Abstract
Purpose
The standard treatment of hypothyroidism is levothyroxine (LT4), which is available as tablets or soft-gel capsules in Denmark. This study aimed to investigate Danish endocrinologists’ use of thyroid hormones in hypothyroid and euthyroid patients.
Methods
An e-mail with an invitation to participate in an online survey investigating practices about substitution with thyroid hormones was sent to all members of the Danish Endocrine Society (DES).
Results
Out of 488 eligible DES members, a total of 152 (31.2%) respondents were included in the analysis. The majority (94.1%) of responding DES members use LT4 as the treatment of choice. Other treatment options for hypothyroidism are also used, as 58.6% prescribe combination therapy with liothyronine (LT3) + LT4 in their clinical practice. LT4 + LT3 combination is preferred in patients with persistent symptoms of hypothyroidism despite biochemical euthyroidism on LT4 treatment. Over half of the respondents answered that thyroid hormone therapy is never indicated for euthyroid patients, but 42.1% will consider it for euthyroid infertile women with high antibody levels. In various conditions that could interfere with the absorption of LT4, most responding Danish endocrinologists prefer tablets and do not expect a significant difference when switching from one type of tablet formulation to another.
Conclusion
The treatment of choice for hypothyroidism is LT4. Combination therapy with LT4 + LT3 is considered for patients with persistent symptoms. Even in the presence of conditions affecting bioavailability, responding Danish endocrinologists prefer LT4 tablets rather than newer LT4 formulations, such as soft-gel capsules.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-021-01555-y.
Keywords: Levothyroxine, Liothyronine, Hypothyroidism, Euthyroidism, Survey, Danish Endocrine Society
Introduction
Hypothyroidism is a common disease affecting approximately 3% of the European population [1]. Levothyroxine (LT4) is standard therapy for hypothyroidism and different types of LT4 formulations are available: tablets, soft-gel capsules, and liquid solutions. Soft-gel capsules and liquid solution are relatively new LT4 formulations and were manufactured to overcome some of the bioavailability issues in tablets. The bioavailability may be reduced when tablets are administered simultaneously with food and beverages, other types of medication (e.g., proton-pump inhibitors, phosphate binders, calcium carbonate, and iron supplements), or concurrent gastrointestinal conditions (e.g., Helicobacter pylori infection, coeliac disease, bariatric surgery, and atrophic gastritis) [2].
While all available LT4 formulations are regarded as effective in treating hypothyroidism, pre-existing morbidity is increased in hypothyroid individuals, and post-diagnosis co-morbidity from both somatic [3] and psychiatric [4] disorders is higher than in euthyroid controls. This eventually is accompanied by a higher rate of disability pension, loss of labor market income [5], and excess mortality from natural as well as unnatural causes [6, 7]. The aforementioned, together with the fact that quality of life is reported as still impaired, and may not be fully restored despite treatment with LT4 [8], is one among many factors leading to the pursuit of better treatment options for hypothyroid individuals. The evidence suggests differences in bioavailability in favor of liquid solution and soft-gel capsules compared to tablets both in patients with and without impaired absorption [2, 9, 10]. Soft-gel capsules and liquid solution are more expensive than tablets, and the cost-effectiveness of switching from tablets to these new formulations remains unsettled [11].
Before LT4 was synthesized, desiccated thyroid extract (DTE) was standard therapy. DTE is derived from the thyroid glands of animals and contains both T4 and T3. Combination therapy can also consist of the synthetic preparation of T3, liothyronine (LT3), and LT4. Thyroid guidelines uniformly favor LT4 as standard therapy, while attitudes diverge regarding combination therapy [12]. Some patients suffering from thyroid failure have persistent symptoms despite adequate thyroid hormone replacement therapy with LT4 [8]. According to current Danish and international guidelines, combination therapy with LT3 and LT4 may be considered in such individuals [13, 14].
LT4 prescriptions are increasing in Denmark [15]. The reasons for this include iodine fortification in food, leading to a higher incidence of thyroid autoimmunity [15, 16], a decreasing TSH threshold for initiating treatment [17], and increasing diagnostic activity [16, 17]. Likewise, combination therapy with LT4 + LT3 or DTE is also gaining popularity in Denmark [18], mostly due to growing demands from patients dissatisfied with the LT4 treatment [19, 20]. However, prescribing T3-containing preparations is not without potential risks. Side effects due to possible iatrogenic hyperthyroidism are a concern, and the extensive evidence for non-superiority of combination therapy over LT4 does not support this trend [12].
This Danish survey was part of an ongoing international incentive referred to as THESIS (Treatment of Hypothyroidism in Europe by Specialists: An International Survey). We aimed to investigate the use of thyroid hormones for hypothyroid and euthyroid patients by Danish endocrinologists. In Denmark, hypothyroid patients are managed in primary and secondary care using the different available LT4 formulations.
Method
The questionnaire was developed to evaluate European endocrinologists’ attitude regarding the treatment of hypothyroidism and substitution with thyroid hormones in euthyroid individuals. The survey was developed in English to ensure comparability between the participating European countries. Survey completion took 5–10 min. An open-access survey platform, Lime-Survey, was used to build and distribute the questionnaire, and a written introduction preceded the survey. Eight questions about demographic data (section A) were followed by twenty-three questions about treating hypothyroid and euthyroid patients (section B) (supplementary 1). Space for comments was available at the end.
An e-mail with an electronic link leading to the voluntary and anonymized questionnaire was sent to all members of the Danish Endocrine Society (DES) on 27th February 2020, followed by three reminders between February and May 2020, where after it was closed. Survey responses were collected and electronically stored by the Lime-Survey service, where the data was accessible by password. Repeat submissions from the same IP-address were automatically blocked.
Statistical analysis
Descriptive statistics were prepared for responses to all questions. Only respondents who had completed all questions about demographic data were considered valid for statistical analysis. In all analyses, respondents stating that they did not know the answer to a given question were pooled in the category with respondents who did not provide an answer. The goodness of fit χ2-test was used to compare frequencies between the categorical variables. Pearson’s χ2-test was used to test if variables in the demographic data (section A) were independent of the outcome in questions in section B. If any variable was not independent of the outcome in any question in section B, a logistic regression analysis was done. A two-sided p value of < 0.05 was considered statistically significant. All analyses were conducted using IBM SPSS statistics software version 25 (SPSS Inc., Chicago, IL, USA).
Results
Sample characteristics
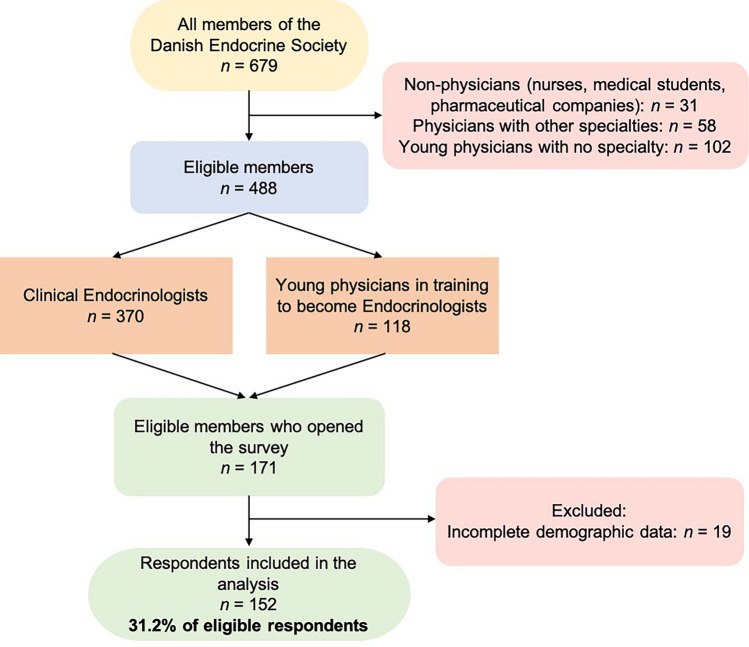
Figure 1 shows a flowchart of the DES members and the respondents. The demographic data of the respondents are compiled in Table 1. Besides being members of DES, two respondents (1.3%) are members of the American Thyroid Association (ATA), 26 (17.1%) are members of the European Thyroid Association (ETA), and 125 (82.2%) are members of additional Danish scientific societies.
Fig. 1.
Flowchart illustrating members of the Danish Endocrine Society and the Danish respondents of “Survey on Current Use of Levothyroxine in Europe”
Table 1.
Characteristics of the 152 Danish respondents of the questionnaire: “Survey on current use of levothyroxine in Europe”
| n (%) | |
|---|---|
| Sex | |
| Female | 67 (44.1) |
| Male | 85 (55.9) |
| Age in years | |
| 20–30 | 2 (1.3) |
| 31–40 | 33 (21.7) |
| 41–50 | 37 (24.3) |
| 51–60 | 48 (31.6) |
| 61–70 | 26 (17.1) |
| 70+ | 6 (3.9) |
| Years in medical practice | |
| 0–10 | 22 (14.5) |
| 11–20 | 48 (31.6) |
| 21–30 | 43 (28.3) |
| 31–40 | 34 (22.4) |
| 40+ | 5 (3.3) |
| Specialtya | |
| Endocrinology | 148 (97.4) |
| Internal medicine | 86 (56.6) |
| Othersb | 4 (2.6) |
| Place of employmenta | |
| University center | 98 (64.5) |
| Regional hospital | 62 (40.8) |
| Private clinic | 5 (3.3) |
| General practice | 0 (0) |
| Basic researcher | 0 (0) |
aThe sum of percentages exceeds 100% because some respondents had more than one specialty/employment
bOne endocrinologist was also accredited in family medicine. One respondent was a pediatric endocrinologist. Two respondents declared not to have a specialty yet (in training to become endocrinologists)
Twenty-four (15.8%) respondents treat thyroid patients rarely, while 52 (34.2%) do so daily, and 76 (50%) treat thyroid patients weekly. Forty-three (28.3%) members treat >100 hypothyroid patients annually, 63 (41.4%) care for 51–100 annually, 34 (22.4%) manage 10–50 per year, and only 12 (7.9%) members rarely treat hypothyroid patients.
Treating hypothyroid patients
Almost all respondents (94.1%) use LT4 as the first treatment of choice for patients with hypothyroidism. Two respondents (1.3%) favor combination therapy with LT4 + LT3, no respondents suggest DTE or LT3 monotherapy as the first treatment of choice, and seven did not provide an answer (LT4 vs. other thyroid hormone regimens; p < 0.001). Although combination therapy is not their first treatment of choice, 58.6% of the respondents may also prescribe LT4 + LT3, 2.6% DTE, and 23.0% LT3 in their daily clinical practice. The rest (4.6%) did not provide an answer.
Using different LT4 formulations
Most of the respondents (89.5%) indicate that the dispensed LT4 formulation is dictated by themselves, while only 2.0% indicate that they have no influence on this matter. Two respondents (1.3%) answer that general practitioners mostly choose the type of LT4 dispensed, while four (2.6%) respond that they do have control, still the type of LT4 dispensed requires justification to the regulatory authorities (LT4 dispensed as prescribed + justified by authorities vs. chosen by general practitioners + no control; p < 0.001).
In the survey, five questions explore the use of different LT4 formulations in specific situations (Table 2). Most Danish endocrinologists prefer LT4 tablets to soft-gel capsules or liquid LT4 for the treatment of hypothyroidism, and they do not expect any major difference when switching from one type of formulation to another. The same attitude applies to situations with interfering drugs, intolerance to various foods, unexplained poor biochemical control, or persistent symptoms despite reasonable biochemical control. Only a minority of responding DES members use the new LT4 formulations in situations with an expected lower absorption and reduced bioavailability of LT4 tablets (tablets + “no major change expected” vs. soft-gel capsules + liquid solution; p < 0.001).
Table 2.
Preferred levothyroxine formulations by members of the Danish Endocrine Society in different clinical situations
| I expect no major changes with the different formulations, n (%) | Tablets/tablets from another manufacturer, n (%) | Soft-gel capsules, n (%) | Liquid solutions, n (%) | Not sure/no answer, n (%) | ||
|---|---|---|---|---|---|---|
| B5. Interfering drugs may influence the stability of therapy. Which LT4 preparation is in your experience least likely to be subject to variable absorption? | 79 (52.0) | 41 (27.0) | 21 (13.8) | 2 (1.3) | 9 (5.9) | |
| B6. Which of the following preparations of LT4 would you prescribe in case of the first diagnosis of hypothyroidism when the patient self-reports intolerance to various foods raising the possibility of celiac disease, malabsorption, lactose intolerance, or intolerance to common excipients? | 28 (18.4) | 97 (63.8) | 14 (9.2) | 1 (0.7) | 12 (7.9) | |
| B7. Which of the following preparations of LT4 would you prescribe for a patient established on LT4 who has unexplained poor biochemical control of hypothyroidism? | 30 (19.7) | 87 (57.2) | 22 (14.5) | 0 (0) | 13 (8.6) | |
| B8. Which of the following preparations of LT4 would you prescribe for a patient with poor biochemical control who is unable (due to busy lifestyle) to take LT4 fasted and separate from food/drink? | 30 (19.7) | 69 (45.4) | 34 (22.4) | 1 (0.7) | 18 (11.8) | |
| B9. Which of the following preparations of LT4 would you prescribe for a patient established on LT4 tablets who have good biochemical control of hypothyroidism but continues to have symptoms? | 46 (30.3) | 68 (44.7) | 18 (11.8) | 1 (0.7) | 19 (12.5) | |
B# refers to the number of the question in the questionnaire
LT4 levothyroxine, n numbers
In a logistic regression, it was demonstrated that male physicians are 4.8 times more likely to indicate “tablets” or “no major change expected” than female physicians by answering the question “Which of the following preparations of LT4 would you prescribe for a patient established on LT4 tablets who have good biochemical control of hypothyroidism but continues to have symptoms?” (p = 0.005, question #B9 in Table 2). To the questions covering poor biochemical control of hypothyroidism, respondents without membership of either ETA or ATA are 3.6 times (p = 0.013, question #B7 in Table 2) and 2.9 times (p = 0.028, question #B8 in Table 2) more likely to stick to “tablets” or “no major change expected”, as compared to colleagues with a membership of one of these associations. In fact, respondents with an international affiliation have an equal preference for tablets, soft-gel capsules, and liquid solution in these two scenarios.
Monitoring thyroid hormone treatment
After initiating LT4 treatment, 109 (71.7%) DES members will re-check serum TSH after 4–6 weeks, and 13.2% after 8 weeks. A minority (1.3%) will re-check after only 2 weeks, one member will rely on clinical evaluation, and 20 (13.2%) did not provide an answer (2 weeks + 4–6 weeks vs. 8 weeks; p < 0.001).
Most respondents will re-check serum TSH after 4–6 weeks (55.9%) when switching to another formulation or changing to another manufacturer, and 39 (25.7%) will re-check after 8 weeks. If the dosage is unchanged, five members (3.3%) state that there is no need for TSH monitoring when switching from one preparation to another, two members (1.3%) rely on clinical evaluation, and 13.8% did not answer (4–6 weeks vs. 8 weeks; p < 0.001). The outcome in questions regarding monitoring does not depend on any demographic variable of the respondents such as sex, age, years in medical practice, membership of ETA/ATA, or the number of patients treated annually.
Treating euthyroid patients with thyroid hormones
This section explored physicians attitude towards prescribing thyroid hormone substitution to treatment-naïve euthyroid patients in different situations. 51.3% of the respondents state that thyroid hormone substitution in such patients is never indicated (never indicated vs. others; p = 0.56). 42.1% indicate that thyroid hormone substitution could be considered in female infertility with high levels of thyroid autoantibodies (indicated in female infertility vs. no indication; p = 0.12). 12.5% will also consider thyroid hormone substitution in patients with a simple goiter growing over time. Only a minority of respondents will consider such treatment in subjects with treatment-resistant depression (5.9%), unexplained fatigue (4.6%), obesity resistant to lifestyle interventions (3.9%), or severe hypercholesterolemia as a complementary treatment (2.6%). Five DES members (3.3%) did not respond to this question.
Combination therapy with LT4 and LT3
A majority of respondents (108; 71.1%) consider switching to combination therapy for patients treated with LT4 with normal serum TSH and persistent symptoms suggestive of hypothyroidism. Seventeen (11.2%) DES members will never use combination therapy due to the low quality of evidence. 2.6% will recommend it to patients with normal serum TSH and unexplained weight gain, while twenty-three (15.1%) did not provide an answer (combination therapy vs. “never use”; p < 0.001). No respondent considers combination therapy in patients recovering from protracted hypothyroidism. Similar to thyroid hormone monitoring, the outcome in questions regarding combination therapy does not depend on any of the demographic variables stated above.
Persistent symptoms in LT4 treated patients
Two (1.3%) DES members estimate that 11–30% of hypothyroid patients experience persistent symptoms despite biochemical euthyroidism on LT4 therapy, while some members (11.2%) claim that > 30% of the patients have persistent symptoms. The majority of the respondents estimate this fraction of patients to be 6–10% (n = 57, 37.5%) or less than 5% (n = 35, 23.0%); ≤ 10% vs. > 10%, p < 0.001). The remaining respondents (27.0%) are not sure. Eighty-nine (58.6%) answer that this trend has increased over the past five years, 21 (13.8%) cannot detect any change, 10 (6.6%) declare fewer such cases, and the rest (21.1%) are not sure/did not answer (more cases vs. fewer cases; p < 0.001). The outcome of these questions does not depend on any of the demographic variables stated above.
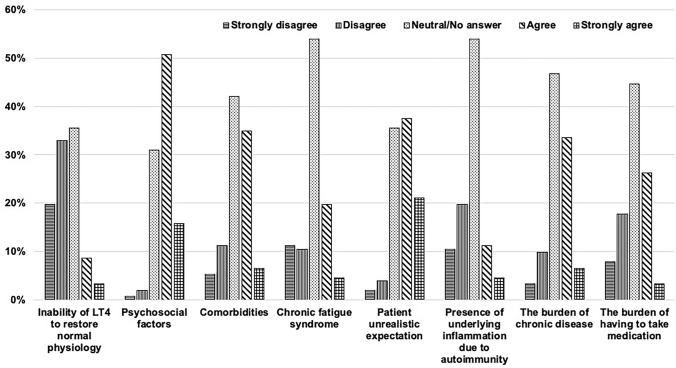
Due to the ongoing speculation on the causes of persistent hypothyroid symptoms, Danish endocrinologists were asked to comment on eight possible causes of this clinical condition (Fig. 2). While most respondents are neutral or did not answer, a few endocrinologists agree that persistent symptoms could be due to psychosocial factors, comorbidities, unrealistic expectations, chronic fatigue syndrome, inflammation due to autoimmunity, the burden of chronic disease, or the burden of taking medication. Only a minority state that symptom persistence might be due to LT4’s inability to restore normal physiology.
Fig. 2.
Danish endocrinologists’ speculation concerning possible factors explaining persistent symptoms of hypothyroidism despite biochemical euthyroidism in patients treated with LT4
Supplementation with selenium and iodine
The Danish endocrinologists are nearly equally divided into two categories regarding supplementation; 57 (37.5%) answer that supplementation with selenium or iodine could be used if requested by the patient, while 62 (40.8%) state that such supplementation should never be used. Ten respondents (6.6%) answer that selenium or iodine could be used in patients with co-existing autoimmune thyroiditis. Only one member (0.7%) recommends supplementation to patients with subclinical hypothyroidism, while twenty-two (14.5%) are not sure/did not provide an answer (“never use” vs. others; p = 0.599). The outcome in questions regarding supplementations does not depend on any of the demographic variables stated above.
Endocrinologists with hypothyroidism
Only two (1.3%) DES members who completed the survey are diagnosed with hypothyroidism. These two hypothyroid physicians are not experiencing excessive tiredness and declare they have not tried combination therapy with LT4 + LT3.
One-hundred-and-nine respondents claim not to be diagnosed with hypothyroidism; of these, 89 (81.7%) will not consider combination therapy, with either LT3 + LT4 or DTE in case of hypothyroidism. Twenty (18.3%) members might consider combination therapy, and 28.9% did not provide an answer to this question.
Discussion
Treatment with LT4
This survey among Danish endocrinologists confirms that LT4, in accordance with the Danish guidelines, is the treatment of choice for newly diagnosed hypothyroid patients [13]. Both tablets and soft-gel capsules are commercially available in Denmark. However, only one in five DES members would consider prescribing soft-gel capsules to patients for whom it is inconvenient to separate LT4 and food intake. In such a situation, some patients might benefit from switching to soft-gel capsules, although the evidence is weak [11]. Danish endocrinologists are also reluctant to use soft-gel capsules in other situations, e.g., interfering drug intake, gastrointestinal disease, poor biochemical control on tablets, or persistent symptoms despite biochemical control. The reason for this attitude is unknown, but probably more data on cost-effectiveness is needed to allow evidence-based use of these new LT4-formulations, as reviewed by Nagy et al. [11]. Interestingly, respondents with a membership of ETA and/or ATA are more prone to prescribe liquid solutions or soft-gel capsules, as compared to colleagues without such international affiliations.
In the Italian THESIS investigation [21], 75% recommended soft-gel capsules or liquid solution to patients established on LT4 but having poor biochemical control of their hypothyroidism. Interestingly, only 14.5% of the Danish endocrinologists agree with this strategy. Liquid thyroid hormone solutions are not available in Denmark, while soft-gel capsules have been on the Italian market for a longer time than in Denmark. Thus, Italian endocrinologists are probably more familiar with other formulations of LT4 than tablets. Other factors explaining why soft-gel capsules are more frequently prescribed in Italy than in Denmark could be differences in cost and marketing and the fact that most studies of efficacy, safety and bioavailability of soft-gel capsules have been conducted in Italy. Another hindrance for the more widespread use of soft-gel capsules in Denmark is the requirement for the Danish Medicines Agency to verify the indication for this formulation to allow reimbursement of expenses. Finally, the lack of evidence for or against the cost-effectiveness of soft-gel capsules compared to tablets may promote diverse practices.
Thyroid hormone therapy in euthyroid patients
In accordance with current guidelines, most respondents agree that thyroid hormone substitution is never indicated in treatment-naïve euthyroid patients. Two out of five Danish endocrinologists would consider thyroid hormone supplementation for infertile women with high levels of thyroid autoantibodies. According to current Danish guidelines, treatment with LT4 might be considered in some euthyroid anti-TPO positive women undergoing fertility treatment, in whom plasma TSH is within the upper normal reference range (i.e., 2.5–4.0 mIU/L). The rationale being to secure euthyroidism in case of pregnancy, although LT4 treatment neither promotes conception nor reduces the risk of pregnancy complications in such individuals [22].
Only one in ten DES members suggests treating a growing goiter with LT4. A previous questionnaire study demonstrated that the preferred treatment for nontoxic goiter in Denmark is radioiodine [23]. In addition, earlier studies have shown that only a minority of Danish patients with goiter are eligible for LT4 therapy [24]. Accordingly, current guidelines discourage its use [25].
Combination therapy with LT4 and LT3
According to Danish guidelines [13], a LT4 + LT3 treatment trial is suggested as an alternative to LT4 in patients with persistent symptoms of hypothyroidism despite stable biochemical control for at least six months, and after the exclusion of interfering conditions. If improvement is not achieved after 3–6 months, the combination therapy should be discontinued. DTE is not recommended [13].
In agreement with current guidelines, the majority of Danish endocrinologists prescribe LT4 + LT3 in selected cases [13], while a minority claimed not to use this treatment regimen due to low-quality evidence. Though genetic variants in deiodinases or thyroid hormone transporters are associated with variability in thyroid hormone plasma levels, their clinical importance in hypothyroid patients remains unsettled and controlled studies are needed to confirm these findings [14].
Interestingly, Danish endocrinologists seem more inclined to prescribe LT4 + LT3 combination therapy to patients with persistent symptoms (71%) than Italian (40%) [21] or Bulgarian (6%) endocrinologists [26]. This different attitude seems not to consider the risks related to the hazard of LT3 induced subclinical hyperthyroidism due to less stable plasma levels of thyroid hormones, which potentially leads to increased morbidity and mortality [27].
Finally, only a small minority of respondents consider the use of desiccated thyroid tablets. This approach appears appropriate as thyroid extracts, characterized by a high content of T3, have not shown better outcomes compared to synthetic LT4 in a randomized controlled trial [28].
Supplementation with iodine and selenium
The Danish guidelines offer no recommendation regarding supplementation with iodine or selenium (7). In line with the ambiguous evidence regarding such supplementation, the Danish endocrinologists are divided, stating that either dietary supplements should never be used or that they could be used at the request of the patients.
Mandatory iodine fortification of salt was implemented in Denmark in 2000 because of mild to moderate iodine deficiency. In some areas, iodine sufficiency was achieved [15], but the iodine fortification resulted in increasing levels of thyroid autoantibodies in the population and a higher incidence of hypothyroidism [29]. Thus, further iodine supplementation in hypothyroid patients in Denmark seems unjustified, unless there is overt iodine deficiency.
The evidence regarding selenium supplementation is more controversial. A recent review concluded that there is no evidence of clinical improvement by selenium supplementation in patients with autoimmune thyroiditis [30], despite an effect on thyroid autoantibodies [31]. The results from the Danish and Italian THESIS surveys indicate that selenium is recommended to patients with hypothyroidism by some endocrinologists, regardless of the selenium status of the patient, and without sufficient evidence to support this approach.
Treatment of physicians with hypothyroidism
Most respondents indicated that they would not consider combination therapy for themselves if they were to develop hypothyroidism, primarily based on lack of evidence. Those considering combination therapy would prefer LT4 + LT3, while DTE is rejected due to the non-physiologically high T3:T4-ratio. Interestingly, 71% of the respondents would prescribe combination therapy for their patients, but only 20% would consider this treatment for themselves.
Strengths and limitations
The strength of this survey is the relatively high response rate, as compared to similar others [21]. We excluded physicians with other specialties than endocrinology to focus on those who manage thyroid patients regularly. In Denmark, a large proportion of endocrinologists manage only patients with diabetes. By circulating the questionnaire to all Danish Endocrine Society members, we obtained 152 responses (31.2% response rate), but also targeted many who do not see such patients. Most DES members who are clinically active within the thyroid field are organized within the Danish Thyroid Association, comprising 115 members. The fact that the number of respondents was considerably higher (i.e. 152) indicates that most endocrinologists, being de facto eligible for this survey, were included. Thus, the response rate may be well above 31.2%, taking the relevant target group into account.
Limitations include the virtual patient cases with loss of nuances potentially interfering with the interpretation. The fact that less than a third of eligible clinicians completed the survey questions the generalizability of conclusions to that of all Danish endocrinologists. Our questionnaire was distributed at the beginning of the Covid-19 pandemic, which might have impacted response rates negatively.
In conclusion, in Denmark the treatment of choice for hypothyroidism is LT4 tablets, also when conditions affecting bioavailability are present. Combination therapy with LT4 + LT3 is considered for patients treated with LT4 with persistent symptoms and stable TSH within the reference range, while only a few DES members prescribe DTE. Notably, the outcome in questions regarding treatment and monitoring does not generally depend on the demographic variables of the respondents, with the exception of the use of soft-gel capsules. LT4 treatment of infertile euthyroid women harboring thyroid antibodies and a wish for pregnancy is still considered by a substantial fraction of Danish endocrinologists. The attitude toward selenium and iodine supplementation in patients with hypothyroidism remains largely controversial.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all members of the Danish Endocrine Society, who contributed to the study by answering the questionnaire.
Author contributions
EVN, EP, PP, RN, RA, and LH contributed to the study conception, design and creation of the questionnaire. JSF was responsible for material preparation and data collection. KRR and SJB were responsible for data evaluation and analysis. KRR wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The first author is supported by a Ph.D. scholarship funded by the Danish Medicines Agency and the Region of Southern Denmark. The survey investigation, THESIS (Treatment of Hypothyroidism in Europe by Specialists: an international Survey), is supported by IBSA Institute Biochimique SA.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available but will be provided by the corresponding author upon reasonable request.
Declarations
Conflict of interest
EVN, EP, PP, and LH have undertaken consultancy work for IBSA. All other authors have no conflicts of interest to declare that are relevant to the content of this article. IBSA had no role in the design of the survey, data analysis, data presentation, data interpretation, or writing the manuscript; the authors did not receive remuneration by IBSA.
Ethics approval
Ethical approval was not required.
Consent to participate
A signed informed consent was not required from participants in this anonymous and voluntary questionnaire survey.
Consent for publication
Not applicable.
Footnotes
THESIS: Treatment of Hypothyroidism in Europe by Specialists: An International Survey.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Madariaga AG, Santos Palacios S, Guillén-Grima F, et al. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99(3):923–931. doi: 10.1210/jc.2013-2409. [DOI] [PubMed] [Google Scholar]
- 2.Fallahi P, Ferrari SM, Ruffilli I, et al. Advancements in the treatment of hypothyroidism with L-T4 liquid formulation or soft gel capsule: an update. Expert Opin Drug Deliv. 2017;14(5):647–655. doi: 10.1080/17425247.2016.1227782. [DOI] [PubMed] [Google Scholar]
- 3.Thvilum M, Brandt F, Almind D, et al. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. A nationwide register study. PLoS ONE. 2013;8(9):1–6. doi: 10.1371/journal.pone.0075789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thvilum M, Brandt F, Almind D, et al. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid. 2014;24(5):802–808. doi: 10.1089/thy.2013.0555. [DOI] [PubMed] [Google Scholar]
- 5.Thvilum M, Brandt F, Brix TH, et al. Hypothyroidism is a predictor of disability pension and loss of labor market income: a Danish register-based study. J Clin Endocrinol Metab. 2014;99(9):3129–3135. doi: 10.1210/jc.2014-1407. [DOI] [PubMed] [Google Scholar]
- 6.Thvilum M, Brandt F, Almind D, et al. Excess mortality in patients diagnosed with hypothyroidism: a nationwide cohort study of singletons and twins. J Clin Endocrinol Metab. 2013;98(3):1069–1075. doi: 10.1210/jc.2012-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiberg Brix T, Ferløv-Schwensen C, Thvilum M, et al. Death by unnatural causes, mainly suicide, is increased in patients with Hashimoto’s thyroiditis, a nationwide Danish register study. Endocrine. 2019;65(3):616–622. doi: 10.1007/s12020-019-01946-5. [DOI] [PubMed] [Google Scholar]
- 8.Winther KH, Cramon P, Watt T, et al. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS ONE. 2016;11(6):e0156925. doi: 10.1371/journal.pone.0156925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimboli P, Scappaticcio L, De Bellis A, et al. Different formulations of levothyroxine for treating hypothyroidism: a real-life study. Int J Endocrinol. 2020;2020:4–9. doi: 10.1155/2020/4524759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virili C, Trimboli P, Romanelli F, et al. Liquid and softgel levothyroxine use in clinical practice: state of the art. Endocrine. 2016;54:3–14. doi: 10.1007/s12020-016-1035-1. [DOI] [PubMed] [Google Scholar]
- 11.Nagy EV, Perros P, Papini E, et al. New formulations of levothyroxine in the treatment of hypothyroidism: trick or treat? Thyroid. 2020;31:1–27. doi: 10.1089/thy.2020.0515. [DOI] [PubMed] [Google Scholar]
- 12.Kraut E, Farahani P. A systematic review of clinical practice guidelines’ recommendations on levothyroxine therapy alone versus combination therapy (LT4 plus LT3) for hypothyroidism. Clin Invest Med. 2015;38(6):E305–E313. doi: 10.25011/cim.v38i6.26194. [DOI] [PubMed] [Google Scholar]
- 13.Bjergved Sigurd L, Karmisholt J, Ryom Riis K et al (2020) Hypothyroidisme—Danish Endocrine Societies [Internet]. https://endocrinology.dk/. 2020. Available from: https://endocrinology.dk/nbv/thyroideasygdomme/hypothyroidisme/
- 14.Wiersinga WM, Duntas L, Fadeyev V, et al. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira C, Knudsen N, Ovesen L, et al. Doubling in the use of thyroid hormone replacement therapy in Denmark: association to iodization of salt? Eur J Epidemiol. 2011;26(8):629–635. doi: 10.1007/s10654-011-9590-5. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen IB, Laurberg P, Knudsen N, et al. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007;92(8):3122–3127. doi: 10.1210/jc.2007-0732. [DOI] [PubMed] [Google Scholar]
- 17.Medici BB, Nygaard B, La Cour JL, et al. Changes in prescription routines for treating hypothyroidism between 2001 and 2015: an observational study of 929,684 primary care patients in Copenhagen. Thyroid. 2019;29(7):910–919. doi: 10.1089/thy.2018.0539. [DOI] [PubMed] [Google Scholar]
- 18.Michaelsson LF, Medici BB, la Cour JL, et al. Treating hypothyroidism with thyroxine/triiodothyronine combination therapy in Denmark: following guidelines of following trends? Eur Thyroid J. 2015;4:174–180. doi: 10.1159/000437262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. 2018;28(6):707–721. doi: 10.1089/thy.2017.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell AL, Hegedüs L, Žarković M, et al. Patient satisfaction and quality of life in hypothyroidism: an online survey by the british thyroid foundation. Clin Endocrinol. 2020;94:513–520. doi: 10.1111/cen.14340. [DOI] [PubMed] [Google Scholar]
- 21.Negro R, Attanasio R, Nagy EV, et al. Use of thyroid hormones in hypothyroid and euthyroid patients; the 2019 Italian Survey. Eur Thyroid J. 2020;9:25–31. doi: 10.1159/000502057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Zhang Y, Tan H, et al. Effect of levothyroxine on pregnancy outcomes in women with thyroid autoimmunity: a systematic review with meta-analysis of randomized controlled trials. Fertil Steril. 2020;37:1–8. doi: 10.1016/j.fertnstert.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Bonnema SJ, Bennedbæk FN, Wiersinga WM, et al. Management of the nontoxic multinodular goitre: a European questionnaire study. Clin Endocrinol. 2000;53(1):5–12. doi: 10.1046/j.1365-2265.2000.01060.x. [DOI] [PubMed] [Google Scholar]
- 24.Fast S, Bonnema SJ, Hegedüs L. The majority of Danish nontoxic goitre patients are ineligible for levothyroxine suppressive therapy. Clin Endocrinol. 2008;69(4):653–658. doi: 10.1111/j.1365-2265.2008.03241.x. [DOI] [PubMed] [Google Scholar]
- 25.Gharib H, Papini E, Garber JR, et al. American association of Clinical Endocrinologists, American college of endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr Pract. 2016;22(May):1–60. doi: 10.4158/EP161208.GL. [DOI] [PubMed] [Google Scholar]
- 26.Borissova A, Boyanov MA, Attanasio R, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS* questionnaire survey of Bulgarian physicians. *THESIS: Treatment of Hypothyroidism in Europe by Specialists: an International Survey. Endocrinologia. 2020;25(4):299–309. [Google Scholar]
- 27.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, et al. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid. 2018;28(5):566–574. doi: 10.1089/thy.2017.0517. [DOI] [PubMed] [Google Scholar]
- 28.Hoang TD, Olsen CH, Mai VQ, et al. Dessicated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):1982–1990. doi: 10.1210/jc.2012-4107. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen IB, Knudsen N, Carlé A, et al. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol. 2011;75(1):120–126. doi: 10.1111/j.1365-2265.2011.04008.x. [DOI] [PubMed] [Google Scholar]
- 30.Winther KH, Rayman MP, Bonnema SJ, et al. Selenium in thyroid disorders—essential knowledge for clinicians. Nat Rev Endocrinol. 2020;16(3):165–176. doi: 10.1038/s41574-019-0311-6. [DOI] [PubMed] [Google Scholar]
- 31.Wichman J, Winther KH, Bonnema SJ, et al. Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta-analysis. Thyroid. 2016;26(12):1681–1692. doi: 10.1089/thy.2016.0256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available but will be provided by the corresponding author upon reasonable request.