diabetes Spectrum 32, 215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaykov AN, Mayer JP, DiMarchi RD, Pursuit of a perfect insulin. Nature Reviews Drug Discovery 15, 425–439 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Home P, Kurtzhals P, Insulin detemir: from concept to clinical experience. Expert Opinion on Pharmacotherapy 7, 325–343 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Porksen NK, Linnebjerg H, Lam ECQ, Garhyan P, Pachori A, Pratley RE, Smith SR, Basal insulin peglispro increases lipid oxidation, metabolic flexibility, thermogenesis and ketone bodies compared to insulin glargine in subjects with type 1 diabetes mellitus. Diabetes, Obesity and Metabolism 20, 1193–1201 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Jacober SJ, Prince MJ, Beals JM, Hartman ML, Qu Y, Linnebjerg H, Garhyan P, Haupt A, Basal insulin peglispro: Overview of a novel long-acting insulin with reduced peripheral effect resulting in a hepato-preferential action. Diabetes Obes Metab 18, 3–16 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Qiu Y, Agrawal R, Chen D, Zheng N, Durupt G, Kim JH, Fisher SJ, Chou D. H. c., Long‐Lasting Designer Insulin with Glucose‐Dependent Solubility Markedly Reduces Risk of Hypoglycemia. Adv. Ther 2, 1900128 (2019). [Google Scholar]
- 64.Jensen MH, Wahlund P-O, Jacobsen JK, Vestergaard B, van de Weert M, Havelund S, Self-association of long-acting insulin analogues studied by size exclusion chromatography coupled to multi-angle light scattering. Journal of Chromatography B 879, 2945–2951 (2011). [DOI] [PubMed] [Google Scholar]
- 65.do Nascimento CVMF, Sinezia C, Sisnande T, Lima LMTR, Lacativa PGS, BZ043, a novel long-acting amylin analog, reduces gastric emptying, food intake, glycemia and insulin requirement in streptozotocin-induced diabetic rats. Peptides, (2019). [DOI] [PubMed] [Google Scholar]
- 66.Khodaverdi E, Heidari Z, Tabassi SAS, Tafaghodi M, Alibolandi M, Tekie FSM, Khameneh B, Hadizadeh F, Injectable supramolecular hydrogel from insulin-loaded triblock PCL-PEG-PCL copolymer and γ-cyclodextrin with sustained-release property. AAPS PharmSciTech 16, 140–149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravaine V, Ancla C, Catargi B, Chemically controlled closed-loop insulin delivery. Journal of Controlled Release 132, 2–11 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Yu J, Wang J, Zhang Y, Chen G, Mao W, Ye Y, Kahkoska AR, Buse JB, Langer R, Gu Z, Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nature Biomedical Engineering 4, 499–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou DH-C, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG, Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl. Acad. Sci. U. S. A 112, 2401–2406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Wong BCK, Chen H, Zhang S, Bian Z, Zhang G, Lin C, Riaz MK, Tyagi D, Lu A, Yang Z, Long-lasting Insulin Treatment Via a Single Subcutaneous Administration of Liposomes in Thermoreversible Pluronic® F127 Based Hydrogel. Curr. Pharm. Des 23, 6079–6085 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Yu J, Zhang Y, Bomba H, Gu Z, Stimuli-responsive delivery of therapeutics for diabetes treatment. Bioengineering & Translational Medicine 1, 323–337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakh NA, Cortinas AB, Weiss MA, Langer RS, Anderson DG, Gu Z, Dutta S, Strano MS, Glucose-responsive insulin by molecular and physical design. Nat. Chem 9, 937–943 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Yu J, Zhang Y, Kahkoska AR, Wang Z, Fang J, Whitelegge JP, Li S, Buse JB, Gu Z, Glucose transporter inhibitor-conjugated insulin mitigates hypoglycemia. Proc. Natl. Acad. Sci. U. S. A 116, 10744–10748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Yu J, Zhang Y, Zhang X, Kahkoska AR, Chen G, Wang Z, Sun W, Cai L, Chen Z, Qian C, Shen Q, Khademhosseini A, Buse JB, Gu Z, Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Science Advances 5, eaaw4357 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z, Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proceedings of the National Academy of Sciences 112, 8260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chap T Fau - Ishida Z, Ishida J Fau - Chou T, Chou CJ Fau - Hartley J, Hartley ML Fau - Entman Cj, Entman D Fau - Brandenburg Ml, Brandenburg RH Fau - Jones D, Jones JB Fau - Field Rh, Field JB, First-pass hepatic extraction and metabolic effects of insulin and insulin analogues. Am J Physiol 252, 209–217 (1987). [DOI] [PubMed] [Google Scholar]
- 77.Meier JJ, Veldhuis JD, Butler PC, Pulsatile Insulin Secretion Dictates Systemic Insulin Delivery by Regulating Hepatic Insulin Extraction In Humans. Diabetes 54, 1649 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Moore MC, Smith MS, Sinha VP, Beals JM, Michael MD, Jacober SJ, Cherrington AD, Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes 63, 494–504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knadler MP, Nguyen T-H, Campanale K, De Veer MJ, Beals JM, Li S, Hansen R, Siesky A, Michael MD, Porter CJH, Addition of 20-kDa PEG to Insulin Lispro Alters Absorption and Decreases Clearance in Animals. Pharm. Res 33, 2920–2929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owens RA, Hansen RJ, Kahl SD, Zhang C, Ruan X, Koester A, Li S, Qian H-R, Farmen MW, Michael MD, Moyers JS, Cutler GB Jr., Vick A, Beals JM, In Vivo and In Vitro Characterization of Basal Insulin Peglispro: A Novel Insulin Analog. J. Pharmacol. Exp. Ther 357, 459–465 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Tiffner K, Boulgaropoulos B, Höfferer C, Birngruber T, Porksen N, Linnebjerg H, Garhyan P, Lam ECQ, Knadler MP, Pieber TR, Sinner F, Quantification of Basal Insulin Peglispro and Human Insulin in Adipose Tissue Interstitial Fluid by Open-Flow Microperfusion. Diabetes Technol. Ther 19, 305–314 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Abramson A, Halperin F, Kim J, Traverso G, Quantifying the Value of Orally Delivered Biologic Therapies: A Cost-Effectiveness Analysis of Oral Semaglutide. J. Pharm. Sci 108, 3138–3145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajracharya R, Song JG, Back SY, Han H-K, Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Owens DR, Zinman B, Bolli G, Alternative routes of insulin delivery. Diabet. Med 20, 886–898 (2003). [DOI] [PubMed] [Google Scholar]
- 85.Whitehead K, Shen Z, Mitragotri S, Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J. Control. Release 98, 37–45 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Cheng H, Zhang X, Qin L, Huo Y, Cui Z, Liu C, Sun Y, Guan J, Mao S, Design of self-polymerized insulin loaded poly(n-butylcyanoacrylate) nanoparticles for tunable oral delivery [DOI] [PubMed]
- 87.Martínez-López AL, Carvajal-Millan E, Sotelo-Cruz N, Micard V, Rascón-Chu A, López-Franco YL, Lizardi-Mendoza J, Canett-Romero R, Enzymatically cross-linked arabinoxylan microspheres as oral insulin delivery system. International Journal of Biological Macromolecules 126, 952–959 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Banerjee A, Ibsen K, Brown T, Chen R, Agatemor C, Mitragotri S, Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. U. S. A 115, 7296–7301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamson NG, Berger A, Fein KC, Whitehead KA, Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng 4, 84–96 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han X, Lu Y, Xie J, Zhang E, Zhu H, Du H, Wang K, Song B, Yang C, Shi Y, Cao Z, Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nature Nanotechnology 15, 605–614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abramson A, Caffarel-Salvador E, Khang M, Dellal D, Silverstein D, Gao Y, Frederiksen MR, Vegge A, Hubálek F, Water JJ, Friderichsen AV, Fels J, Kirk RK, Cleveland C, Collins J, Tamang S, Hayward A, Landh T, Buckley ST, Roxhed N, Rahbek U, Langer R, Traverso G, An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banerjee A, Ibsen K, Iwao Y, Zakrewsky M, Mitragotri S, Transdermal Protein Delivery Using Choline and Geranate (CAGE) Deep Eutectic Solvent. Advanced Healthcare Materials 6, 1601411 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Tanner EEL, Ibsen KN, Mitragotri S, Transdermal insulin delivery using choline-based ionic liquids (CAGE). Journal of Controlled Release 286, 137–144 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Abramson A, Caffarel-Salvador E, Soares V, Minahan D, Tian RY, Lu X, Dellal D, Gao Y, Kim S, Wainer J, Collins J, Tamang S, Hayward A, Yoshitake T, Lee H-C, Fujimoto J, Fels J, Frederiksen MR, Rahbek U, Roxhed N, Langer R, Traverso G, A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med 25, 1512–1518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hadebe SI, Ngubane PS, Serumula MR, Musabayane CT, Transdermal delivery of insulin by amidated pectin hydrogel matrix patch in streptozotocin-induced diabetic rats: effects on some selected metabolic parameters. PLoS One 9, e101461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Marwah H, Garg T, Rath G, Goyal AK, Development of transferosomal gel for trans-dermal delivery of insulin using iodine complex. Drug Deliv 23, 1636–1644 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Pettis RJ, Hirsch L, Kapitza C, Nosek L, Hövelmann U, Kurth H-J, Sutter DE, Harvey NG, Heinemann L, Microneedle-based intradermal versus subcutaneous administration of regular human insulin or insulin lispro: pharmacokinetics and postprandial glycemic excursions in patients with type 1 diabetes. Diabetes Technol. Ther 13, 443–450 (2011). [DOI] [PubMed] [Google Scholar]
- 98.Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD, Insulin delivery systems combined with microneedle technology. Advanced Drug Delivery Reviews 127, 119–137 (2018). [DOI] [PubMed] [Google Scholar]
- 99.GhavamiNejad A, Li J, Lu B, Zhou L, Lam L, Giacca A, Wu XY, Glucose-Responsive Composite Microneedle Patch for Hypoglycemia-Triggered Delivery of Native Glucagon. Adv. Mater 31, e1901051 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Tanner EEL, Curreri AM, Balkaran JPR, Selig-Wober NC, Yang AB, Kendig C, Fluhr MP, Kim N, Mitragotri S, Design Principles of Ionic Liquids for Transdermal Drug Delivery. Advanced Materials 31, 1901103 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z, Advances in transdermal insulin delivery. Advanced Drug Delivery Reviews 139, 51–70 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, Dua K, Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomedicine & Pharmacotherapy 109, 1249–1258 (2019). [DOI] [PubMed] [Google Scholar]
- 103.Chen BZ, Liu JL, Li QY, Wang ZN, Zhang XP, Shen CB, Cui Y, Guo XD, Safety Evaluation of Solid Polymer Microneedles in Human Volunteers at Different Application Sites. ACS Applied Bio Materials 2, 5616–5625 (2019). [DOI] [PubMed] [Google Scholar]
- 104.Oleck J, Kassam S, Goldman JD, Commentary: Why Was Inhaled Insulin a Failure in the Market? Diabetes Spectr 29, 180–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heinemann L, Baughman R, Boss A, Hompesch M, Pharmacokinetic and Pharmacodynamic Properties of a Novel Inhaled Insulin. [DOI] [PMC free article] [PubMed]
- 106.Boss AH, Petrucci RF, Lorber D, Coverage of prandial insulin requirements by means of an ultra-rapid-acting inhaled insulin. J Diabetes Sci Technol 6, 773–779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.King AJF, The use of animal models in diabetes research. Br. J. Pharmacol 166, 877–894 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Renner S, Dobenecker B, Blutke A, Zols S, Wanke R, Ritzmann M, Wolf E, Comparative aspects of rodent and nonrodent animal models for mechanistic and translational diabetes research. Theriogenology 86, 406–421 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Wu KK, Huan Y, Streptozotocin-induced diabetic models in mice and rats. Current Protocols in Pharmacology, 5.47.41–45.47.14 (2008). [DOI] [PubMed] [Google Scholar]
- 110.Plum A, Agersø H, Andersen L, Pharmacokinetics of the Rapid-Acting Insulin Analog, Insulin Aspart, in Rats, Dogs, and Pigs, and Pharmacodynamics of Insulin Aspart in Pigs. Drug Metabolism and Disposition 28, 155 (2000). [PubMed] [Google Scholar]
- 111.Larsen MO, Rolin B, Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J 45, 303–313 (2004). [DOI] [PubMed] [Google Scholar]


