Abstract
Background and aim
COVID-19 infection predisposes to diabetic ketoacidosis(DKA); whether glucocorticoids enhances this risk is unknown.We aimed to study the occurrence of DKA after initiating glucocorticoids in patients with type 2 diabetes mellitus(T2DM) and moderate-to-severe COVID-19, and identify predictors for it.
Methods
Patients with T2DM and moderate or severe COVID-19 infection were prospectively observed for development of new-onset DKA for one week following initiation of parenteral dexamethasone. Clinical and biochemical parameters were compared between those who developed DKA (Group A) and those who didnot (Group B). Logistic regression was done to identify independent risk-factors predicting DKA; ROC-curve analysis to determine cut-offs for the parameters in predicting DKA.
Results
Amongst 302 patients screened, n = 196 were finally included, of whom 13.2% (n = 26,Group A) developed DKA. Patients in Group A were younger, had lower BMI, increased severity of COVID-19 infection, higher HbA1c%, CRP, IL-6, D-dimer and procalcitonin at admission (pall < 0.02). Further, admission BMI (OR: 0.43, CI: 0.27–0.69), HbA1c % (OR: 1.68, CI: 1.16–2.43) and serum IL-6 (OR: 1.02, CI: 1.01–1.03) emerged as independent predictors for DKA. Out of these, IL-6 levels had the highest AUROC (0.93, CI: 0.89–0.98) with a cut-off of 50.95 pg/ml yielding a sensitivity of 88% and specificity of 85.2% in predicting DKA.
Conclusion
There is significant incidence of new-onset DKA following parenteral glucocorticoids in T2DM patients with COVID-19, especially in those with BMI <25.56 kg/m2, HbA1c% >8.35% and IL-6 levels >50.95 pg/ml at admission.
Keywords: COVID-19, Diabetic ketoacidosis, Dexamethasone, IL-6, HbA1c%
1. Introduction
The bidirectional relationship between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19) and diabetes mellitus (DM) is now well established. While DM increases the risk for Acute Respiratory Distress Syndrome (ARDS) and multisystem complications related to COVID-19 infection, SARS-CoV2 infection has been found to precipitate new onset diabetes and severe metabolic complications in patients with pre-existing DM [1,2]. In India, Type 2 diabetes mellitus (T2DM) has emerged as the single largest underlying comorbidity contributing to mortality in patients with COVID-19, and the presence of either or both of the two comorbidities, hypertension and DM, are present in up to 55% of COVID-19 related deaths [3].
Following the preliminary results of the RECOVERY trial, dexamethasone is recommended in the treatment of cases with severe COVID-19 infection or moderate cases with persistent hypoxia [4]. The occurrence of diabetic ketoacidosis (DKA) in patients with T2DM is rare, and though viral infections and glucocorticoids are known to act as precipitating factors, the occurrence of DKA following glucocorticoid therapy has been sparsely reported in literature [5,6]. There have been several reports of ketoacidosis precipitated by COVID-19 infection in patients with known DM and even in previously normoglycemic individuals [[7], [8], [9], [10]], with impairment in both insulin secretion and sensitivity being implicated in the pathogenesis [9,10]. Whether initiation of parenteral glucocorticoids can potentially worsen the ketoacidotic milieu and increase the risk of DKA in these patients remains unknown.
Increased mortality and RAAS-mediated fluid and electrolyte alterations reported with DKA in COVID-19 patients present unique clinical challenges necessitating meticulous management [[11], [12], [13]]. Thus, analysing the association of parenteral glucocorticoids with new-onset DKA and identifying its potential predictors can have significant therapeutic ramifications.
The current study was done with the primary objective of evaluating the occurrence of new-onset DKA with initiation of parenteral glucocorticoid therapy in patients with T2DM and moderate to severe COVID-19 infection, and to identify clinical and biochemical factors that influence the development of DKA in them. The secondary objective was to derive cut-off values for these factors that can help in predicting the increased risk of DKA at admission.
2. Materials and methods
2.1. Subjects
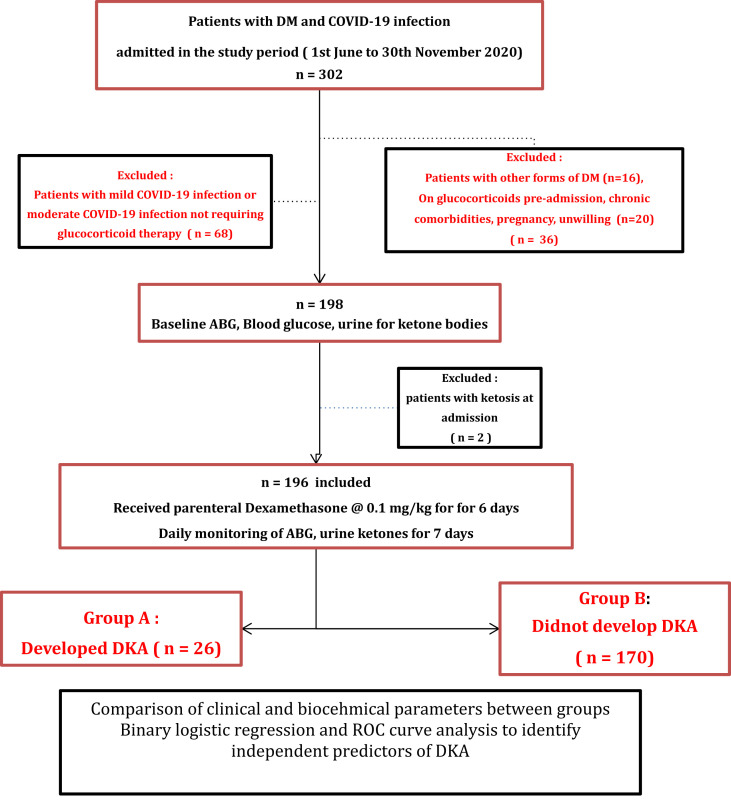
The screening population comprised of patients with known T2DM and rt-PCR confirmed SARS-CoV2 admitted to a tertiary care hospital of Eastern India between June 1, 2020 to November 30, 2020 (n = 302).
2.2. Materials and methods
For the current study, we screened consecutive patients with T2DM and rt-PCR confirmed SARS-CoV2. As outlined in Fig. 1 , patients conforming to the inclusion criteria and filling the informed consent forms, underwent baseline clinical and biochemical evaluation and, once initiated on parenteral dexamethasone therapy, were monitored with capillary blood glucose levels (done before and 2 h after every meal), Arterial Blood Gas analysis (ABG) and urine ketones done daily for the subsequent one week to detect onset of DKA. Clinical and biochemical parameters were eventually compared between those who developed DKA (n = 26) and those who did not develop DKA (n = 170) after dexamethasone therapy. Ethical clearance for the current project was obtained from the institutional ethical committee reference no HWH/IEC-BMHR/001/2020.
Fig. 1.
Study design.
DKA = Diabetic ketoacidosis, DM = Diabetes Mellitus, ABG = Arterial Blood Gas.
Consecutive patients with T2DM and severe COVID-19 infection or moderate COVID-19 infection fulfilling the criteria for glucocorticoid therapy were included. Severity of COVID-19 pneumonia was graded as per clinical protocol laid down by the Ministry of Health and Family Welfare (MoHFW), India [14]. Moderate COVID-19 infection was defined as clinical features of dyspnea and or hypoxia, fever, cough, respiratory rate > = 24/minute or SpO2 < 94% but > 90% at room air. Severe COVID-9 infection was defined was defined as the presence of severe pneumonia, ARDS or MODS. Severe pneumonia was defined as clinical signs of pneumonia with respiratory rate > 30 breaths/min, severe respiratory distress, SpO2 < 90% at room air. ARDS was defined as clinically new or worsening symptoms within one week of onset of symptoms, imaging evidence suggestive of pulmonary infiltrates not explained by cardiac failure or fluid overload, and oxygenation impairment. MODS was defined as signs of acute life-threatening organ dysfunction in the form of altered mental status, difficult or fast breathing, low SpO2, oliguria, tachycardia, weak pulse, hypotension, cold extremities or skin mottling, or laboratory evidence of coagulopathy, thrombocytopenia, acidosis, high serum lactate or hyperbilirubinemia. Rigorous inclusion and exclusion criteria, as summarized in Fig. 1, were followed to select the study subjects. Patients requiring immediate endotracheal intubation or mechanical ventilation, those with known auto-antibody positivity for GAD-65 or IA-2, secondary or pancreatic diabetes and those who had already received or were on concurrent glucocorticoids prior to admission were excluded. Further, patients with prolonged starvation, chronic alcoholism, decompensated chronic liver disease or advanced chronic kidney disease (CKD stages 4 and 5), known malignancies, pregnancy and patients unable/unwilling to give informed consent were also excluded. All the patients received usual standard of care for management of moderate to severe COVID-19 infection as per Indian national guidelines [14]. Additionally, parenteral dexamethasone was administered to all at a standard dose of 0.1 mg/kg for six consecutive days following admission. DKA and severity of DKA were defined using standard criteria [15]. Management of hyperglycemia and DKA was done in accordance with standard of care recommendations [15].
Biochemical investigations done at admission included serum levels of C reactive protein (CRP), Interleukin-6 (IL-6), D-dimer and Procalcitonin, in addition to complete blood count, electrolytes, renal and liver function tests. Serum CRP was measured using particle-enhanced immunoturbidimetry by Integra 400 + analyser (Roche Diagnostics, Rotkreuz, Switzerland), serum IL-6 and procalcitonin by electrochemiluminiscence assay using Cobas e411 analyser (Roche Diagnostics) and D –dimer using quantitative ELISA by Vidas D-dimer (Biomerieux). Semiquantitative estimation of urine ketones was done using KetoDiastix reagent strips by Bayer Diagnostics, India and simultaneous urine sample analysis for ketone body estimation using Cobas - u411 analyser. Capillary blood glucose measurements were done before and 2 h after every meals using Accu-Check Active glucometer and strips following standard measures and precautions. ABG analysis was done using Gem Premier 3500 from Instrumentation Laboratory.
2.3. Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc.,Chicago, Illinois). The two groups were compared using unpaired t-test or Mann-Whitney’s U test for quantitative variables and Chi-square test, with Fisher’s correction where appropriate, for categorical variables. A p value≤0.05 was considered significant. Multiple logistic regression to identify the independent predictors of DKA was done in two steps - univariate analysis (step 1) followed by multivariate analysis using independent variables which were found to be significant (p < 0.05) or suggestive of significance (p < 0.100) in step 1. ROC curves were constructed for variables that can predict DKA and cut-offs were determined using Youden’s index in conjunction with ROC analysis. AUC≥0.5 was considered significant.
3. Results
For the current study, n = 302 patients with T2DM and COVID-19 infection were screened during the study period of six months, of which n = 196 were included for the final analysis Overall, the mean age of the study population (n = 196) was 59·4 (±10·8) years and 37·9% were females. The mean baseline HbA1c was 8·1 (±1·7) % (65 mmol/mmol). The average time duration between the onset of symptoms of COVID-19 to admission was 8·7 ( ±3·4) days. At admission, forty nine (28·9%) of the patients had severe COVID-19 infection.
All these subjects (n = 196) were initiated on parenteral dexamethasone and monitored for development of new-onset DKA. During one week of dexamethasone therapy, n = 26 (13·2%) developed DKA (Group A) while n = 170 (86·8%) had no DKA (Group B). Comparative analysis of clinical and biochemical characteristics were made between these two groups. (Table 1 ). Those in Group A were younger in age, had lower BMI and higher mean HbA1c% than group B (54·8 vs 60·1 years, p = 0·02; 24·85 vs 27·36 kg/m2, p < 0·0001 and 10·11% vs 7·72% [87 mmol/mol vs 61 mmol/mol], p < 0·0001 respectively). Severe COVID-19 infection was more common in group A (n = 11, 42·3%) than in group B (n = 38, 22·8%). The median CRP (mg/l), IL-6 (pg/ml), procalcitonin (ng/ml) and D-dimer levels (ng/ml) at admission were all higher in group A than group B (p all < 0·001). The SpO2% at admission of the patients and the PaO2, PaCO2 in the initial ABG in the two groups were not significantly different between the two groups. There were no differences in the rates of pre-admission use of insulin or any oral hypoglycemic agents including SGLT2 inhibitors in the two groups (Table 1).
Table 1.
Comparison of parameters between those who developed diabetic ketoacidosis (DKA) and those who didnot develop DKAa.
| Parameter | Patients developing DKA (n = 26) | Patients not developing DKA (n = 170) | p |
|---|---|---|---|
| Age (years) | 54·81 (11·7) | 60·1 (10·6) | 0·02 |
| BMI (kg/m2) | 24·85 (1·92) | 27·36 (1·66) | <0·0001 |
| Duration of DM (years) | 8·1 (2·1) | 11·04 (3·2) | 0·07 |
| HbA1c(%) at admission | 10·11 (1·92) | 7·7 (1·47) | <0·0001 |
| Prevalence of macrovascular complications related to DM | 6 (23·1%) | 48 (28·2%) | 0·58 |
| Prevalence of microvascular complications related to Diabetes | 7 (26·9%) | 52 (30·5%) | 0·7 |
| Prior insulin therapy | 8 (30·8%) | 36 (21·1%) | 0·27 |
| Prior oral anti-diabetic medications Metformin DPP4i Sulfonylureas Pioglitazone Alpha-glucosidase inhibitor SGLT2i |
25 (96·2%) 18 (69·2%) 13 (50%) 9 (34·6%) 8 (30·8%) 11 (42·3%) |
152 (84·4%) 13 (76·5%) 99 (58·2%) 45 (26·4%) 82 (48·2%) 45 (26·5%) |
0·48 0·73 0·43 0·39 0·09 0·09 |
| Duration since onset of symptoms of COVID-19 to admission (days) | 9 (2) | 8 (1) | 0·27 |
| Prevalence of severe COVID-19 infection n (%) | 11(42·3%) | 38 (22·3%) | 0·01 |
| CRP at admission (mg/L) | 103·40 (52·44) | 34·70 (22·15) | <0·0001 |
| IL-6 at admission (pg/ml) | 141·70 (71·90) | 12·80 (7·20) | <0·0001 |
| Procalcitonin at admission (ng/ml) | 0·45 (0·20) | 0·14 (0·08) | <0·0001 |
| D - dimer levels at admission (ng/ml) | 3579 (666·20) | 1323 (134·70) | <0·0001 |
| SpO2% at admission | 77 (7.4) | 80 (12.2) | 0.87 |
| PaO2 at admission | 73.6 (8.9) | 77.4 (10.3) | 0.74 |
| Outcome | Recovery: 23 (88·4%) Death: 3 (11·5%) |
Recovery:162 (95·3%) Death: 8 (4·7%) |
0·16 |
Values are mentioned in mean (SD) for quantitative or n (%) for prevalence.The values of CRP, IL-6 and procalcitonin are expressed in median (IQR). DM = Diabetes Mellitus, OHA = Oral hypoglycemic agents, DPP4i = Dipeptidyl peptidase-4 inhibitor, SGLT2i = Sodium-Glucose co-transporter-2,CRP = C reacrive protein, IL-6 = Interleukin-6. p < 0.05 taken as significant.
Amongst those in group A, DKA was severe in n = 4 (15.3%), moderate in n = 8 (30.7%) and mild in n = 14 (53.8%) subjects. Twenty three of them (88.4%) had complete recovery while three patients (11.5%) died during admission. The median (IQR) time to occurrence of DKA following initiation of parenteral glucocorticoids was 18 (IQR: 15–26) hours and the median time to resolution of DKA was 43 (IQR: 18–52) hours . The mean total daily dose of insulin requirement in this group till resolution of DKA was 2.1 (±0.1) units/kg body weight .
3.1. Regression analysis
Multiple logistic regression analysis was performed to assess the impact of multiple parameters on the likelihood of occurrence of DKA following parenteral dexamethasone administration. Univariate analysis revealed six parameters, namely BMI, severity of COVID-19 infection, HbA1c %, serum levels of CRP, IL-6 and D-dimer at admission to be significantly associated with the occurrence of new-onset DKA (pall < 0·01). Subsequently, multivariate analysis revealed that the full model containing all the parameters was statistically significant in predicting the occurrence of DKA following dexamethasone administration [ χ2 (5, n = 170) = 97·6, p < 0·001]. Further, only three of the initial six independent variables made a statistically significant contribution to the model, namely BMI (OR: 0·43, CI: 0·27–0·69) , HbA1c % (OR: 1·68, CI: 1·16–2·43) and serum IL-6 levels at admission (OR: 1·02, CI: 1·01–1·03).
3.2. ROC curve analysis
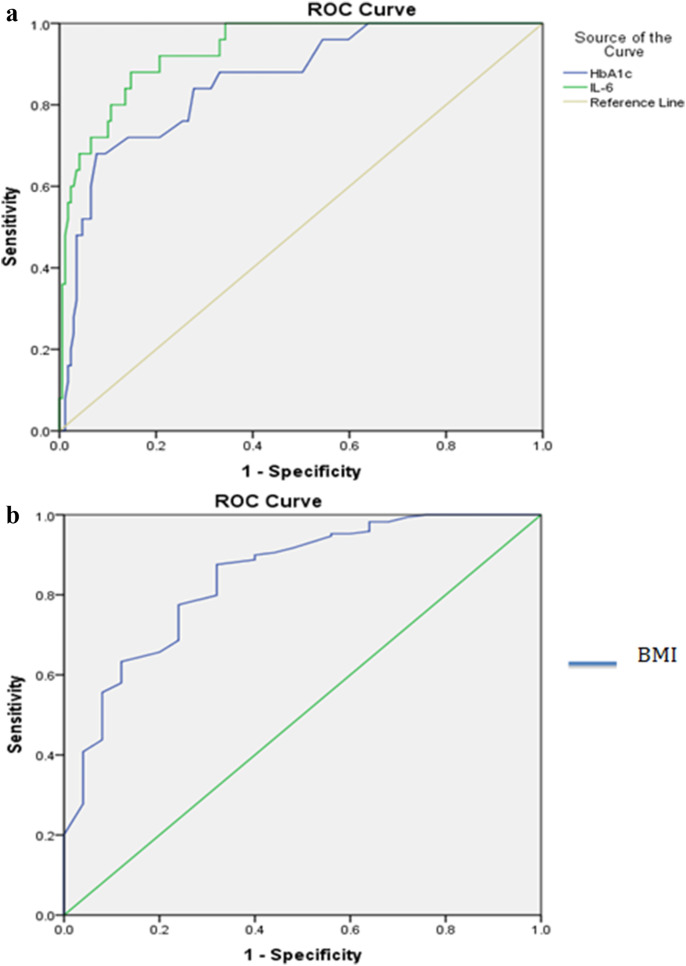
ROC curves were constructed for BMI, HbA1c % and serum IL-6 levels as predictors of DKA at admission. Serum IL- 6 levels showed the highest AUROC of 0·93 (CI: 0·89–0·98, p = 0·001) amongst the three [Table 2 ]. At a cut-off of 50·95 pg/ml, serum IL-6 levels at admission predicted the occurrence of DKA with a sensitivity of 88%, specificity of 85·2% and LR of 5·95. Similarly, cut-offs were derived for HbA1c% and BMI, though they displayed lower sensitivity and specificity than serum IL-6 levels (Table 2, Fig. 2 ).
Table 2.
Comparison of AUROC, sensitivity and specificity of HbA1c%, serum IL-6 levels and BMI in predicting DKA in patients with T2DM and COVID-19 infection receiving glucocorticoids.
| Parameter | AUROC (CI) | Cut-off | Sensitivity (%) | Specificity (%) | Youden’s index | LR |
|---|---|---|---|---|---|---|
| IL-6 levels at admission (pg/ml) | 0·93 (0·89–0·98) | 50·95 | 88 | 85·2 | 0·73 | 5·95 |
| HbA1c (%) | 0·86 (0·75–0·94) | 8·35 | 84 | 72·2 | 0·56 | 4·51 |
| BMI (kg/m2) | 0·84 (0·76–0·93) | 25·56 | 87·6 | 68 | 0·56 | 2·73 |
AUROC = Area under Receiver Operating Characteristic Curve, CI = Confidence Interval, BMI = Body Mass Index, LR = Likelihood ratio.
Fig. 2.
ROC curves of HbA1c% and serum IL-6 levels (1a) and BMI (1b) at admission.
4. Discussion
In our cohort of 196 Indian T2DM patients with moderate or severe COVID-19 infection, 13·2% (n = 26) developed new-onset DKA after initiation of parenteral dexamethasone therapy. To the best of our knowledge, there is no previously reported study in literature evaluating the occurrence of new-onset DKA with parenteral glucocorticoid use in this cohort of patients. A high incidence of DKA has been reported in other studies in patients hospitalised with COVID-19 infection [10,16]. In a hospital based retrospective review of 218 patients with COVID-19 in the UK, the authors have reported an overall prevalence of 2% (4 cases), but the prevalence increases to around 7% amongst subjects with DM [16]. However, this study considered patients of DM with COVID-19 infection of any severity and had no separate subset of glucocorticoid associated DKA. In most reported case series of COVID-19 with DKA published till date, around 75–90% had T2DM while few had new onset DM following COVID- 19 infection [10,13]. We have purposefully excluded patients with all other forms of DM including new onset DM following COVID-19 in order to ensure homogeneity of the study population.
SARS-CoV-2 is known to enter pancreatic β cells via ACE2 receptor and cause downregulation of ACE2, which in turn potentiates Ang-II levels, with the latter exerting pro-inflammatory responses and beta cell cytotoxic responses through its receptors (AT1 or AT2) to impede insulin secretion [[20], [21], [22]]. Moderate to severe COVID 19 infection further increases the susceptibility to DKA through deterioration in peripheral glucose uptake mediated by release of pro-inflammatory cytokines. Though glucocorticoids are known to cause hyperglycemia, the risk of DKA following glucocorticoid use is mostly limited to case reports and is seen more with acute than chronic use [5,6,17].. Glucocorticoids worsen insulin resistance through a myriad of pathways involving reduced muscle GLUT4 transporters, increased lipolysis and proteolysis and enhanced effects of counter-regulatory hormones such as glucagon and epinephrine [18,19]. Simultaneously, there is also downgradation of the hyperinsulinemic response which is usually seen in the pancreatic β cells as a compensatory mechanism to the increase in insulin resistance. In extreme settings, such as those with moderate to severe COVID-19 infection, the absence of the anti-lipolytic effect of insulin may result in DKA. Thus the combined adverse metabolic effects of glucocorticoids and COVID-19 infection can explain the increased propensity of DKA seen in our study population.
In our study, the patients who developed DKA following parenteral dexamethasone had lower BMI, higher HbA1c %, higher prevalence of severe COVID-19 infection and higher serum levels of pro-inflammatory cytokines including CRP, IL-6 and D-dimer. Low BMI, high HbA1c % and serum IL-6 levels at admission were independent predictors of the risk for DKA. Low BMI and higher HbA1c % have previously been identified as risk factors for DKA in young adults with type 1 DM [23,24]. Another study on DKA in COVID-19 patients highlighted that patients were younger and had higher rates of ARDS, acute liver injury and requirement for mechanical ventilation, underpinning more severe COVID-19 infection in those with DKA than in those without [10]. Severe COVID-19 disease is accompanied by higher levels of inflammatory cytokines, which are also elevated in the setting of DKA, independent of accompanying illnesses [25,26]. IL-6 has been postulated to play a very important role in the maladaptive immune response to SARS-CoV-2 virus and is being studied as a possible treatment target [27]. Our finding of IL-6 as an independent predictor of DKA strongly suggests that in patients with moderate to severe COVID-19 infection, pro-inflammatory cytokines serve as one of the most important pathogenetic factors. Elevated IL-6 levels can impair insulin sensitivity, through SOCS- mediated pathways, as also insulin secretion, predominantly through macrophage-induced inflammation and subsequent beta-cell apoptosis, amyloidosis and fibrosis. A longer time to resolution of DKA, as seen in the current study, has been reported in previous studies in COVID-19 patients [13,28]. The daily insulin requirement for resolution of DKA in our study population was higher than the usually recommended dosage required in type 1 DM subjects [15]. This can probably be attributed to increased insulin resistance in our patients as a result of the combined effects of parenteral glucocorticoid therapy and elevated pro-inflammatory cytokines.
Our results suggest that duration of diabetes, duration of COVID-19 related symptoms, the presence of preexisting macrovascular or microvascular complications of DM or prior antihyperglycemic pharmacotherapy do not influence development of DKA with dexamethasone use. Though there were no significant differences in pre-admission use of insulin, SGLT-2 inhibitors (SGLT2i) or any other oral anti-diabetic medications between the two groups, caution should be exercised before initiating SGLT2i in these patients, especially if they have any of the risk predictors, namely BMI < 25.56 kg/m2, HbA1c% > 8.35 and IL-6 > 50.95 pg/ml at admission.
Upon ROC-analysis, we found admission serum IL-6 levels were superior to HbA1c% or BMI in predicting the occurrence of DKA. Further, we demonstrated that admission serum IL-6 greater than 50·95 pg/ml, HbA1c % greater than 8·35% and BMI less than 25·56 kg/m2 can aid in early identification of patients at higher risk for DKA, who in turn would require more stringent monitoring and intensive care for better outcomes. Previous studies done amongst children and young adults with type 1 DM have demonstrated the association of lower BMI with increased risk for DKA, but failed to provide definite cut-off values [29,30]. Similarly, raised levels of IL-6 have shown predictive potential in adult patients with DKA, especially in the presence of an underlying infection [31,32]. Though decline in IL-6 levels have been seen with correction of DKA, there is a lack of established cut-off values that can predict DKA [26,30]. In a study on patients with T2DM developing DKA, ketoacidosis was more likely with HbA1c values at ≥10·1% in patients with newly diagnosed T2DM and at ≥8·6% in patients with a known history of T2DM respectively [32]. However, such HbA1c% cut-offs have not previously been reported for T2DM patients with concomitant COVID-19 infection.
The strengths of our study include a prospective design, stringent inclusion and exclusion criteria and meticulous monitoring of all study subjects for the development of DKA during the entire duration of parenteral dexamethasone use. We have put forth a set of clinically relevant predictors with suitable cut-off values that will aid in early identification and management of those susceptible to develop DKA following parenteral dexamethasone. A single-centre study with relatively smaller sample size and shorter study duration are potential limitations that need to be offset in future larger, multicentric studies. Moreover, comparison with patients with T2DM and COVID-19 of similar severity but not receiving glucocorticoids and with those receiving parenteral glucocorticoids for non-COVID related illnesses, inclusion of a subgroup receiving oral glucocorticoids and elucidation of underlying metabolic pathways of altered insulin secretion and sensitivity, would help in understanding this unique clinical entity better. The effect of preexisting undetected severe hyperglycemia leading to a catabolic state on the onset of DKA after glucocorticoid therapy needs further evaluation in samples involving cases with new onset Diabetes Mellitus following admission.
Funding sources
None.
Author contributions
Author 1 (SM) was involved in formulating the concept of the project, analysis of raw data with interpretation and formulating the initial draft. Author 2 (RD) contributed to the concept and design of the project, critical analysis of data and revising and editing the draft incorporating important intellectual content. Author 3 (ML) was involved in conduct of all relevant biochemical investigations in the laboratory and interpretation of data. Author 4 (RG), author 5 (BC) and author 6 (AH) were actively involved rigid implementation of the study methodology and protocol in hospital wards and ICUs and raw data collection and data verification. Author 7 (AG) was involved in data verification, supervising the entire project and revising the data and draft critically.
Data availability
The raw data file and analysed datasets can be available from the corresponding author on reasonable request by e-mail to sunetra59@gmail.com.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank all the nurses, critical care technicians and laboratory technicians working in the critical care settings and emergency wards dedicated to management of COVID-19 infected patients in our hospital.
References
- 1.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet. Respiratory Medicine. 2020 Apr;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker D.J. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr Rev. 2020 Jun;41(3):bnaa011. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.shortpedia.com/en-in/miscellaneous-news/hypertension-diabetes-among-major-comorbidities-in-coronavirus-deaths-in-mumbai-1602829161 Available from:
- 4.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19-preliminary report. N Engl J Med. 2020 Jun doi: 10.1056/NEJMoa2021436. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwari A., Al-Robeh H., Sharma H., Ammari Z., Khan M.S., Jaume J.C. Steroid-induced diabetic ketoacidosis in a patient with type 2 diabetes mellitus. AACE Clinical Case Reports. 2018 Mar;4(2):e131–e133. [Google Scholar]
- 6.Jabbar A., Farooqui K., Habib A., Islam N., Haque N., Akhter J. Clinical characteristics and outcomes of diabetic ketoacidosis in Pakistani adults with Type 2 diabetes mellitus. Diabet Med. 2004 Aug;21(8):920–923. doi: 10.1111/j.1464-5491.2004.01249.x. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P.K., Kuchay M.S., Mehta Y., Mishra S.K. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes & Metabolic Syndrome: Clin Res Rev. 2020 Sep 1;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oriot P., Hermans M.P. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg. 2020 Jun 18:1–5. doi: 10.1080/17843286.2020.1780390. [DOI] [PubMed] [Google Scholar]
- 9.Chee Y.J., Ng S.J., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020 Jun;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020 Oct;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metabol. 2020 Aug;105(8):2819–2829. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamorro-Pareja N, Parthasarathy S, Annam J, Hoffman J, Coyle C, Kishore P. Unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism. [DOI] [PMC free article] [PubMed]
- 13.Pal R., Banerjee M., Yadav U., Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes & Metabolic Syndrome: Clin Res Rev. 2020 Nov 1;14(6):1563–1569. doi: 10.1016/j.dsx.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf Available from:
- 15.Kohler K., Levy N. Management of diabetic ketoacidosis: a summary of the 2013 Joint British Diabetes Societies guidelines. Journal of the Intensive Care Society. 2014 Jul;15(3):222–225. [Google Scholar]
- 16.Goldman N., Fink D., Cai J., Lee Y.N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020 Aug 1;166:108291. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alakkas Z., Alzaedi O.A., Somannavar S.S., Alfaifi A. Steroid-induced diabetes ketoacidosis in an immune thrombocytopenia patient: a case report and literature review. The American Journal of Case Reports. 2020;21 doi: 10.12659/AJCR.923372. e923372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez A., Jansen-Chaparro S., Saigi I., Bernal-Lopez M.R., Miñambres I., Gomez-Huelgas R. Glucocorticoid-induced hyperglycemia (glucocorticoid-induced hyperglycemia) J Diabetes. 2014 Jan;6(1):9–20. doi: 10.1111/1753-0407.12090. [DOI] [PubMed] [Google Scholar]
- 19.Clore J., Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009 Jul 1;15(5):469–474. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 20.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 May;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 21.Obukhov A.G., Stevens B.R., Prasad R., Calzi S.L., Boulton M.E., Raizada M.K., Oudit G.Y., Grant M.B. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020 Sep 1;69(9):1875–1886. doi: 10.2337/dbi20-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010 Sep 1;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usher-Smith J.A., Thompson M.J., Sharp S.J., Walter F.M. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. Br Med J. 2011 Jul 7;343:d4092. doi: 10.1136/bmj.d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstock R.S., Xing D., Maahs D.M., Michels A., Rickels M.R., Peters A.L., Bergenstal R.M., Harris B., DuBose S.N., Miller K.M., Beck R.W. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metabol. 2013 Aug 1;98(8):3411–3419. doi: 10.1210/jc.2013-1589. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed G.A., Abd-Elrahman M.Z., Bahriz R., Albehairy A. Inflammatory cytokine and plasma C-reactive protein response to ketoacidosis in adults with type 1 diabetes: Egyptian multicenter study. The Egyptian Journal of Internal Medicine. 2020 Dec;32(1):1–8. [Google Scholar]
- 26.Hoffman W.H., Burek C.L., Waller J.L., Fisher L.E., Jr., Khichi M., Mellick L.B. Cytokine response to diabetic ketoacidosis and its treatment. Clin Immunol. 2003 Sep 1;108(3):175–181. doi: 10.1016/s1521-6616(03)00144-x. [DOI] [PubMed] [Google Scholar]
- 27.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat Rev Immunol. 2020 Jun;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armeni E., Aziz U., Qamar S., Nasir S., Nethaji C., Negus R., Murch N., Beynon H.C., Bouloux P., Rosenthal M., Khan S. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. The Lancet Diabetes & Endocrinology. 2020 Aug 1;8(8):660–663. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schober E., Rami B., Waldhoer T. Austrian Diabetes Incidence Study Group. Diabetic ketoacidosis at diagnosis in Austrian children in 1989–2008: a population-based analysis. Diabetologia. 2010 Jun 1;53(6):1057–1061. doi: 10.1007/s00125-010-1704-1. [DOI] [PubMed] [Google Scholar]
- 30.Hekkala A., Reunanen A., Koski M., Knip M., Veijola R., Finnish Pediatric Diabetes Register Age-related differences in the frequency of ketoacidosis at diagnosis of type 1 diabetes in children and adolescents. Diabetes Care. 2010 Jul 1;33(7):1500–1502. doi: 10.2337/dc09-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogos C.A., Giali S., Paliogianni F., Dimitracopoulos G., Bassaris H.P., Vagenakis A.G. Interleukin-6 and C-reactive protein as early markers of sepsis in patients with diabetic ketoacidosis or hyperosmosis. Diabetologia. 2001 Aug 1;44(8):1011–1014. doi: 10.1007/s001250100592. [DOI] [PubMed] [Google Scholar]
- 32.Zhu B., Bu L., Zhang M., Gusdon A.M., Zheng L., Rampersad S., Li J., Qu S. HbA 1c as a screening tool for ketosis in patients with type 2 diabetes mellitus. Sci Rep. 2016 Dec 23;6:39687. doi: 10.1038/srep39687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data file and analysed datasets can be available from the corresponding author on reasonable request by e-mail to sunetra59@gmail.com.